ABSTRACT
We investigated the amyloidogenic potential of inactivated vaccines and the localized production of serum amyloid A (SAA) at the injection site in white layer chickens. Hens in the treated group were injected intramuscularly three times with high doses of inactivated oil-emulsion Salmonella Enteritidis vaccine and multivalent viral and bacterial inactivated oil-emulsion vaccines at two-week intervals. Chickens in the control group did not receive any inoculum. In the treated group, emaciation and granulomas were present, while several chickens died between 4 and 6 weeks after the first injection. Hepatomegaly was seen at necropsy, and the liver parenchyma showed inconsistent discolouration with patchy green to yellowish-brown areas, or sometimes red-brown areas with haemorrhage. Amyloid deposition in the liver, spleen, duodenum, and at injection sites was demonstrated using haematoxylin and eosin staining, Congo red, and immunohistochemistry. The incidence of chicken amyloid A (AA) amyloidosis was 47% (28 of 60) in the treated group. In addition, RT-PCR was used to identify chicken SAA mRNA expression in the liver and at the injection sites. Furthermore, SAA mRNA was detected by in situ hybridization in fibroblasts at the injection sites, and also in hepatocytes. We believe that this is the first report of the experimental induction of systemic AA amyloidosis in white layer chickens following repeated inoculation with inactivated vaccines without the administration of amyloid fibrils or other amyloid-enhancing factors.
Introduction
Amyloidosis is a general term for diseases associated with local or systemic extracellular deposition of misfolded insoluble proteins that by electron microscopy contain non-branching fibrils (Cohen & Calkins, Citation1959) with a size of 7–10 nm (Glenner, Citation1980). Amyloid fibrils show a high content of β-pleated sheets (Eanes & Glenner, Citation1968), but also have α-helices and random coils (Turnell et al., Citation1986). Based on the precursor protein and clinical signs, 36 extracellular fibril proteins that may cause amyloidosis have been described in humans, but only nine of them have been recognized in animals (Sipe et al., Citation2016). Amyloid A (AA) amyloidosis is a systemic amyloidosis which is most frequently found in animals and the only type found in birds (Guo et al., Citation1996). It is frequently associated with chronic inflammatory diseases (Pérez-Villa et al., Citation1989), and its precursor protein is serum amyloid A (SAA) (Benditt & Eriksen, Citation1977; Hoffman et al., Citation1984).
The synthesis and secretion of SAA are stimulated by several cytokines, mainly interleukin-1, interleukin-6, and tumour necrosis factor-α, in response to inflammation (Betts et al., Citation1993; Husby et al., Citation1994). SAA concentration in plasma may increase by up to 1000-fold within 24–48 h of trauma, infection, inflammation, or neoplasia (McAdam & Sipe, Citation1976; Meek & Benditt, Citation1986; Epstein et al., Citation1999).
Some chronic inflammatory or neoplastic diseases in animals stimulate stable high levels of SAA in the plasma, making them susceptible to development of AA amyloidosis (Johan et al., Citation1998; Landman et al., Citation1998). The diagnosis of AA amyloidosis required identification of amyloid deposits in the affected tissues (Benson & Cohen, Citation1979; Woldemeskel, Citation2012).
SAA is a high-density lipoprotein-associated apolipoprotein in the plasma, which is divided into two types (Whitehead et al., Citation1992), acute phase SAA (A-SAA) which is mainly made by hepatocytes during the acute phase response, and constitutive SAA (C-SAA) which is associated with high-density lipoprotein under normal conditions. The level of C-SAA in plasma does not change significantly during the acute phase response (Whitehead et al., Citation1992; Husby et al., Citation1994). Only one type of SAA, which corresponds to the mammalian A-SAA (chicken SAA), has been described in birds (Guo et al., Citation1996). SAA can be produced in vivo and in vitro in hepatocytes and some other cell types such as rheumatoid arthritis synoviocytes, fibroblasts, macrophages, endothelial cells, epithelial cells of several normal tissues such as breast lobule, colon mucosa, prostate gland, kidney tubules, and lung (Upragarin et al., Citation2005a).
AA amyloidosis in brown layer hens is well known as an amyloid arthropathy, which is associated with infection by Enterococcus faecalis (Landman et al., Citation1994, Citation1997; Landman, Citation1999). In spontaneous outbreaks of AA amyloidosis in flocks, the morbidity rate is between 1 and 4% but may increase to 20% (Landman et al., Citation1994). Other researchers have reported that the mortality rate in flocks can be up to 2% (Murakami et al., Citation2013a; Ibi et al., Citation2015). Bacterial oil-emulsion vaccines have been associated with systemic amyloidosis in chickens, and they are strongly suspected as a cause of outbreaks in flocks but this has not yet been confirmed experimentally (Von Rampin et al., Citation1989).
AA amyloidosis can be induced experimentally by repeated inflammatory stimulations such as a mixture of killed malaria parasites and Freund’s adjuvant (Thompson et al., Citation1947), Staphylococcus aureus (MacDonald & Carlson, Citation1969), Mycoplasma synoviae (Maestrini & Pascucci, Citation1970), Escherichia coli and crude endotoxin (Ling et al., Citation1991), crude extracts of E. coli and Salmonella Typhimurium (Ling, Citation1992), Salmonella Pullorum (Wang & Di, Citation1992), E. faecalis (Landman et al., Citation1997), methylcholanthrene (Rigdon, Citation1960), and azo-casein (Druet & Janigan, Citation1966). Recent research has shown that chickens can develop AA amyloidosis at any age (Ibi et al., Citation2015).
The main organs involved in amyloid deposition in chickens are liver and spleen, but depositions have also been reported in the intestines, kidney, heart, gonads, endocrine organs, brain, lungs, and skin (Landman et al., Citation1998). Deposition of amyloid fibrils at the injection site in vaccinated chickens has also been reported (Murakami et al., Citation2013a; Murakami et al., Citation2013b; Ibi et al., Citation2015).
In this study, the amyloidogenic potential of viral and bacterial inactivated oil-emulsion vaccines in white layer hens and the localized production of SAA at the injection site were investigated. Hereto, we inoculated white layers with commercial inactivated oil-emulsion Salmonella Enteritidis vaccine (IOSEV) and commercial multivalent viral and bacterial inactivated oil-emulsion vaccine without administration of any additional amyloid-enhancing factors such as amyloid fibrils or other chemicals.
Material and methods
Experimental birds and housing
Sixty-eight 43-day-old commercial female Julia LSL layer chickens (Lohmann Tierzucht™, Cuxhaven, Germany) that had been vaccinated according to the vaccination programme () of the provider farm (Akagi Multifood Service™, Miyazaki, Japan) were used in this experiment. Chickens were placed in adjacent cages within the same house under a 12 h light:12 h dark lighting cycle. The chickens were fed ad libitum on commercial layer food (Powerchick ZK™, Japan Agricultural Cooperatives, Japan) and fresh tap water was provided.
Table 1. Vaccination schedule of the chickens before being used in the experiment presented in this studya.
Vaccine
In this experiment, two types of commercially available inactivated oil-emulsion vaccines were used: (1)-IOSEV (made by company A) which includes strain 54 (2 × 108 colony-forming units (CFU)/dose), strain 25 (1 × 108 CFU/dose), strain 22 (2 × 108 CFU/dose), and strain 41 (1 × 108 CFU/dose and (2) multivalent viral and bacterial inactivated oil-emulsion vaccine (made by company B) which includes Newcastle disease (Ishii strain 1011.4 EID50/1000 doses), infectious bronchitis (Nerima E10 strain containing 109.4 EID50/1000 doses and TM-86EC strain containing 109.4 EID50/1000 doses), egg drop syndrome-1976 (KE-80 strain containing 109.7 TCID50/1000 doses), M. gallisepticum (containing 1010.4 CFU/1000 doses), and infectious coryza (type A strain 221 containing 1010.7 CFU/1000 doses and type C strain 53–47 containing 1011.3 CFU/1000 doses).
Experimental design
The chickens did not receive any medicines or vaccines between 40 and 70 days of age. At 70 days of age the chickens were divided into two groups. The treated group of 60 chickens received four doses (1 ml) of IOSEV and four doses (2 ml) of multivalent viral and bacterial inactivated oil-emulsion vaccine. The injections into the right and left pectoral muscles were repeated three times at two-week intervals at 70, 91, and 112 days of age, respectively. Eight control chickens did not receive any inoculum.
Bodyweight was measured biweekly for up to 10 weeks after the first injection. All procedures used in this experiment were approved by the Animal Care Committee of the University of Miyazaki, Japan (Experimental number: 2014-024-2).
Post-mortem and histopathology
Half of the surviving chickens were sacrificed at 126 days of age and the remaining half at 140 days of age. Liver, spleen, duodenum, and pectoral muscle samples were collected from all chickens, fixed in 10% neutral buffered formalin or 4% paraformaldehyde (PFA) for two days, and then embedded in paraffin using standard methods. Tissue sections were cut at 4 μm for haematoxylin and eosin (HE) staining, immunohistochemistry (IHC), and in situ hybridization (ISH), and 8 μm for Congo red staining. IHC was performed using an EnVision™+ Dual Link System-HRP (Dako™, Tokyo, Japan). The primary antibodies were as follows: mouse anti-human AA amyloid monoclonal antibody (MX-AA) (Kyowa™, Tokyo, Japan; 1:1000 dilution), anti-Iba-1 polyclonal antibody (Wako Chemicals USA Inc.™, Richmond, VA; 1:250 dilution), and monoclonal mouse anti-vimentin (Dako™; ready to use). The antibodies were diluted with antibody diluent (Dako REAL™).
SAA mRNA expression and ISH
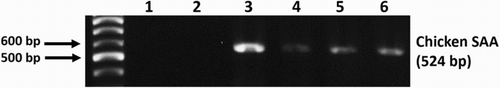
The expression of chicken SAA mRNA was examined by RT-PCR. RNA from fresh samples was extracted using TRIzol® Reagent (Ambion™, Foster City, CA). The chicken SAA mRNA primer sequences were: Sense (5'-GGGGCTTCACTTCCACCTGACCTCC-3') and antisense (5'-CCAAACGCAGCAGTTTCTTTATTGGGC -3') (Upragarin et al., Citation2005a). RT-PCR was performed using an AccessQuick™ RT-PCR System (Promega™, Madison, WI, USA). RT-PCR for chicken SAA mRNA for a 524-bp (Upragarin et al., Citation2005a) product was carried out with 1 cycle of reverse transcription at 45°C for 45 min, inactivation at 94°C for 2 min, 40 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 1 min, and a final incubation at 72°C for 5 min.
An antisense complementary (c) RNA probe specific for chicken SAA mRNA was synthesized from the RT-PCR product as previously described (Shin & Molitor, Citation2002). A digoxigenin-labelled cRNA probe was prepared using a commercial RNA labelling kit (Roche™, Tokyo, Japan). ISH was performed as previously described with slight modifications (Tanizaki et al., Citation2006; Trang et al., Citation2014, Citation2015). Briefly, after deparaffinization and rehydration, the sections were digested with 10 μg/ml of proteinase K at 37°C for 30 min, post-fixed in 4% paraformaldehyde, washed in three changes of phosphate buffer (PB), treated with 0.2 N HCl, and acetylated with 0.25% acetic anhydride in 0.1 M triethanolamine (pH 8.0) for 10 min each. Sections were then treated with 3% H2O2 for 1 h at room temperature, dehydrated, and air-dried. Each section was hybridized with 50 µl of hybridization solution (Maxim Biotech Inc.TM, Rockville, MD, USA) containing 50 ng cRNA probe for 16–18 h at 50°C. After hybridization, the sections were immersed in two changes of 50% formamide/2 × SSC (Saline sodium citrate buffer) for 1 h at 60°C, followed by washing in 1 × buffer containing 1 mM Tris aminomethane (pH 7.5), 0.5 M NaCl, and 1 mM EDTA for 10 min at 37°C. The sections were washed sequentially in 2 × SSC, 0.2 × SSC, and 0.1 × SSC for 40 min each at 60°C. Sections were incubated with 0.5% casein at room temperature for 10 min. The biotinyl–tyramide signal was amplified by serial application of 1:400 diluted anti-digoxigenin horseradish peroxidase (Roche™), 0.07 μM biotinylated tyramide solution (Kerstens et al., Citation1995), and 1:500 diluted streptavidin antibody for 15 min each at room temperature.
After each incubation period, the sections were washed three times with 0.01 M Tris–HCl (pH 7.5), 300 mM NaCl, and 0.5% Tween-20 for 5 min. Finally, colour was developed using liquid DAB+ substrate chromogen system (Dako™) and sections were counterstained with haematoxylin, dehydrated through a graded series of ethanol, cleared with three changes of xylene, and mounted using a mounting solution.
Results
Post-mortem and histopathology
There were significant differences in the average bodyweights of chickens in the treated group compared to the control group (). Within the experimental period, 12 chickens died at 98, 99, 100, 105, 108, 109, 111, 114, 118, and 136 days of age. In the control group, the pectoral muscle was clear and normal. However, in all of the treated chickens (60/60), there was moderate to severe swelling with oedema, abscessation, and presence of granuloma-like structures and petechiae, which changed the colour of the injected muscles to yellowish-white ().
Figure 1. Injection site (pectoral muscle) of a treated chicken. Note the moderate to severe swelling, the presence of granuloma-like structures and petechiae.
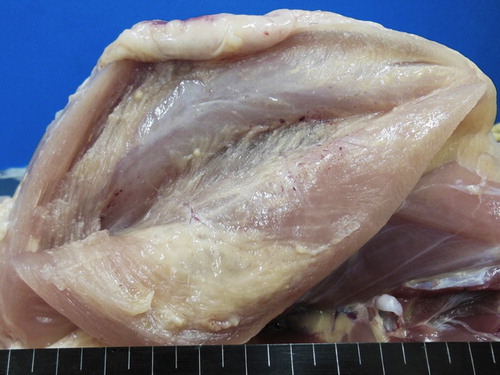
Table 2. Clinical and gross findings of experimental AA amyloidosis in chickens.
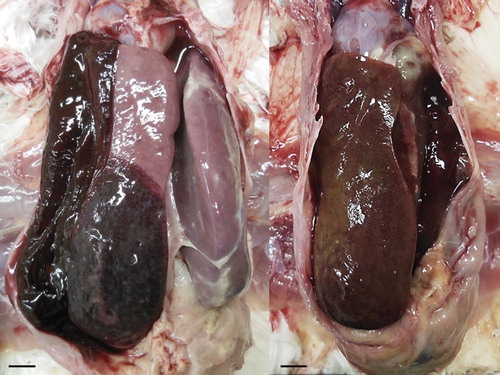
The liver of the treated chickens appeared firm, friable, and the capsular surface was smooth. In nine of the 60 treated chickens, severe hepatomegaly was present and hepatic parenchyma showed inconsistent discolouration with patchy green to yellowish-brown areas, or sometimes red-brown areas with haemorrhage. Haemorrhage was observed in the hepatoperitoneal sac in some of these chickens because the liver had ruptured ().
Figure 2. Livers of treated chickens. Severe hepatomegaly and haemorrhages are present and hepatic parenchyma showed inconsistent discolouration with patchy green to yellowish-brown areas, or sometimes red-brown areas; scale bar = 1 cm.
Intestinal haemorrhage was observed in four of the treated chickens and, additionally, some showed different degrees of splenomegaly. Another notable finding was undeveloped ovaries in the treated group. Ovaries were developed and active in the control group with mature eggs present in the cloaca whereas they were not developed in the treatment group. Lameness or arthritis was not observed and other visceral organs were not visibly affected.
HE staining of liver sections from the treated chickens revealed atrophy and separation of hepatocytes due to amyloid deposition in the perisinusoidal space of Disse, and the hepatic cords had disappeared ((a)). Amyloid deposition in the space of Disse was confirmed by Congo red staining and IHC ((b)). In addition, vacuolation of hepatocytes, multifocal coagulative necrosis, lymphocyte and plasma cell infiltration, haemorrhage, and deposition of hyaline material were also observed ().
Figure 3. (a) Histopathological changes in the liver of a treated chicken showing amyloid deposition in the space of Disse and hepatic cord atrophy. HE stain. (b) Amyloid shown in the space of Disse in a liver section of a treated chicken. Immunohistochemistry with MX-AA antibodies. (c) SAA mRNA appears strongly positive in hepatocytes of a treated chicken. In situ hybridization.
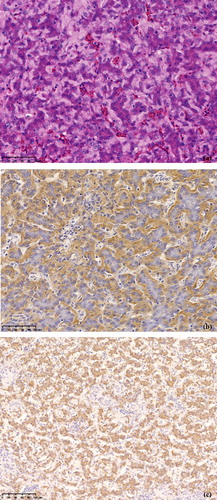
Table 3. Distribution and severity of amyloid deposition in affected chickens, vaccinated with high doses of inactivated oil-emulsion vaccines.
Lesions in the pectoral muscles were associated with the sites of injections. They consisted of granulomatous inflammation with an eosinophilic oil adjuvant component, surrounded by macrophages, multinucleated giant cells, plasma cells, lymphocytes, and heterophils with fibrosis ((a)). Oil components of the inactivated vaccines were separated and scattered in the interstitium of the injection sites. In addition, muscle at the injection sites had degenerated and amyloid fibrils were detected by IHC ((b)). In the Congo red-stained slides, amyloid appeared as apple-green birefringence under a polarized light microscope ((c)).
Figure 4. (a) Large vacuoles likely representing remnants of inactivated oil-emulsion vaccine surrounded by macrophages, multinucleated giant cells, plasma cells, lymphocytes, and heterophils with fibrosis. HE stain. (b) Amyloid shown in the surrounding of the large vacuoles likely representing remnants of inactivated oil-emulsion vaccine at the injection sites of treated chickens. Immunohistochemistry with MX-AA antibodies. (c) Amyloid appears as apple-green birefringence in a tissue sample of the injection site of a chicken when viewed with polarized light under a light microscope. Congo red stain. Scale bar = 100 μm. (d) SAA mRNA is abundantly shown in the periphery of granulomas at the injection site in treated chickens. In situ hybridization. (e) High magnification of (d) shows that the positive signals are detected in spindle-shaped cells. In situ hybridization.
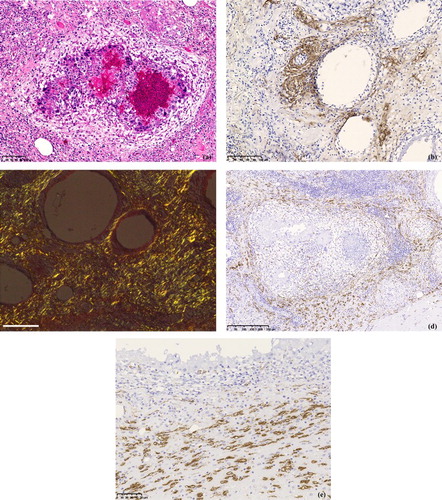
Congo red staining and IHC confirmed that amyloid was deposited in the vessel walls and lamina propria of the duodenum and it was observed in the vessel walls, trabeculae, and interstitial tissue in the spleen. Chickens in the control group did not show any macroscopic or microscopic abnormalities.
SAA mRNA expression and ISH
Chicken SAA mRNA was continuously detected in the pectoral muscles () and livers of the treated group by RT-PCR. In the ISH sections, SAA mRNA was shown to be expressed in hepatocytes ((c)) and in the spindle-shaped cells in the peripheral part of granulomas of the pectoral muscle of the treated chickens ((d) and (e)). IHC results in the target cells were positive for vimentin, and negative for Iba-1 antibody.
Discussion
Brown layer chickens have been shown to be more susceptible to AA-amyloidosis due to a shifted T-helper-cell response (Zekarias et al., Citation2000). There are several studies related to amyloid arthropathy in brown layers (Landman et al., Citation1994; Landman et al., Citation1997; Landman, Citation1999) and broiler breeders (Steentjes et al., Citation2002) whereas it has never been reported in white layer chickens. After the spontaneous outbreak of AA amyloidosis in a white layer flock, AA amyloidosis was recently induced experimentally in white layer chickens (comprising four breeds: Julia, Julia-Lite, P2, and Line-M) by inoculating them with two doses of inactivated Salmonella Enteritidis vaccine and chicken amyloid fibrils as amyloid-enhancing factors, orally, and intravenously (Murakami et al., Citation2013b). However, AA amyloidosis was not induced in chickens that did not receive amyloid fibrils and amyloid arthropathy was not observed. In our study, systemic AA amyloidosis was successfully induced in white layer chickens by inoculation of viral and bacterial inactivated oil-emulsion vaccines alone. Twelve of the treated chickens died within the experimental period and necropsy showed a severe hepatomegaly in nine of them. Rupture of the liver and haemorrhage in the hepatoperitoneal sac were observed in six of these birds. In another three dead chickens, amyloid deposition in the liver, spleen, duodenum, and pectoral muscles was confirmed by Congo red and IHC, and overall, the incidence of AA amyloidosis was 47% (28/60). However, swelling and arthritis of the hock joint, a characteristic feature of amyloid arthropathy in chickens, were not observed in any of the chickens in this experiment. We believe that this is the first study to show the experimental induction of systemic AA amyloidosis in white layer chickens by inoculation with viral and bacterial inactivated oil-emulsion vaccines without administration of additional amyloid fibrils or any other chemicals as amyloid-enhancing factors.
We conclude that, as reported previously by others, induction of AA amyloidosis requires repeated antigenic stimulations. In addition, AA amyloidosis was observed 3–4 weeks after vaccination in spontaneous outbreaks (Shibatani et al., Citation1984; Von Rampin et al., Citation1989; Murakami et al., Citation2013a). In this study, the injections were repeated three times at two-week intervals and the chickens were sacrificed at 4 and 6 weeks after the last injection. The repeated antigen stimulations and sustained chronic inflammatory reactions successfully induced AA amyloidosis in this study. It can be concluded that viral and bacterial inactivated oil-emulsion vaccines have enough amyloidogenic potential to cause an AA amyloidosis outbreak in a flock.
Extrahepatic production of SAA in chickens has only been confirmed in synoviocytes and in the vessel walls of the synovium in chickens that suffered from amyloid arthropathy to date (Ovelgönne et al., Citation2001) and in vitro, it can be produced in primary cultures of chicken synoviocytes (Upragarin et al., Citation2002). In this study, ISH revealed that SAA mRNA was expressed in the spindle-shaped cells in the peripheral part of granulomas at the injection sites of the treated chickens. Based on the morphology of the positive cells and the IHC results, ISH-positive cells were considered to be fibroblasts. This is the first report of extrahepatic production of chicken SAA in fibroblasts other than synoviocytes and vessel walls of the synovium in chickens with amyloid arthropathy. In addition, IHC and Congo red staining confirmed amyloid deposition at the injection sites even in chickens without systemic amyloidosis. Other researchers have suggested that the localized production of SAA may stimulate amyloid formation and deposition at the site of production (Upragarin et al., Citation2005b; Murakami et al., Citation2013b). Our results strongly support this hypothesis.
Amyloid deposits have been found in the livers of market chickens indicating that amyloid contaminated organs and meat may be entering the food chain (Ishiguro et al., Citation2014). Transmission of amyloidosis from animals to humans may be possible as it has been shown previously that AA-amyloidosis can be transmitted orally within and between species (Murakami et al., Citation2013a, Citation2013b, Citation2014) or similar to prion diseases, through a seeding-nucleation process (Murakami et al., Citation2015). Transmission of amyloidosis from animals to humans has been a concern in recent years and although it has not yet been confirmed, it should be considered a public health issue (Benditt & Eriksen, Citation1977; Higuchi, Citation2013; Murakami et al., Citation2013a; Murakami et al., Citation2013b; Ibi et al., Citation2015). Amyloid fibril deposition was detected in the liver, spleen, duodenum, and at the injection sites as reported previously (Murakami et al., Citation2013a, Citation2013b; Citation2015). Amyloid deposition in the ovaries of humans (Mount et al., Citation2002), mice (Frith & Chandra, Citation1991), and cows (Yamada et al., Citation2006) has been confirmed in previous studies but was not examined in this study. It should certainly be investigated in future studies.
Haemorrhage and amyloid deposition in the intestines of four of the 60 treated chickens were observed and were also reported in previous studies (Murakami et al., Citation2013a, Citation2014). Amyloid deposition in the intestine can cause malabsorption, intestinal dysmotility, ulcers, and bleeding (Jarnum, Citation1965; Levy et al., Citation1982; Leong et al., Citation2014). The relationship between systemic amyloidosis and gastrointestinal disorders is well known in humans and requires further investigation in other birds with systemic amyloidosis.
This study provides useful information about the amyloidogenic potential of inactivated vaccines, and the local production of SAA in fibroblasts at injection sites. It should be noted that in this study, the chickens received high doses of inactivated oil-emulsion vaccines three times, whereas normally only one vaccine dose is given. The amyloidogenic potential of other types of vaccines in both experimental and natural conditions, and the role of localized SAA production in the development of chicken AA amyloidosis require further investigation.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Benditt, E.P. & Eriksen, N. (1977). Amyloid protein SAA is associated with high density lipoprotein from human serum. Proceedings of the National Academy of Sciences of the United States of America, 74, 25–28. doi: 10.1073/pnas.74.9.4025
- Benson, M.D. & Cohen, A.S. (1979). Serum amyloid A protein in amyloidosis, rheumatic, and neoplastic diseases. Arthritis & Rheumatology, 22, 36–42. doi: 10.1002/art.1780220106
- Betts, J.C., Cheshire, J.K., Akira, S., Kishimoto, T. & Woo, P. (1993). The role of NF-kappa B and NF-IL6 transactivating factors in the synergistic activation of human serum amyloid A gene expression by interleukin-1 and interleukin-6. The Journal of Biological Chemistry, 268, 25624–25631.
- Cohen, A.S. & Calkins, E. (1959). Electron microscopic observations on a fibrous component in amyloid of diverse origins. Nature, 183, 1202–1203. doi: 10.1038/1831202a0
- Druet, R.L. & Janigan, D.T. (1966). Experimental amyloidosis: amyloid induction with a soluble protein antigen in intact, bursectomized and thymectomized chickens. The American Journal of Pathology, 49, 3–23.
- Eanes, E.D. & Glenner, G.G. (1968). X-ray diffraction studies on amyloid filaments. Journal of Histochemistry & Cytochemistry, 16, 673–677. doi: 10.1177/16.11.673
- Epstein, F.H., Gabay, C. & Kushner, I. (1999). Acute-phase proteins and other systemic responses to inflammation. The New England Journal of Medicine, 340, 448–454. doi: 10.1056/NEJM199902043400522
- Frith, C.H. & Chandra, M. (1991). Incidence, distribution, and morphology of amyloidosis in Charles Rivers CD-1 mice. Toxicologic Pathology, 19, 123–127. doi: 10.1177/019262339101900206
- Glenner, G.G. (1980). Amyloid deposits and amyloidosis: the beta-fibrilloses (second of two parts). The New England Journal of Medicine, 302, 1333–1343. doi: 10.1056/NEJM198006123022403
- Guo, J.T., Aldrich, C.E., Mason, W.S. & Pugh, J.C. (1996). Characterization of serum amyloid A protein mRNA expression and secondary amyloidosis in the domestic duck. Proceedings of the National Academy of Sciences of the United States of America, 93, 14548–14553. doi: 10.1073/pnas.93.25.14548
- Higuchi, K. (2013). Transmission of AA amyloidosis may cause outbreaks of amyloid A amyloidosis in chickens. Amyloid: The Journal of Protein Folding Disorders, 20, 59–60. doi: 10.3109/13506129.2013.792802
- Hoffman, J.S., Ericsson, L.H., Eriksen, N., Walsh, K.A. & Benditt, E.P. (1984). Murine tissue amyloid protein AA. NH2-terminal sequence identity with only one of two serum amyloid protein (ApoSAA) gene products. The Journal of Experimental Medicine, 159, 641–646. doi: 10.1084/jem.159.2.641
- Husby, G., Marhaug, G., Downton, B., Sletten, K. & Sipe, J.D. (1994). Serum amyloid A (SAA): biochemistry, genetics and the pathogenesis of AA amyloidosis. Amyloid: The Journal of Protein Folding Disorders, 1, 119–137. doi: 10.3109/13506129409148635
- Ibi, K., Murakami, T., Goda, W.M., Kobayashi, N., Ishiguro, N. & Yanai, T. (2015). Prevalence of amyloid deposition in mature healthy chickens in the flock that previously had outbreaks of vaccine-associated amyloidosis. The Journal of Veterinary Medical Science, 77, 1241–1245. doi: 10.1292/jvms.15-0029
- Ishiguro, N., Murakami, T., Elhelaly, A.E. & Inoshima, Y. (2014). Surveillance of amyloid deposition and bacterial contamination in chicken liver from meat market. The Journal of Poultry Science, 51, 104–107. doi: 10.2141/jpsa.0130028
- Jarnum, S. (1965). Gastrointestinal haemorrhage and protein loss in primary amyloidosis. Gut, 6, 14–18. doi: 10.1136/gut.6.1.14
- Johan, K., Westermark, G., Engström, U., Gustavsson, Å., Hultman, P. & Westermark, P. (1998). Acceleration of amyloid protein A amyloidosis by amyloid-like synthetic fibrils. Proceedings of the National Academy of Sciences of the United States of America, 95, 2558–2563. doi: 10.1073/pnas.95.5.2558
- Kerstens, H.M., Poddighe, P.J. & Hanselaar, A.G. (1995). A novel in situ hybridization signal amplification method based on the deposition of biotinylated tyramine. Journal of Histochemistry & Cytochemistry, 43, 347–352. doi: 10.1177/43.4.7897179
- Landman, W.J. (1999). Amyloid arthropathy in chickens. The Veterinary Quarterly Journal, 21, 78–82. doi: 10.1080/01652176.1999.9694998
- Landman, W.J., Gruys, E. & Dwars, R.M. (1994). A syndrome associated with growth depression and amyloid arthropathy in layers: a preliminary report. Avian Pathology, 23, 461–470. doi: 10.1080/03079459408419016
- Landman, W.J., Gruys, E. & Gielkens, A.L. (1998). Avian amyloidosis. Avian Pathology, 27, 437–449. doi: 10.1080/03079459808419367
- Landman, W.J.M., Peperkamp, N.H.M.T., Koch, C.A.M., Tooten, P.C.J., Crauwels, P.A.P. & Grays, E. (1997). Induction of amyloid arthropathy in chickens. Amyloid: The Journal of Protein Folding Disorders, 4, 87–97. doi: 10.3109/13506129708995276
- Leong, R.Y., Nio, K., Plumley, L., Molmenti, E. & Klein, J.D. (2014). Systemic amyloidosis causing intestinal hemorrhage and pseudo-obstruction. Journal of Surgical Case Reports, 10, 2014–2019.
- Levy, D.J., Franklin, G.O. & Rosenthal, W.S. (1982). Gastrointestinal bleeding and amyloidosis. The American Journal of Gastroenterology, 77, 422–426.
- Ling, Y.S. (1992). Experimental production of amyloidosis in ducks. Avian Pathology, 21, 141–145. doi: 10.1080/03079459208418827
- Ling, Y.S., Mao, H.P., Zhong, A.C. & Guo, Y.C. (1991). The effects of Escherichia coli and its endotoxin on amyloidosis in ducks. Veterinary Pathology, 28, 519–523. doi: 10.1177/030098589102800609
- MacDonald, D.W. & Carlson, H.C. (1969). Attempted experimental induction of amyloidosis in chickens. Poultry Science, 48, 71–76. doi: 10.3382/ps.0480071
- Maestrini, N. & Pascucci, S. (1970). Amyloidosis in guinea fowl. Atti della SocietaÁ Italiana delle Scienze Veterinarie, 24, 485–486.
- McAdam, K.P. & Sipe, J.D. (1976). Murine model for human secondary amyloidosis: genetic variability of the acute-phase serum protein SAA response to endotoxins and casein. The Journal of Experimental Medicine, 144, 1121–1127. doi: 10.1084/jem.144.4.1121
- Meek, R.L. & Benditt, E.P. (1986). Amyloid A gene family expression in different mouse tissues. The Journal of Experimental Medicine, 164, 2006–2017. doi: 10.1084/jem.164.6.2006
- Mount, S.L., Eltabbakh, G.H. & Hardin, N.J. (2002). Beta-2 microglobulin amyloidosis presenting as bilateral ovarian masses: a case report and review of the literature. The American Journal of Surgical Pathology, 26, 130–133. doi: 10.1097/00000478-200201000-00018
- Murakami, T., Inoshima, Y. & Ishiguro, N. (2015). Systemic AA amyloidosis as a prion-like disorder. Virus Research, 207, 76–81. doi: 10.1016/j.virusres.2014.12.019
- Murakami, T., Inoshima, Y., Sakamoto, E., Fukushi, H., Sakai, H., Yanai, T. & Ishiguro, N. (2013a). AA amyloidosis in vaccinated growing chickens. Journal of Comparative Pathology, 149, 291–297. doi: 10.1016/j.jcpa.2013.02.002
- Murakami, T., Ishiguro, N. & Higuchi, K. (2014). Transmission of systemic AA amyloidosis in animals. Veterinary Pathology, 51, 363–371. doi: 10.1177/0300985813511128
- Murakami, T., Muhammad, N., Inoshima, Y., Yanai, T., Goryo, M. & Ishiguro, N. (2013b). Experimental induction and oral transmission of avian AA amyloidosis in vaccinated white hens. Amyloid: The Journal of Protein Folding Disorders, 20, 80–85. doi: 10.3109/13506129.2013.783474
- Ovelgönne, J.H., Landman, W.J., Gruys, E., Gielkens, A.L. & Peeters, B.P. (2001). Identical amyloid precursor proteins in two breeds of chickens which differ in susceptibility to develop amyloid arthropathy. Amyloid: The Journal of Protein Folding Disorders, 8, 41–51. doi: 10.3109/13506120108993813
- Pérez-Villa, F., Campistol, J.M., Ferrando, J. & Botey, A. (1989). Renal amyloidosis secondary to acne conglobata. International Journal of Dermatology, 28, 132–133. doi: 10.1111/j.1365-4362.1989.tb01336.x
- Rigdon, R.H. (1960). Experimental amyloid production in ducks with methylcholanthrene. Texas Reports on Biology and Medicine, 18, 93–102.
- Shibatani, M., Imoto, H., Suzuki, T., Hasegawa, I. & Itakura, T. (1984). Amyloidosis in a layer chicken flock. Journal of the Japanese Veterinary Medical Association, 37, 787–792. doi: 10.12935/jvma1951.37.787
- Shin, J.H. & Molitor, T.W. (2002). Localization of porcine reproductive and respiratory syndrome virus infection in boars by in situ riboprobe hybridization. Journal of Veterinary Science, 3, 87–96.
- Sipe, J.D., Benson, M.D., Buxbaum, J.N., Ikeda, S.I., Merlini, G., Saraiva, M.J. & Westermark, P. (2016). Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid: The Journal of Protein Folding Disorders, 23, 209–213. doi: 10.1080/13506129.2016.1257986
- Steentjes, A., Veldman, K.T., Mevius, D.J. & Landman, W.J. (2002). Molecular epidemiology of unilateral amyloid arthropathy in broiler breeders associated with Enterococcus faecalis. Avian Pathology, 31, 31–39. doi: 10.1080/03079450120106606
- Tanizaki, Y., Sato, Y., Oka, H., Utsuki, S., Kondo, K., Miyajima, Y., Nagashio, R. & Fujii, K. (2006). Expression of autocrine motility factor mRNA is a poor prognostic factor in high-grade astrocytoma. Pathology International, 56, 510–515. doi: 10.1111/j.1440-1827.2006.01999.x
- Thompson, K.J., Freund, J., Sommer, H.E. & Walter, A.W. (1947). Immunization of ducks against malaria by means of killed parasites with or without adjuvant. American Journal of Tropical Medicine, 27, 79–105. doi: 10.4269/ajtmh.1947.s1-27.79
- Trang, N.T., Hirai, T., Ngan, P.H., Lan, N.T., Fuke, N., Toyama, K., Yamamoto, T. & Yamaguchi, R. (2015). Enhanced detection of porcine reproductive and respiratory syndrome virus in fixed tissues by in situ hybridization following tyramide signal amplification. Journal of Veterinary Diagnostic Investigation, 27, 326–331. doi: 10.1177/1040638715579260
- Trang, N.T., Hirai, T., Yamamoto, T., Matsuda, M., Okumura, N., Giang, N.T., Lan, N.T. & Yamaguchi, R. (2014). Detection of porcine reproductive and respiratory syndrome virus in oral fluid from naturally infected pigs in a breeding herd. Journal of Veterinary Science, 15, 361–367. doi: 10.4142/jvs.2014.15.3.361
- Turnell, W.G., Sarra, R., Baum, J.O., Caspi, D., Baltz, M.L. & Pepys, M.B. (1986). X-ray scattering and diffraction by wet gels of AA-amyloid fibrils. Molecular Biology and Medicine, 3, 409–424.
- Upragarin, N., Asten, A.J., Tooten, P.C.J., Landman, W.J.M. & Gruys, E. (2005a). Serum amyloid A production by chicken fibroblast-like synoviocytes. Veterinary Immunology and Immunopathology, 106, 39–51. doi: 10.1016/j.vetimm.2005.01.004
- Upragarin, N., Landman, W.J., Gaastra, W. & Gruys, E. (2005b). Extrahepatic production of acute phase serum amyloid A. Journal of Histology & Histopathology, 20, 1295–1307.
- Upragarin, N., Landman, W.J., van Asten, A.J.A.M., Toussaint, M.J.M., van Ederen, A.M. & Gruys, E. (2002). Serum amyloid A (SAA) mRNA and protein expression in primary culture chicken synoviocytes. In Proceedings of The Third European Colloquium on Food Safety and Acute Phase Proteins (p. 72). Utrecht.
- Von Rampin, T., Sironi, G. & Gallazzi, D. (1989). Amyloidoseepisoden bei Junghennen nach wiederholter Anwendung van antibakteriellen Ölemulsionvakzinen. Deutsche Tierärztliche Wochenschrift, 96, 168–172.
- Wang, D.H. & Di, B.X. (1992). Pathological study on amyloidosis in chickens. Acta Veterinaria Zootechnica Sinica, 23, 256–261.
- Whitehead, A.S., de Beer, M.C., Steel, D.M., Rits, M., Lelias, J.M., Lane, W.S. & de Beer, F.C. (1992). Identification of novel members of the serum amyloid A protein superfamily as constitutive apolipoproteins of high density lipoprotein. The Journal of Biological Chemistry, 267, 3862–3867.
- Woldemeskel, M. (2012). A concise review of amyloidosis in animals. Veterinary Medicine International, 42, 72–96.
- Yamada, M., Kotani, Y., Nakamura, K., Kobayashi, Y., Horiuchi, N., Doi, T., Suzuki, S., Sato, N., Kanno, T. & Matsui, T. (2006). Immunohistochemical distribution of amyloid deposits in 25 cows diagnosed with systemic AA amyloidosis. Journal of Veterinary Medical Science, 68, 725–729. doi: 10.1292/jvms.68.725
- Zekarias, B., Landman, W.J., Tooten, P.C. & Gruys, E. (2000). Leukocyte responses in two breeds of layer chicken that differ in susceptibility to induced amyloid arthropathy. Veterinary Immunology and Immunopathology, 77, 55–69. doi: 10.1016/S0165-2427(00)00233-6