Abstract
Virus and virus-like diseases are major production constraints in vegetatively propagated crops; once a plant is infected, it remains so throughout its life. This work was undertaken to accelerate the process of eliminating apple chlorotic leaf spot virus (ACLSV) from explants obtained from an infected plum tree by in vitro heat therapy combined with meristem tip culture. After a minimum of 1 week of in vitro heat treatment followed by microdissection of meristematic tissues, trees were propagated successfully into virus-free plants. The treatment procedure, from infected plant to virus-free plantlets, takes a minimum of 14 weeks and could be applied to other fruit tree crops. The results of a one-step reverse transcription PCR analysis of treated plants after three accelerated growth cycles indicated the absence of ACLSV. Heat treatment of the test plants, followed by three rounds of indexing, was accomplished in about 28 months, a procedure that could take ≥36 months using conventional methods. The techniques described in this work may help to speed up the treatment of infected plants and screening of planting materials. The application of these procedures will benefit regulatory agencies, allow stakeholders to have timely access to virus-tested material, and facilitate domestic and international trade.
Résumé
Les maladies virales, ainsi que celles analogues aux maladies virales, constituent d’importantes contraintes à la production chez les cultures propagées végétativement: une fois la plante infectée, elle l’est pour toute la durée de sa vie. Cette étude a été entreprise afin d’accélérer l’élimination, par thermothérapie in vitro combinée à la culture de méristèmes, du virus de la tache chlorotique du pommier (VTCP) d’anciens plants obtenus d’un prunier infecté. Au bout d’au moins une semaine de thermothérapie in vitro suivie de la microdissection des tissus méristématiques, des arbres ont été propagés avec succès en tant que plants exempts de virus. La procédure du traitement, de la plante infectée à la plantule exempte de virus, prend au moins 14 semaines et pourrait être appliquée à d’autres cultures d’arbres fruitiers. Le résultat d’une PCR par transcriptase inverse en une étape des plants traités à la suite de trois cycles de croissance accélérée a confirmé l’absence du VTCP. Le traitement par thermothérapie des plants tests, suivi de trois séances d’indexage, a été effectué en à peu près 28 mois, une procédure qui prendrait 36 mois ou plus en utilisant les techniques traditionnelles. Les techniques décrites dans le cadre de cette étude peuvent contribuer à accélérer le traitement des plants infectés et le criblage du matériel végétal. L’application de ces procédures profitera aux organismes de réglementation, permettra aux parties concernées d’accéder, en temps opportun, à du matériel exempt de virus et facilitera le commerce intérieur et extérieur.
Introduction
Plants that are propagated vegetatively from virus-infected parent plants usually pass on the infection because viruses are capable of infecting their host in a systemic manner (Hipper et al. Citation2013). The systemic nature of viral infection is a major concern of the global fruit industry because it costs producers billions of dollars (Cembali et al. Citation2003; Gergerich et al. Citation2015). Most tree fruit crops, including plum, are propagated vegetatively and, despite the risk, this technique remains the preferred method even in some species that produce viable seeds (Hartmann et al. Citation2011; Westerfield and Krewer Citation2015). To take advantage of the benefits offered by vegetative propagation without the concomitant possible spread of virus infection, it is important to obtain virus-free planting material. Propagation of perennial crop plants using clean stocks is important not only for optimum production (Speiegel Citation1998; Anonymous Citation2017; Monis Citation2020) but also for physiological studies in breeding programmes, since the physiology of diseased plants differs greatly from those of healthy plants (Diener Citation1963; Pallas and Garcıia Citation2011). Most clean planting stock programmes rely on techniques that detect, isolate, and eliminate pathogens to produce, maintain, and propagate healthy planting materials. The demand for clean planting materials is ever-increasing; this necessitates the development of techniques that are effective, reliable, and fast.
With variable success rates, different methods, including chemotherapy, thermotherapy, meristem culture and their variants, have been employed to eliminate viruses from systemically infected plants (Verma et al. Citation2005; Wang et al. Citation2009; Zhao et al. Citation2018). Thermotherapy is the most widely used of these methods; it involves application of heat by hot water or hot air (Welsh and Nyland Citation1965; Grondeau et al. Citation1994) under controlled conditions. The difference between the host and virus in their tolerance to high temperature is the basis for the successful heat inactivation of the virus (Kunkel Citation1936; Barba et al. Citation1992). However, a review of several reports (Wang et al. Citation2018b) has shown that viral invasiveness of meristematic tissue determines the ease of virus elimination. To obtain virus-free explants from plants infected with heat-stable viruses, heat therapy is often combined with meristem and tip culture. This strategy has been employed for the production of foundation stocks in clean plant programmes (Golino et al. Citation2017). Sometimes antiviral agents are added to growth media to increase the success rate of virus elimination, but the phytotoxicity of such treatments often results in prolonged recovery time and poor plant survival (Hansen Citation1989). Cold temperature treatment has also been found to be effective against some viral pathogens (Wang and Valkonen Citation2009; Wang et al. Citation2018a). The key to the success of any meristem culture method is the size of the explant (Sim et al. Citation2019), which is usually composed of the apical dome and a limited number of young leaf primordia, and excludes any differentiated vascular tissues. Typically, the smallest explants have the lowest survival rates during in vitro culture (Vivek and Modgil Citation2018), but when mature they produce the highest proportion of virus-free materials. The efficiency of virus elimination also depends on the type of virus, plant host and virus combinations in mixed infection (Knapp et al. Citation1995). Temperature regime has also been found to affect the success rate (Lozoya-Saldana and Dawson Citation1982; Wang et al. Citation2018b).
An important step in virus elimination evaluations is indexing, which determines whether the virus has been eliminated from the host plant. Observing treated plants for symptom development is not always a reliable method of assessment since most plant viruses are either latent or incite morphological changes in their host at a later stage (Roossinck Citation2010; Richert-Pöggeler and Minarovits Citation2014). Other indexing methods, which have been used for decades, include grafting or mechanical inoculation onto host indicators (bioassay) and serological and nucleic acid-based tests. While these methods have proven to be useful, they are not without shortcomings. The woody bioassay, considered the gold standard in plant virus certification programmes, requires extensive field areas and takes up to 3 years to complete, depending on the virus and indicator plant. Molecular techniques based on serology depend on the availability of suitable antibodies, while PCR relies on optimum reaction conditions including primer design (Bustin and Huggett Citation2017). Next-generation sequencing (NGS), a metagenomic approach that does not require prior knowledge of the pathogen, is increasingly being used as a detection tool (Adams et al. Citation2009; Rott et al. Citation2017; Bomer et al. Citation2019), but requires more research to determine sensitivity. While NGS is more expensive and involves downstream bioinformatics analysis, its usage in indexing/diagnostics is justifiable when dealing with multiple or unknown pathogens (Al Rwahnih et al. Citation2015; Rott et al. Citation2017). The choice of any of the above methods depends on the reliability and efficiency of the test available. To ensure complete elimination of the pathogen from fruit tree crops, treated plants are usually maintained and retested in each growing season for up to 3 years when a traditional method is used (Wood Citation1973; Westwood Citation1989). Notwithstanding the indexing method used, multiple post-treatment testing based on the phenological cycle of the plant is required (Anonymous Citation2017).
The objectives of this study were twofold: (1) to use an efficient treatment method to eliminate apple chlorotic leaf spot virus (ACLSV) from infected plant material; and (2) to develop an accelerated indexing procedure for post-treatment screening to ensure virus-free plants for propagation. The application of these procedures will benefit regulatory agencies, allow stakeholders to have timely access to virus-tested material, and facilitate domestic and international trade.
Materials and methods
Plant materials and pre-treatment testing
A ‘Shiro’ plum (Prunus salicina) tree that was approximately 3 years old was obtained from the virus collection at the Centre for Plant Health (CPH). The ‘Shiro’ plum is a small tree (at maturity, reaching up to 6 m in height with a spread of up to 6 m) that is typically grown for its edible fruit qualities as well as for landscaping. The mother plant was indexed using a range of herbaceous and woody bioassays as well as specific reverse transcription (RT)-PCR and ELISA assays for over 20 different viruses, viroids and phytoplasmas. ACLSV, being tested for via RT-PCR as described below, was the only pathogen detected in the plant. The bioassay and ELISA procedures are not described in this report.
Total nucleic acid was extracted from leaves using a Kingfisher mL magnetic particle processor (Thermo Fisher Scientific, Burlington, ON). The sample preparation protocol was customized and the reagents were optimized as recommended by the manufacturer as follows: plant tissues were homogenized (1:10 tissue-to-buffer ratio) in lysis buffer (4 M guanidine isothiocyanate, 0.2 M sodium acetate-trihydrate, 2.5% (wt/vol) PVP and 25 mM EDTA) and 500 µL of the homogenates were incubated with 50 µL of 20% sarkosyl for 10 min at 70°C. After incubation and spinning briefly, 230 µL of the sample lysates (supernatant) were mixed with 20 µL magnetic beads (MagSi DNA, Cedarlane, Burlington, ON), 220 µL lysis buffer, and 470 µL isopropanol in the binding well (the first tube) of the five-well Kingfisher strip tube. To the second well of the strip tube was added 800 µL VHB wash buffer, and 800 µL of RWB wash buffer to each of the third and fourth wells. To the fifth well (elution well) was added 200 µL of nuclease-free water. The Kingfisher strip tubes were transferred into the Kingfisher magnetic particle processor, and the extraction run was initiated. After the run, the nucleic acid collected in the elution well was diluted 1:10 with 10 mM Tris pH 8.0 and used as a template in RT-PCR. The wash buffers were purchased from Omega Bio-Tek (Norcross, GA).
The primers CLS6860 5ʹ-TTCATGGAAAGACAGGGGCAA-3ʹ and CLS7536 5ʹ-AAGTCTACAGGCTATTTATTATAAGTCTAA-3ʹ (Menzel et al. Citation2002) were used to amplify 678bp fragment in the movement protein. The test was done in a 25 μL reaction volume using 0.1 μM (homologous) and 0.15 μM (complementary) of the primers, 1× PCR buffer, 1× sucrose/cresol solution, 2.3 mM MgCl2, 0.2 mM dNTP mix, 5 mM DTT, 0.1 U AMV reverse transcriptase and 1.25 U Taq polymerase. Reverse transcription was done for 30 min at 54°C, and PCR amplification was done for 35 cycles with a temperature cycling profile of 95°C for 30 s, 62°C for 30 s, 72°C for 30 s, followed by a final extension at 72°C for 10 min. A nested RT-PCR with polyvalent degenerate oligonucleotides (PDO), which can detect tree fruit viruses in the family Betaflexiviridae, including ACLSV (Foissac et al. Citation2005), was also used for indexing. While the ACLSV-specific RT-PCR amplifies sequences in the coat protein region, the PDO primers target conserved motifs in the viral polymerase. PCR amplicons (10 μL) were analyzed using agarose gel electrophoresis with ethidium bromide staining. All five replicates per treatment group (described below) were tested with this RT-PCR.
Tissue culture
The experimental plant was initiated in artificial growth media following standard tissue culture procedures (Bhojwani and Razdan Citation1996). All the media used were prepared in-house using reagents supplied by Sigma (Oakville, ON). The media used included MA (rooting medium), MB (root elongation medium) and JCH (initiation medium), which contain all the nutrients and organic supplements required by the plant for in vitro growth and morphogenesis. The MA medium consisted of the major and minor elements of Murashige and Skoog (Citation1962) with some modifications as follows (in milligrams per litre): 950 KNO3, 825 NH4NO3, 220 CaCl2.2H2O, 185 MgSO4.7H2O, 85 KH2PO4, 8.45 MnSO4.H2O, 4.3 ZnSO4.7H2O, 3.1 H3BO3, 0.41 KI, 0.125 Na2MoO4.2H2O, 0.0125 CoCl2.6H2O, 0.0125 CuSO4.5H2O, 13.9 FeSO4.7H2O, 18.65 Na2EDTA.2H2O, 0.4 thiamine HCl and 1.5 indole 3-butyric acid (3-IBA). Sucrose 30 g L−1, inositol 0.1 g L−1 and 0.6% agar were also added. The pH was adjusted to 5.7 with KOH. The MB medium was the same as MA except that 3-IBA was left out. In the preparation of JCH medium, the concentration of micro- and macronutrients was doubled, and 1 g L−1 3-IBA, 1 g L−1 benzylaminopurine and 0.1 mg L−1 gibberellic acid (GA3) were added. All other nutrients and additives including thiamine HCl, inositol, sucrose and agar were the same as in MA, but the pH was adjusted to 5.2. Pyridoxine (HCl), nicotinic acid and glycine were not added to any of the medium.
Surface sterilization
Axillary buds were excised from vigorously growing plants and subjected to a sterilization procedure under aseptic conditions as follows: the buds were transferred and stirred for 2 min in sterilization solution (6% PerCeptTM), followed by stirring twice in sterile reverse osmosis water for 2 min to wash off the sterilization solution.
Initiation of tissues in vitro
All the work was performed in a laminar airflow hood under aseptic conditions. The sterilized buds were placed onto a sterile glass surface and dissected to remove all cut surfaces that were exposed to the sterilization solution. At the initiation stage, the objective was to get the plant culture established in vitro. Thus, the explants were excised (5–8 mm in length) to contain relatively large numbers of developing leaf primordia so as to achieve maximum survival of growing tissue. The excised tissues were transferred into a sterile 50-mL tube containing 15 mL of the JCH initiation medium. Sterility was maintained throughout this procedure to ensure the survival of the explant. The cultures were maintained in this medium for 3–4 weeks for the shoot to grow. Bacterial or fungal contamination in tissue culture is not uncommon, and the failure of surface sterilization procedures to produce aseptic cultures is a major problem with woody plants. As such, infected plant cultures were isolated and discarded. Non-infected tissues were sub-cultured onto fresh JCH medium as necessary to allow the shoots to proliferate. Cultured tissues were maintained with a 16-h photo-period, provided by warm white fluorescent tubes (4000 lx at culture level), and a constant temperature of 24 ± 1°C.
Thermotherapy
The shoot tissues proliferating on JCH medium were divided into three groups: (1) the first group was propagated into mature plants without further treatment (no-treatment control, NTC); (2) the second group was subjected to microdissection (zero heat treatment, ZHT); and (3) the third group was subjected to four different periods of heat treatment before microdissection (VHT). Five plants per treatment group, for a total of 30 plants, were selected from those that survived the treatment procedures described below.
NTC
To propagate the shoot tissues into mature trees, the NTC cultures were maintained in the growth room until the leaves were well-formed and shoot internodes sufficiently elongated to allow dissection into distinct rootless plants. After dissection, rootless plants were transferred onto MA medium and then onto MB medium. Once the plantlets were well established (with fully developed roots, stems and leaves) in MB medium, they were subjected to a hardening procedure in the greenhouse. As per this procedure, agar material was removed carefully from the base of each plantlet and the plantlets transferred into peat pots containing perlite soil mix. The plants were placed into a plastic bin (clear 25 L plastic box measuring 22”L × 15”W × 6”H, with a lid) under high humidity (70%–80%). The humidity in the bin was gradually reduced during a 1-week transition to greenhouse conditions. Once the plantlets were well established, they were transplanted into 1-gallon pots containing composted bark mulch.
ZHT
Shoot tissues were removed from the tube, placed on the stage of a microscope (LEICA M125, Leica Microsystems Inc., Concord, ON) and dissected to harvest the meristem. To achieve the smallest size possible (~0.3–0.4 mm) without compromising the survival of the tissue, buds (axillary or tip) were excised from shoot tissues and dissected carefully under magnification (20×), removing progressively smaller developing leaves to expose the apical meristem of the bud with the youngest of the leaf primordia. Dissected meristems were transferred onto a sterile Petri dish containing JCH medium. The culture was maintained in the growth room to initiate callus formation and then transferred into a 50 mL tube containing fresh JCH medium to proliferate. Proliferating shoots were propagated into mature trees as described above for the NTC.
VHT
The tubes containing the VHT shoot tissues were placed in a growth chamber and the temperature in the chamber ramped up at the rate of 0.5°C day−1 from 25°C to 37°C. Conditions in the chamber were set to provide 37°C day and 32°C night temperature, with 16-h-light at an intensity of 3000–4000 lx from white fluorescent tubes. Recording of the period of heat treatment started as soon as the temperature in the chamber had reached 37°C. Batches of five heat-treated shoots were removed from the chamber after 1, 2, 4 and 8 weeks, and subjected to microdissection and propagation as described above (ZHT). Each treated shoot was placed on JCH medium in the growth room (24°C) to recover. The shoots were propagated into mature trees as described for the NTC above.
Accelerated growth cycle
After reaching maturity (about 6 months after potting in the greenhouse), the treated plants and their controls were subjected to three cycles of accelerated growth in a growth chamber (Conviron E15, Controlled Environment Ltd, Winnipeg, MB). Each growth cycle consisted of 4 months of growth and 3 months of forced dormancy. Sometimes, the dormancy period was shorter than 3 months depending on when budburst was observed. To initiate dormancy, the temperature in the chamber was ramped down at the rate of 0.5°C day−1 from 25°C to 10°C (day/night), and finally to 4°C. The daylight hour was also reduced at an average rate of 0.3 h day−1 from 16-h-light to 8-h-light. The 4-month growth period started after the temperature was ramped up to 25°C/22°C (day/night), and daylight hours increased to 16-h-light. The ramp-up rates for both temperature and daylight hours were the same as for ramp-down. Treated plants and their controls were retested after each growth cycle.
Results and discussion
Establishment of cultures prior to microdissection
The initiation of tissue cultures was generally successful, and cultures that were initiated prior to microdissection were established with almost 100% survival. The success of initiation in micropropagation is typically influenced by many variables. The initiation medium used, the efficacy of sterilization, i.e. potential microbial contamination, and the size of the explant are among the key factors (Dziedzic Citation2008; Vivek and Modgil Citation2018). The ~7 mm size of explant that we used for initiation in this work, coupled with strict sterilization procedures, resulted in almost 100% survival and establishment of an in vitro culture. Contamination was observed only when cultures were maintained for over 3 months. Sometimes contaminated plants may not have any visible symptoms, and have reduced multiplication and rooting rates or even die. Although poor aseptic techniques or improperly sterilized equipment are frequently the cause of contamination, sometimes even with the strictest procedures contamination can be difficult to eliminate, especially when caused by endophytes. While trying to eliminate ACLSV in apple plants, Vivek and Modgil (Citation2018) found 0.2–0.3-mm-sized meristems to be relatively less prone to contamination, but they were comparatively difficult to establish.
Microdissection and establishment of heat-treated plants
All the heat-treated plants, prior to microdissection, survived (). Many factors affect the survival of in vitro plants during heat treatment: host plant species/variety, temperature, exposure time and light are among the important factors. Elsewhere, Dziedzic (Citation2008) recorded a < 10% survival when ‘Earliblue’ plum was heat treated for 2 weeks at 24/25°C with a light intensity of 54.3 µmol s−1 m−2 (2932.2 lx). Plants did not survive when temperatures were set at 32°C with a light intensity of 934 lx, suggesting a minimum light requirement for the survival of in vitro culture. Light affects shoot regeneration and secondary metabolite biosynthesis during micropropagation (Chen et al. Citation2019), and it can influence the efficacy of plant growth regulators as well as endogenous hormone levels (Halliday et al. Citation2009). The light (4000 lx) conditions used in this work were adequate for healthy growth and development, and the temperature (37°C) was tolerable for the survival of the plants. At the CPH, these light and temperature settings are used as default conditions for in vitro work on tree fruit and grapevine species. During heat therapy for virus elimination, the tendency is to use the upper end of the heat tolerance of the plant to maximize the inactivation of virus. While a constant temperature may be used, Tan et al. (Citation2010) have reported alternating night/day temperatures of 32°C and 38°C to be more favourable for the survival of pear than a constant temperature of 37°C.
Table 1. Survival rate and RT-PCR test results for apple chlorotic leaf spot virus on heat-treated ‘Shiro’ plum (Prunus salicina)
Microdissection aimed at getting the meristem as small as possible is technically challenging, i.e. removing potentially infected leaves yet ensuring tissue survival. On average, up to 80% of the heat-treated tissues that were microdissected survived (). Survival did not appear to be correlated with treatment period. Considering that multiple plants can be propagated from a single culture, this survival rate was deemed sufficient. After testing, only five plants from each treatment group were maintained for indexing.
Time required: initiation to indexing
Propagation of tissue cultures into mature plantlets was achieved in about 3 months (). The longest time was for the initiation of virus-free culture after microdissection; on average cultures were ready for transfer onto MA medium for rooting after about 2 months. The time period between rooting (on both MA and MB media) and transferring into a greenhouse to acclimatize was about 4 weeks. Prunus salicina ‘Shiro’ roots readily on MA medium and can be transferred directly to pots in the greenhouse when the roots are long enough (~1 cm). Transferring it to MB medium (hormone free) allows the root to elongate and the shoot to grow, resulting in the production of healthier, more robust plantlets. After transplanting from MB to pots in the glasshouse, plants were sprayed once or twice with 300 ppm gibberellic acid in order to prevent dormancy, in which the roots continue to grow in the pots but the shoots will cease growth, turn brown and eventually drop their leaves. The growth medium is a key factor for successful rooting of micropropagated shoots; the type of vessel used is also important as it affects the relative humidity and rate of gas exchange (Nissen and Sutter Citation1990; Gribble Citation1999).
Fig. 1 (Colour online) Timeline of virus elimination by heat treatment (two growth cycles, April 2017–August 2019); traditional (in vivo) method compared with in vitro treatment. STC = shoot tip culture, in vitro method: shoot tip meristem was microdissected and propagated in culture media, * in vivo: shoot tip was excised and grafted onto a root stock. Est. = Establishment of treated plant, in vitro: tissue cultured plantlets were rooted in 1-gallon pots and maintained in a growth chamber, *in vivo: shoot tips grafted onto rootstock were maintained in a growth chamber. Growth cycle, in vitro: treated plants were subjected to a 7-month accelerated growth cycle, in vivo: shoot tips grafted onto root stock were maintained and allowed to undergo the natural growth cycle. RT-PCR testing (▼). Note that *the number of days includes the waiting period for a successful graft take which, eventually, add up to Growth Cycle 1.
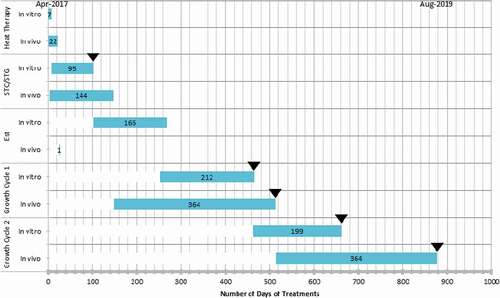
The length of time from explant initiation into tissue culture to greenhouse-growing plantlets was 14 weeks for the plants exposed to 1 week of heat therapy. The first testing for the virus, prognostic, was performed on proliferating tissues without compromising their survival. In approximately 9 months (from initiation) plants were well established in pots and ready for the accelerated growth cycle treatment. The routine virus elimination method used at the CPH involves a minimum of 3 weeks of heat treatment in vivo at 37°C followed by shoot tip grafting onto a root stock. Because these activities have to be synchronized with the natural growth cycle of the plants, the heat therapy usually starts in April, followed by shoot tip grafting (in vivo) in June (Gratz Citation2020). The first testing of heat therapy grafted plants is done in the second growth season (), which occurs approximately 17 months after treatment (August).
The in vitro technique combined with the accelerated growth cycle described in this report and outlined in required ~22 months (for a two-season cycle) () or ~28 months (three-season cycle) to complete. One major advantage of this procedure is the fact that the experiment can be conducted at any time of the year because it does not depend on the natural growth cycle of the plant. Testing at the CPH is performed after each growth cycle for two seasons (), and elsewhere under some certification schemes it is three to four seasons depending on the crop involved (Martin and Tzanetakis Citation2014). Based on the 12-month natural phenological cycle, the time required for the completion of heat treatment and indexing will be 26–38 months, and in some cases up to 48 months depending on the certification programme. Although the goals and methods of achieving them are similar, for various reasons, there is no international standard; treatment and indexing standards are programme- or country-specific (Martin and Tzanetakis Citation2014).
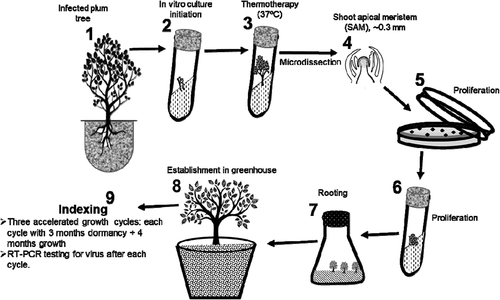
Fig. 2 Flow chart (not drawn to scale) of accelerated in vitro thermotherapy and indexing against apple chlorotic leaf spot virus. (1) Axillary buds were excised from the infected tree and sterilized. (2) Sterile buds were transferred into culture tubes to initiate proliferation. (3) Proliferating tissues were heat treated in a growth chamber at 37°C. (4) Heat-treated tissues were microdissected to obtain ~0.3 mm shoot apical meristems (SAM) that are potentially virus free. (5) SAM were transferred onto media in Petri dishes to initiate proliferation. (6) Proliferating tissues were transferred into tubes to continue growth. (7) Proliferating tissues were transferred into rooting medium and then into root elongation medium. (8) Rooted plantlets were transferred into pots to initiate hardening in the greenhouse and produce mature plants. (9) Mature plants were moved into a growth chamber and subjected to three cycles of accelerated growth. Leaf tissues were collected from mature plants after every growth cycle for virus testing.
Indexing
To determine the success of the virus elimination, the treated plants and controls were tested using RT-PCR. While PCR is known to be a sensitive test, its ability to detect extremely low-level infection in plants cannot be guaranteed. Under error-free conditions, the limit of detection of PCR at 95% confidence has been estimated at three molecules (Stahlberg and Kubista Citation2014). However, since one viral particle is capable of replicating and inciting a new infection, a negative test result soon after heat treatment is not a guarantee that the virus has been eliminated completely from the host plant. Although ACLSV is not known to be heat-tolerant, viruses/strains may remain undetected during one or more testing seasons after treatment. Consequently, it is necessary to retest treated plants after multiple growth cycles. Monitoring the plant through multiple growth cycles allows the viruses that may have been reduced to undetectable levels by therapy to build up in the plant prior to indexing. The minimum time to verify the success of virus elimination after treatment will depend on the virus and host plant. The experiment was designed to retest treated plants at the end of each growing season for three consecutive seasons. To expedite the testing period an accelerated growth cycle of 7 months, a replica of the natural growth cycle of fruit trees (Kurokura et al. Citation2013), was implemented. The results of indexing of the treated plants are presented below.
Effect of heat treatment
Zero heat-treated plants could not be freed of the virus after microdissection. All the ZHT plants tested positive () for ACLSV in RT-PCR. Although it is possible to eliminate viruses from plants following meristem tip culture alone (Sim et al. Citation2019), this procedure is almost always combined with heat therapy or chemotherapy to increase the success rate.
Since meristematic tissue should be free of virus, the success of this method depends on the size of the explant and the plant and virus species (Spiegel et al. Citation1993). In this study, the size to which the ZHT explants were trimmed (~0.3–0.4 mm) was probably not small enough to have removed all infected tissues. However, studies have shown that, regardless of the size of the shoot tip, meristem tip culture alone cannot eliminate some viruses (Wang et al. Citation2016; Li et al. Citation2016). While trying to achieve the minimum size possible for tissue survival and at the same time eliminate any virus present, it is important to consider that regeneration time of meristem cultures is inversely related to the size of the explant. To optimize the benefits of the in vitro method, it might be worthwhile to use larger meristem sizes combined with heat treatment. Whether heat treatment is more important than the size of the starting explant in virus therapy will depend on the specific virus and its invasiveness within the host.
Regardless of treatment duration, all heat-treated plants (VHT) tested negative for ACLSV in RT-PCR (, ). During heat therapy, the temperature and exposure time are very important because the difference between the heat inactivation point of the virus and the maximum tolerance of the host plant is insignificant. In this work, heat treatment at 37°C for 7 days was enough to eliminate the virus. The VHT tissues that were tested included the proliferating shoot tissues in culture tubes and leaf tissues sampled from mature trees after each growth cycle. The success of the heat treatment can be determined as early as 2 months after microdissection. Elsewhere, Gella and Errea (Citation1998) observed a 60%–100% success rate in eliminating ACLSV from three Prunus species using 37°C for between 15 and 36 days. At the CPH, ACLSV was eliminated from different Malus species with an average success rate of ~80% after 3 weeks of traditional heat treatment, and the rate reached 100% when treatment was extended to 4 weeks. While in vitro therapy is more efficient, it is not easy to develop the optimal conditions for successful regeneration of all plant species. Phenotypically, no obvious differences were observed between the heat- (VHT) and non-heat-treated plants (ZHT). However, elsewhere, thermotherapy has been reported to cause phenotypic modifications such as double nodes, leaf shape and fruit quality (Guidoni et al. Citation1997).
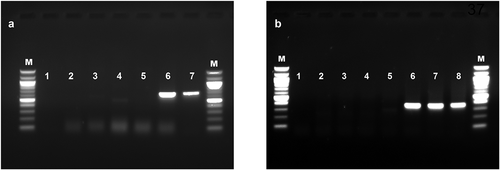
Fig. 3 Reverse transcription-polymerase chain reaction (RT-PCR) detection of (a) apple chlorotic leaf spot virus (ACLSV) and (b) viruses in the Betaflexiviridae family (Foissac et al. Citation2005) in plum plants that were subjected to thermotherapy and meristem microdissection to eliminate viruses. Plants were tested after three accelerated growth cycles. Lanes M = 100bp marker, 1 = no template control, 2 = VHT-8, 3 = VHT-4, 4 = VHT-2, 5 = VHT-1, 6 = NTC, 7 = positive control (ACLSV), 8 = positive control (mixed infection; ACLSV and apple stem grooving virus). Abbreviations: VHT = in vitro heat treated, numbers after hyphen represent treatment duration (weeks), NTC = meristem was microdissected without heat treatment.
A review of virus elimination experiments conducted between 1991 and 2010 showed that thermal cycles of between 35°C and 38°C were frequently used (Panattoni et al. Citation2013). However, information on the minimum temperature⎯time combinations necessary to free plants from many viruses is hard to find. Apart from temperature during heat treatment, host-induced metabolic changes also affect the success of virus elimination (Obrępalska-Stęplowska et al. Citation2015).
Conclusions
The combination of in vitro heat therapy and an accelerated growth cycle can efficiently eliminate viral infection(s) in fruit tree crops intended for propagation. On average, the accelerated growth cycle, which was designed to mimic the natural phenology cycle of fruit tree crops under controlled environmental conditions, reduced the test period by almost 50%. The accelerated growth cycle implemented in this work could be applied to other temperate fruit tree crops as they all undergo similar metabolic and physiological processes during their phenology cycle, including hormonal signalling, carbohydrate metabolism, mitochondrial respiration and oxidative stress (Kurokura et al. Citation2013; Beauvieux et al. Citation2018). Currently, the most common methods of virus elimination in fruit tree planting materials are a combination of in vivo thermotherapy and shoot tip grafting or meristem shoot tip culture. In these traditional methods, a significantly larger space is required for handling plants during heat treatment, and treatment periods are comparatively longer. Moreover, the requirement for preparing scion and rootstock, with the associated challenges of obtaining a successful graftage, makes the traditional methods somewhat challenging.
In summary, the in vitro method described in this work requires minimal space during thermotherapy and eliminates infection in days as opposed to weeks or months in the traditional methods. This is especially so in infections involving heat-stable viruses/viroids. In terms of throughput within a given time period, significantly more virus-free plants can be produced by in vitro than in vivo heat therapy, and meristem tip can be used as a propagule for cryopreservation. In cross-border exchange of planting materials, on account of the reduced risk some countries are less restrictive in their quarantine regulations of in vitro cultures (Salih et al. Citation2001). Nonetheless, the challenges of optimizing in vitro conditions, in particular the growth media, should be acknowledged. Some crop species/varieties are recalcitrant to growth under in vitro conditions (Sriskandarajah et al. Citation1982), and differing genotype-specific responses are common in virus elimination experiments. Moreover, despite its advantages, the application of in vitro techniques is limited owing to the initial cost of the laboratory facilities. That being said, many stakeholders could benefit from implementing these techniques thereby enabling an increase in the timely movement of materials, facilitating domestic and international trade, and accelerating research into these commodities.
Acknowledgements
We would like to thank Dr Thomas Niederberger, Allison Gratz and Michelle Cooper for reading our manuscript and giving constructive comments and criticism.
Additional information
Funding
References
- Adams IP, Glover RH, Monger WA, Mumford R, Jackeviciene E, Navalinskiene M, Samuitiene M, Boonham N. 2009. Next-generation sequencing and metagenomics analysis: a universal diagnostic tool in plant virology. Mol Plant Pathol. 10:537–545. doi:https://doi.org/10.1111/j.1364-3703.2009.00545.x.
- Al Rwahnih M, Daubert S, Golino D, Islas C, Rowhani A. 2015. Comparison of next-generation sequencing versus biological indexing for the optimal detection of viral pathogens in grapevine. Phytopathology. 105:758–763. doi:https://doi.org/10.1094/PHYTO-06-14-0165-R.
- Anonymous. 2017. National clean plant network. [accessed 2020 Mar 16]. http://nationalcleanplantnetwork.org/files/264521.pdf.
- Barba M, Martino L, Lauretti F. 1992. Comparison of different methods to produce virus free stone fruits. Acta Hortic. 309:385–392. doi:https://doi.org/10.17660/ActaHortic.1992.309.57.
- Beauvieux R, Wenden B, Dirlewanger E. 2018. Bud dormancy in perennial fruit tree species: a pivotal role for oxidative cues. Front Plant Sci. 9:657. doi:https://doi.org/10.3389/fpls.2018.00657.
- Bhojwani SS, Razdan MK. 1996. Plant tissue culture: theory and practice. Amsterdam: Elsevier.
- Bomer M, Rathnayake AI, Visendi P, Sewe SO, Sicat JPA, Silva G, Kumar PL, Seal SE. 2019. Tissue culture and next-generation sequencing: a combined approach for detecting yam (Dioscorea spp.) viruses. Physiol Mol Plant Pathol. 105:54–66. doi:https://doi.org/10.1016/j.pmpp.2018.06.003.
- Bustin S, Huggett J. 2017. qPCR primer design revisited. Biomol Detect Quantif. 14:9–28. doi:https://doi.org/10.1016/j.bdq.2017.11.001.
- Cembali T, Folwell RJ, Wandschneider P, Eastwell KC, Howell WE. 2003. Economic implications of a virus prevention program in deciduous tree fruits in the US. Crop Prot. 22:1149–1156. doi:https://doi.org/10.1016/S0261-2194(03)00156-X.
- Chen Y, Huang J, Hou T, Pan I. 2019. Effects of light intensity and plant growth regulators on callus proliferation and shoot regeneration in the ornamental succulent Haworthia. Bot Stud. 60(1):10. doi:https://doi.org/10.1186/s40529-019-0257-y
- Diener TO. 1963. Physiology of virus-infected plants. Annu Rev Phytopathol. 1(1):197–218. doi:https://doi.org/10.1146/annurev.py.01.090163.001213
- Dziedzic E. 2008. Elimination of Prunus necrotic ring spot virus (PNRSV) from plum ‘Earliblue’ shoots through thermotherapy in vitro. J Fruit Ornam Plant Res. 16:101–109.
- Foissac X, Svanella-Dumas L, Gentit P, Dulucq MJ, Marais A, Candresse T. 2005. Polyvalent degenerate oligonucleotides reverse transcription-polymerase chain reaction: a polyvalent detection and characterization tool for trichoviruses, capilloviruses, and foveaviruses. Phytopathology. 95:617–625. doi:https://doi.org/10.1094/PHYTO-95-0617.
- Gella R, Errea P. 1998. Application of In Vitro therapy for Ilarvirus elimination in three Prunus species. J Phytopathol. 146:445–449. doi:https://doi.org/10.1111/j.1439-0434.1998.tb04779.x.
- Gergerich RC, Welliver RA, Gettys G, Osterbauer NK, Kamenidou S, Martin RR, Golino DA, Eastwell K, Fuchs M, Vidalakis G, et al. 2015. Safeguarding fruit crops in the age of agricultural globalization. Plant Dis. 99(2):176–187. doi:https://doi.org/10.1094/PDIS-07-14-0762-FE
- Golino DA, Fuchs M, Sim S, Farrar K, Martelli GP. 2017. Improvement of grapevine planting stock through sanitary selection and pathogen elimination. In: Meng B, Martelli GP, Golino DA, Fuchs M, editors. Grapevine viruses: molecular biology, diagnostics and management. Cham: Springer International Publishing; p. 561–579.
- Gratz A. 2020. WERA_OLD20: virus and virus-like diseases of fruit trees, small fruits, and grapevines. [accessed 2020 May 22]. https://www.nimss.org/projects/13496.
- Gribble K. 1999. The influence of relative humidity on vitrification, growth and morphology of Gypsophila paniculata L. Plant Growth Regul. 27:181–190. doi:https://doi.org/10.1023/A:1006235229848.
- Grondeau C, Samson R, Sands DC. 1994. A review of thermotherapy to free plant materials from pathogens, especially seeds from bacteria. CRC Crit Rev Plant Sci. 13(1):57–75. doi:https://doi.org/10.1080/07352689409701908
- Guidoni S, Mannini F, Ferrandino A, Argamante N, Di Stefano R. 1997. The effect of grapevine leafroll and rugose wood sanitation on agronomic performance and berry and leaf phenolic content of a Nebbiolo clone (Vitis vinifera). Am J Enol Vitic. 48:438–442.
- Halliday KJ, Martínez-García JF, Josse EM. 2009. Integration of light and auxin signaling. Cold Spring Harb Perspect Biol. 1:a001586–a001586. doi:https://doi.org/10.1101/cshperspect.a001586.
- Hansen J. 1989. Antiviral chemicals for plant disease resistance. Crit Rev Plant Sci. 8:45–88. doi:https://doi.org/10.1080/07352688909382270.
- Hartmann HT, Kester DE, Davies FT, Geneve RL. 2011. Hartmann and Kester’s plant propagation: principles and practices. 8th ed. Upper Saddle River: Prentice Hall.
- Hipper C, Brault V, Ziegler-Graff V, Revers F. 2013. Viral and cellular factors involved in phloem transport of plant viruses. Front Plant Sci. 4:154. doi:https://doi.org/10.3389/fpls.2013.00154.
- Knapp E, Hanzer V, Weiss H, da Câmara Machado A, Weiss B, Wang Q, Katinger H, Laimer da Câmara Machado M. 1995. New aspects of virus elimination in fruit trees. Acta Hort. 386:409–418. doi:https://doi.org/10.17660/ActaHortic.1995.386.56.
- Kunkel LO. 1936. Heat treatments for the cure of yellows and other virus diseases of peach. Phytopathology. 26:809–830.
- Kurokura T, Mimida N, Battey NH, Hytönen T. 2013. The regulation of seasonal flowering in the Rosaceae. J Exp Bot. 64(14):4131–4141. doi:https://doi.org/10.1093/jxb/ert233
- Li B, Feng C, Hu L, Wang M, Wang Q. 2016. Shoot tip culture and cryopreservation for eradication of apple stem pitting virus (ASPV) and apple stem grooving virus (ASGV) from apple rootstocks ‘M9ʹ and ‘M26ʹ. Ann Appl Biol. 168:142–150. doi:https://doi.org/10.1111/aab.12250.
- Lozoya-Saldana H, Dawson WO. 1982. Effect of alternating temperature regimes on reduction or elimination of viruses in plant tissues. Phytopathology. 72:1059–1064. doi:https://doi.org/10.1094/Phyto-72-1059.
- Martin RR, Tzanetakis IE. 2014. Pathogen-tested planting material. Encycl Agric Food Syst 4:304–312.
- Menzel W, Jelkman W, Maiss E. 2002. Detection of four apple viruses by multiplex RT-PCR assays with coamplification of plant mRNA as internal control. J Virol Methods. 99:81–92. doi:https://doi.org/10.1016/S0166-0934(01)00381-0.
- Monis J. 2020. Red blotch disease: update & importance of producing clean planting stock. [accessed 2020 Mar 16]. https://thegrapevinemagazine.net/2020/01/red-blotch-disease-update-importance-of-producing-clean-planting-stock/.
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 15(3):473–497. doi:https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
- Nissen S, Sutter E. 1990. Stability of IAA and IBA in nutrient medium to several tissue culture procedures. HortScience. 25:800–802. doi:https://doi.org/10.21273/HORTSCI.25.7.800.
- Obrępalska-Stęplowska A, Renaut J, Planchon S, Przybylska A, Wieczorek P, Barylski J, Palukaitis P. 2015. Effect of temperature on the pathogenesis, accumulation of viral and satellite RNAs and on plant proteome in peanut stunt virus and satellite RNA-infected plants. Front Plant Sci. 6:903. doi:https://doi.org/10.3389/fpls.2015.00903.
- Pallas V, Garcıia JA. 2011. How do plant viruses induce disease? Interactions and interference with host components. J Gen Virol. 92:2691–2705. doi:https://doi.org/10.1099/vir.0.034603-0.
- Panattoni A, Luvisi A, Triolo E. 2013. Review. Elimination of viruses in plants: twenty years of progress. Span J Agric Res. 11:173–188. doi:https://doi.org/10.5424/sjar/2013111-3201.
- Richert-Pöggeler KR, Minarovits J. 2014. Diversity of latent plant-virus interactions and their impact on the virosphere. Plant Virus Host Interact. 14:263–275.
- Roossinck MJ. 2010. Lifestyles of plant viruses. Philos Trans R Soc Lond B Biol Sci. 365:1899–1905. doi:https://doi.org/10.1098/rstb.2010.0057.
- Rott M, Xiang Y, Boyes I, Belton M, Saeed H, Kesanakurti P, Hayes S, Lawrence T, Birch C, Bhagwat B, et al. 2017. Application of next generation sequencing for diagnostic testing of tree fruit viruses and viroids. Plant Dis. 101:1489–1499. doi:https://doi.org/10.1094/PDIS-03-17-0306-RE
- Salih S, Waterworth H, Thompson DA. 2001. Role of plant tissue cultures in international exchange and quarantine of germplasm in the United States and Canada. HortScience. 36(6):1015–1021. doi:https://doi.org/10.21273/HORTSCI.36.6.1015
- Sim ST, Khuu N, Shoulders JR, Pudlo W, Hoang NH, Golino DA. 2019. Elimination of rose viruses using microshoot tip tissue culture. Acta Hortic. 1232:241–246. doi:https://doi.org/10.17660/ActaHortic.2019.1232.35.
- Speiegel S. 1998. Virus certification of strawberries. In: Hadidi A, Khetarpal RK, Hoganezawa H, editors. Plant virus disease control. St. Paul (MN): APS Press; p. 320–324.
- Spiegel S, Frison E, Converse R. 1993. Recent developments in therapy and virus-detection procedures for international movement of clonal plant germplasm. Plant Dis. 77(12):1176–1180. doi:https://doi.org/10.1094/PD-77-1176
- Sriskandarajah S, Mullins MG, Nair Y. 1982. Induction of adventitious rooting in vitro in difficult-to-propagate cultivars of apple. Plant Sci Lett. 24:1–9. doi:https://doi.org/10.1016/0304-4211(82)90002-5.
- Stahlberg A, Kubista M. 2014. The workflow of single-cell expression profiling using quantitative real-time PCR. Expert Rev Mol Diagn. 14(3):323–331. doi:https://doi.org/10.1586/14737159.2014.901154
- Tan R, Wang L, Hong N, Wang G. 2010. Enhanced efficiency of virus eradication following thermotherapy of shoot-tip cultures of pear. Plant Cell Tiss Organ Cult. 101:229–235. doi:https://doi.org/10.1007/s11240-010-9681-0.
- Verma N, Ram R, Zaidi AA. 2005. In vitro production of Prunus necrotic ringspot virus-free begonias through chemo- and thermotherapy. Sci Hortic. 103:239–247. doi:https://doi.org/10.1016/j.scienta.2004.05.005.
- Vivek M, Modgil M. 2018. Elimination of viruses through thermotherapy and meristem culture in apple cultivar ‘Oregon Spur-II’. Virus Dis. 29:75–82. doi:https://doi.org/10.1007/s13337-018-0437-5.
- Wang M, Li B, Feng C, Wang Q. 2016. Culture of shoot tips from adventitious shoots can eradicate apple stem pitting virus but fails in apple stem grooving virus. Plant Cell Tiss Organ Cult. 125:283–291. doi:https://doi.org/10.1007/s11240-016-0948-y.
- Wang MR, Chen L, Zhang Z, Blystad DR, Wang QC. 2018a. Cryotherapy: a novel method for virus eradication in economically important plant species. Methods Mol Biol. 1815:257–268.
- Wang MR, Cui ZH, Li JW, Hao XY, Zhao L, Wang QC. 2018b. In vitro thermotherapy-based methods for plant virus eradication. Plant Methods. 14:87. doi:https://doi.org/10.1186/s13007-018-0355-y.
- Wang Q, Valkonen JPT. 2009. Cryotherapy of shoot tips: novel pathogen eradication method. Trends Plant Sci. 14(3):119–122. doi:https://doi.org/10.1016/j.tplants.2008.11.010
- Wang QC, Panis B, Engelmann F, Lambardi M, Valkonen JPT. 2009. Cryotherapy of shoot tips: a technique for pathogen eradication to produce healthy planting materials and prepare healthy plant genetic resources for cryopreservation. Ann Appl Biol. 154:351–363. doi:https://doi.org/10.1111/j.1744-7348.2008.00308.x.
- Welsh MF, Nyland G. 1965. Elimination and separation of viruses in apple clones by exposure to dry heat. Can J Plant Sci. 45:443–454. doi:https://doi.org/10.4141/cjps65-087.
- Westerfield B, Krewer GW 2015. Propagating deciduous fruit plants common to Georgia. University of Georgia Extension. [accessed 2020 Mar 2]. https://secure.caes.uga.edu/extension/publications/files/pdf/B%20818_5.PDF.
- Westwood MN. 1989. Maintenance and storage: clonal germplasm. Plant Breed Rev. 7:111–128.
- Wood GA. 1973. Application of heat therapy for the elimination of viruses from pip and stone fruit trees in New Zealand. New Zealand J Agric Res. 16(2):255–262. doi:https://doi.org/10.1080/00288233.1973.10421144
- Zhao L, Wang MR, Cui ZH, Chen L, Wang QC. 2018. Combining thermotherapy with cryotherapy for efficient eradication of apple stem grooving virus (ASGV) from infected apple in vitro shoots. Plant Dis. 102:1574–1580. doi:https://doi.org/10.1094/PDIS-11-17-1753-RE.

