Abstract
The use of modified carbohydrates, such as sugar amino acids (SAA), iminosugars and policyclic derivatives, as scaffolds for the generation of bioactive compounds, and the use of carbohydrates as building blocks or ligands for the production of polymers for biomedical applications, is reviewed.
Introduction
Monosaccharides are one of the relevant classes of natural compounds that, like amino acids, constitute the building blocks for the generation of the polymers of life. It is well known that, through the variation of the anomeric configuration and the position of the hydroxyl group involved as acceptor in glycosidic linkages, carbohydrates exploit their great diversity potential, exerting an impressive role in biological recognition phenomena.
As a matter of fact, carbohydrates present unique features widely exploited by nature: (1) their cyclic structure guarantees an adequate conformational rigidity, (2) the presence of multiple hydroxyl groups provides different positions for linkages, and (3) the chirality provides different orientations of the hydroxyl groups and therefore different directions for the substituents linked to them. In other words, nature exploits carbohydrates as “scaffolds” to build up natural molecular architectures.
Taking advantage of this concept, recently synthetic chemists started to exploit carbohydrates as scaffolds for the generation of a variety of non‐natural potentially bioactive compounds.
Using carbohydrates as scaffolds, nature is very heterogeneous: other sugars, but also lipids, peptides, phosphates, and sulphates, are linked to the different hydroxyl groups providing the required diversity as exemplified in .
Figure 1: Examples of how nature exploits carbohydrates as scaffolds that link other sugars, lipids, peptides, and phosphates in well‐defined positions and orientations.
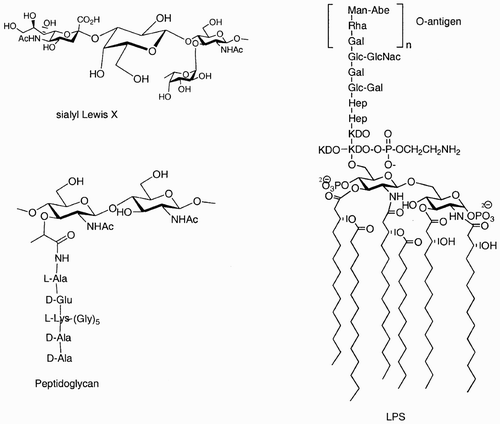
In sialyl Lewis X, a monomer such as galactose, highlighted in the figure, links sugar residues in two different positions. In peptidoglycan, N‐acetylmuramic acid links a sugar (GlcNAc) and a peptide. In lipopolysaccharide (LPS), a glucosamine scaffold links in different positions not only another glucosamine and a complex saccharidic chain, but also lipidic chains and a phosphate.
Also, synthetic chemists exploited their fantasy, using natural and modified monosaccharides, oligosaccharides, and glycomimetics as scaffolds for the generation of libraries of compounds for pharmacological screening, as well as for the production of biomaterials for tissue engineering and as molecular tools for the generation of nanostructures. This review will provide an overview of the products of this fantasy. It does not have the ambition to be exhaustive.
Scaffolds Derived from Natural Monosaccharides and their use for the Generation of Libraries of Bioactive Compounds
The use of carbohydrates as building blocks for the generation of libraries of biologically active compounds is relatively recent, Hirschmann, et al. using for the first time in 1992 a β‐D‐glucoside scaffold as a peptidomimetic targeting the somatostatin receptors.Citation1 The field has known a rapid and diverse development, partially covered in some reviews.Citation2
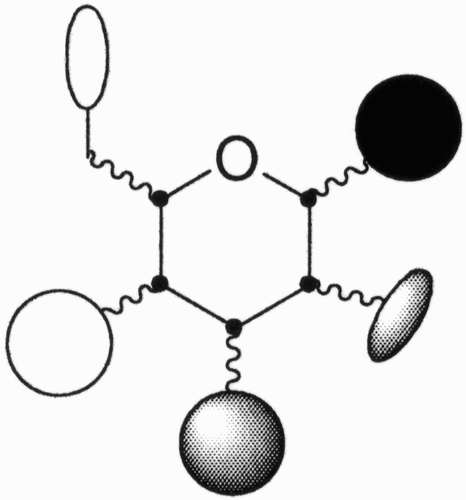
There are a number of factors that make sugars, monosaccharides in particular, attractive molecular scaffolds: availability, high functionalization, chirality, and structural rigidity. Schematically, in an hexopyranosidic scaffold (), diversity can be generated by the five functional groups present at carbon atoms C(1)–C(4) and C(6) as well as by the five contiguous stereocenters at carbon atoms C(1)–C(5).
These characteristics have been exploited in the construction of bioactive compounds following two different (we would say opposite) philosophies. For one side, well‐defined mimics of known bioactive compounds have been built up, properly exploiting the structure of the sugar scaffold; from the other side, libraries of diverse compounds bearing various pharmacophores in a combinatorial approach have been produced, exploiting the points of diversity intrinsic in the sugar structure.
Target Oriented Synthesis of Bioactive Compounds using Natural Monosaccharide Scaffolds
Examples of application of the first “philosophy,” which can be defined as target oriented synthesis of bioactive compounds using carbohydrate scaffolds, have been described in the field of peptidomimetics.
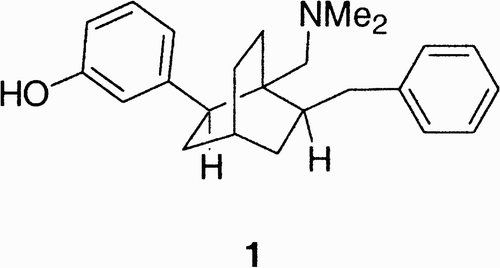
There are a large number of peptides with potential therapeutic interest that display limited biostability (due to proteases hydrolysis) and poor oral activity, limiting thus the application of peptide drugs.Citation3 One solution to this problem is the design of peptidomimetics in which the amide backbone is substituted with a different skeleton while maintaining the proper orientation of the amino acidic substituents.Citation4 While the design of nonpeptide peptidomimetics using novel scaffolds was anticipated by Farmer in 1980,Citation5 when he proposed the attachment of side chains to a cyclohexane ring, the first to synthesize a non peptide peptidomimetic (1, ) were Belanger and DuFresne.Citation6
Compound 1 featured a bicyclooctane core with novel scaffolding, being recognized by the opiate receptor for which it was designed.
PapageorgiouCitation7 and Hirschmann et al.Citation1 Citation8 first introduced peptidomimetics that possessed a carbohydrate backbone. In both cases it was desired to create mimics of the hormone somatostatin (SRIF), a cyclic tetradecapeptide with a wide variety of biological activity, most of it inhibitory in nature,Citation9 but that displays a very short biological half‐life.Citation10
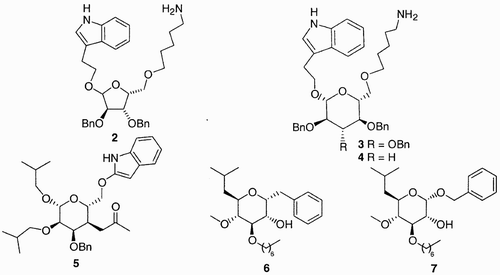
Papageorgiou et al. utilized the tetrasubstituted xylofuranose 2 () as scaffold for mimicking somatostatin,Citation7 while Hirschmann et al. attached the amino acid side chains of somatostatin and its analogs to a glucose scaffold, compounds 3 and 4, that would maintain the functional groups in the bioactive conformation.Citation8
Since these original exploitations of carbohydrates as templates for peptidomimetics, the sugar skeleton, mostly in its pyranosidic form, has been largely utilized as scaffold for the design of various bioactive compounds. Glucose‐based mimics of the depsipeptide hapalosin, such as 6 and 7, were also synthesized.Citation11 Glucose and allose scaffolds were used for the design and synthesis of mimics of the cyclic peptide endothelin antagonist BQ123 (5, ).Citation12 Other examples are reported by Murphy et al.,Citation13 Locardi et al.,Citation14 Le Diguarher,Citation12 Wessel et al.,Citation15 and Hanessian et al., Citation16 who synthesized mimics of other pharmacologically relevant peptides, taking advantage of the multifunctionality of a sugar.
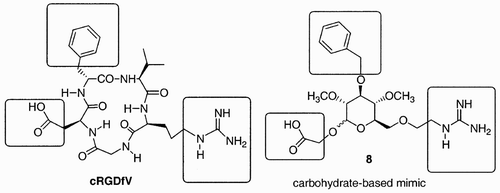
Another example of target oriented synthesis of bioactive molecules based on a carbohydrate scaffold is that reported by Nicolaou et al.,Citation17 who designed carbohydrate mimics of the cyclic peptide cRGDFV (), an antagonist of vitronectins, αvβ3, natural ligands to integrins, which are a class of extracellular proteins that facilitate cell‐cell recognition.
Target Oriented Synthesis of Bioactive Compounds using Sugar Amino Acids
A different approach to the generation of peptidomimetics emerged, in which various synthetic strategies were used to attach the amino acid functionalities directly to the carbohydrate skeleton and to generate thus synthetic sugar amino acids (SAAs).Citation18 Citation19 SAAs are well spread in nature,Citation20 and a well‐known example is the sialic acid family widely found peripherically on glycoproteins.
SAAs have been synthesized since the 1950s,Citation21 but were utilized as biopolymer building blocks to mimic oligo‐ and polysaccharide structures. A great variety of these examples have been reportedCitation22 and reviewedCitation23, which take advantage of the fact that well‐established peptide synthesis methodologies both in solid phase and in solution can be exploited for the synthesis of carbopeptoids oligomers. Furthermore, the folding properties of those oligomeric carbopeptoids have attracted interest.Citation24
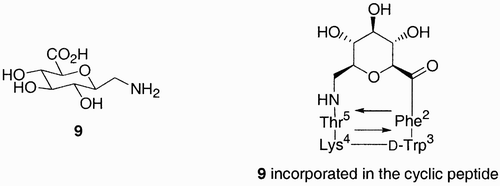
The first example of a sugar amino acid synthesized to be used as a peptidomimetic comes from the work of von Roedern and Kessler.Citation25 Glucosyluronic acid 9, () was incorporated into a cyclic peptide with the β‐turn motif of the somatostatin containing tetrapeptide Phe‐D‐Trp‐Lys‐Thr.
Starting from sugar β‐amino acids, in which the β‐carbon is the anomeric center of a furanoid sugar,Citation26 Taillefumier et al. recently reported the first synthesis of anomeric spiroannelated glycodiazepines,Citation27 compounds discussed in “Diversity in spirocyclic systems,” as potential new templates for biological tools and peptidomimetic scaffolds.
Libraries Generated from Carbohydrate Scaffolds
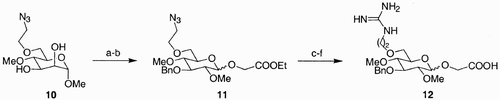
The generation of a library of compounds can follow two different approaches: it can be constructed through a parallel synthesis of individual targets or adopt a combinatorial approach. Initial efforts in this area were dedicated mostly to designing and synthesizing building blocks that would be then incorporated in cyclic peptides; the possibility of molecular scaffolding (i.e., construction of libraries from the structural variety present in the building block) being somewhat less explored. An example of the first type of approach is that reported by Nicolaou et al. Citation28 for the generation of carbohydrate mimetics of the cyclic peptide cRGDFV. Using molecular calculations (Insight‐Discover, CV‐Force Field), several structures of carbohydrate‐based mimics were minimized, and as a result, a small library of nine compounds emerged. Each of the components of the library was then subjected to synthesis, and an example is outlined in . Starting with methyl α‐D‐mannopyranoside, compound 10 was prepared in a sequence of selective protection/deprotection reactions. Methyl mannoside 10 was selectively O‐benzylated at C(3) and then exposed to diethylaminosulfur trifluoride (DAST), to provide 11 after treatment with HOCH2CO2Et. During the latter process, the C(1) methoxide migrated to C(2), with simultaneous inversion of configuration, and a 2:3 mixture of α‐ and β‐anomeric fluorides was formed. When this mixture was subjected to excess of 2‐hydroxyethyl acetate, in the presence of Cp2ZrCl and AgClO4, glycosides 11 were obtained, precursors to the targeted mimetics. Further manipulations of the azide and ethyl ester moieties provided scaffolds 12, which contained on the side chains the necessary guanidine and carboxylic acid functionalities.
Scheme 1: Reagents and conditions: a) i. n‐Bu2SnO, MeOH, Δ; ii. BnBr, CsF, DMF, 25°C, 14 h, 93%; b) DAST, CH2Cl2, 40°C, 4 h, 63% (α/β, 2:3); c) Cp2ZrCl2, AgClO4, HOCH2CO2Et, 4 Å mol. sieves, PhH, 25°C, 4 h, 55% (α/β, 2:3); d) Ph3P, THF, H2O, 65°C, 4 h, 85%; e) LiOH, THF/H2O (8:1), 25°C, 4 h; f) 1H‐pyrazole‐1‐carboxamidine · HCl, i‐Pr2NEt, DMF, 25°C, 16 h, 80% over two steps.
The previously described results open the way to the second “philosophy” concerning the use of carbohydrate scaffolds, which takes advantage of the sugar diversity and multifunctionality to generate libraries of compounds for high throughput screening in drug research. By manipulations of the diversity points present in a monosaccharide, through both a careful choice of orthogonal protecting groups and configurational interconversions, libraries of structurally related compounds (same molecular weight, same pharmacophoric groups, comparable solubility, different spacial orientation, hence different biological properties) can be achieved. Citation2c Citation29 Citation30 Since the diversity at the chiral centers is readily available from nature, most of the studies to date explored the diversity offered by the selective functional group of protection/deprotection, at three,Citation31 Citation32 four, and all five positions.Citation33 Citation34
The preferred way of introducing three pharmacophores on a sugar monomer is by having two functionalities orthogonally protected, while the third functionality (usually at C(1) or at C(6)) is linked to a solid support and is removed last.Citation32 An example in which the C(6)‐functionality of a glucoside‐based scaffold is linked to a polymer comes from Sofia et al.'s work.Citation31 Amino glucoside 13 (), containing three points of diversity, at C(2), C(4) and C(6), was prepared from glucose in seven steps and an overall 22% yield.
Scheme 2: Reagents and conditions: a) HATU, DIPEA, DMF, rt 100%; b) 0.5 M i‐PrNCO/DMF, Cu(I)Cl, rt 100%; c) 20% piperidine/DMF, rt d) 4‐NO2C6H4COOH, HATU, DIPEA, DMF, rt e) 10% TFA/1,2‐dichloroethane, rt 100%.
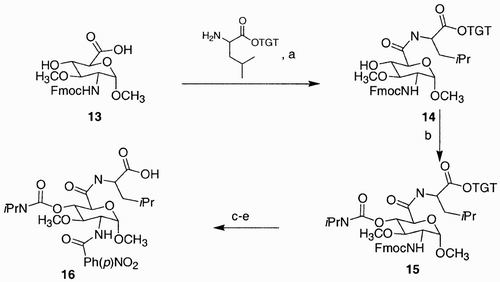
Coupling of 13 with eight amino acid‐functionalized trityl‐Tentagel resins, using O‐(7‐azabenzotriazol‐1‐yl)‐1,1,3,3‐tetramethyluronium hexafluorophosphate (HATU) as coupling agent, in the presence of diisopropylethyl amine, generated new scaffolds of type 14, featuring two points of diversity, at C(2) and at C(4). Treatment of 14 with isopropyl isocyanate introduced the carbamate pharmacophore at C(4); subsequent deprotection of the fluorenylmethoxy carbonyl group using piperidine and condensation of the resulting free amine moiety with p‐nitrobenzoic acid introduced the last pharmacophore at C(2). Final cleavage from the solid support generated compound 16. In this manner, a 1,648‐member sublibrary of biologically active compounds was created.
A different perspective on creating molecular diversity is given by Emmerson et al.,Citation35 who rather than manipulating only the functional diversity present in monosaccharides, chose to exploit diversification through chirality interconversion as well. They prepared 4,6‐O‐benzylidene derivative 18 from readily available N‐acetyl‐D‐glucosamine 17 (). Manipulation of the stereochemistry at C(1)–C(3), and of the protecting groups at the amine moiety, generated 24 related compounds, out of which only three are depicted in . Compounds 19–21 were then used as chiral ligands for the asymmetric reduction of aldehydes with dialkyl zinc.
Scheme 3: Reagents and conditions: a) MeOH, AcCl, 100%; b) PhCH(OMe)2, p‐TsOH, DMF, 70°C, 69%; c) DMSO, (CF3CO)2O, Et3N, CH2Cl2, −78°C, 75%; d) L‐selectride, THF, −78°C, 60%; e) N2H4, 130°C, 88%; f) 1.1 eq. PrI, K2CO3, MeCN, reflux, 63%; g) H2O2, Na2WO4, MeOH, H2O, 46% then LiAlH4, THF, 0–50 °C, 28%.
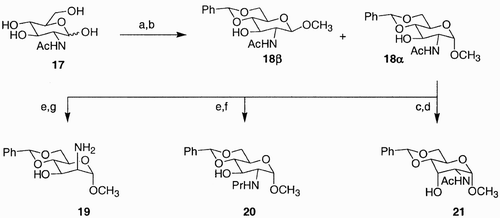
From the combinatorial chemistry point of view, compound 18 is a molecular scaffold with diversification of three sites. Stereochemical manipulations of 18 generate new scaffolds 19, 20, and 21, containing now two points of diversity, at C(2), and C(3), which could be further exploited through selective protection/deprotection strategies.
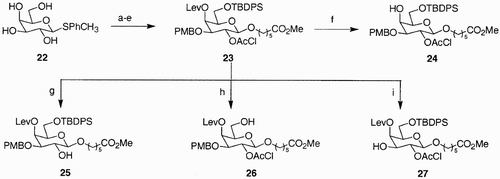
While linking one of the functionalities present in a sugar scaffold to a polymer support is a method mostly used when creating diversification at four sites of the scaffold,Citation9a Citation33 other approaches were also exploited, due to inconveniences of working in solid phase. Limitations in choice of the functional groups due to (a) sensitivity of linkers and pharmacophores to the deprotection conditions, (b) need of Williamson etherification as means of functionalization of free hydroxyl groups, and hence the stability to strong bases, are examples of such inconveniences, apart from the usual difficulties of solid phase (solubility, linker lability). In particular, orthogonality of protecting groups is particularly problematic when the points of diversity are expanded; acidic and/or basic conditions, oxidations, catalytic hydrogenations, use of fluoride ions to deprotect silyl ethers, isomerizations of double bonds, and photolysis can be exploited for deprotection, but very often protecting groups are labile to more than one of these treatments. Furthermore, after deprotection, a derivatization must be effected in experimental conditions, not interfering with the remaining protecting groups and with the linkages of the already introduced substituents. An approach coming from Wong et al.'s workCitation36 avoids the use of polymer support and exploits the different reactivity of the four hydroxyl groups of a thiol glycoside in the solution phase chemistry. For instance, orthogonally protected galactoside 23 () was rapidly synthesized by introducing the four orthogonal protecting groups, t‐butyldiphenylsilyl at C(6), followed by p‐methoxybenzyl at C(3), chloroacetyl at C(2), and finally, levulinyl at C(4). Final glycosylation with methyl 6‐hydroxyhexanoate gave the desired scaffold, which upon selective deprotection and subsequent glycosylation with seven donors generated a library of 45 protected oligosaccharides.
Scheme 4: Reagents and conditions: a) TBPSCl, imidazole, DMF, 100%; b) i. Bu2SnO, toluene, benzene, reflux; ii. PMBCl, Bu4NI, DMF, 60°C, 49%; c) ClCH2COCl, Et3N, CH2Cl2, −20°C to rt 52%; d) levulinic acid, DCC, DMAP, CH2Cl2, 83%, e) i. HO(CH2)5CO2Me, NIS, TMSOTf, 4 Å molecular sieves, CH3CN, −20°C to rt; ii. HgBr2, toluene, CH3NO2, 60°C, 85%; f) NH2‐NH2 AcOH, THF/MeOH, 10:1, 90%; g) NaHCO3, MeOH/H2O, 5:1, 60°C, 99%; h) HF‐pyridine, AcOH/THF, 1:4, 98%; i) CF3COOH, CH2Cl2, −20°C, 97%.
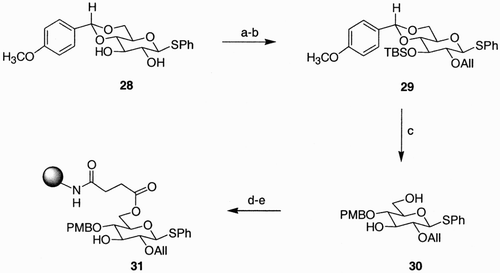
In our group, a glucoside scaffold presenting four points of diversity was efficiently prepared after linking the C(6) functionality to a polymer support.Citation32c Thus, treatment of the easily available benzylidene acetal 28 with t‐butyldimethylsilyl chloride in the presence of imidazole selectively provided the silyl ether at C(3) (). Subsequent allylation gave compound 29, which was then exposed to a LiAlH4‐AlCl3‐reducing system to afford reductive opening of the acetal with simultaneous hydrolysis of the silyl ether. Due to the low reactivity of the carboxy‐polystyrene resin toward compound 30, the latter was functionalized at C(6) with a succinate linker and then linked to an amino‐polystyrene resin, finally providing 31 with an acceptable loading value (0.9 mmol/g). In compound 31 the four diversity sites were generated by the thiol moiety at C(1), the allyl ether at C(2), the secondary unprotected hydroxyl group at C(3), and the p‐methoxybenzyl ether at C(4).
Scheme 5: Reagents and conditions: a) TBDMSCl, Imidazole, CH2Cl2, 94%; b) AllBr, NaH, DMF, 70%; c) LiAlH4‐AlCl3, CH2Cl2, Et2O, 82%; d) succinic anhydride, pyridine, DMAP; e) NH2‐PS/DV, HOBt, HBTU, DIPEA, DMF.
Most recently, Hunger et al. employed 6‐azido‐6‐deoxyglucosamine to generate diversity at four sites of the building block.Citation37 In scaffold 34 (), the anomeric center was linked to a polymeric support through a thioglycoside anchor and was not used as a source of diversity. Any of the protecting groups present in the molecule, an Alloc group at C(2), a tert‐butyldimethylsilyl ether at C(3), a p‐methoxybenzyl ether at C(4), and an azide at C(6), were selectively removed in the presence of the others, independently of the deprotection sequence and in quantitative yields.
Scheme 6: Reagents and conditions: a) TBAF, THF; b) DDQ, CH2Cl2, H2O (10 vol%); c) i. Bu3P, DMF; ii. Et3N, DMF, H2O; d) [Pd2(dba)3], pTsOH, DMF; all yields are quantitative.
![Scheme 6: Reagents and conditions: a) TBAF, THF; b) DDQ, CH2Cl2, H2O (10 vol%); c) i. Bu3P, DMF; ii. Et3N, DMF, H2O; d) [Pd2(dba)3], pTsOH, DMF; all yields are quantitative.](/cms/asset/6a4b407e-ab21-4454-a349-0d4d60bdf777/lcar_a_173266_o_sch0006g.gif)
The novelty brought by this approach is that a second generation of scaffolds could be created by replacing any of the protecting groups with a molecule containing a functionality corresponding to that of the replaced protecting group. This approach limits the request of orthogonalities as for each point of diversity, and protecting group and substituents are labile and stabile to the same experimental conditions.
An example of a glucoside scaffold in which all five hydroxyl groups were used to create diversity comes from the work of Opatz et al.Citation33c The previously synthesized thioglycoside 37 was converted into building block 38 in three steps (), involving hydrolysis of the succinimide ring, linkage of the resulting carboxylic acid to polymer support, and a final acetylation of the aminofunctions present on the polymer. In this manner, scaffold 38 presents an anomeric center anchored to polymer support, easily convertible into a O‐, N‐, or S‐glycoside and four orthogonal protecting groups, a t‐butyldiphenyl silyl ether at C(6), a 1‐ethoxyethyl group at C(4), an allyl ether at C(3), and an acetate at C(2). Using the sequence of deprotections depicted in , a library of 36 compounds type 40 was generated.
Scheme 7: Reagents and conditions: a) LiOH, THF, H2O, quant.; b) TOTU, HOBt, DIPEA, DMF, aminomethylpolystyrene; c) Ac2O, pyridine, dioxane, 98%; d) NH2‐NH2 H2O, DMF, e) KOtBu, DMF; f) MeBr, DMF; g) TBAF, THF; h) FC6H4NCO, DMAP, dioxane; i) PPTS, MeOH, dioxane; j) Steglich esterification at position 4; k) p‐TsOH, [Pd(PPh3)]4, DME, dioxane; l) NBS, EtOH, DTBP, CH2Cl2; m) Et4NBr, cyclohexene, CH2Cl2, 79% yield.
![Scheme 7: Reagents and conditions: a) LiOH, THF, H2O, quant.; b) TOTU, HOBt, DIPEA, DMF, aminomethylpolystyrene; c) Ac2O, pyridine, dioxane, 98%; d) NH2‐NH2 H2O, DMF, e) KOtBu, DMF; f) MeBr, DMF; g) TBAF, THF; h) FC6H4NCO, DMAP, dioxane; i) PPTS, MeOH, dioxane; j) Steglich esterification at position 4; k) p‐TsOH, [Pd(PPh3)]4, DME, dioxane; l) NBS, EtOH, DTBP, CH2Cl2; m) Et4NBr, cyclohexene, CH2Cl2, 79% yield.](/cms/asset/89939021-7bf3-4e10-84b9-9a2e3a926807/lcar_a_173266_o_sch0007g.gif)
Moitessier et al. constructed stereodiverse libraries based on a glycodic scaffold in which an amino group is linked through an appendage at the anomeric carbon, whereas a carboxylic function decorates one of the other hydroxyl groups of the sugar.Citation38 Compounds 41 (), based on a xylopyranosidic backbone, are mimetics of the RGD sequence, aiming to reproduce the RGD loop both in fibronectin (αΠb β3 integrin receptors) and vitronectin (αvβ3 integrin receptor).
The scaffold used for the construction of the library was D‐xylose, which possesses three equatorial hydroxyl groups with the same type of reactivity, and hence one source of diversity. The anomeric hydroxyl, the second diversity point, was selectively transformed into an allyl ether, and then the free hydroxyl groups of the resulting xyloside were subjected to alkylation with different stoichiometries of benzyl bromide.
Libraries Generated from Modified Sugar Amino Acid Scaffolds
We have already mentioned that the presence of a carboxylic and an amino function in a sugar molecule (SAA) is employed in the synthesis of pseudopeptides mimicking well‐defined bioactive peptides. SAAs have also found application in the synthesis of both small and big libraries of potentially bioactive compounds, exploiting not only the amino and acidic function in peptide synthesis, but also the diversity derived from chirality and derivatization of the different hydroxyl groups.
The earliest example of the potential of SAAS as building blocks for the creation of libraries comes from the work of McDevitt and Lansbury.Citation39 Twelve sugar amino acids were synthesized, from which 45–48 are depicted in and , and were then used to generate a small library of oligomers (such as 48) via oligomerization.
Scheme 8: Reagents and conditions: a) i. O3, MeOH, THF, −70°C; ii. NaBH4; b) PPh3, NBS, DMF, 50°C, c) C3H7NH2, DMF, 70°C, d) (Boc)2O, NEt3, CH2Cl2, e) TFA/H2O, 3:1.
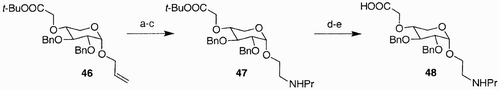
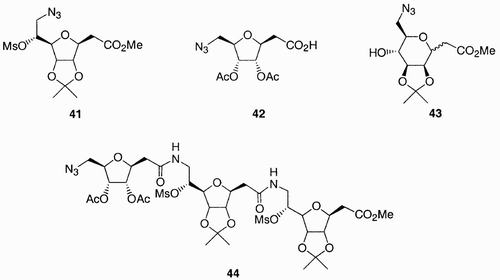
Both furanosidic and pyranosidic forms of sugars were used, and the library was created by manipulations of the azide and carboxylic acid moieties. The advantage of these types of compounds is that they may not be susceptible to proteases cleavage, due to altered backbone relative to natural peptides.
Edwards et al.Citation40 recently synthesized a library of 99 compounds from 3‐deoxy L‐lyxose scaffold 51 (), which was in turn prepared from the L‐gulonolactone monoacetonide derivative 49. Compound 51 contained three points of diversity generated by the azide moiety at C(4), the ester group at C(2), and the primary alcohol at C(5). Since the diversification at the primary hydroxyl group through alkylation was found to be problematic for a parallel synthetic approach, the alkyl group was introduced prior to diversification at the remaining functionalities.
Scheme 9: Reagents and conditions: a) 5 steps, 32%; b) Tf2O, Py, DCM; c) CsO2CCF3, butanone; d) MsCl, DMAP, pyridine, 100%; e) NaN3, DMF, 70%; HCl, MeOH, 78%; f) HCl, MeOH, rt, 73%; g) MeI/CH3CN (1:1), Ag2O, 80°C, 99%; h) H2, Pd/C 10%, EtOAc, i) i. ClC6H4NCO, CH2Cl2, rt, 16 h; ii. AMP's, rt, 16 h; j) PhC3H6NH2, MeOH, 60°C, 24 h, 83%.
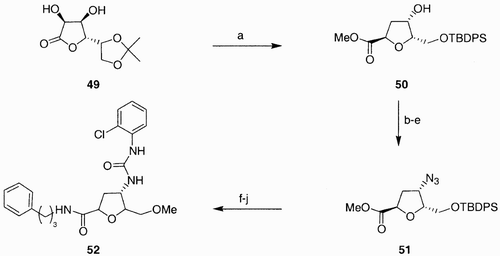
Thus, compound 51 was first methylated, and then the azide converted into urea, after hydrogenation, and finally the ester into an amide. Variation of the alkyl groups on the urea and on the ester generated the library. This work stresses again the value of furanose sugar amino acids as stereodiverse building blocks.
Scaffolds from Polycyclic Carbohydrate Derivatives
As outlined before, the conformational rigidity of carbohydrates represents one of the attractive features for using them as scaffolds for the design of bioactive molecules. By increasing the conformational rigidity of the parent carbohydrates, the generation of new classes of compounds with better scaffolding qualities is possible. Furthermore, the structural originality obtained by proper manipulations of the natural sugars allows patenting the obtained scaffold, which is of fundamental importance for using it in drug research. One intensively investigated way of reducing the molecular flexibility and generating original structures is the introduction of a second and even a third ring on the sugar backbone, generating a fused or a spirocyclic system.Citation41–43 Molecular diversity, and thus creation of libraries of such compounds, is obtained in most cases by exploitation of the functionalities originally present in the parent sugar, through generation of new functionalities during the construction of the polycycle, or both.
Diversity in Fused Polycyclic Systems
Timmer et al. recently synthesized a small library of pyranofurans from a mannitol‐derived scaffold (54, ) containing two points of diversity, originally present in the parent sugar.Citation44 The cis‐fused pyranofuran systems were prepared using a solid‐phase ring‐closing metathesis (RCM) strategy. Thus, diene 53, prepared in 12 steps from D‐(+)‐mannitol, was bound to a Rink amine resin, in the presence of BOP and diisopropyl amine. At this point, the molecular diversity present in 54 was exploited by first condensing the free secondary hydroxyl group with various isocyanates to the corresponding carbamates. Subsequent Staudinger reduction of the azide moiety followed by condensation with various acyl chlorides generated fully functionalized resins type 55. Exposure of 55 to 5 mol% of Grubbs catalyst (56) triggered not only the RCM, but also the cleavage of the products from the polymer. A small library of nine cis‐fused pyranofuranes of type 57 was created in this manner.
Scheme 10: Reagents and conditions: a) BOP, DIPEA, 16 h; b) Bn‐N˭C˭O, Et3N, 16 h; c) i. Me3P, THF, 1 h, then H2O/dioxane 2 h; ii. Ph2NCOCl, DIPEA, 16 h; d) 56 (5 mol%), CH2Cl2, reflux, 16 h, 98% overall yield.
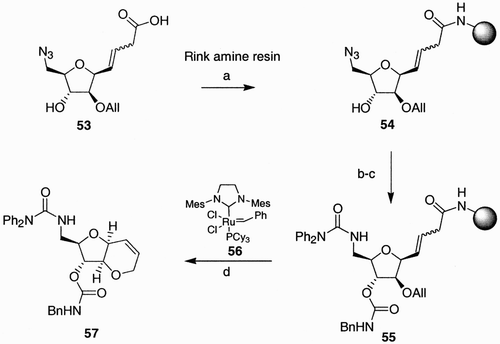
In our group, molecular diversity was introduced during the construction of the polycyclic system.Citation45 Bi‐ and tricyclic azido acids scaffolds were synthesized in liquid phase, starting from fructose. Thus, C‐allyl fructose derivative 58 (), prepared in 98% yield and 60% de upon treatment of methyl O‐tetrabenzyl fructoside with allyltrimethylsilane in the presence of borontrifluoride etherate, was submitted to iodocyclization conditions, by treatment with iodine in tetrahydrofurane, at low temperatures.
Scheme 11: Reagents and conditions: a) I2, THF; b) Zn, AcOH, EtOH/Et2O, 1:1; c) DMSO/Ac2O, 2:1; d) CH2˭CHCH2MgBr, THF; e) I2, THF, 0°C; f) I2, CH2Cl2, rt; g) NaN3, Bu4NI, DMF; h) NaClO2, NaH2PO4, CH3CN.
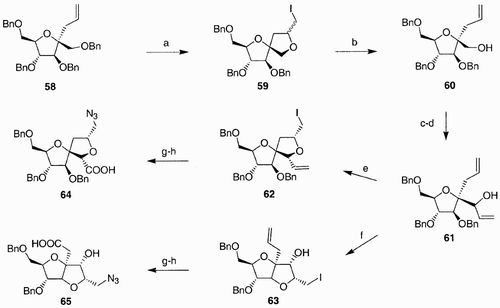
Compound 59 was then obtained as a mixture of diastereomers, which was further exposed to Zn in acetic acid, to generate the derivative 60 by reductive elimination. The oxidation of the free hydroxyl group in 60 to the corresponding aldehyde was followed by stereoselective addition of vinylmagnesium bromide, to generate the R allylic alcohol 61 in good yield and excellent diastereoselectivity. Compound 61 represented the key intermediate for the generation of both spiro (64) and fused (65) bicyclic scaffolds. Notably, when the 5‐exo‐trig cyclization was carried out in tetrahydrofuran, it involved the free hydroxyl group of 61 and generated the spirane 62, whereas in dichloromethane the iodocyclization afforded the fused compound 63. Manipulations of the iodine and olefin moieties in compounds 62 and 63 afforded finally bicyclic derivatives 64 and 65. Molecular diversity was generated in scaffolds 64 and 65 by the different spatial arrangement of the azido groups with respect to the carboxyl terminus, allowing thus the possible introduction of various pseudopeptide secondary structures.
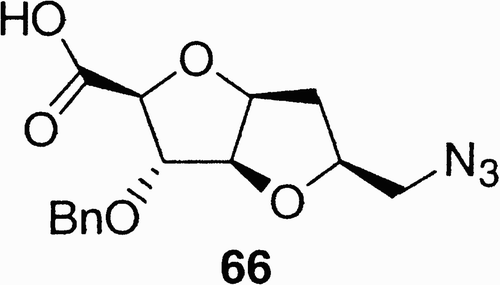
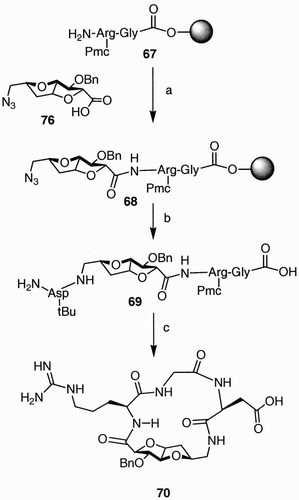
Using a similar approach, the fused bicyclic azido acid 66 () was obtained from D‐(+)‐arabinofuranose.Citation46 In compound 66, the secondary benzyl ether, the azide, and the carboxylic acid moieties create three points of molecular diversity in a conformationally constrained structure.
Once more the presence of the carboxylic and azido functions allowed the use of compound 66 in the synthesis of conformational constrained pseudopeptides. It has been calculated that the distance between these functions in compounds 66 (6 Å) corresponds to that of a β‐turn peptide structure; therefore, the synthesis of pseudopeptide 70 with the biologically relevant RGD sequence constrained in a clinked structure has been affected, exploiting the carbohydrate scaffold 66 as a peptide building block, as reported in .
Diversity in Spirocyclic Systems
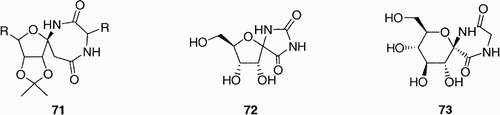
Taillefumier et al. recently reported the first synthesis of anomeric spiroannelated glycodiazepines,Citation47 of the general structure 71 (), as potential new templates for biological tools and peptidomimetic scaffolds. These compounds belong to the class of spironucleosides, which includes the naturally occurring (+)‐hydantocidin 72 Citation48 and the spirodiketopiperazine glucopyranose 73 Citation49 as depicted in .
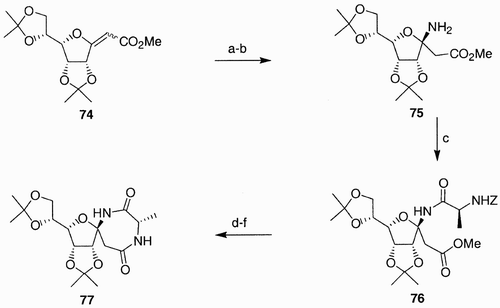
The preparation of structures like 71 is exemplified by the synthesis of glycodiazepine 77. The exo‐glycal 74 (), upon treatment with benzyl amine and then exposure to an atmosphere of hydrogen, provided the β‐amino acid 75. Coupling of the free amine in 75 with a range of N‐benzyloxycarbonyl (Z) α‐amino acids (in the example shown in , with α‐alanine) provided dipeptide 76, which was then converted to the target spirane following a sequence of reactions that included saponification of the methyl ester, hydrogenolysis of the Z group, and diphenylphosphoryl azide (DPPA) base cyclization.
Scheme 13: Reagents and conditions: a) BnNH2 (neat), 48 h; b) H2, 10% Pd/C, EtOAc; c) Z‐NHCH(CH3)CO2H, PyBOP, Et3N, DMF, rt, 14 h, 92% from 74; d) K2CO3, MeOH/H2O (10:1), rt, 48 h; e) H2, 10% Pd/C, EtOH/EtOAc (1.5:1); f) DPPA, Et3N, DMF, 0°C to rt, 14 h.
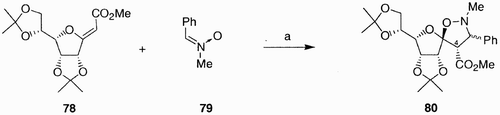
A different class of spiroheterocyclic compounds was obtained with good stereoselectivity and yields upon [3+2] cycloaddition of exo‐glycals with nitrones and nitrile oxides.Citation50 For instance, microwave‐activated cycloaddition of compound 78 () with nitrone 79 provided a spiro‐isoxazolidines 80 (ratio at C(3), S/R, 2:1). The formation of the new C(4) chiral center occurred with complete stereo control, the absolute (S) configuration at this center being determined by the (Z) geometry of the starting olefin.
The most used methods for the generation of spiro‐carbohydrate derivatives seem to be the ring‐closing metathesis and the Pauson‐Khand reaction.Citation51 In these cases, the spirane ring was created at the anomeric center of the sugar.
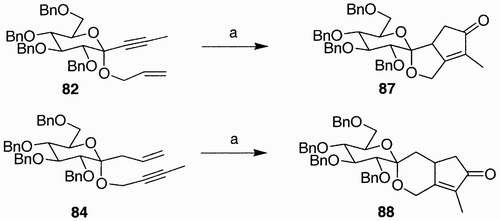
An illustrative example in which of spirosugar building blocks were generated via both ring‐closing metathesis and Pauson‐Khand reaction comes from the work of Leeuwenburgh et al.Citation52 Starting from tetra‐O‐benzyl gluconolactone, the synthesized ketoglycosidic enynes 82 and 84 ( Citation53) are common starting materials for the generation of two different types of oxacyclic scaffolds. On one hand, treatment of 82 and 84 with 5 to 7 mol% of Grubbs catalyst, in toluene, at 60°C, generated spiroacetals 85 and 86.
Scheme 15: Reagents and conditions: a) i. BrHC˭CHCH3, n‐BuLi, THF, −78°C; ii. AllBr, HMPA, −78°C to rt; b) ref. [51]; c) 2‐butyn‐1‐ol, K‐10, mol. sieves, CH2Cl2; d) Ru‐catalyst (5–7 mol%), toluene, 60°C.
![Scheme 15: Reagents and conditions: a) i. BrHC˭CHCH3, n‐BuLi, THF, −78°C; ii. AllBr, HMPA, −78°C to rt; b) ref. [51]; c) 2‐butyn‐1‐ol, K‐10, mol. sieves, CH2Cl2; d) Ru‐catalyst (5–7 mol%), toluene, 60°C.](/cms/asset/8d3e6c02-7be5-400a-ac6f-9391fb9fcd98/lcar_a_173266_o_sch0015g.gif)
Exposing compounds 82 and 84 to the Co(IV) complex and NMO, the more complex spiroacetals 87 and 88 were synthesized ().
Further exploitation of compounds 85–88 could lead to more complex spiroacetals by manipulation of the functionalities present in these molecules.
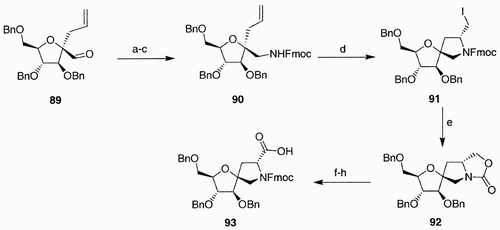
In our group, the spirane moiety was introduced at the anomeric center using the iodocyclization approach earlier described.Citation54 Starting from aldehyde 89 (), a sequence of reactions involved its conversion to the protected amine derivative 90, and iodocyclization of the latter provided the spirane 91. Hydrolysis of the fluorenyl ester generated spontaneously oxazilidinone 92, which, after ring opening upon Fmoc protection and Jones oxidation, afforded the spiro‐annulated D‐proline mimetic 93.
Scheme 17: Reagents and conditions: a) NH2OMe, THF, EtOH; b) LiAlH4, THF; c) FmocCl, DIPEA, CH3CN; d) I2, DME; e) NaOH 1 M, CH3CN; f) NaOH 3 M, EtOH reflux; g) FmocCl, DIPEA, CH3CN; h) Jones oxidation.
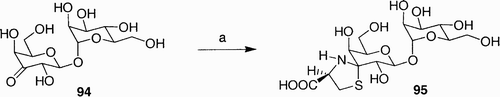
Shibuta et al. reported the preparation of two diastereomeric 1,1-linked galactosyl mannosides possessing a spiro-thiazine ring, which act as sialyl Lewis mimetics.[55] For example, when the unprotected ulo-disaccharide 94 was coupled with S-cysteine (), in the absence of an acid catalyst, diastereomer 95 was obtained in good yield.
Scaffolds from Iminosugars
Along with structurally modified carbohydrates, bioactive glycomimetics such as iminosugars, which are known to be potent inhibitors of carbohydrate‐processing enzymes, have been synthesized and used as scaffolds for the generation of libraries in search of improved selectivity and activity. These compounds are relatively laborious to synthesize, so the creation of a library based on iminosugars, rather than a parallel synthesis of individual structures, could facilitate the search for compounds with improved activity.
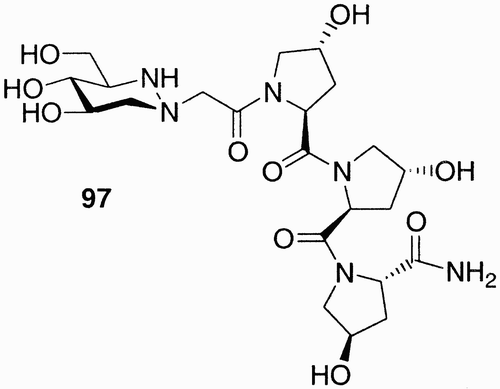
The first example of a library built on an iminosugar is the one reported by Lohse et al.Citation56 (). An analog of 1‐azafagomine, a potent glycosidase inhibitor, is linked to tripeptides obtained from five aminoacids using a combinatorial approach.
A library of 125 compounds was synthesized and investigated toward β‐glycosidase inhibition. One of the members of the library, compound 97 (), showed to be an inhibitor having a Ki value of 20 µM.
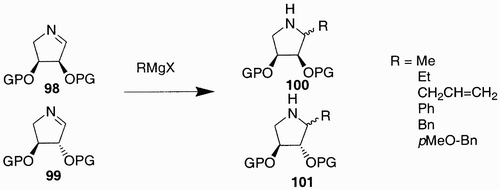
Chapman et al. realized a pyrrolidine derived libraryCitation57 by reacting different Grignard reagents with two cyclic imines 98 and 99 that can be considered sugar‐derived scaffolds. The diastereoisomeric iminosugars 100 and 101 were obtained ().
The Grignard addition allowed the introduction of a hydrophobic substituent, which in some cases proved to increase enzyme inhibition and bioavailability.
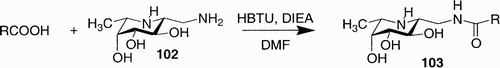
Wu et al.Citation58 realized a fuconojirimycin‐based library in search of fucosidase inhibitors. They obtained 60 compounds by condensing the amino group of fuconojirimycin derivative 102 with different carboxylic acids (). The reaction was carried out in the presence of HBTU (1 equiv.) DIEA (2 equiv.) in DMF, and the products were screened after dilution with H2O without further purification.
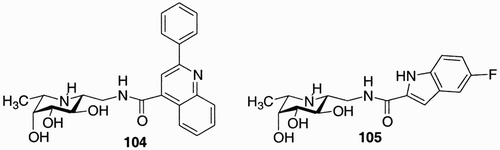
Within the library two compounds (104 and 105, ) showed the most potent inhibitor properties toward fucosidase known so far.
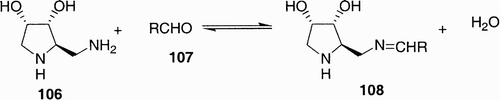
A very original “dynamic” library was that produced by Gerber‐Lemair et al.,Citation59 which is based on an equilibration between an iminosugar scaffold 106 and different aldehydes 107 to form the corresponding imines 108 (). The originality stays in the fact that all members of a library of aldehydes are reacted simultaneously with amine 106, generating a dynamic library of imines, which are incubated with the enzyme. The imine that is the best inhibitor is expected to bind preferentially to the enzyme, thus making possible a rapid assay of a large number of imines.
The library was tested as inhibitors towards α‐mannosidase, and the highest activities were found for aromatic aldehydes and in particular benzaldehyde and its substituted derivatives.
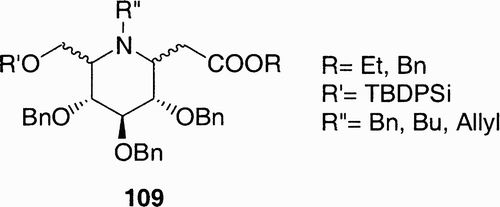
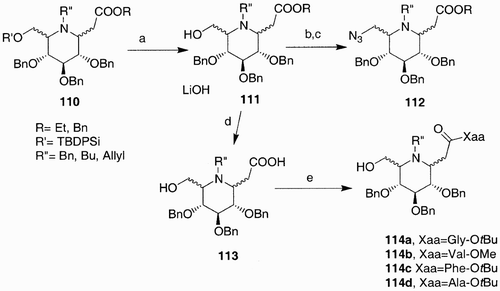
In these examples the iminosugar was first synthesized and subsequently derivatized in one position; more recently weCitation60 reported the synthesis of a small library of iminosugar scaffolds having various points of diversity: variable stereochemistry on ring substituents and on the nitrogen atom, a carboxymethyl functional group and orthogonal protections on the hydroxyl groups ().
With this approach a library based on eight scaffolds was obtained. Each member could then be selectively derivatized on the primary hydroxyl group, on the carboxylic function, and on the nitrogen, for the scaffolds bearing an N‐Bn or N‐allyl group after removal of the N‐substituents. Examples of scaffold derivatization are reported in ; an azido group was introduced exploiting the primary hydroxyl affording derivatives 110. The ester group was hydrolyzed and the carboxylic acid so obtained was condensed with different aminoacids affording derivatives 114.
Scheme 22: Reagents and conditions: a) TBAF, THF; b) MsCl, Py, CH2Cl2; c) NaN3, DMF; d) LiOH, MeOH/H2O/THF; e) HBTU, HOBT, DIPEA, DMF, Xaa.
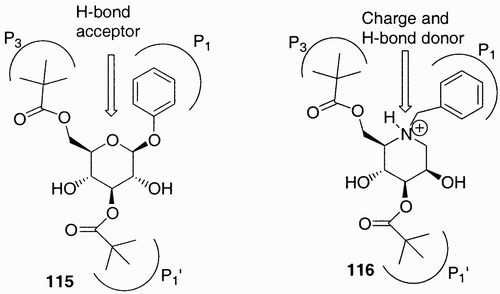
An iminosugar has been also used by Chery et al.Citation61 as scaffold in the synthesis of a peptidomimetic analog of a known HIV‐1 protease inhibitor. A β‐D‐glucopyranoside‐based scaffold (115, ) reported previously showed an activity that was hypothesized by the authors being related to a competitive binding in the enzyme active site. On this basis the authors designed and synthesized the 1‐deoxymannonojirimycin‐based analog (116, ) where the ring nitrogen, which is believed to be protonated at physiological pH, becomes a hydrogen bond donor that, from molecular modeling indications, could bind with a carbonyl group of HIV‐protease amide backbone.
Carbohydrate Scaffolds in Polymers
Synthetic polymers based on carbohydrate scaffolds have recently gained interest not only for their mechanic properties combined with the biodegradability, but also as biomaterials for biomedical applications.
Polymer Scaffolds for Tissue Engineering
Carbohydrate‐based polymer scaffolds have been and are extensively used in the field of tissue engineering. Every year millions of patients suffer the loss or failure of an organ or tissue as a result of accident or disease, and a revolutionary strategy to treat these patients is engineering a manmade organ or tissue. Polymer scaffolds, in this kind of strategy, are used to repair and regenerate tissue as they serve to support, reinforce, and organize regenerating tissue, and in some cases they also serve to release bioactive substances. For these applications it's fundamental that these polymer scaffolds display certain characteristics such as biocompatibility, low toxicity, a precise three‐dimensional microstructure, certain mechanical and physical properties, and biodegradability.
Among the great number of natural and synthetic polymers studied and developed for tissue engineering, carbohydrate‐based scaffolds, especially chitosan, hyaluronate, alginate, and agarose, find extensive application due to the fact that they frequently show adequate biocompatibility, are often biodegradable matrices, and can be derivatized to modulate their mechanical and physical properties for specific purpose.
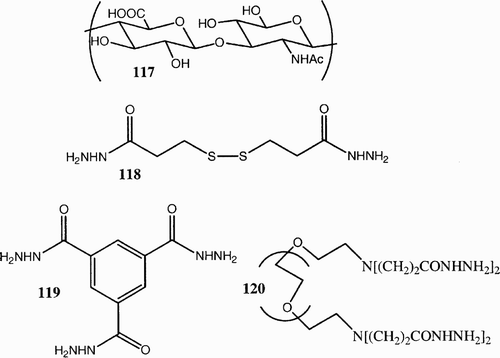
Hyaluronate 117 is one of the glycosaminoglycan components of extracellular matrix and has shown excellent potential for tissue‐engineering applications. Its structure () can be modeled through cross‐linking with various kind of hydrazide derivativesCitation62 Citation63 118–120 to form hydrogels useful as artificial skin,Citation64 wound healing,Citation65 facial dermal implants,Citation66 etc. Hyaluronate gels typically possess low mechanical properties, which have in part limited their application, but they can be manipulated in order to improve their properties. A very significant example is an esterified form of hyaluronan, Hyaff‐11®.
Figure 16: 117, Hyaluronic acid; 118, 3,3′‐dithiobis(propanoic dihydrazide); 119, 1,3,5‐benzene(tricarboxylic trihydrazide); 120, poly(ethylen glycol)‐diamine tetrapropanoic tetrahydrazide.
This biomaterial was obtained from the total esterification of hyaluronate with benzyl alcohol, and it consists of a linear polymer with high‐molecular‐weight distribution. It is insoluble in aqueous solution and ideal for cell growth. Its degradation time is around 40 days, and upon degradation it gives a gel similar to the native hyaluronan found in extracellular matrix. This scaffold has already proved to be an effective scaffold for skin and cartilage tissue engineering,Citation67–71 and seems to be a promising vascular scaffold.Citation72
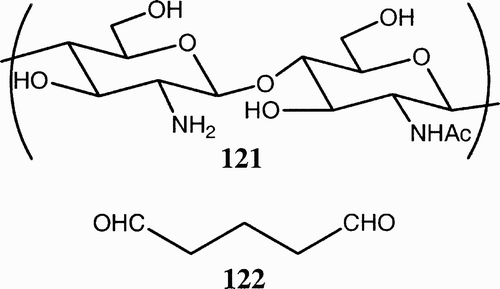
Chitosan (121, ), an amino polysaccharide (poly (1,4)‐d‐glucosamine), is a broadly applicable biomaterial. It is prepared by N‐deacetylation of chitin and usually contains less than 40% of N‐acetyl‐d‐glucosamine residues. Like hyaluronate, chitosan displays a good biocompatibility and low toxicity, structural similarity to natural glycosaminoglycans, and biodegradability operated by chitosanase and lysozyme. Many derivatives have also been developed to enhance solubility and processability of the polymer, such as cross‐linkage with glutaraldehydeCitation73 (122, ) and derivatization with azide,Citation14 or to enhance biological functions such as cellular interaction; this is the case of fructose‐or galactose‐modified chitosan for culture of hepatocytes.Citation75 In addition, a methylpyrrolidone‐derivatized chitosan has been reported to promote bone formation.Citation76
Alginate and agarose are both marine algae polysaccharides also widely used in the field. Alginate () has found use to date in many applications, but, despite its advantageous features of biocompatibility, low toxicity, and low cost, as such it is a poor biomaterial since it degrades easily by losing its cations in the surrounding medium and subsequent dissolution. To overcome the problem it has been covalently cross‐linked with various molecules.Citation77 Another limitation of alginate is its poor cellular interaction; therefore, it has been modified with lectins to enhance ligand‐specific binding properties.Citation78 Cell adhesion peptides, such as CDPGYIGSR, have been covalently coupled to agarose to enhance, like for alginate, the interaction with cells.Citation79
Polymeric Materials from Sugar‐Based Monomers
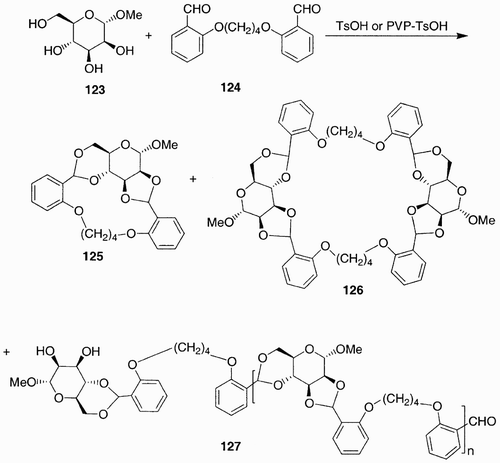
The preparation of synthetic polymers containing carbohydrate‐derived monomers has been a topic of interest since the 1970s, but has gained particular attention in the last decade due to the characteristics these monomers are able to confer to classical polyamide and polyester polymers. Synthetic polymers containing a sugar residue in the main chain have been obtained from the polycondensation of different saccharide monomers with several dicarboxylic derivatives.Citation80 For example 1,4:3,6‐dianhydro‐d‐glucitol and 1,4:3,6‐dianhydro‐d‐mannitol have been used in the preparation of polyesters.Citation81 Also, the polycondensation of d‐glucosamine derivatives has been explored.Citation82 More recently the polycondensation of α‐d‐mannopyranoside (123, ) with different dialdehydes has been investigated,Citation83 and afforded interesting polymeric compounds such as 127 or macromolecules such as 126.
Scheme 23: Polyacetylation of methyl α‐d‐mannopyranoside (123) with 1,4‐bis(2‐formyl‐phenoxy)butane (124).
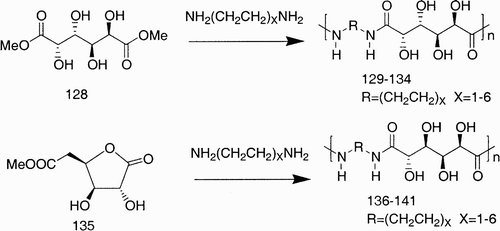
Quite recently a great effort has been devoted to modifying polyamides and polyesters to extend their applications to new fields demanding materials with lower environmental impact, that are more biodegradable, and water‐soluble, and that display biocompatible properties. Linear polyamides, also known as polyhydroxypolyamides (PHPAs), and poly(ester amide)s are among the carbohydrate‐derived polymers that have encountered major interest in the field as analogous of industrial nylons.Citation84 Esterified aldaric acids, d‐glutaric, d‐galactaric, d‐mannaric, meso‐xylaric, or the corresponding alkyl‐aldaric‐1,4‐lactones, have been polymerized with different chain length alkylidenediamines to investigate and determine the differences in polymer properties, expecially intramolecular attractive forces and hydrophilicity/hydrophobicity ().Citation84c Citation85
Scheme 24: Examples of PHPAs form the polymerization of dimethyl galactarate (128) and d‐glucarate 1,4‐lactone (135) with diamines.
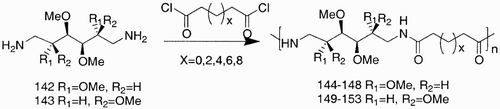
Not only aldaric acids but many other carbohydrate‐derived monomers have been used. Among them are the diamine derivatives such as the 1,6‐diamino derivatives of d‐mannitol (142, ) and l‐iditol (143), which have been condensed with different diacyl chlorides affording, once again, polyamides with novel characteristics.Citation86
Both the described monomers, aldaric acid esters and diamino derivatives, have also been used in combination for the synthesis of regular polyamides analogous to Nylon 66.Citation87 Carbohydrate units have also been employed in the synthesis of copoly(ester amide)s (), abbreviated as PVGAn, where n indicates the percentage of carbohydrate monomer incorporated in the copoly(ester amide) chain, VG indicates the 5‐aminopentyl glutarate unit (155), and A the 5‐aminoarabinitol succinate unit (154).
Scheme 26: Reagents and conditions: a) N‐methyl‐2‐pyrrolidinone, ethyldiisopropylamine, 15 days, 25°C.
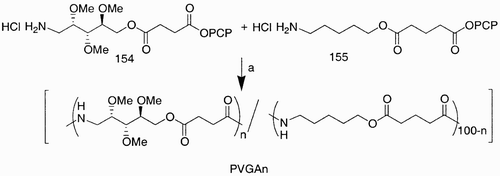
For the preparation of these polymers, a nonsymmetrical carbohydrate monomer, like 154, must be previously prepared; these copolymers are expected to improve properties such as biodegradability and biocompatibility.
Polymers with Glycidic Appendages
Cell‐surface oligosaccharides perform fundamental functions in biological recognition processes, which are based mainly on carbohydrate‐protein interaction.Citation88 Glycopolymers consisting of sugar residues attached to a polymer backbone emerged recently as important tools for the investigation of sugar‐protein interactions.Citation89 An array of synthetic methods for the polymerization of sugar‐based monomers was developed, including ionic polymerization, controlled radical polymerization, and atom transfer radical polymerization (ATRP).
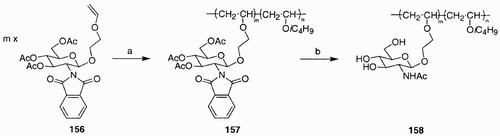
An illustrative example for the preparation of controlled‐structure sugar polymers comes from the work of Yamada et al.Citation90 The block copolymer 158, containing an N‐acetyl‐D‐glucosamine residue, was synthesized via living cationic polymerization starting from vinyl ether building block 156 (). Treatment of 156 with isobutyl vinyl ether in the presence of trifluoroacetic acid/ethylaluminum dichloride and 1,4‐dioxane, in toluene, at 0°C, initiated the living cationic polymerization and afforded the block copolymer 157. Removal of the acetyl protecting groups in 157, using hydrazine monoacetate, and subsequent acetylation of the resulting aminogroup at C(2) provided the target molecule.
Scheme 27: Reagents and conditions: a) CF3COOH, n x CH2˭CHOiC4H9, EtAlCl2, 1,4‐dioxane, 0°C; b) i. NH2‐NH2 H2O, 1,4‐dioxane, 60°C, 4 h; ii. Ac2O, MeOH, rt, 1.5 h, 65%.
The GlcNAc carrying polymer 158 was investigated in its binding properties toward wheat germ agglutinin (WGA) lectin, and showed a much increased recognition ability compared to monovalent GlcNAc itself and its β‐1,4‐linked oligomers.
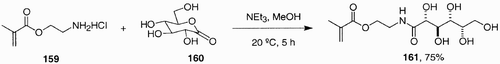
Notably, Narain and Armes recently synthesized low‐dispersity, sugar‐based polymers starting from unprotected carbohydrate‐based building blocks.[91] The starting unit, 2‐gluconamidoethyl methacrylate (GAMA), was synthesized by condensation of 2‐aminoethylmethacrylate with D‐gluconolactone in methanol and in the presence of triethylamine ().
Monofunctional GAMA was then polymerized using atom transfer radical polymerization (ATRP), at 20°C and in protic media, to generate a series of controlled‐structure sugar polymers. Various biomedical applications (drug delivery, cell targeting, adhesion, etc.) have been suggested for these sugar polymersCitation89 Citation90 Citation92 thanks to the characteristics conferred by the carbohydrate residue.
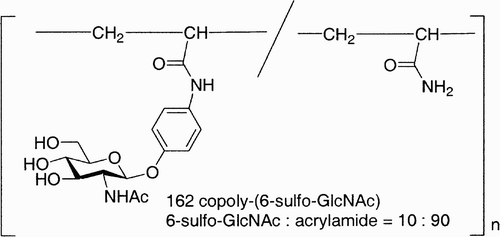
Most of the glycopolymers prepared are made up of simple mono‐and disaccharides, even if glycopolymers carrying cell surface oligosaccharides such as sialyl Lewisx (sLex)Citation93 and globosyl oligosaccharidesCitation94 have potentially greater biological significance. The synthesis of the latter is seriously restricted by the difficulty in preparing the oligosaccharide in sufficient amount. Sasaki et al.Citation95 found that acrylamide copolymers carrying only α‐l‐fucoside and 3‐sulfo‐β‐d‐galactoside residues showed strong activity in blocking the L‐selectin/sLex tetrasaccharide adhesion, which was ascribed to a cooperative binding to L‐selectin. This finding suggested the development of a “carbohydrate module method,” which involves the segmentation of a targeted oligosaccharide into smaller sugars, synthesis of the glycosylated monomers (“carbohydrate modules”), and their reassembly by copolymerization. This approach was employed for sLex, and different glycopolymers carrying different modules have been prepared and analyzed. Glycopolymer 162 () carrying a 6‐sulfo‐GlcNAc cluster was found to serve as one of the most promising agents for its potent activity in blocking the L‐selectin/sLex binding.
Conclusions
This review clearly shows how, emulating and even overcoming nature, chemists have used carbohydrates as fundamental scaffolds for a variety of purposes. Carbohydrates have so unique and precious features, such as chirality, conformational rigidity, polyfunctionality, and biocompatibility, that there is no limit to the fantasy in their use, in the natural form or after proper modifications, as scaffolds for original and useful molecular architectures that can find application in quite different fields.
References
- Hirschmann , R. , Nicolaou , K. C. , Pietranico , S. , Salvino , J. , Leahy , E. M. , Sprengeler , P. A. , Furst , G. and Smith III , A. B. 1992 . Nonpeptidal peptidomimetics with a β‐glucose scaffolding. A partial somatostatin agonist bearing a close structural relationship to a potent, selective substrate P antagonist . J. Am. Chem. Soc. , 114 : 9217 – 9228 .
- Cipolla , L. , Peri , F. , La Ferla , B. , Redaelli , C. and Nicotra , F. 2005 . Glycidic scaffolds for the production of bioactive compounds . Curr. Org. Synthesis , 2 : 153 – 173 .
- Gruner , S. A.W. , Locardi , E. , Lohof , E. and Kessler , H. 2002 . Carbohydrate‐based mimetics in drug design: sugar amino acids and carbohydrate scaffolds . Chem. Rev. , 102 : 491 – 514 .
- Le , G. T. , Abbenante , G. , Becker , B. , Grathwohl , M. , Halliday , J. , Tometzki , G. , Zuegg , J. and Meutermans , W. 2003 . Molecular diversity through sugar scaffolds . Drug Discovery Today , 8 : 701 – 709 .
- Oliver , S. F. and Abell , C. 1999 . Combinatorial synthesis of carbohydrates . Curr. Opin. Chem. Biol. , 3 : 291 – 298 .
- Plattner , J. J. and Norbeck , D. W. 1990 . “ Obstacles to drug development from peptide leads ” . In Drug Discovery Technologies 92 – 126 . Chichester, , UK : Horwood .
- Ringrose , P. S. and Humphrey , M. J. 1986 . Peptides and related drugs: a review of their absorption, metabolism, and excretion . Drug Metab. Rev. , 17 : 283 – 310 .
- Olson , G. L. , Bolin , D. R. , Bonner , M. P. , Bos , M. , Cook , C. M. , Fry , D. C. , Graves , B. J. , Hatada , M. , Hill , D. E. , Kahn , M. , Madison , V. S. , Rusiecki , V. K. , Sarabu , R. J.S. , Vincent , J. P. and Voss , M. E. 1993 . Concepts and progress in the development of peptide mimetics . J. Med. Chem. , 36 : 3039 – 3049 .
- Schneider , J. P. and Kelly , J. W. 1995 . Templates that induce α‐helical, β‐sheet, and loop conformations . Chem. Rev. , 95 : 2169 – 2187 .
- Walter , R. 1977 . Identification of sites in oxytocin involved in uterine receptor recognition and activation . Fed. Proc. , 36 : 1872 – 1878 .
- Farmer , P. S. 1980 . “ Bridging the gap between bioactive peptides and nonpeptides: some perspectives in design ” . In Medicinal Chemistry Edited by: Ariens , E. J. Vol. X (Drug Design) , 119 – 143 . New York : Academic Press .
- Belanger , P. C. and DuFresne , C. 1986 . Preparation of exo‐6‐benzyl‐exo‐2‐(m‐hydroxyphenyl)‐1‐dimethylaminomethylbicyclo[2.2.2]octane. A non‐peptide mimic of enkephalins. Can . J. Chem. , 64 : 1514 – 1520 .
- Papageorgiou , C. , Haltiner , R. , Bruns , C. and Petcher , T. J. 1992 . Design, synthesis, and binding affinity of a nonpeptide mimic of somatostatin . Biorg. Med. Chem. Lett. , 2 : 135 – 140 .
- Hirschmann , R. , Nicolaou , K. C. , Pietranico , S. , Leahy , E. M. , Salvino , J. , Arison , B. , Cichy , M. A. , Spoors , P. G. , Shakespeare , W. C. , Sprengeler , P. A. , Hamley , P. , Smith III , A. B. , Reisine , T. , Raynor , K. , Maechler , L. , Donaldson , C. , Vale , W. , Freidinger , R. M. , Cascieri , M. R. and Strader , C. D. 1993 . De novo design and synthesis of somatostatin non‐peptide peptidomimetics utilizing β‐D‐glucose as a novel scaffolding . J. Am. Chem. Soc. , 115 : 12550 – 12568 .
- Reichlin , S. and Somatostatin . 1983 . New Engl. J. Med. , 309 ( Part 1 ) : 1495 – 1501 . Part 2. 1556–1563; Moreau, J.-P.; DeFeudis, F.V. Pharmacological studies of somatostatin and somatostatin‐analogs: therapeutic advances and perspectives. Life Sci. 40, 419–437;
- Schally , A. V. 1988 . Oncological applications of somatostatin analogues . Cancer Res. , 48 : 6977 – 6985 .
- Schally , A. V. , Coy , D. H. and Meyers , C. A. 1978 . Hypothalamic regulatory hormones . Ann. Rev. Biochem. , 47 : 89 – 128 .
- Dinh , T. Q. , Smith , C. D. , Du , X. and Armstrong , R. W. 1998 . Design, synthesis, and evaluation of the multidrug resistance‐reversing activity of d‐glucose mimetics of hapalosin . J. Med. Chem. , 41 : 981 – 987 .
- Le Diguarher , T. , Boudon , A. , Elwell , C. , Paterson , D. E. and Billington , D. C. 1996 . Synthesis of potential peptidomimetics based on highly substituted glucose and allose scaffolds . Biorg. Med. Chem. Lett. , 6 : 1983 – 1988 .
- Murphy , P. V. , O'Brien , J. L. , Gorey‐Feret , L. J. and Smith III , A. B. 2003 . Synthesis of novel HIV‐1 protease inhibitors based on carbohydrate scaffolds. Tetrahedron , 59 : 2259 – 2271 .
- Locardi , E. , Boer , J. , Modlinger , A. , Schuster , A. , Holzmann , B. and Kessler , H. 2003 . Synthesis and structure‐activity relationship of mannose‐based peptidomimetics selectively blocking integrin α4β7 binding to mucosal addressin cell adhesion molecule‐1 . J. Med. Chem. , 46 : 5752 – 5762 .
- Wessel , H. P. , Banner , D. , Gubernator , K. , Hilpert , K. , Muller , K. and Tschopp , T. 1997 . 6‐Guanidinopyranoses: novel carbohydrate‐based peptidomimetics . Angew. Chem. Int. Ed. Engl. , 36 : 751 – 752 .
- Hanessian , S. , Saavedra , O. M. , Xie , F. , Amboldi , N. and Battistini , C. 2000 . Design and synthesis of functionalized glycomers as non‐peptidic ligands for SH2 binding and as inhibitors of A‐431 human epidermoid and HT‐29 colon carcinoma cell lines . Bioorg. Med. Chem. Lett. , 10 : 439 – 442 .
- Nicolaou , K. C. , Trujillo , J. I. and Chibale , K. 1997 . Design, synthesis and biological evaluation of carbohydrate‐based mimetics of cRGDFV . Tetrahedron , 53 : 8751 – 8778 .
- Suhara , Y. , Izumi , M. , Ichikawa , M. , Penno , M. B. and Ichikawa , Y. 1997 . Peptide‐sugar hybrids: like peptide, like oligosaccharide . Tetrahedron Lett. , 38 : 7167 – 7170 .
- Cipolla , L. , Lay , L. and Nicotra , F. 1997 . New and easy access to C‐glycosides of glucosamine and mannosamine . J. Org. Chem. , 62 : 6678 – 6681 .
- Smith , M. D. , Long , D. D. , Marquess , D. G. , Claridge , T. D.W. and Fleet , G. W.J. 1998 . Synthesis of oligomers of tetrahydrofuran amino acids: furanose carbopeptoids . J. Chem. Soc. Chem. Commun. , 18 : 2039 – 2040 .
- Chakraborty , T. K. , Jayaprakash , S. , Diwan , P. V. , Nagaraj , R. , Jampani , S. R.B. and Kunwar , A. C. 1998 . Folded conformation in peptides containing furanoid sugar amino acids . J. Am. Chem. Soc. , 120 : 12962 – 12963 .
- Smith , M. D. , Long , D. D.A.M. , Marquess , D. G. , Claridge , T. D.W. and Fleet , G. W.J. 1999 . Absence of secondary structure in a carbopeptoid tetramer of a trans‐5‐aminomethyl‐tetrahydrofuran‐2‐carboxylate . Tetrahedron Lett. , 40 : 2191 – 2194 .
- Long , D. D. , Hungerford , N. L. , Smith , M. D. , Brittain , D. E.A. , Marquess , D. G. , Claridge , T. D.W. and Fleet , G. W.J. 1999 . From sequencamers to foldamers? Tetrameric furanose carbopeptoids from cis‐ and trans‐5‐aminomethyl‐tetrahydrofuran‐2‐carboxylates . Tetrahedron Lett. , 40 : 2195 – 2198 .
- Schrey , A. , Osterkamp , F. , Struadi , A. , Rickert , C. , Wagner , H. , Koert , U. , Herrschaft , B. and Harms , K. 1999 . Synthesis of enantiomerically pure amino acids containing 2,5‐disubstituted THF rings in the molecular backbone . Eur. J. Org. Chem. , 1999 : 2977 – 2990 .
- Overkleeft , H. S. , Verhelst , S. H.L. , Pieterman , E. , Meeuwenoord , N. J. , Overhand , M. , Cohen , L. H. , van der Marel , G. A. and Van Boom , J. H. 1999 . Design and synthesis of a protein: farnesyltransferase inhibitor based on sugar amino acids . Tetrahedron Lett. , 40 : 4103 – 4106 .
- Chakraborty , T. K. , Ghosh , S. , Jayaprakash , S. , Sharma , J. A.R.P. , Ravikanth , V. , Diwan , P. V. , Nagaraj , R. and Kunwar , A. C. 2000 . Synthesis and conformational studies of peptidomimetics containing furanoid sugar amino acids and a sugar diacid . J. Org. Chem. , 65 : 6441 – 6457 .
- Peri , F. , Cipolla , L. , La Ferla , B. and Nicotra , F. 2000 . Synthesis of bicyclic sugar azido acids and their incorporation in cyclic peptides . J. Chem. Soc. Chem. Commun. , : 2303 – 2304 .
- Gruner , S. A.W. , Locardi , E. , Lohof , E. and Kessler , H. 2002 . Carbohydrate‐based mimetics in drug design: sugar amino acids and carbohydrate scaffolds . Chem. Rev. , 102 : 491 – 514 .
- Peri , F. , Cipolla , L. , Forni , E. , La Ferla , B. and Nicotra , F. 2001 . Sugar‐derived amino acids: powerful secondary structure inducing elements in the design of novel peptidomimetics . Chemtracts: Organic Chem. , : 481 – 499 .
- Fox , J. J. , Kuwada , Y. and Watanabe , K. A. 1968 . Nucleosides LVI. On the structure of the nucleotise antibiotic, gougerotin . Tetrahedron Lett. , 2 : 6029 – 6032 .
- Isono , K. , Asahi , K. and Suzuki , S. 1969 . Polyoxins, antifungal antibiotics XIII. Structure of polyoxins . J. Am. Chem. Soc. , 91 : 7490 – 7505 .
- Lichtenthaler , F. W. , Morino , T. and Menzel , H. M. 1975 . Nucleosides XXV. Structure of aspiculamycin. Its identity with the nucleoside antibiotic gougerotin . Tetrahedron Lett. , 9 : 665 – 668 .
- Waltho , J. P. , Williams , D. H. , Selva , E. and Ferrari , P. 1987 . Structure elucidation of the glycopeptide antibiotic complex A40926 . J. Chem. Soc. Perkin Trans. 1 , 9 : 2103 – 2107 .
- Heyns , K. and Paulsen , H. 1955 . Oxidative transformation of carbohydrates IX. Synthesis of 2‐amino‐2‐deoxy‐D‐glucuronic acid . Chem. Ber. , 88 : 188 – 195 .
- Müller , C. , Kitas , E. and Wessel , H. P. 1995 . Novel oligosaccharide mimetics by solid‐phase synthesis . J. Chem. Soc. Chem. Commun. , : 2425 – 2426 .
- Wessel , H. P. , Mitchell , C. M. , Lobato , C. M. and Schmid , G. 1995 . Saccharice‐peptide hybrids as novel oligosaccharide mimetics . Angew. Chem. Int. Ed. Engl. , 34 : 2712 – 2713 .
- Suhara , Y. , Izumi , M. , Ichikawa , M. , Penno , M. B. and Ichikawa , Y. 1997 . Peptide‐sugar hybrids: like peptide, like oligosaccharides . Tetrahedron Lett. , 38 : 7167 – 7170 .
- Smith , M. D. , Long , D. D. , Marquess , D. G. , Claridge , T. D.W. and Fleet , G. W.J. 1998 . Synthesis of oligomers of tetrahydrofuran amino acids: furane carbopeptoids . Chem. Commun. , : 2039 – 2040 .
- Moreno‐Vergas , A. J. , Robina , I. , Petricci , E. and Vogel , P. 2004 . Synthesis of D‐ and L‐2,3‐trans‐3,4‐cis‐4,5‐trans‐3,4‐dihydroxy‐5‐hydroxymethylproline and tripeptides containing them . J. Org. Chem. , 69 : 4487 – 4491 .
- Moreno‐Vergas , A. J. , Jimenez‐Barbero , J. and Robina , I. 2003 . Hetaryleneaminopolyols and hetarylenecarbopeptoids: a new type of glyco‐ and peptidomimetics. Syntheses and studies on solution conformation and dynamics . J. Org. Chem. , 68 : 4138 – 4150 .
- Wessel , H. P. 2001 . “ Non‐sugar glycomimetics ” . In Glycoscience: Chemistry and Chemical Biology Edited by: Fraser‐Reid , B. , Tatsuta , K. and Thiem , J. Vol. III , 2725 – 2752 . Heidelberg : Springer Verlag .
- Schweizer , F. 2002 . Glycosamino acids: building blocks for combinatorial synthesis—implications for drug discovery . Angew. Chem. Int. Ed. , 41 : 230 – 253 .
- Long , D. D. , Hungerfor , N. L. , Smith , M. D. , Brittain , D. E.A. , Marquess , D. G. , Claridge , T. D.W. and Fleet , G.W. J. 1999 . From sequencamers to foldamers? Tetrameric furanose carbopeptoids from cis‐ and trans‐5‐aminomethyl‐tetrehydrofuran‐2‐carboxylates . Tetrahedron Lett. , 40 : 2195 – 2198 . , and ref. cited therein
- von Roedern , E. G. and Kessler , H. 1994 . Sugar amino acid as a novel peptide mimetic . Angew. Chem. Int. Ed. Engl. , 33 : 687 – 689 .
- von Roedern , E. G. , Lohof , E. , Hessler , G. , Hoffmann , M. and Kessler , H. 1996 . Synthesis and conformational analysis of linear and cyclic peptides containing sugar amino acids . J. Am. Chem. Soc. , 118 : 10156 – 10167 .
- Taillefumier , C. , Lakhrissi , Y. , Lakhrissi , M. and Chapleur , Y. 2002 . Facile synthesis of fused furanosyl β‐amino acids from protected sugar lactones: incorporation into a peptide chain . Tetrahedron: Asymm. , 13 : 1707 – 1711 .
- Taillefumier , C. , Thiegles , S. and Chapleur , Y. 2004 . Anomeric spiro‐annelated 1,4‐diazepine 2,5‐diones from furano exo‐glycals: towards a new class of spiro‐nucleosides . Tetrahedron , 60 : 2213 – 2224 .
- Nicolaou , K. C. , Trujillo , J. I. and Chibale , K. 1997 . Design, synthesis and biological evaluation of carbohydrate‐based mimetics of cRGDFV . Tetrahedron , 53 : 8751 – 8778 .
- Thomson , L. A. and Ellman , J. A. 1996 . Synthesis and applications of small molecule libraries . Chem. Rev. , 96 : 555 – 600 . For earlier references to carbohydrate libraries see:
- Balkenhohl , F. , Bussche‐Huennefeld , C. , Lansky , A. and Zecchel , C. 1996 . Combinatorial synthesis of small organic molecules . Angew. Chem. Int. Ed. Eng. , 35 : 2288 – 2337 .
- Barkley , A. and Arya , P. 2001 . Combinatorial chemistry toward understanding the function(s) of carbohydrates and carbohydrate conjugates . Chem. Eur. J. , 7 : 555 – 563 .
- Kahne , D. 1997 . Combinatorial approaches to carbohydrates . Curr. Opin. Chem. Biol. , 1 : 130 – 135 .
- Sofia , M. , Hunter , R. , Chan , T. Y. , Vaughan , A. , Dulina , R. , Wang , H. and Gange , D. 1998 . Carbohydrate‐based small‐molecule scaffolds for the construction of universal pharmacophore mapping libraries . J. Org. Chem. , 63 : 2802 – 2803 .
- Opatz , T. , Kallus , C. , Wunberg , T. , Schmidt , W. , Henke , S. and Kunz , H. 2002 . d‐Glucose as a multivalent chiral scaffold for combinatorial chemistry . Carbohydr. Res. , 337 : 2089 – 2110 .
- Schweizer , F. and Hindsgaul , O. 1999 . Combinatorial synthesis of carbohydrates . Curr. Opin. Chem. Biol. , 3 : 291 – 298 .
- Peri , F. , Nicotra , F. , Leslie , C. P. , Micheli , F. , Seneci , P. and Marchioro , C. 2003 . D‐glucose as a regioselectively addressable scaffold for combinatorial chemistry on solid phase . J. Carbohydr. Chem. , 22 : 57 – 71 .
- Hirschmann , R. , Ducry , L. and Smith III , A. B. 2000 . Development of an efficient regio‐ and stereoselective route to libraries based on the β‐D‐glucose scaffold . J. Org. Chem. , 65 : 8307 – 8316 .
- Brill , W. K.‐D. , De Mesmaeker , A. and Wendeborn , S. 1998 . Solid‐phase synthesis of levoglucosan derivatives . Synlett , 10 : 1085 – 1090 .
- Wunberg , T. , Kallus , C. , Opatz , T. , Henke , S. , Schmidt , W. and Kunz , H. 1998 . Carbohydrates as multifunctional chiral scaffolds in combinatorial synthesis . Angew. Chem., Int. Ed. Eng. , 37 : 2503 – 2505 .
- Kallus , C. , Opatz , T. , Wunberg , T. , Schmidt , W. , Henke , S. and Kunz , H. 1999 . Combinatorial solid‐phase synthesis using D‐galactose as a chiral five‐dimension‐diversity scaffold . Tetrahedron Lett. , 40 : 7783 – 7786 .
- Opatz , T. , Kallus , C. , Wunberg , T. , Schmidt , W. , Henke , S. and Kunz , H. 2003 . D‐Glucose as a pentavalent chiral scaffold . Eur. J. Org. Chem. , 8 : 1527 – 1536 .
- Marcaurelle , L. A. and Seeberger , P. H. 2002 . Combinatorial carbohydrate chemistry . Curr. Opin. Chem. Biol. , 6 : 289 – 296 .
- Emmerson , D. P.G. , Villard , R. , Mugnaini , C. , Batsanov , A. , Howard , J. A.K. , Hems , W. P. , Tooze , R. P. and Davis , B. G. 2003 . Precise structure activity relationships in asymmetric catalysis using carbohydrate scaffolds to allow ready fine tuning: dialkylzinc‐aldehyde additions . Org. Biomol. Chem. , 1 : 3826 – 3838 .
- Wong , C.‐H. , Ye , X.‐S. and Zhang , Z. 1998 . Assembly of oligosaccharide libraries with a designed building block and an efficient orthogonal protection‐deprotection strategy . J. Am. Chem. Soc. , 120 : 7137 – 7138 .
- Hunger , U. , Ohnsmann , J. and Kunz , H. 2004 . Carbohydrate scaffolds for combinatorial syntheses that allow selective deprotection of all four positions independent of the sequence . Angew. Chem. Int. Ed. Eng. , 43 : 1104 – 1107 .
- Moitessier , N. , Dufour , S. , Chretien , F. , Thiery , J. P. , Maigret , B. and Chapleur , Y. 2001 . Design, synthesis and preliminary biological evaluation of a focused combinatorial library of stereodiverse carbohydratescaffold‐based peptidomimetics . Bioorg. Med. Chem. , 9 : 511 – 523 .
- McDevitt , J. P. and Lansbury , P. T. Jr. 1996 . Glycosamino acids: new building blocks for combinatorial synthesis . J. Am. Chem. Soc. , 118 : 3818 – 3828 .
- Edwards , A. A. , Ichihara , O. , Murfin , S. , Wilkes , R. , Whittaker , M. , Watkin , D. J. and Fleet , G. W.J. 2004 . Tetrahydrofuran‐based amino acids as library scaffolds . J. Comb. Chem. , 6 : 230 – 238 .
- Bonnert , R. V. and Jenkins , P. R. 1987 . The first example of a Robinson annulation on a carbohydrate derivative . Chem. Commun. , : 6 – 7 . For examples of used ring formation via radical cyclization, see
- Bonnert , R. V. , Davies , M. J. , Howarth , J. , Jenkins , P. R. and Lawrence , N. J. 1992 . Stereoselective reductive hydroxymethylation of an annulated sugar derivative via radical cyclization . J. Chem. Soc. Perkin Trans. 1 , : 27 – 29 .
- Wood , A. J. and Jenkins , P. R. 1997 . The first example of a highly stereoselective intramolecular radical cyclization of a cyclopentenol derivative . Tetrahedron Lett. , 38 : 1853 – 1856 .
- Lopez , J. C. and Fraser‐Reid , B. 1997 . Parlaying C‐O chirality into C‐C chirality: improving the cost/benefit ratio of carbohydrate templates . J. Chem. Soc. Chem. Comm. , : 2251 – 2257 .
- Bonnert , R. V. and Jenkins , P. R. 1987 . The first example of a Robinson annulation on a carbohydrate derivative . Chem. Commun. , : 6 – 7 . For other examples of used‐ring formation, see
- Bonnert , R. V. , Howarth , J. , Jenkins , P. R. and Lawrence , N. J. 1991 . Robinson annulation on a carbohydrate derivative . J. Chem. Soc. Perkin Trans. 1 , : 1225 – 1229 .
- Marco‐Contelles , J. 1996 . Asymmetric Pauson‐Khand reaction Cobalt‐mediated cycloisomerization of 1,6‐enynes in carbohydrate templates: synthesis of bis‐heteroannulated pyranosides . J. Org. Chem. , 61 : 7666 – 7670 .
- Wood , A. J. , Jenkins , P. R. , Fawcett , J. and Russell , D. R. 1995 . The synthesis and x‐ray crystal structure of a cyclopenta‐annulated sugar; the first example of an intramolecular aldol cyclopenta‐annulation in carbohydrate chemistry . Chem. Commun. , : 1567 – 1568 .
- Wood , A. J. , Holt , D. J. , Dominguez , M.‐C. and Jenkins , P. R. 1998 . The stereoselective preparation of an enantiomerically pure cyclopentane using intramolecular aldol cyclopentannulation of a glucose derivative . J. Org. Chem. , 63 : 8522 – 8529 .
- Sinou , D. and Bedjeguelal , K. 2001 . Metal‐catalyzed cyclization on carbohydrate templates. A stereoselective access to enantiopure polycyclic compounds . J. Carbohydr. Chem. , 20 : 335 – 337 .
- Holt , D. J. , Barker , W. D. , Jenkins , P. R. , Panda , J. and Ghosh , S. 2000 . Stereoselective preparation of enantiomerically pure annulated carbohydrates using ring‐closing metathesis . J. Org. Chem. , 65 : 482 – 493 .
- Holt , D. J. , Barker , W. D. , Jenkins , P. R. , Davies , D. L. , Garratt , S. , Fawcett , J. , Russell , D. R. and Ghosh , S. 1998 . Ring‐closing metathesis in carbohydrate annulation . Angew. Chem. Int. Ed. , 37 : 3298 – 3300 .
- Reynolds , D. D. and Kenyon , W. O. 1950 . Preparation and reactions of sulfonic esters IV. Preparation of cyclic ethers . J. Am. Chem. Soc. , 72 : 1593 – 1596 . For example of spiro‐cyclic ring formation see
- Picard , P. , Leclercq , D. and Moulines , J. 1975 . Preparation of tetrahydropyrans from 1,5‐diols Improvement of the method with sulfonic esters . Tetrahedron Lett. , 16 : 2731 – 2732 .
- Canonne , P. , Foscolos , G. B. and Belanger , D. 1980 . One‐step annulation. A convenient method for the preparation of diols, spirolactones, and spiroethers from lactones . J. Org. Chem. , 45 : 1828 – 1835 .
- Piers , E. and Ashvinikumar , V. G. 1990 . A (Z)‐ethylidenecyclopentane annulation method Total syntheses of (±)‐anhydrooplopanone, (±)‐oplopanone, and (±)‐8‐epi‐oplopanone . J. Org. Chem. , 55 : 2380 – 2390 .
- Middleton , D. S. and Simpkins , N. S. 1988 . Facile synthesis of functionalized spiro ethers via radical cyclizations . Tetrahedron Lett. , 29 : 1315 – 1318 .
- Lesueur , C. , Nouguier , R. , Bertrand , M. P. , Hoffmann , P. and De Mesmaeker , A. 1994 . Stereocontrol in radical cyclizations on sugar templates . Tetrahedron , 50 : 5369 – 5380 .
- Bertrand , M. P. , Lesueur , C. and Nouguier , R. 1995 . Sulfonyl radical‐induced cyclization of 1,6‐dienes: synthesis of 2‐C‐branched, 3,4‐annulated sugars . Carbohydr. Lett. , 1 : 393 – 398 .
- Marco‐Contelles , J. , Alhambra , C. and Martinez‐Grau , A. 1998 . Carbocycles from carbohydrates via free radical cyclizations Synthesis and manipulation of annulated furanoses . Synlett , : 693 – 699 . , and references therein
- Paquette , L. A. and Tae , J. 1996 . Stereocontrolled preparation of spirocyclic ethers by intramolecular trapping of oxonium ions with allylsilanes . J. Org. Chem. , 61 : 7860 – 7866 .
- Timmer , M. S.M. , Verdoes , M. , Sliedregt , L. A.J.M. , Marel , G. A.v.d. , Boom , J. H.V. and Overkleeft , H. S. 2003 . The use of a mannitol‐derived fused oxacycle as a combinatorial scaffold . J. Org. Chem. , 68 : 9406 – 9411 .
- Cipolla , L. , Forni , E. , Barbero , J. J. and Nicotra , F. 2002 . Synthesis and conformational analysis of fructose‐derived scaffolds: molecular diversity from a single molecule . Chem. Eur. J. , 8 : 3976 – 3983 .
- Peri , F. , Bassetti , R. , Caneva , E. , De Gioia , L. , La Ferla , B. , Presta , M. , Tanghetti , E. and Nicotra , F. 2002 . Arabinose‐derived bicyclic amino acids: synthesis, conformational analysis and construction of an αvβ3‐selective RGD peptide . J. Chem. Soc. Perkin 1 , : 638 – 644 .
- Taillefumier , C. , Thiegles , S. and Chapleur , Y. 2004 . Anomeric spiro‐annelated 1,4‐diazepine 2,5‐diones from furano exo‐glycals: towards a new class of spiro‐nucleosides . Tetrahedron , 60 : 2213 – 2224 .
- Nakajima , M. , Itoi , K. , Takamatsu , Y. , Kinoshita , T. , Okazaki , T. , Kawakubo , K. , Shindo , M. , Nonma , T. , Tohjigamori , M. and Haneishi , T. 1991 . Hydantocidin: a new compound with herbicidal activity from Streptomyces hygroscopicus . J. Antibiot. , 44 : 293 – 300 .
- Pachlatko , J. P. and Zähner , H. New streptomyces hygroscopicus TU‐2474‐for prodn. of the herbicide hydantocidin and the bactericide homomycin Ciba‐Geigi AG . DE Pat. 4,129,616 A1 . 1992 .
- Krülle , T. M. , Watson , K. A. , Gregoriou , M. , Johnson , L. N. , Crook , S. , Watkin , D. J. , Griffiths , R. C. , Nash , R. J. , Tsitsanou , K. E. , Zographos , S. E. , Oikonomakos , N. G. and Fleet , G. W.J. 1995 . Specific inhibition of glycogen phosphorylase by a spirodiketopiperazine at the anomeric position of glucopyranose . Tetrahedron Lett. , 36 : 8291 – 8294 .
- Taillefumier , C. , Enderlin , G. and Chapleur , Y. 2005 . Cycloaddition of nitrones and nitrile oxides to activated Exo‐glycals . Lett. Org. Chem. , 2 : 226 – 230 .
- Pauson , P. L. 1985 . The Khand reaction. A convenient and general route to a wide range of cyclopentenone derivatives . Tetrahedron , 41 : 5855 – 5860 .
- Schore , N. E. 1991 . The Pauson‐Khand cycloaddition reaction for synthesis of cyclopentenones . Org. React. , 40 : 1 – 90 .
- Leeuwenburgh , M. A. , Appeldoorn , C. C.M. , van Hooft , P. A.V. , Overkleeft , H. S. , van derMarel , G. A. and van Boom , J. H. 2000 . Synthesis of highly functionalised carbohydrate‐derived spiroacetals by ring‐closing metathesis and Pauson‐Khand reaction of ketoglycosidic enynes . Eur. J. Org. Chem. , 2000 : 873 – 877 .
- Trost , B. M. and Edstrom , E. D. 1990 . Synthesis of (+)‐phyllanthocin via a metal‐catalyzed cycloreduction . Angew. Chem. , 102 : 541 – 543 .
- Forni , E. , Cipolla , L. , Caneva , E. , La Ferla , B. , Peri , F. and Nicotra , F. 2002 . Polycyclic scaffolds from fructose . Tetrahedron Lett. , 43 : 1355 – 1357 .
- Shibata , K. , Hiruma , K. , Kanie , O. and Wong , C.‐H. 2000 . Synthesis of 1,1‐linked galactosyl mannosides carrying a thiazine ring as mimetics of sialyl Lewis X antigen: investigation of the effect of carboxyl group orientation on P‐selectin inhibition . J. Org. Chem. , 65 : 2393 – 2398 .
- Lohse , A. , Jensen , K. B. and Bols , M. 1999 . The first combinatorial library of azasugar glycosidase inhibitors . Tetrahedron Lett. , 40 : 3033 – 3036 .
- Chapman , T. M. , Courtney , S. , Hay , P. and Davis , B. G. 2003 . Highly diastereoselective additions to polyhydroxylated pyrrolidine cyclic imines: ready elaboration of aza‐sugar scaffolds to create diverse carbohydrate‐processing enzyme probes . Chem. Eur. J. , 9 : 3397 – 3414 .
- Wu , C.‐Y. , Chang , C.‐F. , Chen , J. S.‐Y. , Wong , C.‐H. and Lin , C.‐H. 2003 . Rapid diversity‐oriented synthesis in microtiter plates for in situ screening: discovery of potent and selective α‐fucosidase inhibitors . Angew. Chem. Int. Ed. , 42 : 4661 – 4664 .
- Gerber‐Lemair , S. , Popowycz , F. , Rodriguez‐Garcia , E. , Asenjo , A. T.C. , Robina , I. and Vogel , P. 2002 . An efficient combinatorial method for the discovery of glycosidase inhibitors . Chem. Bio. Chem. , 3 : 466 – 470 .
- La Ferla , B. , Bugada , P. , Cipolla , L. , Peri , F. and Nicotra , F. 2004 . Synthesis of iminosugar scaffolds for the generation of glycosidase inhibitor libraries . Eur. J. Org. Chem. , : 2451 – 2470 .
- Chery , F. and Murphy , P. V. 2004 . Synthesis of an iminosugar based peptidomimetic analogue . Tetrahedron Lett. , 45 : 2067 – 2069 .
- Vercruysse , K. P. , Marecak , D. M. , Marecej , J. F. and Prestwich , G. D. 1997 . Synthesis and in vitro degradation of new polyvalent hydrazide cross‐linked hydrogels of hyaluronic acid . Bioconjugate Chem. , 8 : 686 – 694 .
- Pouyani , T. , Harbison , G. S. and Prestwich , G. D. 1994 . Novel hydrogels of hyaluronic acid: synthesis, surface morphology, and solid‐state NMR. J . Am. Chem. Soc. , 116 : 7515 – 7522 .
- Choi , Y. S. , Hong , S. R. , Lee , Y. M. , Song , K. W. , Park , M. H. and Nam , Y. S. 1999 . Studies on gelatin‐containing artificial skin: II. Preparation and characterization of crosslinked gelatin‐hyaluronate sponge . J. Biomed. Mater. Res. , 48 : 631 – 639 .
- Radomsky , M. L. , Aufdemorte , T. B. , Swain , L. D. , Fox , W. C. , Spiro , R. C. and Poster , J. W. 1999 . Novel formulation of fibroblast growth factor‐2 in a hyaluronan gel accelerates fracture healing in nonhuman primates . J. Orthopaed. Res. , 17 : 607 – 614 .
- Duranti , F. , Salti , G. , Bovani , B. , Calandra , M. and Rosati , M. L. 1998 . Injectable hyaluronic acid gel for soft tissue augmentation. A clinical and histological study . Dermatol. Surg. , 24 : 1317 – 1325 .
- Caravaggi , C. , De Giglio , R. , Tritelli , C. , Sommaria , M. , Dalla , N. S. , Faglia , E. , Mantero , M. , Clerici , G. , Fratino , P. , Dalla , P. L. , Mariani , G. and Morabito , A. 2003 . HYAFF 11‐based autologous dermal and epidermal grafts in the treatment of noninfected diabetic plantar and dorsal foot ulcers: a prospective, multicenter, controlled, randomized clinical trial . Diabetes Care , 26 : 2853 – 2859 .
- Grigolo , B. , Roseti , L. , Fiorini , M. , Fini , M. , Giavaresi , G. , Aldini , N. N. , Giardino , R. and Facchini , A. 2001 . Transplantation of chondrocytes seeded on a hyaluronan derivative (Hyaff®‐11) into cartilage defects in rabbits . Biomaterials , 22 : 2417 – 2424 .
- Tonello , C. , Zavan , B. , Cortivo , R. , Brun , P. , Panfilo , S. and Abatangelo , G. 2003 . In vitro reconstruction of human dermal equivalent enriched with endothelial cells . Biomaterials , 24 : 1205 – 1211 .
- Galassi , G. , Brun , P. , Radice , M. , Cortivo , R. , Zanon , G. F. , Genovese , P. and Abatangelo , G. 2000 . In vitro reconstructed dermis implanted in human wounds: degradation studies of the HA‐based supporting scaffold . Biomaterials , 21 : 2183 – 2191 .
- Aigner , J. , Tegeler , J. , Hutzler , P. , Campodoccia , D. , Pavesio , A. , Hammer , C. , Kastenbauer , E. and Neumann , A. 1998 . Cartilage tissue engineering with novel nonwoven structured biomaterial based on hyaluronic acid benzyl ester . J. Biomed. Mater. Res. , 42 : 172 – 181 .
- Turner , N. J. , Kielty , C. M. , Walzer , M. G. and Canfield , A. E. 2004 . A novel hyaluronan‐based biomaterial (Hyaff‐11®) as a scaffold for endothelial cells in tissue engineered vascular grafts . Biomaterials , 25 : 5955 – 5964 .
- Mi , F. L. , Kuan , C. Y. , Shyu , S. S. , Lee , S. T. and Chang , S. F. 2000 . The study of gelation kinetics and chain‐relaxation properties of glutaraldehyde‐cross‐linked chitosan gel and their effects on microspheres preparation and drug release . Carbohydr. Polym. , 41 : 389 – 396 .
- Ono , K. , Saito , Y. , Yura , H. , Ishikawa , K. , Kurita , A. , Akaike , T. and Ishihara , M. 2000 . Photocrosslinkable chitosan as a biological adhesive . J. Biomed. Mater. Res. , 49 : 289 – 295 .
- Yagi , K. , Michibayashi , N. , Kurikawa , N. , Nakashima , Y. , Mizoguchi , T. , Harada , A. , Hagashiyama , S. , Muranaka , H. and Kawase , M. 1997 . Effectiveness of fructose‐modified chitosan as a scaffold for hepatocyte attachment . Biol. Pharm. Bull. , 20 : 1290 – 1294 .
- Muzzarelli , R. A.A. , Zucchini , C. , Ilari , P. , Pugnaloni , A. , Mattioli‐Belmonte , M. , Bigini , G. and Castaldini , C. 1993 . Osteoconductive properties of methylpyrrolidinone chitosan in an animal model . Biomaterials , 14 : 925 – 929 .
- Lee , K. Y. , Rowley , J. A. , Eiselt , P. , Moy , E. M. , Bouhadir , K. H. and Mooney , D. J. 2000 . Controlling mechanical and swelling properties of alginate hydrogels independently by cross‐linker type and cross‐linking density . Macromolecules , 33 : 4291 – 4294 .
- Sultzbaugh , K. J. and Speaker , T. J. 1996 . A method to attach lectins to the surface of spermine/alginate microcapsules based on the avidin biotin interaction . J. Microencapsulation , 13 : 363 – 376 .
- Borkenhagen , M. , Clemence , J. F. , Sigrist , H. and Aebischer , P. 1998 . Three‐dimensional extracellular matrix engineering in the nervous system . J. Biomed. Mater. Res. , 40 : 392 – 400 .
- Thiem , J. and Bachmann , F. 1993 . Syntheses and properties of polyamides from glucosamine and glucose derivatives . Macromol. Chem (Oxford) , 194 : 1035 – 1057 .
- Okada , M. , Okada , Y. and Aoi , J. 1995 . Synthesis and degradabilities of polyesters from 1,4:3,6‐dianhydrohexitols and aliphatic dicarboxylic acids . J. Polym. Sci. Part A: Polym. Chem. , 33 : 2813 – 2820 .
- Kadokawa , J. , Kato , M. , Kobayashi , N. , Karasu , M. , Tagaya , H. and Chiba , K. 1994 . Regioselective polycondensation of N‐carboxyalkanoyl‐D‐glucosamine using the hexachlorotriphosphazene/pyridine system as a condensing agent . Macromol. Rapid Commun. , 15 : 971 – 978 .
- Kadokawa , J. , Horiguchi , T. , Sunaga , E. , Karasu , M. , Tagaya , K. and Chiba , K. 1997 . Regioselective polycondensation of benzyl 2‐amino‐2‐deoxy‐α‐D‐glucopyranoside hydrochloride with carbon dioxide . Acta Polymer. , 48 : 310 – 313 .
- Maślińka‐Solich , J. and Kukowka , S. 2004 . Chiral polymers containing a carbohydrate moiety . Synthesis. Macromo. Biosci. , 4 : 421 – 430 .
- Thiem , J. and Bachmann , F. 1994 . Carbohydrate‐derived polyamides . Trends Polym. Sci. , 2 : 425 – 432 .
- Varela , O. and Orgueira , H. A. 2000 . Synthesis of chiral polyamides from carbohydrate‐derived monomers . Adv. Carbohydr. Chem. Biochem. , 55 : 137 – 174 .
- Kiely , D. E. , Chen , L. and Lin , T‐H. 2000 . Synthetic polyhydroxypolyamides from galactaric, xylaric, D‐glucaric, and D‐mannaric acids and alkylenediamine monomers—some comparisons . J. Polym. Sci. Part A: Polym. Chem. , 38 : 594 – 603 . and references therein
- Styron , S. D. , Kiely , D. E. and Ponder , G. 2003 . Alternating stereoregular head, tail‐tail, head‐poly(alkylene D‐glucaramides) derived from a homologous series of symmetrical diamido‐di‐D‐glucaric acid monomers . J. Carbohydr. Chem. , 22 : 123 – 142 .
- Mancera , M. , Roffé , I. , Al‐Kass , S. S.J. , Rivas , M. and Galbis , J. A. 2003 . Synthesis and characterization of new stereoregular AABB‐type polyamides from carbohydrate‐based monomers having D‐manno and L‐ido configurations . Macromolecules , 36 : 1089 – 1097 .
- Mancera , M. , Roffé , I. , Al‐Kass , S. S.J. , Rivas , M. and Galbis , J. A. 2003 . New derivatives of D‐mannaric and galactaric acids: synthesis of a new stereoregular Nylon 66 analog from carbohydrate‐based monomers having the D‐manno configuration . Carbohydr. Res. , 338 : 1115 – 1119 .
- Kobata , A. 1992 . Structures and functions of the sugar chains of glycoproteins . Eur. J. Biochem. , 209 : 483 – 501 .
- Dwek , R. A. 1996 . Glycobiology: toward understanding the function of sugars . Chem. Rev. , 96 : 683 – 720 .
- Kallin , E. 1994 . Use of glycosylamines in preparation of polyacrilamide copolymers . Meth. Enzymol. Neoglycoconjugates Pt. A , 242 : 221 – 226 .
- Kobayashi , K. , Kobayashi , A. and Akaike , T. 1994 . Culturing hepatocytes on lactose‐carrying polystyrene layer via asialoglycoprotein receptor mediated interactions . Meth. Enzymol. Neoglycoconjugates Pt. B: Biomed. Appl. , 247 : 409 – 418 .
- Lee , Y. C. and Lee , R. T. 1995 . Carbohydrate‐protein interactions: basis of glycobiology . Acc. Chem. Res. , 28 : 321 – 327 .
- Kiessling , L. L. and Pohl , N. L. 1996 . Strength in numbers: non‐natural polyvalent carbohydrate derivatives . Chem. Biol. , 3 : 71 – 77 .
- Roy , R. 1996 . Syntheses and some applications of chemically defined multivalent glycoconjugates . Curr. Opin. Str. Biol. , 6 : 692 – 702 .
- Yamada , K. , Minoda , M. and Miyamoto , T. 1999 . Controlled synthesis of amphiphilic block copolymers with pendant N‐acetyl‐D‐glucosamine residues by living cationic polymerization and their interaction with WGA lectin . Macromolecules , 32 : 3553 – 3558 .
- Narain , R. and Armes , S. P. 2002 . Synthesis of low polydispersity, controlled‐structure sugar methacrylate polymers under mild conditions without protecting group chemistry . Chem. Commun. , : 2776 – 2777 .
- Kobayashi , K. and Tshuchida , A. 1997 . A new type of artificial glycoconjugate polymer: a convenient synthesis and its interaction with lectins . Macromolecules , 30 : 2016 – 2020 .
- Kobayashi , A. , Goto , M. , Kobayashi , K. and Akaike , T. 1994 . Receptor‐mediated regulation of differentiation and proliferation of hepatocytes by a synthetic polymer model of asialoglycoprotein . J. Biomater. Sci. Polymer Edn. , 6 : 325 – 342 .
- Roy , R. , Park , W. K.C. , Srivastava , O. P. and Foxall , C. 1996 . Combined glycomimetic and multivalent strategies for the design of potent selectin antagonists . Bioorg. Med. Chem. Lett. , 6 : 1399 – 1402 .
- Miyauchi , H. , Tanaka , M. , Koike , H. , Kawamura , N. and Hayashi , M. 1997 . Synthesis and inhibitory effect of a sialyl lewis x‐acrylamide homopolymer at preventing cell adhesion . Bioorg. Med. Chem. Lett. , 7 : 985 – 988 .
- Gordon , E. J. , Sanders , W. J. and Kiessling , L. L. 1998 . Synthetic ligands point to cell surface strategies . Nature , 392 : 30 – 31 .
- Thoma , G. , Patton , J. T. , Magnani , J. L. , Ernst , B. , Öhrlein , R. and Duthaler , R. O. 1999 . Versatile functionalization of polylysine: synthesis, characterization, and use of neoglycoconjugates . J. Am. Chem. Soc. , 121 : 5919 – 5929 .
- Dohi , H. , Nishida , Y. , Mizuno , M. , Shinkai , M. , Kobayashi , T. , Takeda , T. , Uzawa , H. and Kobayashi , K. 1999 . Synthesis of an artificial glycoconjugate polymer carrying Pk‐antigenic trisaccharide and its potent neutralization activity against Shiga‐like toxin . Bioorg. Med. Chem. , 7 : 2053 – 2062 .
- Kitov , P. I. , Sadowska , J. M. , Mulvey , G. , Armstrong , G. D. , Ling , H. , Pannu , N. S. , Read , R. J. and Bundle , D. R. 2000 . Shiga‐like toxins are neutralized by tailored multivalent carbohydrate ligands . Nature , 403 : 669 – 672 .
- Sasaki , K. , Nishida , Y. , Tsurumi , T. , Uzawa , H. , Kondo , H. and Kobayashi , K. 2002 . Facile assembly of cell surface oligosaccharide mimics by copolymerization of carbohydrate modules . Angew. Chem. Int. Ed. , 41 : 4463 – 4467 .