Abstract
Plastic biodegradation has emerged as a sustainable approach and green alternative in handling the ever-increasing accumulation of plastic wastes in the environment. The complete biodegradation of polyethylene terephthalate is one of the most recent breakthroughs in the field of plastic biodegradation. Despite the success, the effective and complete biodegradation of a wide variety of plastics is still far from the practical implementation, and an on-going effort has been mainly devoted to the exploration of novel microorganisms and enzymes for plastic biodegradation. However, alternative strategies which enhance the existing biodegradation process should not be neglected in the continuous advancement of this field. Thus, this review highlights various strategies which have shown to improve the biodegradation of plastics, which include the pretreatment of plastics using UV irradiation, thermal, or chemical treatments to increase the susceptibility of plastics toward microbial action. Alternative pretreatment strategies are also suggested and compared with the existing techniques. Besides, the effects of additives such as pro-oxidants, natural polymers, and surfactants on plastic biodegradation are discussed. In addition, considerations governing the biodegradation performance, such as the formulation of biodegradation medium, cell-free biocatalysis, and physico-chemical properties of plastics, are addressed. Lastly, the challenges and future prospects for the advancement of plastic biodegradation are also highlighted.
Introduction
Global production and consumption of plastics have been on the rapid rise in the past decade due to the widespread industrial applications of plastics including: packaging, construction, textiles, and the production of numerous consumer products [Citation1]. However, societal negligence in handling plastic wastes results in their accumulation in the environment, and plastic pollution has now become one of the biggest global environmental concerns. The accumulation of plastic wastes in the environment is predicted to reach 12 000 metric tons by 2050, with the packaging industry contributing as high as 46.7% of the total plastic wastes generated in 2015 [Citation1]. Due to their lightweight and flexible features, fossil fuel-derived plastics, such as high-density polyethylene (HDPE), low-density polyethylene (LDPE), polypropylene (PP), polystyrene (PS), and polyethylene terephthalate (PET) are widely used in the packaging industry [Citation2].
Furthermore, the spread of the coronavirus disease 2019 (COVID-19) threatens the environmental sustainability and brought upon a significant contribution toward plastic pollution. The personal protective equipment and face masks are mostly made of synthetic polymers, and their increased demand by frontline health workers and the general public are likely to persist until the end of this public health crisis [Citation3]. The implementation of lockdown in most areas around the world has also highly fostered the e-commerce platform and the demand of plastics for packaging is expected to rise by 40% [Citation4]. In addition, an increased preference in the usage of single-use plastics during shopping in times of pandemic has been observed due to safety concerns to reduce the risk of contamination [Citation4]. The increasing reliance on food delivery services as a result of the pandemic also contributes toward the greater consumption of single-use plastics for food packaging and their subsequent disposal after usage [Citation4]. The continuous accumulation of plastic wastes in the environment has been posing serious threats toward human health and the marine ecosystem. Plastic debris entering the ocean threatens marine lives primarily through entanglement and ingestion. Besides, the ingestion and transfer of plastic debris along the marine food chain may cause adverse impacts on human health [Citation5]. Therefore, an efficient handling of plastic wastes accumulation is required for the wellbeing of human and the environment.
Although the reuse, reduce, and recycle (3R) concept has been widely implemented across the globe for plastic waste management, this initiative alone is insufficient to overcome the problem of accumulating plastic wastes in the environment as several challenges still prevail, especially in times of pandemic when the risk of contamination reduces the likelihood of recycling or reusing plastics. Furthermore, out of 6300 metric tons of plastic wastes generated in 2015, only 9% of the wastes were recycled whereas 79% of wastes were accumulated in landfills, and the remaining 12% of plastic wastes were incinerated [Citation1], releasing numerous harmful compounds into the environment, further contributing toward environmental pollution [Citation6]. Recently, there is a shift of focus toward the biodegradation of plastic wastes, which is deemed to be a greener alternative due to the milder and less energy-intensive operating conditions as compared to the traditional treatment methods. Moreover, the abundance of microorganisms in nature implies that plastic biodegradation may be an effective strategy and a more sustainable approach for alleviating the accumulation of plastic wastes in the environment. Microorganisms have demonstrated excellent adaptability in harsh environments and they are good cleaning machines in environmental remediation. For instance, the removal of heavy metals from contaminated soil by some species of microorganisms is possible due to their higher tolerance toward the toxicity of heavy metals [Citation7]. Hence, stress-tolerant microorganisms capable of biodegrading plastic wastes naturally would be the ideal candidates for catalyzing the composting of plastics disposed in the environment.
Plastic biodegradation involves the formation of biofilm on polymer surface, where the microbial colony secretes extracellular enzymes to facilitate polymer fragmentation; the monomers or other degradation products released from the fragmentation of polymers become the substrates for the microorganisms [Citation6]. To achieve an economical outcome while being effective in handling the mountainous plastic wastes present in the environment, the biodegradation approach should be implemented at a larger scale, which remains to be fully explored. Aside from the scalability issue of plastic biodegradation, there is only a limited number of PET-degrading enzymes that have been well characterized and heavily studied, whereas the enzymes capable of biodegrading plastics, such as polyvinyl chloride (PVC), HDPE, LDPE, PP, and PS have not been identified [Citation8]. Therefore, huge efforts have been put into the exploration of novel microbial strains and enzymes in the hope of expanding the versatility of biodegradation strategy for tackling various types of plastics. A comprehensive compilation of plastic-degrading microbial strains and their performances in the biodegradation of various plastics has been reported elsewhere [Citation9,Citation10].
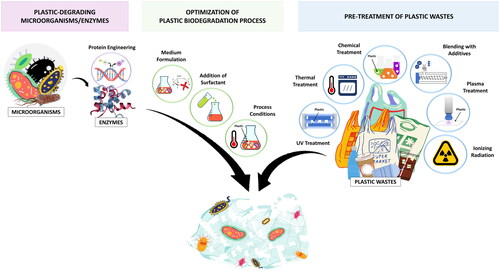
Although the roles of microorganisms and enzymes are indispensable in an effective biodegradation process of plastics, the strategies of enhancing the biodegradation process must not be overlooked because any improvement in the microbial or enzymatic activities could result in a higher rate of plastic biodegradation. Thus, this review aims to provide a holistic view on the methods that enhance the overall efficiency of plastic biodegradation processes, including the optimization of enzymatic activities, the biodegradation process, and the pretreatment of plastic samples. The relevant strategies are depicted in . Besides, alternative methods that improve plastic biodegradation are suggested and discussed along with the process considerations and challenges of the existing strategies. This review aims to inspire future research to look deeper into the field of plastic biodegradation and address the current challenges and inconsistencies in studies related to the biodegradation of plastics. Future prospects of plastic biodegradation are elaborated in the later section of this review.
Pretreatment of plastics
In most of the plastic biodegradation studies, the pretreatment of plastics was first conducted to induce polymer modifications for facilitating plastic biodegradation upon incubation with microorganisms. The widely adopted strategies of plastic pretreatment include UV irradiation, thermal, and chemical treatments. These abiotic factors induce polymer oxidation and create regions within the polymer chain, which increase the susceptibility of polymer toward the action of microorganisms [Citation8]. The pretreatment of plastics is usually performed on HDPE, LDPE, and PP. This is mainly because of their higher resistance toward biodegradation, as their polymeric structures (long hydrocarbon chains) contribute toward their highly hydrophobic nature [Citation11].
Polymer oxidation upon subjection of plastic to the pretreatment is typically evinced through the increase in formation of functional groups, primarily carbonyl, carboxyl, and ester groups within the polymer chain [Citation12]. An increase in the number of functional groups on the pretreated plastics helps in enhancing polymer hydrophilicity, enabling a better adherence of microorganisms and formation of biofilm on polymer surface upon subsequent incubation with microorganisms [Citation12]. Besides, the changes in surface morphology of polymers upon pretreatment can be observed through an increase in their surface roughness [Citation13], accompanied by the formation of cracks, pits, and grooves upon incubation of pretreated polymers with microorganisms [Citation12]. The SEM micrographs showing the effects of UV pretreatment on the surface morphology of LDPE films are depicted in .
Figure 2. Effects of UV pretreatment on the surface morphologies of LDPE films. (a) Control (no UV pretreatment, no incubation with microorganisms). (b) After UV pretreatment for 25 days. (c) Formation of pits and cracks on UV-pretreated LDPE films after incubation with soil microorganisms for 126 days [Citation14].
![Figure 2. Effects of UV pretreatment on the surface morphologies of LDPE films. (a) Control (no UV pretreatment, no incubation with microorganisms). (b) After UV pretreatment for 25 days. (c) Formation of pits and cracks on UV-pretreated LDPE films after incubation with soil microorganisms for 126 days [Citation14].](/cms/asset/c5af0ee7-1f79-4171-ac87-0fca2e36f709/ibty_a_2170861_f0002_b.jpg)
In this section, the impacts of pretreatment (e.g., UV, thermal, and chemical) strategies on the biodegradation of plastics are discussed along with certain process considerations and suggestions to enhance the practicability and effects of these pretreatment techniques on the overall performance of plastic biodegradation. Several emerging or alternative pretreatment techniques that are less explored are also suggested.
UV pretreatment of plastics
The UV pretreatment of plastics involves plastic samples subjected to UV irradiation at wavelengths ranging within 225–312 nm; the UV irradiation is usually performed in a controlled environment using UV chamber, UV lamps, or weathering testers such as the QUV Accelerated Weathering Tester, which simulates the natural weathering conditions by alternating UV and humidity conditions on polymer surface [Citation15,Citation16]. Upon exposure to UV irradiation, plastic samples undergo photooxidative degradation through the excitation of polymer molecules, resulting in the formation of radicals and other active species. Subsequently, these species undergo various reactions (e.g., the abstraction of hydrogen atom or the recombination of radicals) to promote chain scission and/or cross-linking of polymer chains. Functional groups, such as vinyl, aldehydes, and ketones are the common products of polymer photo-oxidation. The evolution of CO and/or CO2 has also been observed in the photo-oxidation of PE, PP, and PET plastics whereas the rapid yellowing of PS and PVC plastics is one of the common damages caused by UV irradiation [Citation17–20]. Detailed mechanisms on the photo-oxidation of various plastics are illustrated in [Citation18–22].
Figure 3. Detailed mechanisms on the photo-oxidation of various plastics [Citation18–22].
![Figure 3. Detailed mechanisms on the photo-oxidation of various plastics [Citation18–22].](/cms/asset/abcc2e87-794e-4c00-90bd-f2d037cf3105/ibty_a_2170861_f0003_b.jpg)
As shown in several past studies on the biodegradation of LDPE [Citation14,Citation16], the large number of carbonyl groups generated from UV pretreatment reduced upon incubation with microorganisms. This phenomenon suggests the initiation of polymer biodegradation occurring at the carbonyl groups. Moreover, the decrease in carbonyl groups was accompanied by a simultaneous increase in the number of double bond groups [Citation13,Citation14]. This observation may be due to the transformation of carbonyl to double-bond groups through the Norrish type II mechanism [Citation13]. However, an opposite trend has been observed in several studies where a continuous increase in carbonyl index in HDPE and LDPE was observed upon biological treatment, indicating the microbial oxidation of short polymer chains [Citation23,Citation24]. Several studies have shown that surface morphology of plastics was affected upon UV treatment [Citation12,Citation13]. An exception was noted in a study reporting the biodegradation of PP film by Pseudomonas sp. and Actinomycetes sp., where the surface morphology of UV-pretreated PP film showed no visible structural changes, and yet the successful colonization of microorganisms on PP films was observed [Citation25].
UV irradiation has also induced changes to the mechanical properties of plastics, which can be determined through a higher degree of reduction in tensile strength of plastics and a slight increase in crystallinity of UV-pretreated plastic [Citation14,Citation23]. Typically, the action of microorganisms is initiated in the amorphous regions of polymer rather than in the crystalline regions because the crystalline region is more resistant to enzymatic attack [Citation23]. However, a higher polymer crystallinity as a result of UV exposure did not seem to hinder the enzymatic actions on the biodegradation of plastics, and this may be due to the other accompanying effects of UV irradiation (e.g., the increased formation of functional groups and a higher hydrophilicity of polymer) which contribute toward the enhanced plastic biodegradation. Nonetheless, an opposite trend of results was observed from the case of PET. The exposure of PET films to UV irradiation resulted in a higher density of carboxyl groups and a higher degree of polymer crystallinity but the overall biodegradation of UV-pretreated PET was lower as compared to the control samples (without UV treatment) [Citation26]. The weight loss of UV-pretreated PET films showed a reduction of approximately 8% compared to untreated films. This may indicate that the biodegradation of PET plastics is largely dependent on its degree of crystallinity because previous studies have reported a reduced level of degradation of a highly crystalline PET as compared to a less crystalline sample [Citation27]. A summary of the effects of UV pretreatment on the biodegradation of plastics is shown in .
Table 1. Effects of UV, thermal, and chemical pretreatments on the biodegradation of plastics.
The optimal operating parameters of UV pretreatment, especially treatment duration, is crucial in the performance of photo-oxidation and the subsequent biodegradation of plastic samples. Hadad et al. [Citation16] have shown the increased gravimetric and molecular weight losses of LDPE films when the UV treatment duration was increased from 60 to 120 h. In addition, another study was conducted by subjecting LDPE powder to 1 h of UV irradiation but no significant effects on the LDPE structure was observed after pretreatment [Citation28]; the absence of formation of carbonyl groups on the LDPE implies the deficiency in the treatment duration (1 h) for inducing any polymer modification. Hence, the UV pretreatment of plastics is typically performed for 3–15 days in most biodegradation studies.
Thermal pretreatment of plastics
The thermal pretreatment of plastics involves heating the plastic samples to induce changes in the polymer’s properties that facilitate its subsequent biodegradation. Thermal pretreatment is often performed in an oven, at temperatures between 70 and 150 °C [Citation23,Citation31]. Nonetheless, a higher pretreatment temperature has led to an increased formation of functional groups and a higher superficial growth of hyphae on the surface of LDPE [Citation23].
The effects of thermal pretreatment on plastic biodegradation are almost similar to those of UV irradiation as summarized in . Thermally pretreated plastic samples also exhibited a higher level of polymer crystallinity due to the annealing effect contributed by the thermal treatment [Citation32]. A further increase in polymer crystallinity was also observed after biological treatment, probably due to the action of microorganisms on the amorphous regions of polymer [Citation15]. However, there was a study reporting a reduction in polymer crystallinity upon thermal treatment of LDPE films, which might be caused by the different cooling rates subjected to the samples after thermal treatment, causing the variation in polymer structure [Citation23]. The duration of thermal pretreatment is also as lengthy as that of UV irradiation treatment period, which typically takes 3–10 days to complete [Citation24,Citation34]. However, a previous study showed that PP films thermally treated at 100 °C for merely 15 min could result in the increased roughness and uneven polymer surface, as evinced by the SEM micrographs [Citation25]. Furthermore, the microbial colonization on the surface of these pretreated PP films indicated that the resultant polymer modifications are inductive to the biodegradation of plastics.
In view of the similarities between UV and thermal pretreatments in terms of treatment duration and their effects on plastic biodegradation, the efficiencies of both UV and thermal pretreatments are worthy of comparison. The UV-pretreated LDPE contained a higher number of carbonyl and hydroxyl groups as compared to the thermally pretreated LDPE [Citation23]. The UV-pretreated PP films were also found to have more significant increase in the polymer hydrophilicity and a higher degradation rate than the thermally pretreated plastics [Citation29]. In addition, the weight loss of plastic samples was noted to be higher in UV-pretreated PP upon biological treatment [Citation12,Citation29]. Generally, UV pretreatment is more effective than the thermal pretreatment for plastic biodegradation but the latter may be more practical in the large-scale biodegradation of plastics as larger number of samples can be subjected to the heat treatment simultaneously using industrial ovens.
Chemical pretreatment of plastics
The chemical pretreatment of plastics involves the exposure of plastic samples to concentrated chemical solutions or acids, which act as oxidizing agents to induce polymer oxidation [Citation30]. The liquid etching of polymeric surfaces caused by the chemical pretreatment increases the surface roughness by the formation of pits and cracks, and promotes the generation of unsaturated and polar functional groups on the polymeric surfaces [Citation36]. Although significant etching effects are observed on PE treated with strong oxidizing acids at temperatures above 60 °C, a lower temperature has been commonly used in the chemical pretreatment of plastic samples before incubation with microorganisms [Citation24,Citation36].
The chemical pretreatment of PP with Fenton’s reagent salt improved the growth of Bacillus subtilis, which was indicated by the higher cell density in the biodegradation medium as compared to un-pretreated PP; this implies the role of chemical pretreatment in rendering the polymer as sole carbon source for microorganisms [Citation30]. However, the pretreatment of PP films with aqua regia solution for 3 days eventually led to a reduction in the colony-forming unit of Bacillus subtilis during biodegradation, despite resulting in a higher weight loss of polymer as compared to un-pretreated samples [Citation30]. This suggests that some chemical compounds may have detrimental effects on cell growth despite demonstrating superior performance in polymer degradation. Moreover, Motta et al. [Citation35] have observed the enhanced degradation effects (e.g., increase in the formation of carbonyl and hydroxyl groups and enhanced colonization by Curvularia sp.) when transition metal or inorganic acid was added along with an oxidizing agent in the chemical pretreatment of PS, as opposed to the application of oxidizing agent solely in the pretreatment process. A summary of the effects of chemical pretreatment on the biodegradation of plastics is shown in .
Chemical pretreatment of plastics is also a feasible pretreatment option in the large-scale biodegradation of plastics because a large quantity of plastic samples can be immersed in the concentrated chemical solutions simultaneously. The reusability of chemicals for multiple rounds of plastic pretreatment, which remains to be explored, may also promote an economical outcome in the scale-up of plastic biodegradation process. Besides, the effects of a cascading pretreatment on the biodegradation of plastics have been investigated whereby HDPE films were subjected to UV irradiation for 60 h, followed by thermal (50 °C) for 72 h and chemical (KMnO4/HCl and citric acid) treatments [Citation24]. For HDPE films subjected to the cascading pretreatment, the highest weight loss of polymer and significant surface modification and corrosion were achieved as compared to similar films subjected to the discrete pretreatment [Citation24]. However, the adoption of several pretreatment techniques in the large-scale biodegradation of plastics may not be practical due to the significantly prolonged treatment duration and the economic considerations, which may not be compensated or justified by the enhancement in the plastic biodegradation.
Alternative pretreatment strategies
There are some emerging or alternative pretreatment approaches that may enhance the overall performance of plastic biodegradation, although these methods are less explored or adopted as compared to the conventional pretreatment technologies as discussed earlier. One of the alternatives is ionizing radiation (e.g., gamma rays), which stimulates the ionization and excitation of polymer to form the reactive intermediates, free radicals, and excited states. These generated species are responsible for the modification of polymer structures based on chain scission or cross-linking mechanisms [Citation37]. Cross-linking of polymer usually takes place under vacuum environments whereas the presence of oxygen in the process of pretreatment enhances chain scission [Citation37]. Sheik et al. [Citation38] evaluated the effects of gamma irradiation on the biodegradation of LDPE and PP films by endophytic fungi Lasiodiplodia theobromae. The increase in weight loss and the formation of carbonyl groups of gamma-irradiated LDPE and PP films were observed, as compared to control samples (without gamma irradiation). Furthermore, the similar study observed the enhanced effects (e.g., larger increase in the formation of carbonyl groups and higher weight losses of LDPE and PP films) at higher gamma dose rates. However, a further increase in gamma dose rate above 100 kGy would bring about the pyrolysis of PP films whereas the cleavage of LDPE films may occur above 1000 kGy. On the other hand, gamma dose rates at 20 kGy and 200 kGy were insufficient to induce weight loss in LDPE and PP films, respectively [Citation38]. Hence, the selection of appropriate gamma dose rates is critical for the optimum pretreatment of plastics using gamma irradiation.
Plasma treatment involves the excitation of gas with electrical energy to generate plasma that reacts with polymer surfaces to induce the formation of functional groups and to modify polymer properties [Citation39,Citation40]. Surface plasma treatment has been performed on polyolefins to increase surface hydrophilicity, roughness, and the formation of radicals on polymeric surfaces [Citation41]. Gómez-Méndez et al. [Citation42] studied the biodegradation of plasma-pretreated LDPE sheets with Pleurotus ostreatus where LDPE sheets were pretreated using oxygen glow discharge plasma at 600 V for 6 min. The pretreated polymer showed a reduction of 76.57% in surface contact angle, indicating a significant improvement in surface hydrophilicity. The surface roughness of pretreated LDPE also increased by 99.81%, and the fungal colonization on the polymer surface was enhanced to 88.72% as compared to untreated samples which showed 45.55% of colonization only. This study has demonstrated the feasibility of plasma treatment in inducing polymer modifications at a significantly shorter treatment duration (i.e., 6 min), as compared to the common plastic pretreatment techniques (UV and thermal pretreatments), which are typically performed for 2–10 days. Further research can be performed to increase the adoptability of plasma pretreatment in enhancing the overall biodegradation of plastics. A comparison among different pretreatment approaches with the advantages and drawbacks associated to each technique is summarized in .
Table 2. Comparison of plastic pretreatment approaches.
Additives to enhance plastic biodegradation
The manufacture of commercial plastics involves the formulation of basic polymer with various chemical compounds known as additives, which improve the overall functionality and performance of the final product [Citation44]. Stabilizers are among the most commonly used additives in polymer formulation to prolong the shelf life of plastics and provide protection from thermal- and UV-associated oxidation [Citation45]. Therefore, the presence of synthetic additives in commercially available plastics reduces the rate of polymer oxidation and the susceptibility of plastics toward biodegradation. Nonetheless, the incorporation of additives, such as pro-oxidants, natural polymers, non-ionic surfactants, and mineral oil, helps in rendering the plastics toward the action of microorganisms in order to initiate and enhance the biodegradation of plastics. In this section, the effects of additives on the biodegradation of plastics are reviewed and discussed.
Incorporation of additives in plastic production
The incorporation of additives such as pro-oxidants and natural polymers into synthetic plastics is a commonly used technique to enhance the biodegradation of plastics. Pro-oxidants initiate and enhance the oxidation of plastics (mainly HDPE, LDPE, and PP) in the presence of light or heat through the generation of free radicals on the polymer surface. These free radicals further react with oxygen to produce oxides or hydroperoxides, thereby resulting in polymer chain scission [Citation46]. As the presence of antioxidants and heat stabilizers in commercial plastics delays the rate of polymer oxidation, the supplementation of pro-oxidant additives in the plastic product helps to counter the effects of stabilizers by initiating and enhancing polymer oxidation in the presence of light or heat [Citation47]. Metal stearates or carboxylates with transition valency are typically added as pro-oxidant additives in plastics [Citation48,Citation49]. On the other hand, the presence of natural polymers such as starch and cellulose in the plastic product enhances the biodegradation of plastics because natural polymers are expected to undergo the biodegradation quicker than the polymer backbone chains, thus leaving a hollow polymer matrix and increasing the surface-to-volume ratio of polymer for the continuous action of microorganisms within the plastic samples [Citation50].
Effects of the incorporation of pro-oxidants and natural polymers in the biodegradation of plastics are shown in . Generally, positive effects were observed in the biodegradation of plastics containing additives (e.g., pro-oxidants and natural polymers). However, several reports did not mention the type or the concentration of pro-oxidants utilized in the respective studies. The type and concentration of pro-oxidants used along with their basis of selection shall be considered in future studies to provide insights into their impact on plastic biodegradation. Nonetheless, an increase in the concentration of pro-oxidants has rendered an enhanced polymer oxidation of plastic upon UV exposure [Citation49]. The increase in the carbonyl index of UV-pretreated plastics and the reductions in both tensile strength and molecular weight of plastics were more significant when a higher concentration of pro-oxidants was mixed into the plastic [Citation49]. Besides, comparative studies have been conducted to evaluate the efficiencies of various transition metal carboxylates in plastic degradation. Based on the reported results, cobalt stearate is generally one of the most effective pro-oxidants that enhanced the polymer oxidation of LDPE upon exposure to light or heat [Citation49,Citation53]. Moreover, the efficiencies of pro-oxidants and natural polymers as additives to enhance plastic biodegradation have been compared. PP samples incorporated with metal ions showed higher levels of carbonyl index, polymer weight loss, and microbial colonization on polymer surface, as compared to the starch-blended PP [Citation12].
Table 3. Effects of additives on plastic biodegradation.
Supplementation of additives in plastic biodegradation media
Surfactant is the most common additive supplemented into plastic biodegradation media; it could modify the interaction forces at the interface between microorganisms and polymer to reduce surface tension and increase the wettability of polymeric surfaces [Citation54], thereby facilitating biofilm formation and secretion of extracellular enzymes for the effective biodegradation of plastics [Citation55]. Non-ionic surfactants such as Tween 20, 60, and 80 are the most commonly used surfactants for plastic biodegradation [Citation16,Citation56]. Besides surfactants, mineral oil has been commonly supplemented into the biodegradation media as an additive to enhance the biodegradation of plastics [Citation16,Citation56].
Supplementation of mineral oil into the biodegradation media has shown positive effects to the biodegradation of HDPE, in which the increased weight loss of polymer was attributed to the enhanced colonization on the surface of HDPE, which led to the accelerated formation of biofilm [Citation57]. Several reports [Citation56,Citation58] have proven that mineral oil is more effective compared to Tween 20 and 80 in the biodegradation of plastics, as the addition of mineral oil showed an increase in biofilm bacterial count and a higher weight loss of plastics. In another study, both mineral oil and Tween 85 enhanced the colonization of Brevibacillus borstelensis on LDPE, as measured through the total extractable protein collected in the first four days of biodegradation. A decrease in the total extractable protein was observed from the 4th day onwards [Citation16]. This may suggest that the addition of non-ionic surfactants and mineral oil facilitated the bacterial colonization on polymer surfaces but were unable to sustain the growth and survival of microorganisms.
Tribedi and Sil [Citation58] have observed the enhanced biodegradation of LDPE when the mineral oil concentration was increased from 0.01 to 0.05%, as evinced by the enhanced colonization of Pseudomonas sp. and the increased weight loss of LDPE. Sodium dodecyl sulfate (SDS), an anionic surfactant, has been applied as an additive in the biodegradation of LDPE by Lysinibacillus fusiformis but its effect on biodegradation efficiency was found to be concentration dependent [Citation59]. The weight loss of LDPE, along with the carbonyl and double bond indices, was the highest at 6% SDS concentration whereas an increase in SDS concentration from 8 to 10% resulted in the decreases in weight loss of LDPE and both carbonyl and double bond indices. This implies that the increase in surfactant concentration does not necessarily promote the biodegradation of plastics, and the optimal surfactant concentration is crucial for the enhancement of plastic biodegradation [Citation59].
Biodegradation media formulation
The optimal formulation of biodegradation media with minimal nutrients, typically carbon and nitrogen, enhances the plastic biodegradation process because the composition of media influences the growth and survival of microorganisms, which eventually impact the action of microorganisms on plastic biodegradation. To attain an effective plastic biodegradation, carbon starvation is often performed to induce the utilization of the target polymer by microorganisms as their sole carbon source [Citation16]. This is because the carbon catabolite repression in microorganisms prevents the utilization of secondary carbon sources in the presence of easily metabolizable carbon sources such as glucose [Citation60,Citation61]. Hence, the absence of alternative carbon sources in the biodegradation media compels the microorganisms to make use of the organic acid and alcohol groups, which were formed in the polymer chain during pretreatment, as the sole carbon source. Furthermore, an overall increase in cell hydrophobicity was observed in the carbon-starved cultures compared to the non-starved cultures [Citation62].
The effects of carbon and nitrogen depletion on the biodegradation of LDPE by Brevibacillus borstelensis were studied using mannitol and potassium nitrate as carbon and nitrogen sources, respectively [Citation16]. The biodegradation of LDPE in mannitol-free media resulted in the highest percentage weight loss of LDPE compared to the media supplemented with 20% or 50% mannitol. It was also reported that the effect of nitrogen depletion on the biodegradation of LDPE is less than that of carbon. However, it is well known that not all microbial species are able to survive under harsh culture conditions, and therefore the absence of carbon sources does not necessarily improve plastic biodegradation. Mukherjee et al. [Citation59] have observed no biodegradation activity (e.g., no changes in carbonyl index, crystallinity and weight loss) by Lysinibacillus fusiformis on the thermally oxidized LDPE when the biodegradation media was not supplemented with glucose. However, a decrease in both carbonyl index and crystallinity accompanied with an increase in polymer weight loss was observed in the oxidized LDPE when 1% w/v of glucose was added to the biodegradation media. This study showed that glucose was utilized by Lysinibacillus fusiformis for its initial growth, and, upon glucose exhaustion, the oxidized polymer will be utilized as the carbon source for microorganisms [Citation59]. Several other studies also reported the similar trend of significant improvements in plastic biodegradation when minimal amounts of carbon and nitrogen sources were supplemented into the biodegradation media [Citation24,Citation63,Citation64]. These studies showed the importance of minimal supplementation of carbon and nitrogen sources into the biodegradation media in order to stimulate the action of microorganisms in plastic biodegradation. However, the metabolic rates of microorganisms are species dependent. For example, microorganisms such as Brevibacillus borstelensis may not require the presence of initial nutrient sources due to its higher ability to survive in carbon-deprived media as compared to other microbial species. In addition, the amounts of carbon and nitrogen sources required for the initial growth and adaptation of microorganisms are highly dependent on their species, as shown by the varying amounts of carbon sources used in several plastic biodegradation studies [Citation63,Citation64].
Protein engineering
The biodegradation of plastics by microorganisms involves the secretion of extracellular enzymes which are responsible for the cleavage of polymer chains to form the low-molecular-weight fragments, such as oligomers, dimers, and monomers [Citation65]. Therefore, it is important to engineer an enzyme with desired characteristics that enhance the overall biodegradation process of plastics. To date, the biodegradation of PET has been widely studied due to the discovery of several putative enzymes for its biodegradation [Citation27,Citation66], while the specific enzymes associated with the biodegradation of HDPE, LDPE, PP, PS, and PVC have not been identified and extensively studied [Citation8]. Since the enzymatic degradation of PET plastics is more extensively studied, protein engineering strategies mainly focused on the enhancement of PET-degrading enzymes for biodegradation process. These strategies adopted for the modification of PET-degrading enzymes shall serve as future references for the modification of enzymes capable of degrading other plastics.
In one of the experiments involving protein engineering, site-directed mutagenesis was performed on a putative cutinase from the thermophilic bacterium, Saccharomonospora viridis AHK190 [Citation67]. The thermostability and activity of cutinase were improved by substituting Serine-226 and Arginine-228 with proline and serine, respectively. In another study, single-site substitution of amino acid residues in cutinase derived from Thermobifida fusca KW3 (TfCut2) resulted in a 2.7-fold increase in the weight loss of PET films as compared to the wild-type cutinase [Citation68]. The improvement in PET hydrolysis was a result of relieved product inhibition of the intermediate product, MHET. These studies have proven the potential of protein engineering in improving the catalytic efficiency and stability of enzymes associated with plastic biodegradation. Zhu et al. [Citation69] have provided a critical and holistic review on various rational design strategies for the optimization of plastic-degrading enzymes. Future research in protein engineering shall focus on the reusability of enzymes and the economic readiness of the large-scale enzymatic degradation of plastics to handle the vast accumulation of plastic wastes in the environment.
Process considerations affecting plastic biodegradation
The plastic biodegradation process is governed by several considerations which may affect the overall biodegradation performance of microorganisms and should not be overlooked when conducting biodegradation studies on plastics. One of them includes the purity of plastic samples used in plastic biodegradation studies. The presence of impurities in the polymer chain may act as alternative carbon sources for the microorganisms and prevent the utilization of the polymer backbone chain as the main carbon source for microorganisms [Citation70]. Therefore, it is recommended to conduct multiple quantification techniques (e.g., evaluating the formation of functional groups, changes in surface morphology, release of degradation products, and changes in plastic properties) to confirm the true plastic-degrading ability of microorganisms [Citation70]. Although the adoption of pure plastic samples (without the presence of impurities) in biodegradation studies could confirm the action of microorganisms in breaking down the main polymer chain, the degrading performance of microbial strains may not provide an exact representation of the biodegradation of consumer-end plastics, which are the main contributors toward plastic wastes pollution. In the effort to improve the biodegradation of commercial plastics, benzene and alcohol were successfully used as solvents to remove the synthetic additives such as plasticizers, coloring agents, and fillers from the plastics [Citation33].
Factors such as shapes and sizes of plastic samples also contribute toward the efficiency of biodegradation process. Most biodegradation studies involve the preparation of plastic samples into smaller pieces, usually in the form of films, pellets, or powder, for easier adherence and consumption by microorganisms [Citation28,Citation71]. An experiment conducted on the biodegradation of LDPE, HDPE, and PP plastics has shown a higher weight loss of plastics in the form of films as compared to the pelleted plastics [Citation71]. The greater surface area available in plastic films enhances the rate of plastic biodegradation. Another important consideration in biodegradation studies is the total surface area of plastic samples exposed toward the action of microorganisms. Since there is no guideline on the recommended size and thickness of plastic samples for the biodegradation process, varying sizes of plastic samples have been utilized in biodegradation experiments. Due to this reason, it is difficult to perform a critical comparison to evaluate the plastic biodegradation performances of various microbial strains because the total surface area available for microbial actions varied from experiment to experiment.
The adoption of purified enzymes in the biodegradation of plastics has been explored in a recent study [Citation72]. Fungal peroxidases were purified from Phanerochaete chrysosporium using gel filtration chromatography and were used in the biodegradation of PVC. The study has concluded that the application of purified fungal peroxidases in the biodegradation of PVC was more effective compared to a traditional whole cell approach due to the higher rate of enzymatic reaction [Citation72]. Moreover, the comparative study showed that the conventional whole cell approach took a longer processing time [Citation72]. However, the cost of enzyme purification remains an unattractive factor in the large-scale enzymatic degradation of plastics, which led to the adoption of crude enzymes in plastic biodegradation, with the aim of reducing process complexity and the processing cost required. Several attempts have been made for the application of crude enzymes in plastic biodegradation studies, and the positive results observed have confirmed the viability of using crude enzymes in plastic biodegradation [Citation73–75]. However, the daily supplementation of crude enzymes is required for the biodegradation of plastics, which may also restrain its application in the large-scale biodegradation of plastics. Nonetheless, the adoption of crude enzymes in plastic biodegradation still requires further studies because the performance and stability of crude enzymes in the biodegradation process are yet to be fully understood. To alleviate the long culture period needed for the preparation of a microbial culture, it is desirable to make use of the prepared cultures for multiple runs of biodegradation. A past study demonstrated the improvement in PE biodegradation through the incubation of acclimated biofilm communities with the naturally weathered PE samples. In that study, the biofilm formed on PE samples was harvested and incubated again with the new PE samples. Results showed that the acclimated biofilm communities were able to colonize and degrade PE samples faster, resulting in a significantly higher weight loss of polymer [Citation76]. This study unlocked opportunities for reusing biofilms in subsequent biodegradation processes and reducing the need for sustaining massive volume of microbial culture, which can be advantageous especially in the large-scale biodegradation of plastics. Further research on the reusability of enzymes in plastic biodegradation will also be helpful to achieve an economical outcome in the industrialization of plastic biodegradation.
In addition, temperature is another process condition affecting the performances of microorganisms in plastic biodegradation. The biodegradation of LDPE, HDPE, and PP films by Brevibacillus sp. and Aneurinibacillus sp. was conducted at temperatures ranging from 5 to 55 °C; the highest weight loss of plastics was achieved at 50 °C, which is the optimum temperature for bacterial growth of the respective species [Citation71]. A further increase in the working temperature reduced the weight loss of plastics, suggesting the inhibitive effect of a temperature beyond optimal level on the catalytic activities. Furthermore, a significant increase in the biodegradation of PET plastics was observed at 70 °C, which is the glass transition temperature of PET plastics [Citation77]. At process temperatures near the glass transition temperature, the mobility of PET chains is enhanced and the polymers become more susceptible toward the action of enzymes in the process of plastic biodegradation [Citation77]. This shows that the thermostability of enzymes is also critical for the effective biodegradation of plastics at temperatures near the glass transition temperature. A critical review on the approaches to enhance the thermostability of enzymes has been reported elsewhere by other researchers [Citation69].
Future perspectives of plastic biodegradation
The biodegradation approach has been widely proclaimed as a more environmental route to the mitigation of plastic wastes accumulation. However, the slow biodegradation process remains a barrier to the industrialization of plastic biodegradation because the overall process, beginning from the pretreatment of plastics to the mineralization of plastic wastes into carbon dioxide and water, takes up to several months. Therefore, the adoption of process integration and intensification may contribute toward a significant reduction in the overall process duration. By comparing several pretreatment strategies that have been discussed earlier, thermal pretreatment is considered one of the most practical pretreatment strategies to be integrated with the biological treatment of plastics due to its ease of scalability and process simplicity. Plastic samples incubated with microbial strains can be subjected to thermal treatment simultaneously at temperatures up to 70–100 °C, which are the commonly used temperatures in the thermal pretreatment of plastics [Citation12,Citation31]. The simultaneous action of heat and biological factors may accelerate the biodegradation process of plastics since the changes in polymers induced by thermal treatment (e.g., improvement in surface hydrophilicity, reduction in polymer mechanical strength, and the formation of functional groups) will be able to immediately facilitate the microbial actions, leading to an accelerated biodegradation process. However, this approach requires the utilization of thermophilic microbial strains which are able to withstand extreme temperatures (∼100 °C). Hence, further exploration for novel thermophilic microbial strains or enzymes for the biodegradation of plastics is necessary. Relevant protein engineering strategies can also be adopted to enhance enzyme thermostabilities at higher temperatures.
Plastic degradation or conversion could play a part in the circular economy whereby plastic wastes are degraded into monomers or functional chemicals to be utilized in other applications. The conversion of LDPE into functional chemicals, such as succinic, glutaric, and adipic acids, has been achieved through chemical routes that have a high energy demand (operating temperature up to 180 °C) [Citation78]. Therefore, the strategy of biodegrading and converting plastic wastes into their monomers or other functional chemicals is viewed as a greener and less energy-intensive approach. This approach does not necessitate the complete assimilation and mineralization of plastic wastes by microbial strains, and therefore the processing time could be less. The adoption of PET biodegradation in circular economy has been demonstrated through the synthesis of virgin PET from the terephthalic acid monomers biodegraded by PET hydrolase [Citation79]. The percentage of depolymerization of PET into its monomers was as high as 90% after 10 h of biocatalytic reaction, and the virgin PET synthesized using the resultant terephthalic acid monomers has the average molecular weight and intrinsic viscosity similar to the PET manufactured using petrochemical terephthalic acid. In addition, the cost of enzymes required for the biodegradation of one ton of PET plastics was approximately 4% of the price per ton of virgin PET [Citation79]. This hints that the enzyme is not a strong economic barrier to the commercialization of PET biodegradation. This successful case study also highlights the importance of identifying the relevant enzymes associated with the biodegradation of other plastics into their respective monomers. It is anticipated that the discovery of novel plastic-degrading enzymes could accelerate the commercialization of plastic biodegradation or propel the circular economy of plastics via the bioconversion of plastics into useful products or new plastics.
Furthermore, the discovery of novel microbial strains or enzymes for the effective biodegradation of plastics may contribute significantly toward the in situ bioremediation of plastic pollution. Recombinant DNA and genetic engineering technologies offer a promising approach to equip the bacteria growing in the plastic-polluted areas with the ability to secrete various plastic-degrading enzymes. By releasing these genetically modified (GM) bacteria with enhanced plastic-degrading abilities to their habitat polluted by plastics, an environmental bioremediation could be realized. The release of these plastic-eating GM bacteria to the environment is perceived to be less concerning compared to the farming of GM crops in agricultural land [Citation80], or the release of the Aedes aegypti mosquito infected by Wolbachia pipientis bacteria for attenuating mosquito-borne diseases [Citation81]. Nonetheless, the safety and biohazard aspects of the potentially released GM bacteria have to be considered meticulously. Although the release of GM bacteria for plastic pollution bioremediation can be targeted toward the existing landfills that are heavily contaminated with plastics, the interaction of GM bacteria with other biological systems where the bacteria may disseminate, and the stability of engineered gene in GM bacteria, have to be extensively studied prior to their release. Furthermore, the complete mineralization of plastic wastes into carbon dioxide and water by GM bacteria is crucial because it ensures that microplastics are not generated from the incomplete degradation of plastics. The formation of microplastics (i.e., plastic particles smaller than 5 mm) poses a serious threat to the marine ecosystem as the ingestion of microplastics by zooplankton species has shown to cause detrimental effects on their feeding behavior, growth, development, reproduction ability, and lifespan [Citation82]. Therefore, these considerations have to be addressed carefully prior to the realization of plastic pollution bioremediation by GM bacteria.
On the other hand, future research shall focus on the formulation of an enzyme cocktail for the effective biodegradation of mixed plastics. Since there is no single enzyme capable of biodegrading various types of plastics, the formulation of a cocktail consisting of the associated enzymes aids in the effective biodegradation of various plastic wastes simultaneously. Although the successful development of an enzyme cocktail for the biodegradation of plastics has not yet been reported, the adoption of enzyme cocktail has been well established in other fields, such as the hydrolysis of lignocellulosic biomass which requires multiple enzymes in optimum quantities at operating pH and temperature of 4.8 and 50 °C, respectively [Citation83]. The enzyme cocktail was optimized based on its synergism with the substrate, and the hydrolysis of lignocellulosic biomass was significantly improved by 75% using the optimized enzyme cocktail. The formulation of enzyme cocktail for the biodegradation of mixed plastic wastes will contribute toward an enhanced process efficiency because the preliminary sorting of plastic wastes will not be required. This approach may also be helpful in the bioremediation of plastic pollution, as the existing landfills are often filled with various plastic wastes. Further studies on the potential of enzymes in the biodegradation of colored plastics shall be emphasized because the commercially available plastics typically contain dyes and pigments that may impact the enzymatic activities on plastics. The adoption of protein engineering on leaf-branch compost cutinase has shown promising results in the biodegradation of colored PET flakes [Citation79]. This breakthrough provides significant insights into future research for the biodegradation of other colored plastics. Nonetheless, this approach also requires further exploration on the optimization of process conditions for the synergistic actions of various enzymes. The risk of inhibition of enzyme catalytic activities due to the formation of plastic biodegradation products should also not be overlooked in the effort to accomplish the effective biodegradation of mixed plastic wastes simultaneously.
Recently, a novel approach to accelerate the biodegradation of plastics was reported whereby plastic-degrading enzymes were incorporated into the polymer matrix of plastics. By embedding enzymes responsible for the degradation of biodegradable polyesters into the vegetable-based polylactic acid (PLA) and polycaprolactone (PCL) biodegradable plastics, the biodegradation of PCL was achieved in two days under industrial composting conditions at 40 °C whereas the disintegration of PLA took six days at 50 °C [Citation84]. This radical approach was claimed to be cost-effective because the confinement of enzymes into the respective polymer matrix would only contribute toward a slight increase in the production cost of respective plastics [Citation85]. The applicability of such approach in the biodegradation of other synthetic plastics shall be further explored.
Conclusions
Throughout the years of extensive research in the biodegradation of plastics, various novel enzymes originated from microorganisms have been screened for their abilities to degrade plastic. However, until now, only the complete biodegradation of PET in considerably short durations has been achieved whereas the effective biodegradation of the other five main categories of plastics still requires intensive research efforts. Hence, this review has highlighted various strategies and valuable insights which may contribute toward the advancement in the biodegradation of other plastics. As the accumulation of plastic wastes in the environment increases, there is a pressing need for gearing up the biodegradation strategy for a shift toward a sustainable management of plastic wastes.
Nonetheless, the challenges and inconsistencies in the current studies of plastic biodegradation shall also be addressed. Extensive studies on identification and characterization of the enzymes responsible for the degradation of other plastics and their mechanisms of degradation shall be emphasized before the optimization and industrialization of plastic biodegradation process can be realized.
Disclosure statement
All the authors agree with the submission and declare no financial interests or personal conflicts.
Additional information
Funding
References
- Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3(7):e1700782.
- Salwa HN, Sapuan SM, Mastura MT, et al. Green bio composites for food packaging. Int J Recent Technol Eng. 2019;8:450–459.
- Parashar N, Hait S. Plastics in the time of COVID-19 pandemic: protector or polluter? Sci Total Environ. 2021;759:144274.
- Patrício Silva AL, Prata JC, Walker TR, et al. Increased plastic pollution due to COVID-19 pandemic: challenges and recommendations. Chem Eng J. 2021;405:126683.
- Sharma S, Chatterjee S. Microplastic pollution, a threat to marine ecosystem and human health: a short review. Environ Sci Pollut Res. 2017;24(27):21530–21547.
- Zhang J, Wang X, Gong J, et al. A study on the biodegradability of polyethylene terephthalate fiber and diethylene glycol terephthalate. J Appl Polym Sci. 2004;93(3):1089–1096.
- González Henao S, Ghneim-Herrera T. Heavy metals in soils and the remediation potential of bacteria associated with the plant microbiome. Front Environ Sci. 2021;9:604216.
- Mohanan N, Montazer Z, Sharma PK, et al. Microbial and enzymatic degradation of synthetic plastics. Front Microbiol. 2020;11:580709.
- Shilpa NB, Meena SS. Microbial biodegradation of plastics: challenges, opportunities, and a critical perspective. Front Environ Sci Eng. 2022;16:161.
- Zhang Y, Pedersen JN, Eser BE, et al. Biodegradation of polyethylene and polystyrene: from microbial deterioration to enzyme discovery. Biotechnol Adv. 2022;60:107991.
- Liu X, Gao C, Sangwan P, et al. Accelerating the degradation of polyolefins through additives and blending. J Appl Polym Sci. 2014;131(18):40750.
- Jeyakumar D, Chirsteen J, Doble M. Synergistic effects of pretreatment and blending on fungi mediated biodegradation of polypropylenes. Bioresour Technol. 2013;148:78–85.
- Kundungal H, Gangarapu M, Sarangapani S, et al. Role of pretreatment and evidence for the enhanced biodegradation and mineralization of low-density polyethylene films by greater waxworm. Environ Technol. 2021;42:717–730.
- Esmaeili A, Pourbabaee AA, Alikhani HA, et al. Biodegradation of low-density polyethylene (LDPE) by mixed culture of Lysinibacillus xylanilyticus and Aspergillus Niger in soil. PLOS One. 2013;8(9):e71720.
- Arkatkar A, Arutchelvi J, Bhaduri S, et al. Degradation of unpretreated and thermally pretreated polypropylene by soil consortia. Int Biodeterior Biodegrad. 2009;63(1):106–111.
- Hadad D, Geresh S, Sivan A. Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis. J Appl Microbiol. 2005;98(5):1093–1100.
- Fernando SS, Christensen PA, Egerton TA, et al. Carbon dioxide evolution and carbonyl group development during photodegradation of polyethylene and polypropylene. Polym Degrad Stab. 2007;92(12):2163–2172.
- Yousif E, Hasan A. Photostabilization of poly(vinyl chloride) – still on the run. J Taibah Univ Sci. 2015;9(4):421–448.
- Yousif E, Haddad R. Photodegradation and photostabilization of polymers, especially polystyrene: review. Springerplus. 2013;2(1):398.
- Chamas A, Moon H, Zheng J, et al. Degradation rates of plastics in the environment. ACS Sustain Chem Eng. 2020;8:3494–3511.
- Oswald HJ, Turi E. The deterioration of polypropylene by oxidative degradation. Polym Eng Sci. 1965;5(3):152–158.
- Sang T, Wallis CJ, Hill G, et al. Polyethylene terephthalate degradation under natural and accelerated weathering conditions. Eur Polym J. 2020;136:109873.
- Manzur A, Limón-González M, Favela-Torres E. Biodegradation of physicochemically treated LDPE by a consortium of filamentous fungi. J Appl Polym Sci. 2004;92(1):265–271.
- Balasubramanian V, Natarajan K, Rajeshkannan V, et al. Enhancement of in vitro high-density polyethylene (HDPE) degradation by physical, chemical, and biological treatments. Environ Sci Pollut Res. 2014;21(21):12549–12562.
- Margandan MM. Growth of Actinomycetes and Pseudomonas sp., biofilms on abiotically pretreated polypropylene surface. Eur J Zool Res. 2014;3:6–17.
- Falkenstein P, Gräsing D, Bielytskyi P, et al. UV pretreatment impairs the enzymatic degradation of polyethylene terephthalate. Front Microbiol. 2020;11:689.
- Yoshida S, Hiraga K, Takehana T, et al. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science. 2016;351(6278):1196–1199.
- Montazer Z, Najafi MBH, Levin DB. Microbial degradation of low-density polyethylene and synthesis of polyhydroxyalkanoate polymers. Can J Microbiol. 2019;65(3):224–234.
- Aravinthan A, Arkatkar A, Juwarkar AA, et al. Synergistic growth of Bacillus and Pseudomonas and its degradation potential on pretreated polypropylene. Prep Biochem Biotechnol. 2016;46:109–115.
- Arkatkar A, Juwarkar AA, Bhaduri S, et al. Growth of Pseudomonas and Bacillus biofilms on pretreated polypropylene surface. Int Biodeterior Biodegrad. 2010;64(6):530–536.
- Awasthi S, Srivastava N, Singh T, et al. Biodegradation of thermally treated low density polyethylene by fungus Rhizopus oryzae NS 5. 3 Biotech. 2017;7(1):73.
- Sudhakar M, Doble M, Murthy PS, et al. Marine microbe-mediated biodegradation of low- and high-density polyethylenes. Int Biodeterior Biodegrad. 2008;61(3):203–213.
- Chatterjee S, Roy B, Roy D, et al. Enzyme-mediated biodegradation of heat treated commercial polyethylene by Staphylococcal species. Polym Degrad Stab. 2010;95(2):195–200.
- Awasthi S, Srivastava P, Singh P, et al. Biodegradation of thermally treated high-density polyethylene (HDPE) by Klebsiella pneumoniae CH001. 3 Biotech. 2017;7(5):332.
- Motta O, Proto A, De Carlo F, et al. Utilization of chemically oxidized polystyrene as co-substrate by filamentous fungi. Int J Hyg Environ Health. 2009;212(1):61–66.
- Mijovic JS, Koutsky JA. Etching of polymeric surfaces: a review. Polym Plast Technol Eng. 1977;9(2):139–179.
- Abiona A, Osinkolu AG. Gamma-irradiation induced property modification of polypropylene. Int J Phys Sci. 2010;5:960–967.
- Sheik S, Chandrashekar KR, Swaroop K, et al. Biodegradation of gamma irradiated low density polyethylene and polypropylene by endophytic fungi. Int Biodeterior Biodegrad. 2015;105:21–29.
- Abourayana H, Dowling D. Plasma processing for tailoring the surface properties of polymers. In: Surf energy; 2015. p. 123–152.
- Ebnesajjad S. Chapter 8 – surface treatment of polyvinyl fluoride films and coatings. In: Ebnesajjad S, editor. Polyvinyl fluoride. Norwich: William Andrew Publishing; 2013. p. 193–212.
- Pionteck J, Wypych G. Chapter 10 – antistatic agent incorporation method and its performance. In: Pionteck J, Wypych G, editors. Handbook of antistatics. 2nd ed. Toronto: ChemTec Publishing; 2016. p. 129–139.
- Gómez-Méndez LD, Moreno-Bayona DA, Poutou-Piñales RA, et al. Biodeterioration of plasma pretreated LDPE sheets by Pleurotus ostreatus. PLOS One. 2018;13(9):e0203786.
- Lee B, Pometto AL, Fratzke A, et al. Biodegradation of degradable plastic polyethylene by Phanerochaete and Streptomyces species. Appl Environ Microbiol. 1991;57(3):678–685.
- Hahladakis JN, Velis CA, Weber R, et al. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater. 2018;344:179–199.
- Hunt TP. Polymer additives: supercritical fluid chromatography. In: Wilson ID, editor. Encyclopedia of separation science. Oxford: Academic Press; 2000. p. 3901–3906.
- Chiellini E, Corti A, D'Antone S, et al. Oxo-biodegradable carbon backbone polymers – oxidative degradation of polyethylene under accelerated test conditions. Polym Degrad Stab. 2006;91(11):2739–2747.
- Lukanina YK, Popov AA, Khvatov AV. Biodegradation of polymer compositions with pro-oxidants. Proceedings of the IOP Conference Series: Materials Science and Engineering; 2020. p. 12016.
- Roy PK, Titus S, Surekha P, et al. Degradation of abiotically aged LDPE films containing pro-oxidant by bacterial consortium. Polym Degrad Stab. 2008;93(10):1917–1922.
- Roy PK, Surekha P, Rajagopal C, et al. Effect of cobalt carboxylates on the photo-oxidative degradation of low-density polyethylene. Part-I. Polym Degrad Stab. 2006;91(9):1980–1988.
- Erlandsson B, Karlsson S, Albertsson A-C. The mode of action of corn starch and a pro-oxidant system in LDPE: influence of thermo-oxidation and UV-irradiation on the molecular weight changes. Polym Degrad Stab. 1997;55(2):237–245.
- Corti A, Muniyasamy S, Vitali M, et al. Oxidation and biodegradation of polyethylene films containing pro-oxidant additives: synergistic effects of sunlight exposure, thermal aging and fungal biodegradation. Polym Degrad Stab. 2010;95(6):1106–1114.
- Kaczmarek H, Bajer K. Biodegradation of plasticized poly(vinyl chloride) containing cellulose. J Polym Sci B Polym Phys. 2007;45(8):903–919.
- Roy PK, Surekha P, Raman R, et al. Investigating the role of metal oxidation state on the degradation behaviour of LDPE. Polym Degrad Stab. 2009;94(7):1033–1039.
- Mulligan CN. Chapter 15 – rhamnolipid biosurfactants: solubility and environmental issues. In: Letcher TM, editor. Thermodynamics, solubility and environmental issues. Amsterdam: Elsevier; 2007. p. 279–298.
- Ganesh Kumar A, Anjana K, Hinduja M, et al. Review on plastic wastes in marine environment – biodegradation and biotechnological solutions. Mar Pollut Bull. 2019;150:110733.
- Mor R, Sivan A. Biofilm formation and partial biodegradation of polystyrene by the actinomycete Rhodococcus ruber: biodegradation of polystyrene. Biodegradation. 2008;19(6):851–858.
- Devi RS, Kannan VR, Nivas D, et al. Biodegradation of HDPE by Aspergillus spp. from marine ecosystem of Gulf of Mannar, India. Mar Pollut Bull. 2015;96(1–2):32–40.
- Tribedi P, Sil AK. Low-density polyethylene degradation by Pseudomonas sp. AKS2 biofilm. Environ Sci Pollut Res. 2013;20(6):4146–4153.
- Mukherjee S, RoyChaudhuri U, Kundu PP. Anionic surfactant induced oxidation of low density polyethylene followed by its microbial bio-degradation. Int Biodeterior Biodegrad. 2017;117:255–268.
- Brückner R, Titgemeyer F. Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol Lett. 2002;209(2):141–148.
- Galinier A. Carbon catabolite repression or how bacteria choose their favorite sugars. Med Sci. 2018;34(6–7):531–539.
- Sanin SL, Sanin FD, Bryers JD. Effect of starvation on the adhesive properties of xenobiotic degrading bacteria. Process Biochem. 2003;38(6):909–914.
- Sekhar VC, Nampoothiri KM, Mohan AJ, et al. Microbial degradation of high impact polystyrene (HIPS), an e-plastic with decabromodiphenyl oxide and antimony trioxide. J Hazard Mater. 2016;318:347–354.
- Volke-Sepúlveda T, Saucedo-Castañeda G, Gutiérrez-Rojas M, et al. Thermally treated low density polyethylene biodegradation by Penicillium pinophilum and Aspergillus Niger. J Appl Polym Sci. 2002;83(2):305–314.
- Fesseha H, Abebe F. Degradation of plastic materials using microorganisms: a review. Public Health Open J. 2019;4(2):57–63.
- Sulaiman S, Yamato S, Kanaya E, et al. Isolation of a novel cutinase homolog with polyethylene terephthalate-degrading activity from leaf-branch compost by using a metagenomic approach. Appl Environ Microbiol. 2012;78(5):1556–1562.
- Kawai F, Oda M, Tamashiro T, et al. A novel Ca2+-activated, thermostabilized polyesterase capable of hydrolyzing polyethylene terephthalate from Saccharomonospora viridis AHK190. Appl Microbiol Biotechnol. 2014;98(24):10053–10064.
- Wei R, Oeser T, Schmidt J, et al. Engineered bacterial polyester hydrolases efficiently degrade polyethylene terephthalate due to relieved product inhibition. Biotechnol Bioeng. 2016;113(8):1658–1665.
- Zhu B, Wang D, Wei N. Enzyme discovery and engineering for sustainable plastic recycling. Trends Biotechnol. 2021;40(1):22–37.
- Montazer Z, Najafi MBH, Levin D. Challenges with verifying microbial degradation of polyethylene. Polymers. 2020;12(1):123.
- Skariyachan S, Patil AA, Shankar A, et al. Enhanced polymer degradation of polyethylene and polypropylene by novel thermophilic consortia of Brevibacillus sps. and Aneurinibacillus sp. screened from waste management landfills and sewage treatment plants. Polym Degrad Stab. 2018;149:52–68.
- Khatoon N, Jamal A, Ali MI. Lignin peroxidase isoenzyme: a novel approach to biodegrade the toxic synthetic polymer waste. Environ Technol. 2019;40:1366–1375.
- Santo M, Weitsman R, Sivan A. The role of the copper-binding enzyme – laccase – in the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. Int Biodeterior Biodegrad. 2013;84:204–210.
- Fujisawa M, Hirai H, Nishida T. Degradation of polyethylene and nylon-66 by the laccase-mediator system. J Polym Environ. 2001;9(3):103–108.
- Zhang H, Kong D, Wang L, et al. Degradation of UV-pretreated polyolefins by latex clearing protein from Streptomyces sp. strain K30. Sci Total Environ. 2022;806:150779.
- Syranidou E, Karkanorachaki K, Amorotti F, et al. Development of tailored indigenous marine consortia for the degradation of naturally weathered polyethylene films. PLOS One. 2017;12(8):e0183984.
- Alisch M, Feuerhack A, Müller H, et al. Biocatalytic modification of polyethylene terephthalate fibres by esterases from actinomycete isolates. Biocatal Biotransform. 2004;22(5–6):347–351.
- Bäckström E, Odelius K, Hakkarainen M. Trash to treasure: microwave-assisted conversion of polyethylene to functional chemicals. Ind Eng Chem Res. 2017;56(50):14814–14821.
- Tournier V, Topham CM, Gilles A, et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature. 2020;580(7802):216–219.
- Kumar K, Gambhir G, Dass A, et al. Genetically modified crops: current status and future prospects. Planta. 2020;251(4):91.
- Murray JV, Jansen CC, Barro PD. Risk associated with the release of Wolbachia-infected Aedes aegypti mosquitoes into the environment in an effort to control dengue. Front Public Health. 2016;4:43.
- Botterell ZLR, Beaumont N, Dorrington T, et al. Bioavailability and effects of microplastics on marine zooplankton: a review. Environ Pollut. 2019;245:98–110.
- Agrawal R, Semwal S, Kumar R, et al. Synergistic enzyme cocktail to enhance hydrolysis of steam exploded wheat straw at pilot scale. Front Energy Res. 2018;6:122.
- DelRe C, Jiang Y, Kang P, et al. Near-complete depolymerization of polyesters with nano-dispersed enzymes. Nature. 2021;592(7855):558–563.
- Plastics Today. Plastics-eating enzymes gain renewed interest as solution to waste problem; 2021; [cited 2021 Aug 12]. Available from: https://www.plasticstoday.com/materials-research/plastics-eating-enzymes-gain-renewed-interest-solution-waste-problem