Abstract
Microalgae have long been regarded as a promising solution for biological carbon abatement from the power industry, offering renewable biomass without competing for land or water resources used for food crops. In this study, we extensively examined the application of photosynthetic microorganisms for closing carbon, nitrogen, and micronutrient loops in the power industry. Subsequently, we explored the bottom-up integration of algal biorefineries into power industry waste streams for increased economic benefits and reduced environmental impacts. Analysis of the available data indicated that microalgae integration with the power industry is primarily performed using flue-gas-assisted cultivation. This approach allows for carbon sequestration typically below one gram per liter per day, too low to significantly impact carbon abatement at achievable scales of microalgae cultivation. Alternative approaches are also being explored. For example, soluble bicarbonate platforms allow for higher biomass productivity and temporary carbon storage. Meanwhile, the use of ashes and waste heat and thermophilic strains can result in lower cultivation costs and better control of cultivation conditions. These approaches offer further incremental improvement to microalgae-based carbon abatement systems in the power industry but are unlikely to be an umbrella solution for carbon reduction. Consequently, in the near term, microalgae-based carbon valorization systems are likely to be limited to niche applications involving the synthesis of high-value products. For microalgae to truly transform carbon abatement processes radical improvements in both biology and engineering approaches are urgently needed.
Introduction
Our modern life is fundamentally connected with resources and their utilization. The availability of abundant energy and chemicals is the major factor that has shaped the development of our civilization in the post-industrial age. Since the beginning of the industrial revolution, the use of fossil resources allowed unprecedented growth of the world economy, population, and life quality. Exponential economic growth was fueled by soaring consumption of fossil resources that in turn resulted in rapid environmental deterioration, as well as conditions adverse to human health and the climate. If our modern societies are to survive, it is imperative to decarbonize our economies and look for alternatives to fossil-derived fuels and chemicals in all aspects of life.
Decarbonization of the economy can be achieved through multiple approaches, and clearly, a combination of many technologies will be required to meet this ambitious goal. However, energy production is only one of the ways we employ fossil resources. Chemicals that we currently derive from such material are also major contributors to climate change and environmental pollution [Citation1]. This pool of carbon will be much more difficult to replace than that associated with fossil fuel energy production. Among all proposed renewable energy sources, only biomass-derived alternatives are based on carbon and, as such, can contribute to replacing an array of our needs not only in terms of energy but, more important, renewable carbon.
Microalgae have been long considered the future of bioenergy, the third-generation feedstock that would not compete with food crops for land or water resources [Citation2,Citation3] and would provide renewable biomass in sufficient quantities to replace fossil feedstocks. In this manuscript, the term microalgae is used broadly and encompasses both eukaryotic microalgae (Chlorophyta, Rhodophyta, and Bacillariophyta) and cyanobacteria (Cyanophyta). When either of the groups is considered individually, the respective terms are used instead.
Despite years of research and technology development, the deployment of economically sound microalgae to the biofuel production process is yet to materialize. It is believed that major low-energy breakthrough technologies in microalgae cultivation, dewatering, and harvesting must be developed before one can consider the cultivation of microalgae for the sole production of biofuels [Citation4]. Further, factors such as temperature, essential nutrients (N/P), CO2, and light availability are all cost-generating and critical for any successful microalgal technology process.
Despite these difficulties, microalgae are considered a promising group of organisms if we examine a broader context of their use in the transition toward a bio-based economy. First, these microorganisms are capable of oxygenic photosynthesis, i.e., they harvest light energy and utilize water and carbon dioxide to build their cellular structures. Because of their autotrophic metabolism, microalgae are not only one of the most important contributors to the O2 levels in the atmosphere but also a promising solution for reducing the CO2 levels in the atmosphere and climate change mitigation. One kilogram of microalgae biomass requires 1.83 kg of CO2 to be reduced during autotrophic growth [Citation5]. Due to their unicellular structure and lack of non-photosynthetic tissues, microalgae have greater light conversion efficiency per unit of biomass and possess a higher growth rate than terrestrial plants, and many strains can double their cell numbers several times in a single day [Citation6,Citation7]. Doubling times of some cyanobacteria take 2–3 h and approach those of heterotrophic cells [Citation7]. Second, they are capable of harvesting energy in myriad ways and thus can be grown in a photo, hetero, and mixotrophic conditions [Citation8], which open a variety of possibilities for use as microbial cell factories with a range of different feedstocks containing carbon and nitrogen at varying levels of oxidation (). Some cyanobacterial strains, such as Nostoc, Anabaena, Aphanizomenon, Microcystis, Oscillatoria, Gloeocapsa, etc., are also capable of atmospheric nitrogen fixation [Citation9]. Third, microalgae do not require arable land and do not directly compete with food crops for this resource. Fourth, microalgae can be cultivated in waste, saline, or brackish water which significantly lowers their water footprint and increases the sustainability of their cultivation [Citation10]. Researchers have also explored the potential of reusing growth medium in microalgae cultivation through a closed-loop system that involves nutrient replenishment. This approach can significantly lower the costs and environmental impact associated with microalgae cultivation. However, it is important to note that reusing the medium may lead to the accumulation of harmful compounds and pathogens that can hinder the growth and development of microalgae [Citation11].
Figure 1. Overview of generic microalgal metabolism. Green shaded area – chloroplast; pink folded area – mitochondrion; yellow area – cytoplasm. Membrane transporters of carbon, nitrogen, and sulfur compounds have been marked with corresponding green, blue, and yellow icons.
3PG – 3-phosphoglycerate; BPG – Bisphoglyceric acid; GAP – Glyceraldehyde 3-phosphate; Ru5P – Ribulose 5-phosphate; RuBP – Ribulose-1,5-disphosphate; Xu5P – Xylulose 5-phosphate; R5P – Ribulose 5-phosphate; S7P – Sedoheptulose 7-phosphate; DHAP – Dihydroxyacetone phosphate; SBP – Sedoheptulose 1,7-bisphosphate; E4P – Erythrose 4-phosphate; F6P – Fructose 6-phosphate; G6P – Glucose 6-phosphate; G1P – Glucose 1-phosphate; ADPG – Adenosine diphosphate glucose; Gluc – Glucose; Fru – Fructose; Suc – Sucrose; UDPG – Uridine diphosphate glucose; FBP – Fructose bisphosphate; PA – Phosphatidic acid; TAG – Triacylglycerol; FFA – Free fatty acid; FFA-CoA – free fatty acid-CoA; tE-ACP – Trans enolyl-ACP (acyl carrier protein); 3H-ACP – 3-hydroxyacyl ACP; 3KA-ACP – 3-ketoxyacyl ACP; acyl-ACP – Acyl-acyl carrier protein; Mal-CoA – Malonyl-CoA; Mal-ACP – Malonyl-ACP; Ac-CoA – Acetyl-CoA; PYR – Pyruvate; IPP – Isopentenyl pyrophosphate; DXP – 2,3-dihydroxy-4-oxo-pentoxy; DMAPP – Dimethylallyl pyrophosphate; GGPP – Geranylgeranyl pyrophosphate; CIT – Citrate; OAA – Oxaloacetic acid; 2-OG – 2-Oxoglutaric acid; SU – Succinic acid ; APS – 2-aminophenol 4-sulfonic acid; Cys – Cysteine; Glu – Glutamate; Gln – Glutamine; ICIT – Isocitrate; SU-CoA – Succinate-CoA; 4HB – 4-hydroxybutyric acid; GLY – Glycine; MAL – Malate; FUM – Fumarate; AcAc-CoA – Aceto-acetyl-CoA; 3HB-CoA – 3-Hydroxybutyric Acid-CoA; Poly-3HB – poly-3-hydroxybutyrate; Poly(3HB-Co-4HB) – Poly (3-hydroxybutyrate co 4-hydroxybutyrate); 4HB-CoA – 4-hydroxybutyric acid-CoA. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.

Finally, microalgae are naturally capable of producing numerous products ranging from food and feed, to nutraceuticals, and to chemicals and fuels, all of which will be required as the transition progresses from fossil-based to a renewable-based circular economy [Citation10]. These features made microalgae promising organisms for the valorization of waste streams from numerous industries and important candidates for closing carbon and nutrient loops of many processes. Production of microalgal biomass is usually achieved using one of several methods such as open ponds or raceways, closed ponds, closed photobioreactors (PBRs), plastic bag systems, immobilized or attached cultivation systems, etc., and their selection is performed case-by-case. Until now, open ponds/raceways and PBRs are the best-established algae cultivation methods. The choice of the most suitable cultivation system is situation-dependent, dictated by both the desired species of algae and the final intended purpose [Citation12].
The ability of microalgae to utilize a variety of organic carbon substrates, waste CO2, N, P, and other compounds may significantly enhance the sustainability of algal biomass production and have a profound effect on the development of algal technologies [Citation13]. Many waste-producing industries could be, at least in principle, integrated with microalgal cultivation to minimize carbon and nutrient wastes from these processes and provide low-cost inputs to microalgae development. Due to the complex composition of wastes, it is essential to consider their constituents and biological effects on a given strain. Shortage or biological unavailability of even one of the essential nutrients results in growth retardation [Citation13], whilst the presence of potentially inhibitory compounds can have deleterious effects on growth.
Biorefineries are integrated facilities that aim to maximize the output from biological raw materials [Citation14,Citation15]. The principles underlying their operation are analogous to those of traditional petroleum refineries, i.e., maximizing output in monetary and volumetric terms and minimizing waste to an absolute minimum. By combining relevant processes on the same site, significant reductions in equipment, operating and personnel costs can be achieved. Additionally, different biorefinery modules can synthesize chemicals required to produce more sophisticated and higher-value products, thus limiting transportation costs. Although large-scale integrated biorefineries are still the technology of the future, numerous biomass conversion processes already demonstrate certain features of biorefining. For example, the corn wet-milling process focuses on maximizing the value of raw material, fractionating it into several streams such as starch (used for ethanol fermentation, high fructose corn syrup, or modified starches); germs (corn oil); kernels and glutens (corn gluten meal) [Citation16]. Similarly, the sugarcane industry often combines three processes, sugar production (high-value product), ethanol fermentation, and energy generation from sugar bagasse in one facility. [Citation17]. An ideal biorefinery is a zero or near-zero waste facility maximizing economic output from biomaterial whilst keeping the carbon, nitrogen, and nutrient waste at a minimum.
To date, numerous authors have suggested that microalgae are well-suited for developing the third generation of biorefineries while simultaneously producing multiple products, mainly a high-value product (HVP) and a biofuel from a microalgal strain of choice [Citation4, Citation18]. Two approaches could be utilized for the design of such a facility. A top-down approach focuses on the de novo design of facilities to maximize the value of a selected microalgal strain. Such an approach, although quite attractive, would require the simultaneous development of numerous technologies, their integration and optimization to bring the costs down. In other words, it is likely to face the same type of problems that in previous years hindered the development of algae to biofuel processes, magnified by the need to integrate them into a functional unit. An alternative, bottom-up, approach would focus on integrating microalgae into existing industries to maximize the value of their waste streams. By employing such an approach, microalgae could contribute to closing: carbon, nitrogen, phosphorus, and micronutrient loops in numerous industries and substantially impact the development of the closed-cycle economy.
Three almost universal features of microalgae metabolism are particularly interesting. First, the flexibility of their metabolism allows for utilizing different forms of carbon, such as gaseous CO2, carbonates, acetates, or carbohydrates [Citation19]. These compounds can originate from numerous wastes that could be valorized using microalgae. The two former, gaseous carbon dioxide and soluble carbonates () are particularly attractive when valorizing power industry waste. Second, microalgae can assimilate many nitrogen and phosphorous compounds. Most anthropogenic nutrient-rich waste streams contain significant amounts of nitrogen and phosphorus that must be carefully handled to avoid uncontrolled environmental release and eutrophication. Microalgae appear to be an ideal solution for decreasing the nutrient load of these waste streams and producing valuable products at the same time. Microalgae’s metabolic features harmonize with the highly water-soluble nitrogen and sulfur oxides released from the power industry. Thirdly, microalgae are aquatic organisms naturally suitable for the diluted waste streams characteristic of many industries.
This manuscript explores waste streams from the power industry and their suitability as carbon and/or nutrient sources for microalgae cultivation. It specifically focuses on the actual use of these waste streams and not their proxies, wherever such data were available. It also explores proposed applications of microalgae for closing carbon, nitrogen, and micronutrient loops in these processes. It further explores promising starting points for the bottom-up integration of algal biorefineries in current waste streams for the increased value of the process and reduced environmental impacts.
Power industry overview
Currently, the combustion of fossil fuels is still the primary source of global energy and the major source of total anthropogenic CO2 emissions estimated at 36.6 Gt in 2021 [Citation20]. Meanwhile, the amount of CO2 emissions from coal-fired power plants reached an all-time high, with a surge of almost 6.6% compared to the previous year. This increase was more than 100 Mt higher than the previous peak in 2018 [Citation20,Citation21]. In addition to coal, oil and gas are still predominant fuels in the energy and heavy-industry sectors worldwide [Citation20].
Therefore, the development of efficient CO2 capture, utilization, and sequestration methods is required to reduce and mitigate anthropogenic CO2 emissions. Furthermore, besides flue gases, there are three other main waste streams resulting from power and heavy industries that can be potentially explored in microalgae cultivation, namely ash, cooling water, and waste heat.
Integration of microalgae for closing carbon and nutrient loops in the power industry
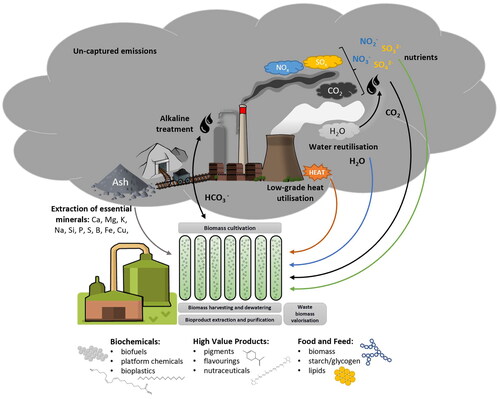
As previously stated, four main power industry waste streams can be utilized to support the cultivation of microalgae: flue gasses, ashes, water, and heat (). Each is described in a subsequent section of this manuscript.
Figure 2. Power industry waste microalgal valorization scheme taking into consideration closing carbon (black), water (blue), nutrient (green), and low-grade heat (orange) loops and indicating processing steps (grey boxes) and potential products from algal biomass.

Flue gases
Flue gasses, resulting from the combustion of fossil resources, can be used for the autotrophic cultivation of photosynthetic microorganisms. There are two approaches to delivering this carbon to microalgal culture, directly as gasses with or without pretreatment or as a bicarbonate solution resulting from the alkaline treatment of carbon dioxide. In addition to those fully oxidized compounds, CO2 electro and photochemical conversion methods allow for the synthesis of alternative soluble C1 compounds, like formate or methanol [Citation22], but these compounds cannot be directly accessed by the native metabolism.
Direct flue gas utilization
Flue gasses generated during the combustion of fuels are hot and rich in carbon dioxide. Due to the large-scale boilers typically used in the power industry, flue gasses represent a significant challenge but also an opportunity for carbon recycling technologies. Detailed analysis of flue gas composition indicates that it varies significantly with the type of fuel, boiler, and gas cleaning facilities. Typical flue gas emitted from a boiler using low-sulfur heavy-oil fuel contains 10–15% CO2 and 100–300 ppm NOx and SOx [Citation23], while those from coal-fired power plants were found to have the following composition: CO2 10%–20%, O2 5%, SOx (SO2) 10–500 ppm, and NOx (NO2) 150–850 ppm [Citation24–26]. Flue gas from natural gas boilers is characterized by a lower concentration of CO2 (6–8%), a concentration of NO2 around 25 ppm [Citation27] and is typically regarded as the most suitable for cultivating microalgae. Flue gasses of other notable CO2 – emitting industries have distinctive compositions, i.e., coke oven of a steel plant (CO2 23%, O2 4%, NO 78 ppm, and SO2 87 ppm) [Citation28], silicomanganese smelters (CO2 17 %v/v, NOx 100 ppm, and SO2 1 ppm) [Citation29], and cement kilns (15–25% CO2) [Citation30], etc. In recent years, biomass co-firing with coal has been introduced in several countries to limit fossil CO2 emissions. Compared with coal-fired plants, flue gases from co-firing are characterized by lower CO2 and SOx concentrations. Meanwhile, no general conclusion can be drawn on NOx emission because it can increase or decrease depending on biomass characteristics and its harvest time [Citation31].
The utilization of flue gases as a carbon source for autotrophic cultivation of microalgae has long been considered among other carbon mitigation strategies [Citation32–34]. Numerous recent studies have attempted to utilize actual and simulated flue gasses for cultivating cyanobacteria and eukaryotic microalgae (). Significant differences between various microalgae strains concerning their preference for certain forms of inorganic carbon have been found in these studies. Regarding CO2 uptake by photosynthetic organisms, the transfer of gaseous inorganic carbon is typically driven by diffusion () [Citation58]. If the pH of the growth medium stays within the range that does not affect the physiology of the organism, the higher concentration of CO2 may result in a higher accumulation of cellular inorganic carbon and higher productivity. Most microalgae prefer elevated, albeit lower than 5%, concentrations of CO2, but only a few can grow in high concentrations approaching 100% [Citation59], and some strains are equally adept in employing dissolved inorganic carbon as their gaseous form [Citation60,Citation61] ( and ).
Table 1. Characteristics of microalgae-based flue gas sequestration.
Table 2. Application of bicarbonate for the cultivation of microalgae and cyanobacteria.
All these factors can determine the combination of the strain and carbon delivery method for a particular application. The direct injection of flue gas without prior pretreatment into the culture medium (apart from heat exchangers) has the potential to reduce cultivation costs. However, it can also result in unfavorable growth conditions due to excessive CO2 content, the presence of NOx, SOx, CxHy, and CO, particulate matter, halogen acids, and heavy metals [Citation30, Citation73]. All these factors bring significant challenges. First, due to poor CO2 mass transfer between gas and liquid phases, long tubular systems are preferred to maximize a gas residence time. Second, directly injecting flue gasses into an unbuffered culture medium results in a pH drop and cellular stress [Citation54]. Third, contaminants in flue gasses, such as SOx and NOx, result in a further pH drop. Among strategies to overcome the toxic effects of NOx and SOx in flue gas, pH control and high initial cell concentration are typically recommended [Citation74]. More recently, adaptive laboratory evolution and genetic engineering approaches have also been explored. The former was successfully applied to enhance the growth rate of a marine diatom Phaeodactylum tricornutum at low pH resulting from the inflow of the above-mentioned acidic gases [Citation75]. Meanwhile, the latter showed that a deleterious intracellular pH drop can be alleviated with an overexpression of H+-ATPase [Citation54]. Adding flue gas to the culture is frequently performed intermittently, or the flue gasses are diluted to tackle these problems. It has been reported that growth rates were over fivefold higher when intermittent flue gas was employed instead of a continuous supply [Citation28].
An analysis of successful cases of the application of actual flue gasses for microalgae cultivation allows for drawing the following conclusions. Almost all successful applications of flue gasses use photobioreactors, mostly the airlift type made of acrylate or glass or alternatively, disposable polyethylene bags (). This approach increases the gas residence time and resultant mass transfer from the gaseous phase to the liquid phase and, ultimately, to the cell. Reports on the application of a raceway design for the sequestration of CO2 from real flue gasses are available but are arguably less successful [Citation76,Citation77]. Flue gas composition has a profound impact on microalgae growth. It has been shown that many strains can provide good tolerance to high CO2 (2–20%), NO concentrations of 150–500 ppm, and SO2 concentrations of up to 100 ppm [Citation78] (). Meanwhile, flue gasses are typically rich in CO2 (10 to 15%) and frequently contain SOx and NOx at concentrations of 100–300 ppm SOx and 50–400 ppm of NOx () [Citation27]. This indicates that SOx is the more problematic of the two. Wild-type microalgae strains that can withstand such conditions include: Monoraphidium, Nannochloris sp, Tetraselmis sp, and Scenedesmus dimorphus [Citation78,Citation79]. Interestingly, some acidophilic species isolated from hot springs (e.g., Cyanidium caldarium) have shown exceptional thermal and CO2 tolerance up to 100% of CO2 in a gas stream [Citation79–81]. SOx pose more severe problems for microalgae cultivation using flue gases due to rapid acidification of the growth medium beyond the physiological optimum of microalgae. For example, Lee et al. reported that the microalgae strain Chlorella sp. KR-1 grown with 80 ppm SOx exhibited 70% of the control’s growth [Citation23]. Elsewhere, high sulfur concentration did not inhibit Scenedesmus obliquus growth when the pH of the culture was neutralized by oil shale ash, which indicates that SOx causes indirect effects inhibiting the growth by lowering the pH [Citation82]. This suggests the possibility of direct CO2 fixation from the flue gases of modern power stations equipped with flue gas desulfurization (average concentration of SOx after treatment 70 ppm) with: Chlorella sp. KR-1 [Citation23], Desmodesmus abundans, Scenedesmus, and Botryococcus [Citation74], providing adjustments to pH are implemented.
Other flue gas impurities, such as: CO, CxHy, particulate matter (PM), halogen acids, and heavy metals can also impact microalgae cultivation [Citation73], but their effect has not been studied thoroughly to date. Early studies have not observed the deleterious effects of the toxic compounds present in the flue gas on Chlorella vulgaris growth [Citation83]. The typical heavy metals in concentrations expected from a coal-fired power plant (in μM As 1.04, Cd 0.13, Co 0.27, Cr 2.50, Cu 2.06, Hg 0.05, Ni 0.05, Pb 0.26, Se 0.13, and Zn 6.73) [Citation84]. At micromolar concentrations, these compounds did positively affect the microalgal growth of Scenedesmus obliquus, as many of these metals are essential for enzymatic processes in microalgae, while at higher concentrations, negative effects were observed [Citation84].
Interestingly, despite typical flue gas temperatures exceeding 120 °C [Citation44], most biosequestration studies were performed at 20–30 °C. This suggests that extensive gas cooling must be performed before this source of CO2 can be utilized for microalgae cultivation. Typically, Chlorella strains are cultivated under 25 °C, Scenedesmus between 25 and 30 °C, and Arthrospira (Spirulina) between 30 and 34 °C. Surprisingly, only one study suggests employing thermophilic strains of the genus Thermosynechococcus, capable of growing in the range of 45–57 °C, to reduce the need for cooling and control the contamination of the sample with undesired bacteria and eukaryotic predators [Citation67].
The analysis of biomass productivity across different cultivation methods shows that the productivities of 0.05 g L-1 d-1 to 0.5 g L-1 d-1 are typically obtained. The highest biomass productivities in the range of 0.41 to 0.528 g L-1 d-1 are typically achieved for various Chlorella strains [Citation41, Citation43,Citation44]. That translates into maximal photosynthetic carbon fixation of 0.8 to 0.9 g L-1 d-1. Such low values imply that current productivities are insufficient to have any noticeable impact on CO2 sequestration from flue gasses using the direct injection approach. Notably, these productivities are lower than 2.11 g L-1 d-1 achieved with C. vulgaris UTEX26 strain grown in the 9.5% CO2 in flashing LED [Citation85]. This indicates that microalgae grown with actual flue gasses still need to bridge the gap to the cultivation setups that utilize purified CO2 as a carbon source. Even when the four-fold increase of productivity matching that value is implemented, the productivity is unlikely to be sufficient for large-scale deployment of such systems for global carbon abatement but may be sufficient for high-value biomass niche production.
Biomass composition produced from CO2 sequestration fundamentally affects its application. Both the strain type and cultivation conditions impact final algal biomass composition. Prior studies with heavy metals typically present in flue gasses revealed that while mercury and selenium are mostly lost, other metals accumulate in biomass [Citation84]. Moreover, the utilization of cement-kiln flue gasses resulted in algal biomass lead accumulation. This indicates that cultures aerated with such flue gas should not be used as food supplements or animal feed [Citation30]. Consequently, the type of impurities determines the application of algal biomass. High-energy molecules such as lipids or hydrocarbons suitable for biofuels are preferred when the strain is cultivated using flue gasses. Such preference is most likely due to the potentially harmful carryover of toxic contaminants such as heavy metals that prevent the production of higher-end food and feed products from flue gas-cultured algae. Although it has been suggested that the proper conditioning of flue gases prevents toxicity and ensures efficient growth of high-quality microalgal biomass [Citation83], additional studies on this topic are required to ensure biomass product safety. Nevertheless, despite meeting the foodstuff requirements, even high-quality biomass can be rejected by potential customers only because of the flue gas origin of CO2.
Analysis of the effect of flue gasses on the composition of cellular lipids that can be used for biodiesel production is inconclusive. CO2 concentration ranging from 2.5 to 5% was optimal for lipid productivities of Chlorella minutussima and Scenedesmus sp. without significantly affecting the lipid composition and its suitability for biodiesel production [Citation86,Citation87]. Conflicting studies were reported for Chlorella sorokiniana. During the cultivation of this strain, replacing CO2 with real flue gasses resulted in significant alteration of the fatty acid profile, decreased content of saturated fatty acids, and increased monounsaturated ones [Citation88]. Meanwhile, the total content of lipids remained relatively stable. Lipid content stability between cultivation in CO2-enriched air and flue gasses has also been found in Chlorella MTF-7 [Citation28]. Another study has demonstrated that the source of flue gasses, such as a coke oven, hot stove or power plant, impacts flue gas composition and subsequent biomass productivity and cellular composition [Citation44]. Results showed that the highest lipid content was achieved using hot stove flue gas and the lowest using coke oven flue gas. The composition of fatty acid profiles varied considerably. This indicates that there is no obvious correlation between CO2 content resulting from flue gas use and either decreased or increased lipid content or lipid composition modification. The differences in these studies are mostly associated with nitrogen availability in the flue gasses and follow an established correlation of carbon allocation to growth or lipid production depending on nitrogen availability.
Soluble carbonate platform
Indirect methods of carbon dioxide delivery are also considered. Soluble inorganic carbon delivery has numerous advantages over the gaseous approach. It allows the decoupling of carbon capture and utilization and relatively straightforward transport of the captured carbon to different microalgae cultivation and processing sites [Citation89]. There are also biological reasons for using the bicarbonate platform. Unlike gaseous CO2 transport driven primarily by concentration, the soluble carbonate transport is active and governed by carbon uptake and carbon concentration mechanisms [Citation90]. In cyanobacteria, the bicarbonate uptake can be either driven with ATP (BCT1) or ion gradient (SbtAB, BicAB) [Citation91]. Analogous mechanisms govern bicarbonate delivery into the pyrenoid in eukaryotic microalgae [Citation92]. This allows the cells to better adjust the uptake of inorganic carbon depending on the metabolic needs and avoid the repetitive interconversion of carbon species between gaseous (carbon fixation) and soluble forms (cellular storage).
To date, the utilization of a soluble carbonate platform is less prevalent than direct flue gas injection, and its application to sequester carbon dioxide from actual flue gasses is rare. Interestingly, employing purified sodium bicarbonate to enhance cyanobacteria growth is widespread (). When bicarbonate supplementation is used for eukaryotic microalgae cultivation the concentrations of dissolved inorganic carbon (DIC) generally do not exceed 0.1–0.2 M and result in maximal productivities of 0.5 g L-1 d-1. The highest productivities among microalgae are achieved by fast-growing freshwater strains like Chlorella, where bicarbonate is supplemented along with gaseous CO2 [Citation66]. When exogenous gaseous CO2 is not introduced, eukaryotic microalgae show significantly lower productivity (). The situation is markedly different in cyanobacteria. The productivity of these blue-green algae achieved using the bicarbonate platform typically exceeds that of eukaryotic microalgae and is also superior to that of cyanobacteria grown with flue gasses ( and ), indicating the preference of these photosynthetic phototrophs for soluble inorganic carbon.
The cultivation modes applicable to the bicarbonate platform are also more diverse than direct injection methods. Flask, bioreactor, and even floating designs have been successfully applied (). The constant availability of carbon in the cultivation medium can also significantly save costs associated with pumping CO2-rich gas to the cultures. Many cyanobacteria, marine and freshwater alike, can grow in elevated temperatures and concentrations of bicarbonate higher than 0.2 M [Citation93]. Meanwhile, their biomass productivities often exceed 0.5 g L-1 d-1, even in bicarbonate concentrations higher than 0.5 M. The highest daily productivity reported to date (1.123 g L-1 d-1) was achieved for a marine strain, Synechococcus PCC 7002, recently delineated as Picosynechococcus PCC 7002 [Citation94]. Other strains, including easy-to-harvest filamentous Arthrospira (Spirulina) and Leptolyngbya that do not require the complex nutritional inputs of PCC 7002, also exhibit promising productivities exceeding 0.5 g L-1 d-1 [Citation70]. The corresponding carbon fixation values are approximately double the biomass productivity and in most successful studies reach 1 to 2 g L-1 d-1 ().
A combination of flue gas injection and bicarbonate supplementation is often used in conjunction, allowing better carbon uptake and growth medium pH control. Both addition of bicarbonate and the process of photosynthesis alkalinizes the cultivation medium. Flue gas supplementation can assist in lowering its pH to physiological levels. Typically, when carbon dioxide and bicarbonate were used as combined carbon sources, the microalgae growth rate was faster than that of a single carbon source. However, with the consumption of bicarbonate, the solution pH increases, and subsequently, the supply of gaseous CO2 can buffer this effect [Citation95]. Considering the relative ease of how bicarbonate can be obtained from CO2 and its compatibility with increasingly popular direct air capture approaches that rely on carbonate chemistry [Citation20], it is surprising that this method has received relatively low attention, and hopefully, studies on the larger-scale deployment will emerge in the future.
Alternative soluble C1 platforms
In recent years, it has become increasingly common to look beyond gaseous carbon dioxide as a C1 substrate for microbial production. While bicarbonate is one of the alternatives discussed above, more reduced C1 alternatives have also been hotly debated. Among proposed C1 platforms carbon monoxide, formate, methanol, and methane have been at the forefront of discussion [Citation22]. The application of soluble alternatives such as formate and methanol appears particularly promising due to their complete miscibility with water and relatively low toxicity [Citation22]. These properties facilitate storage and transport using conventional means, such as pipelines or tankers [Citation96]. The application of soluble C1 intermediates alleviates the major problem associated with the mass transfer of carbon dioxide to microbial cultures that hinders almost every biochemical carbon valorization approach. Since eukaryotic microalgae and cyanobacteria are not naturally capable of methanol and formate assimilation, and their natural metabolism of these C1 compounds is directed toward dissimilation toward CO2, extensive genetic engineering would be required. Currently, there is only a single example of introducing the formate entry point to the metabolism of a photosynthetic microorganism, model cyanobacterium, Synechocystis sp. PCC 6803 attempting to generate a C1 mixotrophic strain [Citation97]. This conceptually ambitious work introduced the assimilatory route for formate by expressing formate-tetrahydrofolate ligase (FTL) originating from methylotrophic M. extorquens AM1 into cyanobacterial cells. The initial results were largely unsatisfactory, and the transgenic strain did not show any improvement in its growth parameters in the presence of an additional C1 carbon source. Instead, it exhibited an altered carbon and nitrogen metabolism signaling. The underlying causes are not yet known, but the concept, whilst very challenging, is promising and worth exploring further. The approach could expand the possibility of using alternative, soluble and storable C1 platforms for carbon photo-valorization. The soluble C1 delivery, either through bicarbonate or more reduced compounds, such as formate or methanol, has numerous advantages over the gaseous approach. It allows the decoupling of carbon capture and utilization and relatively straightforward transport of the captured carbon to different microalgae cultivation and processing sites [Citation60].
Ash
The total annual solid waste production of so-called coal combustion products is around 750 million tonnes [Citation98]. Every 4 tons of burned coal led to approximately 1 ton of fly ash [Citation99]. In 2010, Greenpeace pointed out that fly ash is regarded as the largest single source of pollution of industrial solid wastes in China, and its accumulation in 2020 was estimated to be 3Gt [Citation99]. Using coal fly ash (e.g., in the construction industry) varies significantly around the world, fluctuating from around 60% in India, and reaching up to 100% in some European countries. The characteristics of each type of coal-burning by-product (fly ash, bottom ash, and ash water, i.e., water used to remove fly ash from flue gases) depend on various parameters such as coal quality or boiler type. Generally, the main components (over 90%) of coal ash are the oxides of silicon, aluminum, iron, and calcium. Magnesium, potassium, sodium, titanium, and sulfur are minor constituents (about 8%). Trace elements, such as arsenic, cadmium, lead, mercury, and selenium, make up 1% of the total composition. Coal fly ash also contains essential nutrients, such as calcium, magnesium, potassium, phosphorus, sulfur, boron, iron, copper, and zinc. Most of these nutrients are in forms readily available apart from phosphorus due to its very low solubility [Citation100,Citation101]. In addition to nutrients, coal fly ash contains trace elements, such as cadmium, lead, nickel, selenium, and mercury, at a concentration below 1%, which may be of environmental concern and can enter the food chain through microalgae. Nevertheless, they are present in small concentrations and often characterized by low solubility and, thus, mobility [Citation100]. However, no focused research on their accumulation and transfer in the case of fly ash is available.
Biomass combustion and coal-biomass co-firing are predominant methods in many countries to increase the share of renewable energy. This is particularly applicable to regions with a high percentage of coal-derived energy in the national energy mix and a relatively large amount of available biomass. Inorganic constituents, such as calcium, potassium, sodium, silicon, and phosphorus, predominantly affect the chemical composition of biomass ash. The composition of ash itself strongly depends on biomass type, its harvesting time, and processing. For example, wood is typically characterized by high alkali metal content, and rice husk contains high silica content. As expected, the co-combustion of biomass results in the fly ash’s altered chemical composition, leading to an increase in the content of P, Ca, and Mg combined with higher bioavailability of calcium and magnesium [Citation100, Citation102]. In addition, the fact that harmful substances, like As, Cd, Cr, Cu, Ni, Pb, and Zn, have low bioavailability means that fly ash produced from burning wood and peat could serve as a viable fertilizer for agricultural or forestry purposes. [Citation103]. The number of studies on the application of fly ash from biomass combustion as a source of inorganic nutrients for microalgae cultivation is somewhat limited to date. There were, however, successful attempts to use charcoal ash and charcoal ash leachate as an inorganic nutrient source for microalgae cultivation, achieving productivity comparable with cultivation on Guillard’s f/2 media. Moreover, the addition of dry ash to water was a more effective nutrient source than the preparation of leachate solutions and allowed for a continuous supply of necessary nutrients during the growth phase [Citation104]. Rai et al. [Citation101] investigated the application of selected nitrogen-fixing blue-green algae to ameliorate fly ash. The N2-fixing strains grew onto water-soaked, sterilized fly ash in Petri plates. The fly ash from coal combustion contains all necessary macro and micronutrients, except for nitrogen which diazotrophic organisms could fix from the atmosphere.
Among strains tested, only one Anabaena doliolum demonstrated prolific growth. According to the authors, the high pH (up to 9) of fly ash ameliorated metal toxicity. Generally, cyanobacteria growth improved the physicochemical properties of fly ash, e.g., lower pH increased available phosphorus. The physicochemical properties of fly ash were typically improved by cyanobacteria growth. For instance, the availability of phosphorus increased due to the lower pH. However, the accumulation of metals in cells, with the order of Ni > Fe > Mn > Zn > Cu, resulted in a reduction in the toxicity of fly ash, but consequently had a negative impact on the quality of biomass [Citation101]. It could be useful to further investigate the use of ash as a source of nutrients in algae cultivation. For instance, the addition of kiln dust or oil shale ash to growth media could be taken into consideration due to its beneficial growth components and pH buffering effect [Citation74, Citation82]. However, even though nutrients from biomass and coal combustion ash might support microalgae cultivation, the biomass quality and potential use must be carefully considered. Furthermore, there are technical limits in applying any ash, e.g., how to deliver nutrients from fly ash and then separate them from biomass after cultivation, or, in the case of pre-leaching, deliver them in sufficient amounts to support growth and simultaneously prevent leaching of unwanted metals. Generally, there is a strong need for research on the application of ash in microalgae cultivation, as current data are insufficient.
Waste heat and cooling water
Power and heavy industries produce massive quantities of waste heat and cooling water, yet these two streams are sparingly discussed in the available literature. It is estimated that 20–50% of industrial energy input is lost as waste heat, mostly of low grade between 30 °C and 100 °C, that has limited use for other applications [Citation105,Citation106]. Furnaces, exhaust gas and steam, cooling fluids, and wastewater from: washing, drying, or cooling processes, refrigeration systems, motors, or the exhaust air from production halls could all be considered potential waste heat sources [Citation107]. Wet closed-loop systems where freshwater is withdrawn and passed through a steam condenser are also examples. Instead of being discharged downstream, the heated water is cooled in a wet tower or pond [Citation108]. The cooling ponds could be used for microalgae cultivation [Citation109], and water, after proper conditioning, is reused again as cooling water. Water availability becomes a serious challenge when scaling up algae cultivation in the vicinity of large CO2 emitters that also are typically large water consumers. One way to mitigate the high water demands of microalgae cultivation is introducing technological advancements like nanoporous ceramic membrane capillary condensation to extract high-quality water and its latent heat from flue gases [Citation110]
To date, heat utilization to support microalgae growth is scarcely documented. For instance, Laamanen et al. [Citation106] demonstrated using waste heat for year-round microalgae cultivation with an optimal growth range of 15–25 °C, in colder regions where ambient temperatures fall below 15 °C. In warm climates, however, the cultivation of mesophilic microalgae usually requires cooling. Therefore, waste heat could be directed toward downstream biomass processing, such as drying, lipid extraction, harvesting, etc. [Citation111–113]. Waste heat could also support the cultivation of thermophilic microalgae. Combining low-cost waste heat with thermophilic strain cultivation could be economically feasible. Extremophilic microalgae have been gaining more interest due to several advantageous cultivation characteristics such as the high growth rate of some strains, low contamination, high biomass, and lipid productivities [Citation80], and as sources of thermostable enzymes [Citation114] and pigments [Citation115,Citation116]. Thermophilic microalgae and cyanobacteria can achieve peak biomass productivities of 2.5–3.5 g L-1 d-1 at temperatures exceeding 50 °C under optimized conditions [Citation117,Citation118]. Elevated cultivation temperatures, ranging from 50 °C (Cyanidium [Citation119], Thermosynechococcus [Citation120]) to the maximal temperature of photosynthetic growth of 72 °C (Thermostichus [Citation121]), are high enough to seriously limit culture contamination with undesired organisms and low enough to effectively employ waste heat generated by the power industry. One added advantage of thermophilic cyanobacterial strains is their capacity to actively uptake bicarbonate with a variety of transporters [Citation91]. This makes them a promising solution for utilizing both direct flue gases and the bicarbonate platform. However, only a single study has explored their effectiveness in flue gas sequestration [Citation122].
Conclusions and outlook
Biological carbon abatement remains a relevant topic due to the ongoing and projected use of fossil fuels in the power industry. This study examined significant discoveries in implementing photosynthetic microorganisms to close biorefinery loops in the power industry. We investigated the feasibility of using flue gases (both directly and indirectly), combustion ashes, and cooling water as additional streams to support the growth of photosynthetic microbes, including microalgae and cyanobacteria. Since there are already ample reviews of the bioproducts from microalgae, natural and engineered alike [Citation123,Citation124], this study focuses on the upstream segment of the valorization process.
Critical analysis of the available literature reveals that not all flue gasses are equally applicable as carbon sources for microalgal cultures. Natural gas post-combustion exhausts are preferred for most strains because of the negligible concentration of SOx and heavy metals [Citation73]. However, when fossil feedstocks rich in sulfur and nitrogen, such as coal, are used, the effects of their combustion gasses on microalgae should be evaluated case-by-case.
The bicarbonate platform is often overlooked when discussing microalgae’s potential to mitigate power industry emissions. Unfortunately, studies that focus on the capture of flue gasses in the alkaline solution and its subsequent utilization for cultivating photosynthetic microorganisms are sparse [Citation54, Citation95]. Whilst the productivity of C. reinhardtii strain used in this work was markedly lower than the average, the results of cyanobacteria cultivation in technical- or laboratory-grade scales indicate a potential for improvement. At least two-fold higher biomass productivities than those of microalgae used in direct flue gas injection were observed, and combinatorial approaches are even more promising. High biomass productivity combined with a simplified carbon distribution and delivery system provides a promising solution for a bicarbonate platform that allows flexible, temporary storage, transportation, and effective oxidized carbon-stream management. Furthermore, the intensified deployment of direct air capture systems that largely rely on carbonate chemistry creates further synergies with this approach looking into the future [Citation20].
Whether carbon sequestration by microalgae coupled with fossil-fuel power plants can make a significant impact on curbing CO2 emissions remains debatable. The maximum CO2 uptake rates have been reported as three to four grams of CO2 per liter culture per day [Citation125] in addition to NOx/SOx abatement [Citation126]. It is highly questionable if those peak productivities can be maintained daily throughout the year. Assuming an optimistic scenario that they can, such productivities would require dedicating almost 3,000 hectares of installations in the form of raceway ponds (Supplementary File) to sequester 0.1% of 9.7 Gt of carbon dioxide emitted by the coal power industry alone [Citation20,Citation21].
When realistic scales of algal cultivation, biomass productivity, and cultivation costs are evaluated, the sequestration or valorization of any meaningful content of CO2 produced by a power industry with microalgae biomass cultivation is difficult, extremely expensive, or even impossible to execute. There may be some niche applications where the addition of industrial waste CO2 can assist in producing valuable components, but microalgal sequestration as an umbrella solution for carbon capture is somewhat unrealistic for several reasons. First, point CO2 sources are either baseload facilities (coal, oil, and natural gas) or intermittent peaking or compensating sources (usually natural gas). The former generates CO2 constantly, even at night when photosynthetic capture is impossible. The latter is not predictable enough due to its intermittent character. Second, the gaseous nature of flue gasses complicates storing and delivering this carbon source. Third, the high temperature of flue gasses combined with typically used mesophilic strains requires extensive gas cooling before flue gasses can be utilized as a carbon source.
Employing dissolved inorganic carbon, such as bicarbonate, mitigates some of these challenges. First, carbon dioxide can be stored as a bicarbonate solution and transported easily. Second, even CO2 emissions from intermittent sources can be captured. Third, the issues of temperature and carbon delivery are decoupled in the bicarbonate system, as the heat exchange and carbon feeding can be adjusted depending on the actual needs of a microalgal or cyanobacterial system. This research gap should be filled in the future due to the apparent potential of soluble carbonates as a platform for photobioconversion. These benefits are further amplified in the proposed soluble C1 photovalorisation approach using formate or methanol as substrates. It should be highlighted however that this concept requires significant conceptual and technological advances before it can be considered for mitigating emissions from large sources, as none of the native microalgal strains can utilize these carbon compounds, and transgenic approaches explored to date were largely unsuccessful.
Another neglected area appears to be waste heat use. A study focusing on year-round microalgae cultivation in a cold climate is promising. However, the potential applications of waste heat are much broader and include the widespread deployment of thermophilic strains for carbon valorization and the production of thermostable products. Whilst it is not a norm, there are successful examples of highly productive strains among thermophilic microalgae. This, combined with their metabolic flexibility for carbon sources, the ability to synthesize thermostable pigments of high value, and to be protected from eukaryotic grazers that cannot withstand elevated temperatures makes them underutilized bioresource for industrial CO2 valorization.
Another area of potential interest is the utilization of post-combustion ash and its leachates as a nutrient source for microalgae cultivation. The most challenging aspect of this waste stream is its contamination with heavy metals, so the carryover of pollutants to the final products should be closely monitored before large-scale deployment could be introduced.
Finally, considering the magnitude of the challenge associated with carbon abatement from the power industry, it is essential to focus on basic research to understand microalgal metabolism and improve it. Whilst there is significant of research on the development of new carbon fixation pathways for originally heterotrophic microbes [Citation127,Citation128], there is little focus on autotrophic organism improvement beyond photorespiratory bypasses [Citation129].
To achieve a significant reduction in carbon emissions in the power industry, a revolutionary breakthrough that dramatically improves microalgae productivity is needed. Whilst incremental improvements generated to date are valuable, researchers and industry professionals should now divert their attention to more substantial interventions in the fundamental process of photosynthetic carbon fixation.
To summarize, it is unlikely that the current approaches based on direct flue gas injection are likely to have a major effect on carbon dioxide mitigation in the power industry. In the mid-term, developing alternative, soluble capture and utilization platforms allowing for decoupling of capture and valorization processes, such as bicarbonate, better integration of thermophilic strains combined with recycling of water and nutrients from the process, could bring near-term incremental gains. But for microalgae to have a major impact on carbon abatement from the power industry more ambitious, disruptive research lines that target the very foundation of photosynthetic carbon fixation may be required.
Supplemental Material
Download MS Word (132 KB)Acknowledgements
The authors would like to thank Priscilla L. Young for her valuable comments about the manuscript and proofreading.
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
References
- Levi PG, Cullen JM. Mapping global flows of chemicals: from fossil fuel feedstocks to chemical products. Environ Sci Technol. 2018;52:1725–1734. doi: 10.1021/acs.est.7b04573.
- Debnath C, Bandyopadhyay TK, Bhunia B, et al. Microalgae: sustainable resource of carbohydrates in third-generation biofuel production. Renew Sustain Energy Rev. 2021;150:111464. doi: 10.1016/j.rser.2021.111464.
- Sheehan J, Dunahay T, Benemann J, et al. A look back at the US Department of Energy’s Aquatic Species Program: biodiesel from algae. Close out report. National Renewable Energy Lab, Department of Energy, Golden; 1998. (Report Number NREL/TP-580-24190).
- Li J, Liu Y, Cheng JJ, et al. Biological potential of microalgae in China for biorefinery-based production of biofuels and high value compounds. N Biotechnol. 2015;32:588–596. doi: 10.1016/j.nbt.2015.02.001.
- Khoobkar Z, Delavari Amrei H, Heydarinasab A, et al. Biofixation of CO2and biomass production from model natural gas using microalgae: an attractive concept for natural gas sweetening. J Co2 Util. 2022;64:102153. doi: 10.1016/j.jcou.2022.102153.
- Bhola V, Swalaha F, Kumar RR, et al. Overview of the potential of microalgae for CO2 sequestration. Int J Environ Sci Technol. 2014; 11:2103–2118. doi: 10.1007/s13762-013-0487-6.
- Yu J, Liberton M, Cliften PF, et al. Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO2. Sci Rep. 2015;5:8132. doi: 10.1038/srep08132.
- Khoobkar Z, Delavari Amrei H. Effect of photo, hetero and mixotrophic conditions on the growth and composition of Anabaena variabilis: an energy nexus approach. Energy Nexus. 2021;2:100010. doi: 10.1016/j.nexus.2021.100010.
- Grizeau D, Bui LA, Dupré C, et al. Ammonium photo-production by heterocytous cyanobacteria: potentials and constraints. Crit Rev Biotechnol. 2016;36:607–618. doi: 10.3109/07388551.2014.1002380.
- Leong YK, Chew KW, Chen WH, et al. Reuniting the biogeochemistry of algae for a low-carbon circular bioeconomy. Trends Plant Sci. 2021;26:729–740. doi: 10.1016/j.tplants.2020.12.010.
- Lu Z, Loftus S, Sha J, et al. Water reuse for sustainable microalgae cultivation: current knowledge and future directions. Resour Conserv Recycl. 2020;161:104975. doi: 10.1016/j.resconrec.2020.104975.
- Sirohi R, Kumar Pandey A, Ranganathan P, et al. Design and applications of photobioreactors – a review. Bioresour Technol. 2022;349:126858. doi: 10.1016/j.biortech.2022.126858.
- Chen J, Dai L, Mataya D, et al. Enhanced sustainable integration of CO2 utilization and wastewater treatment using microalgae in circular economy concept. Bioresour Technol. 2022;366:128188. doi: 10.1016/j.biortech.2022.128188.
- 't Lam GP, Vermuë MH, Eppink MHM, et al. Multi-product microalgae biorefineries: from concept towards reality. Trends Biotechnol. 2018;36:216–227. doi: 10.1016/j.tibtech.2017.10.011.
- Chew KW, Yap JY, Show PL, et al. Microalgae biorefinery: high value products perspectives. Bioresour Technol. 2017;229:53–62. doi: 10.1016/j.biortech.2017.01.006.
- Rosentrater KA, Evers AD. Chapter 14 – Wet milling: separating starch, gluten (protein) and fibre. In: Rosentrater KA, Evers ADBT-KT of C, editors. Woodhead publishing series in food science, technology and nutrition. Duxford (UK): Woodhead Publishing; 2018. p. 839–860.
- Vaz SJ. Sugarcane-biorefinery. Adv Biochem Eng Biotechnol. 2019;166:125–136.
- Jacob-Lopes E, Guillermo L, Mérida R, et al. Microalgal biorefineries. In: Jacob-Lopes E, editor. Biomass production and uses. London (UK): Intechopen; 2015. p. 938–965.
- Turon V, Baroukh C, Trably E, et al. Use of fermentative metabolites for heterotrophic microalgae growth: yields and kinetics. Bioresour Technol. 2015;175:342–349. doi: 10.1016/j.biortech.2014.10.114.
- International Energy Agency. World Energy Outlook 2022 [Internet]. Available from: www.iea.org/t&c/.
- Coal. 2022 [Internet]. Available from: www.iea.org.
- Cotton CA, Claassens NJ, Benito-Vaquerizo S, et al. Renewable methanol and formate as microbial feedstocks. Curr Opin Biotechnol. 2020;62:168–180. doi: 10.1016/j.copbio.2019.10.002.
- Lee JS, Kim DK, Lee JP, et al. Effects of SO2 and NO on growth of Chlorella sp. KR-1. Bioresour Technol. 2002;82:1–4. doi: 10.1016/s0960-8524(01)00158-4.
- Maeda K, Owada M, Kimura N, et al. Pergamon CO2 fixation from the flue gas on coal-fired thermal power plant by microalgae To screen microalgac which arc suitable for direct COZ fixation, microalgae were sampled from. Energy Convers Mgmi. 1995;36:717–720. doi: 10.1016/0196-8904(95)00105-M.
- Roberts DA, Paul NA, Bird MI, et al. Bioremediation for coal-fired power stations using macroalgae. J Environ Manage. 2015;153:25–32. doi: 10.1016/j.jenvman.2015.01.036.
- McGinn PJ, Dickinson KE, Bhatti S, et al. Integration of microalgae cultivation with industrial waste remediation for biofuel and bioenergy production: opportunities and limitations. Photosynth Res. 2011;109:231–247. doi: 10.1007/s11120-011-9638-0.
- Doucha J, Straka F, Lívanský K. Utilization of flue gas for cultivation of microalgae (Chlorella sp.) in an outdoor open thin-layer photobioreactor. J Appl Phycol. 2005;17:403–412. doi: 10.1007/s10811-005-8701-7.
- Chiu S-YY, Kao C-YY, Huang T-TT, et al. Microalgal biomass production and on-site bioremediation of carbon dioxide, nitrogen oxide and sulfur dioxide from flue gas using Chlorella sp. cultures. Bioresour Technol. 2011;102:9135–9142. doi: 10.1016/j.biortech.2011.06.091.
- Mortensen LM, Gislerød HR. The growth of Chlamydomonas reinhardtii as influenced by high CO2 and low O2 in flue gas from a silicomanganese smelter. J Appl Phycol. 2015;27:633–638. doi: 10.1007/s10811-014-0357-8.
- Borkenstein CG, Knoblechner J, Frühwirth H, et al. Cultivation of Chlorella emersonii with flue gas derived from a cement plant. J Appl Phycol. 2011;23:131–135. doi: 10.1007/s10811-010-9551-5.
- Sahu SG, Chakraborty N, Sarkar P. Coal-biomass co-combustion: an overview. Renew Sustain Energy Rev. 2014;39:575–586. doi: 10.1016/j.rser.2014.07.106.
- Hughes E, Benemann JR. Biological fossil CO2 mitigation. Energy Convers Mgmt. 1997;38:S467–S473. doi: 10.1016/S0196-8904(96)00312-3
- Benemann JR. CO2 mitigation with microalgae systems. Energy Convers Mgmt. 1997;38:s475–S479. doi: 10.1016/S0196-8904(96)00313-5.
- Negoro M, Hamasaki A, Ikuta Y, et al. Carbon dioxide fixation by microalgai photosynthesis using actual flue gas discharged from a boiler. Appl Biochem Biotechnol. 1993;39–40:643–653. doi: 10.1007/BF02919025.
- Miranda AM, Sáez AA, Hoyos BS, et al. Improving microalgal biomass production with industrial CO2 for bio-oil obtention by hydrothermal liquefaction. Fuel. 2021;302:121236. doi: 10.1016/j.fuel.2021.121236.
- Aslam A, Thomas-Hall SR, Mughal T, et al. Heavy metal bioremediation of coal-fired flue gas using microalgae under different CO 2 concentrations. J Environ Manage. 2019;241:243–250. doi: 10.1016/j.jenvman.2019.03.118.
- Aslam A, Thomas-Hall SR, Mughal TA, et al. Selection and adaptation of microalgae to growth in 100% unfiltered coal-fired flue gas. Bioresour Technol. 2017;233:271–283. doi: 10.1016/j.biortech.2017.02.111.
- Aslam A, Thomas-Hall SR, Manzoor M, et al. Mixed microalgae consortia growth under higher concentration of CO2 from unfiltered coal fired flue gas: fatty acid profiling and biodiesel production. J Photochem Photobiol B. 2018;179:126–133. doi: 10.1016/j.jphotobiol.2018.01.003.
- Choi SY, Sim SJ, Ko SC, et al. Scalable cultivation of engineered cyanobacteria for squalene production from industrial flue gas in a closed photobioreactor. J Agric Food Chem. 2020;68:10050–10055. doi: 10.1021/acs.jafc.0c03133.
- Ji MK, Yun HS, Hwang JH, et al. Effect of flue gas CO2 on the growth, carbohydrate and fatty acid composition of a green microalga Scenedesmus obliquus for biofuel production. Environ Technol. 2017;38:2085–2092. doi: 10.1080/09593330.2016.1246145.
- García-Cubero R, Moreno-Fernández J, García-González M. Potential of chlorella vulgaris to abate flue gas. Waste Biomass Valor. 2018;9:2015–2019. doi: 10.1007/s12649-017-9987-9.
- Rossi RA, Camargo EC, Crnkovic PCGM, et al. Physiological and biochemical responses of Chlorella vulgaris to real cement flue gas under controlled conditions. Water Air Soil Pollut. 2018;229:259. doi: 10.1007/s11270-018-3914-y.
- Pavlik D, Zhong Y, Daiek C, et al. Microalgae cultivation for carbon dioxide sequestration and protein production using a high-efficiency photobioreactor system. Algal Res. 2017;25:413–420. doi: 10.1016/j.algal.2017.06.003.
- Kao CY, Chen TY, Chang YB, et al. Utilization of carbon dioxide in industrial flue gases for the cultivation of microalga Chlorella sp. Bioresour Technol. 2014;166:485–493. doi: 10.1016/j.biortech.2014.05.094.
- Zhao Y, Li J, Ma X, et al. Screening and application of Chlorella strains on biosequestration of the power plant exhaust gas evolutions of biomass growth and accumulation of toxic agents. Environ Sci Pollut Res Int. 2022;29:6744–6754. doi: 10.1007/s11356-021-15950-8.
- Wang B, Xu YF, Sun ZL. Mass transfer characteristics and effect of flue gas used in microalgae culture. Appl Microbiol Biotechnol. 2022;106:7013–7025. doi: 10.1007/s00253-022-12206-4.
- Olofsson M, Lindehoff E, Legrand C. Production stability and biomass quality in microalgal cultivation – contribution of community dynamics. Eng Life Sci. 2019;19:330–340. doi: 10.1002/elsc.201900015.
- Yu BS, Sung YJ, Hong ME, et al. Improvement of photoautotrophic algal biomass production after interrupted co2 supply by urea and kh2 po4 injection. Energies (Basel). 2021;14:778. doi: 10.3390/en14030778.
- Song Y, Cheng J, Miao Y, et al. SO2 impurity in simulated flue gas with 15% CO2 affects dynamic bubble dissolution and arthrospira photosynthetic growth. ACS Sustain Chem Eng. 2021;9:5580–5589. doi: 10.1021/acssuschemeng.0c09197.
- Premaratne M, Liyanaarachchi VC, Nishshanka GKSH, et al. Nitrogen-limited cultivation of locally isolated Desmodesmus sp. for sequestration of CO2from simulated cement flue gas and generation of feedstock for biofuel production. J Environ Chem Eng. 2021;9:105765. doi: 10.1016/j.jece.2021.105765.
- Olofsson M, Lindehoff E, Frick B, et al. Baltic Sea microalgae transform cement flue gas into valuable biomass. Algal Res. 2015;11:227–233. doi: 10.1016/j.algal.2015.07.001.
- Cho SJ, Sung YJ, Lee JS, et al. Robust cyst germination induction in Haematococcus pluvialis to enhance astaxanthin productivity in a semi-continuous outdoor culture system using power plant flue gas. Bioresour Technol. 2021;338:125533. doi: 10.1016/j.biortech.2021.125533.
- Lage S, Gentili FG. Chemical composition and species identification of microalgal biomass grown at pilot-scale with municipal wastewater and CO2 from flue gases. Chemosphere. 2023;313:137344. doi: 10.1016/j.chemosphere.2022.137344.
- Choi HI, Hwang SW, Kim J, et al. Augmented CO2 tolerance by expressing a single H+-pump enables microalgal valorization of industrial flue gas. Nat Commun. 2021;12:6049. doi: 10.1038/s41467-021-26325-5.
- Yadav G, Dubey BK, Sen R. A comparative life cycle assessment of microalgae production by CO2 sequestration from flue gas in outdoor raceway ponds under batch and semi-continuous regime. J Clean Prod. 2020;258:120703. doi: 10.1016/j.jclepro.2020.120703.
- Jebali A, Acién FG, Rodriguez Barradas E, et al. Pilot-scale outdoor production of Scenedesmus sp. in raceways using flue gases and centrate from anaerobic digestion as the sole culture medium. Bioresour Technol. 2018;262:1–8. doi: 10.1016/j.biortech.2018.04.057.
- Cheng J, Yang Z, Zhou J, et al. Improving the CO2 fixation rate by increasing flow rate of the flue gas from microalgae in a raceway pond. Korean J Chem Eng. 2018;35:498–502. doi: 10.1007/s11814-017-0300-1.
- Sanaye Mozaffari Sabet N, Golzary A. CO2 biofixation at microalgae photobioreactors: hydrodynamics and mass transfer study. Int J Environ Sci Technol. 2022;19:11631–11648. doi: 10.1007/s13762-022-04286-6.
- Vuppaladadiyam AK, Yao JG, Florin N, et al. Impact of flue gas compounds on microalgae and mechanisms for carbon assimilation and utilization. ChemSusChem. 2018;11:334–355. doi: 10.1002/cssc.201701611.
- Chi Z, Elloy F, Xie Y, et al. Selection of microalgae and cyanobacteria strains for bicarbonate-based integrated carbon capture and algae production system. Appl Biochem Biotechnol. 2014;172:447–457. doi: 10.1007/s12010-013-0515-5.
- Kim G, Roh K, Han JI. The use of bicarbonate for microalgae cultivationand its carbon footprint analysis. Green Chem. 2019;21:5053–5062. doi: 10.1039/C9GC01107B.
- Ming C, Ta H, Hui H, et al. Chemosphere effects of dissolved inorganic carbon and nutrient levels on carbon fixation and properties of Thermosynechococcus sp. in a continuous system. Chemosphere. 2012;88:706–711.
- Zhu C, Zhai X, Jia J, et al. Seawater desalination concentrate for cultivation of Dunaliella salina with floating photobioreactor to produce β-carotene. Algal Res. 2018;35:319–324. doi: 10.1016/j.algal.2018.08.035.
- Zhai X, Zhu C, Zhang Y, et al. Seawater supplemented with bicarbonate for efficient marine microalgae production in floating photobioreactor on ocean: a case study of Chlorella sp. Sci Total Environ. 2020;738:139439. doi: 10.1016/j.scitotenv.2020.139439.
- Zhu C, Zhai X, Xi Y, et al. Efficient CO2 capture from the air for high microalgal biomass production by a bicarbonate pool. J Co2 Util. 2020;37:320–327. doi: 10.1016/j.jcou.2019.12.023.
- Nayak M, Suh WI, Lee B, et al. Enhanced carbon utilization efficiency and FAME production of Chlorella sp. HS2 through combined supplementation of bicarbonate and carbon dioxide. Energy Convers Manag. 2018;156:45–52. doi: 10.1016/j.enconman.2017.11.002.
- Liang Y, Tang J, Luo Y, et al. Thermosynechococcus as a thermophilic photosynthetic microbial cell factory for CO2 utilisation. Bioresour Technol. 2019;278:255–265. doi: 10.1016/j.biortech.2019.01.089.
- Zhu C, Zhai X, Wang J, et al. Large-scale cultivation of Spirulina in a floating horizontal photobioreactor without aeration or an agitation device. Appl Microbiol Biotechnol. 2018;102:8979–8987. doi: 10.1007/s00253-018-9258-0.
- Chen Z, Li T, Yang B, et al. Isolation of a novel strain of Cyanobacterium sp. with good adaptation to extreme alkalinity and high polysaccharide yield. J Ocean Limnol. 2021;39:1131–1142. doi: 10.1007/s00343-020-0113-7.
- Zhu C, Che J, Zhai X, et al. Cost-effective and efficient production of carbohydrates from an Alkalihalophilic Leptolyngbya sp. in a photobioreactor with periodical mixing. ACS Sustain Chem Eng. 2020;8:15310–15316. doi: 10.1021/acssuschemeng.0c05499.
- De Farias Silva CE, Grisa B, Sforza E, et al. Effects of sodium bicarbonate on biomass and carbohydrate production in synechococcus PCC 7002. Chem Eng Trans. 2016;49:241–246.
- Chi Z, Xie Y, Elloy F, et al. Bicarbonate-based integrated carbon capture and algae production system with alkalihalophilic cyanobacterium. Bioresour Technol. 2013;133:513–521. doi: 10.1016/j.biortech.2013.01.150.
- Van Den Hende S, Vervaeren H, Boon N. Flue gas compounds and microalgae: (bio-)chemical interactions leading to biotechnological opportunities. Biotechnol Adv. 2012;30:1405–1424. doi: 10.1016/j.biotechadv.2012.02.015.
- Lara-Gil JA, Álvarez MM, Pacheco A. Toxicity of flue gas components from cement plants in microalgae CO2 mitigation systems. J Appl Phycol. 2014;26:357–368. doi: 10.1007/s10811-013-0136-y.
- Su Y, Xu M, Brynjólfsson S, et al. Physiological and molecular insights into adaptive evolution of the marine model diatom Phaeodactylum tricornutum under low-pH stress. J Clean Prod. 2023;412:137297. doi: 10.1016/j.jclepro.2023.137297.
- Pawlowski A, Mendoza JL, Guzmán JL, et al. Effective utilization of flue gases in raceway reactor with event-based pH control for microalgae culture. Bioresour Technol. 2014;170:1–9. doi: 10.1016/j.biortech.2014.07.088.
- de Godos I, Mendoza JL, Acién FG, et al. Evaluation of carbon dioxide mass transfer in raceway reactors for microalgae culture using flue gases. Bioresour Technol. 2014;153:307–314. doi: 10.1016/j.biortech.2013.11.087.
- Jiang Y, Zhang W, Wang J, et al. Utilization of simulated flue gas for cultivation of Scenedesmus dimorphus. Bioresour Technol. 2013;128:359–364. doi: 10.1016/j.biortech.2012.10.119.
- Kumar K, Dasgupta CN, Nayak B, et al. Development of suitable photobioreactors for CO2 sequestration addressing global warming using green algae and cyanobacteria. Bioresour Technol. 2011;102:4945–4953. doi: 10.1016/j.biortech.2011.01.054.
- Onay M, Sonmez C, Oktem HA, et al. Thermo-resistant green microalgae for effective biodiesel production: isolation and characterization of unialgal species from geothermal flora of Central Anatolia. Bioresour Technol. 2014;169:62–71. doi: 10.1016/j.biortech.2014.06.078.
- Miari S. CO2 Assimilation in a thremopkilic cyanobacterium. Energy Convers Manag. 1995;36:763–766.
- Podkuiko L, Olt J, Kikas T. Growth of Scenedesmus obliquus under artificial flue gas with a high sulphur concentration neutralized with oil shale ash. Proc Estonian Acad Sci. 2017;66:51. doi: 10.3176/proc.2017.2.03.
- Douskova I, Doucha J, Livansky K, et al. Simultaneous flue gas bioremediation and reduction of microalgal biomass production costs. Appl Microbiol Biotechnol. 2009;82:179–185. doi: 10.1007/s00253-008-1811-9.
- Napan K, Teng L, Quinn JC, et al. Impact of heavy metals from flue gas integration with microalgae production. Algal Res. 2015;8:83–88. doi: 10.1016/j.algal.2015.01.003.
- Fu W, Gudmundsson O, Feist AM, et al. Maximizing biomass productivity and cell density of Chlorella vulgaris by using light-emitting diode-based photobioreactor. J Biotechnol. 2012;161:242–249. doi: 10.1016/j.jbiotec.2012.07.004.
- Dineshkumar R, Dash SK, Sen R. Process integration for microalgal lutein and biodiesel production with concomitant flue gas CO2 sequestration: a biorefinery model for healthcare, energy and environment. RSC Adv. 2015;5:73381–73394. doi: 10.1039/C5RA09306F.
- Nayak M, Karemore A, Sen R. Sustainable valorization of flue gas CO2 and wastewater for the production of microalgal biomass as a biofuel feedstock in closed and open reactor systems. RSC Adv. 2016;6:91111–91120. doi: 10.1039/C6RA17899E.
- Kumar K, Banerjee D, Das D. Carbon dioxide sequestration from industrial flue gas by Chlorella sorokiniana. Bioresour Technol. 2014;152:225–233. doi: 10.1016/j.biortech.2013.10.098.
- Zhu C, Chen S, Ji Y, et al. Progress toward a bicarbonate-based microalgae production system. Trends Biotechnol. 2022;40:180–193. doi: 10.1016/j.tibtech.2021.06.005.
- Singh SK, Sundaram S, Sinha S, et al. Recent advances in CO2 uptake and fixation mechanism of cyanobacteria and microalgae. Crit Rev Environ Sci Technol. 2016;46:1297–1323. doi: 10.1080/10643389.2016.1217911.
- Tang J, Zhou H, Yao D, et al. Comparative genomic analysis revealed distinct molecular components and organization of CO2-concentrating mechanism in thermophilic cyanobacteria. Front Microbiol. 2022;13:876272. doi: 10.3389/fmicb.2022.876272.
- Tomar V, Sidhu GK, Nogia P, et al. Regulatory components of carbon concentrating mechanisms in aquatic unicellular photosynthetic organisms. Plant Cell Rep. 2017;36:1671–1688. doi: 10.1007/s00299-017-2191-3.
- Tang J, Jiang D, Luo Y, et al. Potential new genera of cyanobacterial strains isolated from thermal springs of western Sichuan, China. Algal Res. 2018;31:14–20. doi: 10.1016/j.algal.2018.01.008.
- Komárek J, Johansen JR, Šmarda J, et al. Phylogeny and taxonomy of Synechococcus–like cyanobacteria. Fottea. 2020;20:171–191. doi: 10.5507/fot.2020.006.
- Choi YY, Joun JM, Lee J, et al. Development of large-scale and economic pH control system for outdoor cultivation of microalgae Haematococcus pluvialis using industrial flue gas. Bioresour Technol. 2017;244:1235–1244. doi: 10.1016/j.biortech.2017.05.147.
- Alliance Consulting International. Methanol Safe Handling Manual. Methanol Institute. 2008;5:1–37. Available from: https://www.methanol.org/wp-content/uploads/2020/03/Safe-Handling-Manual_5th-Edition_Final.pdf
- Song S, Timm S, Lindner SN, et al. Expression of formate-tetrahydrofolate ligase did not improve growth but interferes with nitrogen and carbon metabolism of Synechocystis sp. PCC 6803. Front Microbiol. 2020;11:1–14.
- Bielecka A, Kulczycka J. Coal combustion products management toward a circular economy—a case study of the coal power plant sector in Poland. Energies (Basel). 2020;13:3603. doi: 10.3390/en13143603.
- Wang P, Wang J, Qin Q, et al. Life cycle assessment of magnetized fly-ash compound fertilizer production: a case study in China. Renew Sustain Energy Rev. 2017;73:706–713. doi: 10.1016/j.rser.2017.02.005.
- Shaheen SM, Hooda PS, Tsadilas CD. Opportunities and challenges in the use of coal fly ash for soil improvements–a review. J Environ Manage. 2014;145:249–267. doi: 10.1016/j.jenvman.2014.07.005.
- Rai UN, Tripathi RD, Singh N, et al. Amelioration of fly-ash by selected nitrogen fixing blue green algae. Bull Environ Contam Toxicol. 2000;64:294–301. doi: 10.1007/s001289910043.
- Pöykiö R, Nurmesniemi H, Keiski RL. Heavy metals in flue gas cleaning residue. Environ Chem Lett. 2010;8:295–300. doi: 10.1007/s10311-009-0220-3.
- Pesonen J, Kuokkanen T, Rautio P, et al. Bioavailability of nutrients and harmful elements in ash fertilizers: effect of granulation. Biomass Bioenergy. 2017;100:92–97. doi: 10.1016/j.biombioe.2017.03.019.
- Sandoval R MA, Flores E MF, Narváez C RA, et al. Phototrophic culture of Chlorella sp. using charcoal ash as an inorganic nutrient source. Algal Res. 2015;11:368–374. doi: 10.1016/j.algal.2015.07.008.
- Le VL, Kheiri A, Feidt M, et al. Thermodynamic and economic optimizations of a waste heat to power plant driven by a subcritical ORC (Organic Rankine Cycle) using pure or zeotropic working fluid. Energy. 2014;78:622–638. doi: 10.1016/j.energy.2014.10.051.
- Laamanen CA, Shang H, Ross GM, et al. A model for utilizing industrial off-gas to support microalgae cultivation for biodiesel in cold climates. Energy Convers Manag. 2014;88:476–483. doi: 10.1016/j.enconman.2014.08.047.
- Brückner S, Liu S, Miró L, et al. Industrial waste heat recovery technologies: an economic analysis of heat transformation technologies. Appl Energy. 2015;151:157–167. doi: 10.1016/j.apenergy.2015.01.147.
- IEA. World Energy Outlook 2012. Paris (France): IEA; 2012. Chapter 17, Water for energy: is energy becoming a thirstier resource?; p. 501–528.
- Covarrubias Y, Cantoral-Uriza EA, Casas-Flores JS, et al. Thermophile mats of microalgae growing on the woody structure of a cooling tower of a thermoelectric power plant in Central Mexico. Rev Mex Biodivers. 2016;87:277–287. doi: 10.1016/j.rmb.2016.04.001.
- Wang D, Bao A, Kunc W, et al. Coal power plant flue gas waste heat and water recovery. Appl Energy. 2012;91:341–348. doi: 10.1016/j.apenergy.2011.10.003.
- Song C, Chen G, Ji N, et al. Biodiesel production process from microalgae oil by waste heat recovery and process integration. Bioresour Technol. 2015;193:192–199. doi: 10.1016/j.biortech.2015.06.116.
- Zou X, Xu K, Xue Y, et al. Interactions of Chlorella vulgaris and fly ash cenospheres in heat-aided ballasted flotation. Algal Res. 2020;46:101813. doi: 10.1016/j.algal.2020.101813.
- Laamanen CA, Scott JA. Development of heat-aided flocculation for flotation harvesting of microalgae. Biomass Bioenergy. 2017;107:150–154. doi: 10.1016/j.biombioe.2017.09.020.
- Mehetre GT, Zothanpuia, Deka P, et al. Chapter 6 – Thermophilic and thermotolerant cyanobacteria: environmental and biotechnological perspectives. In: Singh P, Fillat M, Kumar ABT-CL and its A in B, editors. Cyanobacterial Lifestyle and its Applications in Biotechnology. Cambridge (UK): Academic Press; 2022. p. 159–178.
- Varshney P, Mikulic P, Vonshak A, et al. Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour Technol. 2015;184:363–372. doi: 10.1016/j.biortech.2014.11.040.
- Liang Y, Kaczmarek MB, Kasprzak AK, et al. Thermosynechococcaceae as a source of thermostable C-phycocyanins: properties and molecular insights. Algal Res. 2018;35:223–235. doi: 10.1016/j.algal.2018.08.037.
- Bergmann P, Trösch W. Repeated fed-batch cultivation of Thermosynechococcus elongatus BP-1 in flat-panel airlift photobioreactors with static mixers for improved light utilization: influence of nitrate, carbon supply and photobioreactor design. Algal Res. 2016;17:79–86. doi: 10.1016/j.algal.2016.03.040.
- Hsueh HT, Chu H, Chang CC. Identification and characteristics of a cyanobacterium isolated from a hot spring with dissolved inorganic carbon. Environ Sci Technol. 2007;41:1909–1914. doi: 10.1021/es0620639.
- Eisele LE, Bakhru SH, Liu X, et al. Studies on C-phycocyanin from cyanidium caldarium, a eukaryote at the extremes of habitat. Biochim Biophys Acta. 2000;1456:99–107. doi: 10.1016/s0005-2728(99)00110-3.
- Riaz S, Xiao M, Chen P, et al. The genome copy number of the thermophilic Cyanobacterium Thermosynechococcus elongatus E542 is controlled by growth phase and nutrient availability. Appl Environ Microbiol. 2021;87:1–13. doi: 10.1128/AEM.02993-20.
- Pedersen D, Miller SR. Photosynthetic temperature adaptation during niche diversification of the thermophilic cyanobacterium Synechococcus A/B clade. Isme J. 2017;11:1053–1057. doi: 10.1038/ismej.2016.173.
- Liang Y, Tang J, Luo Y, et al. Thermosynechococcus as a thermophilic photosynthetic microbial cell factory for CO2 utilisation. Bioresour Technol. 2019;278:255–265. doi: 10.1016/j.biortech.2019.01.089.
- Chen J, Huang Y, Shu Y, et al. Recent progress on systems and synthetic biology of diatoms for improving algal productivity. Front Bioeng Biotechnol. 2022;10:908804. doi: 10.3389/fbioe.2022.908804.
- Cao K, Cui Y, Sun F, et al. Metabolic engineering and synthetic biology strategies for producing high-value natural pigments in Microalgae. Biotechnol Adv. 2023;68:108236. doi: 10.1016/j.biotechadv.2023.108236.
- Skjanes K, Lindblad P, Muller J. BioCO2 – a multidisciplinary, biological approach using solar energy to capture CO2 while producing H2 and high value products. Biomol Eng. 2007;24:405–413. doi: 10.1016/j.bioeng.2007.06.002.
- Yen H-W, Ho S-H, Chen C-Y, et al. CO 2, NO x and SO x removal from flue gas via microalgae cultivation: a critical review. Biotechnol J. 2015;10:829–839. doi: 10.1002/biot.201400707.
- Gleizer S, Ben-Nissan R, Bar-On YM, et al. Conversion of Escherichia coli to generate all biomass carbon from CO2. Cell. 2019;179:1255–1263.e12. doi: 10.1016/j.cell.2019.11.009.
- Gassler T, Sauer M, Gasser B, et al. The industrial yeast Pichia pastoris is converted from a heterotroph into an autotroph capable of growth on CO2. Nat Biotechnol. 2020;38:210–216. doi: 10.1038/s41587-019-0363-0.
- Shih PM, Zarzycki J, Niyogi KK, et al. Introduction of a synthetic CO2-fixing photorespiratory bypass into a cyanobacterium. J Biol Chem. 2014;289:9493–9500. doi: 10.1074/jbc.C113.543132.