Abstract
Heme, an iron-containing tetrapyrrole in hemoproteins, including: hemoglobin, myoglobin, catalase, cytochrome c, and cytochrome P450, plays critical physiological roles in different organisms. Heme-derived chemicals, such as biliverdin, bilirubin, and phycocyanobilin, are known for their antioxidant and anti-inflammatory properties and have shown great potential in fighting viruses and diseases. Therefore, more and more attention has been paid to the biosynthesis of hemoproteins and heme derivatives, which depends on the adequate heme supply in various microbial cell factories. The enhancement of endogenous biosynthesis and exogenous uptake can improve the intracellular heme supply, but the excess free heme is toxic to the cells. Therefore, based on the heme-responsive regulators, several sensitive biosensors were developed to fine-tune the intracellular levels of heme. In this review, recent advances in the: biosynthesis, acquisition, regulation, and upcycling of heme were summarized to provide a solid foundation for the efficient production and application of high-value-added hemoproteins and heme derivatives.
Introduction
Heme, the cofactor of hemoproteins, plays essential roles in prokaryotic and eukaryotic cells, including: oxygen transport [Citation1], electron transfer [Citation2], gas sensing [Citation3], signal transduction [Citation4], biological clock [Citation5], and microRNA processing [Citation6]. Currently, hemoproteins have been widely applied in the fields of food and medicine. For example, bovine myoglobin (bovine-Mb) and soy hemoglobin (soy-Hb) can be used as food-grade coloring and flavoring agents [Citation7,Citation8]; lactoperoxidase can be used as a bioavailable bacteriostatic agent [Citation9]; human hemoglobin (human-Hb) can be used as an acellular oxygen carrier [Citation10]; and P450 enzymes can be used as efficient biocatalysts [Citation11]. In addition, heme-derived compounds, such as biliverdin (BV, usually refers to BVIXα), bilirubin (BR), and phycocyanobilin (PCB), have received considerable attention for their potential in fighting various viruses and diseases, especially COVID-19 [Citation12–14]. Due to their antioxidant [Citation15,Citation16], anti-inflammatory [Citation17,Citation18], and neuroprotective effects [Citation19], these compounds can enhance antioxidant enzymic activity, upregulate anti-inflammatory cytokines, and inhibit the formation of α-synuclein and amyloid-β fibril.
All these biotechnological applications rely on the sufficient supply of heme in microbial cell factories. Heme is incorporated into hemoproteins during the co-translational folding process and assists in polypeptide-folding [Citation20]. The insufficient intracellular heme can cause the degradation of subunits or apoproteins [Citation21]. In addition, the biosynthesis of heme-derived chemicals starts from the catabolism of heme by heme oxygenase (HO), which leads to the generation of BV. Subsequently, biliverdin reductase (BvdR) and phycocyanobilin: ferredoxin oxidoreductase (PcyA) utilize BV as a substrate to generate BR and PCB, respectively (). Therefore, the intracellular availability of heme is crucial for producing highly active hemoproteins [Citation22,Citation23] and high-value-added heme derivatives [Citation24–26].
Figure 1. Schematic overview of the biosynthetic pathway of heme (a) and its derivatives (b). G-6-P: glucose-6-phosphate; 3-PG: 3-phosphoglycerate; Pyr: pyruvate; CoA: coenzyme A; A-CoA: acetyl-CoA; CIT: citrate; ICT: isocitrate; α-KG: α-ketoglutarate; SUC: succinate; MAL: malate; TCA: tricarboxylic acid cycle; GLO: glyoxylate; LDH: lactate dehydrogenase; PTA: phosphate acetyltransferase; KDH: α-ketoglutarate dehydrogenase; SCS: succinyl‑CoA synthase; ICL: isocitrate lyase; Fdred: reduced Fd (ferredoxin); Fdox: oxidized Fd; Fnr: Fd-NADP+ reductase.

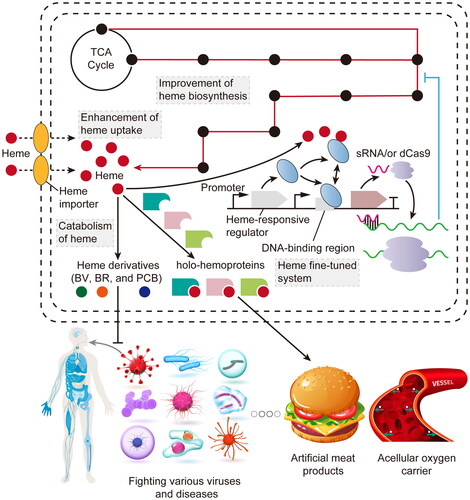
There are two ways to improve intracellular heme supply. The first approach is the enhancement of endogenous heme biosynthesis. Although heme biosynthesis can be enhanced by the exogenous addition of 5-aminolevulinic acid (ALA, the key precursor of heme), the high cost of ALA makes it unsuitable for large-scale industrial production [Citation23]. Therefore, many researchers have tried to increase the intracellular accumulation of ALA and activate the downstream enzymes responsible for heme synthesis. The second method is the supplementation of heme during the synthesis of hemoproteins or heme-derived chemicals. However, the ability of heme uptake for common microbial hosts (Saccharomyces cerevisiae, Pichia pastoris, Escherichia coli, and Corynebacterium glutamicum) is extremely weak. Therefore, it is necessary to introduce heme importers in these hosts to overcome this drawback [Citation27–30].
With the increase of intracellular heme levels, the production of heme derivatives and hemoproteins can be improved. However, the excess free heme that exceeds the demand for product synthesis and cell growth is cytotoxic [Citation23], which has an adverse effect on the sustainable production of heme derivatives and hemoproteins. Hence, the heme-responsive biosensor was designed by the incorporation of a heme-sensing regulator with Clustered Regularly Interspaced Short Palindromic Repeats interference (CRISPRi) or small regulatory RNA (sRNA) systems to fine-tune intracellular heme levels [Citation23, Citation31].
With the rapid development of synthetic biology and system biology in recent years, many new advances have been reported in the: biosynthesis, acquisition, regulation, and upcycling of heme. Many new heme importers and heme-sensing regulators were discovered. Based on these discoveries, a series of strategies were developed to enhance the intracellular heme supply for synthesizing highly active hemoproteins and high-value-added heme derivatives. However, these advances have not been comprehensively reviewed. Thus, this review provides an overall summary in the: biosynthesis, acquisition, regulation, and upcycling of heme, laying a solid foundation for the development of valuable hemoproteins and heme derivatives.
Enhanced heme biosynthesis and its application in hemoprotein expression
Heme biosynthetic pathway
Heme biosynthesis begins with ALA, which has two main synthetic pathways depending on the species, C4 or C5 pathways (). In the C4 pathway that exists in: humans, animals, fungi, and α-proteobacteria, ALA synthase (ALAS) catalyzes the condensation of glycine (Gly) and succinyl-CoA (Suc-CoA) to form ALA. In the C5 pathway found in archaea, plants, and many bacteria, ALA is synthesized from l-glutamate (l-Glu) via a series of enzymes containing: glutamyl-tRNA synthetase (GluRS), glutamyl-tRNA reductase (GluTR), and glutamate-1-semialdehyde 2,1-aminomutase (GSAM). Then, ALA is condensed into porphobilinogen (PBG) by porphobilinogen synthase (PBGS; also named as ALA dehydratase, ALAD). Next, PBG undergoes a polymerization reaction triggered by porphobilinogen deaminase (PBGD) to form an unstable linear tetrapyrrole 1-hydroxymethylbilane (HMB), which is utilized as a substrate by uroporphyrinogen III synthase (UROS) and cyclized to produce uroporphyrinogen III (UPG III). In the classical protoporphyrin-dependent pathway (PPD) found in eukaryotes and α-proteobacteria, UPG III is converted into heme through the consecutive generation of intermediates, including: coproporphyrinogen III (CPG III), protoporphyrinogen IX (PPG IX), and protoporphyrin IX (PP IX), which are synthesized by: the uroporphyrinogen III decarboxylase (UROD), coproporphyrinogen III oxidase (CPO), protoporphyrinogen oxidase (PPO), and ferrochelatase (FECH) catalyze, respectively. In the noncanonical coproporphyrin-dependent pathway (CPD) found in Actinobacteria and Firmicutes, the initial step for the conversion of UPG III into heme is similar with the classical pathway. Subsequently, CPG III is transformed into coproporphyrin III (CP III) by CPO, which is gradually converted into coproheme and heme through two sequential reactions catalyzed by coproporphyrin ferrochelatase (CPFC) and hydrogen peroxide-dependent heme synthase (HS), respectively [Citation32,Citation33].
The strategies of metabolic engineering to synthesize ALA
The key precursor ALA is essential for the biosynthesis of heme [Citation23]. Therefore, the recent metabolic engineering strategies for ALA production were summarized (). Initially, the researchers focused on engineering the shorter and simpler C4 pathway to improve ALA production, but it encounters a critical requirement of supplementing main precursors (Gly and Suc-CoA), which limits the productivity and yield of ALA [Citation36]. For example, the titer of ALA reached 2.46 g/L in E. coli by co-expressing ALASRS (derived from Rhodobacter sphaeroides) and ALA exporter RhtA with the addition of 3 g/L Gly and 1 g/L Suc [Citation34]. To overcome this problem, the biosynthesis of CoA and precursors (Gly and Suc-CoA) was enhanced and the expression of ALAD was downregulated, resulting in the titer of ALA increased to 2.81 g/L [Citation35]. Based on these strategies and further utilization of 30 g/L glycerol as carbon source, 6.93 g/L of ALA was synthesized in E. coli, reaching 50.9% of the theoretical maximum yield [Citation38]. In addition, the soluble expression and activity of ALAS were improved by the fusion expression with TrxA tag or co-expression of GroEL/ES chaperones, leading to a significant enhancement in ALA production from 1.21 to 3.67 g/L [Citation37]. Meanwhile, the supplementation of 30 μM PLP (the cofactor of ALAS) was applied to increase the ALA titer from 1.4 to 2.41 g/L [Citation39]. Furthermore, ALA can cause severe damage to E. coli cells and alters their morphology by generating reactive oxygen species [Citation40], thus it is necessary to strengthen the antioxidant defense system of hosts by co-expressing catalase KatE and superoxide dismutase SodB, resulting in an improved ALA titer of 11.50 g/L. Another research showed that the removal of NCgl0580 that encodes a permease of the drug/metabolite transporter in C. glutamicum can redistribute the central carbon metabolism fluxes and increase the supply of Suc-CoA. As a result, there was a 2.49-fold increase in the titer of ALA, which opens up a new avenue for achieving high-level ALA production [Citation54]. Finally, in the latest research, the highest titer of ALA (30.70 g/L) in E. coli was reported by developing a C75A/R365K variant of Rhodopseudomonas palustris ALAS with the property of anti-feedback inhibition and enhanced catalytic activity and discovering a new ALA exporter (EamA) [Citation41].
Table 1. Metabolic engineering strategies to synthesize ALA and heme in recent years.
Alternatively, the C5 pathway has attracted more attention to produce ALA from glucose. It has been shown that N-terminal modification of the GluTR increases its stability and reduces the effect of feedback inhibition from heme. Indeed, the feedback-resistant version of GluTR resulted in a 1.76-fold increase in ALA production compared to its wild-type [Citation42]. Based on this research, ALA production was further improved by weakening the expression of ALAD or pushing the flux of the TCA cycle toward l-Glu [Citation43,Citation44]. However, the titer of ALA remained low, ranging between 2.0 and 3.0 g/L. Therefore, the following studies combined the previous strategies and focused on regenerating the cofactors of GluRS, GluTR, and GSAM (ATP, NADPH, and PLP) and activating the glyoxylate cycle, which increased the titer of ALA to 3.0–5.0 g/L [Citation45–47]. Additionally, due to more steps involved in the C5 pathway, spatial assembly of GluRS, GluTR, and GSAM using the protein scaffold was applied to eliminate the obstacles in the transfer of intermediates, resulting in a significant increase in ALA titer to 11.40 g/L [Citation48]. In the future, the useful strategies utilized in various species could be integrated together to further boost ALA production.
The strategies of metabolic engineering to synthesize heme
Based on the accumulation of ALA, the enhancement of the downstream heme biosynthetic pathway has been achieved in various microorganisms (). At first, an engineered E. coli HAEM7 strain that can efficiently synthesize and secrete heme was constructed by enhancing the C5 pathway and the other subsequent seven genes, inhibiting the formation of byproducts and the degradation of heme, and overexpressing the heme exporter CcmABC, producing 87.8 mg/L intracellular and 151.4 mg/L extracellular heme in the 6.6 L fed-batch fermentation [Citation49]. In the following, the same group further optimized the fermentation conditions and developed a novel feeding strategy, leading to the highest reported titer of heme (1.03 g/L) in E. coli [Citation50]. In addition, another recent study focused on the elimination of competitive pathways (the formation of siroheme and heme O) and the reinforcement of heme efflux pathway (Dpp and Ccm), increasing the titer of heme by 2.08-fold to 28.20 mg/L in E. coli [Citation51].
Furthermore, the metabolic engineering of C. glutamicum has shown promising results in heme synthesis. Through combining the C4 and C5 pathways, optimizing the noncanonical CPD pathway, reducing the accumulation of intermediate (UPG III), knocking out the heme-binding proteins (HBPs) (HrrS, HtaA, and HmuT) in the cell membrane, and overexpressing the heme exporter HrtBA, the engineered strain produced 66.23 mg/L intracellular and 242.95 mg/L extracellular heme in the 2 L fed-batch fermentation [Citation52]. Moreover, S. cerevisiae was considered as a suitable food-grade host for the production of Hb and Mb to develop artificial meat. Applying the genome-scale metabolic models (Yeast8 and ecYeast8) to identify the genes that significantly affect heme biosynthesis, the engineered S. cerevisiae strain showed a 70-fold increase in the intracellular titer of heme (53.5 mg/L), which can be used as a powerful heme-supplying chassis [Citation53].
Efficient expression of hemoproteins through enhancing heme biosynthesis
Recently, enhancing heme biosynthetic pathway has emerged as a promising approach for synthesizing highly active hemoproteins (). Soy-Hb and bovine-Mb, synthesized in an engineered P. pastoris strain overexpressing eight genes involved in heme biosynthesis, have been approved by the USA Food and Drug Association (FDA) as flavoring and coloring agents (GRN nos. 737 and 1001), and applied by Impossible Foods Inc. (Redwood City, CA) and Motif Food Works Inc. (Boston, MA) to develop the popular artificial meat products of “Impossible Burgers” and “HEMAMI”, respectively. The following research used the similar strategy to increase intracellular availability of heme in P. pastoris, increasing the titer of soy-Hb to 3.5 g/L [Citation55]. However, a recent study revealed that there was a noticeable accumulation of heme intermediates (PBG, HMB, CPG III, PPG IX, and PP IX) rather than heme in the engineered P. pastoris strain overexpressing all heme biosynthetic enzymes [Citation61]. Therefore, in our latest result, the intracellular supply of heme was moderately enhanced in S. cerevisiae by improving ALA synthesis (multi-copy integrated expression of ALAS), removing the spatial barrier during heme synthesis (the assembly of CPO with mitochondrial localization signal truncated PPO and FECH), and knocking out the HMX1(HO). Applying this engineered S. cerevisiae strain, several highly active Hb and Mb with a superior heme-binding ratio were successfully synthesized, including: soy-Hb, clover-Hb, bovine-(Hb/Mb), and porcine-(Hb/Mb) [Citation22]. Based on these engineering strategies, the rate-limiting steps in heme biosynthesis mediated by PBGS, PBGD, and UROS were further eliminated in our subsequent study, resulting in the enhanced synthesis of high-activity porcine-Mb, soy-Hb, and Vitreoscilla-Hb in P. pastoris [Citation56]. In addition, a metabolically engineered S. cerevisiae strain produced human-Hb at the level of 18% total intracellular proteins by overexpressing PBGD and deleting HMX1 and the heme-dependent repressor ROX1 [Citation57]. Furthermore, in C. glutamicum, the latest study showed that the heme-binding ratio of synthesized soy-Hb could reach 28% by adding 1.0 g/L ALA [Citation58].
Table 2. Enhanced hemoprotein expression through improved intracellular heme biosynthesis.
In the field of whole-cell P450s catalysis, 1,12-dodecanediol synthesized by CYP153A33 increased from 0.26 to 0.58 mM with the supplementation of 0.5 mM ALA [Citation59], the co-expression of ALAS improved the heme-binding ratio in CYP119 from 66 to 99% [Citation62], as well as the synthesis of lutein and apigenin by P450s increased by 71.5% and 99.0%, respectively, when GluTRfbr (a feedback-resistant version) and GSAM were overexpressed [Citation60]. Moreover, the combination of assembling three rate-limiting enzymes (PBGS, PBGD, and UROS) and integrally expressing another three biosynthetic enzymes (GluTR, GSAM, and FECH) enhanced the enzymic activity of mutated P450-BM3 by approximately 2.0-fold [Citation23], overexpression of: GluRS, GluTR, GSAM, UROD, CPO, PPO, and FECH, could increase the whole-cell catalytic efficiencies of three P450 enzymes (from Mycobacterium marinum, Polaromonas sp. JS666, and Marinobacter aquaeolei) by 1.8- to 6.5-fold [Citation61], and: overexpression of ALAS, assembly of PBGS, PBGD, and UROS using protein scaffolds, cytoplasmic localization of PPO and FECH, and inhibition of heme degradation, improved the whole-cell catalytic activity of mutated P450-BM3 by 7.5-fold [Citation56].
Improving intracellular heme supply by introducing heme importers
Improving the ability of heme uptake is beneficial for increasing intracellular heme supply, producing high-activity hemoproteins and high-value-added heme derivatives [Citation23, Citation30]. In this section, the new discovered heme importers and their latest applications in biotechnology were introduced.
The mechanism of heme acquisition in pathogenic bacteria and fungal
Most of heme importers were identified in pathogenic bacteria, fungal, parasites, and mammals (). In Gram-negative bacteria, the classic heme uptake system (HUS) consists of TonB-dependent heme receptors (TBDRs) in the outer membrane, the TonB–ExbB–ExbD complex, periplasmic HBP and ATP-binding cassette (ABC) transporters [Citation89] (). The TBDRs can either bind free heme or accept heme from Hb and hemophore (HMP), and then internalize it into the periplasm through the TonB–ExbB–ExbD complex [Citation66]. Subsequently, heme is transferred by the periplasmic HBP to ABC transporters in the inner membrane and is further carried to the cytoplasm for the synthesis of hemoprotein or the utilization of iron [Citation89].
Figure 2. The mechanism of heme uptake and its diverse applications in biotechnology. (a) The HUS in Gram-negative and Gram-positive bacteria. There are both Isd and non-Isd systems present in Gram-positive bacteria, such as S. aureus and C. diphtheriae. OM: outer membrane; IM: inner membrane; CW: cell wall. (b) The applications of heme importers in the production of hemoprotein and heme-derived compounds, the assembly of ArMs, and the development of vaccines and antibodies.

Table 3. The latest identified heme importers and their potential targets for fighting pathogens.
In Gram-positive bacteria, classic HUSs are mainly divided into iron-regulated surface determinants (Isd) and non-Isd systems (). In the Isd system of Staphylococcus aureus, the cell-wall-anchored surface receptors (IsdH, IsdB, and IsdA) contain the special domain of heme-binding near transporter (NEAT) [Citation90]. These receptors are the main binding proteins for haptoglobin (HP)–Hb complexes, Hb, and heme, respectively. IsdA can bind free heme or receive it from IsdH and IsdB and transfer it to the NEAT-containing IsdC buried in the cell wall [Citation91]. IsdC then delivers heme to the membrane-embedded transporter IsdDEF that transports heme across the membrane and into the cytoplasm [Citation91]. As for non-Isd systems, taking Corynebacterium diphtheriae as an example, it primarily includes the cell-wall-anchored surface receptors HtaA and HtaB, as well as the ABC transporter HmuTUV [Citation92]. HtaA possesses two heme-binding conserved regions (CR1 and CR2) and can acquire heme from Hb [Citation93]. Additionally, ChtA and ChtC, which contain a single CR domain, require HtaA for the uptake of heme from HP–Hb complexes [Citation92]. HtaB also harbors a single CR domain and can bind free heme or accept heme attached to HtaA and transport it to HmuTUV membrane transporters, which internalize heme into the cytoplasm [Citation94].
The general model of heme uptake in fungal pathogens is quite different, including Candida albicans, Schizosaccharomyces pombe, and Cryptococcus neoformans. The mechanism is that the cell wall surface-anchored binding proteins of heme or hemoprotein (Rbt5/Pga7/Csa2 in C. albicans, Shu1 in S. pombe, and Cig1 in C. neoformans) capture free heme or extract heme from hemoproteins and steer it across the cell wall to the plasma membrane, where it is endocytosed into the cytoplasm with the aid of ubiquitinated proteins and the endosomal sorting complex required for transport (ESCRT) [Citation95–97].
The newly discovered heme importers
With the development of genomics and protein engineering, more and more heme importers have been identified, especially in pathogens (). In Gram-negative bacteria, it was found that a HMP-like protein HmuY is essential for acquiring heme and increasing virulence in the main periodontopathogen (Porphyromonas gingivalis). The heterologous co-expression of HmuY and HmuR (TBDR) in a hemA mutant E. coli strain where the endogenous heme biosynthesis pathway is blocked, can promote the utilization of exogenous heme and growth recovery [Citation98]. The homologous proteins of HumY have been recently identified in other periodontopathogens, such as Tfo in Tannerella forsythia [Citation63], PinO/PinA in Prevotella intermedia [Citation64], and Bvu in Bacteroides vulgatus [Citation65], which display different modes of heme coordination with HmuY (heme ligands: His134/His166 in HmuY, Met120/Met149 in Tfo, Met119 in PinO, Met129/Met158 in PinA, and Met145/Met172 in Bvu). In addition, a recent study found that the outer membrane HBP RhuA can transfer heme to the RhuR (TBDR) to facilitate heme utilization in Riemerella anatipestifer. The heme binding ability of RhuA is stronger than that of HemR (TBDR) derived from Serratia marcescens in ΔhemA mutant E. coli strain [Citation66].
In Gram-positive bacteria, the discovery of new energy coupling factor (ECF) transporters attracted more attention. It is a noncanonical ABC transporter comprised of a substrate-specific component (EcfS), a transmembrane protein (EcfT), and a pair of cytosolic ATPases (EcfA) [Citation76]. It can acquire heme from free heme and hemoproteins and independently functions without the Isd system. The latest identified ECF transporters include LhaSTA and its homologs SLUG_19150/19160/19170 in Staphylococcus lugdunensis [Citation74,Citation75], SiaFGH in group A Streptococcus [Citation76], and Lsa_1836-1840 in Lactobacillus sakei [Citation77]. Among them, the LhaS (EcfS) could be heterologously expressed in E. coli and absorb heme from the growth media [Citation74]. In eukaryotes, previous studies have shown that the S. cerevisiae mutant (Δhem1) can uptake heme and the growth can be restored by introducing these heme importers derived from parasites, including: TbHRG in Trypanosoma brucei [Citation99], BmHRG-1 in Brugia malayi [Citation100], CeHRG1 in Caenorhabditis elegans [Citation101], LHR1 in Leishmania [Citation102], and TcHTE in Trypanosoma cruzi [Citation103]. The latest discovered heme importers include: Str3 in S. pombe [Citation78], IrHRG in Ixodes ricinus [Citation79], LmFLVCRb in Leishmania [Citation80], and SLCO2B1 and SLC46A1 in mammals [Citation81,Citation82]. Among them, besides Shu1, Str3 is the second cell-membrane surface heme importer in S. pombe. The increasing expressional level of Str3 restored the growth of S. pombe Δhem1Δshu1 mutant when hemin was supplemented [Citation78]. Moreover, the heterologous expression of Str3 enhanced the ability of S. cerevisiae Δhem1 mutant to absorb exogenous heme [Citation78].
The latest applications of heme importers
The application of heme importers has shown great potential in synthesizing hemoproteins and heme derivatives, assembling artificial metalloenzymes (ArMs), and developing vaccines and antibodies (). Regarding the synthesis of hemoproteins and heme derivatives, a previous study showed that the introduction of the entire HUS hug operon derived from Plesiomonas shigelloides into E. coli resulted in a 30-fold increase in the expression of human-Hb compared to the control strain without the transport system when supplemented with 2.3 mM hemin [Citation27]. Later, it was proposed that the E. coli strain Nissle 1917 (EcN) strain bearing the ChuA (TBDR) might be an ideal host for the production of hemoproteins. It was found that the EcN strain produced a higher titer (over 82.36%) and heme-binding ratio of the protein sGAF2 than the control strain E. coli BL21(DE3) when 10 μM hemin was added [Citation29]. However, the EcN strain lacks the T7 RNA polymerase, which limits the use of common commercial vectors based on the T7 promoter. Therefore, the gene encoding T7 RNA polymerase was integrated into the genome of EcN strain to construct the EcN(T7) strain, which exhibited more efficient expressional or synthetic efficiency of hemoprotein, BV, and PCB when hemin was supplemented [Citation28]. In addition, the recent result showed that the co-expression of ChuA in E. coli C41(DE3) increased the whole-cell catalytic efficiency of mutated P450-BM3 and P450-sca-2 by 16.1% and 29.4%, respectively [Citation23].
In terms of ArMs assembly, the EcN strain showed a two-fold increase in Ir content in the supernatant of cell lysates compared to the control BL21 (DE3) strain, indicating that EcN is more efficient in exogenously utilizing the cofactor Ir(Me)MPIX (porphyrin compounds) of Ir-CYP119 [Citation104]. However, the EcN strain may not be the optimal choice. When Ir(Me)MPIX was supplemented, the co-expression of the hug operon in BL21(DE3) resulted in a significant increase in the assembly and catalytic efficiency of Ir-CYP119 compared to the co-expression of ChuA and the blank control [Citation105]. Therefore, whether to introduce the heme receptor in the out membrane alone or the entire HUS depends on the homology of HUS components between the expression host and the original source strain, which has also been demonstrated in our recent study [Citation23]. In addition, HUS components are crucial for the survival of pathogens that cannot synthesize heme as an internal iron source, which can be targeted for developing vaccines and antibody-based immunotherapies (), including: IsdA for S. aureus [Citation83], NEAT proteins (IsdX1, IsdX2, and Bslk) for Bacillus anthracis [Citation84], and Shr and HtsA for group A Streptococcus [Citation87,Citation88].
Dynamically regulating heme synthetic pathway by biosensor
The excess intracellular heme is cytotoxic because it can cause lipid peroxidation and trigger ROS formation [Citation23]. To control the concentration of heme at the lower level, there are heme-responsive regulators existing in various organisms. Heme can bind to the heme-responsive regulators (such as HrtR in Lactococcus lactis), altering their conformation and the ability of DNA binding to regulate the transcription of downstream genes involved in heme synthesis [Citation106]. Based on the special property of heme-binding, many heme-sensing regulators have been employed to develop fine-tuning systems to dynamically control the moderate supply of heme for the sustainable production of hemoproteins and heme-derived compounds. In this section, the new discovered heme-responsive regulators and several fluorescently labeled heme sensors were introduced, providing a foundation to develop further effective heme-regulating systems.
The latest discovered heme-responsive regulators include: HatR in Clostridium difficile [Citation107], FhtR in Enterococcus faecalis [Citation108], ChuP in Chromobacterium violaceum [Citation72], and PhuS in Pseudomonas aeruginosa [Citation109] (). HatR and FhtR belong to the TetR family of transcriptional regulators and function as transcriptional repressors of the hatRT and hrtBAEf operons, respectively [Citation107,Citation108]. When intracellular free heme is abundant, HatR and FhtR sense and bind to heme, inhibiting their function as repressors and increasing the transcription of hatRT and hrtBAEf, respectively. Next, HatT and HrtBAEf control the efflux of intracellular heme, thereby reducing heme toxicity in the cells. Unlike HatR and FhtR, ChuP can bind heme and activate the expression of the ChuR (TBDR) and siderophore VbuA by the regulation of post-transcription under the limitation of iron, thereby increasing the uptake of iron [Citation72]. In addition, PhuS is a special regulator that controls the intracellular homeostasis of iron through its dual function. It can transfer heme to HO and repress the transcription of PrrH sRNA [Citation109] that is involved in the degradation of mRNA encoding non-essential iron-containing proteins such as pyochelin siderophore [Citation110]. Apo-PhuS binds to the PrrH promoter, but this binding is inhibited in the presence of heme. In iron-limited conditions, the equilibrium shifts toward apo-PhuS, which downregulates the relative level of PrrH; while under heme-replete conditions, the equilibrium shifts toward holo-PhuS, leading to the increased expression of PrrH.
Table 4. The new discovered heme-responsive regulators in recent years.
Based on the heme-sensing regulators, various regulatory systems have been developed to synthesize heme and its precursors, and hemoproteins. HrrSA, a two-component heme-responsive system in C. glutamicum, comprises a histidine kinase HrrS and a regulator HrrA [Citation111]. HrrS responds to the extracellular heme by phosphorylating HrrA, which binds to the promoter of the hmuO gene encoding HO (PhmuO) and upregulates its expression. In a previous study, the titer of ALA in C. glutamicum increased by 32.8% using PhmuO to express the ALA exporter RhtA [Citation45]. In addition, another study used a regulator HrtR derived from L. lactis, a hybrid promoter containing HrtR DNA-binding site (hrtOL) and CRISPRi technique to construct a heme-responsive regulatory system in E. coli [Citation31]. The expression of ALAD, PBGD, and FECH was dynamically downregulated using this system, respectively, resulting in the increase of 54.0%, 428.4%, and 65.0% in the titers of ALA, PBG, and porphyrins. Considering the long regulatory period (>6 h) of this CRISPRi-based system for the fine-tuning of heme synthetic pathway and the potential off-target effect of dCas9, a modified heme biosensor (regulatory period of 2–3 h) was developed in E. coli using a mutated HrtR regulator and its binding site hrtOL, and sRNA technique [Citation23]. Using this sensitive system to regulate the enhanced heme biosynthetic pathway, the whole-cell enzymic activity of mutated P450-BM3 increased by 1.4-fold.
The design of pathways to efficiently synthesize heme-derived compounds
As for the production of heme derivatives, although BV and BR, as well as PCB can be extracted from mammalian bile and Spirulina, respectively [Citation24,Citation25,Citation112], these methods suffer from: the shortage of raw materials, unsustainability, difficulty in purification, etc. Hence, the effective biosynthetic approach is selected based on the adequate intracellular supply of heme. In the recent years, with the development of systems and synthetic biology, some novel strategies have been used to enhance the synthetic level of heme-derived compounds ().
Figure 3. The strategies used for enhancing the production of heme derivatives (BV, BR, and PCB). (a) Metabolic engineering for predicting and designing the rate-limiting steps in the BV biosynthetic pathway [Citation24]. MetaCyC: a website (https://metacyc.org/) used for the thermodynamic analysis. (b) Bioconversion of heme into BV and BV into BR in engineered EcN(T7) and E. coli BL21(DE3) strains, respectively [Citation25, Citation30]. ChuA: an outer membrane heme receptor. (c) Modular optimization for the expression of the ho1 and pcyA genes in recombinant E. coli [Citation26, Citation113]. T: T. elongatus; S: Synechocystis. (d) Spatial assembly of HO1 and PcyA by the short peptide tags and DNA scaffolds to optimize substrate trafficking and facilitate metabolic flux [Citation26, Citation113]. RIDD/RIAD: the short peptide tags; ADB1/2: the artificial DNA binding domains. (e) Response surface methodology for optimizing the fermentation conditions of PCB in engineered E. coli strains. (f) Enhancement of PCB biosynthesis for optogenetic control of cell signal pathways in mammalian and B. subtilis [Citation114–116]. Pr: red-absorbing ground state; Pfr: (far-red)-absorbing ground state; Pg: green-absorbing ground state.
![Figure 3. The strategies used for enhancing the production of heme derivatives (BV, BR, and PCB). (a) Metabolic engineering for predicting and designing the rate-limiting steps in the BV biosynthetic pathway [Citation24]. MetaCyC: a website (https://metacyc.org/) used for the thermodynamic analysis. (b) Bioconversion of heme into BV and BV into BR in engineered EcN(T7) and E. coli BL21(DE3) strains, respectively [Citation25, Citation30]. ChuA: an outer membrane heme receptor. (c) Modular optimization for the expression of the ho1 and pcyA genes in recombinant E. coli [Citation26, Citation113]. T: T. elongatus; S: Synechocystis. (d) Spatial assembly of HO1 and PcyA by the short peptide tags and DNA scaffolds to optimize substrate trafficking and facilitate metabolic flux [Citation26, Citation113]. RIDD/RIAD: the short peptide tags; ADB1/2: the artificial DNA binding domains. (e) Response surface methodology for optimizing the fermentation conditions of PCB in engineered E. coli strains. (f) Enhancement of PCB biosynthesis for optogenetic control of cell signal pathways in mammalian and B. subtilis [Citation114–116]. Pr: red-absorbing ground state; Pfr: (far-red)-absorbing ground state; Pg: green-absorbing ground state.](/cms/asset/6f3b8f37-1552-4d08-9d30-767719157690/ibty_a_2291339_f0003_c.jpg)
Regarding the production of BV, a previous study identified the rate-limiting enzymes (GluTR and GSAM) in the heme biosynthetic pathway using thermodynamic analysis and revealed a novel Fe-coproporphyrin III decarboxylase (HemQ)-mediated coproporphyrin dependent pathway in C. glutamicum () [Citation24]. Subsequently, the expression of: GluTRfbr (the feedback-resistant version of GluTR, derived from Salmonella typhimurium), GSAM (derived from E. coli), HemQ, and HmuO (hereafter HO1) was optimized in different plasmids, resulting in the highest reported titer of BV at 68.74 mg/L during 5 L fed-batch fermentation. Next, another study used the engineered EcN(T7) strain overexpressing the ho1 gene to produce BV (BVIXα) with the supplementation of 10.0 μM hemin () [Citation30]. It displayed that the yield of BVIXβ and -δ isomers (26% and 24%, respectively) were improved by over 700-fold and 400-fold than the yield obtained by the chemical coupled oxidation method (0.035% and 0.054%, respectively).
As for the microbial production of BR in recent years, only one research reported the biotransformation of BV using the engineered E. coli strain overexpressing the bvdR gene from cyanobacterium Synechocystis () [Citation25]. Using 450 mg/L of BV as the substrate, it exhibited a conversion rate of 72.3% and a yield of 20.8 mg/g (DCW), which is 35-fold higher than the traditional method extracted from pig bile.
Along with the growing demand for PCB in the: food, pharmaceutical, cosmetic, and optogenetic fields [Citation117], the synthesis of PCB in different organisms has become a hot topic, including: E. coli, Bacillus subtilis, and mammalian cells. In E. coli, a previous study found that monocistronic expression of the ho1 and pcyA genes (derived from Synechocystis sp. PCC6803) could produce 6.64 mg/L PCB, but the upregulation of: GluTR, GSAM, PBGS, PPO, and FECH and the increased copy number of ho1 and pcyA could not significantly further improve the titer of PCB [Citation118]. Another study achieved the PCB titer of 13 mg/L in the engineered E. coli overexpressing ho1 and pcyA (derived from Synechocystis sp. PCC6803) at the: optimized concentration of lactose (4 mmol/L), induction temperature (24.69 °C), induction time (4.6 h), and induction duration (13.57 h) obtained by response surface methodology () [Citation119]. Nevertheless, the PCB titers remain low due to the low expressional level of ho1 and pcyA from Synechocystis sp. and the insufficient supply of the cofactors (NADPH and Fd) for PCB biosynthesis. Therefore, our recent study obtained a higher PCB titer of 28.32 mg/L during 5 L fed-batch fermentation through the spatial assembly of the highly active ho1 and pcyA (ho1 from Thermosynechococcus elongatus and pcyA from Synechocystis sp. PCC6803) using proper DNA scaffolds, the supplementation of 200 mg/L ALA, 20 mg/L FeSO4·7H2O, and 5 g/L vitamin C (reducing agent), and the overexpression of PBGS, FECH, and NadK (NAD kinase) () [Citation26]. Subsequently, the PCB titer was increased to the highest reported record of 147.0 mg/L in our latest study by selecting the better chassis E. coli BL21(DE3), enhancing heme supply using the overexpression of all genes involved in heme biosynthesis, assembling HO1 and PcyA (derived from Synechocystis sp. PCC6803) using the short peptide tags RIAD-RIDD () [Citation113].
Furthermore, the biosynthesis of PCB in B. subtilis and mammalian cells was mainly used as the optogenetic control of cell signaling. The genetically encoded PhyB (phytochrome B)-PIF (phytochrome-interacting factor) LID system for manipulating cellular signaling in mammalian cells was developed based on the optical properties of PCB. PhyB can ligate with PCB to form a photoabsorbing chromophore and can rapidly bind to PIF when exposed to red light, while the PhyB–PIF complex promptly dissociates when exposed to far-red light (). In the previous reported PhyB–PIF LID system, the co-expression of: MTS-PcyA-P2A, MTS-HO1-P2A, MTS-Fd-P2A, and MTS-Fnr (MTS, mitochondria-targeting sequence; P2A, self-cleaving 2A peptide; pcyA and ho1 derived from T. elongatus BP-1; Fd and Fnr derived from Synechocystis sp. PCC 6803) produced a red fluorescent intensity equivalent to 2.5 μM PCB, which was further enhanced by knocking out/or down for BvdR [Citation114]. Subsequently, the PhyB–PIF LID system was improved by the polycistronic co-expression of: MTS-tFnr-Fd-P2A, MTS-PcyA-P2A, and MTS-HO1 (tFnr-Fd, a CpcD-like domain-truncated version of Fnr fused with Fd; pcyA, ho1, Fd, and Fnr from T. elongatus BP-1), increasing the synthesis of PCB by 3.9-fold [Citation115]. In B. subtilis, the binding of Cph1 (a phytochrome from Synechocystis sp. PCC 6803) with PCB resulted in weak red fluorescence. Furthermore, the fusion expression of ho1*-pcyA** (ho1*, codon-optimized initial 15 codons of ho1; pcyA**, codon-optimized complete pcyA with a transcriptional terminator to eliminate potential mRNA instability) led to a significant 80-fold increase in red fluorescence [Citation116]. The enhancement of the PCB synthesis could support the robust function of CcaSR (a transcriptional regulatory pathway driven by optogenetic) in B. subtilis ().
Conclusions and future perspectives
The bioproduction of heme derivatives and hemoproteins depends on the availability of intracellular heme. Over the past five years, various approaches, including systems metabolic engineering, genome-scale metabolic modeling, and pathway reconstruction, have been used to increase intracellular heme levels in different microorganisms, resulting in the successful synthesis of heme derivatives and hemoproteins. However, the titer, yield, and productivity of these products in the recombinant strains are still low for industrial applications, and several challenges are required to be overcome. First, the regulation of heme synthesis varied a lot in different microorganisms, and the effective strategies cannot be universally applied [Citation55]. Second, the heme biosynthetic pathway is long and complex, and the overexpression of all necessary genes can result in the accumulation of heme intermediates rather than heme [Citation61]. Third, the ability of heme uptake is too weak in the commercial microbial hosts and are required to be further enhanced [Citation23]. Fourth, the novel regulatory elements are limited to balance the heme supply and the production of heme derivatives/or hemoproteins in different microorganisms [Citation23, Citation31]. Fifth, the activities of several enzymes related to the synthesis of heme derivatives are relatively low [Citation26, Citation113].
To address these issues, five potential directions can be attempted. At first, the metabolic flux analysis can be used to identify the rate-limiting steps of heme synthesis in diverse microbial hosts [Citation120]. Then, the variants of rate-limiting enzymes with high activities can be obtained through de novo design based on the predictive tools such as AlphaFold or the insertion of nonstandard amino acids [Citation121,Citation122]. In addition, the directed evolution method [Citation123] or the saturation mutagenesis for the key residues of heme‐binding can be used to enhance the capacity of heme importers to absorb exogenous heme. Furthermore, the transcriptomic analysis can be utilized to analyze the transcriptional changes under the different levels of intracellular heme to develop an increasing number of regulatory elements for heme biosynthesis, such as hybrid promoters and DNA adapters. Finally, highly active enzymes for the synthesis of heme derivatives (HO, etc.) can be obtained from algae, microorganisms, plants, and animals by the combination of machine learning and high-throughput screening [Citation124]. By employing these strategies, the production of heme derivatives and hemoproteins can be significantly enhanced through the fine-tuning the enhanced heme biosynthetic pathway.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Shibayama N, Sato-Tomita A, Ohki M, et al. Direct observation of ligand migration within human hemoglobin at work. Proc Natl Acad Sci USA. 2020;117:4741–4748. doi: 10.1073/pnas.1913663117.
- Blumberger J. Electron transfer and transport through multi-heme proteins: recent progress and future directions. Curr Opin Chem Biol. 2018;47:24–31. doi: 10.1016/j.cbpa.2018.06.021.
- Sarkar A, Carter EL, Harland JB, et al. Ferric heme as a CO/NO sensor in the nuclear receptor Rev-Erbβ by coupling gas binding to electron transfer. Proc Natl Acad Sci U S A. 2021;118:e2016717118. doi: 10.1073/pnas.2016717118.
- Jentho E, Ruiz-Moreno C, Novakovic B, et al. Trained innate immunity, long-lasting epigenetic modulation, and skewed myelopoiesis by heme. Proc Natl Acad Sci USA. 2021;118:e2102698118. doi: 10.1073/pnas.2102698118.
- Murray MH, Valfort AC, Koelblen T, et al. Structural basis of synthetic agonist activation of the nuclear receptor REV-ERB. Nat Commun. 2022;13:7131. doi: 10.1038/s41467-022-34892-4.
- Nguyen TA, Park J, Dang TL, et al. Microprocessor depends on hemin to recognize the apical loop of primary microRNA. Nucleic Acids Res. 2018;46:5726–5736. doi: 10.1093/nar/gky248.
- Simsa R, Yuen J, Stout A, et al. Extracellular heme proteins influence bovine myosatellite cell proliferation and the color of cell-based meat. Foods. 2019;8:521. doi: 10.3390/foods8100521.
- Fraser RZ, Shitut M, Agrawal P, et al. Safety evaluation of soy leghemoglobin protein preparation derived from Pichia pastoris, intended for use as a flavor catalyst in plant-based meat. Int J Toxicol. 2018;37:241–262. doi: 10.1177/1091581818766318.
- Xiong L, Li C, Boeren S, et al. Effect of heat treatment on bacteriostatic activity and protein profile of bovine whey proteins. Food Res Int. 2020;127:108688. doi: 10.1016/j.foodres.2019.108688.
- Belcher DA, Lucas A, Cabrales P, et al. Polymerized human hemoglobin facilitated modulation of tumor oxygenation is dependent on tumor oxygenation status and oxygen affinity of the hemoglobin-based oxygen carrier. Sci Rep. 2020;10:11372. doi: 10.1038/s41598-020-68190-0.
- Hu B, Zhao X, Wang E, et al. Efficient heterologous expression of cytochrome P450 enzymes in microorganisms for the biosynthesis of natural products. Crit Rev Biotechnol. 2023;43:227–241. doi: 10.1080/07388551.2022.2029344.
- Singh D, Wasan H, Reeta KH. Heme oxygenase-1 modulation: a potential therapeutic target for COVID-19 and associated complications. Free Radic Biol Med. 2020;161:263–271. doi: 10.1016/j.freeradbiomed.2020.10.016.
- Pentón-Rol G, Marín-Prida J, McCarty MF. C-phycocyanin-derived phycocyanobilin as a potential nutraceutical approach for major neurodegenerative disorders and COVID-19-induced damage to the nervous system. Curr Neuropharmacol. 2021;19:2250–2275. doi: 10.2174/1570159X19666210408123807.
- Kim DH, Ahn HS, Go HJ, et al. Hemin as a novel candidate for treating COVID-19 via heme oxygenase-1 induction. Sci Rep. 2021;11:21462. doi: 10.1038/s41598-021-01054-3.
- Baylor JL, Butler MW. Immune challenge-induced oxidative damage may be mitigated by biliverdin. J Exp Biol. 2019;222:200055. doi: 10.1242/jeb.200055.
- Yao Q, Jiang X, Huang ZW, et al. Bilirubin improves the quality and function of hypothermic preserved islets by its antioxidative and anti-inflammatory effect. Transplantation. 2019;103:2486–2496. doi: 10.1097/TP.0000000000002882.
- Tran DT, Jeong YY, Kim JM, et al. The anti-inflammatory role of bilirubin on "two-hit" sepsis animal model. Int J Mol Sci. 2020;21:8650. doi: 10.3390/ijms21228650.
- Shiels RG, Hewage W, Pennell EN, et al. Biliverdin and bilirubin sulfonate inhibit monosodium urate induced sterile inflammation in the rat. Eur J Pharm Sci. 2020;155:105546. doi: 10.1016/j.ejps.2020.105546.
- Liu Y, Jovcevski B, Pukala TL. C-phycocyanin from Spirulina inhibits α-synuclein and amyloid-β fibril formation but not amorphous aggregation. J Nat Prod. 2019;82:66–73. doi: 10.1021/acs.jnatprod.8b00610.
- Komar AA, Kommer A, Krasheninnikov IA, et al. Cotranslational folding of globin. J Biol Chem. 1997;272:10646–10651. doi: 10.1074/jbc.272.16.10646.
- Weickert MJ, Curry SR. Turnover of recombinant human hemoglobin in Escherichia coli occurs rapidly for insoluble and slowly for soluble globin. Arch Biochem Biophys. 1997;348:337–346. doi: 10.1006/abbi.1997.0410.
- Xue J, Zhou J, Li J, et al. Systematic engineering of Saccharomyces cerevisiae for efficient synthesis of hemoglobins and myoglobins. Bioresour Technol. 2023;370:128556. doi: 10.1016/j.biortech.2022.128556.
- Hu B, Yu H, Zhou J, et al. Whole-cell P450 biocatalysis using engineered Escherichia coli with fine-tuned heme biosynthesis. Adv Sci. 2023;10:e2205580.
- Seok J, Ko YJ, Lee ME, et al. Systems metabolic engineering of Corynebacterium glutamicum for the bioproduction of biliverdin via protoporphyrin independent pathway. J Biol Eng. 2019;13:28. doi: 10.1186/s13036-019-0156-5.
- Mei J, Wu X, Zheng S, et al. Production of bilirubin by biotransformation of biliverdin using recombinant Escherichia coli cells. Bioprocess Biosyst Eng. 2022;45:563–571. doi: 10.1007/s00449-021-02679-4.
- Zhao X, Gao H, Wang Y, et al. Efficient synthesis of phycocyanobilin by combinatorial metabolic engineering in Escherichia coli. ACS Synth Biol. 2022;11:2089–2097. doi: 10.1021/acssynbio.2c00016.
- Smith BJ, Gutierrez P, Guerrero E, et al. Development of a method to produce hemoglobin in a bioreactor culture of Escherichia coli BL21(DE3) transformed with a plasmid containing Plesiomonas shigelloides heme transport genes and modified human hemoglobin genes. Appl Environ Microbiol. 2011;77:6703–6705. doi: 10.1128/AEM.05712-11.
- Fiege K, Frankenberg-Dinkel N. Construction of a new T7 promoter compatible Escherichia coli Nissle 1917 strain for recombinant production of heme-dependent proteins. Microb Cell Fact. 2020;19:190. doi: 10.1186/s12934-020-01447-5.
- Fiege K, Querebillo CJ, Hildebrandt P, et al. Improved method for the incorporation of heme cofactors into recombinant proteins using Escherichia coli Nissle 1917. Biochemistry. 2018;57:2747–2755. doi: 10.1021/acs.biochem.8b00242.
- Robinson EA, Frankenberg-Dinkel N, Xue F, et al. Recombinant production of biliverdin IXβ and δ isomers in the T7 promoter compatible Escherichia coli Nissle. Front Microbiol. 2021;12:787609. doi: 10.3389/fmicb.2021.787609.
- Zhang J, Wang ZG, Su TY, et al. Tuning the binding affinity of heme-responsive biosensor for precise and dynamic pathway regulation. Iscience. 2020;23:101067. doi: 10.1016/j.isci.2020.101067.
- Swenson SA, Moore CM, Marcero JR, et al. From synthesis to utilization: the ins and outs of mitochondrial heme. Cells. 2020;9:579. doi: 10.3390/cells9030579.
- Yang Q, Zhao J, Zheng Y, et al. Microbial synthesis of heme b: biosynthetic pathways, current strategies, detection, and future prospects. Molecules. 2023;28:3633. doi: 10.3390/molecules28083633.
- Tan SI, You SC, Shih IT, et al. Quantification, regulation and production of 5-aminolevulinic acid by green fluorescent protein in recombinant Escherichia coli. J Biosci Bioeng. 2020;129:387–394. doi: 10.1016/j.jbiosc.2019.10.005.
- Ding W, Weng H, Du G, et al. 5-Aminolevulinic acid production from inexpensive glucose by engineering the C4 pathway in Escherichia coli. J Ind Microbiol Biotechnol. 2017;44:1127–1135. doi: 10.1007/s10295-017-1940-1.
- Zou Y, Chen T, Feng L, et al. Enhancement of 5-aminolevulinic acid production by metabolic engineering of the glycine biosynthesis pathway in Corynebacterium glutamicum. Biotechnol Lett. 2017;39:1369–1374. doi: 10.1007/s10529-017-2362-x.
- Yu TH, Yi YC, Shih IT, et al. Enhanced 5-aminolevulinic acid production by co-expression of codon-optimized hemA gene with chaperone in genetic engineered Escherichia coli. Appl Biochem Biotechnol. 2020;191:299–312. doi: 10.1007/s12010-019-03178-9.
- Miscevic D, Mao JY, Kefale T, et al. Strain engineering for high-level 5-aminolevulinic acid production in Escherichia coli. Biotechnol Bioeng. 2021;118:30–42. doi: 10.1002/bit.27547.
- Shih IT, Yi YC, Ng IS. Plasmid-free system and modular design for efficient 5-aminolevulinic acid production by engineered Escherichia coli. Appl Biochem Biotechnol. 2021;193:2858–2871. doi: 10.1007/s12010-021-03571-3.
- Zhu C, Chen J, Wang Y, et al. Enhancing 5-aminolevulinic acid tolerance and production by engineering the antioxidant defense system of Escherichia coli. Biotechnol Bioeng. 2019;116:2018–2028. doi: 10.1002/bit.26981.
- Pu W, Chen J, Zhou Y, et al. Systems metabolic engineering of Escherichia coli for hyper-production of 5‑aminolevulinic acid. Biotechnol Biofuels Bioprod. 2023;16:31. doi: 10.1186/s13068-023-02280-9.
- Zhang J, Weng H, Ding W, et al. N-terminal engineering of glutamyl-tRNA reductase with positive charge arginine to increase 5-aminolevulinic acid biosynthesis. Bioengineered. 2017;8:424–427. doi: 10.1080/21655979.2016.1230572.
- Su T, Guo Q, Zheng Y, et al. Fine-tuning of hemB using CRISPRi for increasing 5-aminolevulinic acid production in Escherichia coli. Front Microbiol. 2019;10:1731. doi: 10.3389/fmicb.2019.01731.
- Ko YJ, You SK, Kim M, et al. Enhanced production of 5-aminolevulinic acid via flux redistribution of TCA cycle toward l-glutamate in Corynebacterium glutamicum. Biotechnol Bioproc E. 2019;24:915–923. doi: 10.1007/s12257-019-0376-z.
- Zhang C, Li Y, Zhu F, et al. Metabolic engineering of an auto-regulated Corynebacterium glutamicum chassis for biosynthesis of 5-aminolevulinic acid. Bioresour Technol. 2020;318:124064. doi: 10.1016/j.biortech.2020.124064.
- Noh MH, Lim HG, Park S, et al. Precise flux redistribution to glyoxylate cycle for 5-aminolevulinic acid production in Escherichia coli. Metab Eng. 2017;43:1–8. doi: 10.1016/j.ymben.2017.07.006.
- Zhang J, Weng H, Zhou Z, et al. Engineering of multiple modular pathways for high-yield production of 5-aminolevulinic acid in Escherichia coli. Bioresour Technol. 2019;274:353–360. doi: 10.1016/j.biortech.2018.12.004.
- Luo Z, Pan F, Zhu Y, et al. Synergistic improvement of 5-aminolevulinic acid production with synthetic scaffolds and system pathway engineering. ACS Synth Biol. 2022;11:2766–2778. doi: 10.1021/acssynbio.2c00157.
- Zhao XR, Choi KR, Lee SY. Metabolic engineering of Escherichia coli for secretory production of free haem. Nat Catal. 2018;1:720–728. doi: 10.1038/s41929-018-0126-1.
- Choi KR, Yu HE, Lee H, et al. Improved production of heme using metabolically engineered Escherichia coli. Biotechnol Bioeng. 2022;119:3178–3193. doi: 10.1002/bit.28194.
- Geng Z, Ge J, Cui W, et al. Efficient de novo biosynthesis of heme by membrane engineering in Escherichia coli. Int J Mol Sci. 2022;23:15524. doi: 10.3390/ijms232415524.
- Ko YJ, Kim M, You SK, et al. Animal-free heme production for artificial meat in Corynebacterium glutamicum via systems metabolic and membrane engineering. Metab Eng. 2021;66:217–228. doi: 10.1016/j.ymben.2021.04.013.
- Ishchuk OP, Domenzain I, Sánchez BJ, et al. Genome-scale modeling drives 70-fold improvement of intracellular heme production in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2022;119:e2108245119. doi: 10.1073/pnas.2108245119.
- Wu J, Jiang M, Kong S, et al. Unveiling the effect of NCgl0580 gene deletion on 5-aminolevulinic acid biosynthesis in Corynebacterium glutamicum. Fermentation. 2023;9:213. doi: 10.3390/fermentation9030213.
- Shao Y, Xue C, Liu W, et al. High-level secretory production of leghemoglobin in Pichia pastoris through enhanced globin expression and heme biosynthesis. Bioresour Technol. 2022;363:127884. doi: 10.1016/j.biortech.2022.127884.
- Yu F, Zhao XR, Zhou JW, et al. Biosynthesis of high-active hemoproteins by the efficient heme-supply Pichia pastoris chassis. Adv Sci. 2023;10:e2302826.
- Ishchuk OP, Frost AT, Muñiz-Paredes F, et al. Improved production of human hemoglobin in yeast by engineering hemoglobin degradation. Metab Eng. 2021;66:259–267. doi: 10.1016/j.ymben.2021.05.002.
- Wang M, Shi Z, Gao N, et al. Sustainable and high-level microbial production of plant hemoglobin in Corynebacterium glutamicum. Biotechnol Biofuels Bioprod. 2023;16:80. doi: 10.1186/s13068-023-02337-9.
- Park HA, Choi KY. α,ω-Oxyfunctionalization of C12 alkanes via whole-cell biocatalysis of CYP153A from Marinobacter aquaeolei and a new CYP from Nocardia farcinica IFM10152. Biochem Eng J. 2020;156:107524. doi: 10.1016/j.bej.2020.107524.
- Park SY, Eun H, Lee MH, et al. Metabolic engineering of Escherichia coli with electron channelling for the production of natural products. Nat Catal. 2022;5:726–737. doi: 10.1038/s41929-022-00820-4.
- Ge J, Wang X, Bai Y, et al. Engineering Escherichia coli for efficient assembly of heme proteins. Microb Cell Fact. 2023;22:59. doi: 10.1186/s12934-023-02067-5.
- Honda Y, Nanasawa K, Fujii H. Coexpression of 5-aminolevulinic acid synthase gene facilitates heterologous production of thermostable cytochrome P450, CYP119, in holo form in Escherichia coli. Chembiochem. 2018;19:2156–2159. doi: 10.1002/cbic.201800331.
- Bielecki M, Antonyuk S, Strange RW, et al. Tannerella forsythia Tfo belongs to Porphyromonas gingivalis HmuY-like family of proteins but differs in heme-binding properties. Biosci Rep. 2018;38:20181325.
- Bielecki M, Antonyuk S, Strange RW, et al. Prevotella intermedia produces two proteins homologous to Porphyromonas gingivalis HmuY but with different heme coordination mode. Biochem J. 2020;477:381–405. doi: 10.1042/BCJ20190607.
- Siemińska K, Cierpisz P, Śmiga M, et al. Porphyromonas gingivalis HmuY and Bacteroides vulgatus Bvu-A novel competitive heme acquisition strategy. Int J Mol Sci. 2021;22:2237. doi: 10.3390/ijms22052237.
- Liu MF, Liu SQ, Huang M, et al. An exposed outer membrane hemin-binding protein facilitates hemin transport by a TonB-dependent receptor in Riemerella anatipestifer. Appl Environ Microbiol. 2021;87:e0036721. doi: 10.1128/AEM.00367-21.
- Balhesteros H, Shipelskiy Y, Long NJ, et al. TonB-dependent heme/hemoglobin utilization by Caulobacter crescentus HutA. J Bacteriol. 2017;199:e00723-16. doi: 10.1128/JB.00723-16.
- Hogle SL, Brahamsha B, Barbeau KA. Direct heme uptake by phytoplankton-associated Roseobacter bacteria. mSystems. 2017;2:e00124-16. doi: 10.1128/mSystems.00124-16.
- Kawano H, Miyamoto K, Yasunobe M, et al. Identification of the heme acquisition system in Vibrio vulnificus M2799. Microb Pathog. 2018;117:100–108. doi: 10.1016/j.micpath.2018.02.022.
- Ramezanalizadeh F, Rasooli I, Owlia P. Protective response against Acinetobacter baumannii with ferric iron receptors HemTR-BauA in a murine sepsis model. Future Microbiol. 2021;16:143–157.
- Bateman TJ, Shah M, Ho TP, et al. A Slam-dependent hemophore contributes to heme acquisition in the bacterial pathogen Acinetobacter baumannii. Nat Commun. 2021;12:6270.
- de Lima VM, Batista BB, da Silva Neto JF. The regulatory protein ChuP connects heme and siderophore-mediated iron acquisition systems required for Chromobacterium violaceum virulence. Front Cell Infect Microbiol. 2022;12:873536. doi: 10.3389/fcimb.2022.873536.
- Cao K, Zhang T, Li N, et al. Identification and tetramer structure of hemin-binding protein SPD_0310 linked to iron homeostasis and virulence of Streptococcus pneumoniae. mSystems. 2022;7:e0022122. doi: 10.1128/msystems.00221-22.
- Jochim A, Adolf L, Belikova D, et al. An ECF-type transporter scavenges heme to overcome iron-limitation in Staphylococcus lugdunensis. Elife. 2020;9:e57322. doi: 10.7554/eLife.57322.
- Aubourg M, Gravey F, Dhalluin A, et al. Identification of the iron-limitation stimulon in Staphylococcus lugdunensis. Arch Microbiol. 2021;203:3687–3694. doi: 10.1007/s00203-021-02342-2.
- Chatterjee N, Cook LCC, Lyles KV, et al. A novel heme transporter from the energy coupling factor family is vital for group A Streptococcus colonization and infections. J Bacteriol. 2020;202:e00205-20. doi: 10.1128/JB.00205-20.
- Verplaetse E, André-Leroux G, Duhutrel P, et al. Heme uptake in Lactobacillus sakei evidenced by a new energy coupling factor (ECF)-like transport system. Appl Environ Microbiol. 2020;86:e02847-19. doi: 10.1128/AEM.02847-19.
- Normant V, Mourer T, Labbé S. The major facilitator transporter Str3 is required for low-affinity heme acquisition in Schizosaccharomyces pombe. J Biol Chem. 2018;293:6349–6362. doi: 10.1074/jbc.RA118.002132.
- Perner J, Hatalova T, Cabello-Donayre M, et al. Haem-responsive gene transporter enables mobilization of host haem in ticks. Open Biol. 2021;11:210048. doi: 10.1098/rsob.210048.
- Cabello-Donayre M, Orrego LM, Herráez E, et al. Leishmania heme uptake involves LmFLVCRb, a novel porphyrin transporter essential for the parasite. Cell Mol Life Sci. 2020;77:1827–1845. doi: 10.1007/s00018-019-03258-3.
- Unlu G, Prizer B, Erdal R, et al. Metabolic-scale gene activation screens identify SLCO2B1 as a heme transporter that enhances cellular iron availability. Mol Cell. 2022;82:2832–2843.e7. doi: 10.1016/j.molcel.2022.05.024.
- Li H, Wang D, Wu H, et al. SLC46A1 contributes to hepatic iron metabolism by importing heme in hepatocytes. Metabolism. 2020;110:154306. doi: 10.1016/j.metabol.2020.154306.
- Bennett MR, Bombardi RG, Kose N, et al. Human mAbs to Staphylococcus aureus IsdA provide protection through both heme-blocking and Fc-mediated mechanisms. J Infect Dis. 2019;219:1264–1273. doi: 10.1093/infdis/jiy635.
- Jelinski J, Terwilliger A, Green S, et al. Progress towards the development of a NEAT vaccine for anthrax II: immunogen specificity and alum effectiveness in an inhalational model. Infect Immun. 2020;88:e00082-20. doi: 10.1128/IAI.00082-20.
- Mitra A, Ko YH, Cingolani G, et al. Heme and hemoglobin utilization by Mycobacterium tuberculosis. Nat Commun. 2019;10:4260. doi: 10.1038/s41467-019-12109-5.
- Medha, Priyanka, Sharma S, et al. Design of a peptide-based vaccine from late stage specific immunogenic cross-reactive antigens of PE/PPE proteins of Mycobacterium tuberculosis. Eur J Pharm Sci. 2022;168:106051. doi: 10.1016/j.ejps.2021.106051.
- Chatterjee N, Huang YS, Lyles KV, et al. Native human antibody to Shr promotes mice survival after intraperitoneal challenge with invasive group A Streptococcus. J Infect Dis. 2021;223:1367–1375. doi: 10.1093/infdis/jiaa540.
- Song YL, Zhang XL, Cai MH, et al. Active and passive immunizations with HtsA, a streptococcal heme transporter protein, protect mice from subcutaneous group A Streptococcus infection. J Microbiol Immunol Infect. 2020;53:87–93. doi: 10.1016/j.jmii.2018.03.002.
- Richard KL, Kelley BR, Johnson JG. Heme uptake and utilization by Gram-negative bacterial pathogens. Front Cell Infect Microbiol. 2019;9:81. doi: 10.3389/fcimb.2019.00081.
- Conroy BS, Grigg JC, Kolesnikov M, et al. Staphylococcus aureus heme and siderophore-iron acquisition pathways. Biometals. 2019;32:409–424. doi: 10.1007/s10534-019-00188-2.
- Valenciano-Bellido S, Caaveiro JMM, Morante K, et al. Structure and role of the linker domain of the iron surface-determinant protein IsdH in heme transportation in Staphylococcus aureus. J Biol Chem. 2022;298:101995. doi: 10.1016/j.jbc.2022.101995.
- Allen CE, Schmitt MP. Utilization of host iron sources by Corynebacterium diphtheriae: multiple hemoglobin-binding proteins are essential for the use of iron from the hemoglobin–haptoglobin complex. J Bacteriol. 2015;197:553–562. doi: 10.1128/JB.02413-14.
- Uluisik RC, Akbas N, Lukat-Rodgers GS, et al. Characterization of the second conserved domain in the heme uptake protein HtaA from Corynebacterium diphtheriae. J Inorg Biochem. 2017;167:124–133. doi: 10.1016/j.jinorgbio.2016.11.027.
- Draganova EB, Akbas N, Adrian SA, et al. Heme binding by Corynebacterium diphtheriae HmuT: function and heme environment. Biochemistry. 2015;54:6598–6609. doi: 10.1021/acs.biochem.5b00666.
- Kornitzer D, Roy U. Pathways of heme utilization in fungi. Biochim Biophys Acta Mol Cell Res. 2020;1867:118817. doi: 10.1016/j.bbamcr.2020.118817.
- Roy U, Kornitzer D. Heme-iron acquisition in fungi. Curr Opin Microbiol. 2019;52:77–83. doi: 10.1016/j.mib.2019.05.006.
- Labbé S, Mourer T, Brault A, et al. Machinery for fungal heme acquisition. Curr Genet. 2020;66:703–711. doi: 10.1007/s00294-020-01067-x.
- Olczak T, Sroka A, Potempa J, et al. Porphyromonas gingivalis HmuY and HmuR: further characterization of a novel mechanism of heme utilization. Arch Microbiol. 2008;189:197–210. doi: 10.1007/s00203-007-0309-7.
- Cabello-Donayre M, Malagarie-Cazenave S, Campos-Salinas J, et al. Trypanosomatid parasites rescue heme from endocytosed hemoglobin through lysosomal HRG transporters. Mol Microbiol. 2016;101:895–908. doi: 10.1111/mmi.13430.
- Luck AN, Yuan X, Voronin D, et al. Heme acquisition in the parasitic filarial nematode Brugia malayi. FASEB J. 2016;30:3501–3514. doi: 10.1096/fj.201600603R.
- Yuan X, Protchenko O, Philpott CC, et al. Topologically conserved residues direct heme transport in HRG-1-related proteins. J Biol Chem. 2012;287:4914–4924. doi: 10.1074/jbc.M111.326785.
- Huynh C, Yuan X, Miguel DC, et al. Heme uptake by Leishmania amazonensis is mediated by the transmembrane protein LHR1. PLoS Pathog. 2012;8:e1002795. doi: 10.1371/journal.ppat.1002795.
- Merli ML, Pagura L, Hernández J, et al. The Trypanosoma cruzi protein TcHTE is critical for heme uptake. PLoS Negl Trop Dis. 2016;10:e0004359. doi: 10.1371/journal.pntd.0004359.
- Liu Z, Huang J, Gu Y, et al. Assembly and evolution of artificial metalloenzymes within E. coli Nissle 1917 for enantioselective and site-selective functionalization of C─H and C=C bonds. J Am Chem Soc. 2022;144:883–890. doi: 10.1021/jacs.1c10975.
- Huang J, Liu Z, Bloomer BJ, et al. Unnatural biosynthesis by an engineered microorganism with heterologously expressed natural enzymes and an artificial metalloenzyme. Nat Chem. 2021;13:1186–1191. doi: 10.1038/s41557-021-00801-3.
- Sawai H, Yamanaka M, Sugimoto H, et al. Structural basis for the transcriptional regulation of heme homeostasis in Lactococcus lactis. J Biol Chem. 2012;287:30755–30768. doi: 10.1074/jbc.M112.370916.
- Knippel RJ, Zackular JP, Moore JL, et al. Heme sensing and detoxification by HatRT contributes to pathogenesis during Clostridium difficile infection. PLoS Pathog. 2018;14:e1007486. doi: 10.1371/journal.ppat.1007486.
- Saillant V, Lipuma D, Ostyn E, et al. A novel Enterococcus faecalis heme transport regulator (FhtR) senses host heme to control its intracellular homeostasis. mBio. 2021;12:e03392-20. doi: 10.1128/mBio.03392-20.
- Wilson T, Mouriño S, Wilks A. The heme-binding protein PhuS transcriptionally regulates the Pseudomonas aeruginosa tandem sRNA prrF1,F2 locus. J Biol Chem. 2021;296:100275. doi: 10.1016/j.jbc.2021.100275.
- Hoang TM, Huang W, Gans J, et al. The heme-responsive PrrH sRNA regulates Pseudomonas aeruginosa pyochelin gene expression. bioRxiv. 2023.
- Keppel M, Piepenbreier H, Gätgens C, et al. Toxic but tasty – temporal dynamics and network architecture of heme-responsive two-component signaling in Corynebacterium glutamicum. Mol Microbiol. 2019;111:1367–1381. doi: 10.1111/mmi.14226.
- Roda-Serrat MC, Christensen KV, El-Houri RB, et al. Fast cleavage of phycocyanobilin from phycocyanin for use in food colouring. Food Chem. 2018;240:655–661. doi: 10.1016/j.foodchem.2017.07.149.
- Wang Y, Li N, Shan X, et al. Enhancement of phycocyanobilin biosynthesis in Escherichia coli by strengthening the supply of precursor and artificially self-assembly complex. Synth Syst Biotechnol. 2023;8:227–234. doi: 10.1016/j.synbio.2023.02.005.
- Uda Y, Goto Y, Oda S, et al. Efficient synthesis of phycocyanobilin in mammalian cells for optogenetic control of cell signaling. Proc Natl Acad Sci U S A. 2017;114:11962–11967. doi: 10.1073/pnas.1707190114.
- Uda Y, Miura H, Goto Y, et al. Improvement of phycocyanobilin synthesis for genetically encoded phytochrome-based optogenetics. ACS Chem Biol. 2020;15:2896–2906. doi: 10.1021/acschembio.0c00477.
- Castillo-Hair SM, Baerman EA, Fujita M, et al. Optogenetic control of Bacillus subtilis gene expression. Nat Commun. 2019;10:3099.
- Pagels F, Guedes AC, Amaro HM, et al. Phycobiliproteins from cyanobacteria: chemistry and biotechnological applications. Biotechnol Adv. 2019;37:422–443. doi: 10.1016/j.biotechadv.2019.02.010.
- Ge BS, Chen Y, Yu Q, et al. Regulation of the heme biosynthetic pathway for combinational biosynthesis of phycocyanobilin in Escherichia coli. Process Biochem. 2018;71:23–30. doi: 10.1016/j.procbio.2018.05.011.
- Ma CB, Li WJ, Ge BS, et al. Biosynthesis of phycocyanobilin in recombinant Escherichia coli. J Ocean Limnol. 2020;38:529–538. doi: 10.1007/s00343-019-9060-6.
- Antoniewicz MR. A guide to metabolic flux analysis in metabolic engineering: methods, tools and applications. Metab Eng. 2021;63:2–12. doi: 10.1016/j.ymben.2020.11.002.
- Jumper J, Evans R, Pritzel A, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2.
- Li H, Wang R, Wang A, et al. Rapidly and precisely cross-linked enzymes using bio-orthogonal chemistry from cell lysate for the synthesis of (S)-1-(2,6-dichloro-3-fluorophenyl) ethanol. ACS Sustain Chem Eng. 2020;8:6466–6478. doi: 10.1021/acssuschemeng.0c00987.
- Packer MS, Liu DR. Methods for the directed evolution of proteins. Nat Rev Genet. 2015;16:379–394. doi: 10.1038/nrg3927.
- Vanella R, Kovacevic G, Doffini V, et al. High-throughput screening, next generation sequencing and machine learning: advanced methods in enzyme engineering. Chem Commun. 2022;58:2455–2467. doi: 10.1039/d1cc04635g.