ABSTRACT
Chronotype or diurnal preference is a questionnaire-based measure influenced both by circadian period and by the sleep homeostat. In order to further characterize the biological determinants of these measures, we used a hypothesis-free approach to investigate the association between the score of the morningness-eveningness questionnaire (MEQ) and the Munich chronotype questionnaire (MCTQ), as continuous variables, and volumetric measures of brain regions acquired by magnetic resonance imaging (MRI). Data were collected from the Baependi Heart Study cohort, based in a rural town in South-Eastern Brazil. MEQ and anatomical 1.5-T MRI scan data were available from 410 individuals, and MCTQ scores were available from a subset of 198 of them. The average MEQ (62.2 ± 10.6) and MCTQ (average MSFsc 201 ± 85 min) scores were suggestive of a previously reported strong general tendency toward morningness in this community. Setting the significance threshold at P > .002 to account for multiple comparisons, we observed a significant association between lower MEQ score (eveningness) and greater volume of the left anterior occipital sulcus (β = −0.163, p = .001) of the occipital lobe. No significant associations were observed for MCTQ. This may reflect the smaller dataset for MCTQ, and/or the fact that MEQ, which asks questions about preferred timings, is more trait-like than the MCTQ, which asks questions about actual timings. The association between MEQ and a brain region dedicated to visual information processing is suggestive of the increasingly recognized fluidity in the interaction between visual and nonvisual photoreception and the circadian system, and the possibility that chronotype includes an element of masking.
Introduction
The average human endogenous period, or tau, is 24.2 h, with marked individual stability and age independence, but inter-individual variability (Czeisler et al. Citation1999). Tau is one of the determinants of the phase angle of entrainment to the external light-dark cycle and, therefore, influences the timing of circadian rhythms. For example, longer tau associates with preferences for activity in the evening and a later sleep onset time in humans (Duffy et al. Citation2001). Chronotype is a concept that refers to such individual differences in the expression of circadian rhythms (Adan et al. Citation2012). Rather than being measured through expensive and intrusive laboratory investigations, it is determined through questionnaires that can be administered to a large number of participants. A number of self-assessment tools have been developed to measure chronotype. Horne and Östberg’s Morningness-eveningness Questionnaire (MEQ) (Horne and Östberg Citation1976) and the Munich Chronotype Questionnaire (MCTQ) (Roenneberg et al. Citation2003) are the two most widely used by some considerable margin (Panjeh et al. Citation2020). The MEQ asks about the respondent’s preferred time to perform physical and intellectual activities, as well as sleep and wake-up times. Scores range from 16 (extreme evening type) to 86 points (extreme morning type). The MCTQ, on the other hand, focuses on actual sleep and wake-up times for work/study and free days; chronotype is calculated based on the mid-sleep phase on free days (corrected for the sleep debt accumulated over the week, MSFsc), ranging from 0 (extreme early chronotype) to 12 h (extreme late chronotype). Reflecting preferred (MEQ) or actual (MCTQ) sleep-wake timing, chronotype presents as a continuum, which may be classified into early, intermediate, and late chronotypes. The dimensionality of the MEQ scale has been probed in relation to the two-process model of sleep regulation (Borbely Citation1982), and found to relate both to the circadian process of regulation (Process C) and the homeostatic process that determines sleep propensity (Process S). Early chronotypes have a faster dissipation of sleep pressure after sleep onset (Mongrain et al. Citation2006) and an accelerated buildup of sleepiness after waking (Mongrain and Dumont Citation2007), as observed in individuals homozygous for the longer allele of the PER3 variable number tandem repeat polymorphism (Viola et al. Citation2007). All chronotype scales show consistent lifespan effects such that eveningness is prevalent in youth, peaking in late adolescence (Roenneberg et al. Citation2004), followed throughout the lifespan with a linear shift toward morningness (Carrier et al. Citation1997; Robilliard et al. Citation2002). Chronotype shows high heritability, ranging between 21% and 52% (von Schantz et al. Citation2015), and genome-wide association studies (GWAS) have associated chronotype with 351 genes to date (Jones et al. Citation2019). Thus, chronotype associates with preferred sleep-wake timing, although wake time, in particular, often has to be reconciled with social demands. This presents particular difficulties for late chronotypes, who experience sleep deprivation and social jetlag when trying to conform to schedules more suited for early chronotypes (Wittmann et al. Citation2006), likely explaining the higher prevalence of metabolic disorders (Yu et al. Citation2015), cardio-vascular disease (Knutson and von Schantz Citation2018), and depression (Drennan et al. Citation1991) observed in late chronotypes.
Circadian rhythmicity is evident in brain activity, with consequences for behavior across the 24 h cycle. Time-of-day effects on glucose metabolism have been found in areas that promote arousal (hypothalamus and brainstem) as well as cortical regions (temporal cortex and occipital lobe) (Buysse et al. Citation2004). Interactions between chronotype, time of day, and task-related brain activity have been shown using fMRI: late chronotypes exhibit higher thalamic and brainstem activity in the evening hours (Schmidt et al. Citation2009, Citation2015). The effects of chronotype on brain activation patterns have important broader implications for the interpretation of functional MRI findings. However, the question of whether chronotype is associated with features of brain structure has received relatively less attention. One study performed on young males (Rosenberg et al. Citation2014) reported evidence of lower white matter integrity in specific fiber tracts of late chronotypes, with chronotype defined categorically by the MCTQ. Other studies have examined the effects on brain volume. Using voxel-based morphometry and a whole-brain approach, Takeuchi and colleagues (Takeuchi et al. Citation2015) assessed correlations between MEQ score and brain region volumes. Eveningness was associated with higher volumes in the precuneus, middle and superior occipital lobe, and reduced volume of the orbitofrontal cortex. Rosenberg and colleagues returned to the issue in a study focusing on cortical thickness as well as regional volumes (Rosenberg et al. Citation2018), utilizing a whole-brain analysis and categorizing chronotype using the MCTQ in 48 young adult males aged 18–35 y of age. The late chronotype group showed greater cortical thickness in the inferior parietal cortex, insula, and prefrontal regions; volumetric findings corroborated those of Takeuchi et al., with late chronotypes, compared to early chronotypes, showing higher volume in the lateral occipital cortex and precuneus areas. Norbury used a hypothesis-driven approach in a large sample (UK Biobank) and focused on the precuneus as a region of interest (ROI), with chronotype based on a single question regarding diurnal preference (Norbury Citation2020). Individuals who reported being a definite evening type had greater gray matter volume in the precuneus ROI, compared to definite morning types. A study with different aims (Horne and Norbury Citation2018) focused only on the hippocampus and showed that the degree of eveningness, measured using the reduced form of the MEQ, correlated with focal atrophy in a specific hippocampal subregion.
Thus, within this limited literature, there appears to be some consistent suggestions of a possible link between eveningness and a higher brain volume in regions within the occipital lobe and precuneus. Other associations might be less robust, although conclusions are hampered by a limited number of studies. For example, Rosenberg et al. (Citation2018) studied only 16 individuals per chronotype group. Also, previous studies have often relied on restricted age ranges. substantially between populations Although Takeuchi et al. used a large sample size (Takeuchi et al. Citation2015), it included young adults, only, as did the studies by Rosenberg et al. The divergent use of chronotype measures, handled as continuous or as categorical variables, is also a limitation within the previously published literature. Although these measures correlate with one another (Zavada et al. Citation2005), it has been argued that they measure constructs that do not entirely overlap. Here, we took advantage of the availability of structural MRI for a subset of the Baependi Heart Study cohort, which is located in a rural town in the Brazilian South-East (Egan et al. Citation2016), and from which chronotype instrument data are also available (Ruiz et al. Citation2020; von Schantz et al. Citation2015). The primary goal of the present study was to explore the brain structural associations of chronotype estimated through both the morningness-eveningness questionnaire and the Munich chronotype questionnaire in a Brazilian population sample.
Materials and methods
Study population
The Baependi Heart Study is an ongoing Brazilian genetic epidemiological cohort study, with a longitudinal family-based design that was established in 2005. Baependi is a small rural town in the State of Minas Gerais in the South-East of Brazil. It has limited inbound migration and a high degree of admixture between European, African, and Native American ancestries (Egan et al. Citation2016). The methodology for recruitment has been described in detail previously (Egan et al. Citation2016). This study protocol conformed to international ethics standards based on the Declaration of Helsinki and was approved by the local ethics committee (Hospital das Clínicas, University of São Paulo, Brazil number 0494/10). Each volunteer provided informed consent before participation. The inclusion criteria for the present analysis include complete MRI scan without image artifacts (image quality and consistency inspected by experts) and having completed at least one chronotype questionnaire (MEQ and MCTQ). No exclusion criteria were applied beyond the standard exclusion criteria (e.g. metal implants, claustrophobia) for suitability for MRI scanning. From the original sample, 410 volunteers met the criteria and were included in the present analysis.
Instruments
Diurnal preference was assessed by the Brazilian Portuguese version of the MEQ (Benedito-Silva et al. Citation1990), a scale containing 19 questions. Chronotype scores that range from 16 to 86 points, with higher scores indicating morningness. MEQ data were collected during the second wave of the study (May 2013 – May 2016). In addition, a subset of volunteers (n = 198) also completed the Brazilian Portuguese version of the MCTQ, an instrument used to assess sleep-wake patterns on workdays and free days. Chronotypes were defined as a function of a phase of sleep, i.e., mid-sleep phase corrected for sleep debt accumulated over the workdays (Roenneberg et al. Citation2003). The collection of MCTQ was performed during a more limited period of the study (January 2016 – November 2018).
MRI acquisition and processing
Data were collected from March 2015 to December 2017. MRI scans were obtained at the Hospital Cônego Monte Raso in Baependi utilizing a 1.5-T MAGNETOM (Siemens, Munich, Germany). A high-resolution T1-weighted structural image was acquired using a three-dimensional fast spoiled gradient echo T1-weighted sequence with the following parameters: Voxel size 1 mm3, 160 slices, Matrix Size 256 × 256, TR 1700 ms, TE 5.1 ms, flip angle 120, inversion time 850 ms. A previous analysis using this dataset to examine associations with metabolic syndrome has recently been published (Alkan et al. Citation2019).
Volumetric analyses
All raw images were visually inspected for quality. Cortical reconstruction and volumetric segmentation were performed using the Freesurfer 6.0.0 image analysis suite (http://surfer.nmr.mgh.harvard.edu). The reconstruction pipeline employed by Freesurfer, which includes intensity normalization, motion correction, and the exclusion of non-brain tissue, was performed using a hybrid watershed/surface deformation procedure. The images are transformed to Talairach space and the subcortical white matter and deep gray matter structures are segmented (Dale et al. Citation1999; Fischl et al. Citation2002) and the subcortical volumetric estimates calculated. The FreeSurfer reconstructions were checked visually for errors, and where necessary the Pial and White Matter Surfaces were manually corrected. In total, scans from three individuals were excluded due to poor image quality or excess movement. Cortical volume estimates were calculated based on the Destrieux atlas (Destrieux et al. Citation2010). In total, volume estimates for 235 (subcortical and cortical) regions were extracted and these were all included in the analyses. To account for differences in head size, we then normalized the calculated volume of each of the 235 selected regions. The volume for each region was divided by the estimated total intracranial volume (ICV) for the participant. These normalized volumes were used as the basis for subsequent analyses.
Statistical analysis
We analyzed each continuous outcome (MEQ and MCTQ scores) as the dependent variables in polygenic linear mixed models. The fixed effects were age, gender, and the volume of brain areas. An additive genetic relationship matrix (kinship matrix) was included in each of the two models we developed to estimate how chronotype is related to the volume of brain areas. To generate the matrix, we used the package kinship2 version 1.8.5 (Sinnwell et al. Citation2014) (https://CRAN.R-project.org/package=kinship2). To run the polygenic linear mixed models taking into account the family structure, we used the package lme4qtl version 0.2.1 (Ziyatdinov et al. Citation2018) (https://github.com/variani/lme4qtl) and Coxme version 2.2–16 (R package) (https://CRAN.R-project.org/package=coxme) (T Therneau). All analyses were performed using the computing environment R version 3.6.3 and Python version 3.7.8.
Results
Sample characteristics
Data were obtained from 410 volunteers (62.9% women) aged 18–87 y (mean ± SD age 46.6, SD 15.4 y) who completed the MRI exam and the MEQ. A subgroup of 198 volunteers (66.2% women) aged 18–73 y (mean age 43.4, SD 13.3 y) also completed the MCTQ. MEQ scores ranged from 22 to 85 (theoretical range: 16–86). The average score in the total population was 62.2 ± 10.6. MEQ score increased as a function of age (r2 = 0.64, p = 2.6 x 10−22). MCTQ score, represented by MSFsc, ranged from 9 min to 591 min. The average mid-sleep phase in the total population was 201 ± 85 min. The distribution of MSFsc within the sample was not normal according to the Shapiro-Wilk test (p = 1.4e−10). The descriptive analysis of the distribution reveals that the median is 193 min, lower and upper quartiles are 147 min and 242 min, respectively. MSFsc changed as a function of age (r2 = 0.42, p = 1.3 x 10−03).
Multiple regression analyses controlling for age, sex, and family structure
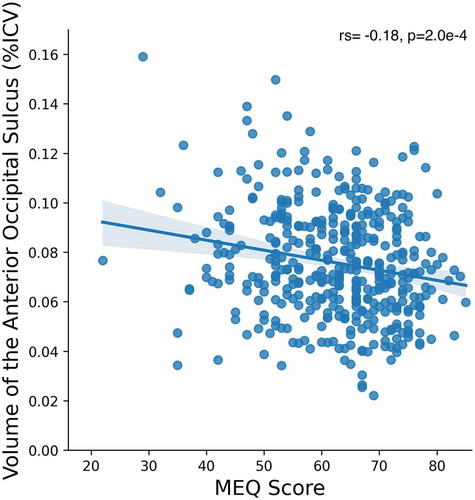
Firstly, we analyzed MEQ and MCTQ scores as dependent variables using polygenic linear mixed models. The ICV-corrected volume of all 235 cortical and subcortical brain regions calculated by Freesurfer were entered as predictors, and age and gender were entered as control variables. The additive genetic relationship matrix was included as a random effect. Considering the correction for multiple comparisons, the significance threshold of the p-value was set at p = .002. Two models were developed: the first using the results from the MEQ and the second one with the results from MCTQ. For MEQ, the volume of the left anterior occipital sulcus (Ih_S_occipital_ant_volume), was found to be the only brain region whose volume explained significant variance in the MEQ score (β = −0.163, p = .001). The relationship between the MEQ score and the volume of the left anterior occipital sulcus was further explored by simple bivariate correlation analysis (rs = −0.18, p = 2.0e−4) (). In contrast, MCTQ score was not significantly associated with volume in any brain region.
Discussion
The current study set out to comprehensively, and without prior hypothesis, explore inter-individual brain structural differences (as measured by regional brain volumes), associated with chronotype. Few previous studies have addressed this question, and this is the first study to include both of the most widely used measures of chronotype. Previous studies have relied solely on one or the other. Choice of scale might be critical given that the MEQ assesses subjective preferences for the timing of activities and is thus closer to a trait-construct of chrono-type (Randler et al. Citation2017), while the MCTQ relies on actual sleep timing, therefore representing more of a state-construct (Roenneberg et al. Citation2019).
This study advances the literature by utilizing a large sample that spans the adult age range, enhancing the generalizability of the findings. Also, analyses of the brain images were based on Freesurfer’s whole-brain cortical parcellation approach. This offers advantages over previous studies that focused only on specific brain regions or used voxel-based morphometry (VBM). FreeSurfer calculates the total volume of a cortical parcellation, while VBM assesses gray matter volume on a voxel-by-voxel basis. VBM is more susceptible to partial volume effects, particularly if anatomically defined masks are not used, which can lead to an overestimation of volume differences (Kennedy et al. Citation2009). Thus, the Freesurfer approach employed here might be more robust.
Notably, we observed a highly significant (p = .001) relationship between MEQ score and volume in the left occipital lobe, specifically and the anterior region of the left occipital cortex, such that greater eveningness correlated with increased volume in this region. The fact that this relationship was highly significant even after correcting for multiple comparisons (accounting for the large number of brain regions included in the model) underscores the strength of the identified association, although an r value of −0.18 equates to a small to medium effect size. Imaging studies often make use of a priori hypotheses and adopt a region of interest approach which relaxes the multiple comparisons constraints on statistical significance. Thus, the significant effect obtained here in spite of a whole-brain, hypo-thesis-free approach, is worthy of note.
Takeuchi et al. found that eveningness in young adults correlated with volume in the middle and superior occipital lobe, although that study was based on VBM (Takeuchi et al. Citation2015). The current study represents an important replication and extension of that being based on regional brain volume parcellation and on a sample spanning the entire adult age range. On the other hand, no significant associations were observed with MCTQ. This conflicts with a previous study using categorized MCTQ chronotype in young adults, which found higher gray matter volume in the lateral occipital cortex and precuneus of late chronotypes (Rosenberg et al. Citation2018). It is plausible that the chronotype measured by MCTQ (which asks questions about actual timings) is closer to a state construct, as argued by Roenneberg and colleagues who devised the MCTQ (Roenneberg et al. Citation2019). In contrast, the MEQ (which asks questions about preferred timings) has more trait-like qualities (Randler et al. Citation2017). However, it is important to note MCTQ data were only available for about half of the study sample. This is a limitation of the current work, as indeed is the use of questionnaire data rather than objective measures of circadian period or phase (which would not be feasible within a larger population sample). The limitation of exclusion criteria to the ability to undergo an MRI scan and respond to the questionnaires may be viewed as a weakness. Previous publications have often applied exclusion criteria such as psychiatric conditions. However, the distinctively asymmetric distribution of multiple morbidities across the chronotype spectrum means that this feature of the current sample could equally be viewed as a strength.
Our finding suggests that the constructs captured by the MEQ scale have the potential to yield more detailed information on anatomical associations in studies with larger participant numbers and higher scanner resolution. However, while the MEQ scale’s interaction with gender (Duarte et al. Citation2014) and age (Carrier et al. Citation1997; Robilliard et al. Citation2002) are linear, the way in which the distribution curve of the MEQ score is centered varies quite substantially between populations (von Schantz et al. Citation2015), making pooling of different population samples problematic. On the other hand, replication studies in populations drawn from other geographical locations can now be attempted using a hypothesis-driven, region of interest approach, to further explore the findings presented here.
The anterior occipital sulcus, also known as the preoccipital sulcus (sulcus preoccipitalis) or the ascending branch of the inferior temporal sulcus, is a superficial anatomical feature at the border with the temporal lobe; in the majority of cases, it is a continuation of inferior temporal sulcus (Malikovic et al. Citation2012). Although the anterior occipital sulcus has been suggested to contain a region specialized for motion detection (V5/MT+) (Malikovic et al. Citation2007), it likely incorporates regions specialized for other higher-order visual functions as well. As such, its involvement will be upstream of the optic radiation from retinal ganglion cells to the primary visual cortex via the lateral geniculate nucleus, and unconnected to the retino-hypothalamic pathway from intrinsically photosensitive retinal ganglion cells (ipRGCs) to the suprachiasmatic nucleus. How, then, might a size difference in a cortical surface area upstream of the visual cortex relate to diurnal preference/chronotype, the main biological substrates of which, Process C and Process S, are correlated to deep-lying areas of the brain? One possibility is that the effect of chronotype is connected to masking. This term is used to describe the acute behavioral response that modulates the circadian output signal in response to events that the circadian oscillator had not predicted (Mrosovsky Citation1999; Rietveld et al. Citation1993). It has been suggested based on observations in diurnal rodent models that different “chronotypes” (here understood as animals exhibiting diurnal or nocturnal behavior) exhibit different masking responses (Langel et al. Citation2014; Vivanco et al. Citation2010). We hypothesize that masking is also associated with higher cortical areas receiving input from the visual cortex. Masking is generally viewed as a phenomenon that is principally generated by the hypothalamus. Lesions of the suprachiasmatic nuclei (SCN) have been demonstrated to impair masking responses in Syrian golden hamsters (Li et al. Citation2005). However, lesions in the lateral geniculate nuclei (LGN), the gateway to the visual cortex, in mice abolished positive masking in response to dim light (Edelstein and Mrosovsky Citation2001), while ablation of the visual cortex was found to enhance negative masking (Redlin et al. Citation2003). Our findings suggest the possibility that daily or frequent positive masking, as experienced by late chronotype or shift workers, could lead to expansion in the size of the post-visual cortical areas. The sensory input to such cortical areas, mediated by the visual cortex, could be informed by signals either from classical visual photoreceptors (rods and cones) or nonvisual photoreceptors, and/or by (intrinsically photosensitive ganglion cells, ipRGCs), which are now known to contribute to subjective vision, influencing perception of changes in scene brightness and low-frequency patterns (Lucas et al. Citation2020).
Late chronotypes have a stronger blue-light ipRGC response (Van Der Meijden et al. Citation2016). Since ipRGC responses influence occipital activity (Vandewalle et al. Citation2018), this could contribute to the structural differences identified here. Furthermore, fMRI findings reveal significant circadian modulation of occipital cortex activity. Occipital activity is modulated to compensate for lower visual signal quality at dawn and dusk (Cordani et al. Citation2018). Activation patterns show significant interactions between circadian signals and sleep debt, specifically in occipital areas (Muto et al. Citation2016). Inconsistent sleep timing has also been linked to reduced occipital cortex activity during an attention task (Zhang et al. Citation2020). Thus, evidence points to altered input and activation patterns within the occipital cortex of late chronotypes. These could underlie the observed structural differences: input to, and activity within, the occipital cortex is critical for shaping its structural properties during neural development, and plasticity in this region also persists into adulthood (Castaldi et al. Citation2020). Thus, the chronotype-related volumetric differences identified here could potentially be explained on this basis. In late chronotypes, a long-term pattern of daily or near-daily masking in visual cortex activation could also contribute. The unilaterality of the association is unexpected but may relate to the equally unexpected observation that prior exposure to melanopic light has an asymmetric effect on light-evoked right-hemisphere parieto-occipital α-power (Newman et al. Citation2016), leaving open the question of whether other types of responses to melanopic light also have unilateral effects.
In conclusion, the current study revealed a modest but persistent association between the MEQ score and brain volume, localized to a region of the left occipital cortex. This both confirms and builds on previous findings. This study is the first to utilize both the MEQ and MCTQ to explore chronotype and brain structure; results suggest that the more trait-like MEQ might be the more sensitive instrument for structural MRI investigations. The fact that the structural association with diurnal preference was restricted to a specific occipital region suggests an involvement of post-visual mechanisms that could provide hypotheses for future human and animal studies on the biological basis of chronotype.
Additional information
Funding
References
- Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, Randler C. 2012. Circadian typology: a comprehensive review. Chronobiol Int. 29(9):1153–1175. doi:10.3109/07420528.2012.719971
- Alkan E, Taporoski TP, Sterr A, von Schantz M, Vallada H, Krieger JE, Pereira AC, Alvim R, Horimoto ARVR, Pompéia S, et al. 2019. Metabolic syndrome alters relationships between cardiometabolic variables, cognition and white matter hyperintensity load. Sci Rep. 9(1):4356. doi:10.1038/s41598-019-40630-6
- Benedito-Silva AA, Menna-Barreto L, Marques N, Tenreiro S. 1990. A self-assessment questionnaire for the determination of morningness-eveningness types in Brazil. Prog Clin Biol Res. 341B:89–98.
- Borbely AA. 1982. A two process model of sleep regulation. Hum Neurobiol. 1(3):195–204.
- Buysse DJ, Nofzinger EA, Germain A, Meltzer CC, Wood A, Ombao H, Kupfer DJ, Moore RY. 2004. Regional brain glucose metabolism during morning and evening wakefulness in humans: preliminary findings. Sleep. 27(7):1245–1254. doi:10.1093/sleep/27.7.1245
- Carrier J, Monk TH, Buysse DJ. 1997. Sleep and morningness‐eveningness in the “middle”years of life (20–59y). J Sleep Res. 6:230–237. doi:10.1111/j.1365-2869.1997.00230.x
- Castaldi E, Lunghi C, Morrone MC. 2020. Neuroplasticity in adult human visual cortex. Neurosci Biobehav Rev. 112:542–552. doi:10.1016/j.neubiorev.2020.02.028
- Cordani L, Tagliazucchi E, Vetter C, Hassemer C, Roenneberg T, Stehle JH, Kell CA. 2018. Endogenous modulation of human visual cortex activity improves perception at twilight. Nat Commun. 9(1):1274. doi:10.1038/s41467-018-03660-8
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, et al. 1999. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 284(5423):2177–2181. doi:10.1126/science.284.5423.2177
- Dale AM, Fischl B, Sereno MI. 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 9(2):179–194. doi:10.1006/nimg.1998.0395
- Destrieux C, Fischl B, Dale A, Halgren E. 2010. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 53(1):1–15. doi:10.1016/j.neuroimage.2010.06.010
- Drennan MD, Klauber MR, Kripke DF, Goyette LM. 1991. The effects of depression and age on the Horne-Östberg morningness-eveningness score. J Affect Disord. 23(2):93–98. doi:10.1016/0165-0327(91)90096-b
- Duarte LL, Menna-Barreto L, Miguel MAL, Louzada F, Araújo J, Alam M, Areas R, Pedrazzoli M. 2014. Chronotype ontogeny related to gender. Braz J Med Biol Res. 47(4):316–320. doi:10.1590/1414-431x20143001
- Duffy JF, Rimmer DW, Czeisler CA. 2001. Association of intrinsic circadian period with morningness–eveningness, usual wake time, and circadian phase. Behav Neurosci. 115(4):895–899. doi:10.1037/0735-7044.115.4.895
- Edelstein K, Mrosovsky N. 2001. Behavioral responses to light in mice with dorsal lateral geniculate lesions. Brain Res. 918(1–2):107–112. doi:10.1016/s0006-8993(01)02966-3
- Egan KJ, von Schantz M, Negrão AB, Santos HC, Andréa RV, Duarte NE, Gonçalves GC, Soler JMP, de Andrade M, Lorenzi-Filho G, et al. 2016. Cohort profile: the Baependi Heart Study—a family-based, highly admixed cohort study in a rural Brazilian town. BMJ Open. 6(10):e011598. doi:10.1136/bmjopen-2016-011598
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, et al. 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 33(3):341–355. doi:10.1016/s0896-6273(02)00569-x
- Horne CM, Norbury R. 2018. Exploring the effect of chronotype on hippocampal volume and shape: a combined approach. Chronobiol Int. 35(7):1027–1033. doi:10.1080/07420528.2018.1455056
- Horne JA, Östberg O. 1976. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 4(2):97–110.
- Jones SE, Lane JM, Wood AR, Van Hees VT, Tyrrell J, Beaumont RN, Jeffries AR, Dashti HS, Hillsdon M, Ruth KS, et al. 2019. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun. 10(1):343. doi:10.1038/s41467-018-08259-7
- Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, Acker JD, Raz N. 2009. Age-related differences in regional brain volumes: a comparison of optimized voxel-based morphometry to manual volumetry. Neurobiol Aging. 30(10):1657–1676. doi:10.1016/j.neurobiolaging.2007.12.020
- Knutson KL, von Schantz M. 2018. Associations between chronotype, morbidity and mortality in the UK Biobank cohort. Chronobiol Int. 35(8):1045–1053. doi:10.1080/07420528.2018.1454458
- Langel J, Yan L, Nunez AA, Smale L. 2014. Behavioral masking and cFos responses to light in day- and night-active grass rats. J Biol Rhythms. 29(3):192–202. doi:10.1177/0748730414533289
- Li X, Gilbert J, Davis FC. 2005. Disruption of masking by hypothalamic lesions in Syrian hamsters. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 191(1):23–30. doi:10.1007/s00359-004-0569-5
- Lucas RJ, Allen AE, Milosavljevic N, Storchi R, Woelders T. 2020. Can We See with Melanopsin? Annu Rev Vis Sci. http://dx.doi.org/10.1146/annurev-vision-030320-041239
- Malikovic A, Amunts K, Schleicher A, Mohlberg H, Eickhoff SB, Wilms M, Palomero-Gallagher N, Armstrong E, Zilles K. 2007. Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT+: a probabilistic, stereotaxic map of area hOc5. Cereb Cortex. 17(3):562–574. doi:10.1093/cercor/bhj181
- Malikovic A, Vucetic B, Milisavljevic M, Tosevski J, Sazdanovic P, Milojevic B, Malobabic S. 2012. Occipital sulci of the human brain: variability and morphometry. Anat Sci Int. 87(2):61–70. doi:10.1007/s12565-011-0118-6
- Mongrain V, Carrier J, Dumont M. 2006. Difference in sleep regulation between morning and evening circadian types as indexed by antero-posterior analyses of the sleep EEG. Eur J Neurosci. 23(2):497–504. doi:10.1111/j.1460-9568.2005.04561.x
- Mongrain V, Dumont M. 2007. Increased homeostatic response to behavioral sleep fragmentation in morning types compared to evening types. Sleep. 30(6):773–780. doi:10.1093/sleep/30.6.773
- Mrosovsky N. 1999. Masking: history, definitions, and measurement. Chronobiol Int. 16(4):415–429. doi:10.3109/07420529908998717
- Muto V, Jaspar M, Meyer C, Kussé C, Chellappa SL, Degueldre C, Balteau E, Shaffii-Le Bourdiec A, Luxen A, Middleton B, et al. 2016. Local modulation of human brain responses by circadian rhythmicity and sleep debt. Science. 353(6300):687–690. doi:10.1126/science.aad2993
- Newman DP, Lockley SW, Loughnane GM, Martins ACP, Abe R, Zoratti MTR, Kelly SP, O’Neill MH, Rajaratnam SMW, O’Connell RG, et al. 2016. Ocular exposure to blue-enriched light has an asymmetric influence on neural activity and spatial attention. Sci Rep. 6:27754. doi:10.1038/srep27754
- Norbury R. 2020. Diurnal preference and grey matter volume in a large population of older adults: data from the UK Biobank. J Circadian Rhythms. 18:3. doi:10.5334/jcr.193
- Panjeh S, Pompeia S, Archer SN, Pedrazzoli M, von Schantz M, Cogo-Moreira H. 2020. What are we measuring with the morningness-eveningness questionnaire? Exploratory factor analysis across four samples from two countries. Chronobiol Int. 1–14. doi:10.1080/07420528.2020.1815758
- Randler C, Faßl C, Kalb N. 2017. From Lark to Owl: developmental changes in morningness-eveningness from new-borns to early adulthood. Sci Rep. 7(1):45874. doi:10.1038/srep45874
- Redlin U, Cooper HM, Mrosovsky N. 2003. Increased masking response to light after ablation of the visual cortex in mice. Brain Res. 965(1–2):1–8. doi:10.1016/s0006-8993(02)03844-1
- Rietveld WJ, Minors DS, Waterhouse JM. 1993. Circadian rhythms and masking: an overview. Chronobiol Int. 10(4):306–312. doi:10.1080/07420529309059713
- Robilliard DL, Archer SN, Arendt J, Lockley SW, Hack LM, English J, Leger D, Smits MG, Williams A, Skene DJ, et al. 2002. The 3111 Clock gene polymorphism is not associated with sleep and circadian rhythmicity in phenotypically characterized human subjects. J Sleep Res. 11(4):305–312. doi:10.1046/j.1365-2869.2002.00320.x
- Roenneberg R, Pilz P, Zerbini Z, Winnebeck W. 2019. Chronotype and Social Jetlag: a (Self-) Critical Review. Biology. 8(3):54. doi:10.3390/biology8030054
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. 2004. A marker for the end of adolescence. Curr Biol. 14(24):R1038–9. doi:10.1016/j.cub.2004.11.039
- Roenneberg T, Wirz-Justice A, Merrow M. 2003. Life between clocks: daily temporal patterns of human chronotypes. Journal of Biological Rhythms. 18(1):80–90. doi:10.1177/0748730402239679
- Rosenberg J, Jacobs HIL, Maximov II, Reske M, Shah NJ. 2018. Chronotype differences in cortical thickness: grey matter reflects when you go to bed. Brain Struct Funct. 223(7):3411–3421. doi:10.1007/s00429-018-1697-y
- Rosenberg J, Maximov II, Reske M, Grinberg F, Shah NJ. 2014. “Early to bed, early to rise”: diffusion tensor imaging identifies chronotype-specificity. Neuroimage 84:428–434. doi:10.1016/j.neuroimage.2013.07.086
- Ruiz FS, Beijamini F, Beale AD, Gonçalves BDSB, Vartanian D, Taporoski TP, Middleton B, Krieger JE, Vallada H, Arendt J, et al. 2020. Early chronotype with advanced activity rhythms and dim light melatonin onset in a rural population. Journal of Pineal Research. 69(3):e12675. doi:10.1111/jpi.12675
- Schmidt C, Collette F, Leclercq Y, Sterpenich V, Vandewalle G, Berthomier P, Berthomier C, Phillips C, Tinguely G, Darsaud A, et al. 2009. Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area. Science. 324(5926):516–519. doi:10.1126/science.1167337
- Schmidt C, Collette F, Reichert CF, Maire M, Vandewalle G, Peigneux P, Cajochen C. 2015. Pushing the limits: chronotype and time of day modulate working memory-dependent cerebral activity. Front Neurol. 6:199. doi:10.3389/fneur.2015.00199
- Sinnwell JP, Therneau TM, Schaid DJ. 2014. The kinship2 R package for pedigree data. Human Heredity. 78(2):91–93. doi:10.1159/000363105
- Takeuchi H, Taki Y, Sekiguchi A, Nouchi R, Kotozaki Y, Nakagawa S, Miyauchi CM, Iizuka K, Yokoyama R, Shinada T, et al. 2015. Regional gray matter density is associated with morningness–eveningness: evidence from voxel-based morphometry. Neuroimage. 117:294–304. doi:10.1016/j.neuroimage.2015.05.037
- Van Der Meijden WP, Van Someren JL, Te Lindert BHW, Bruijel J, Van Oosterhout F, Coppens JE, Kalsbeek A, Cajochen C, Bourgin P, Van Someren EJW. 2016. Individual differences in sleep timing relate to melanopsin-based phototransduction in healthy adolescents and young adults. sleep. 39(6):1305–1310. doi:10.5665/sleep.5858
- Vandewalle G, Van Ackeren MJ, Daneault V, Hull JT, Albouy G, Lepore F, Doyon J, Czeisler CA, Dumont M, Carrier J, et al. 2018. Light modulates oscillatory alpha activity in the occipital cortex of totally visually blind individuals with intact non-image-forming photoreception. Sci Rep. 8(1):16968. doi:10.1038/s41598-018-35400-9
- Viola AU, Archer SN, James LM, Groeger JA, Lo JCY, Skene DJ, von Schantz M, Dijk D-J. 2007. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. 17(7):613–618. doi:10.1016/j.cub.2007.01.073
- Vivanco P, Rol MÁ, Madrid JA. 2010. Pacemaker phase control versus masking by light: setting the circadian chronotype in dual octodon degus. Chronobiol Int. 27(7):1365–1379. doi:10.3109/07420528.2010.502984
- von Schantz M, Taporoski TP, Horimoto ARVR, Duarte NE, Vallada H, Krieger JE, Pedrazzoli M, Negrão AB, Pereira AC. 2015. Distribution and heritability of diurnal preference (chronotype) in a rural Brazilian family-based cohort, the Baependi study. Sci Rep. 5(1):9214. doi:10.1038/srep09214
- Wittmann M, Dinich J, Merrow M, Roenneberg T. 2006. Social jetlag: misalignment of biological and social time. Chronobiol Int. 23(1–2):497–509. doi:10.1080/07420520500545979
- Yu JH, Yun C-H, Ahn JH, Suh S, Cho HJ, Lee SK, Yoo HJ, Seo JA, Kim SG, Choi KM, et al. 2015. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J Clin Endocrinol Metab. 100(4):1494–1502. doi:10.1210/jc.2014-3754
- Zavada A, Gordijn MCM, Beersma DGM, Daan S, Roenneberg T. 2005. Comparison of the munich chronotype questionnaire with the Horne-Östberg’s morningness-eveningness score. Chronobiol Int. 22(2):267–278. doi:10.1081/CBI-200053536
- Zhang R, Tomasi D, Shokri-Kojori E, Wiers CE, Wang G-J, Volkow ND 2020. Sleep inconsistency between weekends and weekdays is associated with changes in brain function during task and rest. Sleep 43(10). doi:10.1093/sleep/zsaa076
- Ziyatdinov A, Vázquez-Santiago M, Brunel H, Martinez-Perez A, Aschard H, Soria JM. 2018. lme4qtl: linear mixed models with flexible covariance structure for genetic studies of related individuals. BMC Bioinform. 19(1):68. doi:10.1186/s12859-018-2057-x