Abstract
Background: The visceral adiposity index (VAI) is a mathematical tool that reflects a patient’s visceral adiposity and insulin resistance. Recent studies have noted an association between VAI and cardiovascular event. We analyzed the association between VAI and coronary artery calcium score (CACS) in Korean adults.
Methods: For 33,468 participants (mean age 42 yrs) in a health screening program, VAI was calculated using the following formulae: [waist circumference (WC)/{39.68 + (1.88 * body mass index (BMI))}] * (triglyceride/1.03) * {1.31/high-density lipoprotein cholesterol (HDL-C)} for men and [WC/{36.58 + (1.89 * BMI)}] * (triglyceride/0.81) * (1.52/HDL-C) for women. Coronary artery calcium scores were measured with multi-detector computed tomography.
Results: CACS was positively correlated with VAI (r = 0.027, p < 0.001). Subjects with 0 < CACS <100 and CACS ≥ 100 had significantly higher VAI compared to those with CACS = 0 (2.04 ± 1.97, 2.08 ± 1.67 vs. 1.68 ± 1.50, p < 0.001). In logistic regression analyses with CACS >0 as the dependent variable, subjects in the highest tertile of VAI (>1.777) had significantly increased odds ratio for CACS >0 compared to subjects in the lowest tertile (<0.967), even after adjusting for confounding variables, including BMI (OR 1.26, 95% CI 1.147–1.381).
Conclusions: Subjects with high VAI had increased risk for subclinical atherosclerosis, as assessed by CACS.
Recent studies have noted an association between visceral adiposity index (VAI) and cardiovascular event.
Subjects with coronary artery calcification (CAC) showed significantly higher VAI compared to those without CAC.
The subjects with high VAI showed increased odds ratio for CAC as compared to subjects with low VAI, suggesting high VAI reflects increased risk for subclinical atherosclerosis
Key messages
Introduction
The absolute risk of cardiovascular disease (CVD) is directly connected to well-established risk factors, such as hypertension, diabetes, age, smoking, etc. Better global risk assessment systems, such as so-called cardiometabolic risk, have emerged to quantify CVD risk because abdominal obesity and insulin resistance-related metabolic markers are also thought to be causal risk factors of CVD (Citation1–4).
Visceral obesity, the most prevalent manifestation of metabolic syndrome, is a marker of dysfunctional adipose tissue and ectopic fat infiltration (Citation5–8). Visceral obesity is related to increase in adipocytokines, proinflammatory activity, exacerbation of insulin sensitivity, diabetes risk, dyslipidemia with high triglyceride (TG)/low high-density lipoprotein cholesterol (HDL-C), hypertension, atherosclerosis, and mortality (Citation9–12). Therefore, measuring visceral adiposity is important in individuals who are at risk for CVD. Computed tomography (CT) scan and magnetic resonance imaging (MRI) at the umbilical level are the standard techniques used to measure visceral fat accumulation (Citation13–18). Several surrogate markers have been routinely used as indicators of visceral adipose function. Increased waist circumference (WC) and increased waist-to-hip ratio, which are the indicators of central obesity, have been used as the predictors of obesity-related metabolic abnormalities, and are more accurate than body mass index (BMI) (Citation19).
Recently, the visceral adipose index (VAI) was developed as a sex-specific mathematical scoring system that uses simple, classical anthropometric (BMI and WC) and metabolic parameters (TG and HDL-C) to identify insulin resistance and cardiometabolic risk (Citation20). In several studies, VAI has been found to be independently associated with visceral adiposity, making it a useful substitute for visceral CT scan (Citation20–22). One study reported that VAI is a reliable indicator of visceral fat function and insulin sensitivity (Citation22). Some studies have reported that VAI is a valuable indicator of visceral adipose function, insulin sensitivity, and cardiometabolic risk (Citation20,Citation23,Citation24). Another study found that higher VAI was positively associated with pre-hypertension and hypertension in both men and women (Citation25).
Coronary artery calcium score (CACS), calculated from computed tomography scans, correlates closely with plaque burden and is a reliable surrogate marker of atherosclerosis (Citation25,Citation26). CACS measurement improves risk prediction for cardiovascular events and mortality beyond traditional risk factors and outperforms other risk markers. However, there are no studies that have examined the association of VAI with subclinical atherosclerosis as determined by CACS. Herein, we evaluate the association between VAI and CACS in apparently healthy Korean subjects in order to determine whether VAI is a reliable indicator of subclinical atherosclerosis.
Materials and methods
Study participants
This is a cross-sectional study of subjects from the Kangbuk Samsung Health Study, a large database of a medical health check-up program at the Health Promotion Center of Kangbuk Samsung Hospital, Sungkyunkwan University, Seoul, Korea. The purpose of the program is to promote the health of employees of the industrial companies through a regular health check-up and to improve early detection of existing diseases.
Of the 34,462 subjects who participated in the medical check-up program between January 2010 and December 2012, we excluded subjects with a self-reported history of ischemic heart disease (n = 419) or ischemic stroke (n = 270) and subjects who were using aspirin (n = 38) or a statin (n = 1,249). Final analyses were performed in 33,468 subjects (26,836 men and 6,632 women) who had a mean age of 41.5 years.
Participants provided written informed consent to use their medical check-up data in the research. The design, protocol, and the consent procedure were reviewed and approved by the Institutional Review Board of Kangbuk Samsung Hospital (KBS12089) and are in accordance with the Helsinki Declaration of 1975.
Anthropometric and laboratory measurements
Height and weight were measured by experienced nurses while the subjects were wearing lightweight gowns. Each participant’s WC was measured at the midpoint between the top of the iliac crest and the lower border of the last palpable rib. BMI was calculated by dividing the participant’s weight (kg) by the square of height (m). Blood pressure was measured using a standardized sphygmomanometer after the patient had been at rest for 5 min. Systolic blood pressure and diastolic blood pressure were measured three times with the participant in a seated position, with 1 min of rest between each measurement. The average of the second and third measurements was used in our analyses.
Blood samples were collected from the antecubital vein after an overnight fast. The hexokinase method was used to test fasting glucose concentrations (Modular D2400; Hitachi, Tokyo, Japan). Fasting insulin concentrations were determined using an electrochemiluminescence immunoassay (Modular E170; Hitachi). An enzymatic calorimetric test was used to measure total cholesterol and TG concentrations. The selective inhibition method was used to measure HDL-C levels, and a homogeneous enzymatic calorimetric test was used to measure low-density lipoprotein cholesterol (LDL-C) levels.
Lifestyle habits were assessed by self-report. A patient was classified as a smoker if he or she had smoked at least five packs of cigarettes in his or her lifetime. If a patient drank more than 20 g of alcohol daily, he or she was classified as an alcohol drinker. Having a regular exercise habit was defined as exercising with at least moderate intensity at least three times every week.
Diabetes mellitus status was determined by self-report, fasting blood glucose level, and glycated hemoglobin (HbA1c) level, according to the American Diabetes Association guidelines (Citation27).
Definition of VAI score
The VAI was calculated using the following sex-specific formula (Citation24):
Subjects were divided into tertiles according to VAI: <0.967, 0.967–1.777, >1.777.
Measurement of CACS
Multi-detector computed tomography (MDCT) for coronary calcium scoring was calculated from images generated from a 64-slice, spiral computed tomograph (GE Healthcare, Little Chalfont, Buckinghamshire, UK) and using HeartBeat-CS software (Philips, Amsterdam, The Netherlands). The 64-slice MDCT followed this protocol: 0.625 mm slice thickness, 120kVP, 800 effective mAs, and 400ms rotational speed. Severity of coronary artery calcification was assessed using the Agatston score (Citation28). Total CACS was determined based on the sum of the individual scores of four major epicardial coronary arteries: left main, left anterior descending, left circumflex, and right coronary artery. The technicians who performed MDCT were blinded to all patient information, and CACS was automatically detected with the HeartBeat-CS software.
The presence of coronary artery calcification was defined as CACS >0 and was further subdivided by severity: 0 < CACS <100 or CACS ≥100. CACS was analyzed in natural logarithm form plus 1: ln(CACS +1).
Statistical analysis
All data were analyzed using SPSS for Windows, version 18.0 (IBM, Armonk, NY). Bivariate correlation analyses were performed to identify associations between VAI and candidate independent variables; as all the variables including CACS did not show normal distribution and were skewed, Spearman’s correlation analysis was used to analyze the correlations. As for CACS, since 85.9% of the participants had CACS of zero, natural logarithmic transformation of CACS +1 was used for the bivariate correlation analyses instead of raw CACS values (Citation29–31). Student’s t-test was used to compare the mean values of the parameters for subjects with and without CACS >0. Comparisons of the proportion of subjects in the groups with and without CACS >0 and of the proportion of subjects with CACS >0 among the VAI tertiles were performed using the Chi-square test. Logistic regression analyses were performed with CACS >0 as the dependent variable after adjusting for age and sex in the model 1. In the model 2, the analysis was performed with adjustment for age, sex, total cholesterol, systolic blood pressure, smoking, fasting glucose and fasting insulin. In the model 3, the analysis was performed with adjustment for the variables in the model 2 plus TG and HDL-C, which are the lipid parameters for dyslipidemia. In the final model, the analysis was performed with the adjustment for the variables in the model 3 plus BMI, which is the most influential variable.
Results
shows the general and metabolic characteristics of all study participants (n = 33,468). The mean participant age was 41.5 years, 80.2% of total participants were men, and the mean BMI was 24.3 kg/m2. The mean VAI was 1.73 ± 1.58 and the mean CACS was 11.2 ± 72.0.
Table 1. General characteristics of all participants and comparisons among groups with tertiles of VAI.
When the mean values of the metabolic parameters were compared among the groups of VAI tertiles, the subjects in the 3rd tertile of VAI was the eldest and the mostly obese (). Mean values of fasting blood glucose and HbA1c increased from 1st to 3rd tertile groups of VAI. All other metabolic parameters worsened from 1st to 3rd tertiles of VAI (). Mean CACS increased from 1st to 3rd tertile groups of VAI ().
shows results from bivariate correlations between VAI and the metabolic values, including CACS. Subjects with CACS >0 had worse metabolic parameters compared to subjects with CACS = 0. CACS and ln(CACS +1) were weakly positively correlated with VAI (r = 0.027, p < 0.001 and r = 0.070, p < 0.001), respectively.
Table 2. Bivariate correlations between metabolic parameters and visceral adiposity index.
shows the comparisons of the metabolic parameter values among the groups divided degree of CACS. Of all the subjects, only 14.9% had CACS >0. The mean metabolic parameters showed significant differences among the three groups, with the values in group with CACS ≥100 the highest. Mean VAI was the highest in subjects with CACS ≥100 among the groups (). The subjects with CACS >0 were least represented in the lowest VAI tertile group (<0.967) and most represented in the highest VAI tertile group (>1.777, ).
Figure 1. Comparison of mean visceral adiposity index between subjects divided by coronary artery calcium score severity.
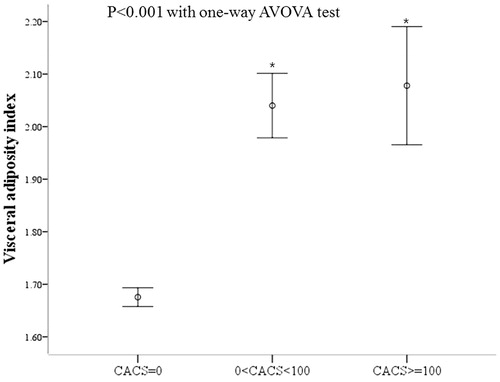
Figure 2. Comparison of the proportion of subjects with coronary artery calcium score >0 according to tertiles of visceral adiposity index.
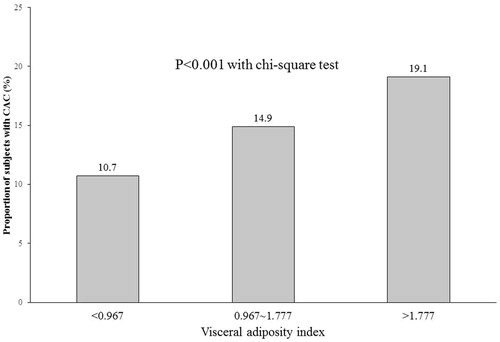
Table 3. Comparison of metabolic parameters according to presence/absence of coronary artery calcification.
shows risk for CAC according to VAI tertile. In logistic regression analyses with presence of CACS >0 as the dependent variable, the highest VAI tertile group (>1.777) had significantly higher odds ratio (OR) for having CAC compared to the lowest tertile group (<0.967), even after adjusting for age and sex (Model 1: odds ratio (OR) = 1.81, 95% CI: 1.669–1.972). After further adjustment for total cholesterol, systolic blood pressure, smoking, fasting blood glucose, and fasting insulin (Model 2), the OR for CAC was 1.39 (95% CI: 1.267–1.517) in the highest tertile compared to the lowest tertile. Although further adjustment, BMI showed attenuated, but still statistically significant OR for CACS >0 (Model 3) (OR = 1.26, 95% CI: 1.147–1.381). When similar analyses were performed with CACS >100 as the dependent variable, similar results were observed, although the ORs were attenuated (). When similar analyses were performed with VAI included in the models as the continuous variable, VAI showed positive correlation with the presence of CAC even after adjustment for confounding variables (Supplemental Table).
Table 4. Odds ratios (95% CI) for coronary artery calcification according to tertiles of visceral adiposity index.
Discussion
In this study of apparently healthy Korean subjects, VAI showed weak but statistically significant correlation with CACS, and the subjects who had higher CACS had significantly higher VAI values. In logistic regression analyses, the highest VAI tertile had significantly increased odds of having CACS >0 after adjusting for confounding variables, including BMI. Although the risk was attenuated after adjustment for confounding variables, similar results were observed with CACS >100 as the dependent variable.
Visceral adiposity rather than mere obesity, which is frequently observed in patients with hypertriglyceridemia and low HDL-C (Citation32), is known to affect the development of CVD through increased adipokine production, proinflammatory milieu, and deterioration of insulin sensitivity (Citation9–12). This is why the assessment of visceral adiposity is important in the identification of patients at high risk for CVD. Several surrogate markers, such as BMI, WC, and dyslipidemia, have been used as the markers of visceral adiposity. The VAI, which was first developed by Amato et al., is a sex-specific scoring tool based on WC, BMI, TG, and HDL-C and offers a quantitative estimate of visceral adiposity and insulin sensitivity (Citation20). Our results partly support the reports by Amato et al. in that VAI is a novel indicator of visceral obesity, and that it has high correlation with the classic markers of cardiometabolic risk (Citation20–22).
Herein, we found a significant association between VAI and CAC, which, to our knowledge, is the first report of this association in the literature. Furthermore, subjects with higher CACS had a significantly higher VAI compared to those without CAC. The risk of CACS >0 was higher in the upper VAI tertiles compared to the lowest tertile, even after adjusting for several factors, including BMI. These results are consistent with previous results that showed a strong association between VAI and CVD (Citation20,Citation24). VAI increased significantly in metabolically healthy obese individuals compared to metabolically healthy normal-weight individuals and was a novel risk factor of CVD (Citation33). In another study, VAI increased the cardiometabolic risk of type 2 diabetes and decreased significantly after 12 months of intervention (Citation34). VAI is also known to be a predictive marker of CKD or CV events in hemodialysis patients and patients with polycystic ovary syndrome (Citation22,Citation35), as well as in patients with pre-hypertension or hypertension (Citation21). The direct mechanism for the association between VAI, which reflects visceral fat and insulin resistance, and CACS, which is a marker for subclinical atherosclerosis and reflects cardiovascular burden, could be the explained through the condition called, ‘adiposopathy’, which is featured by increased circulating free fatty acid, systemic inflammation and vasculopathy in subjects with abdominal obesity and insulin resistance (Citation36).
Excess visceral adiposity, featured as adiposity dysfunction, increased adipocytokine production and pro-inflammatory activity that might cause the insulin resistance, and atherogenic dyslipidemia with high TG and low HDL-C, could be the main reason for the link between pathologic adipose tissue and cardiometabolic risk in human body (Citation37). There are many surrogate markers or methods to measure visceral adiposity in vivo (Citation38). In several studies, visceral obesity, as assessed by CT scan, correlated well with diabetogenic, atherogenic, prothrombotic, and proinflammatory metabolic abnormalities—also known as metabolic syndrome; metabolic syndrome is also known to be associated with increased risk of CVD (Citation9–12,Citation39). However, CT and MRI imaging are not frequently used to measure visceral fat because they are not cost-effective and the measurement protocols are complex. As the calculation for VAI contains TG and WC, the main components of visceral obesity and insulin resistance, VAI could be considered as an accurate marker for cardiometabolic risk. However, further studies are needed to evaluate the effectiveness of newly developed surrogate markers.
This study has some limitations. First, it is a cross-sectional study; thus, we cannot imply causality between VAI and CACS. Second, subjects of this study were all Korean, so we cannot generalize the results to the general population. Third, there is no appropriate VAI cut-off value to estimate cardiovascular risk; we used tertile values and compared CACS or the proportion of subjects with CACS >0. Fourth, there was gender imbalance in our study population. Fifth, the correlation coefficient between absolute CACS value and VAI was 0.027, somewhat weak to interpret as a significant correlation. In a large sample, almost any correlation coefficient could be significantly different from zero. Lastly, failure to consider the menopausal status of the subjects, other disease status such as hypertension, the lack of adjustment for diet pattern and socioeconomic status could weaken the plausibility of the results. However, this is first large study to explore the relationship between VAI and subclinical atherosclerosis using CACS. Therefore, despite these limitations, our exploratory study is valuable.
In conclusion, VAI had a significantly positive association with subclinical atherosclerosis, as assessed by CACS. Furthermore, VAI was an independent risk factor of CAC. Our results suggest that VAI could be a useful clinical marker of cardiometabolic risk, at least in the Korean population.
Disclosure statement
The authors report no conflicts of interest.
References
- Eckel RH, Kahn R, Robertson RM, Rizza RA. Preventing cardiovascular disease and diabetes: a call to action from the American diabetes association and the American heart association. Circulation. 2006;113:2943–6.
- Grundy SM. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol. 2006;47:1093–100.
- Ferrannini E. Is insulin resistance the cause of the metabolic syndrome? Ann Med. 2006;38:42–51.
- Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–19.
- Oh JY. Regional adiposity, adipokines, and insulin resistance in type 2 diabetes. Diabetes Metab J. 2012;36:412–14.
- Despres JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med. 2006;38:52–63.
- Bjorntorp P. Metabolic implications of body fat distribution. Diabetes Care. 1991;14:1132–43.
- Lebovitz HE, Banerji MA. Point: visceral adiposity is causally related to insulin resistance. Diabetes Care. 2005;28:2322–5.
- Pascot A, Lemieux S, Lemieux I, Prud'homme D, Tremblay A, Bouchard C, et al. Age-related increase in visceral adipose tissue and body fat and the metabolic risk profile of premenopausal women. Diabetes Care. 1999;22:1471–8.
- Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7.
- Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003;23:15–39.
- DeNino WF, Tchernof A, Dionne IJ, Toth MJ, Ades PA, Sites CK, et al. Contribution of abdominal adiposity to age-related differences in insulin sensitivity and plasma lipids in healthy nonobese women. Diabetes Care. 2001;24:925–32.
- Tokunaga K, Matsuzawa Y, Ishikawa K, Tarui S. A novel technique for the determination of body fat by computed tomography. Int J Obes. 1983;7:437–45.
- Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metab Clin Exp.1987;36:54–9.
- Seidell JC, Bakker CJ, van der Kooy K. Imaging techniques for measuring adipose-tissue distribution-a comparison between computed tomography and 1.5-T magnetic resonance. Am J Clin Nutr. 1990;51:953–7.
- Ryo M, Kishida K, Nakamura T, Yoshizumi T, Funahashi T, Shimomura I. Clinical significance of visceral adiposity assessed by computed tomography: a Japanese perspective. World J Radiol. 2014;6:409–16.
- Examination Committee of Criteria for 'Obesity Disease' in Japan; Japan Society for the Study of Obesity. New criteria for 'obesity disease' in Japan. Circ J. 2002;66:987–92.
- Despres JP, Lamarche B. Effects of diet and physical activity on adiposity and body fat distribution: implications for the prevention of cardiovascular disease. Nutr Res Rev. 1993;6:137–59.
- Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J. The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb. 2005;12:295–300.
- Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33:920–2.
- Ding Y, Gu D, Zhang Y, Han W, Liu H, Qu Q. Significantly increased visceral adiposity index in prehypertension. PLoS One. 2015;10:e0123414.
- Oh JY, Sung YA, Lee HJ. The visceral adiposity index as a predictor of insulin resistance in young women with polycystic ovary syndrome. Obesity (Silver Spring, Md). 2013;21:1690–4.
- Han L, Fu KL, Zhao J, Wang ZH, Tang MX, Wang J, et al. Visceral adiposity index score indicated the severity of coronary heart disease in Chinese adults. Diabetol Metab Syndr. 2014;6:143
- Salazar MR, Carbajal HA, Espeche WG, Aizpurúa M, Maciel PM, Reaven GM. Identification of cardiometabolic risk: visceral adiposity index versus triglyceride/HDL cholesterol ratio. Am J Med. 2014;127:152–7.
- Alluri K, Joshi PH, Henry TS, Blumenthal RS, Nasir K, Blaha MJ. Scoring of coronary artery calcium scans: history, assumptions, current limitations, and future directions. Atherosclerosis. 2015;239:109–17.
- Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45.
- American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care. 2016;39 Suppl 1:S13–22.
- Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74:243–52.
- Ishiyama M, Suzuki E, Katsuda J, Murase H, Tajima Y, Horikawa Y, et al. Associations of coronary artery calcification and carotid intima-media thickness with plasma concentrations of vascular calcification inhibitors in type 2 diabetic patients. Diabetes Res Clin Pract. 2009;85:189–96.
- Mikami S, Hamano T, Fujii N, Nagasawa Y, Isaka Y, Moriyama T, et al. Serum osteoprotegerin as a screening tool for coronary artery calcification score in diabetic pre-dialysis patients. Hypertens Res. 2008;31:1163–70.
- Han D, Ó Hartaigh B, Gransar H, Yoon JH, Kim KJ, Kim MK, et al. Incremental benefit of coronary artery calcium score above traditional risk factors for all-cause mortality in asymptomatic Korean adults. Circ J. 2015;79:2445–51.
- Unger RH, Scherer PE. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol Metab. 2010;21:345–52.
- Du T, Zhang J, Yuan G, Zhang M, Zhou X, Liu Z, et al. Nontraditional risk factors for cardiovascular disease and visceral adiposity index among different body size phenotypes. Nutr Metab Cardiovasc Dis. 2015;25:100–7.
- Russo GT, Labate AM, Giandalia A, Romeo EL, Villari P, Alibrandi A, et al. Twelve-month treatment with liraglutide ameliorates visceral adiposity index and common cardiovascular risk factors in type 2 diabetes outpatients. J Endocrinol Invest. 2015;38:81–9.
- Chen HY, Chiu YL, Chuang YF, Hsu SP, Pai MF, Yang JY, et al. Visceral adiposity index and risks of cardiovascular events and mortality in prevalent hemodialysis patients. Cardiovasc Diabetol. 2014;13:136.
- Bays HE. “Sick fat,” metabolic disease, and atherosclerosis. Am J Med. 2009;122:S26–S37.
- Lim S. Ectopic fat assessment focusing on cardiometabolic and renal risk. Endocrinol Metab (Seoul). 2014;29:1–4.
- Naboush A, Hamdy O. Measuring visceral and hepatic fat in clinical practice and clinical research. Endocr Pract. 2013;19:587–9.
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7.

