Abstract
Background: The BASE ACS trial demonstrated an outcome of titanium-nitride-oxide-coated bioactive stents (BAS) that was non-inferior to everolimus-eluting stents (EES) in patients with acute coronary syndrome (ACS). We performed a post-hoc analysis of diabetic versus non-diabetic patients from the trial.
Methods: We randomised 827 patients (1:1) with ACS to receive either BAS or EES. The primary endpoint was major adverse cardiac events (MACE): a composite of cardiac death, non-fatal myocardial infarction (MI) or ischaemia-driven target lesion revascularisation (TLR). Follow-up was planned yearly through 7 years.
Results: Of 827 patients, 140 (16.9%) were diabetic; of these, 36 (25.7%) were insulin-treated. Mean follow-up duration was 4.2 ± 1.9 years. MACE was more frequent in diabetics versus non-diabetics (23.6% versus 13.7%, respectively, p = 0.003), mainly driven by more frequent cardiac death (7.9% versus 2.2%, respectively, p = 0.002). The rates of non-fatal MI, ischaemia-driven TLR were comparable (p > 0.05 for all). In diabetic patients, MACE was comparable between the two stent arms (18.5% versus 28.0%, for BAS versus EES, respectively, p = 0.18).
Conclusions: Diabetic patients treated with early percutaneous coronary intervention for ACS had worse long-term outcome, compared with non-diabetics, mainly driven by more frequent cardiac death. The long-term outcome of BAS was comparable to EES in diabetics.
Diabetic patients presenting with acute coronary syndrome who were treated with early percutaneous coronary intervention had worse long-term clinical outcome, compared with non-diabetics, mainly driven by a high incidence of cardiac death.
Age independently predicted both major adverse cardiac events and cardiac death in diabetic patients.
The long-term clinical outcome of titanium-nitride-oxide-coated bioactive stents was comparable to that of everolimus-eluting stents in the diabetic, as well as in the non-diabetic subgroup.
Key Messages
Introduction
First-generation drug-eluting stents (DES) significantly reduced in-segment late lumen loss and angiographic restenosis, compared with bare-metal stents (BMS), in patients with diabetes mellitus (DM) (Citation1,Citation2). However, among patients treated with first-generation DES, diabetics continued to have worse efficacy and safety outcome, compared with non-diabetics; the outcome was particularly worse in insulin-treated diabetics (Citation3). Currently, second-generation DES are the standard-of-care for percutaneous coronary intervention. Yet, the outcome of everolimus-eluting stents (EES) implantation in diabetic patients is controversial. In a small randomised study, and pooled data from 4 randomised trials, EES resulted in an angiographic and clinical outcome similar to that of first-generation DES, respectively (Citation4,Citation5). In the diabetic patient subset from the SCAAR registry, EES was associated with improved safety outcome, but with a similar efficacy outcome, compared with first-generation DES (Citation6).
The safety of titanium-nitride-oxide-coated bioactive stents (BAS) was demonstrated in real-world unselected cohorts (Citation7,Citation8), and randomised clinical trials of patients treated for acute coronary syndrome (ACS) (Citation9,Citation10). The adequately powered BASE ACS trial, showed non-inferiority of BAS versus EES, for the primary endpoint of major adverse cardiac events (MACE) in patients with ACS, both at 1- and 2-year follow-up (Citation10,Citation11). Yet, the comparative outcome of BAS versus EES in diabetic patients presenting with ACS remains unclear. In a post-hoc analysis, we sought to present the long-term clinical outcome of the BASE ACS trial in diabetic versus non-diabetic patients, with stent-based analysis of the outcome in the diabetic and non-diabetic subgroups.
Materials and methods
Patient selection and study design
The trial design was previously described (Citation10). Briefly, the BASE ACS was a prospective single-blinded randomised controlled trial conducted in 14 centres. From January 2009 to September 2010, we randomised 827 patients (1:1) presenting with ACS who underwent early percutaneous coronary intervention to receive either Titan-2® BAS (Hexacath, Paris, France) or Xience V® EES (Abbott Vascular, Santa Clara, CA). Follow-up was planned at 12 months, and yearly thereafter through 7 years.
Ethical issues
The trial was initiated by the investigators and conducted according to the ethical guidelines of the 1964 Declaration of Helsinki, as revised in 2013. Informed written consent was obtained from every patient after explanation of the trial protocol; the protocol was approved by the ethics committees of the coordinating center (Satakunta Central Hospital) and the other participating centres. The trial is registered with ClinicalTrials.gov, number NCT00819923.
Pharmacological interventions
Patients already maintained on aspirin received no additional aspirin loading dose. Those not maintained on aspirin were pretreated with aspirin at a loading dose of 250 mg orally or 250–500 mg intravenously during the procedure, and continued at a dose of 75–150 mg daily indefinitely. Oral clopidogrel was initiated at a loading dose of 300–600 mg before or immediately after the procedure and continued at a dose of 75 mg daily. According to the protocol, patients in either group were prescribed oral clopidogrel for a minimum of 6 months, and thereafter, for extended periods (maximum 12 months) at operator’s discretion. During the procedure, low-molecular-weight heparin (enoxaparin sodium) or unfractionated heparin was administered intravenously in the standard dosage recommended by the guidelines. Use of glycoprotein IIb IIIa inhibitors or bivalirudin was left to operator’s discretion.
Definitions and study endpoints
DM was defined as previous diagnosis of DM treated with pharmacological or non-pharmacological measures. The patients were assigned as insulin-treated if they received insulin. The diagnostic criteria for non-ST-segment elevation ACS and ST-segment elevation myocardial infarction (MI) were previously described (Citation10). The primary endpoint was the first occurrence of MACE: a composite of cardiac death, non-fatal MI, or ischaemia-driven target lesion revascularisation (TLR). Secondary endpoints included non-cardiac death, and definite stent thrombosis (ST). Cardiac death was defined as death from cardiovascular causes or any death without known cause. ST was adjudicated according to the criteria of definite ST described by the Academic Research Consortium (Citation12). An independent clinical events committee whose members were blinded to stent group allocation adjudicated all the individual endpoints according to the prespecified definitions.
Statistical analysis
Continuous variables were presented as mean ± standard deviation, whereas categorical variables were described with absolute and relative (percentage) frequencies. Comparisons between the two subgroups (diabetics versus non-diabetics) were performed using the unpaired t-test for continuous variables, and the Pearson chi-square test or Fisher's exact test for categorical variables, as appropriate. Data analysis was based on the intention-to-treat principle. We observed significant, differences between the two subgroups in several baseline characteristics. Therefore, we performed a propensity score-matched analysis of the two subgroups in order to estimate the impact of diabetes on the clinical outcome. We calculated the propensity score using a logistic regression model in which we included – as covariates – all the baseline clinical, angiographic, and procedural variables with a difference between the two subgroups as indicated by a p < 0.1 in univariate analysis. Hosmer–Lemeshow test was used to assess the fit of the logistic regression model (chi-square: 6.188, p = 0.626). Matching was performed based on an estimated caliper width of 0.2 the standard deviation of the propensity score logit. Comparisons of clinical outcome were also performed based on the stent group allocation within either subgroup individually (diabetics and non-diabetics). Time-to-event curves were constructed using Kaplan–Meier estimates, based on all the available follow-up data for MACE, and were compared with the log-rank test. In order to identify the independent predictors of outcome (MACE and cardiac death) within the diabetic subgroup, univariate logistic regression was initially performed for each of the baseline clinical, angiographic, and procedural variables. Then, the variables significantly associated (2-sided p < 0.1) with the dependent variable in univariate analysis were included as covariates in a multivariable Cox regression hazard model in which the dependent variable was the outcome variable (MACE or cardiac death). The results of multivariable regression were presented as hazard ratio (HR) with 95% confidence interval (CI). All tests were two-sided and statistical significance was set at 5%. Data were analysed with SPSS version 16.
Results
Baseline clinical, angiographic, and procedural data
Of the 827 patients enrolled in the BASE ACS trial, 140 (16.9%) were diabetic; of these, 36 (25.7%) were insulin-treated. Mean follow-up duration was 4.2 ± 1.9 years. Diabetic patients were older, more likely females, with more frequent risk factors, and more prior coronary events, versus non-diabetics (p < 0.05 for all). The other baseline clinical, angiographic, and procedural data were balanced between the two subgroups (p > 0.05 for all) ().
Table 1. Baseline clinical, angiographic and procedural characteristics of the two study groups.
Long-term clinical outcome in crude subgroups
The cumulative incidence of MACE at long-term follow-up was higher in diabetics versus non-diabetics (23.6% versus 13.7%, respectively, p = 0.003) ( and ). This was driven by more cardiac death events (p = 0.002), and a trend toward more non-fatal MI events (p = 0.095). The other endpoints were comparable between the two subgroups (p > 0.05 for all) (). Interestingly, insulin-treated diabetic patients had similar rates of adverse outcome at long-term follow-up compared with non-insulin-treated ones (p > 0.05 for all).
Figure 1. Kaplan–Meier estimates of the primary endpoint (a composite of cardiac death, non-fatal myocardial infarction, or ischaemia-driven target lesion revascularization) in the two subgroups at long-term follow-up. MACE: major adverse cardiac events.
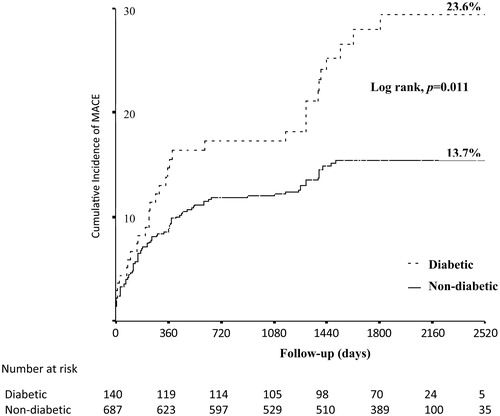
Table 2. Clinical outcome in the two study groups at long-term follow-up.
Long-term clinical outcome in matched subgroups
Propensity score matching yielded 266 patients (133 pairs) with balanced baseline characteristics (). In the propensity score-matched pairs, the incidence of cardiac death was higher in diabetics versus non-diabetics (7.5% versus 0.8%, respectively, p = 0.006). MACE and the other individual endpoints were comparable between the two subgroups (p > 0.05 for all) ().
Table 3. Baseline clinical, angiographic and procedural characteristics of the two matched-pair subgroups.
Table 4. Clinical outcome in the two matched-pair subgroups at long-term follow-up.
Stent-based analysis of the two subgroups
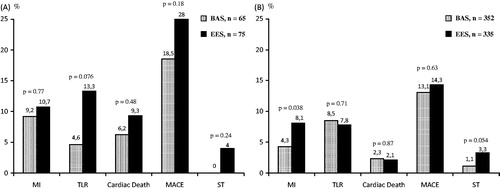
In the diabetic patients, MACE was comparable between the two stent arms (18.5% versus 28.0%, for BAS versus EES, respectively, p = 0.18). Likewise, the other individual endpoints were comparable (p > 0.5 for all) (). Similarly, in the non-diabetic patients, MACE and the other individual endpoints were comparable between the two stent arms (p > 0.5 for all); however, there was a trend toward less definite ST events in patients who received BAS versus EES (1.1% versus 3.3%, respectively, p = 0.054) ().
Figure 2. Stent-based analyses of the two subgroups at long-term follow-up: (A) Diabetic subgroup; (B) Non-diabetic subgroup. BAS: bioactive stents; EES: everolimus-eluting stents; MACE: major adverse cardiac events; MI: myocardial infarction; ST: stent thrombosis; TLR: target lesion revascularisation.
Predictors of adverse outcome in the diabetic subgroup
In univariate analysis, the predictors of MACE in the diabetic subgroup were age (p = 0.068), hypertension (p = 0.077), and the number of vessels treated (p = 0.008). In multivariable analysis, the only independent predictor of MACE was age (HR 1.038, 95% CI 1.002–1.070, p = 0.037). Similarly, in univariate analysis, the predictors of cardiac death were age (p = 0.095), gender (p = 0.089), hypertension (p = 0.035), and hyperlipidemia (p = 0.015). In multivariable analysis, the only independent predictor of cardiac death was age (HR 1.083, 95% CI 1.006–1.166, p = 0.033).
Discussion
Main findings
The current post-hoc analysis of the BASE ACS trial demonstrated that diabetic patients presenting with ACS who were treated with early percutaneous coronary intervention had worse long-term clinical outcome, compared with non-diabetics, mainly driven by a high incidence of cardiac death. Age independently predicted both MACE and cardiac death in such patient subset. Moreover, the long-term clinical outcome of BAS was comparable to that of EES in the diabetic, as well as in the non-diabetic subgroup. The current report is the first to address the comparative outcome of BAS versus EES in diabetic patients presenting with ACS.
Outcome of percutaneous coronary intervention in diabetic patients
Previous studies reported worse short- and long-term clinical outcome of percutaneous coronary intervention in patients with versus those without DM, in the era of BMS (Citation13–15). DM was independently associated with 5-year adverse events (both restenotic and non-restenotic) in low-risk patients who received BMS (Citation16). These findings were corroborated by the results of a small study and two post-hoc analyses from randomised controlled trials of first-generation DES (Citation3,Citation17,Citation18). Possible underlying mechanisms of adverse outcome in diabetic patients include endothelial dysfunction, accelerated platelet deposition, overexpression of growth factors, in addition to promotion of inflammatory cell recruitment and smooth muscle cell proliferation (Citation19). Additionally, in a cohort of patient who underwent DES implantation for stable angina, diabetics had a higher on-clopidogrel platelet reactivity versus non-diabetics; type 2 DM was an independent predictor of high on-clopidogrel platelet reactivity (Citation20). The results of the current post-hoc analysis support the previous data, and extend the findings to diabetic patients presenting with ACS; yet, after propensity score matching, only the incidence of cardiac death remained higher in diabetic patients. Paradoxically, the rates of ischaemia-driven TLR and definite ST were numerically lower in the matched diabetic, versus non-diabetic, subgroup. This might be due to the censoring effect of the high rate of cardiac death in the diabetic subgroup (7.5%); in addition, owing to the small size of the matched subgroups, we cannot rule out the play of chance.
Effect of insulin requirement
The high cardiac mortality in the diabetic subgroup in the current study might be related to the high percentage of patients with insulin-treated DM (25.7%). The incidence of cardiac death was numerically higher in diabetic patients treated with versus without insulin (data not shown). Some prior studies stratified outcome based on insulin requirement. In post-hoc analyses from two randomised trials of first-generation DES, insulin-treated diabetic patients had higher rates of MACE, compared with diabetic patients treated without insulin (Citation17,Citation18). Although some registry reports of first-generation DES failed to demonstrate a significant difference in outcome between insulin-treated and non-insulin-treated diabetic patients (Citation21,Citation22), data from the Bern-Rotterdam registry showed that insulin-treated diabetics had higher cardiac and all-cause mortality (Citation23). Moreover, insulin-treated DM independently predicted both MACE and ST in the e-Cypher registry (Citation24). Additionally, in a small observational study of patients treated with sirolimus-eluting stents, insulin-treated DM (39% of all diabetics) was independently associated with TLR, and with the composite of death/MI (Citation3). Furthermore, in a pooled analysis of data from five controlled studies of zotarolimus-eluting stents, insulin-treated diabetics had a higher incidence of target lesion failure, compared with non-insulin-treated diabetics (Citation25). Insulin treatment was associated with increased platelet aggregation in patients with type 2 DM receiving dual antiplatelet therapy (Citation26). Insulin requirement might reflect more advanced disease state, with long-standing hyperglycaemia, and consequently, failure of other measures of glycaemic control.
Choice of stent in diabetic patients treated for ACS
Patients with DM have increased 1-year mortality after ACS, compared with non-diabetics (Citation27). In a substudy of diabetic patients presenting with ST-elevation MI in the HORIZONS AMI trial, treatment with paclitaxel-eluting stents, versus BMS, was associated with reduction of ischaemia-driven TLR, with comparable safety outcome (Citation28). Similar results were obtained from a pooled analysis of diabetic patients from 7 randomised trials (not including the HORIZONS AMI) comparing first-generation DES with BMS in the setting of ST-elevation MI (Citation29). However, in a recent report of diabetic patients presenting with the full spectrum of ACS, implantation of DES was associated with lower all-cause mortality and MACE, with comparable rates of target vessel revascularisation and non-fatal MI (Citation30). In the current post-hoc analysis, implantation of BAS, versus EES, in diabetic patients presenting with ACS was associated with comparable rates of both safety and efficacy outcome. Similarly, the composite MACE rates were comparable between the 2 stent arms in the 1- and 2-year reports of the trial (Citation10,Citation11). Interestingly, the incidence of non-fatal MI was lower in the BAS arm at both time points (Citation10,Citation11). At 4-year follow-up, the incidence of MACE was again comparable; the rates of both non-fatal MI and definite ST were lower in the BAS arm (Citation31). It is noteworthy that dual antiplatelet therapy is needed for one month only following BAS implantation.
Limitations of the study
The BASE ACS trial was not designed a priori to explore specific differences in outcome based on the presence of diabetes. Due to the retrospective nature of this post-hoc analysis, some data relevant to the outcome might have been missed. In addition, the trial may be underpowered for specific subgroup analysis; therefore, we cannot rule out a type II error as the cause for failure to demonstrate significant difference between the subgroups, or between the stent arms in either subgroup. Moreover, analysis of patient data in one subgroup who received different stent designs should also be interpreted with caution. Finally, the current post-hoc analysis was a non-randomised subgroup analysis; this might limit the conclusiveness of the results, even after propensity score matched analysis.
Conclusion
The diabetic patients presenting with ACS who were treated with early percutaneous coronary intervention had worse long-term clinical outcome, compared with the non-diabetics, mainly driven by a high incidence of cardiac death. The long-term clinical outcome of BAS was comparable to that of EES in the diabetic subgroup.
Acknowledgements
The authors thank Tuija Vasankari, Eija Niemelä, and Minna Ampio, for their support in the conduct of this study.
Disclosure statement
None of the authors declared any conflict of interests.
References
- Sabaté M, Jiménez-Quevedo P, Angiolillo DJ, Gómez-Hospital JA, Alfonso F, Hernández-Antolín R, et al. Randomized comparison of sirolimus-eluting stent versus standard stent for percutaneous coronary revascularization in diabetic patients: the diabetes and sirolimus-eluting stent (DIABETES) trial. Circulation. 2005;112:2175–83.
- Ortolani P, Balducelli M, Marzaroli P, Piovaccari G, Menozzi A, Guiducci V, et al. Two-year clinical outcomes with drug-eluting stents for diabetic patients with de novo coronary lesions: results from a real-world multicenter registry. Circulation. 2008;117:923–30.
- Kumar R, Lee TT, Jeremias A, Ruisi CP, Sylvia B, Magallon J, et al. Comparison of outcomes using sirolimus-eluting stenting in diabetic versus nondiabetic patients with comparison of insulin versus non-insulin therapy in the diabetic patients. Am J Cardiol. 2007;100:1187–91.
- Stone GW, Kedhi E, Kereiakes DJ, Parise H, Fahy M, Serruys PW, et al. Differential clinical responses to everolimus-eluting and paclitaxel-eluting coronary stents in patients with and without diabetes mellitus. Circulation. 2011;124:893–900.
- Kim WJ, Lee SW, Park SW, Kim YH, Yun SC, Lee JY, et al. Randomized comparison of everolimus-eluting stent versus sirolimus-eluting stent implantation for de novo coronary artery disease in patients with diabetes mellitus (ESSENCE-DIABETES): results from the ESSENCE-DIABETES trial. Circulation. 2011;124:886–92.
- Kedhi E, Gomes ME, Lagerqvist B, Smith JG, Omerovic E, James S, et al. Clinical impact of second-generation everolimus-eluting stent compared with first-generation drug-eluting stents in diabetes mellitus patients: insights from a nationwide coronary intervention register. JACC Cardiovasc Interv. 2012;5:1141–9.
- Mosseri M, Miller H, Tamari I, Plich M, Hasin Y, Brizines M, et al. The titanium-NO stent: results of a multicenter registry. Eurointervention. 2006;2:192–6.
- Karjalainen PP, Ylitalo A, Airaksinen KEJ, Nammas W. Five-year clinical outcome of titanium nitride-oxide-coated bioactive stent implantation in a real world population: a comparison with paclitaxel-eluting stents: the PORI registry. J Interv Cardiol. 2011;24:1–8.
- Tuomainen PO, Ylitalo A, Niemelä M, Kervinen K, Pietilä M, Sia J, et al. Five-year clinical outcome of titanium-nitride-oxide-coated bioactive stents versus paclitaxel-eluting stents in patients with acute myocardial infarction: long-term follow-up from the TITAX AMI trial. Int J Cardiol. 2013;168:1214–9.
- Karjalainen PP, Niemelä M, Airaksinen JK, Rivero-Crespo F, Romppanen H, Sia J, et al. A prospective randomised comparison of titanium-nitride-oxide-coated bioactive stents with everolimus-eluting stents in acute coronary syndrome: the BASE-ACS trial. EuroIntervention. 2012;8:306–15.
- Romppanen H, Nammas W, Kervinen K, Airaksinen JK, Pietilä M, Rivero-Crespo F, et al. Stent-oriented versus patient-oriented outcome in patients undergoing early percutaneous coronary intervention for acute coronary syndrome: 2-year report from the BASE-ACS trial. Ann Med. 2013;45:488–93.
- Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51.
- Lee TT, Feinberg L, Baim DS, Holmes DR, Aroesty JM, Carrozza JP Jr, et al. Effect of diabetes mellitus on five-year clinical outcomes after single-vessel coronary stenting (a pooled analysis of coronary stent clinical trials). Am J Cardiol. 2006;98:718–21.
- Abizaid A, Kornowski R, Mintz GS, Hong MK, Abizaid AS, Mehran R, et al. The influence of diabetes mellitus on acute and late clinical outcomes following coronary stent implantation. J Am Coll Cardiol. 1998;32:584–9.
- Laskey WK, Selzer F, Vlachos HA, Johnston J, Jacobs A, King SB III, et al. Comparison of in-hospital and one-year outcomes in patients with and without diabetes mellitus undergoing percutaneous catheter intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol. 2002;90:1062–7.
- Cutlip DE, Chhabra AG, Baim DS, Chauhan MS, Marulkar S, Massaro J, et al. Beyond restenosis: five-year clinical outcomes from second-generation coronary stent trials. Circulation. 2004;110:1226–30.
- Moussa I, Leon MB, Baim DS, O’Neill WW, Popma JJ, Buchbinder M, et al. Impact of sirolimus-eluting stents on outcome in diabetic patients: a SIRIUS (SIRolImUS-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) substudy. Circulation. 2004;109:2273–8.
- Hermiller JB, Raizner A, Cannon L, Gurbel PA, Kutcher MA, Wong SC, et al. Outcomes with the polymer-based paclitaxel-eluting TAXUS stent in patients with diabetes mellitus: the TAXUS-IV trial. J Am Coll Cardiol. 2005;45:1172–9.
- Aronson D, Bloomgarden Z, Rayfield EJ. Potential mechanisms promoting restenosis in diabetic patients. J Am Coll Cardiol. 1996;27:528–35.
- Feldman L, Tubach F, Juliard JM, Himbert D, Ducrocq G, Sorbets E, et al. Impact of diabetes mellitus and metabolic syndrome on acute and chronic on-clopidogrel platelet reactivity in patients with stable coronary artery disease undergoing drug-eluting stent placement. Am Heart J. 2014;168:940–7.
- Ong AT, Aoki J, van Mieghem CA, Rodriguez Granillo GA, Valgimigli M, Tsuchida K, et al. Comparison of short- (one month) and long- (twelve months) term outcomes of sirolimus- versus paclitaxel-eluting stents in 293 consecutive patients with diabetes mellitus (from the RESEARCH and T-SEARCH registries). Am J Cardiol. 2005;96:358–62.
- Berenguer A, Mainar V, Bordes P, Valencia J, Gomez S. Efficacy of sirolimus-eluting stents in diabetics with complex coronary lesions. Rev Esp Cardiol. 2006;59:117–24.
- Simsek C, Räber L, Magro M, Boersma E, Onuma Y, Stefanini GG, et al. Long-term outcome of the unrestricted use of everolimus-eluting stents compared to sirolimus-eluting stents and paclitaxel-eluting stents in diabetic patients: the Bern-Rotterdam diabetes cohort study. Int J Cardiol. 2013;170:36–42.
- Urban P, Gershlick AH, Guagliumi G, Guyon P, Lotan C, Schofer J, et al. Safety of coronary sirolimus-eluting stents in daily clinical practice: one-year follow-up of the e-Cypher registry. Circulation. 2006;113:1434–41.
- Silber S, Serruys PW, Leon MB, Meredith IT, Windecker S, Neumann FJ, et al. Clinical outcome of patients with and without diabetes mellitus after percutaneous coronary intervention with the resolute zotarolimus-eluting stent: 2-year results from the prospectively pooled analysis of the international global RESOLUTE program. J Am Coll Cardiol Intv. 2013;6:357–68.
- Angiolillo DJ, Bernardo E, Ramirez C, Costa MA, Sabate M, Jimenez-Quevedo P, et al. Insulin therapy is associated with platelet dysfunction in patients with type 2 diabetes mellitus on dual oral antiplatelet treatment. J Am Coll Cardiol. 2006;48:298–304.
- Hasin T, Hochadel M, Gitt AK, Behar S, Bueno H, Hasin Y. Comparison of treatment and outcome of acute coronary syndrome in patients with versus patients without diabetes mellitus. Am J Cardiol. 2009;103:772–8.
- Witzenbichler B, Wöhrle J, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, et al. Paclitaxel-eluting stents compared with bare metal stents in diabetic patients with acute myocardial infarction: the harmonizing outcomes with revascularization and stents in acute myocardial infarction (HORIZONS-AMI) trial. Circ Cardiovasc Interv. 2011;4:130–8.
- Iijima R, Byrne RA, Dibra A, Ndrepepa G, Spaulding C, Laarman GJ, et al. Drug-eluting stents versus bare-metal stents in diabetic patients with ST-segment elevation acute myocardial infarction: a pooled analysis of individual patient data from seven randomized trials. Rev Esp Cardiol. 2009;62:354–64.
- Lai CC, Lin TH, Yip HK, Liu CP, Li AH, Shyu KG, et al. One-year cardiovascular outcomes of drug-eluting stent versus bare-metal stent implanted in diabetic patients with acute coronary syndrome. J Chin Med Assoc. 2016;79:239–47.
- Karjalainen PP, Niemelä M, Pietilä M, Sia J, de Belder A, Rivero-Crespo F, de Bruyne B, Nammas W. 4-year outcome of bioactive stents versus everolimus-eluting stents in acute coronary syndrome. Scand Cardiovasc J. 2016. [Epub ahead of print]. doi:10.1080/14017431.2016.1177198.

