Abstract
Purpose: We aimed to evaluate the joint impact of cardiorespiratory fitness (CRF) and frequency of sauna bathing (FSB) on the risk of cardiovascular and all-cause mortality.
Design: CRF measured by respiratory gas analyses and sauna exposure were assessed at baseline in a prospective study of 2277 men. CRF was categorized as low and high (median cut-offs) and FSB as low and high (≤2 and 3–7 sessions/week, respectively).
Results: During a median follow-up of 26.1 years, 520 cardiovascular and 1124 all-cause deaths occurred. Comparing high versus low CRF, the multivariate-adjusted hazard ratios (HRs) 95% CIs for cardiovascular and all-cause mortality were 0.51 (0.41–0.63) and 0.65 (0.57–0.75), respectively. Comparing high versus low FSB, the corresponding HRs were 0.74 (0.59–0.94) and 0.84 (0.72–0.97), respectively. Compared to low CRF & low FSB, the HRs of CVD mortality for high CRF & high FSB; high CRF & low FSB; and low CRF & high FSB were 0.42 (0.28–0.62), 0.50 (0.39–0.63) and 0.72 (0.54–0.97), respectively. For all-cause mortality, the corresponding HRs were 0.60 (0.48–0.76), 0.63 (0.54–0.74) and 0.78 (0.64–0.96), respectively.
Conclusions: A combination of high CRF and frequent sauna bathing confers stronger long-term protection on mortality outcomes compared with high CRF or high FSB alone.
Cardiorespiratory fitness (CRF) and frequency of sauna bathing are independently associated with reduced mortality risk; a combination of good CRF and frequent sauna bathing may confer additional survival benefits.
In a population-based prospective cohort study, a combination of high CRF levels and frequent sauna bathing (3–7 sessions per week) was associated with a substantial risk reduction in fatal cardiovascular and all-cause mortality events compared with good CRF or frequent sauna bathing alone.
A combination of good fitness levels produced by aerobic exercises and frequent sauna bathing may have added health benefits and confer more protection on the risk of mortality.
KEY MESSAGES
Introduction
Physical activity is well established to be associated with reduced risk of adverse vascular and non-vascular outcomes [Citation1]. Cardiorespiratory fitness (CRF), as assessed by maximal oxygen uptake (VO2max) during maximal-effort graded exercise testing, is a measure of the level of aerobic fitness and an index of cardiac and respiratory functioning. It is regarded as the gold standard for assessing aerobic capacity [Citation2]. Cardiorespiratory fitness is consistently inversely and independently associated with the risk of cardiovascular disease (CVD) and all-cause mortality [Citation3]. An increasing broad body of evidence also suggests that CRF improves CVD risk prediction beyond that of traditional cardiovascular risk factors [Citation3,Citation4]. Sauna bathing, a form of passive heat therapy, is a traditional Finnish activity which is commonly used for the purposes of pleasure, relaxation and wellness. Sauna bathing is becoming a popular activity in many other countries [Citation5,Citation6] and it has been associated with several health benefits [Citation7–11]. Recent observational epidemiological evidence has shown that having frequent sauna baths was independently associated with a reduced risk of fatal cardiovascular and all-cause mortality outcomes [Citation12].
Although CRF and sauna bathing, a measure of the level of aerobic fitness and a healthy recreational activity, respectively, are both individually associated with reduced risks of cardiovascular and all-cause mortality; it remains unknown whether these factors interact together to further decrease mortality risk. Our question was whether sauna bathing confers additional survival benefits in those with highest levels of fitness at baseline or not. In this context, using a population-based prospective cohort of 2277 Caucasian men, our primary objective was to evaluate the joint effects of CRF and frequency of sauna bathing (FSB) on the risk of fatal CVD and all-cause mortality events. We initially assessed the separate associations of CRF and FSB with risk of each outcome in the same set of participants to enable direct comparisons.
Materials and methods
Study design and participants
Study participants were part of the Kuopio Ischemic Heart Disease (KIHD) risk factor study, which is an ongoing representative population-based study comprising a representative sample of middle-aged men aged 42–61 years recruited from eastern Finland. Details of recruitment methods and assessment of risk markers have been described in previous reports [Citation13,Citation14]. Briefly, baseline examinations were conducted between March 1984 and December 1989. Of 3433 potentially eligible and randomly selected men; 3235 were found to be eligible, and of this number, 2682 (78%) volunteered to participate; 186 provided no response to the invitation and 367 declined to give informed consent. This dataset analysed comprised of 2277 men who had complete information on exposures, relevant risk markers and outcomes. The study protocol was approved by the Research Ethics Committee of the University of Eastern Finland and each participant gave written informed consent. All study procedures were conducted according to the Declaration of Helsinki.
Baseline characteristics and ascertainment of outcomes
For blood specimen collection, participants were instructed to fast overnight, abstain from alcohol consumption for at least 3 d, and to keep away from smoking for at least 12 h prior to the assessments. The cholesterol contents of serum lipoprotein fractions and triglycerides were measured enzymatically (Boehringer, Mannheim, Germany). Serum high-density lipoprotein cholesterol was separated from fresh serum samples using ultracentrifugation and precipitation [Citation12]. Serum C-reactive protein (CRP) was assessed with an immunometric assay (Immulite High Sensitivity C-Reactive Protein Assay; DPC, Los Angeles, CA). Participants completed self-administered health and lifestyle questionnaire for the assessment of age, smoking, alcohol consumption, level of physical activity, socio-economic status (SES), baseline diseases and medication history [Citation15]. The energy expenditure of physical activity was assessed using the validated KIHD 12-month leisure-time physical activity history questionnaire [Citation16,Citation17]. Adulthood socioeconomic status (SES) was assessed as a combined measure of income, education, occupation, occupational prestige, material standard of living and housing conditions, all of which were assessed with self-reported questionnaires. Values for SES ranged from 0 to 25, with a higher value indicating lower levels of SES [Citation18,Citation19]. Alcohol consumption was assessed using the Nordic Alcohol Consumption Inventory. History of type 2 diabetes was defined as having a clinical diagnosis of diabetes and regular treatment with diet, oral hypoglycaemic agents or insulin therapy, fasting plasma glucose ≥7.0 mmol/l, or according to self-reports. History of coronary heart disease (CHD) was based on a previous myocardial infarction, angina pectoris, the use of nitroglycerin for chest pain once a week or more frequently or chest pain. Resting blood pressure was measured between 8 and 10 a.m. with a random-zero sphygmomanometer. Maximal oxygen uptake was used as a measure of CRF, which was assessed using respiratory gas exchange analysers (Medical Graphics, MCG, St. Paul, MN) during cycle ergometer exercise testing as described in detail elsewhere [Citation13]. Briefly, a maximal symptom-limited exercise tolerance test was performed between 08:00 and 10:00 h using an electrically braked cycle ergometer. The standardized testing protocol comprised of a 3-min warm-up at 50 W followed by a step-by-step increase in workload by 20 W/min with direct analyses of respiratory gases. The exercise tests were conducted under the supervision of an experienced physician and assisted by an experienced nurse to ensure safety. Assessment of sauna bathing was based on a traditional Finnish sauna which consists of dry air (10–20%) with relatively high temperature. The recommended temperature for a sauna bath is from 80 to 100 °C at the level of the bather’s head, but the temperature is much lower at the floor-level which keeps ventilation of sauna room efficient and sauna condition comfortable for sauna bathers. Baseline sauna bathing habits were assessed by a self-administered questionnaire and these represented typical weekly sauna bathing sessions as well as the temperature in the sauna room [Citation7,Citation20]. The questionnaires were checked by an experienced nurse at the time of baseline examination. All deaths that occurred by the end of 2014 were used and were ascertained from hospital documents, health centre wards and death certificates and medico-legal reports. Outcomes were coded using the International Classification of Diseases codes.
Statistical analysis
Descriptive data are presented as means (SD) or medians (interquartile range, IQR) for continuous variables and percentages for categorical variables. Hazard ratios (HRs) with 95% confidence intervals (CIs) for outcomes were calculated using Cox proportional hazard models, after confirmation of the assumptions of the proportionality of hazards [Citation21]. Cardiorespiratory fitness was categorised into low and high CRF based on median cut-offs of CRF, whiles FSB was categorized into low and high frequencies (defined as ≤2 and 3–7 sauna sessions per week, respectively). These categories were created based on the distribution of the data and findings from our previous studies conducted on the topic [Citation11–13]. For the associations of one exposure (e.g. CRF) with mortality outcomes, formal tests of interaction were used to assess if the associations were modified by the other exposure (e.g. FSB). The joint associations of CRF and FSB with outcomes were based on the following four possible combinations: high CRF & high FSB; low CRF & high FSB; high CRF & low FSB; and low CRF & low FSB. To put our findings into clinical context, we also calculated the number needed to treat (NNT) associated with high CRF & high FSB using the formula proposed by Altman and Anderson [Citation22]: NNT (t) = 1/[SB(t))HR – SB(t)], where SB(t) denotes the Kaplan–Meier survival probability in the reference group (low CRF & low FSB) at time t and HR refers to the Cox regression estimate comparing the exposure group with the reference group. All statistical analyses were conducted using Stata version 14 (Stata Corp, College Station, TX).
Results
Baseline characteristics
Baseline characteristics of study participants are summarized in . The mean (SD) age of study participants at baseline was 53 (5) years. The mean (SD) of CRF and median (interquartile range, IQR) FSB was 30.3 (8.0) ml/kg/min and 2 (1–2) sessions per week, respectively.
Table 1. Baseline characteristics of study participants.
Associations of CRF and frequency of sauna bathing with mortality outcomes
During a median (IQR) follow-up of 26.1 (19.0–28.1) years, a total of 520 CVD deaths and 1124 all-cause deaths occurred. Cumulative hazard curves showed reduced risks of CVD death and all-cause mortality among participants with high CRF & high FSB compared with other groups (p value for log-rank test < .0001 for all; ). In age-adjusted analyses, compared to men with low CRF, the HR of CVD mortality for men with high CRF was 0.34 (95% CI: 0.28–0.42). The HR was minimally attenuated on further adjustment for several established cardiovascular risk factors (body mass index, smoking status, systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, history of type 2 diabetes, prevalent CHD, alcohol consumption) 0.49 (95% CI: 0.39–0.60) and remained consistent on additional adjustment for socio-economic status and C-reactive protein 0.51 (95% CI: 0.41–0.63). The corresponding adjusted HRs for all-cause mortality were 0.51 (95% CI: 0.45–0.58), 0.63 (95% CI: 0.55–0.72) and 0.65 (95% CI: 0.57–0.75) comparing high versus low CRF (). On further adjustment for FSB, the HRs for cardiovascular and all-cause mortality remained persistent comparing high versus low CRF; 0.51 (95% CI: 0.41–0.63) and 0.66 (95% CI: 0.57–0.75), respectively.
Figure 1. Cumulative Kaplan–Meier curves for cardiovascular and all-cause mortality during follow-up according to CRF and sauna bathing frequency groups. CRF: cardiorespiratory fitness; CVD: cardiovascular disease; FSB: frequency of sauna bathing.
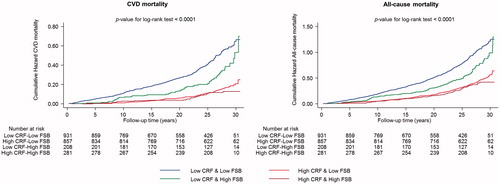
Table 2. Associations of cardiorespiratory fitness with risk of cardiovascular and all-cause mortality.
The age-adjusted HR for CVD mortality comparing men with high versus low FSB was 0.67 (95% CI: 0.53–0.85). The HR was attenuated to 0.74 (95% CI: 0.59–0.94) on further adjustment for several established and emerging cardiovascular risk factors. The corresponding adjusted HRs for all-cause mortality were 0.77 (95% CI: 0.66–0.90) and 0.84 (95% CI: 0.72–0.97) comparing high versus low FSB (). Comparing high versus low FSB, the HRs for cardiovascular and all-cause mortality remained consistent on additional adjustment for CRF; 0.76 (95% CI: 0.60–0.96) and 0.85 (95% CI: 0.73–0.99), respectively.
Table 3. Association of frequency of sauna bathing with risk of cardiovascular and all-cause mortality.
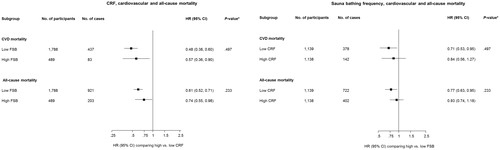
The associations of CRF and mortality outcomes were not modified by FSB (p values for interactions >.10) and neither were the associations of FSB with mortality outcomes modified by CRF (p values for interactions >.10) ().
Figure 2. Interactions of the associations of CRF and sauna bathing frequency with cardiovascular and all-cause mortality. CI: confidence interval; CRF: cardiorespiratory fitness; CVD: cardiovascular disease; FSB: frequency of sauna bathing; HR: hazard ratio; HRs are adjusted for age, body mass index, smoking status, systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, history of type 2 diabetes, prevalent coronary heart disease and alcohol consumption; *, p values for interaction.
Joint associations
reports the HRs for the joint associations of CRF and FSB sessions with the risk of cardiovascular and all-cause mortality. Compared with men with low CRF & low FSB, the age-adjusted HRs of CVD mortality for the following groups: high CRF & high FSB; low CRF & high FSB; and high CRF & low FSB were 0.27 (95% CI: 0.19–0.40), 0.65 (95% CI: 0.49–0.86), and 0.33 (95% CI: 0.27–0.41), respectively. The HRs were 0.42 (95% CI: 0.28–0.62), 0.72 (95% CI: 0.54–0.97) and 0.50 (95% CI: 0.39–0.63), respectively, after further adjustment for several established and emerging cardiovascular risk factors. For all-cause mortality, the age-adjusted HRs for high CRF & high FSB; low CRF & high FSB; and high CRF & low FSB were 0.45 (95% CI: 0.36–0.56), 0.72 (95% CI: 0.59–0.88) and 0.49 (95% CI: 0.42–0.56), respectively, when compared with men with low CRF & low FSB. After further adjustment for several established risk factors and potential confounders, the corresponding HRs were 0.60 (95% CI: 0.48–0.76), 0.78 (95% CI: 0.64–0.96) and 0.63 (95% CI: 0.54–0.74), respectively.
Table 4. Joint associations of cardiorespiratory fitness and frequency of sauna bathing with risk of cardiovascular and all-cause mortality.
The absolute risk reduction of CVD mortality associated with high CRF & 3–7 sauna sessions per week was 0.36 during the entire duration of follow-up, which translated into a NNT of 4 (95% CI: 3–7) to prevent one cardiovascular death. For all-cause mortality, the corresponding NNT was 5 (95% CI: 4–7).
Discussion
Consistent with results of previously published population-based prospective studies [Citation3,Citation12], our findings show that CRF and FSB are each inversely and independently associated with cardiovascular and all-cause mortality. The associations of each exposure with mortality outcomes remained independent of and were not modified by one another. In our further evaluation of the joint impact of CRF and FSB on risk of mortality outcomes, the associations were strongest for men with high CRF & high FSB, followed by high CRF & low FSB and then low CRF & high FSB. The combined exposures of CRF and FSB (in any combination of categories) were more strongly associated with mortality outcomes compared with the separate associations for each exposure. These results suggest a multiplicative effect of both exposures. However, the overall findings suggest CRF to be a stronger indicator of mortality outcomes compared with FSB. Finally, our findings suggest that the NNT for high aerobic fitness levels and frequent sauna bathing to prevent one death over long-term follow-up ranged from 4 to 5 in approximately healthy middle-aged men.
The mechanistic pathways underlying the protective effects of high aerobic capacity or fitness levels (as defined objectively by CRF) on vascular and non-vascular outcomes have been discussed extensively in the literature and these involve both physiological and metabolic processes [Citation23]. These include reduction in inflammation [Citation24,Citation25]; reduction in oxidative stress; beneficial modulation of circulating cardiovascular risk markers, such as lipids, glucose, natriuretic peptides and cardiac troponin T [Citation26,Citation27]; increase in cardiac output and the formation of collateral vessels [Citation28,Citation29]; and reductions in body weight and blood pressure as well as improvement in endothelial function [Citation28,Citation30]. Sauna bathing may confer its protective effects on CVD and mortality via several mechanisms and these include (i) improvement in cardiovascular function [Citation31–33]; (ii) reduction in systemic blood pressure [Citation20]; (iii) increased cardiac output as a result of increased body temperature which causes increased skin blood flow, whereas there is a decrease in blood flow to the internal organs [Citation32]; (iv) improvement in endothelial function [Citation34,Citation35]; (v) increase in left ventricular ejection fraction [Citation34]; and (vi) reductions in oxidative stress [Citation36,Citation37]; a recent study has shown that a single Finnish sauna bath is able to reduce the oxidative stress caused by 30 min of aerobic exercise in healthy men [Citation36]. Other effects of regular sauna bathing which might contribute to its risk reduction of mortality outcomes include positive alterations of the autonomic nervous system, reduced levels of natriuretic peptides, and improved arterial stiffness and intima media thickness [Citation36,Citation38–40]. Indeed, evidence suggests the responses produced by an ordinary sauna bath correspond to that produced by moderate or high intensity physical activity such as walking [Citation41]. In contrast to the training response experienced during physical activity, there is no active function of skeletal muscles during the sauna bathing. However, sauna induced increased heart rate increases myocardial workload and oxygen demand similarly to physical exercise. It also significantly increases the endurance of the musculoskeletal and cardiorespiratory system [Citation42].
Given the common pathways postulated to underpin the associations of CRF and sauna bathing on CVD and mortality risk, the strong protective association observed as a result of the combination of both exposures (high CRF & high FSB) may be as a result of the multiplicative effect of these pathways. For example, it has been demonstrated that there is enhanced metabolism when isotonic exercise is performed during sauna exposure [Citation43]. It has also been shown that the heart rate response to a subsequent bout of exercise is higher when it is preceded by a combination of exercise and sauna than just by the sauna [Citation44]. Though sauna exposure produces some adaptations that are similar to the effects induced by exercise; whether the beneficial effects of sauna are dependent on baseline physical capacity or CRF is not very clear. In an investigation on the effects of sauna bathing on athletes, the authors demonstrated an augmentation in acute physiological responses when sauna exposure followed exercise [Citation44]. In another study in which six male distance runners completed three weeks of post-training sauna bathing, study participants experienced an enhancement in endurance running performance [Citation42]. Taking the whole evidence together suggests that beneficial effects of sauna bathing may be more marked in people with good fitness levels at baseline. However, further evaluation is still needed.
These results highlight a substantial risk reduction of cardiovascular and all-cause mortality events in middle-aged men with high CRF levels as well as frequent sauna bathing (3–7 sessions per week). Though high aerobic fitness levels are well established to be linked with health benefits and longevity, our findings suggest that a combination of regular aerobic exercise activity leading to improved CRF and frequent sauna bathing has added benefits and confers more protection on mortality outcomes. Indeed, our absolute risk reduction estimates translate to only 4–5 middle-aged men who need to be both aerobically fit and frequent sauna bathers, to prevent one death over their life time. However, the NNT estimate needs to be interpreted with caution as it assumes that the effect of the intervention or exposure (fitness levels and frequent sauna bathing) is constant over time and mortality events occur at a constant rate over time [Citation37]. Sauna bathing is a well-tolerated recreational activity, can be used after an exercise session and has a good safety profile [Citation31]. Sauna bathing seems to be safe even among patients who have recovered from myocardial infarction (MI) and patients with stable angina pectoris or heart failure. However, individuals who are prone to orthostatic hypotension, unstable angina pectoris, recent MI or severe aortic stenosis should be cautious while using the sauna [Citation31]. Further studies into the biological processes underlying the protective effects of sauna bathing on adverse health outcomes and its added role in CVD and mortality risk prevention in general populations are warranted. We acknowledge that the public health implications of the current findings may seem limited in populations where sauna use is not common. However, given the emerging evidence on the potential health benefits of sauna bathing, it is expected that sauna use will increase in many other populations in the near future. Further research should replicate the current findings in other populations.
Strengths and limitations
Strengths of this study include the first prospective evaluation of the joint effect of CRF and FSB on cardiovascular and all-cause mortality risk, the recruitment of a representative sample of men who were well characterized, the long-term and complete follow-up of the cohort, and the adjustment for several cardiovascular risk factors. Some limitations of this study also merit consideration. Though CRF was objectively measured by VO2max, which is an accurate measure of aerobic fitness; sauna bathing frequency was assessed using self-reported questionnaires, which may have introduced the possibility of misclassification bias. The findings were based on a sample of Caucasian men and therefore cannot be generalized to women and other populations. There was also possibility of residual confounding due to the observational study design.
Conclusions
In a general male Caucasian population, a combination of high CRF levels and regular sauna bathing confers stronger long-term protection on cardiovascular and all-cause mortality outcomes compared with good CRF or frequent sauna bathing alone. However, objectively measured CRF remained a stronger indicator of cardiovascular and all-cause mortality outcomes.
Acknowledgements
The authors are grateful to the staff of the Kuopio Research Institute of Exercise Medicine and the Research Institute of Public Health and University of Eastern Finland, Kuopio, Finland for the data collection in the study.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Kokkinos P. Physical activity, health benefits, and mortality risk. ISRN Cardiol. 2012;2012:718789.
- Noonan V, Dean E. Submaximal exercise testing: clinical application and interpretation. Phys Ther. 2000;80:782–807.
- Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–2035.
- Laukkanen JA, Kurl S, Salonen R, et al. The predictive value of cardiorespiratory fitness for cardiovascular events in men with various risk profiles: a prospective population-based cohort study. Eur Heart J. 2004;25:1428–1437.
- Perasalo J. Traditional use of the sauna for hygiene and health in Finland. Ann Clin Res. 1988;20:220–223.
- Valtakari P. The sauna and bathing in different countries. Ann Clin Res. 1988;20:230–235.
- Laukkanen T, Kunutsor S, Kauhanen J, et al. Sauna bathing is inversely associated with dementia and Alzheimer’s disease in middle-aged Finnish men. Age Ageing. 2017;46:245–249.
- Laitinen LA, Lindqvist A, Heino M. Lungs and ventilation in sauna. Ann Clin Res. 1988;20:244–248.
- Cox NJ, Oostendorp GM, Folgering HT, et al. Sauna to transiently improve pulmonary function in patients with obstructive lung disease. Arch Phys Med Rehabil. 1989;70:911–913.
- Nurmikko T, Hietaharju A. Effect of exposure to sauna heat on neuropathic and rheumatoid pain. Pain. 1992;49:43–51.
- Kunutsor SK, Laukkanen T, Laukkanen JA. Sauna bathing reduces the risk of respiratory diseases: a long-term prospective cohort study. Eur J Epidemiol. 2017 [cited Sep 13]. DOI:10.1007/s10654-017-0311-6
- Laukkanen T, Khan H, Zaccardi F, et al. Association between sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Intern Med. 2015;175:542–548.
- Kunutsor SK, Kurl S, Khan H, et al. Associations of cardiovascular and all-cause mortality events with oxygen uptake at ventilatory threshold. Int J Cardiol. 2017;236:444–450.
- Kunutsor SK, Khan H, Nyyssonen K, et al. Lipoprotein(a) and risk of sudden cardiac death in middle-aged Finnish men: a new prospective cohort study. Int J Cardiol. 2016;220:718–725.
- Salonen JT, Nyyssonen K, Korpela H, et al. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992;86:803–811.
- Laukkanen JA, Laaksonen D, Lakka TA, et al. Determinants of cardiorespiratory fitness in men aged 42 to 60 years with and without cardiovascular disease. Am J Cardiol. 2009;103:1598–1604.
- Lakka TA, Venalainen JM, Rauramaa R, et al. Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction. N Engl J Med. 1994;330:1549–1554.
- Yang S, Lynch JW, Raghunathan TE, et al. Socioeconomic and psychosocial exposures across the life course and binge drinking in adulthood: population-based study. Am J Epidemiol. 2007;165:184–193.
- Lynch JW, Kaplan GA, Cohen RD, et al. Childhood and adult socioeconomic status as predictors of mortality in Finland. Lancet. 1994;343:524–527.
- Zaccardi F, Laukkanen T, Willeit P, et al. Sauna bathing and incident hypertension: a prospective cohort study. Am J Hypertens. 2017 [cited Jun 13]. DOI:10.1093/ajh/hpx102
- Therneau TM, Grambsch PM. Modeling survival data: extending the cox model. New York (NY): Springer; 2000.
- Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319:1492–1495.
- Physical activity and cardiovascular health. NIH Consensus Development Panel on Physical Activity and Cardiovascular Health. JAMA. 1996;276:241–246.
- Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology. 2002;13:561–568.
- Church TS, Barlow CE, Earnest CP, et al. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol. 2002;22:1869–1876.
- deFilippi CR, de Lemos JA, Tkaczuk AT, et al. Physical activity, change in biomarkers of myocardial stress and injury, and subsequent heart failure risk in older adults. J Am Coll Cardiol. 2012;60:2539–2547.
- Lin X, Zhang X, Guo J, et al. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2015;4:e002014.
- Hambrecht R, Wolf A, Gielen S, et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454–460.
- Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801.
- Niebauer J, Hambrecht R, Velich T, et al. Attenuated progression of coronary artery disease after 6 years of multifactorial risk intervention: role of physical exercise. Circulation. 1997;96:2534–2541.
- Hannuksela ML, Ellahham S. Benefits and risks of sauna bathing. Am J Med. 2001;110:118–126.
- Kukkonen-Harjula K, Kauppinen K. Health effects and risks of sauna bathing. Int J Circumpolar Health. 2006;65:195–205.
- Crandall CG, González-Alonso J. Cardiovascular function in the heat-stressed human. Acta Physiol (Oxf). 2010;199:407–423.
- Ohori T, Nozawa T, Ihori H, et al. Effect of repeated sauna treatment on exercise tolerance and endothelial function in patients with chronic heart failure. Am J Cardiol. 2012;109:100–104.
- Imamura M, Biro S, Kihara T, et al. Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J Am Coll Cardiol. 2001;38:1083–1088.
- Sutkowy P, Woźniak A, Boraczyński T, et al. The effect of a single Finnish sauna bath after aerobic exercise on the oxidative status in healthy men. Scand J Clin Lab Invest. 2014;74:89–94.
- Masuda A, Miyata M, Kihara T, et al. Repeated sauna therapy reduces urinary 8-epi-prostaglandin F(2alpha). Jpn Heart J. 2004;45:297–303.
- Radtke T, Poerschke D, Wilhelm M, et al. Acute effects of Finnish sauna and cold-water immersion on haemodynamic variables and autonomic nervous system activity in patients with heart failure. Eur J Prev Cardiolog. 2016;23:593–601.
- Miyata M, Kihara T, Kubozono T, et al. Beneficial effects of Waon therapy on patients with chronic heart failure: results of a prospective multicenter study. J Cardiol. 2008;52:79–85.
- Brunt VE, Howard MJ, Francisco MA, et al. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol (Lond). 2016;594:5329–5342.
- Vuori I. Sauna bather’s circulation. Ann Clin Res. 1988;20:249–256.
- Scoon GS, Hopkins WG, Mayhew S, et al. Effect of post-exercise sauna bathing on the endurance performance of competitive male runners. J Sci Med Sport. 2007;10:259–262.
- Iwase S, Kawahara Y, Nishimura N, et al. Effects of isotonic and isometric exercises with mist sauna bathing on cardiovascular, thermoregulatory, and metabolic functions. Int J Biometeorol. 2014;58:1109–1117.
- Ridge BR, Pyke FS. Physiological responses to combinations of exercise and sauna. Aust J Sci Med Sport. 1986;18:25–28.

