Abstract
Background: Individuals ascending to high altitude are at a risk of getting acute mountain sickness (AMS). The present study is a network meta-analysis comparing all the interventions available to prevent AMS.
Methods: Electronic databases were searched for randomized clinical trials evaluating the use of drugs to prevent AMS. Incidence of AMS was the primary outcome and incidence of severe AMS, paraesthesia (as side effect of acetazolamide use), headache and severe headache, and oxygen saturation were the secondary outcomes. Odds ratio [95% confidence interval] was the effect estimate for categorical outcomes and weighted mean difference for oxygen saturation. Random effects model was used to derive the direct and mixed treatment comparison pooled estimates. Trial sequential analysis and grading of the evidence for key comparisons were carried out.
Results: A total of 24 studies were included. Acetazolamide at 125, 250 and 375 mg twice daily, dexamethasone and ibuprofen had statistically significant lower incidence of AMS compared to placebo. All the above agents except ibuprofen were also observed to significantly reduce the incidence of severe AMS. Acetazolamide alone or in combination with Ginkgo biloba were associated with lower incidence of headache, but higher risk of paraesthesia. Acetazolamide at 125 mg and 375 mg twice daily significantly reduce the incidence of severe headache as like ibuprofen. Trial sequential analysis indicates that the current evidence is adequate for the incidence of AMS only for acetazolamide 125 and 250 mg twice daily. Similarly, the strength of evidence for acetazolamide 125 and 250 mg twice daily was moderate while it was either low or very low for all other comparisons.
Conclusions: Acetazolamide at 125, 250 and 375 mg twice daily, ibuprofen and dexamethasone significantly reduce the incidence of AMS of which adequate evidence exists only for acetazolamide 125 and 250 mg twice daily therapy. Acetazolamide 125 mg twice daily could be the best in the pool considering the presence of enough evidence for preventing AMS and associated with lower incidence of paraesthesia.
Acetazolamide 125, 250 and 375 mg twice daily, dexamethasone and ibuprofen reduce the incidence of AMS in high altitudes.
Adequate evidence exists supporting the use of acetazolamide 125 mg and 250 mg twice daily for preventing AMS of which acetazolamide 125 mg twice daily could be the best.
Key messages
Introduction
Acute mountain sickness (AMS) is characterized by headache, light-headedness, nausea and insomnia occurring due to rapid ascent to great height without acclimatization [Citation1]. AMS has been reported to occur between 15 and 80% [Citation2]. The incidence of AMS has been reported to be proportionate with the rate of ascent; 54% reported AMS at a mean ascent rate of 91 m/h and 73% at 1268 m/h [Citation3]. The main pathophysiologic event of AMS is hypoxia with low barometric pressure that can potentially lead to acute cerebral oedema [Citation4].
Ascending slowly at a rate of 300–500 m a day is the best measure to prevent AMS, gradually improves comfort, well-being and sleep and forms the physiological basis of acclimatization [Citation5]. Carbonic anhydrase inhibitor such as acetazolamide, corticosteroid like dexamethasone and analgesic such as ibuprofen has been proven to be useful in preventing AMS [Citation6]. A recent direct pair wise meta-analysis of randomized controlled clinical trials has confirmed the efficacy of acetazolamide in preventing AMS [Citation7]. However, there is a lack of systematic compilation and application of meta-analytic principles for interventions other than acetazolamide in the prevention of AMS. The present study is a network meta-analysis of pharmacological interventions available for preventing AMS in individuals ascending high altitudes.
Methods
Information sources and search strategy
We registered the protocol with PROSPERO, the registration number is CRD42017071272. A thorough literature search was conducted and completed on 11 July 2017 with the following search strategy: (((((((acetazolamide [tiab] OR diamox [tiab] OR dexamethasone [tiab] OR steroids [tiab] OR corticosteroids [tiab] OR budesonide [tiab] OR carbonic anhydrase inhibitor* [tiab] OR ibuprofen [tiab] OR carbasalate [tiab] OR NSAID* [tiab])) AND (mountain sickness [tiab] OR high altitude [tiab]))) AND random* [tiab]) NOT (review [pt] OR review [tiab]). We included studies published from the year 2000 and no limits were placed with regard to language of publication.
Eligibility criteria
We included only randomized controlled clinical trials evaluating prophylactic effects of active medications in preventing AMS following ascent to a high altitude. We included studies that compared one active drug with another or with placebo. We excluded studies that have recruited individuals with past history of AMS or with any other significant medical conditions. Incidence of AMS was considered as the primary outcome variable while incidence of severe AMS, paraesthesia (as side effect of acetazolamide use), headache, severe headache, adverse events and oxygen saturation were the secondary outcomes.
Study procedure and statistical analysis
Two authors carried out an independent literature search and extracted the following details: trial site, year, trial methods, participants, interventions and outcomes. Any disagreement between the authors was resolved through discussion. The present systematic review and network meta-analysis was conducted and reported according to preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines [Citation8]. Risk of bias of the included studies was assessed using Cochrane risk of bias tool [Citation9]. Publication bias was assessed for the comparison of acetazolamide with placebo using Funnel plot, Trim and Fill method of analysis, Eggers regression analysis and Begg and Mazumdar rank correlation tests. Odds ratio [95% confidence interval] was used to assess categorical outcomes while weighted mean difference [95% confidence interval] was used for assessing the difference in oxygen saturation. Random effects model was used to derive the pooled estimates by direct and mixed treatment comparison methods. Inconsistency between direct and indirect pooled effect estimates was assessed using ¯H statistics wherein a value of <3 was considered as minimal, 3–6 as modest and >6 as gross [Citation10]. Most of the included studies have defined AMS as Lake Louise AMS score ≥3 with headache and presence of one of the following symptoms: nausea, fatigue, dizziness and difficulty sleeping. Sensitivity analysis was carried out by removing studies that defined AMS by other criteria and also trials with high risk of bias in at least one domain. We also carried out trial sequential analysis for the comparison of acetazolamide with placebo for the primary outcome variable by adjusting the direct pooled estimate to the information size accrued till date. O’Brien-Fleming method of alpha-spending function was used with 5% alpha error and 80% power for assessing the statistical significance of the estimate. MetaXL was used for the analysis of mixed treatment comparison estimates, Meta-Essentials for assessing publication bias and trial sequential analysis software for analysing the adjusted pooled estimates [Citation11–13]. We graded the evidence for key comparisons for the primary outcome variable using Grades of Recommendation, Assessment, Development and Evaluation (GRADE) working group approach [Citation9].
Results
Search results
A total of 114 articles were obtained with the search strategy of which finally 24 [Citation14–37] were included. The study flow diagram as per PRISMA requirement is shown in . Key characteristics of the included studies are mentioned in Supplementary Table S1. A summary of the risk of bias of the individual studies for each domain is depicted in Supplementary Figure S1 and overall low risk was observed in most of the domains.
Publication bias
Publication bias was assessable for the comparisons of acetazolamide 125 and 250 mg twice daily with placebo for the incidence of AMS. No missing studies were identified by the Trim and Fill method of analyses for both the comparisons thus the chances of publication bias for these comparisons is slim (Supplementary Figures S2 and S3). Egger’s regression co-efficient test value was 0.38 and p value was .7 for acetazolamide 125 mg compared to placebo and Begg and Mazumdar rank correlation test also revealed absence (p = .5) of publication bias for the same. Egger’s regression co-efficient test value for acetazolamide 250 mg twice daily was –0.82 with p value of .5 and Begg and Mazumdar rank correlation test also did not reveal any publication bias (p = .3).
Pooled estimates
Primary outcome
A total of 21 studies with 3493 participants were pooled for the analysis of AMS incidence. The network plot comparing the interventions is depicted in Supplementary Figure S4. Forest plot of the mixed treatment comparison estimates of the comparisons is depicted in and it can be observed that acetazolamide 375 mg twice daily, dexamethasone, ibuprofen, acetazolamide 125 mg twice daily, combined acetazolamide with Ginkgo biloba and acetazolamide 250 mg twice daily had statistically significant lower incidence of AMS compared to placebo. Additionally, dexamethasone, ibuprofen, acetaminophen, acetazolamide 125 mg, 250 mg and 375 mg twice daily performed better than spironolactone {0.2 [0.1, 0.6], 0.2 [0.1, 0.5], 0.3 [0.1, 0.9], 0.2 [0.1, 0.5], 0.2 [0.1, 0.5] and 0.1 [0.02, 0.3], respectively}. Acetaminophen, methazolamide, budesonide, procaterol, combined budesonide with formoterol, combined acetazolamide with dexamethasone, calcium carbasalate, acetazolamide 85 mg thrice daily, Ginkgo biloba and spironolactone were not observed to possess any significant benefit compared to placebo (). Comparison of the pooled estimates by direct and mixed treatment analyses between the interventions is mentioned in . It can be observed that acetazolamide at 125, 250 and 375 mg twice daily, combined acetazolamide with Ginkgo biloba, ibuprofen and dexamethasone had statistically significant effects in both direct and mixed treatment analyses against placebo. A high degree of consistency was observed between the direct and mixed treatment comparison pooled estimates (¯H=1). However, the mixed treatment comparison pooled estimate was not significant for Ginkgo biloba compared to placebo {0.68 [0.19, 2.45]} and the combination of acetazolamide with Ginkgo biloba was not observed to perform better than acetazolamide alone.
Figure 2. Forest plot of mixed treatment comparison estimates for the incidence of AMS. Acetazolamide 375 mg twice daily, dexamethasone, ibuprofen, acetazolamide 125 mg twice daily, combined acetazolamide with Ginkgo biloba and acetazolamide 250 mg twice daily were observed with significantly lower incidence of AMS compared to placebo.
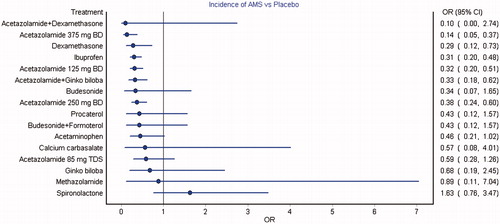
Table 1. Mixed treatment comparison pooled estimates {odds ratio [95% confidence interval]} for the key comparisons for incidence of AMS.
Secondary outcome measures
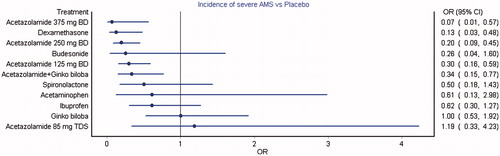
A total of 13 studies with 2548 participants were pooled for the analysis of incidence of severe AMS and the mixed treatment comparison estimates revealed a statistically significant reduction in the incidence with acetazolamide 375 mg twice daily, dexamethasone, acetazolamide 250 mg twice daily, acetazolamide 125 mg twice daily and combination of acetazolamide and Ginkgo biloba compared to placebo (). Ibuprofen, acetazolamide at 85 mg thrice daily, 125 mg and 250 mg twice daily, procaterol, inhalational budesonide, inhalational budesonide with formoterol and acetazolamide with dexamethasone were observed with statistically significant higher oxygen saturation compared to placebo in the pooled analysis (15 studies with 1702 participants) (Supplementary Figure S5). Acetazolamide at all the doses either alone or in combination with Ginkgo biloba was associated with significantly higher incidence of paraesthesia compared to placebo (seven studies; 1603 participants) (Supplementary Figure S6). Incidentally, only the same interventions also were observed with significant reduction in the incidence of headache (nine studies; 2160 participants) (Supplementary Figure S7). Ibuprofen and acetazolamide at 125 mg and 375 mg twice daily were observed with significantly lower incidence of severe headache compared to placebo (six studies; 1279 participants) (Supplementary Figure S8).
Sensitivity analysis
Fourteen of the 21 studies included for analysis of primary outcome variable used the following criteria for diagnosis of AMS: Lake Louise AMS score ≥3 with headache and presence of one of the following symptoms: nausea, fatigue, dizziness and difficulty sleeping. Seven studies [Citation18,Citation20,Citation31–33,Citation35,Citation37] used a different criteria and removal of the estimates from the pooled analysis did not alter the overall interpretation of incidence of AMS significantly (Supplementary Figure S9). Similarly, excluding data from studies with high risk of bias in at least one domain obtained similar results as that of the overall analysis except that acetazolamide at 250 mg twice daily was not observed with significant effects (Supplementary Figure S10).
Trial sequential analysis
Trial sequential analyses were carried out for the comparisons of acetazolamide 125 mg and 250 mg twice daily with placebo for AMS incidence. Enough evidence has accrued till date confirming the protective effect of acetazolamide (at 125 and 250 mg twice daily) against AMS (Supplementary Figures S11 and S12).
Grading the evidence
Grading of the evidence for key comparisons was carried out based on the limitations of precision, publication bias, risk of bias and heterogeneity for the direct comparisons and consistency for the mixed treatment comparisons (). The evidence for acetazolamide at 125 and 250 mg twice daily was moderate and either low or very low for all other comparisons.
Table 2. Grading the evidence for key interventions compared to placebo for the incidence of AMS.
Discussion
The present network meta-analysis was conducted to compare the efficacy and safety of agents used in the prevention of AMS in individuals ascending high altitudes. A total of 24 studies were included in the final analysis. Acetazolamide 375 mg twice daily, dexamethasone, ibuprofen, acetazolamide 125 mg twice daily and acetazolamide 250 mg twice daily had statistically significant lower incidence of AMS compared to placebo. Acetazolamide at 125, 250 and 375 mg twice daily and dexamethasone were also observed to significantly reduce the incidence of severe AMS. Acetazolamide was also observed to lower the incidence of headache but with a higher risk of paraesthesia. Acetazolamide at 125 mg and 375 mg twice daily significantly reduces the incidence of severe headache as like ibuprofen. Trial sequential analysis indicates that the current evidence is adequate for the incidence of AMS only for acetazolamide 125 and 250 mg twice daily. Similarly, the strength of evidence for acetazolamide 125 and 250 mg twice daily was moderate while it was either low or very low for all other comparisons.
High altitude illness ranges from non-serious AMS to life-threatening high altitude pulmonary oedema and cerebral oedema and the symptoms tend to be most severe in the first 24–96 hours of presence in high altitude [Citation38]. The main precipitating factor for high altitude sickness is hypoxia combined with low air pressure that induces cerebral vasodilation and headache. Acetazolamide acidifies the blood thus improving the respiration and the subsequent oxygen saturation [Citation39]. In fact, acetazolamide has been the most common drug evaluated for preventing high altitude sickness. However, due to commonly observed adverse effects with acetazolamide, researchers evaluated various other agents but there is lack of head-to-head clinical trials comparing these interventions. A network meta-analysis offers an advantage to evaluate the available interventions for a disease condition in a single platform. To discuss with an example, A, B and C are three interventions and head-to-head clinical trials have been conducted between A and B and B and C. By using principles of network meta-analysis, the relative effect estimates between A and C can be predicted in the absence of head-to-head clinical trials between A and C. The present study is the first network meta-analysis and we observed a lot of interventions to be useful in preventing AMS with no significant differences between the interventions as assessed by mixed treatment comparison estimates. Recent guidelines for the prevention of AMS and high altitude illness suggest the use of acetazolamide at 125 mg twice daily [Citation40]. In the present network meta-analysis, we observed that acetazolamide at 125, 250 and 375 mg twice daily and in combination with Ginkgo biloba reduces the incidence of AMS with no significant differences in the pooled estimates between the interventions. However, acetazolamide at 125 mg twice daily is associated with the least incidence of paraesthesia compared to other doses. Additionally, acetazolamide at 125 twice daily reduces the incidence of severe headache and the trial sequential analysis showed presence of adequate evidence for 125 mg twice daily regimen in preventing AMS. No conclusion could be drawn for the association of severe headache with acetazolamide 250 mg twice daily due to lack of separate reporting of data on headache. Low et al. [Citation41] systematically analysed the literature and through a pairwise direct meta-analysis, concluded that 125 mg twice daily is the lowest possible dose of acetazolamide that could be effective. Although, the present meta-analysis showed promising therapeutic effects of ibuprofen and the combination of acetazolamide with Ginkgo biloba, the evidence should be considered preliminary as trial sequential analysis could not be performed due to scanty data. Also, the addition of Ginkgo biloba has not been observed to increase the therapeutic effect of acetazolamide and hence it is unsure whether Ginkgo biloba has an independent therapeutic benefit in preventing AMS. This was also reflected by the absence of any significant effect in preventing AMS in the present study for Ginkgo biloba. Recent direct pairwise meta-analyses comparing ibuprofen with placebo established a number needed to treat; six for incidence of AMS, seven for high altitude headache and 13 for severe headache [Citation42,Citation43]. We also observed that the incidence of AMS was not significantly different for ibuprofen and dexamethasone compared to acetazolamide. However, this evidence is entirely based on the estimates obtained indirectly through placebo as the comparator. Hence more caution is needed while interpreting the same as the results is not confirmatory and may change with accruing evidence in the future. Hence, in individuals allergic to acetazolamide, ibuprofen or dexamethasone may be a suitable alternative agent. Although none of the studies have reported any serious adverse event with either ibuprofen or dexamethasone, prescribers need to be more concerned when using in patients with severe peptic ulcer disease or renal failure.
The strength of the study is that this is the first network meta-analysis comparing all the interventions available for preventing AMS and till date has included the maximum number of individuals amongst the meta-analyses in this field; most of the studies have used similar criteria for the diagnosis of AMS and the trial sequential analysis after adjusting for the type 1 error still showed significant benefits for acetazolamide 125 and 250 mg twice daily. From clinical standpoint, it might take several years to generate real time evidence comparing all the interventions included in this meta-analysis. However, the study is limited by the fact that the altitude differences at which participants have been recruited in the included studies were not considered; rate of ascent was different between the studies; lost to follow up observed in few studies; past history of AMS has not been reported in all the included studies and EMBASE could not be searched due to access constraints. However, hand search from potential studies did not reveal the presence of any other study. Sparse data for the medical interventions included in this meta-analysis precluded any analysis on the differences in the adverse event profile.
To conclude, acetazolamide at 125, 250, 375 mg twice daily, ibuprofen and dexamethasone significantly reduce incidence of AMS. However, adequate evidence for recommendation exists only for acetazolamide 125 and 250 mg twice daily. Of these two, acetazolamide 125 mg twice daily is preferable considering the lower risk of paraesthesia. Evidence regarding other interventions is preliminary and should be interpreted with caution as the results might change with the future head-to-head clinical trials.
Kannan_et_al._Supplementary_Material.pdf
Download PDF (744.3 KB)Acknowledgements
We thank PROSPERO for registering the review protocol. We are also grateful to Cochrane for utilizing RevMan 5.3 software for generating risk of bias graph. We are grateful to EpiGear for using MetaXL in generating mixed treatment comparison results for secondary outcomes and Copenhagen Trial Unit for utilizing trial sequential analysis software.
Disclosure statement
The authors do not have any conflict of interest.
Additional information
Funding
References
- Hackett PH, Roach RC. High-altitude illness. N Engl J Med. 2001;345:107–114.
- Mairer K, Wille M, Burtscher M. The prevalence of and risk factors for acute mountain sickness in the Eastern and Western Alps. High Alt Med Biol. 2010;11:343–348.
- Murdoch D. Altitude sickness. BMJ Clin Evid. 2010;2010:1209.
- Basnyat B, Murdoch DR. High-altitude illness. Lancet. 2003;361:1967–1974.
- Bärtsch P, Swenson ER. Clinical practice: acute high-altitude illnesses. N Engl J Med. 2013;368:2294–2302.
- Fiore DC, Hall S, Shoja P. Altitude illness: risk factors, prevention, presentation, and treatment. Am Fam Phys. 2010;82:1103–1110.
- Ritchie ND, Baggott AV, Andrew Todd WT. Acetazolamide for the prevention of acute mountain sickness—a systematic review and meta-analysis. J Travel Med. 2012;19:298–307.
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784.
- Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. 5.1.0 edition; [cited 2017 Jul 14]. Available from: http://handbook-5-1.cochrane.org/
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558.
- Van Rhee HJ, Suurmond R, Hak T. User manual for meta-essentials: workbooks for meta-analysis (Version 1.0). Rotterdam, The Netherlands: Erasmus Research Institute of Management; [cited 2017 Jul 14]. Available from: www.erim.eur.nl/research-support/meta-essentials
- Thorlund K, Engstrom J, Wetterslev J, et al. Trial sequential analysis. Copenhagen trial unit; [cited 2017 Jul 14]. Available from: http://www.ctu.dk/tools-and-links/trial-sequential-analysis.aspx
- Epigear MetaXL; [cited 2017 Jul 14]. Available from: http://www.epigear.com/index_files/contact.html
- Basnyat B, Gertsch JH, Johnson EW, et al. Efficacy of low-dose acetazolamide (125 mg BID) for the prophylaxis of acute mountain sickness: a prospective, double-blind, randomized, placebo-controlled trial. High Alt Med Biol. 2003;4:45–52.
- Basnyat B, Gertsch JH, Holck PS, et al. Acetazolamide 125 mg BD is not significantly different from 375 mg BD in the prevention of acute mountain sickness: the prophylactic acetazolamide dosage comparison for efficacy (PACE) trial. High Alt Med Biol. 2006;7:17–27.
- Basnyat B, Holck PS, Pun M, et al. Spironolactone does not prevent acute mountain sickness: a prospective, double-blind, randomized, placebo-controlled trial by SPACE Trial Group (spironolactone and acetazolamide trial in the prevention of acute mountain sickness group). Wilderness Environ Med. 2011;22:15–22.
- Bradwell AR, Myers SD, Beazley M, et al. Exercise limitation of acetazolamide at altitude (3459 m). Wilderness Environ Med. 2014;25:272–277.
- Carlsten C, Swenson ER, Ruoss S. A dose-response study of acetazolamide for acute mountain sickness prophylaxis in vacationing tourists at 12,000 feet (3630 m). High Alt Med Biol. 2004;5:33–39.
- Chen GZ, Zheng CR, Qin J, et al. Inhaled budesonide prevents acute mountain sickness in young Chinese men. J Emerg Med. 2015;48:197–206.
- Choi PC, Lee JH. Comparison of methazolamide and acetazolamide for prevention of acute mountain sickness in adolescents. J Emerg Med Public Health. 2011;22:523–530.
- Chow T, Browne V, Heileson HL, et al. Ginkgo biloba and acetazolamide prophylaxis for acute mountain sickness: a randomized, placebo-controlled trial. Arch Intern Med. 2005;165:296–301.
- Gertsch JH, Basnyat B, Johnson EW, et al. Randomised, double blind, placebo controlled comparison of ginkgo biloba and acetazolamide for prevention of acute mountain sickness among Himalayan trekkers: the prevention of high altitude illness trial (PHAIT). BMJ. 2004;328:797.
- Gertsch JH, Lipman GS, Holck PS, et al. Prospective, double-blind, randomized, placebo-controlled comparison of acetazolamide versus ibuprofen for prophylaxis against high altitude headache: the Headache Evaluation at Altitude Trial (HEAT). Wilderness Environ Med. 2010;21:236–243.
- Gertsch JH, Corbett B, Holck PS, et al. Altitude Sickness in Climbers and Efficacy of NSAIDs Trial (ASCENT): randomized, controlled trial of ibuprofen versus placebo for prevention of altitude illness. Wilderness Environ Med. 2012;23:307–315.
- Hillenbrand P, Pahari AK, Soon Y, et al. Prevention of acute mountain sickness by acetazolamide in Nepali porters: a double-blind controlled trial. Wilderness Environ Med. 2006;17:87–93.
- Kanaan NC, Peterson AL, Pun M, et al. Prophylactic acetaminophen or ibuprofen result in equivalent acute mountain sickness incidence at high altitude: a prospective randomized trial. Wilderness Environ Med. 2017;28:72–78.
- Kayser B, Hulsebosch R, Bosch F. Low-dose acetylsalicylic acid analog and acetazolamide for prevention of acute mountain sickness. High Alt Med Biol. 2008;9:15–23.
- Ke T, Wang J, Swenson ER, et al. Effect of acetazolamide and gingko biloba on the human pulmonary vascular response to an acute altitude ascent. High Alt Med Biol. 2013;14:162–167.
- Lipman GS, Kanaan NC, Holck PS, et al. Ibuprofen prevents altitude illness: a randomized controlled trial for prevention of altitude illness with nonsteroidal anti-inflammatories. Ann Emerg Med. 2012;59:484–490.
- Lipman GS, Pomeranz D, Burns P, et al. A randomized controlled trial of budesonide versus acetazolamide for the prevention of acute mountain sickness. Am J Med. 2017;13. DOI:10.1016/j.amjmed.2017.05.034
- Moraga FA, Flores A, Serra J, et al. Ginkgo biloba decreases acute mountain sickness in people ascending to high altitude at Ollagüe (3696 m) in northern Chile. Wilderness Environ Med. 2007;18:251–257. Erratum in: Wilderness Environ Med. 2008;19:51.
- Parati G, Revera M, Giuliano A, et al. Effects of acetazolamide on central blood pressure, peripheral blood pressure, and arterial distensibility at acute high altitude exposure. Eur Heart J. 2013;34:759–766.
- Sikri G, Srinivasa AB, Grewal RS. Is concurrent prophylactic use of acetazolamide and dexamethasone superior to acetazolamide alone in un-acclimatized lowlanders on ascent to high altitude? Indian J Physiol Pharmacol. 2014;58:87–91.
- van Patot MC, Leadbetter G, 3rd, Keyes LE, et al. Prophylactic low-dose acetazolamide reduces the incidence and severity of acute mountain sickness. High Alt Med Biol. 2008;9:289–293.
- Wang J, Ke T, Zhang X, et al. Effects of acetazolamide on cognitive performance during high-altitude exposure. Neurotoxicol Teratol. 2013;35:28–33.
- Wright AD, Beazley MF, Bradwell AR, et al. Medroxyprogesterone at high altitude. The effects on blood gases, cerebral regional oxygenation, and acute mountain sickness. Wilderness Environ Med. 2004;15:25–31.
- Zheng CR, Chen GZ, Yu J, et al. Inhaled budesonide and oral dexamethasone prevent acute mountain sickness. Am J Med. 2014;127:1001–1009.e2.
- Imray C, Booth A, Wright A, et al. Acute altitude illnesses. BMJ. 2011;343:d4943.
- Swenson ER. Carbonic anhydrase inhibitors and high altitude illnesses. Subcell Biochem. 2014;75:361–386.
- Luks AM, McIntosh SE, Grissom CK, et al. Wilderness Medical Society. Wilderness Medical Society practice guidelines for the prevention and treatment of acute altitude illness: 2014 update. Wilderness Environ Med. 2014;25:S4–S14.
- Low EV, Avery AJ, Gupta V, et al. Identifying the lowest effective dose of acetazolamide for the prophylaxis of acute mountain sickness: systematic review and meta-analysis. BMJ. 2012;345:e6779.
- Xiong J, Lu H, Wang R, et al. Efficacy of ibuprofen on prevention of high altitude headache: a systematic review and meta-analysis. PLoS One. 2017;12:e0179788.
- Pandit A, Karmacharya P, Pathak R, et al. Efficacy of NSAIDs for the prevention of acute mountain sickness: a systematic review and meta-analysis. J Commun Hosp Intern Med Perspect. 2014;4. doi: 10.3402/jchimp.v4.24927