Abstract
Objectives
To appraise whether plasma exchange (PLEX) effectively improves visual function for acute optic neuritis (ON) in neuromyelitis optica (NMO) or neuromyelitis optica spectrum disorder (NMOSD).
Methods and analysis
We searched Medline, Embase, Cochrane Library, ProQuest Central, and Web of Science to identify relevant articles published between 2006 and 2020.
Eligible studies were in English and evaluated visual outcomes for people with acute ON in NMO or NMOSD treated with PLEX. They also had adequate pre- and posttreatment data. Excluded were studies with 1 or 2 case reports, or incomplete data.
Results
Twelve studies were qualitatively synthesized (1 RCT; 1 controlled NRSI; 10 observational studies). Five before-and-after observational studies were used for quantitative synthesis. The PLEX in the 5 studies (3 to 7 cycles over 2 to 3 weeks) was performed as second-line or adjunctive therapy for acute ON in NMO/NMOSD.
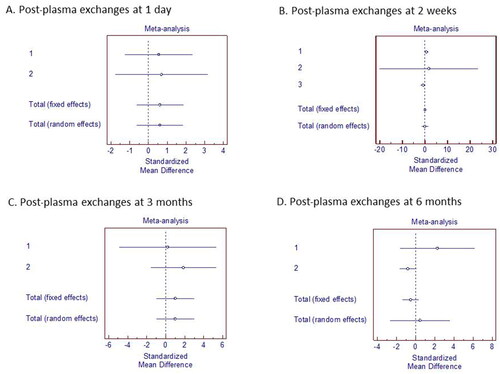
The qualitative synthesis revealed that visual-acuity recovery occurred between one day and 6 months after the first PLEX cycle completion. Thirty-two of 48 participants in the 5 quantitative-synthesis studies received PLEX. Relative to pre-PLEX values, visual-acuity improvements were nonsignificant at these post-PLEX time points: 1 day (SMD 0.611; 95% CI −0.620 to 1.842); 2 weeks (SMD 0.0214; 95% CI −1.250 to 1.293); 3 months (SMD 1.014; 95% CI −0.954 to 2.982); and 6 months (SMD 0.450; 95% CI −2.643 to 3.543).
Conclusions
There were inadequate data to determine whether PLEX effectively treats acute ON in NMO/NMOSD.
Key messages
Aggregate current data of this systematic review is insufficient to definitively conclude whether therapeutic PLEX is effective in improving VA in cases of NMO or NMOSD.
Background
Neuromyelitis optica (NMO) and neuromyelitis optica spectrum disorder (NMOSD) are autoimmune-mediated central nervous system disorders characterized by severe optic neuritis (ON) and transverse myelitis. They result in incomplete recovery, permanent disability, and a high risk of recurrence [Citation1]. Historically, NMO was deemed a subtype of multiple sclerosis (MS) as a result of their similar clinical presentations. Recently, however, NMO has been found to be distinct from MS in terms of ethnic groups, presentation of ON, and neuroimaging and laboratory findings [Citation2].
The appearance of serum aquaporin-4 IgG antibody (AQP4-Ab) distinguishes NMO, which is an astrocytopathic disorder arising from MS and other demyelinating diseases [Citation3]. After the disclosure of the association between AQP4-Ab and NMO, revised diagnostic criteria for NMO were promulgated in 2006 [Citation4]. These criteria had a higher specificity than the preceding criteria of 1999 [Citation2,Citation4]. In 2015, new diagnostic criteria for NMOSD, namely, the International Consensus Diagnostic Criteria for NMOSD, were proposed by Wingerchuk et al. [Citation5] Recurrent loss of vision from ON may initially or solely be found in NMOSD. Compared with MS, the prevalence of NMOSD is markedly higher among Asian than Western populations [Citation4,Citation6]. ON of NMOSD often manifests as severe visual loss and eventually results in complete blindness [Citation4,Citation7].
Because NMO/NMOSD is rare and frequently severe, there is limited evidence for current guideline treatments for NMO/NMOSD. Randomized control trials are not available to evaluate treatment efficacy. Most treatment recommendations have been based on reported cases, case series, and a small number of retrospective and prospective studies [Citation8]. These studies suggest that acute attacks or relapses of ON might be treated with a first-line therapy of a high-dose steroid, intravenous methylprednisolone, at the dosage of 1 g for 3 to 7 consecutive days. Early initiation of an escalation therapy with either plasma exchange (PLEX) or plasmapheresis is recommended as an additional therapy in cases of steroid-unresponsive ON [Citation2]. PLEX is increasingly being used for acute ON in NMOSD, and it has been reported to be effective. However, there are many constraints on performing PLEX, such as it is expensive, has poor accessibility, and has possible side effects [Citation5]. To date, no systematic reviews have evaluated the efficiency of PLEX compared with other treatments for patients with ON in NMO/NMOSD.
Objectives
To determine whether PLEX is effective in improving visual function following acute ON in cases of NMO or NMOSD, based on a literature review.
Methods
The protocol for this systematic review was registered with PROSPERO (CRD42020179901). The literature search was lastly performed in 2021.
Selection criteria
The following criteria were applied to select studies for this systematic review:
Participant types
Participants with acute ON who had been diagnosed with NMO or NMOSD, using standard diagnostic criteria, without any age, gender, or ethnicity restrictions.
Intervention types
Studies using PLEX, plasmapheresis, or apheresis as therapeutic interventions were included. We did not limit the PLEX courses (dose, duration, and onset). The control arms for comparisons could be steroids without PLEX, or any other treatments for acute ON. Moreover, studies without control arms were included.
Outcome measures
The outcome measures were visual functions assessed with any of the following parameters: visual acuity, contrast sensitivity, color vision, visual field, optical coherence tomography, retinal nerve fiber layer, macular ganglion cell layer, or visual evoked potential. These measures varied with each article. The same parameters were recorded before and after PLEX, with no limitations placed on the follow-up periods. We categorized the measurement time points of suitable outcomes if relevant data had been gathered sufficiently.
Study types
We included all types of studies: randomized controlled trials (RCTs), non-randomized studies of interventions (NRSIs), and observational studies. We excluded case reports or case series that had only 2 participants. Full-text studies, abstracts, and results published in trial registries were included if there was adequate information for analysis. Studies with incomplete data were excluded. Given that revised diagnostic criteria for NMO were released in 2006, the included articles were published between 2006 and 2020. There were no restrictions on the country of publication, but English versions of the articles were required.
A comprehensive literature search was conducted using Medline, Embase, Cochrane Library, ProQuest Central, and Web of Science. The search terms were ‘Optic neuritis’ and (‘Neuromyelitis Optica’ or ‘NMO’ or ‘Neuromyelitis Optica Spectrum Disorder’ or ‘NMOSD’ or ‘Devic’s disease’) and (‘Plasma exchange’ or ‘Plasmapheresis’ or ‘Apheresis’). The relevant published articles ranged from 1 January 2006 to 14 April 2020. We hand searched the reference dataset of all described studies, review articles, and practice guidelines from Google Scholar to assess all potentially related studies. In cases where the published data was incomplete, the authors of the publications concerned were contacted to retrieve additional information.
The studies were performed using a search strategy developed by 1 reviewer (YN). The search results were recorded in Endnote library. All records were verified by another reviewer, who is a neuro-ophthalmologist and an expert in systematic-review methodology (WC).
Data collection
We used EndNote X9 Library software to collect data from electronic searches and remove duplicated records.
Selection of studies
Two of the 4 reviewers (YN and WC) independently and carefully assessed the abstracts of the studies identified by the literature search to assess their eligibility for this review. The abstracts were encoded as ‘retrieve’ or ‘do not retrieve.’ Abstracts approved by at least 1 author were deemed eligible. Complete copies of the eligible full-text publications were subsequently obtained for further analysis.
Three reviewers who were neuro-ophthalmologists (WC, NC, and PL), one of whom was also an expert in systematic-review methodology (WC), independently assessed each of the articles. The reviewers resolved any disagreement by consensus. Studies were included for qualitative synthesis if they fulfilled the selection criteria and were accepted by all 3 reviewers.
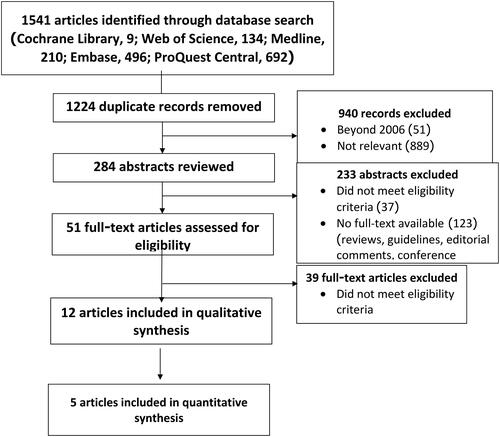
The study recruiting process was guided by the PRISMA statement (Moher 2009) and is illustrated in the flowchart at . The chart details the total numbers for the retrieved references and the included and excluded studies. It also lists the reasons for the exclusion of publications after their abstract or full-text assessments.
Data extraction and management
One reviewer (YN) gathered all data extractions and assessment. Two reviewers (WC and NC) independently evaluated the methodological quality and risk of bias of the eligible studies identified by the selection process. In cases of disagreement, a third reviewer (PL) was involved until consensus was reached.
One reviewer (YN) completed the customized data extraction in accordance with the Cochrane systematic review guidelines. Microsoft Excel software was used for this purpose. The accuracy and the plausibility of the extracted data were verified by another reviewer (either WC or NC).
The extracted data comprised name; authors; publication year; country; study period and design; inclusion and exclusion criteria; participant details; lateralization of eye involvement; criteria for NMO/NMOSD diagnoses; interventions (treatment course, dose, and duration of plasma exchange); outcome parameters; timing of outcome assessments; definition of treatment success; study implications, limitations, and risk of bias; and annotations. The combinations of outcome parameters employed differed from study to study. They consisted of these factors: visual acuity (VA), color vision, contrast sensitivity, visual field, optical coherence tomography, retinal nerve fiber layer assessment, visual evoked potential, and expanded disability status scale.
Three reviewers (WC, NC, and YN) independently determined the risk of bias of each study. If there was disagreement, a fourth reviewer (PL) assessed the study independently and made a final decision. We assessed the risk of bias using tools related to each study design. We used the risk-of-bias visualization tool (robvis) to produce a risk-of-bias summary [Citation9].
Statistical analysis
Eligible studies were included in the quantitative analysis if they had adequate statistical data on the same outcome parameters at both the pre- and posttreatment stages.
VA was the only outcome parameter common to all of the eligible studies. It was converted into a logarithm of minimum angle of resolution (LogMAR) for a continuous statistical analysis. The mean pre- and posttreatment LogMARs, including their standard deviations, were drawn out from each study. The results from the individual studies were grouped by post-plasma exchange periods. They were then analyzed separately at each follow-up time point.
The statistical analyses were performed using MetaEasy software. In the case of continuous outcomes that used the same scales but which were measured in different ways, [their analyses using their standardized mean differences (SMDs) with 95% CI. The total SMDs with 95% CI is assumed for both for the fixed-effects and random-effects models. If the value ‘0’ is not inside the 95% CI, then the SMD is deemed statistically significant at the 5% level (p < 0.05). If the test of heterogeneity is statistically significant (a high Cochran’s Q value, and p < 0.05), more emphasis should be placed on the random-effects model. The assessment of publication bias would be displayed via visualization of the funnel plot if sufficient studies proved to be eligible for the meta-analysis.
Dealing with missing data
We could not obtain missing data from the authors of a few studies [Citation3,Citation10–12]. As we did not collect any data at this stage, it was not necessary to make any conclusion. We decided to exclude those studies from the quantitative synthesis.
Data synthesis
All studies feasible for the quantitative synthesis had observational designs (before-and-after studies). We performed a simple meta-analysis. We separately assessed the standardized mean difference with 95% CI for each post-PLEX period: 1 day, 2 weeks, 1 month, 3 months, and 6 months. If a meta-analysis could not be performed because most of the outcome data did not allow quantitative assessment, we discussed based on the outcome in a narrative with the outcome from all studies shown in tables.
Results
Description of studies
Results of the search
The de-duplication process identified 1541 articles (9 articles from Cochrane Library, 134 from Web of Science, 210 from Medline, 496 from Embase, and 692 from ProQuest Central). After removing those duplicates, 1224 articles underwent rigorous title reviews. Of those, 940 were excluded as they were published before 2006 or did not relate to the current investigation.
An abstract review resulted in a total of 233 of the remaining 284 articles being excluded. In some cases, this was because they did not meet our eligibility criteria. In other cases, they did not have appropriate published data, given that they were either terminated or ongoing projects. The remaining 51 full-text articles were comprehensively assessed; 39 were excluded as they did not fulfil the inclusion criteria. While the remaining 12 articles were included in the qualitative synthesis, only 5 of them were also eligible for the quantitative synthesis as the other records did not present the outcomes of interest. We did not identify additional studies within those eligible studies’ citations. A flowchart depicting the retrieval, review, and selection process is at .
Design and sample size
The combined number of participants in the 12 studies included in the qualitative analysis was 233. Of those, 140 received PLEX. The 12 study types were 1 RCT [Citation12], 1 controlled NRSI [Citation13], and 10 observational studies [Citation3,Citation4,Citation14–20].
Only 5 of the 12 studies were utilized for the quantitative assessment [Citation4,Citation14–16,Citation19]. All had an observational design (before-and-after studies).
Efficacy outcomes
We evaluated the visual outcomes reported by 5 observational (before-and-after) studies.
In all, 48 participants were evaluated; 32 of them received PLEX and had pre- and posttreatment VA (LogMAR) data available.
We could not include the efficacy outcomes of the RCT or the controlled NRSI. This is because they reported only the mean pre- and posttreatment LogMARs for both the interventional and controlled groups, without raw data or standard deviations [Citation12,Citation13].
Of the 5 observational studies, 3 were excluded because only mean or median VAs were reported before and after PLEX [Citation11,Citation17,Citation18]. The other 2 studies were excluded as they did not report our prioritized outcomes [Citation3,Citation20]. Instead, they focused on the progression and treatment outcomes of ON without the specific outcomes for NMOSD [Citation20]. They also focused on the treatment during the maintenance phase without presenting adequate data for the acute phase of NMOSD [Citation3].
Settings
Of the 12 included articles, 6 originated from Europe, 4 from Germany [Citation14–16,Citation19], and 2 from France [Citation13,Citation17], 5 articles originated from Asia, 2 from Thailand [Citation11,Citation12], 2 from Japan [Citation3,Citation18], and 1 from China. [Citation4], and 1 article originated from Argentina [Citation20].
The 1 article from China [Citation4] and the 4 articles from Germany were included in the meta-analysis [Citation14–16,Citation19].
Participants
The 5 articles used for the meta-analysis had a combined total of 48 participants with acute ON. Their mean age was 40 years. Asians represented 31 of the 48 (64.6%) [Citation4], but no race was documented for the remaining 17 participants. Women accounted for 42 of the 48 participants (87.5%). The International Consensus Diagnostic Criteria for NMOSD, proposed by Wingerchuk in 2015, was used to diagnose NMOSD in the majority of participants [Citation5]. Two young participants were diagnosed with NMO using the International Pediatric MS Study Group criteria [Citation16]. Seropositivity (aquaporin-4 water channel- NMO-IgG or myelin oligodendrocyte glycoprotein-MOG-IgG) was found in 137 patients [Citation3,Citation4,Citation12,Citation18,Citation19].
Interventions
The first-line treatment for all participants was a 3- to 7-day course of high-dose, intravenous, methylprednisolone. The intervention of interest, PLEX, was administered to 32 of the 48 participants as second-line therapy in cases of steroid unresponsiveness, or as adjunctive treatment with steroids. The PLEX was performed as 3 to 7 cycles over 2 to 3 weeks. The mean delay from onset to the initiation of PLEX varied from 117 to 6 weeks [Citation14]. The main characteristics of the included studies are summarized in .
Table 1. Main characteristics of studies included in the qualitative synthesis.
Outcomes
Twelve studies reported an improvement in VA among the participants who underwent PLEX. Visual improvement was first recorded between 1 day after the initial PLEX cycle and 6 months following the last PLEX cycle. Only 5 of the 12 studies reported standard deviations in association with mean VA (converted to LogMAR). Posttreatment VA was recorded along with timing after receiving PLEX. A simple meta-analysis could be conducted by separately calculating the SMD with 95% CI at each post-plasma exchange period: 1 day [Citation14,Citation16], 2 weeks [Citation4,Citation15,Citation19], 1 month [Citation15], 3 months [Citation14,Citation16], and 6 months [Citation16,Citation19]. The pre- and posttreatment VAs reported by the 5 studies are detailed in .
Table 2. Details of visual acuity (VA) before and after plasma exchange (PLEX) of studies included in the quantitative synthesis.
Improvements in VA
The included articles consisted of 5 observational (before-and-after) studies that reported VA before PLEX (after receiving steroids) and after PLEX, measured from the last day of the PLEX cycle.
The analysis was carried out using the standardized mean difference as the outcome measure. A random-effects model was fitted to the data. The amount of heterogeneity (i.e. tau [Citation2]), was estimated using the Hedges’ estimator. In addition to the estimate of tau [Citation2], the Q-test for heterogeneity and the I2 statistic are reported. In case any amount of heterogeneity is detected (i.e. tau [Citation2] > 0, regardless of the results of the Q-test), a prediction interval for the true outcomes is also provided. Studentized residuals and Cook’s distances are used to examine whether studies may be outliers and/or influential in the context of the model.
Studies with a studentized residual larger than the 100 × (1–0.05/(2 X k)) the percentile of a standard normal distribution are considered potential outliers (i.e. using a Bonferroni correction with two-sided alpha = 0.05 for k studies included in the meta-analysis). Studies with a Cook’s distance larger than the median plus six times the interquartile range of the Cook’s distances are considered to be influential. The rank correlation test and the regression test, using the standard error of the observed outcomes as predictor, are used to check for funnel plot asymmetry.
A total of k = 3 studies were included in the analysis. The observed standardized mean differences ranged from −1.6459 to 0.7168, with the majority of estimates being negative (67%). The estimated average standardized mean difference based on the random-effects model was\hat{\mu} = −0.3024 (95% CI: −1.5617 to 0.9570). Therefore, the average outcome did not differ significantly from zero (z = −0.4706, p = 0.6379). According to the Q-test, the true outcomes appear to be heterogeneous (Q(2) = 7.8614, p = 0.0196, tau [Citation2] = 0.8580, I2 = 75.9138%). A 95% prediction interval for the true outcomes is given by −2.5119 to 1.9071. Hence, although the average outcome is estimated to be negative, in some studies the true outcome may in fact be positive. An examination of the studentized residuals revealed that one study had a value larger than ± 2.3940 and may be a potential outlier in the context of this model. According to the Cook’s distances, none of the studies could be considered to be overly influential. Neither the rank correlation nor the regression test indicated any funnel plot asymmetry (p = 1.0000 and p = 0.3594, respectively). Forest plots of the simple meta-analysis are presented in .
Risk of bias of included studies
Risk of bias of observational studies
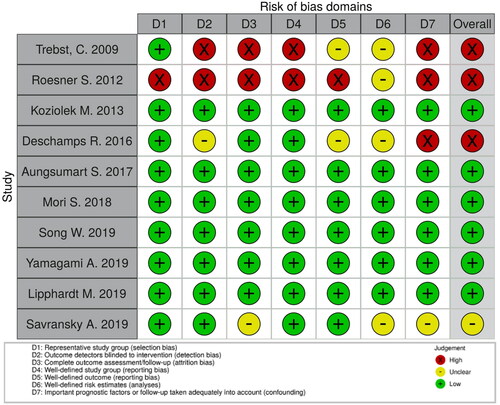
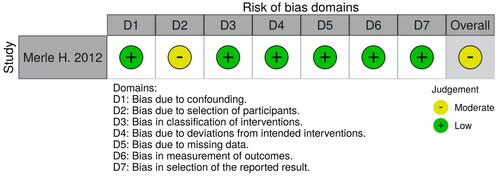
We critically assessed the methodological quality and risk of bias of the 10 observational studies [Citation3,Citation4,Citation11,Citation14–20], using Cochrane’s assessment criteria for observational studies tools. Their overall risks of bias were judged along with the highest risk domain of each study. We judged the overall risks to be ‘low’ for 6 studies [Citation3,Citation4,Citation11,Citation16,Citation18,Citation19], ‘unclear’ for 1 study [Citation19], and ‘high’ for 3 studies [Citation14,Citation15,Citation17]. The main high-risk domains were detection bias, attrition bias, reporting bias, and confounding.
The full judgements per trial and category are depicted in . The overall risks of bias of the included studies in the meta-analysis are summarized in .
Table 3. Overall risk of bias of studies included in the meta-analysis.
Risk of bias of randomized controlled trials
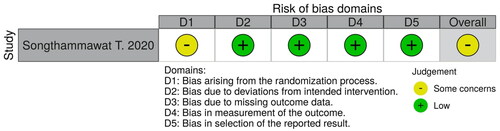
We assessed the methodological quality and risk of bias for 1 RCT, using the Risk of Bias 2.0 (RoB2) tools [Citation12]. Bias arising from the randomization process was the only domain judged to have ‘some concerns.’ This was because the allocation sequences might not have been concealed to protect the intervention assignments of the participants.
The other domains were judged to have a ‘low’ risk of bias. The overall risk of bias, then, was judged as ‘some concerns’ because there was at least 1 ‘some concerns’ domain in the study.
We present the full judgement per category in .
Risk of bias of controlled non-randomized studies of interventions
We assessed the methodological quality and risk of bias for 1 controlled NRSI, using Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tools [Citation13]. Bias due to participant selection was the only concern, and it was judged to be a ‘moderate’ risk. This is because responsiveness to prior high-dose steroids might have influenced the selection of the participants in the plasma exchange group, which is the intervention of interest. The other domains were judged to be of ‘low’ risk. The overall risk of bias, then, was judged to be ‘moderate.’
We present the full judgement per category in .
Discussion
The aim of this systematic review was to assess whether PLEX can effectively improve visual function for acute ON in cases of NMO/NMOSD. This review analyzed data from all available articles, most of which were retrospective in design. All of the studies included in our meta-analysis were observational studies.
NMO/NMOSD is rare and frequently severe. The diagnostic criteria for NMOSD were most recently revised in 2015 (International Consensus Diagnostic Criteria for NMOSD) after the disclosure of serum AQP4-Ab. The worldwide prevalence of NMOSD is 13/100,000 in blacks, compared with 4.0/100,000 in whites [Citation21]. Hence, almost 65% of the participants in our meta-analysis were Asian, despite most of the included studies having originated in Europe.
Heterogeneity was detected across the included studies, with a wide variability in their treatment courses and outcome reporting.
We did not include some relevant articles in the meta-analysis due to their having incomplete data. There were also some ongoing interventional studies as well as 1 systemic review of an RCT [Citation22]. Due to the limitations of the current data, this review will be updated once newly identified evidence becomes available, given that the conclusions may change.
There are presently no definite treatment guidelines for ON in cases of NMO/NMOSD. However, PLEX is widely used for the acute phase of NMOSD in limited settings, and it has been reported to be effective by many studies. This systematic review found that therapeutic PLEX can improve visual function. The VA improvements first became apparent between one day and 6 months after the completion of the course of PLEX cycles for corticosteroid nonresponders or when used as adjunctive treatment with steroids. However, the simple meta-analysis did not establish that the VA improvements were statistically significant at any of the recorded post-PLEX follow-up time points. In addition, although PLEX removes the inflammatory substances from the plasma and reduces damages to the axons. However, no comparative data suggested that IgG antibody status might contribute to the PLEX effectiveness on NMO/NMOSD.
Limitations
Important limitations of this investigation were a few primary literatures being included for meta-analysis, the nature of retrospective observational studies, unpublished articles with negative results and the studies conducted without any control groups. There were large variabilities in the baseline characteristics of the participants, symptom onsets, treatment courses, and outcome measures. This made it difficult to ascertain if the functional improvements observed after the PLEX treatments were influenced by the preceding steroid usage, or whether they simply reflected the natural course of the disease. Despite having a well-organized study, the main risks of bias (detection bias, attrition bias, reporting bias, and confounding) were unavoidable given the aforementioned limitations.
Moreover, there were limited quality data available from the accessible electronic databases. Non-English articles were also excluded due to limitations in the analytic process, even though the highest incidences of NMO/NMOSD are found in Asia. The number of included articles and enrolled participants were so small that conclusions based on the meta-analysis could not be confidently drawn.
Conclusions
Implications for practice and further research
From the current eligible data, there was an inadequate level of evidence to definitively conclude whether therapeutic PLEX is effective in improving VA in cases of NMO or NMOSD. Several questions still remain, for instance, the dosage, timing, and duration of PLEX. The conclusions based on this review are tentative, and they are open to change once more solid evidence becomes available.
Further well-designed, prospective, multicenter, controlled studies with larger numbers of participants and longer follow-up periods are required to accurately determine the efficacy and efficiency of PLEX. Different treatment courses should also be compared to identify the optimal protocols for conducting the PLEX for acute ON in cases of NMO or NMOSD. Since serum AQP4-Ab reactivity influences the disease pathogenesis, future studies should determine the correlation between PLEX and serum AQP4-Ab as well as measure disability-related outcomes.
Author contributions
YN and WC contributed to the review conception and conceived the original protocol. NC and PL revised the protocol. YN and SS performed the search, screening of titles, abstracts, and full-text articles. WC supervised database searches. YN and SS executed the data extraction and WC verified it. YN, WC, NC, PL analyzed the data; SS advocated the analyses. WC confirmed the analyses. YN wrote the original manuscript with the support by SS and supervision from WC. NC, PL and SS reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors particularly thank Professor Pisake Lumbiganon, MD, MS, a convenor of Cochrane Thailand, for his valuable comments and suggestions on the review methodology. The authors also thank Dr. Aitthanatt Chachris Eitivipart, a researcher at the National Science and Technology Development Agency, Thailand, for his critical comments on the search strategy. The authors are also grateful to Mr.David Park for the English-language editing of this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated or analyzed during the study are included in this published article and its supplementary information files. All background or extracted data are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Wuebbolt D, Andrejevic K, Nguyen V, et al. Pregnancy outcomes in patients with neuromyelitis optica. Obstetr Gynecol. 2018;131(1):1–11. doi: 10.1097/01.AOG.0000533052.44223.2d.
- Sellner J, Boggild M, Clanet M, et al. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010;17(8):1019–1032. doi: 10.1111/j.1468-1331.2010.03066.x.
- Yamagami A, Wakakura M, Inoue K, et al. Clinical characteristics of anti-aquaporin 4 antibody positive optic neuritis in Japan. Neuroophthalmology. 2019;43(2):71–80. doi: 10.1080/01658107.2018.1520905.
- Song W, Qu Y, Huang X. Plasma exchange: an effective add-on treatment of optic neuritis in neuromyelitis optica spectrum disorders. Int Ophthalmol. 2019;39(11):2477–2483. doi: 10.1007/s10792-019-01090-z.
- Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–189. doi: 10.1212/WNL.0000000000001729.
- Hickman SJ, Ko M, Chaudhry F, et al. Optic neuritis: an update typical and atypical optic neuritis. Neuro-ophthalmology. 2008;32(5):237–248. doi: 10.1080/01658100802391905.
- Levin MH, Bennett JL, Verkman AS. Optic neuritis in neuromyelitis optica. Prog Retin Eye Res. 2013;36:159–171. doi: 10.1016/j.preteyeres.2013.03.001.
- Likhar N, Mothe RK, Esam H, et al. Epidemiology and current treatment of neuromyelitis optica: a systematic review. Value in Health. 2015;18(7):A750–A751. doi: 10.1016/j.jval.2015.09.2904.
- McGuinness LA, Higgins JP. Risk of bias VISualization (robvis): An R package and shiny web app for visualizing risk of bias assessments. Res Synth Methods. 2021;12(1):55–61. doi: 10.1002/jrsm.1411.
- Bonnan M, Valentino R, Debeugny S, et al. Short delay to initiate plasma exchange is the strongest predictor of outcome in attacks of neuromyelitis optica spectrum disorders. J Neurol Neurosurg Psychiatry. 2018;89(4):346–351. doi: 10.1136/jnnp-2017-316286.
- Aungsumart S, Apiwattanakul M. Clinical outcomes and predictive factors related to good outcomes in plasma exchange in severe attack of NMOSD and long extensive transverse myelitis: case series and review of the literature. Mult Scler Relat Disord. 2017;13:93–97. doi: 10.1016/j.msard.2017.02.015.
- Songthammawat T, Srisupa-Olan T, Siritho S, et al. A pilot study comparing treatments for severe attacks of neuromyelitis optica spectrum disorders: intravenous methylprednisolone (IVMP) with add-on plasma exchange (PLEX) versus simultaneous ivmp and PLEX. Mult Scler Relat Disord. 2020;38:101506. doi: 10.1016/j.msard.2019.101506.
- Merle H, Olindo S, Jeannin S, et al. Treatment of optic neuritis by plasma exchange (add-on) in neuromyelitis optica. Arch Ophthalmol. 2012;130(7):858–862. doi: 10.1001/archophthalmol.2012.1126.
- Trebst C, Reising A, Kielstein JT, et al. Plasma exchange therapy in Steroid-Unresponsive relapses in patients with multiple sclerosis. Blood Purif. 2009;28(2):108–115. doi: 10.1159/000224630.
- Roesner S, Appel R, Gbadamosi J, et al. Treatment of steroid-unresponsive optic neuritis with plasma exchange. Acta Neurol Scand. 2012;126(2):103–108. doi: 10.1111/j.1600-0404.2011.01612.x.
- Koziolek M, Mühlhausen J, Friede T, et al. Therapeutic apheresis in pediatric patients with acute CNS inflammatory demyelinating disease. Blood Purif. 2013;36(2):92–97. doi: 10.1159/000354077.
- Deschamps R, Gueguen A, Parquet N, et al. Plasma exchange response in 34 patients with severe optic neuritis. J Neurol. 2016;263(5):883–887. doi: 10.1007/s00415-016-8073-8.
- Mori S, Kurimoto T, Ueda K, et al. Short-term effect of additional apheresis on visual acuity changes in patients with steroid-resistant optic neuritis in neuromyelitis optica spectrum disorders. Jpn J Ophthalmol. 2018;62(4):525–530. doi: 10.1007/s10384-018-0602-9.
- Lipphardt M, Mühlhausen J, Kitze B, et al. Immunoadsorption or plasma exchange in steroid-refractory multiple sclerosis and neuromyelitis optica. J Clin Apher. 2019;34(4):381–391. doi: 10.1002/jca.21686.
- Savransky A, Rubstein A, Rios MH, et al. Prognostic indicators of improvement with therapeutic plasma exchange in pediatric demyelination. Neurology. 2019;93(22):e2065–e2073. doi: 10.1212/WNL.0000000000008551.
- Flanagan EP, Cabre P, Weinshenker BG, et al. Epidemiology of aquaporin-4 autoimmunity and neuromyelitis optica spectrum. Ann Neurol. 2016;79(5):775–783. doi: 10.1002/ana.24617.
- Wei S. Effectiveness of Plasma Exchange in Treating with Severe Acute AQP4-Ab Positive Optic Neuritis. 2018; NCT03586557: https://clinicaltrials.gov/show/NCT03586557.