Abstract
Background
Atelectasis affects approximately 90% of anaesthetized patients, with laparoscopic surgery and pneumoperitoneum reported to exacerbate this condition. High-frequency oscillation therapy applies continuous positive pressure pulses to oscillate the airway, creating a pressure difference in small airways obstructed by secretions. This process helps reduce peak airway pressure, open small airways, and decrease atelectasis incidence, while also facilitating respiratory tract clearance. This study examines the efficacy of high-frequency oscillation on reduction of atelectasis in laparoscopic cholecystectomy (LC) patients under general anaesthesia, evaluated using lung ultrasound.
Methods
Sixty-four patients undergoing laparoscopic cholecystectomy were randomly divided into a control group and a high-frequency oscillation (HFO) group. Both groups underwent total intravenous anaesthesia under invasive arterial monitoring. The HFO group received a 10-minute continuous high-frequency oscillation therapy during surgery, while the control group received no intervention. Lung ultrasound evaluations were performed three times: five minutes post-intubation (T1), at the end of the surgery (T2), and before leaving the Post-Anaesthesia Care Unit (PACU; T3). Blood gas analysis was performed twice: prior to induction with no oxygen supply and before PACU discharge (oxygen supply off).
Results
The HFO group displayed a significantly lower incidence of atelectasis at T3 (57.5% vs. 90.3%, OR 6.88, 95%CI (1.74 to 27.24)) compared to the control group. Moreover, the HFO group’s PaO2 levels remained consistent with baseline levels before PACU discharge, unlike the control group. Although there was no significant difference in LUS scores between the groups at T1 (8.56 ± 0.15 vs. 8.19 ± 0.18, p = 0.1090), the HFO group had considerably lower scores at T2 (13.41 ± 0.17 vs.7.59 ± 0.17, p < 0.01) and T3 (13.72 ± 0.14 vs.7.25 ± 0.21, p < 0.01).
Conclusion
Our study indicates that high-frequency oscillation effectively reduces atelectasis in patients undergoing laparoscopic cholecystectomy. Additionally, it can mitigate the decline in oxygen partial pressure associated with atelectasis.
Introduction
Postoperative pulmonary complications (PPC), led predominantly by atelectasis, contribute significantly to postoperative morbidity and mortality, with incidence rates ranging from 64% to 90% [Citation1–4]. A complex interplay of factors including general anaesthesia, pneumoperitoneum, and surgical positioning contributes to its occurrence [Citation2]. Current literature indicates that atelectasis occurs in approximately 90% of anaesthetized patients and may persist for several days post-surgery. The prevalent use of high oxygen concentrations during anaesthesia induction and maintenance, coupled with anaesthetic-induced loss of muscle tone and diminished lung functional residual capacity, largely underlie this phenomenon [Citation5,Citation6]. Moreover, laparoscopic surgeries, such as cholecystectomy, heighten the risk of common postoperative pulmonary complications, including atelectasis, pneumonia, and pleural effusion [Citation7]. The necessity for pneumoperitoneum in laparoscopic cholecystectomy augments intra-abdominal pressure, elevates the diaphragm, and exacerbates atelectasis. Atelectasis compromises lung compliance, impairs oxygenation, amplifies patient discomfort, escalates pulmonary vascular resistance, and potentially prolongs hospital stays, thereby increasing overall healthcare costs [Citation8].
High-frequency oscillation, a chest physical therapy utilizing pneumatic forms, produces continuous positive pressure pulses, maintaining oscillation during both inhalation and exhalation [Citation9]. This technique generates a pressure gradient within small, secretion-blocked airways, facilitating secretion mobilization towards larger airways. It also augments lung recruitment and improves oxygenation through the application of continuous positive expiratory pressure [Citation10]. High-frequency oscillation has been proven to enhance pulmonary function in patients on mechanical ventilation [Citation11–13]. However, its efficacy in mitigating atelectasis induced by general anaesthesia and surgery remains to be explored. Past research substantiates the effectiveness of ultrasound-guided alveolar recruitment or sustained adequate positive end-expiratory pressure (PEEP) in preventing perioperative atelectasis [Citation14,Citation15]. Given this, we hypothesized that continuous high-frequency oscillation could likewise reduce perioperative atelectasis.
Lung ultrasonography, a non-invasive, radiation-free, portable, and accurate method, provides a reliable means to assess lung aeration [Citation16]. Ample evidence underscores the high sensitivity and specificity of lung ultrasound in diagnosing perioperative atelectasis [Citation17]. To test our hypothesis, we devised and conducted a randomized controlled trial, leveraging lung ultrasonography to compare the effects of high-frequency oscillation on the reduction of perioperative atelectasis and other outcomes in patients undergoing laparoscopic surgery under general anaesthesia.
Methods
Study design and participants
This controlled, randomized, patient and evaluator-blinded trial aims to assess the impact of high-frequency oscillation on the reduction of perioperative atelectasis. The study protocol received approval from the Ethics Committee of Xinhua Hospital (XHEC-C-2022-102-1), affiliated with Shanghai Jiaotong University School of Medicine. Participant recruitment took place from November the 21st, 2022, finished on January 31st, 2023. All participants provided written informed consent. The study was registered at the Chinese Clinical Trial Registry (http://www.chictr.org.cn) with registration number ChiCTR 2200065673). Eligible individuals were adults aged 25 to 70, with an American Society of Anaesthesiologists (ASA) physical status of 1 or 2, who were scheduled for laparoscopic cholecystectomy under general anaesthesia. We excluded patients with a body mass index (BMI) over 35 kg/m2, a history of chest or lung surgery, or severe underlying diseases.
Anaesthesia protocol
All participants underwent total intravenous anaesthesia under invasive arterial monitoring throughout surgery. The first blood gas analysis was conducted immediately following the establishment of invasive arterial catheterization. After three minutes of denitrogenation and pure oxygen supply, anaesthesia was induced with midazolam 0.03 mg/kg, atropine 0.02 mg/kg, propofol 2 mg/kg, fentanyl 4ug/kg, and cisatracurium besilate 0.15 mg/kg, followed by tracheal intubation. Anaesthesia maintenance was achieved using propofol 2 to 6 μg/kg·min and remifentanil (0.05 to 0.2 μg/kg·min) IV. Mechanical ventilation was performed in volume-controlled mode, with an inspiratory ratio of 1:2 and PEEP 5 cm H2O to prevent atelectasis [Citation18]. The ventilatory settings in both groups were FiO2 0.5; tidal volume 8 ml/kg of predicted body weight. The respiratory rate (RR) was set to 12 breaths/min at first and then adjusted to maintain end tidal carbon dioxide pressure between 35 and 45 mmHg. After extubation, patients were transferred to the Post-Anaesthesia Care Unit (PACU) and received three litres of oxygen per minute through a nasal catheter. In the last 10 minutes in the PACU, patients breathed without supplemental oxygen. A second blood gas analysis was performed one minute before PACU discharge.
Randomization and blind method
Participants were randomly allocated to either the control group or the HFO group using computer-generated randomization software (www.randomization.com) at a 1:1 ratio. The patients, the outcome assessor who scored lung ultrasound clips, and PACU nurses were blinded to group allocation, while the bedside anaesthesiologists and the lung ultrasound operators were aware of the allocation due to procedural needs.
Lung ultrasonography and high-frequency oscillation therapy
All patients underwent three lung ultrasonography scans: five minutes post-intubation (T1), at the end of surgery (T2), and before leaving the PACU (T3). Each lung was examined at 12 sections according to Acosta’s protocol. Perioperative atelectasis was scored between 0 and 4, based on the degree of de-aeration [Citation15,Citation19,Citation20]. We considered anaesthesia-induced atelectasis clinically significant if more than three sections showed any signs of atelectasis (atelectasis score 1 or greater). Ultrasonography was performed by an experienced study team member with the GE Versana ActiveTM ultrasound system, and all clips were stored and scored by the outcome assessor. We choose one skilled anaesthesiologist who had performed at least 100 lung ultrasound examinations in patients as the assessor to eliminate subjective bias.
The HFO group received 10 minutes of continuous high-frequency oscillation therapy (via MetaNeb®) during surgery. We connected the MetaNeb® system to the inspiratory end of the ventilator when gallbladder separation was complete. The patient’s vital signs, lung conditions, and ventilator parameters were evaluated and recorded throughout therapy. In both groups, airway secretions were suctioned at the end of surgery.
Study outcomes
The primary endpoint was the incidence of atelectasis at T3. Secondary outcomes encompassed lung ultrasound scores for consolidation and B-lines, blood gas parameters (PaO2, SO2, etc.) from T1 to T3, respiratory dynamics parameters, haemodynamics, desaturation in the PACU, minimum SpO2 in the PACU, incidence of postoperative respiratory events (including desaturation with pulse oximeter value ≤95% in the PACU, postoperative apnoea, laryngospasm, bronchospasm, and productive cough with high fever), and length of hospital stay.
Data analyses
Sample size calculation was informed by a previous study indicating a 75–90% incidence of atelectasis following laparoscopic surgery under general anaesthesia [Citation1]. Given the high diagnostic accuracy of lung ultrasound for atelectasis (approximately 88%) [Citation20], we hypothesized that the incidence of perioperative atelectasis would be 50% in the HFO group and 85% in the control group. With a significance level (α error) of 0.05 and 80% power, we estimated a required sample size of 27 participants per group using PASS 2008 software (ver. 8.0.16; NCSS statistical software, Kaysville, UT, USA). Accounting for a potential attrition rate of 20%, we determined a final sample size of 33 participants for each group.
All data were analysed using SPSS software (ver. 23.0). The Kolmogorov–Smirnov test was used to assess data normality. Continuous variables are expressed as mean ± standard deviation (SD), while categorical variables are expressed as numbers or percentages. The significance of continuous data was determined using Student’s t-tests or Mann–Whitney rank-sum tests, as appropriate. The x2 test was used to test the significance of categorical data, and Fisher’s exact test was used when any expected frequency was less than 1, or 20% of the expected frequencies were 5 or less. All P-values were two-sided, and only a P value of less than 0.05 was considered statistically significant.
Results
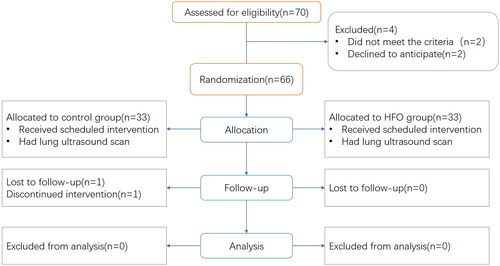
A total of 66 participants were randomized into the control (n = 33) and ultrasound (n = 33) groups. One patient from each group was excluded due to a switch from laparoscopic cholecystectomy to laparotomy and a failure to conduct lung ultrasonography before PACU discharge, respectively. Therefore, data from 64 were analysed (, CONSORT flow diagram). Baseline characteristics were similar across groups (), with no significant differences in the duration of anaesthesia (84.77 ± 2.54 min vs. 84.21 ± 1.49 min) or pneumoperitoneum (53.35 ± 1.80 min vs. 56.03 ± 1.62 min).
Table 1. Baseline characteristics.
Values are shown in number or mean ± SD
Primary outcomes indicated that at T1, atelectasis developed in 22 control group participants and 23 HFO group participants, a difference that was not statistically significant (71.0% vs. 69.7%; OR 1.063; 95% CI 0.363 to 3.110, p = 0.911). However, at T2, the incidence of perioperative atelectasis was significantly higher in the control group (90.3% vs. 51.5%; OR 8.784; 95% CI 2.227 to 34.656; p = 0.002), a difference that persisted at T3 (90.3% vs. 57.5%; OR 6.88; 95% CI1.737 to 27.236; p = 0.006).
With regard to secondary outcomes, comparison of lung ultrasound clips at T1 to T3 is shown in . Lung ultrasound scores did not significantly differ between groups at T1 (8.56 ± 0.15 vs. 8.19 ± 0.18, p = 0.1090). However, significant differences emerged at T2 (13.41 ± 0.17 vs.7.59 ± 0.17, p < 0.01) and persisted at T3 (13.72 ± 0.14 vs.7.25 ± 0.21, p < 0.01). Similarly, both scores were significantly lower in the HFO group than in the control group at T2 (consolidation: 3 [0 to 6] vs. 7 [5 to 9], p < 0.001; B-lines: 6 [1 to 6] vs. 10 [6 to 13]; p < 0.001) and maintained to T3 (consolidation: 2 [0 to 5] vs. 2 [0 to 6], p < 0.001; B-lines, 5 [0 to 7] vs. 9 [5 to 10]; p < 0.001) ().
Figure 3. B-line scores (a) and Consolidation scores (b) from T1 to T3 for patients who received no intervention (control group, black box) or continuous high-frequency oscillation therapy (HFO group, grey box).
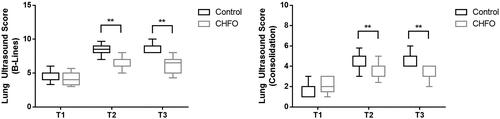
T1: Five minutes post-intubation, T2: Time at the end of surgery. T3: Discharge from the PACU (postanaesthesia care unit)
T1, Five minutes post-intubation; T2, Time at the end of surgery; T3, Discharge from the PACU. The bold black line represents the median value, the ends of the boxes indicate interquartile ranges and error bars indicate 10 and 90 percentiles.
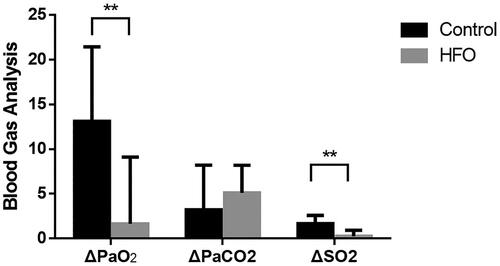
PaO2 in the blood gas analysis did not significantly differ between groups before general anesthesia induction (no oxygen inhalation applied) but was significantly lower in the control group before PACU discharge (oxygen supply off) (80.78 ± 0.62 vs. 91.18 ± 0.81, p < 0.01). The same trend was observed for SO2, while no difference in PaCO2 was observed between groups at any time ().
This value is calculated as the D-value before general anesthesia induction and till to discharge from the PACU of two groups.
also summarizes other secondary outcomes. No patients experienced hemodynamic or pneumodynamics instability during the continuous high-frequency oscillation therapy. There were fewer instances of desaturation in the PACU in the HFO group (3) than in the control group (12), and the median minimum SpO2 in the PACU was also significantly higher in the HFO group (97 [94 to 98] vs. 93 [91 to 96], p = 0.004).
Table 2. Comparison of perioperative variables between groups.
Data is presented in number, median [IQR] or mean ± SD. T1: Time after tracheal intubation, T2: Time at the end of surgery. T3: Discharge from the PACU (postanaesthesia care unit). OR: odds ratio, CI: confidence interval. Desaturation is defined as a pulse oximeter value of ≤95%.
With regard to postoperative respiratory adverse events, three patients in the control group and two in the HFO group developed a productive cough or high fever. All five were successfully treated. The mean length of hospitalization did not significantly differ between the control and HFO groups (3.2 ± 2.0 days vs. 3.0 ± 1.5 days; p = 0.837).
Discussion
Our study demonstrates that continuous high-frequency oscillation therapy for airway clearance can effectively mitigate the incidence of perioperative atelectasis and ameliorate the decrease in oxygen partial pressure resulting from atelectasis. And we hope that the reduction in atelectasis will reduce postoperative pulmonary complications, which needs to be demonstrated in larger trials.
Traditionally, ultrasound has not been the preferred imaging modality for the lung due to the complex air-filled microarchitecture of lung tissue, which limits image clarity [Citation21]. However, recent studies highlight the excellent diagnostic accuracy of lung ultrasound for conditions such as pleural effusion and pneumothorax [Citation22]. Moreover, the typical artifact patterns that emerge during ultrasound can differentiate crucial conditions like lung oedema, consolidation, infiltration, lung congestion, and interstitial lung disease [Citation20,Citation23]. The portability of ultrasound machines enables examinations to be performed at the patient’s bedside and even intraoperatively. These advantages led us to utilize lung ultrasound to assess lung function in our study.
Atelectasis, which occurs in 90% of patients undergoing anesthesia[Citation4], can cause decreased lung compliance, increased pulmonary vascular resistance, impairment of oxygenation, and the development of lung injury. These effects can persist for several days postoperatively, affecting patient recovery. In our study, participants underwent laparoscopic cholecystectomy, a procedure that requires pneumoperitoneum and the Trendelenburg position. Both factors are known to exacerbate atelectasis, rendering our study more clinically relevant. The predominant clinical methods to reduce atelectasis include using PEEP during surgery, performing lung recruitment manoeuvres, and cleaning the respiratory tract to reduce airway resistance [Citation15,Citation24]. The CHFO mode of MetaNeb® system simultaneously clears the airway and applies PEEP. Based on this background, we designed our study to investigate the effect of high-frequency oscillation therapy on the reduction of atelectasis in perioperative patients.
The MetaNeb system provides noninvasive physiotherapy through continuous high-frequency oscillation. This therapy creates a pressure difference in small airways blocked by secretions, generating an accelerated expiratory airflow that moves secretions to the large airways [Citation9,Citation10]. We monitored vital signs and ventilatory parameters to ensure patient safety during therapy and found no patients developed hemodynamic or pneumodynamics instability. Continuous high-frequency oscillation has been shown to enhance mucociliary clearance of secretions and help resolve atelectasis in many cases before [Citation10,Citation25,Citation26]. Our findings demonstrate that the incidence of perioperative atelectasis was significantly lower in the HFO group than the control group (90.3% vs. 51.5%; OR 8.784; 95% CI 2.227 to 34.656; p = 0.002) at the end of surgery (T2), and this difference persisted until discharge from the PACU (T3). This trend was mirrored in the lung ultrasound scores at T1 to T3. Moreover, PaO2 barely decreased from baseline in the HFO group before patients left the PACU compared to the control group (Δ1.648 vs. Δ13.123, p < 0.01).
With respect to secondary outcomes, the control group had a higher incidence of desaturation in the PACU and a significantly lower blood oxygen saturation than the HFO group. This may result from the CHFO therapy opening small airways, reducing the area of atelectasis, and thus improving the patient’s spontaneous respiratory function. However, we did not find significant differences in postoperative respiratory adverse events and length of hospitalization.
Nevertheless, this study still has several limitations. Firstly, lung ultrasound scans and blood gas tests were only monitored up to 1 h after surgery, leaving the long-term effects of HFO therapy unknown. Secondly, this single-centre, small sample-controlled study only involved one single type of operation. The impact of this therapy on other laparoscopic operations with longer operating times needs further investigation. Thirdly, although no hemodynamic or respiratory dynamic instabilities were observed during the continuous high-frequency oscillation treatment, a slight increase in carbon dioxide levels was noticed post-intervention. This increase reversed within minutes, but our observed indicators were limited, and it remains unclear whether continuous high-frequency oscillation therapy might affect other lung function parameters.
In conclusion, our study suggests that using continuous high-frequency oscillation treatment can reduce the incidence of perioperative atelectasis in patients undergoing laparoscopic cholecystectomy and can also alleviate the decrease in oxygen partial pressure induced by atelectasis.
Acknowledgements
The authors thank Yanfei Mao, Ph.D. (Department of Anesthesiology and Surgical Intensive Care Unit, Xinhua Hospital, Shanghai Jiaotong University School of Medicine) for his valuable assistance and dedication in reviewing this manuscript.
Disclosure statement of interest
No potential conflict of interest was reported by the author(s).
Data availability
The data that support the findings of this study are available on request from the corresponding author, S.Y. Li. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Additional information
Notes on contributors
Yuan-jun Qin
Yuan-jun Qin (First Author): Conceptualization, Methodology, Software, Investigation, Formal Analysis, Writing - Original Draft;
Yun-qian Zhang
Yun-qian Zhang (Co-First Author): Data Curation, Software, Writing - Original Draft;
Qi Chen
Qi Chen (Co-First Author): Resources, Investigation, Supervision, Review & Editing;
Yan Wang
Yan Wang (Co-Corresponding Author): Data analysis and interpretation, Visualization, Validation;
Si-yuan Li
Si-yuan Li (Corresponding Author): Conceptualization, Resources, Supervision, Writing - Review & Editing.
Y.-J. Qin, Y.-Q. Zhang and Q. Chen contributed equally to this work and should be considered as co-first authors. And All authors agree to be accountable for all aspects of the work.
References
- Brooks-Brunn JA. Postoperative atelectasis and pneumonia. Heart Lung. 1995;24(2):1–7. doi:10.1016/s0147-9563(05)80004-4.
- Magnusson L, Spahn DR. New concepts of atelectasis during general anaesthesia. Br J Anaesth. 2003;91(1):61–72. doi:10.1093/bja/aeg085.
- Lagier D, Zeng C, Fernandez-Bustamante A, et al. Perioperative pulmonary atelectasis: part II. Clinical implications. Anesthesiology. 2022;136(1):206–236. doi:10.1097/ALN.0000000000004009.
- Duggan M, Kavanagh BP. Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiology. 2005;102(4):838–854. doi:10.1097/00000542-200504000-00021.
- Hedenstierna G, Edmark L. Mechanisms of atelectasis in the perioperative period. Best Pract Res Clin Anaesthesiol. 2010;24(2):157–169. doi:10.1016/j.bpa.2009.12.002.
- Gunnarsson L, Tokics L, Gustavsson H, et al. Influence of age on atelectasis formation and gas exchange impairment during general anaesthesia. Br J Anaesth. 1991;66(4):423–432. doi:10.1093/bja/66.4.423.
- Patel SK, Bansal S, Puri A, et al. Correlation of perioperative atelectasis with duration of anesthesia, pneumoperitoneum, and length of surgery in patients undergoing laparoscopic cholecystectomy. Cureus. 2022;14(4):e24261. doi:10.7759/cureus.24261.
- Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth. 2017;118(3):317–334. doi:10.1093/bja/aex002.
- Junqueira FMD, Nadal JAH, Brandão MB, et al. High-frequency oscillatory ventilation in children: a systematic review and meta-analysis. Pediatr Pulmonol. 2021;56(7):1872–1888. doi:10.1002/ppul.25428.
- Caldwell KB. A novel ventilatory technique in refractory hypoxemic respiratory failure secondary to therapeutic thoracentesis and paracentesis. Am J Case Rep. 2020;21:e924862. doi:10.12659/AJCR.924862.
- Dilday J, Leon D, Kuza CM. A review of the utility of high-frequency oscillatory ventilation in burn and trauma ICU patients. Curr Opin Anaesthesiol. 2023;36(2):126–131. doi:10.1097/ACO.0000000000001228.
- Morgan S, Hornik CP, Patel N, et al. Continuous high-frequency oscillation therapy in invasively ventilated pediatric subjects in the critical care setting. Respir Care. 2016;61(11):1451–1455. doi:10.4187/respcare.04368.
- Bordessoule A, Piquilloud L, Lyazidi A, et al. Imposed work of breathing during high-frequency oscillatory ventilation in spontaneously breathing neonatal and pediatric models. Respir Care. 2018;63(9):1085–1093. doi:10.4187/respcare.05703.
- Yildiz AM, et al. Impact of positive end-expiratory pressure with alveolar recruitment maneuver on respiratory and oxygenation parameters of patients during laparoscopic bariatric surgery. Eur Rev Med Pharmacol Sci. 2022;26(24):9170–9179. doi:10.26355/eurrev_202212_30668.
- Song IK, Kim EH, Lee JH, et al. Effects of an alveolar recruitment manoeuvre guided by lung ultrasound on anaesthesia-induced atelectasis in infants: a randomised, controlled trial. Anaesthesia. 2017;72(2):214–222. doi:10.1111/anae.13713.
- Ntoumenopoulos G, Buscher H, Scott S. Lung ultrasound score as an indicator of dynamic lung compliance during veno-venous extra-corporeal membrane oxygenation. Int J Artif Organs. 2021;44(3):194–198. doi:10.1177/0391398820948870.
- Lee JH, Choi S, Ji SH, et al. Effect of an ultrasound-guided lung recruitment manoeuvre on postoperative atelectasis in children: a randomised controlled trial. Eur J Anaesthesiol. 2020;37(8):719–727. doi:10.1097/EJA.0000000000001175.
- Jeong H, Tanatporn P, Ahn HJ, et al. Pressure support versus spontaneous ventilation during anesthetic emergence-effect on postoperative atelectasis: a randomized controlled trial. Anesthesiology. 2021;135(6):1004–1014. doi:10.1097/ALN.0000000000003997.
- Kim BR, Lee S, Bae H, et al. Lung ultrasound score to determine the effect of fraction inspired oxygen during alveolar recruitment on absorption atelectasis in laparoscopic surgery: a randomized controlled trial. BMC Anesthesiol. 2020;20(1):173. doi:10.1186/s12871-020-01090-y.
- Acosta CM, Maidana GA, Jacovitti D, et al. Accuracy of transthoracic lung ultrasound for diagnosing anesthesia-induced atelectasis in children. Anesthesiology. 2014;120(6):1370–1379. doi:10.1097/ALN.0000000000000231.
- Nelson M, Stankard B, Greco J, et al. Point of care ultrasound diagnosis of empyema. J Emerg Med. 2016;51(2):140–143. doi:10.1016/j.jemermed.2016.05.023.
- Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577–591. doi:10.1007/s00134-012-2513-4.
- Radzina M, Biederer J. Ultrasonography of the lung. Rofo. 2019;191(10):909–923. doi:10.1055/a-0881-3179.
- Spadaro S, Karbing DS, Mauri T, et al. Effect of positive end-expiratory pressure on pulmonary shunt and dynamic compliance during abdominal surgery. Br J Anaesth. 2016;116(6):855–861. doi:10.1093/bja/aew123.
- Ortiz-Pujols S, Boschini LA, Klatt-Cromwell C, et al. Chest high-frequency oscillatory treatment for severe atelectasis in a patient with toxic epidermal necrolysis. J Burn Care Res. 2013;34(2):e112-5–e115. doi:10.1097/BCR.0b013e318257d83e.
- Meyers M, Rodrigues N, Ari A. High-frequency oscillatory ventilation: a narrative review. Can J Respir Ther. 2019;55:40–46. doi:10.29390/cjrt-2019-004.