Abstract
Background
Numerous studies have explored whether the prognostic nutritional index (PNI) can predict the prognosis of cervical cancer (CC); however, their findings remain controversial. This meta-analysis focused on evaluating the relationship between the PNI and the prognosis of patients with CC.
Methods
Relevant articles were collected from specific databases up to March 16, 2023. The relationship between the PNI and survival outcomes in patients with CC was estimated using combined hazard ratios (HRs) and associated 95% confidence intervals (CIs). The association of the PNI with clinicopathological features in patients with CC was assessed by combining odds ratios (ORs) and associated 95% CIs.
Results
Nine articles with 2508 cases were included in the meta-analysis. According to our pooled findings, a decreased PNI showed a significant association with worse overall survival (OS) (HR = 2.98, 95% CI = 2.22–3.99, p < .001) as well as progression-free survival (PFS) (HR = 2.43, 95% CI = 1.92–3.07, p < .001) in patients with CC. The subgroup analysis indicated that the results were reliable. Moreover, the decreased PNI showed a significant association with the presence of lymph node metastasis (LN metastasis, OR = 1.53, 95% CI = 1.04–82.24, p = .030) and maximum tumor size >4 cm (OR = 1.73, 95% CI = 1.21–2.46, p = .002). However, the PNI was not significantly associated with histology, differentiation, or FIGO stage.
Conclusion
In this study, a low PNI predicted dismal OS and PFS in patients with CC, who also tend to suffer from LN metastasis and larger tumor size. PNI is a promising biomarker for predicting the prognosis of patients with CC in clinical practice.
KEY MESSAGES
To our knowledge, the present meta-analysis is the first to explore whether the PNI can be used to predict the prognosis of patients with CC.
In this study, a low PNI predicted dismal OS and PFS in patients with CC, who also tend to suffer from LN metastasis and larger tumor size.
PNI is a promising biomarker for predicting the prognosis of patients with CC in clinical practice.
Introduction
Cervical cancer (CC) is a major global threat to the health of women [Citation1] and ranks fourth among cancers affecting women worldwide [Citation2]. According to the GLOBOCAN estimates, in 2020, 604,127 newly diagnosed cases and 341,831 deaths due to CC occurred worldwide [Citation3]. Despite advances in the treatment of CC, its prognosis remained poor over the past several decades, especially in developing countries. CC has a poor prognosis primarily due to poor screening and treatment of early-stage disease, although it is one of the most curable cancers with a natural history quite well-known. Consequently, the identification of new and effective biomarkers to predict the prognosis of patients with CC is urgently needed.
Accumulating evidence has shown a significant connection between nutritional status, operative complications, and clinical outcomes in patients with cancer [Citation4]. Many inflammatory blood-based parameters such as systemic immune-inflammation index (SII) [Citation5], controlling nutritional status (CONUT) score [Citation6], geriatric nutritional risk index (GNRI) [Citation7], and albumin-to-globulin ratio (AGR) [Citation8] have been reported as effective prognostic markers in patients with cancer. The prognostic nutritional index (PNI) can be computed based on serum albumin levels and peripheral blood lymphocyte counts [Citation9]. The PNI was first proposed by Buzby et al. in 1980 to evaluate the risk of postoperative complications in patients undergoing gastrointestinal surgery [Citation9]. PNI was calculated using the following formula: PNI = peripheral blood (10 × serum albumin [g/dL] + 0.005 × total lymphocyte count [/mm3]) [Citation10]. Recently, numerous studies have indicated that PNI can be used to predict the prognosis of patients with different types of cancer, including gastric cancer [Citation11], hepatocellular carcinoma [Citation12], lymphoma [Citation13], urothelial carcinoma [Citation14], and pancreatic neuroendocrine neoplasms [Citation15]. Specifically, there is evidence that the lower the PNI before treatment, the worse the prognosis of patients with prostate cancer treated with androgen deprivation therapy [Citation16]. The pretreatment-PNI is an independent prognostic factor, irrespective of neoadjuvant chemotherapy, for patients with esophageal squamous cell carcinoma [Citation17]. Current evidence also suggests that the PNI is a significant prognostic marker for disease-specific and event-free survival in patients with soft tissue sarcomas [Citation18]. Additionally, a multivariate model showed that a low PNI was associated with shorter survival duration in patients with follicular lymphoma [Citation19]. Although the effect of PNI on predicting the prognosis of patients with CC has been widely explored [Citation20–28], the findings remain conflicting. Some studies have reported that a decreased PNI predicts worse survival in patients with CC [Citation20,Citation23,Citation24], whereas others have indicated no obvious correlation between PNI and CC prognosis [Citation21]. Therefore, we collected updated data for the present meta-analysis to accurately identify the role of PNI in predicting the prognosis of patients with CC.
Materials and methods
Study guideline
This meta-analysis was performed following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [Citation29].
Search strategy
The Embase, PubMed, Cochrane Library, Web of Science, and China National Knowledge Infrastructure databases were systematically searched. The search terms were as follows: (prognostic nutritional index OR PNI) AND (cervical carcinoma OR cervical neoplasm OR cervical cancer OR cervical tumor). Literature retrieval was updated on March 16, 2023. There were no restrictions on publication language. Additionally, we manually checked the reference lists and related reviews to identify potential inclusions.
Inclusion and exclusion criteria
Patients included should satisfy the following criteria: (1) CC was pathologically diagnosed; (2) articles reporting the relationship of PNI with survival outcomes in patients with CC; (3) cut-off values should be identified to divide low/high PNI; (4) hazard ratios (HRs) and associated 95% confidence intervals (CIs) of survival outcomes should be available or calculable; and (5) available survival outcomes, such as overall survival (OS), progression-free survival (PFS), cancer-specific survival (CSS), and recurrence-free survival (RFS). Studies conforming to the following criteria were excluded: (1) reviews, letters, meeting abstracts, comments, and case reports; (2) duplicates; and (3) animal studies.
Data extraction and quality assessment
Two reviewers (Z.N. and B.Y.) independently assessed and collected information from each included study. Any disagreement was resolved through discussions to reach a consensus. The following information was collected in each study: first author; publication year; country; sample size; age; study period; International Federation of Gynecology and Obstetrics (FIGO) stage; treatment; follow-up; threshold; threshold calculation approach; survival endpoints; survival analysis type; study design; HRs; and 95% CIs. The quality of the studies was evaluated using the Newcastle-Ottawa Quality Assessment Scale (NOS), which includes three aspects: selection, outcomes, and comparability, with a total score of 0–9. Studies with NOS scores ≥6 were considered to be of high quality.
Statistical analysis
This study calculated pooled HRs and 95% CIs to estimate the relationship between the PNI and the survival outcomes of patients with CC. Heterogeneities among articles were assessed by I2 statistics and Cochran’s Q test, with P heterogeneity <0.10 and I2 > 50% indicating heterogeneity. Therefore, the random-effects model was applied to this condition; otherwise, the fixed-effects model was adopted. Possible sources of heterogeneity were detected using subgroup analysis. The association of the PNI with clinicopathological characteristics in patients with CC was evaluated through combining odds ratios (ORs) and 95% CIs. Begg’s and Egger’s tests were used to detect possible publication biases. Stata software (version 12.0; Stata Corp., College Station, TX, USA) with a copyright license obtained was used for the statistical analysis. Statistical significance was set at p < .05 (two-sided).
Results
Process of literature search
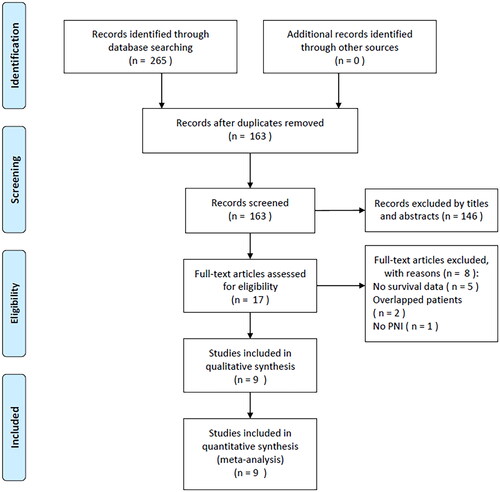
A total of 265 records were detected through the primary search, of which duplicate articles were eliminated, and 163 articles remained (). After reading their titles and abstracts, 146 studies were eliminated because of irrelevance, and 17 studies were further evaluated. The full texts of these 17 articles were read, and eight were eliminated for the following reasons: no survival data provided (n = 5), overlapped patients (n = 2), and no PNI analysis (n = 1). Ultimately, this study included nine articles involving 2508 cases [Citation20–28] ().
Features of the included articles
shows the basic features of the selected articles [Citation20–28] published from 2016 to 2023. Eight studies were performed in China [Citation21–28] and one in Japan [Citation20]. All the studies were retrospective in design. Six studies were published in English [Citation20–22,Citation24,Citation25,Citation28] and three studies were published in Chinese [Citation23,Citation26,Citation27]. The median sample size included in these articles was 229 patients (range, 82–698). Four studies included patients with FIGO stages I–II [Citation22,Citation24,Citation25,Citation28], four studies included those with stages I–IV [Citation20,Citation21,Citation23,Citation27], and one study included those with stages I–III [Citation26]. The median PNI cut-off value was 48.82 (range, 45–52.68). Seven articles [Citation20,Citation22–24,Citation26–28] selected thresholds via receiver operating characteristic (ROC) curves, and two studies [Citation21,Citation25] determined the cut-off values based on the literature. Eight studies reported that the PNI could be used to predict OS [Citation20–26,Citation28], whereas six studies [Citation20,Citation22–25,Citation27] reported a relationship between the PNI and PFS in patients with CC. The median NOS score was 7 (range, 6–9), suggesting the high quality of each included article.
Table 1. Basic characteristics of included studies in this meta-analysis.
PNI and OS of patients with CC
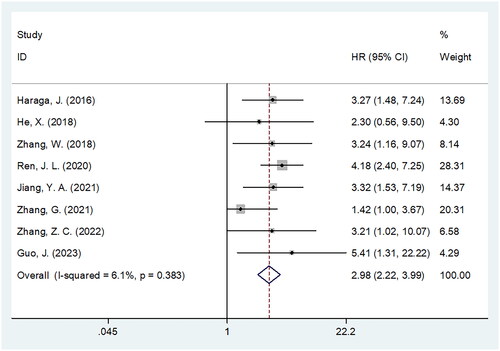
Eight studies involving 2,391 patients [Citation20–26,Citation28] reported a relationship between the PNI and OS in patients with CC. A fixed-effects model was used because of insignificant heterogeneity (I2 = 6.1%, p = .383). According to and , pooled HR = 2.98, 95% CI = 2.22–3.99, and p < .001, which indicated a significant relation between decreased PNI and worse OS among patients with CC. Subgroup analysis stratified by various factors was conducted for OS. As shown in , a decreased PNI significantly predicted OS regardless of country, sample size, FIGO stage, threshold, or survival analysis type (p < .05). Furthermore, decreased PNI markedly predicted dismal OS when cut-off value was determined by ROC analysis (HR = 3.69, 95% CI = 2.63–5.18, p < .001; ).
Table 2. Subgroup analysis of prognostic role of PNI for OS in patients with cervical cancer.
PNI and PFS in patients with CC
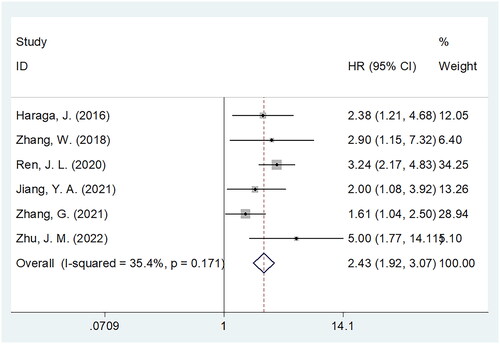
Six studies consisting of 2088 patients [Citation20,Citation22–25,Citation27] provided data on whether the PNI could be used to predict PFS among patients with CC. A pooled HR = 2.43, 95% CI = 1.92–3.07, and p < .001 were obtained, indicating that a low PNI predicted the shortened PFS among patients with CC (; ). As revealed by subgroup analysis, a reduced PNI significantly predicted PFS in all subgroups, including sample size, country, FIGO stage, threshold, threshold calculation, and survival analysis type (all p < .05; ).
Table 3. Subgroup analysis of prognostic role of PNI for PFS in patients with cervical cancer.
Relation of PNI with clinicopathological features
This study investigated the relationship between the PNI and clinicopathological factors of CC using data from four studies involving 1,098 patients [Citation23,Citation24,Citation26,Citation28]. The following factors were analyzed: histology [non-SCC (squamous cell carcinoma) vs SCC], lymph node (LN) metastasis (yes vs no), differentiation (poor vs well/moderate), maximal tumor size (>4 cm vs ≤4 cm), and FIGO stages II vs I and III–IV vs I–II. According to and , the combined results indicated the relation of PNI with the presence of LN metastasis (OR = 1.53, 95% CI = 1.04–82.24, p = .030) and maximum tumor size >4 cm (OR = 1.73, 95% CI = 1.21–2.46, p = .002). However, PNI was not significantly related to histology (OR = 1.41, 95% CI = 0.66–2.98, p = .374), differentiation (OR = 0.76, 95% CI = 0.51–1.12, p = .168), and FIGO stage (I–II) (OR = 0.90, 95% CI = 0.61–1.35, p = .624) or (I–IV) (OR = 1.97, 95% CI = 0.70–5.565, p = .199) (; ).
Figure 4. Forest plots of association between PNI and clinicopathological factors in patients with cervical cancer. (A) histology; (B) lymph node metastasis; (C) differentiation; (D) maximum tumor size; (E) FIGO stage (II vs I); and (F) FIGO stage (III–IV vs I–II).
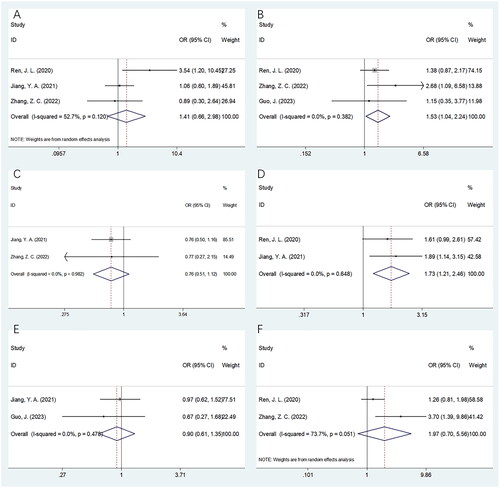
Table 4. The correlation between PNI and clinicopathological features in patients with cervical cancer.
Publication bias
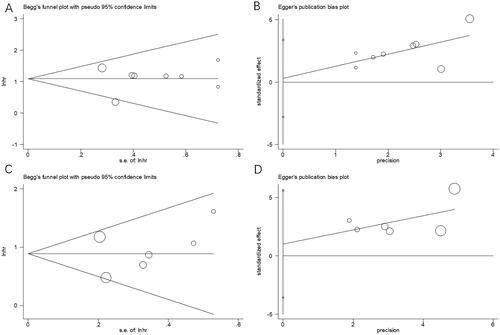
Begg’s and Egger’s tests were used to assess the possible publication bias. Funnel plots did not exhibit any symmetry (), indicating the absence of an obvious publication bias in this meta-analysis: p = .536 and 0.819 upon Begg’s and Egger’s tests separately for OS, p = .452 and .573 for PFS separately.
Discussion
To our knowledge, the present meta-analysis is the first to explore whether the PNI can be used to predict the prognosis of patients with CC. The PNI reflects the nutritional and immune statuses of individual patients. Whether PNI can be used to predict the prognosis of CC remains controversial, according to previous investigations. In this meta-analysis, we collected data from nine studies with 2508 patients to accurately explore the effect of PNI on predicting the prognosis of patients with CC. According to our meta-analysis, a decreased PNI predicted poor OS and PFS among patients with CC. The PNI reliably predicted the prognosis of patients with CC in various subgroups. Moreover, our study also indicated that decreased PNI was associated with LN metastasis and tumor size >4 cm, suggesting that patients with CC with low PNI are prone to tumor metastasis and have a higher tumor burden. Taken together, the current meta-analysis revealed that the PNI is a cost-effective and credible prognostic marker for patients with CC.
Nutrition and inflammation play pivotal roles in tumorigenesis, cancer progression, and metastasis by modulating the tumor microenvironment [Citation30–32]. PNI was calculated using serum albumin levels and lymphocyte counts; therefore, a low PNI can be attributed to a low serum albumin level and lymphocyte count. The potential mechanisms of the prognostic role of PNI in patients with CC are interpreted below. Most serum proteins are composed of albumin, which is produced in the liver. Albumin has a critical effect on human body functions [Citation8]. Serum albumin is an acute-phase protein related to systemic inflammation that reflects nutritional status [Citation33]. The level of serum albumin is related to chronic inflammation, which activates cytokines such as IL-1 and TNF-α [Citation34–37]. Second, the blood lymphocyte levels were calculated as part of the PNI. As a fundamental component of cell-mediated immunity, lymphocytes inhibit the proliferation and invasion of tumor cells via cytokine-mediated cytotoxicity [Citation38,Citation39]. Several lymphocyte subpopulations have also been implicated in the regulation of tumor progression, including B cells, natural killer (NK) cells, and CD4+ and CD8+ T cells [Citation40]. Moreover, malnutrition can further weaken the immune system, increase side effects, and render multiple treatment strategies less effective [Citation41,Citation42]. Therefore, the PNI combines albumin level and lymphocyte count and is a powerful indicator of the prognosis of patients with CC.
Notably, the results indicated that a low PNI was significantly associated with the presence of LN metastasis and tumor size of > 4 cm (). Disease severity is associated with a lower PNI in more advanced tumors. It has long been considered that albumin does not reflect the nutritional status of patients with cancer but rather cancer severity. Therefore, the PNI cannot be considered a tool that can detect advanced cancer stages, but rather a prognostic tool that can correlate cancer severity with a poorer prognosis. However, a worse prognosis is expected in patients with more advanced tumors. Therefore, it is important to investigate the prognostic role of the PNI in survival stratified by tumor staging. The results of the subgroup analysis in our meta-analysis showed that the PNI remained a significant prognostic factor for OS and PFS, irrespective of tumor stage ( and ).
Recently, many meta-analyses have reported that the PNI can be used to predict the prognosis of various solid tumors [Citation43–48]. In a recent meta-analysis of 2, 322 cases of breast cancer, low pretreatment PNI deteriorated OS as well as disease-free survival (DFS) rates [Citation44]. Kang et al. have reported that patients with gastrointestinal stromal tumors who had a decreased PNI showed poor OS and RFS in a meta-analysis of 2307 patients [Citation46]. In another meta-analysis that recruited 3, 631 patients, Tang et al. have showed that patients with nasopharyngeal carcinoma with decreased PNI had poor OS, PFS, and distant metastasis-free survival [Citation48]. In a study conducted by Luan et al. a decreased PNI predicted dismal OS and worse PFS in patients with diffuse large B-cell lymphoma [Citation49].
This study had certain limitations. First, the enrolled articles were from East Asian countries, particularly China. Therefore, our findings apply to Asian patients with CC. Second, the PNI thresholds were not uniform among the included studies. Third, all included studies were retrospective, which may have caused a selection bias. Therefore, large multicenter clinical studies should be conducted for validation.
Conclusions
In conclusion, the PNI significantly predicted poor OS and PFS in patients with CC. Patients with CC and a decreased PNI also tend to suffer from the presence of LN metastasis and larger tumor size. The PNI is a promising prognostic marker for patients with CC in clinical practice.
Authors contributions
ZN contributed the central idea, analyzed most of the data and wrote the main manuscript text. ZN and BY contributed to refining the ideas, collecting the data, carrying out additional analyses and revising the manuscript. All authors contributed to the article and approved the submitted version.
Ethics statement
Ethical approval was not required for this meta-analysis because all the data used in this study were obtained from published articles.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Disclosure statement
The authors declare that there is no conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Zhang S, Xu H, Zhang L, et al. Cervical cancer: epidemiology, risk factors and screening. Chin J Cancer Res. 2020;32(6):1–11. doi:10.21147/j.issn.1000-9604.2020.06.05.
- Hill EK. Updates in cervical cancer treatment. Clin Obstet Gynecol. 2020;63(1):3–11. doi:10.1097/grf.0000000000000507.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660.
- Bailón-Cuadrado M, Pérez-Saborido B, Sánchez-González J, et al. Prognostic nutritional index predicts morbidity after curative surgery for colorectal cancer. Cir Esp. 2019;97(2):71–80. doi:10.1016/j.ciresp.2018.08.015.
- Zhou Y, Dai M, Zhang Z. Prognostic significance of the systemic immune-inflammation index (SII) in patients with small cell lung cancer: a meta-analysis. Front Oncol. 2022;12:814727. doi:10.3389/fonc.2022.814727.
- Niu Z, Yan B. Prognostic and clinicopathological impacts of controlling nutritional status (CONUT) score on patients with gynecological cancer: a meta-analysis. Nutr J. 2023;22(1):33. doi:10.1186/s12937-023-00863-8.
- Zhao H, Xu L, Tang P, et al. Geriatric nutritional risk index and survival of patients with colorectal cancer: a meta-analysis. Front Oncol. 2022;12:906711. doi:10.3389/fonc.2022.906711.
- He J, Pan H, Liang W, et al. Prognostic effect of albumin-to-Globulin ratio in patients with solid tumors: a systematic review and meta-analysis. J Cancer. 2017;8(19):4002–4010. doi:10.7150/jca.21141.
- Buzby GP, Mullen JL, Matthews DC, et al. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139(1):160–167. doi:10.1016/0002-9610(80)90246-9.
- Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–1005.
- Sun H, Chen L, Huang R, et al. Prognostic nutritional index for predicting the clinical outcomes of patients with gastric cancer who received immune checkpoint inhibitors. Front Nutr. 2022;9:1038118. doi:10.3389/fnut.2022.1038118.
- Persano M, Rimini M, Tada T, et al. Role of the prognostic nutritional index in predicting survival in advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab. Oncology. 2023;101(5):283–291. doi:10.1159/000528818.
- Shen Z, Zhang S, Chen X, et al. Prognostic value of prognostic nutritional index on extranodal natural killer/T-cell lymphoma patients: a multicenter propensity score matched analysis of 1022 cases in Huaihai lymphoma working group. Hematol Oncol. 2023;41(3):380–388. doi:10.1002/hon.3124.
- Kageyama S, Yoshida T, Kobayashi K, et al. Prognostic nutritional index of early post-pembrolizumab therapy predicts long-term survival in patients with advanced urothelial carcinoma. Oncol Lett. 2023;25(2):49. doi:10.3892/ol.2022.13635.
- Fu M, Yu L, Yang L, et al. Predictive value of the preoperative prognostic nutritional index for postoperative progression in patients with pancreatic neuroendocrine neoplasms. Front Nutr. 2022;9:945833. doi:10.3389/fnut.2022.945833.
- Li B, Lu Z, Wang S, et al. Pretreatment elevated prognostic nutritional index predicts a favorable prognosis in patients with prostate cancer. BMC Cancer. 2020;20(1):361. doi:10.1186/s12885-020-06879-1.
- Takao K, Konishi H, Fujiwara H, et al. Clinical significance of prognostic nutritional index in the treatment of esophageal squamous cell carcinoma. In Vivo. 2020;34(6):3451–3457. doi:10.21873/invivo.12184.
- Matsuyama Y, Nakamura T, Yoshida K, et al. Role of the prognostic nutritional index in patients with soft-tissue sarcoma. In Vivo. 2021;35(4):2349–2355. doi:10.21873/invivo.12511.
- Mozas P, Rivero A, Rivas-Delgado A, et al. The prognostic nutritional index (PNI) is an independent predictor of overall survival in older patients with follicular lymphoma. Leuk Lymphoma. 2022;63(4):903–910. doi:10.1080/10428194.2021.2010064.
- Haraga J, Nakamura K, Omichi C, et al. Pretreatment prognostic nutritional index is a significant predictor of prognosis in patients with cervical cancer treated with concurrent chemoradiotherapy. Mol Clin Oncol. 2016;5(5):567–574. doi:10.3892/mco.2016.1028.
- He X, Li JP, Liu XH, et al. Prognostic value of C-reactive protein/albumin ratio in predicting overall survival of Chinese cervical cancer patients overall survival: comparison among various inflammation based factors. J Cancer. 2018;9(10):1877–1884. doi:10.7150/jca.23320.
- Zhang W, Liu K, Ye B, et al. Pretreatment C-reactive protein/albumin ratio is associated with poor survival in patients with stage IB-IIA cervical cancer. Cancer Med. 2018;7(1):105–113. doi:10.1002/cam4.1270.
- Ren JL, Lan M, Sun C, et al. Value of prognostic nutrition index in predicting the efficacy and prognosis of concurrent chemoradiotherapy in patients with cervical cancer. J Cancer Control Treat. 2020;33:850–857. doi:10.3969/j.issn.1674-0904.2020.10.006.
- Jiang YA, Gu HF, Zheng XJ, et al. Pretreatment C-reactive protein/albumin ratio is associated with poor survival in patients with 2018 FIGO stage IB-IIA HPV-positive cervical cancer. Pathol Oncol Res. 2021;27:1609946. doi:10.3389/pore.2021.1609946.
- Zhang G, Zhang Y, He F, et al. Preoperative controlling nutritional status (CONUT) score is a prognostic factor for early-stage cervical cancer patients with high-risk factors. Gynecol Oncol. 2021;162(3):763–769. doi:10.1016/j.ygyno.2021.06.012.
- Zhang ZC, Li Y, Huang L. Predictive value of prognostic nutritional index on prognosis of patients with cervical cancer radiotherapy. Oncol Prog. 2022;20:1366–1368. doi:10.11877/j.issn.1672-1535.2022.20.13.18.
- Zhu JM, YY, D Jiang ZD. The value of preoperative prognostic nutritional index in evaluating short-term prognosis of patients with cervical cancer after surgery. J Clin Nurs. 2022;21:30–33. doi:10.3969/j.issn.1671-8933.2022.05.010.
- Guo J, Lv W, Wang Z, et al. Prognostic value of inflammatory and nutritional markers for patients with early-stage poorly-to moderately-differentiated cervical squamous cell carcinoma. Cancer Control. 2023;30:10732748221148913. doi:10.1177/10732748221148913.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi:10.1016/j.jclinepi.2009.06.005.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013.
- Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol. 2017;18(8):843–850. doi:10.1038/ni.3754.
- Saha SK, Lee SB, Won J, et al. Correlation between oxidative stress, nutrition, and cancer initiation. Int J Mol Sci. 2017;18(7):18. doi:10.3390/ijms18071544.
- Caraceni P, Tufoni M, Bonavita ME. Clinical use of albumin. Blood Transfus. 2013;11(Suppl 4):S18–S25. doi:10.2450/2013.005s.
- Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol. 2005;39(4 Suppl 2):S143–S146. doi:10.1097/01.mcg.0000155514.17715.39.
- Oñate-Ocaña LF, Aiello-Crocifoglio V, Gallardo-Rincón D, et al. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. 2007;14(2):381–389. doi:10.1245/s10434-006-9093-x.
- Polterauer S, Grimm C, Zeillinger R, et al. Association of C-reactive protein (CRP) gene polymorphisms, serum CRP levels and cervical cancer prognosis. Anticancer Res. 2011;31:2259–2264. 2011/07/09.
- Bosarge PL, Shoultz TH, Griffin RL, et al. Stress-induced hyperglycemia is associated with higher mortality in severe traumatic brain injury. J Trauma Acute Care Surg. 2015;79(2):289–294. doi:10.1097/ta.0000000000000716.
- Song AJ, Ding K, Alnahhas I, et al. Impact of lymphopenia on survival for elderly patients with glioblastoma: a secondary analysis of the CCTG CE.6 (EORTC 26062-22061, TROG03.01) randomized clinical trial. Neurooncol Adv. 2021;3(1):vdab153. doi:10.1093/noajnl/vdab153.
- Trikha M, Corringham R, Klein B, et al. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res. 2003;9:4653–4665.
- Luvián-Morales J, González-Trejo S, Carrillo JF, et al. Association of the prognostic nutritional index and overall survival in patients with colorectal cancer: a STROBE compliant retrospective cohort study. Cancer Med. 2019;8(7):3379–3388. doi:10.1002/cam4.2212.
- St Paul M, Ohashi PS. The roles of CD8(+) T cell subsets in antitumor immunity. Trends Cell Biol. 2020;30(9):695–704. doi:10.1016/j.tcb.2020.06.003.
- Obermair A, Simunovic M, Isenring L, et al. Nutrition interventions in patients with gynecological cancers requiring surgery. Gynecol Oncol. 2017;145(1):192–199. doi:10.1016/j.ygyno.2017.01.028.
- Tan X, Chen H. The prognostic value of prognostic nutritional index in patients with ovarian cancer: a systematic review and meta-analysis. Nutr Cancer. 2023;75(1):73–81. doi:10.1080/01635581.2022.2104879.
- Hu G, Ding Q, Zhong K, et al. Low pretreatment prognostic nutritional index predicts poor survival in breast cancer patients: a meta-analysis. PLoS One. 2023;18(1):e0280669. doi:10.1371/journal.pone.0280669.
- Xue S, Zhao H, Zhang K, et al. Prognostic and clinicopathological correlations of pretreatment prognostic nutritional index in renal cell carcinoma: a meta-analysis. Urol Int. 2022;106(6):567–580. doi:10.1159/000521353.
- Kang N, Gu H, Ni Y, et al. Prognostic and clinicopathological significance of the prognostic nutritional index in patients with gastrointestinal stromal tumours undergoing surgery: a meta-analysis. BMJ Open. 2022;12(12):e064577. doi:10.1136/bmjopen-2022-064577.
- Zhang Q, Bao J, Zhu ZY, et al. Prognostic nutritional index as a prognostic factor in lung cancer patients receiving chemotherapy: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25(18):5636–5652. doi:10.26355/eurrev_202109_26783.
- Tang M, Jia Z, Zhang J. The prognostic role of prognostic nutritional index in nasopharyngeal carcinoma: a systematic review and meta-analysis. Int J Clin Oncol. 2021;26(1):66–77. doi:10.1007/s10147-020-01791-x.
- Luan C, Wang F, Wei N, et al. Prognostic nutritional index and the prognosis of diffuse large b-cell lymphoma: a meta-analysis. Cancer Cell Int. 2020;20(1):455. doi:10.1186/s12935-020-01535-x.