Abstract
Background
Effective pain control of herpes zoster ophthalmicus (HZO) is not only essential to attenuate the clinical symptoms but to reduce the risk of postherpetic neuralgia development. Recently, neuromodulation therapy has been one promising option for neuropathic pain and increasingly applied in management of zoster-related pain. One key factor of neuromodulation treatment is the therapeutic site for the impaired nerves. In this study we aim to investigate one novel dual-neuromodulation strategy, targeting the level of the peripheral branch and trigeminal ganglion, in the pain management of HZO.
Methods
Dual neuromodulation strategy combining short-term peripheral nerve stimulation (PNS) with pulsed radiofrequency (PRF) of trigeminal ganglion was compared with single PNS treatment for HZO-related pain. Clinical recordings of patients were retrospectively reviewed. The primary outcome was the pain severity, assessed by the visual analogue scale (VAS) before and after neuromodulation therapy.
Results
PNS achieved significant relief of pain with or without PRF treatment before discharge, which provided enduring therapeutic effect up to 12-month follow-up. The mean reduction of VAS was 6.7 ± 1.4 in dual modulation therapy (n = 13) at last follow-up and 5.4 ± 1.5 in PNS subgroup (n = 20), respectively. Moreover, dual modulation strategy provided better control of pain compared with PNS therapy alone at each time point.
Conclusion
It is feasible and effective to combine the PNS and PRF in pain management of HZO. This novel dual modulation strategy of trigeminal pathway may provide additional therapeutic effects of pain symptoms in HZO population.
KEY MESSAGES
Dual neuromodulation strategy for pain management of herpes zoster ophthalmicus is proposed, with regard to stimulation site (peripheral and trigeminal ganglion) and apparatus (electrical nerve stimulation and pulsed radiofrequency).
Superior clinical outcome was associated with novel neuromodulation therapy with dual therapeutic targets, when compared with peripheral nerve stimulation in treatment of herpes zoster ophthalmicus.
We conducted literature review to compare distinct pattern of neuromodulation (peripheral nerve stimulation and radiofrequency) in treatment of trigeminal neuropathic pain caused by herpes zoster.
Introduction
Herpes zoster ophthalmicus (HZO) is caused by the reactivation of latent varicella-zoster virus, typically resulting in painful vesicular rash in the distribution of the ophthalmic division of trigeminal nerve [Citation1]. The estimation of HZO prevalence has accounted for about 10%–20% of the total herpetic infection [Citation2]. Risk factor for herpetic infection includes ageing, immunocompromised status and comorbidities [Citation3]. In addition, recent evidence has indicated a potential causal relationship between HZO and COVID-19 infection or vaccination [Citation4–7]. It has been estimated that total incidence of cutaneous lesion secondary to COVID‐19 ranged between 4% and 20.4%, less risk has been reported in COVID-19 cases caused by vesicular lesions [Citation7, Citation8].
One featured manifestation of HZO is the severe pain affected the ophthalmic dermatome, characterized by stabbing radiating and localized pain during acute phase of vesicular eruption [Citation3]. Moreover, about half of the patients may develop postherpetic neuralgia (PHN) with ophthalmic involvement [Citation2]. PHN is characterized by moderate to severe facial pain which can last for more than 3 months after initial skin lesion [Citation9]. The neuropathic feature of PHN is characterized with continuous or spontaneous burning pain, paroxysmal electric shock-like pain, mechanical allodynia and hyperalgesia [Citation9]. Consequently, the quality of life is significantly impaired in HZO population, and the ongoing requirement of pain management heavens the burden of public health system [Citation10].
The principle of HZO treatment is to apply the anti-viral therapy at early stage of infection; however, it remains controversial that oral acyclovir can reduce the risk of PHN [Citation11]. In addition to anti-viral agents, it is necessary to perform pain therapy in most cases with analgesic medication, including tricyclic antidepressants, antiseizure drugs, opioids and topical analgesics [Citation12]. Interventional procedures may be considered when pain relief is insufficient with oral medicine.
Local nerve block is commonly applied to achieve immediate but short-term pain relief. Recently, we demonstrated that the implantable neuromodulation therapy provided effective and enduring analgesic effect in patients with HZO [Citation13, Citation14]. By placing an electrical nerve stimulator towards the supraorbital and supratrochlear nerves, continuous stimulation can be delivered to cover the painful regions. However, we found that about 22.2% of HZO patients suffered facial pain in the distribution of the second branch of trigeminal nerve (i.e. the maxillary nerve), which cannot be modulated by the peripheral ophthalmic stimulation [Citation13]. In some severe cases, patients may suffer pain involved with all three branches of trigeminal nerve. Thus, in this study, we aim to investigate the clinical effect of one novel dual modulation strategy, targeting the peripheral nerve branch and Gasserian ganglion simultaneously.
Materials and methods
This was a retrospective medical recording review of consecutive patients with HZO orofacial pain presenting at the Department of Pain, the Third Xiangya Hospital, Central South University between January 2018 and June 2022. Surgical protocol was approved by the institutional and ethics board of the Third Xiangya Hospital of Central South University.
Participants
Patients who were diagnosed with HZO were involved in this study. The diagnosis was confirmed consistent with our previous criteria: vesicular rash and dermatomal pain associated with ophthalmic distribution [Citation13]. Phase of HZO-related pain was categorized according to the disease duration, that acute pain indicated pain occurred within 1 month, and those with moderate to severe trigeminal neuropathic pain over 3 months were considered as PHN population. Subacute phase of HZO-related pain was defined as duration of disease ranging between one and 3 months [Citation13]. Patients were classified into two subgroups according to the method of neuromodulation therapy. Patients who underwent the implantation of peripheral nerve stimulation (PNS) were categorized into Group 1, and those who received dual neuromodulation treatment (PNS combined with trigeminal ganglion pulsed radiofrequency [PRF]) were categorized into Group 2, respectively.
Surgery
Peripheral nerve stimulation
The surgical detail of PNS has been described previously [Citation13]. One supine position was used with the head of patient turning slightly to the contralateral side of the surgical region. After local anaesthesia with 1% lidocaine, a 14-G Tuohy needle was introduced into the 2 cm posterolateral junction of the frontal and zygomatic portion of the orbital rim. The cannulation was then penetrated through the subcutaneous tissue and reached the plane of supra-periosteal tissue over the eyebrow in a semilunar path. The tip of cannula was slightly beyond the cranial middle line (). One eight-contact electrode (Model 3873; Medtronic, Minneapolis, MN, USA) was inserted into the Tuohy needle. The distal ending of electrical lead was connected to one extension multi-lead cable (Model: 355531; Medtronic), to adjust the stimulation parameter with one external neurostimulator (Model 37022; Medtronic). The stimulation electrode was programmed with a pulse width of 500 µS at a frequency of 40 Hz, to induce a sensation of paresthaesia covering the painful region. The amplitude of stimulation voltage was set between 0.5 and 3.0 mV according to the pain severity reported by the patient.
Pulsed radiofrequency of Gasserian ganglion
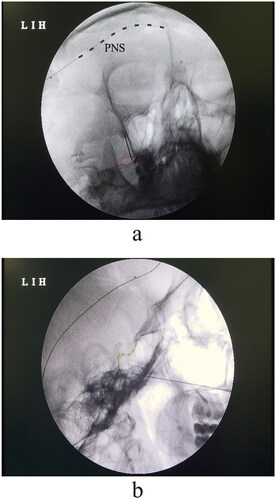
To perform the percutaneous PRF of the trigeminal ganglion, patient was placed in one supine position with the shoulder slightly elevating for better visualization of the oval foramen. To exposure the anterior–posterior view of the oval foramen, the probe of C-arm was set about 15 degrees ipsilaterally and 30 degrees caudally to the surgical site. The entry of puncturing needle was set about 2.5 cm lateral to the corner of the mouth. Local anaesthesia of 1% lidocaine was applied during the puncturing procedure of the oval foramen. The insertion of needle was performed with the submental, oblique view until we reached the bony edge of the foramen (). The depth of cannulation was then confirmed under the lateral view of fluoroscopy. To target the trigeminal ganglion, we aimed to place the tip of needle 2–3 mm within the clivus line (). The parameter of PRF stimulation was consistent with our previous protocol [Citation15], at a frequency of 2 Hz (20 ms pulse width) and temperature of 42 degrees. The duration of stimulation was set to 240 s and repeated for three cycles.
Clinical outcomes and follow-up
The demographic and clinical data were collected with one brief questionnaire according to the medical history by two independent researchers (Y.W. and L.Y.). To investigate the clinical outcome of neuromodulation therapy, one telephone interview was performed at 1-, 3-, 6- and 12-month follow-up. The primary clinical outcome was the pain severity assessed by the visual analogue scale (VAS), ranging from 0 (‘pain free’) to 10 (‘worst pain imaginable’).
Statistical analysis
Statistical analysis was performed by Prism 9.0 software (GraphPad, San Diego, CA, USA). Variables were presented as mean ± standard deviation. Two-way analysis of variance with repeated measures and post-hoc multiple pairwise comparison Sidak’s testing was used to assess the alteration of pain scores between two groups over time. A p-value <.05 was considered statistically significant.
Results
Demographics
A total of 33 patients (18 males and 15 females) were included in this study, with a mean age of 70.2 ± 9.0 years old. Twenty patients (11 male and 9 female) underwent PNS therapy, and 13 (7 male and 6 female) were treated with dual neuromodulation, respectively. The mean age of PNS group and PNS + PRF group were 70.25 ± 9.20 (range, 50–86) and 70.15 ± 9.00 years old (range, 50–80) separately, which had no statistically significant difference (p = .977). Most patients (76%) reported severe facial pain with VAS over 6/10 points in the ophthalmic region at admission (Group 1 vs. Group 2: 7.5 ± 0.9 vs. 7.2 ± 1.4, p = .578). The majority of participants presented with one subacute herpetic lesion, with disease duration ranging between 1 and 3 months. Only five patients were diagnosed with PHN in this study. Before neuromodulation therapy, all patients had failed to control pain with conventional treatment, including oral analgesic agents and nerve block. The clinical features of patients are shown in .
Table 1. General characteristics of patients.
Follow-up
All participants consent to undertake the routine telephone follow-up and accomplished the 1- and 3-month follow-up after their hospital discharge. Fourteen out of 20 patients were accomplished for the final follow-up in Group 1. One patient lost the 6-month follow-up in Group 2, and 8 out of 13 patients finished the 12-month follow-up in Group 2, respectively.
Therapeutic efficacy
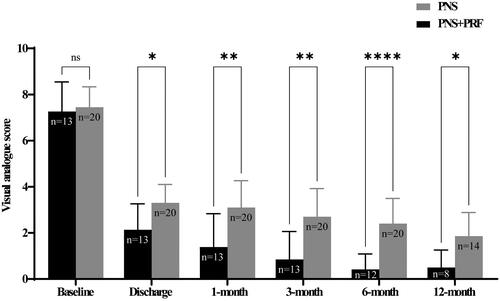
Both groups obtained significant pain relief at discharge, with pain reduction of 4.2 ± 1.0 VAS after PNS treatment and 5.0 ± 1.4 for dual neuromodulation strategy, respectively. The VAS score between Group 1 and Group 2 was 3.1 ± 1.2 and 1.4 ± 1.4 at 1 month, 2.7 ± 1.2 and 0.8 ± 1.2 at 3 months and 2.4 ± 1.1 and 0.4 ± 0.7 at 6 months. The therapeutic effect was enduring up to 12-month follow-up, as the pain scores gradually decreased to 1.6 ± 1.3 in Group 1 and 0.5 ± 0.8 in Group 2 (). Meanwhile, the pain severity was significantly attenuated in Group 2 at each time point of follow-up compared with the other cohort ().
Figure 2. Pain severity assessed by the visual analogue score at each follow-up point. Patients were interviewed at baseline, discharge, 1-, 3-, 6- and 12 months after neuromodulation treatment. Two-way analysis of variance with repeated measures and Bonferroni’s post-test. ns = non-significant, *p < .05, **p < .01, ****p < .0001.
Complications
We did not observe any obvious complications (e.g. infection, haematoma, tetrode migration or nerve injury) in this study.
Literature and discussion
Significant percentages of HZO patients may suffer severe and long-lasting facial pain. Except shortening disease course and preventing ophthalmic complications, sufficient analgesia is necessary to improve the quality of life and reduce the risk of PHN. Here we demonstrated that one novel neuromodulation therapy targeting both peripheral nerve branch and Gasserian ganglion is an effective option for pain management in patients with HZO disease.
In the literature review, a series of studies focused on PRF and/or PNS neuromodulation therapy for pain management of HZO were identified () [Citation13, Citation16–35]. Despite HZO, other indication of neuromodulation usage for facial pain management includes idiopathic, postsurgical, traumatic, post-stroke, multiple sclerosis, radioactive, classic trigeminal neuralgia and other unclassified types of aetiology [Citation16, Citation20–23]. Multiple phases of herpetic disease (acute, subacute and PHN) can be considered to undertake neuromodulation treatment, ranging from 2 weeks to 30 years. The majority of previous data were obtained from observational cohort studies or case series, and about 23% (5 out of 22) were randomized controlled trials [Citation29, Citation30, Citation32, Citation35]. To treat HZO-related neuralgia, supraorbital and infraorbital branches are commonly targeted for stimulator implantation. Unlike classical trigeminal neuralgia, less herpetic cases present with mandibular lesions, and the implantable neuromodulation can also be applied directly to this region [Citation18, Citation19, Citation22] or in the trigeminal ganglion [Citation23].
Table 2. Summary of enrolled literatures..
It is important to apply anti-viral treatment at early stage of herpes zoster to attenuate pain and prevent the development of PHN. However, it remains a great challenge to efficiently control the HZO-related pain with conventional anti-viral agents, which is consistent with our previous report [Citation13]. They may need to take anticonvulsants and opioid analgesics for pain relief, which commonly brings in side effects (e.g. gastrointestinal and psychiatric dysfunction, addiction and tolerance) [Citation36]. Thus, alternative option of pain management in HZO cohort is necessarily needed.
Neuromodulation therapy has been one promising method in treatment of intractable neuropathic pain. One strategy of neuromodulation is to apply PRF approaching the dorsal root ganglion or trigeminal ganglion, which has been applied in treatment of herpetic neuralgia [Citation29, Citation37]. Recently, an emerging neuromodulation of implantable PNS has been introduced to treat trigeminal herpetic pain [Citation14]. Unlike radiofrequency treatment, the implantable device can provide persistent electrical stimulation to the affected nerves. Thus, one advantage of this novel dual neuromodulation therapy is to provide enduring therapeutic effect compared with short-term PRF stimulation. In addition to stimulation duration, the mechanism underlying the analgesic effect may also vary between PNS and PRF due to the site of stimulation. Thus, we think it necessary to investigate the clinical effect of the combination of PNS and PRF in HZO pain management.
Consistent with our previous study [Citation13], PNS achieved significant pain relief of HZO-related pain with average 4.2 VAS reduction at discharge. Moreover, we found that dual neuromodulation treatment achieved better clinical outcome compared with PNS, as demonstrated by the significantly lower pain severity at short- and long-term follow-up (). The estimated recurrence rate of herpetic zoster-related pain was about 37% in one cohort who underwent PRF treatment only [Citation38]. Given the high risk of recurrent pain, we did not recommend the patient to only take PRF procedure according to the clinical routine in our centre, which is consistent with recent findings that combination of PNS and PRF or PNS alone provided superior analgesic function over single PRF treatment [Citation27]. Specifically, pain severity and functional deficiency were both significantly lower in patients treated with PNS combined with or without PRF from 1 to 24 months after treatment, compared with PRF treatment [Citation27].
The well-known gate control theory of pain may play an important role in the analgesic effect of neuromodulation [Citation39]. This theory proposed that the selective stimulation of non-nociceptive afferents (Aβ fiber) can inhibit the nociceptive afferents (Aθ and C fibers) and then decrease the perception of pain [Citation40, Citation41]. Mechanism underlying the analgesic effect of PRF includes inhibition of inflammatory responses and interfering ectopic firing at ganglion [Citation42, Citation43]. Recently, we have demonstrated that supraspinal mechanism may partially mediate the therapeutic function of PNS [Citation44].
There are several limitations in our study. First, this is a single-centre study with relatively small sample number. Second, the retrospective and nonrandomized nature of study design. In addition, we did not enrol one group with only PRF treatment in this study, mainly due to the insufficient therapeutic effect. Finally, we did not compare the effect of different stimulation parameters, including stimulation frequency, voltage and duration on the therapeutic effect. We aim to perform randomized, well-controlled, clinical trials with large sample size in future studies to investigate the optimal strategy of neuromodulation treatment in pain management of HZO.
Conclusions
It is feasible and effective to apply dual neuromodulation techniques at trigeminal ganglion and peripheral nerve branch. Better clinical outcomes may be achieved by this novel neuromodulation therapy in pain control for HZO patients, compared with individual PNS treatment.
Authors contributions
Conceptualization: H.Z. and D.H. Methodology: J.M., Y.W., L.Y., D.H. and H.Z. Validation: J.M. and Y.W. Formal analysis: J.M. Investigation: J.M. and H.Z. Resources: H.Z. and D.H. Data curation: J.M. Writing – original draft preparation: J.M. and H.Z. Writing – review and editing: H.Z. Visualization: H.Z. Supervision: H.Z. Project administration: H.Z. Funding acquisition: H.Z. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
The authors declare no competing interests.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Kedar S, Jayagopal LN, Berger JR. Neurological and ophthalmological manifestations of varicella zoster virus. J Neuroophthalmol. 2019;39(2):1–9. doi: 10.1097/WNO.0000000000000721.
- Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008;115(2 Suppl):S3–S12. doi: 10.1016/j.ophtha.2007.10.009.
- Feller L, Khammissa RAG, Fourie J, et al. Postherpetic neuralgia and trigeminal neuralgia. Pain Res Treat. 2017;2017:1681765. doi: 10.1155/2017/1681765.
- Avallone G, Cavallo F, Astrua C, et al. Cutaneous adverse reactions following SARS-CoV-2 vaccine booster dose: a real-life multicentre experience. J Eur Acad Dermatol Venereol. 2022;36(11):e876–e879. doi: 10.1111/jdv.18386.
- Rallis KI, Fausto R, Ting DSJ, et al. Manifestation of herpetic eye disease after COVID-19 vaccine: a UK case series. Ocul Immunol Inflamm. 2022;30(5):1136–1141. doi: 10.1080/09273948.2022.2046795.
- You IC, Ahn M, Cho NC. A case report of herpes zoster ophthalmicus and meningitis after COVID-19 vaccination. J Korean Med Sci. 2022;37(20):e165. doi: 10.3346/jkms.2022.37.e165.
- Martora F, Fabbrocini G, Picone V. A case of herpes zoster ophthalmicus after third dose of comirnaty (BNT162b2 mRNA) vaccine. Dermatol Ther. 2022;35(5):e15411.
- Martora F, Villani A, Fabbrocini G, et al. COVID-19 and cutaneous manifestations: a review of the published literature. J Cosmet Dermatol. 2023;22(1):4–10. doi: 10.1111/jocd.15477.
- Johnson RW, Rice AS. Clinical practice. Postherpetic neuralgia. N Engl J Med. 2014;371(16):1526–1533. doi: 10.1056/NEJMcp1403062.
- Matthews S, De Maria A, Passamonti M, et al. The economic burden and impact on quality of life of herpes zoster and postherpetic neuralgia in individuals aged 50 years or older in Italy. Open Forum Infect Dis. 2019;6(2):ofz007. doi: 10.1093/ofid/ofz007.
- Chen N, Li Q, Yang J, et al. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2014;2:CD006866.
- Pavan-Langston D. Herpes zoster antivirals and pain management. Ophthalmology. 2008;115(2 Suppl):S13–S20. doi: 10.1016/j.ophtha.2007.10.012.
- Han R, Guo G, Ni Y, et al. Clinical efficacy of short-term peripheral nerve stimulation in management of facial pain associated with herpes zoster ophthalmicus. Front Neurosci. 2020;14:574713. doi: 10.3389/fnins.2020.574713.
- Ni Y, Yang L, Han R, et al. Implantable peripheral nerve stimulation for trigeminal neuropathic pain: a systematic review and meta-analysis. Neuromodulation. 2021;24(6):983–991. doi: 10.1111/ner.13421.
- Yang L, Huang Y, Ma J, et al. Clinical outcome of pulsed-radiofrequency combined with transforaminal epidural steroid injection for lumbosacral radicular pain caused by distinct etiology. Front Neurosci. 2021;15:683298. doi: 10.3389/fnins.2021.683298.
- Manning A, Ortega RG, Moir L, et al. Burst or conventional peripheral nerve field stimulation for treatment of neuropathic facial pain. Neuromodulation. 2019;22(5):645–652. doi: 10.1111/ner.12922.
- Wan CF, Song T. Short-term peripheral nerve stimulation relieve pain for elder herpes zoster ophthalmicus patients: a retrospective study. Neuromodulation. 2021;24(6):1121–1126. doi: 10.1111/ner.13288.
- Liu DY, Chen JS, Lin CY, et al. Subcutaneous peripheral nerve stimulation for treatment of acute/subacute herpes zoster-related trigeminal neuralgia: a retrospective research. Clin J Pain. 2021;37(12):867–871. doi: 10.1097/AJP.0000000000000981.
- Jakobs M, Unterberg A, Treede RD, et al. Subcutaneous trigeminal nerve field stimulation for refractory trigeminal pain: a cohort analysis. Acta Neurochir. 2016;158(9):1767–1774. doi: 10.1007/s00701-016-2881-6.
- Johnson MD, Burchiel KJ. Peripheral stimulation for treatment of trigeminal postherpetic neuralgia and trigeminal posttraumatic neuropathic pain: a pilot study. Neurosurgery. 2004;55(1):135–142. doi: 10.1227/01.NEU.0000126874.08468.89.
- Texakalidis P, Tora MS, Anthony CL, et al. Peripheral trigeminal branch stimulation for refractory facial pain: a single-center experience. Clin Neurol Neurosurg. 2020;194:105819. doi: 10.1016/j.clineuro.2020.105819.
- Klein J, Sandi-Gahun S, Schackert G, et al. Peripheral nerve field stimulation for trigeminal neuralgia, trigeminal neuropathic pain, and persistent idiopathic facial pain. Cephalalgia. 2016;36(5):445–453. doi: 10.1177/0333102415597526.
- Taub E, Munz M, Tasker RR. Chronic electrical stimulation of the Gasserian ganglion for the relief of pain in a series of 34 patients. J Neurosurg. 1997;86(2):197–202. doi: 10.3171/jns.1997.86.2.0197.
- Jia Y, Shen Y, Meng L, et al. Efficacy, safety, and predictors of response to pulsed radiofrequency therapy for acute zoster-related trigeminal neuralgia patients: a multicenter retrospective study. Pain Physician. 2022;25(4):E523–E530.
- Ding Y, Hong T, Li H, et al. Efficacy of CT guided pulsed radiofrequency treatment for trigeminal postherpetic neuralgia. Front Neurosci. 2019;13:708. doi: 10.3389/fnins.2019.00708.
- Zhang H, Ni H, Liu S, et al. Supraorbital nerve radiofrequency for severe neuralgia caused by herpes zoster ophthalmicus. Pain Res Manag. 2020;2020:3191782–3191787. doi: 10.1155/2020/3191782.
- Fan X, Ren H, Xu F, et al. Comparison of the efficacy of short-term peripheral nerve stimulation and pulsed radiofrequency for treating herpes zoster ophthalmicus neuralgia. Clin J Pain. 2022;38(11):686–692. doi: 10.1097/AJP.0000000000001074.
- Liu DY, Chen JS, Fang ZZ, et al. Pulsed radiofrequency of the trigeminal ganglion for treating postherpetic neuralgia of the ophthalmic branch. Pain Res Manag. 2021;2021:9791801. doi: 10.1155/2021/9791801.
- Wan C, Dong DS, Song T. High-voltage, long-duration pulsed radiofrequency on Gasserian ganglion improves acute/subacute zoster-related trigeminal neuralgia: a randomized, double-blinded, controlled trial. Pain Phys. 2019;22(4):361–368. doi: 10.36076/ppj/2019.22.361.
- Wan CF, Song T. Comparison of two different pulsed radiofrequency modes for prevention of postherpetic neuralgia in elderly patients with acute/subacute trigeminal herpes zoster. Neuromodulation. 2022;25(8):1364–1371. doi: 10.1111/ner.13457.
- Li H, Ding Y, Zhu Y, et al. Effective treatment of postherpetic neuralgia at the first branch of the trigeminal nerve by high-voltage pulsed radiofrequency. Front Neurol. 2021;12:746035. doi: 10.3389/fneur.2021.746035.
- Li M, Hu H, Tong SX, et al. The therapeutic efficacy of pulsed radiofrequency alone versus a dexamethasone and pulsed radiofrequency combination in patients with trigeminal postherpetic neuralgia: a double-blind, randomized controlled trial. Pain Phys. 2022;25(4):E543–E549.
- Ding Y, Yao P, Li H, et al. CT-guided stellate ganglion pulsed radiofrequency stimulation for facial and upper limb postherpetic neuralgia. Front Neurosci. 2019;13:170. doi: 10.3389/fnins.2019.00170.
- Zhang JF, Williams JP, Zhao QN, et al. Combined high-voltage pulsed radiofrequency and ozone therapy versus ozone therapy alone in treating postherpetic neuralgia: a retrospective comparison. Med Gas Res. 2023;13(1):15–22. doi: 10.4103/2045-9912.352660.
- Zhang W, He C. Clinical efficacy of pulsed radiofrequency combined with intravenous lidocaine infusion in the treatment of subacute herpes zoster neuralgia. Pain Res Manag. 2022;2022:5299714–5299753. doi: 10.1155/2022/5299753.
- McNicol ED, Midbari A, Eisenberg E. Opioids for neuropathic pain. Cochrane Database Syst Rev. 2006;2013(8):CD006146. doi: 10.1002/14651858.CD006146.
- Wu C-Y, Lin H-C, Chen S-F, et al. Efficacy of pulsed radiofrequency in herpetic neuralgia: a meta-analysis of randomized controlled trials. Clin J Pain. 2020;36(11):887–895. doi: 10.1097/AJP.0000000000000867.
- Luo G, Zhang Z, Zhu J, et al. Association between the risk of relapse and the type of surgical procedure for herpes zoster-related pain. Pain Phys. 2021;24(8):E1227–E1236.
- Schmidt GL. The use of spinal cord stimulation/neuromodulation in the management of chronic pain. J Am Acad Orthop Surg. 2019;27(9):e401–e407. doi: 10.5435/JAAOS-D-17-00829.
- Marchand S. Spinal cord stimulation analgesia: substantiating the mechanisms for neuropathic pain treatment. Pain. 2015;156(3):364–365. doi: 10.1097/01.j.pain.0000000000000089.
- Kaye AD, Ridgell S, Alpaugh ES, et al. Peripheral nerve stimulation: a review of techniques and clinical efficacy. Pain Ther. 2021;10(2):961–972. doi: 10.1007/s40122-021-00298-1.
- Van Boxem K, Huntoon M, Van Zundert J, et al. Pulsed radiofrequency: a review of the basic science as applied to the pathophysiology of radicular pain: a call for clinical translation. Reg Anesth Pain Med. 2014;39(2):149–159. doi: 10.1097/AAP.0000000000000063.
- Berta T, Qadri Y, Tan PH, et al. Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Expert Opin Ther Targets. 2017;21(7):695–703. doi: 10.1080/14728222.2017.1328057.
- Zhou H, Han R, Chen L, et al. Effect of implantable electrical nerve stimulation on cortical dynamics in patients with herpes zoster-related pain: a prospective pilot study. Front Bioeng Biotechnol. 2022;10:862353. doi: 10.3389/fbioe.2022.862353.