Abstract
Introduction
Vaccination hesitancy is an important barrier to vaccination among IBD patients. The development of adverse events is the main concern reported. The purpose of this monocentric study was to assess SARS-CoV-2 vaccination safety in IBD patients by evaluating the postvaccination flare risk and incidence of overall adverse events.
Methods
Surveys were handed out on three consecutive months to each patient presenting at the Crohn-Colitis Centre, where they documented their vaccination status and any side effects experienced after vaccination.
Dates of flares occurring in 2021 were recorded from their electronic medical records. Baseline and IBD characteristics and flare incidence were compared between the vaccinated and unvaccinated patients, and among the vaccinated population before and after their vaccination doses. The characteristics of patients who developed side effects and of those who did not were compared.
Results
We enrolled 396 IBD patients, of whom 91% were vaccinated. The proportion of patients who experienced flares was statistically not different between the vaccinated and the unvaccinated population (1.8 vs 2.6 flares per 100 person-months (p = 0.28)). Among vaccinated patients, there was no difference across the prevaccination, 1 month post any vaccination, and more than 1 month after any vaccination periods, and between the Spikevax and Cominarty subgroups. Overall, 46% of patients reported vaccination side effects, mostly mild flu-like symptoms.
Conclusion
SARS-CoV-2 vaccination with mRNA vaccines seems safe, with mostly mild side effects. The IBD flare risk is not increased in the month following any vaccination.
Keywords:
Introduction
Inflammatory bowel disease (IBD) comprises ulcerative colitis (UC) and Crohn’s disease (CD). Its pathogenesis is complex, multifactorial, and incompletely understood [Citation1]. In addition to genetic and environmental factors, an immune response imbalance seems to be a major contributing factor [Citation2]. It is therefore no surprise that current therapeutic options mainly consist of immunosuppressants and immunomodulators [Citation3].
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) vaccines became a cornerstone of the fight against the Coronavirus Disease 2019 (COVID-19) pandemic but also a major subject of concern. Patients suffering from inflammatory bowel disease were initially thought to be more at risk of severe forms of SARS-CoV-2. However, this fear was only confirmed in certain treatment subgroups, in particular those under systematic corticosteroids and combination therapies, as well as in IBD patients with active disease [Citation4–8]. Despite the clear expert recommendations in favour of a policy of general vaccination of IBD patients against the SARS-CoV-2 virus, vaccination hesitancy persists [Citation9,Citation10]. Vaccination hesitancy is defined as a delay in acceptance or a refusal of vaccination despite the availability of vaccination services [Citation11]. A study from the USA about the perception of SARS-CoV-2 vaccination among IBD patients established that 70% of the patients hesitant to receive SARS-CoV-2 vaccination reported concerns about adverse events, in particular fear of flares [Citation12,Citation13]. This fact was also shown in a Europe-wide survey distributed to IBD patients, where the main concerns were side effects and the flare-up risk [Citation14]. This initial hesitancy is understandable since IBD patients were underrepresented in the trials of the various vaccines [Citation15–18]. Furthermore, the question of inducing flares through vaccine-triggered immune activation arose, even though it has never been recorded for routinely administrated vaccines [Citation12,Citation19–22]. SARS-CoV-2 infections were identified as possible IBD flare inducers, further increasing the fear of SARS-CoV-2 vaccination–induced flares [Citation5,Citation23]. Eighty percent of hesitant IBD patients reported that their health care provider’s recommendation was important in their decision [Citation13]. These statements both emphasize that safety concerns are an important barrier to vaccination and that treating physicians, in particular gastroenterologists, have an important role to play in counselling IBD patients and promoting their vaccination coverage.
The purpose of this paper is to assess the safety and flare risk of SARS-CoV-2 vaccination among an IBD cohort to further guide vaccine-hesitant IBD patients.
Materials and methods
Study design and setting
This study was conducted in the outpatient Crohn-Colitis Centre in Bern. From January to March 2022, questionnaires concerning SARS-CoV-2 vaccination status and experienced post-vaccination side effects were handed out to all IBD patients presenting for their follow-up appointments. These were then filed in their electronic patient medical records (EMR) using Vitomed software. Ethics approval was granted from the local Swiss Human Research Ethics Committee (BASEC ID: 2022-01458). Informed consent was then obtained from all the patients who fulfilled the three criteria below for data abstraction:
filled-out vaccination questionnaire
established IBD diagnosis, as documented in their EMR
follow-up at Bern’s Crohn-Colitis Center for at least one month prior to January 1, 2022
All the information available in each patient’s EMR for the year 2021, including baseline characteristics (age, sex), IBD characteristics (UC vs CD diagnosis, Montreal classification, associated extraintestinal manifestations (EIMs), surgical status, treatment), vaccination status (vaccine scheme, dates), adverse events (occurring in the month following any vaccination), and overall flares (number and dates), was then retrospectively reviewed. The time of observation started at the date of the beginning of the analysis period (January 1, 2021) or at the date of the start of follow-up at our clinic (if later than January 1, 2021) and ended on the date of the end of the study period (December 31, 2021).
Variables
The outcome was the flare incidence after vaccination. A flare was defined as an increased clinical activity of IBD, with a partial Mayo score ≥4 for UC or Harvey-Bradshaw Index score ≥4 for CD, with a compatible physician evaluation. The physician, who abstracted the data of the number and date of flares, reassessed the medical file to confirm the flare-diagnosis in conjunction with the other available data (e.g. calprotectine, exclusion of other causes of symptom exacerbation, endoscopic findings, necessity of therapy escalation/switch). This part of the data abstraction took place separately from the data abstraction of the vaccination information, so that vaccination status could not interfere with the physician evaluation of the presence of a flare. The flare risk was compared among the patients in the vaccinated subgroup overall as well as in three different time periods: pre-vaccination, 1 month after any vaccination, and more than 1 month after any vaccination (illustrated in Supplementary Figure 1).
Statistical methods
We first asked if flare incidence is altered by vaccination. To answer this question, patient characteristics and flare incidence were compared between vaccinated and unvaccinated patients. Frequencies (n), percentages (%), medians and interquartile ranges (IQRs), and P-values were reported from a chi-squared or Wilcoxon rank-sum test. Absolute flare risk was calculated with 95% confidence intervals (CIs) using Poisson rates. The null hypothesis of ‘no period effect’ was tested using a likelihood ratio (LRT) test. Associations of patient and IBD characteristics which could be associated with flare risk were assessed using univariable and multivariable Poisson regression models. Because of the small number of flares, only univariable associations were assessed for period risk analyses. As only three patients had received a Janssen vaccine, these patients were excluded from period-specific analyses to avoid overfitting.
Our second question was to assess the safety of vaccination by analysing the incidence of side effects. We recorded the occurrence of side effects and compared patient, IBD, and vaccination characteristics between patients with side effects and those without. All statistical analyses were performed in R version 4.1.2 (R Core Team, 2021).
Results
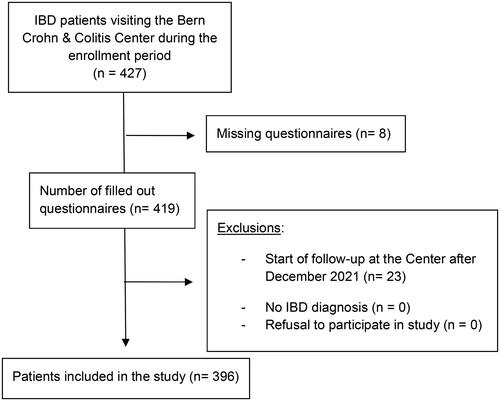
During the 3-month enrollment period, 427 patients presented at our Crohn-Colitis Centre for follow-up. Of these, 419 patients completed the vaccination questionnaire and 396 eventually fulfilled the criteria for enrollment (shown in ).
The study population’s characteristics are summarized in . Their IBD diagnoses include Crohn’s disease (CD; 251 patients, 63.4%), ulcerative colitis (UC; 139 patients, 35.1%), and indeterminate colitis (IC; 6 patients, 1.5%).
Table 1. Baseline characteristics of the study population.
Among the CD patients, most had an ileocolonic location (146/251, 58.2%) with stricturing and/or penetrating behaviours (151/251, 60%). Included UC patients predominantly suffered from extensive colitis (E3) (97/139, 69.8%).
Among all IBD diagnoses combined, 153(38.6%) patients experienced, during their disease activity, at least one extraintestinal manifestation, mostly articular (85%, 130/153) or cutaneous (18%, 28/153). At the time of the first vaccination, most of the IBD patients (324/356, 81.8%) were treated with biologics or small molecules, mainly TNFα antagonists (141, 35.6%).
Of our study population, 362 patients (91%) were vaccinated by the end of 2021: 167 patients (46.1%) received BNT162b2/Cominarty (Pfizer-BioNTech), 188 (51.9%) received mRNA-1273/Spikevax (NIH-Moderna), and 3 (0.8%) received Ad26.COV2-S/Janssen (Johnson & Johnson). The vaccine was not documented in the EMR of 4 (1.1%) patients. Among the vaccinated subgroup, 51.1% (185/362) had already undergone booster vaccination by the end of 2021.
shows the baseline characteristics of the vaccinated and unvaccinated groups. We observed that unvaccinated patients tended to be younger than vaccinated ones (median age of 38 vs 43 years, p = 0.053), however, this difference was not statistically significant. Other characteristics (sex; IBD type, duration, severity, and associated EIMs; and baseline treatment) did not differ significantly between the groups.
Table 2. Patient characteristics by vaccination status.
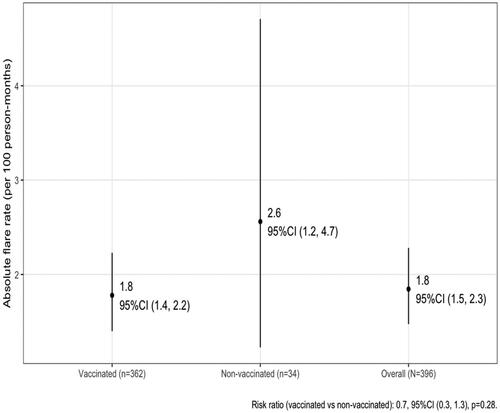
During the one-year study period, 85 flares were noted, with a total follow-up time of 4606 person-months, which corresponds to an absolute flare risk of 1.8 flares per 100 person-months (shown in ). There was no difference between the flare incidence during the year 2021 between patients who received at least one SARS-CoV-2 vaccine shot and those who did not: the overall incidence was 17% vs 21%, respectively (p = 0.3), corresponding to 1.8 flares per 100 person-months vs 2.6 flares per 100 person-months (p = 0.28). This was also confirmed after inverse probability weighing.
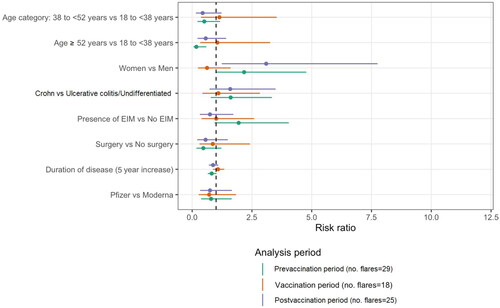
Supplementary Figure 2 illustrates the patient eligibility flow chart for the period-specific analysis. There were 29 flares noted in the pre-vaccination period (flare risk of 2.1, 95% CI 1.4–3.0, per 100 person-months), 18 flares (flare risk of 2.4, 95% CI 1.4–3.7, per 100 person-month) in the vaccination period, and 25 flares in the postvaccination period (flare risk of 1.3, 95% CI 0.8–1.9, per 100 person-months). There is no evidence for a period effect (p = 0.09; ). The flare-risk during the month following vaccination was, in absolute value, higher after Moderna’s (2.1 per 100 person-months) compared with Pfizer’s (1.5 per 100 person-months) vaccine. However, this difference was not statistically significant (risk ratio of 0.7, 95% CI 0.4–1.2, p = 0.21), and the flare risks of both these groups were comparable to the one observed in the unvaccinated group (2.6 per 100 person-months).
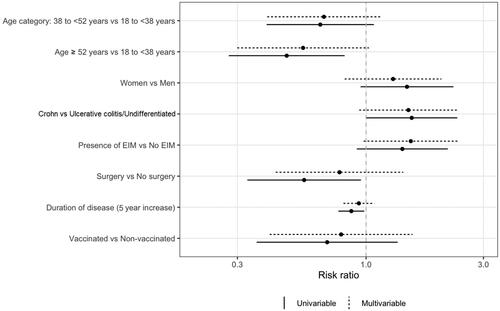
Next, we compared patients who experienced flares with patients who did not. In the univariable model, the eldest one-third of our patients tended to be less likely to experience IBD flares (p = 0.007) than the younger two-thirds. However, this was no longer statistically significant after multivariable analysis (p = 0.06), implicating probable confounding factors. No other baseline or IBD characteristics were identified as being significant risk factors for disease flares (shown in and ).
Table 3. Risk factor analysis of flare risk.
After the initial vaccination, 43.6% (158/362) of patients reported side effects. The most frequent were flu-like symptoms (76.6%, 121/158) and local site reaction (11%). Diarrhoea was reported by 7 patients (4.4%). No patients experienced severe side effects necessitating hospitalization. After the booster vaccination, side effects were reported in 38% (71/185) of patients.
In the subgroup analysis of all the patients who developed side effects (), sex, IBD type (CD, UC, IC), IBD extent, presence of EIM, past surgery, and baseline treatment did not differ significantly from those who did not. However, younger patients tended to report more side effects then older ones (p < 0.001), together with patients diagnosed with IBD at younger age (p < 0.001). There seemed to be significantly more patients who developed side effects in the Moderna compared with the Pfizer group (58% vs 34%, p < 0.001), but only in regards to the mild flu-like symptoms.
Table 4. Side effects.
Discussion
Our results show that SARS-CoV-2 vaccination was, overall, well tolerated in this IBD population. Reported side effects were mild and concerned mostly flu-like symptoms.
This matches well with international data [Citation14,Citation24–29]. In a survey of almost 1000 health care professionals, more side effects were reported after the second vaccination with the Moderna vaccine [Citation30]. Also, in this study where all side effects from the different vaccinations (initial scheme, booster) were analysed as a whole, an increased incidence of them was reported in Moderna-vaccinated compared with Pfizer-vaccinated persons. These findings are concordant with available data on post-vaccination side effects, suggesting a good external validity of our data and study population. The main interest of our study is that we do not show an increased IBD flare risk following SARS-CoV-2 vaccination, regardless of the vaccine product administered.
This conclusion matches well with that of other recent publications [Citation24,Citation31–33]. Interestingly, there was a tendency for the eldest third of our cohort to have fewer flares. This could be explained by the fact that IBD disease tends to become milder in the elderly, particularly in UC patients [Citation34,Citation35].
The strength of our study is that almost 400 consecutive patients were included. The vaccination surveys were handed out systematically to all IBD patients presenting for a follow-up consultation. Only 8 eligible patients’ data were missing. They could either have escaped the distribution process or refused to complete the questionnaire. In some other studies, IBD patients were broadly recruited e.g. via social media and patient support groups, but only a minority of these patients participated, which could lead to a major representation bias as the patient vaccination experience could influence their motivation to participate in these studies [Citation25,Citation33]. Our recruitment strategy clearly limits this bias. However, since questionnaires were handed out only during a 3-month period, patients with stable and mild disease presenting less frequently at the Centre could have missed the enrollment window. Patients benefiting from infusion biological therapies and those with a narrow follow-up because of active IBD could, in contrast, be overrepresented in this study population. Furthermore, being treated at a specialized Crohn-colitis centre, this IBD patient population tends to have more extensive and complicated disease courses than does the overall IBD population. All of these may have resulted in selection bias. This is also highlighted in : most UC patients included were suffering from extensive disease (E3), and over 60% of included CD patients had stricturing (B2) or penetrating (B3) disease.
Another strength of this study is that the documentation of IBD flares is trustworthy. As the study was retrospective, there were no patients lost to follow-up whose post-vaccination flares could have been missed. Flare information was extracted directly from the EMRs of patients. In other studies, IBD flares were self-reported by patients or extrapolated from cortisone prescriptions [Citation25,Citation33]. As a recent study on immune-mediated inflammatory disease highlighted, when patients reported a post-vaccination increased activity of their underlying disease, a physician confirmed this diagnosis only in 40% of cases [Citation32]. As is now well-established, the general population can experience, in over 5% of cases, diarrhoea or abdominal pain after the vaccination [Citation36,Citation37]. This can also occur in IBD patients and should be distinguished from a flare. Basing oneself only on the presence of self-reported increase of abdominal pain or diarrhoea could lead to overestimation of flares. It is therefore a major strength of our study that a physician went through the EMR of each included patient to evaluate for the presence of disease flares during the study period.
Furthermore, our study has the advantage of comparing the flare incidence with an external control (unvaccinated population) and internal control (vaccinated population at time points outside the vaccination period) group. Because of our small non-vaccinated group, this first analysis does not allow us to draw any conclusions, being underpowered. By collecting data over an entire year and calculating the flare incidence before the start of the vaccination, a month following any vaccination, and over a month following any vaccination, we managed to calculate flare-incidence at different time points. Comparing these rates, allowed us to evaluate the impact of vaccination on flare risk, while acknowledging the baseline flare-risk associated with the spontaneous disease evolution of the underlying IBD. This is a strength compared to studies where only an absolute post-vaccination flare risk was evaluated.
Among the included patients, the majority (364 patients, 92%) had been followed in our Crohn-Colitis Centre for the entire year of 2021. For the remaining 32, their data were considered pro rata of their follow-up duration in order not to analyse vaccination periods in which flares could not have been documented in the studied Crohn-Colitis Centre EMR.
The vaccination data (vaccination type and dates) were gathered from the patients’ questionnaires, which were completed with the help of COVID certificates on paper or available on patient’s smartphones, limiting the recall bias. The dates and number of flares were directly abstracted from the EMR and could therefore not be impacted by recall bias.
The main limitation, intrinsic to the study design, is the lack of randomization. This IBD population decided whether they would get vaccinated or not. They were not randomized into “vaccinated” and “unvaccinated” groups and might therefore systematically differ, resulting in a kind of confounding bias. In the end, slightly more of the unvaccinated patients than the vaccinated patients (21 vs 17%) had at least one flare in 2021. This difference is not statistically significant. One hypothesis is that patients with less well-controlled IBD disease, with more tendency to have flares, might be less inclined to get vaccinated. Therefore, the unvaccinated flare risk might be falsely elevated and mask a possible slight difference from the vaccinated flare risk. Younger patients were overrepresented in the “unvaccinated” group. This matches with the Swiss Federal Office of Public Health data where, also in the general population, the vaccination coverage among younger people was lower than coverage among the elderly [Citation38]. Also on a European scale, older IBD patients tended to be more vaccinated than younger ones [Citation14].
As the majority of the patients received a mRNA vaccine, the flare risks and safety profiles of the other types of SARS-CoV-2 vaccines on the international market could not be assessed and should not be extrapolated. In this cohort, 98% (355/362) of the vaccinated patients did indeed receive a mRNA vaccine. The vaccination product was not documented in 4 cases. Only 3 patients were vaccinated with a viral-vector vaccine (Janssen). This could be explained by the delayed arrival of the Johnson & Johnson vaccine on the Swiss market. Despite approval by the Swiss agency for therapeutic products (Swissmedic) in March 2021, doses were only ordered in September 2021 because, until then, the shipping time was too long [Citation39,Citation40]. In comparison, Cominarty (Pfizer-BioNTech) and Spikevax (NIH-Moderna) were already approved by Swissmedic in December 2020 and January 2021, respectively, and readily available for administration since then [Citation41,Citation42]. None of the studied patients received AZD1222 (Oxford-AstraZeneca) or NVX-CoV2373/Nuvaxovid (Novavax), as they were not (yet) approved by Swissmedic [Citation43,Citation44].
Nonetheless, we find it very interesting to have so much data on mRNA-vaccines as they are currently being extensively developed for broader indications for both infectious (Influenza, CMV) and non-infectious (cancer) diseases [Citation45–48]. More vaccinations are recommended in IBD patients as the general population because of their impaired immunity [Citation49]. Having collected data on this ‘newer’ vaccination modality in an IBD population is we think of big relevance for safety concerns for upcoming ARNm vaccines
To conclude, in an IBD cohort of almost 400 consecutive patients, flare risk was not increased during the month following a mRNA SARS-CoV-2 vaccination. Furthermore, both mRNA vaccines seem safe, with mostly mild, flu-like side effects. This was also the case among older patients and among patients with extensive IBD under immunosuppressants.
We hope our study will help promote vaccination coverage among our IBD patients, as well as fight against the common misconception of vaccination-induced flares. Having an initial good safety signal for mRNA vaccines, this could be reassuring for future vaccines produced with this technology.
Author contributions statement
L. Rossier: conception and design, analysis and interpretation of data, drafting of the paper
N. Décosterd: conception and design, analysis and interpretation of data, drafting of the paper
C. Matter: analysis and interpretation of data, critical review of the paper
D. Staudenmann: analysis and interpretation of data, critical review of the paper
A. Moser: analysis and interpretation of data, critical review of the paper
B. Egger: critical review of the paper
F. Seibold: conception and design, analysis and interpretation of data, critical review of the paper
All authors approved the version to be published and agree to be accountable for all aspects of the work.
Funding sources
In preparation of this manuscript, no external funding was received.
Supplemental Material
Download Zip (284 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are not publicly available because this information that could compromise the privacy of research participants. The data will be shared on reasonable request to the corresponding author.
References
- Zhang Y-Z, Li Y-Y. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20(1):1–11. doi: 10.3748/wjg.v20.i1.91.
- Lee SH, Kwon JE, Cho M-L. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 2018;16(1):26–42. doi: 10.5217/ir.2018.16.1.26.
- Misselwitz B, Juillerat P, Sulz MC, Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology., et al. Emerging treatment options in inflammatory bowel disease: janus kinases, stem cells, and more. Digestion. 2020;101(Suppl 1):69–82. doi: 10.1159/000507782.
- Derikx LAAP, Lantinga MA, de Jong DJ, et al. Clinical outcomes of covid-19 in patients with inflammatory bowel disease: a nationwide cohort study. J Crohns Colitis. 2021;15(4):529–539. doi: 10.1093/ecco-jcc/jjaa215.
- Wetwittayakhlang P, Albader F, Golovics PA, et al. Clinical outcomes of COVID-19 and impact on disease course in patients with inflammatory bowel disease. Can J Gastroenterol Hepatol. 2021;2021:7591141–7591149. doi: 10.1155/2021/7591141.
- Macaluso FS, Giuliano A, Fries W, et al. Severe activity of inflammatory bowel disease is a risk factor for severe COVID-19. Inflamm Bowel Dis. 2022;29(2):217–221. doi: 10.1093/ibd/izac064.
- Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159(2):481–491.e3. doi: 10.1053/j.gastro.2020.05.032.
- Ungaro RC, Brenner EJ, Gearry RB, et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut. 2021;70(4):725–732. doi: 10.1136/gutjnl-2020-322539.
- Alexander JL, Moran GW, Gaya DR, et al. SARS-CoV-2 vaccination for patients with inflammatory bowel disease: a british society of gastroenterology inflammatory bowel disease section and IBD clinical research group position statement. Lancet Gastroenterol Hepatol. 2021;6(3):218–224. doi: 10.1016/S2468-1253(21)00024-8.
- Siegel C, Melmed G, McGovern D, et al. SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: recommendations from an international consensus meeting. Gut. 2021;70(4):635–640. doi: 10.1136/gutjnl-2020-324000.
- MacDonald NE, SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–4164. doi: 10.1016/j.vaccine.2015.04.036.
- Zhang E, Gupta A, Al-Ani A, et al. Misconceptions drive COVID-19 vaccine hesistancy in individuals with inflammatory bowel disease. Can J Gastroenterol Hepatol. 2022;2022:4527844–4527847. doi: 10.1155/2022/4527844.
- Dalal RS, McClure E, Marcus J, et al. COVID-19 vaccination intent and perceptions among patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2021;19(8):1730–1732.e2. doi: 10.1016/j.cgh.2021.02.004.
- Ellul P, Revés J, Abreu B, et al. Implementation and short-term adverse events of anti-SARS-CoV-2 vaccines in inflammatory bowel disease patients: an international web-based survey. J Crohns Colitis. 2022;16(7):1070–1078. doi: 10.1093/ecco-jcc/jjac010.
- Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of Single-Dose Ad26.COV2.S vaccine against covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544.
- Wellens J, Colombel J-F, Satsangi JJ, et al. SARS-CoV-2 vaccination in IBD: past lessons, current evidence, and future challenges. J Crohns Colitis. 2021;15(8):1376–1386. doi: 10.1093/ecco-jcc/jjab046.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577.
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389.
- Manser CN, Maillard MH, Rogler G, et al. Vaccination in patients with inflammatory bowel diseases. Digestion. 2020;101 Suppl 1(Suppl 1):58–68. doi: 10.1159/000503253.
- Desalermos A, Pimienta M, Kalligeros M, et al. Safety of immunizations for the adult patient with inflammatory bowel disease—a systematic review and meta-analysis. Inflamm Bowel Dis. 2021;28(9):1430–1442. doi: 10.1093/ibd/izab266.
- Rahier J-F, Papay P, Salleron J, et al. H1N1 vaccines in a large observational cohort of patients with inflammatory bowel disease treated with immunomodulators and biological therapy. Gut. 2011;60(4):456–462. doi: 10.1136/gut.2010.233981.
- Satyam VR, Li P-H, Reich J, et al. Safety of recombinant zoster vaccine in patients with inflammatory bowel disease. Dig Dis Sci. 2020;65(10):2986–2991. doi: 10.1007/s10620-019-06016-4.
- Saifuddin A, Kent AJ, Mehta SJ, et al. Treatment adaptations and outcomes of patients experiencing inflammatory bowel disease flares during the early COVID-19 pandemic: the PREPARE-IBD multicentre cohort study. Aliment Pharmacol Ther. 2022;56(10):1460–1474. doi: 10.1111/apt.17223.
- James D, Jena A, Bharath PN, et al. Safety of SARS-CoV-2 vaccination in patients with inflammatory bowel disease: a systematic review and meta-analysis. Dig Liver Dis. 2022;54(6):713–721. doi: 10.1016/j.dld.2022.03.005.
- Weaver KN, Zhang X, Dai X, et al. Impact of SARS-CoV-2 vaccination on inflammatory bowel disease activity and development of vaccine-Related adverse events: results from PREVENT-COVID. Inflamm Bowel Dis. 2022;28(10):1497–1505. doi: 10.1093/ibd/izab302.
- Spiera E, Ungaro RC, Kornbluth A. Effectiveness and safety of COVID-19 vaccines in patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2022;18(3):145–155.
- Botwin GJ, Li D, Figueiredo J, et al. Adverse events following SARS-CoV-2 mRNA vaccination among patients with inflammatory bowel disease. Am J Gastroenterol. 2021;116(8):1746–1751. doi: 10.14309/ajg.0000000000001342.
- Hadi YB, Thakkar S, Shah-Khan SM, et al. COVID-19 vaccination is safe and effective in patients with inflammatory bowel disease: analysis of a large multi-institutional research network in the United States. Gastroenterology. 2021;161(4):1336–1339.e3. doi: 10.1053/j.gastro.2021.06.014.
- Cannatelli R, Ferretti F, Carmagnola S, et al. Risk of adverse events and reported clinical relapse after COVID-19 vaccination in patients with IBD. Gut. 2022;71(9):1926–1928. doi: 10.1136/gutjnl-2021-326237.
- Melanson SEF, Zhao Z, Kumanovics A, et al. Tolerance for three commonly administered COVID-19 vaccines by healthcare professionals. Front Public Health. 2022;10:975781. doi: 10.3389/fpubh.2022.975781.
- Lev-Tzion R, Focht G, Lujan R, et al. COVID-19 vaccine is effective in inflammatory bowel disease patients and is not associated with disease exacerbation. Clin Gastroenterol Hepatol. 2022;20(6):e1263–82–e1282. doi: 10.1016/j.cgh.2021.12.026.
- van Dam KPJ, Wieske L, Stalman EW, et al. Disease activity in patients with immune-mediated inflammatory diseases after SARS-CoV-2 vaccinations. J Autoimmun. 2023;135:102984. doi: 10.1016/j.jaut.2022.102984.
- Elkharsawi A, Arnim U, von Schmelz R, et al. SARS-CoV-2 vaccination does not induce relapses of patients with inflammatory bowel disease. Z Gastroenterol. 2022;60(1):77–80. doi: 10.1055/a-1710-3861.
- Lakatos PL, David G, Pandur T, et al. IBD in the elderly population: results from a population-based study in Western Hungary, 1977-2008. J Crohns Colitis. 2011;5(1):5–13. doi: 10.1016/j.crohns.2010.08.004.
- Charpentier C, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut. 2014;63(3):423–432. doi: 10.1136/gutjnl-2012-303864.
- Kadali RAK, Janagama R, Peruru S, et al. Non-life-threatening adverse effects with COVID-19 mRNA-1273 vaccine: a randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J Med Virol. 2021;93(7):4420–4429. doi: 10.1002/jmv.26996.
- Dighriri IM, Alhusayni KM, Mobarki AY, et al. Pfizer-BioNTech COVID-19 vaccine (BNT162b2) side effects: a systematic review. Cureus. 2022;14(3):e23526. doi: 10.7759/cureus.23526.
- COVID-19 Switzerland | Coronavirus | Dashboard. n.d. https://www.covid19.admin.ch/en/vaccination/doses. (accessed October 18, 2022).
- Covid-19-Impfstoff: Bund unterzeichnet Vertrag mit Janssen. n.d. https://www.admin.ch/gov/de/start/dokumentation/medienmitteilungen.msg-id-85292.html. (accessed December 11, 2022).
- COVID-19 vaccine from Johnson & Johnson: Swissmedic approves the third vaccine against COVID-19. n.d. https://www.admin.ch/gov/en/start/documentation/media-releases.msg-id-82783.html. (accessed December 8, 2022).
- Swissmedic 2019 © Copyright. Swissmedic autorise le premier vaccin contre le COVID-19 en Suisse n.d. https://www.swissmedic.ch/swissmedic/fr/home/news/coronavirus-covid-19/covid-19-impfstoff_erstzulassung.html. (accessed December 8, 2022).
- Swissmedic 2019 © Copyright. Swissmedic autorise le vaccin de Moderna contre le COVID-19 n.d. https://www.swissmedic.ch/swissmedic/fr/home/news/coronavirus-covid-19/zulassung-covid-19-impfstoff-moderna.html. (accessed December 8, 2022).
- Swissmedic 2019 © Copyright. Swissmedic octroie une autorisation de durée limitée au vaccin Nuvaxovid de Novavax contre le Covid-19 n.d. https://www.swissmedic.ch/swissmedic/fr/home/news/coronavirus-covid-19/zl-nuvaxovid-novovax.html. (accessed October 16, 2022).
- Demande d’autorisation avec soumission des données en continu pour un vaccin contre le Covid-19: Swissmedic exige des données supplémentaires. n.d. https://www.swissmedic.ch/swissmedic/fr/home/news/coronavirus-covid-19/coronavirus-impfstoff-astrazeneca-weitere-daten-verlangt.html. (accessed October 16, 2022).
- Chuluyan E, Davio C, Dusetti N[, et al. The mRNA vaccine for the treatment of pancreatic cancer is here to stay. ]. Medicina (Mex). 2023;83:650–652.
- Reina J. [The new generation of messenger RNA (mRNA) vaccines against influenza]. Enferm Infecc Microbiol Clin. 2023;41(5):301–304. doi: 10.1016/j.eimc.2021.07.009.
- Manus J-M. Un vaccin à ARNm contre le cytomégalovirus. Rev Francoph Lab. 2022;2022(538):10–11. doi: 10.1016/S1773-035X(21)00350-6.
- Galy A, Berkhout B, Breckpot K, et al. Recent advances using genetic therapies against infectious diseases and for vaccination. Hum Gene Ther. 2023;34(17-18):896–904. doi: 10.1089/hum.2023.123.
- Kucharzik T, Ellul P, Greuter T, et al. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis. 2021;15(6):879–913. doi: 10.1093/ecco-jcc/jjab052.