Abstract
Background
Erector spinae plane block is a promising strategy for pain management in some settings. However, the effectiveness of erector spinae plane block versus caudal block in pediatric inguinal hernia repair has yet to be formally investigated.
Methods
One hundred and two patients aged 2–5 years undergoing unilateral open inguinal hernia repair randomly received unilateral erector spinae plane block (0.2% ropivacaine 0.5 mL kg−1), caudal block (0.2% ropivacaine 1 mL kg−1), or no block. The primary outcome was time to the first rescue analgesia, defined as the interval from the end of surgery to the Face, Legs, Activity, Cry, and Consolability scale greater than three. Secondary outcomes included the number of patients requiring rescue analgesia, the area under the curve of pain scores over time, satisfaction of guardians, and adverse events.
Results
The median time to the first rescue analgesia was longer in the erector spinae plane block group than in the caudal block group [10.0 h (interquartile range, 6.6–24.0 h) vs. 5.0 h (interquartile range, 2.9–7.3 h); p < .001]. The Cox regression model demonstrated that the risk of postoperative rescue analgesia requirement was 0.38 in children receiving erector spinae plane block compared with caudal block (95% confidence interval 0.23–0.64; p < .001). Additionally, the area under the curve of the pain scores over time was lower in the erector spinae plane block group than in the caudal block group (44.3 [36.6–50.7] vs. 59.0 [47.1–64.5]; p < .001).
Conclusions
Erector spinae plane block provided superior postoperative analgesia compared to caudal block in children undergoing inguinal hernia repair.
Trial registration: Chinese Clinical Trial Registry; ChiCTR2100048303
KEY MESSAGES
Erector spinae plane block (ESPB) is beneficial for postoperative analgesia in children undergoing inguinal hernia repair.
Ultrasound-guided ESPB provided superior analgesia efficacy to caudal block in the pediatric population.
ESPB is an attractive strategy for pain management after lower abdominal surgical procedures.
Introduction
Lower abdominal surgeries, including inguinal hernia repair, have been performed extensively in the daily practice of pediatric surgeries. Given their predominantly day-case nature, ensuring sufficient postoperative analgesia is crucial for perioperative care. Extensive utilization of lower abdominal surgeries is observed in the routine practice of pediatric surgeries [Citation1]. Failure to adequately control postoperative pain can impede functional recuperation and lead to negative behavioral changes and family dissatisfaction [Citation2]. Various nerve blocks have been developed to improve postoperative analgesia and facilitate recovery in pediatric patients. However, a consensus regarding the most effective strategy for pediatric procedures has yet to be reached [Citation3].
Caudal block is the most frequently performed regional technique for pain management in children undergoing lower abdominal surgeries. The implementation of real-time ultrasound guidance has resulted in enhanced reliability and safety profiles for caudal blocks. However, a notable drawback of this technique is its limited duration of action following a single injection, despite the use of long-acting analgesics or adjuvant coadministration. Thus, several interfacial plane blocks have been suggested as alternatives for postoperative analgesia in children, including quadratus lumborum block, transverse abdominis plane block, and rectus sheath block [Citation4–6].
The erector spinae plane block (ESPB), which has evolved rapidly in the last decade, involves an ultrasound-guided injection of a relatively large volume of local anesthetic into the fascial plane beneath the erector spinae muscle [Citation7]. ESPB is gaining popularity in pediatrics due to the increasing number of studies demonstrating its potential as a practical approach for managing postoperative pain in various surgeries [Citation8–10]. Recent evidence indicates quadratus lumborum block is more effective than caudal block for pediatric inguinal hernia repair and orchiopexy surgeries, while ESPB confers similar analgesic effects to quadratus lumborum block undergoing lower abdominal operations [Citation11,Citation12]. However, no studies have directly compared ESPB and caudal block for pediatric inguinal hernia repair. Therefore, in this randomized trial, we tested the hypothesis that children treated with ESPB would provide better control of acute postoperative pain compared with those treated with caudal block following inguinal hernia repair.
Patients and methods
Study design
This is a prospective, single-center, randomized, double-blinded, placebo-controlled, three-arm parallel-group trial. The Institutional Review Board of Fujian Provincial Hospital reviewed and approved the study protocol (K2021-03-068, March 8, 2021). We registered the study at the Chinese Clinical Trials Registry (https://www.chictr.org.cn, ChiCTR2100048303, July 5, 2021). All legal guardians of participants provided written informed consent during the preoperative interview. Data collection commenced on July 6, 2021, and was completed on January 31, 2022, at Fujian Provincial Hospital, Fuzhou, China. We conducted this trial following the principles of Good Clinical Practice and the Declaration of Helsinki. No changes to the study protocol occurred after trial commencement. The manuscript was presented following the Consolidated Standards of Reporting Trials (CONSORT) 2010 statement [Citation13].
We enrolled 102 American Society of Anesthesiologists (ASA) physical status I–II participants aged 2–5 years undergoing elective unilateral open inguinal hernia repair. Participants with a history of allergies to local anesthetics, bleeding or coagulation disorders, anatomical abnormalities, local infection at the site of the needle puncture area, surgery duration over one hour, and developmental delay were excluded.
Randomization and blinding
The eligible participants were randomly assigned in a 1:1:1 ratio to three groups (ESPB, caudal block, and control) using a computer-generated random allocation sequence (R version 4.0.5, R Foundation for Statistical Computing, Vienna, Austria). A research nurse not involved in the study prepared the randomization list with a block size of 6 and concealed it in sealed, opaque, sequentially numbered envelopes opened on the day of surgery. Sterile stickers were affixed to the puncture sites of all participants to conceal group allocation. The administration of the blocks, whether ESPB or caudal, was conducted by an independent anesthesiologist who was not involved in further intraoperative anesthesia management or data collection, analysis, and interpretation. As a result, the guardians of the children, surgeons, and investigators responsible for data collection were all blinded to the group assignment.
Anesthetic procedure
All children were premedicated using midazolam 0.5 mg kg−1 orally in the preoperative holding area. We induced anesthesia using 5% sevoflurane via a mask in 100% oxygen, and an intravenous cannula was subsequently secured. Afterward, tropisetron 0.1 mg kg−1 plus dexamethasone 0.15 mg kg−1 was intravenously administered for antiemetic prophylaxis. We administered sufentanil 0.2 μg kg−1 and propofol 2.0 mg kg−1 to facilitate laryngeal mask airway insertion. General anesthesia was maintained using 2–3% sevoflurane in a 50% nitrous oxide and 50% oxygen mixture. Following the completion of the regional block, a minimum of 15 min was allowed before the surgical incision was made, with all operations being performed by the same surgical team. Intravenous acetaminophen 15 mg kg−1 was administered to all children for postoperative analgesia before the end of the surgical procedure. Subsequently, the children were transferred to the postanesthetic care unit (PACU) and accompanied by one of their legal guardians for further observation. The assessment of pain intensity in the postoperative period was conducted using the Face, Legs, Activity, Cry, and Consolability (FLACC) scale [Citation14] by an independent researcher unaware of the study protocol and group assignment. Each of the five items of the FLACC scale was scared, ranging from 0 to 2, which were then aggregated to obtain a total score ranging from 0 to 10. Higher scores on the scale indicated a higher level of pain intensity. If the FLACC scale exceeded three, morphine 25 μg kg−1 intravenously in the PACU or ibuprofen 10 mg kg−1 orally in the ward was administered.
Study interventions
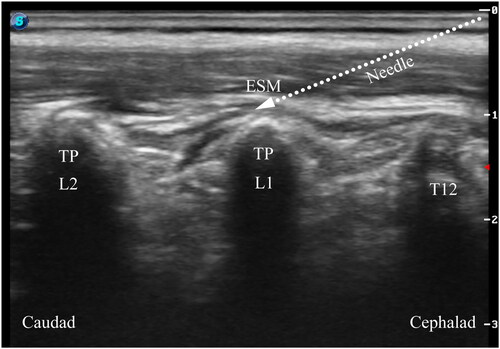
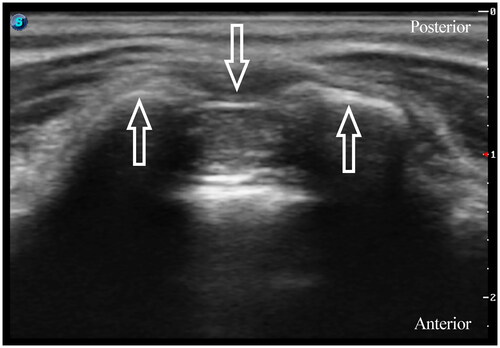
All blocks were performed under general anesthesia before the surgical incision. Patients were placed in the lateral decubitus position for block performance. In the ESPB group, a single injection of ESPB was conducted under ultrasound guidance (Edge, FUJIFILM Sonosite Inc., Bothell, WA, USA). The 6–13 MHz linear probe in a sterile sleeve was applied 1–2 cm lateral to the midline to identify the L1 transverse process by counting upward from the sacrum region. After identifying the erector spinae muscle and transverse processes, a 22-gauge, 50 mm nerve block needle (USG-Type CCR, Hakko Co., Chikuma-Shi, Nagano, Japan) was advanced into the target interfacial plane in the craniocaudal direction using the in-plane technique (). After confirming the needle placement, we injected 0.2% ropivacaine 0.5 mL kg−1 under real-time ultrasound visualization. ESPB was considered successful by the linear spread craniocaudally between the tip of the transverse process and the erector spinae muscle. In the caudal block group, we placed a 13–6 MHz linear array probe at the level of the sacral cornus. After visualization of the sacral hiatus, a 22-gauge intravenous catheter with an inner stylet (Becton Dickinson Infusion Therapy Systems Inc., Sandy, Utah, USA) was inserted using an out-of-plane approach (). Following negative aspiration of blood or cerebrospinal fluid, 0.2% ropivacaine 1 mL kg−1 was administered slowly. In the control group, patients did not receive ESPB or caudal block.
Outcome assessment
The primary outcome was time to the first rescue analgesia, defined as the interval from the end of surgery to the FLACC scale greater than three. Secondary outcomes included the number of patients requiring rescue analgesia, the area under the curve (AUC) of the pain scores over time, guardians’ satisfaction, and adverse events. The number of patients requiring rescue analgesia was recorded postoperatively during the first 24 h. Pain intensity was assessed using the FLACC scale at 0.5, 1, 2, 4, 6, 8, 12, and 24 h postoperatively by an independent researcher blinded to the study protocol and group assignment. The AUCs of the pain scores were calculated using the FLACC scale at multiple time points. The guardians’ satisfaction with postoperative analgesia was self-reported at 24 h using an 11-point NRS score (0 is completely unsatisfied, and 10 is the most satisfied). The anesthesiologist in charge of the block identified any block-related adverse events, such as hematoma, neurological injury, deep visceral injury, or local anesthetic toxicity. Any other potential adverse events, including postoperative nausea or vomiting (PONV), laryngospasm, bradycardia, and hypotension, were also recorded.
Statistical analysis
The study was powered to detect the differences in time to the first rescue analgesia between the ESPB group and the caudal group (the main comparison) using Power Analysis & Sample Size (PASS) software (Version 15; NCSS LLC, Kaysville, USA). Our pilot study showed that time to the first rescue analgesia [mean ± standard deviation (SD)] was 6.4 ± 3.7 h in children receiving caudal block. Using a two-sided two-sample equal-variance t test, 90% power to detect a 50% difference in time to the first rescue analgesia at a two-sided 5% significance level required a sample size of 30 participants per group. Allowing for a 10% loss to follow-up, we recruited 102 participants in this study.
All analyses were conducted using the Statistical Package for Social Science (SPSS) software (version 25; SPSS Inc, Armonk, USA). The primary outcomes were analyzed using the Mann–Whitney U test. The primary comparison was between the ESPB and the caudal groups, with ESPB versus control as a secondary comparison. Secondary outcomes between groups were compared using Mann–Whitney U tests and Chi-square or Fisher’s exact test, as appropriate. Time-to-event data were estimated using Kaplan–Meier survival analysis and compared by the log-rank test. The Cox proportional hazards regression model was used to calculate the hazard ratio (HR) and associated 95% confidence intervals (CI). We determined the AUCs of the FLACC scale using GraphPad Prism 8.0 (GraphPad Software, California, USA) and analyzed them using the Mann–Whitney U test. A linear mixed-effects model for repeated measures was used to compare the evolution over time for the pain FLACC score. The model incorporated three fixed effects: treatment group, time, and the interaction between treatment and time, as well as two random effects, random intercept and slope, to account for subject-specific variability. A 2-sided p value less than .05 was considered statistically significant.
Results
From July 2021 to January 2022, we screened 107 patients undergoing unilateral open inguinal hernia repair for trial eligibility. Five were subsequently excluded for coagulation disorders or refusal to participate. After randomization, four were discontinued for protocol violation or lost to follow-up. Consequently, 33 patients in the caudal block group, 32 in the ESPB group, and 33 in the control group were ultimately analyzed. Details are presented in the CONSORT flow diagram (). The participants’ demographic and clinical characteristics were similar among the three groups ().
Figure 3. Consolidated Standards of Reporting Trials (CONSORT) flow diagram. ESPB: erector spinae plane block.
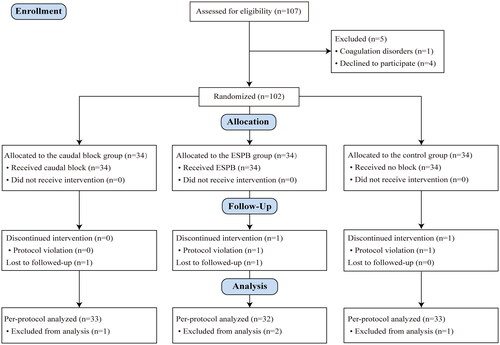
Table 1. Demographic and clinical characteristics of participants.
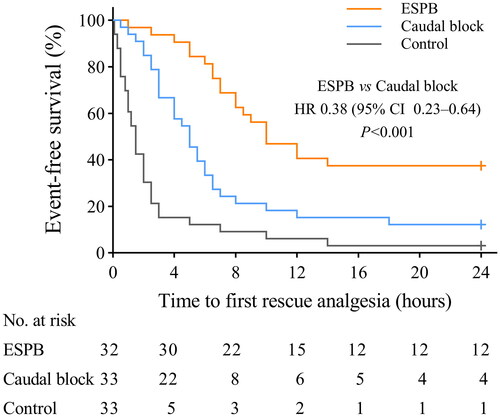
The median time to the first rescue analgesia was longer in the ESPB group than in the caudal block group [10.0 h (IQR, 6.6–24.0 h) vs. 5.0 h (IQR, 2.9–7.3 h); p < .001] and in the control group [10.0 h (IQR, 6.6–24.0 h) vs. 1.5 h (IQR, 0.7–2.6 h); p < .001], . Furthermore, the Cox regression model revealed that the risk of postoperative rescue analgesia requirement in the ESPB group was 0.38 (95% CI: 0.23–0.64, p < .001) compared with the caudal block group and 0.24 (95% CI: 0.13–0.44, p < .001) compared with the control group.
Figure 4. The proportion of participants who did not receive rescue analgesia. Cross marks represent that follow-up was censored at 24 hours postoperatively. ESPB: erector spinae plane block; HR: hazard ratio; CI: confidence interval.
The linear mixed-effects analysis indicated the main effects of treatment group, time, and the interaction effect between group and time (all p < .001, Supplemental Table 1). Compared with the caudal block group, the 6-, 8-, and 12-h postoperative FLACC scale was lower in the ESPB group (p < .001, p < .001, and p = .001, respectively; ). As detailed in , participants in the ESPB group had lower AUCs of the FLACC scale over 24 h than those in the caudal block group [44.3 (36.6–50.7) vs. 59.0 (47.1–64.5), p < .001]. No block-related adverse events or major medical complications were reported in any participants during the study period.
Figure 5. FLACC pain scores during the postoperative 24 h. Data are mean with error bars showing standard deviation. A linear mixed model showed that the FLACC scale was significantly lower in the ESPB group than in the caudal group at 6, 8, and 12 hours postoperatively (p < .001, p < .001, and p = .001, respectively). ESPB: erector spinae plane block; FLACC: face, legs, activity, cry, and consolability.
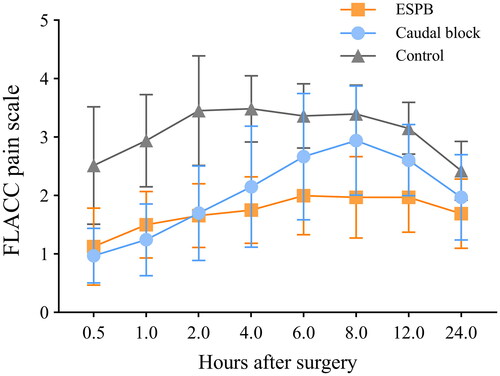
Table 2. Secondary outcomes.
Discussion
This study showed that ultrasound-guided single-shot ESPB provided superior postoperative pain relief to caudal block in children undergoing inguinal hernia repair, as indicated by a prolonged time to the first rescue analgesia, fewer patients requiring rescue analgesia, and lower postoperative pain scores. Given the ease of ultrasound-guided ESPB and the relatively low risk of adverse effects in theory, ESPB could be included in the multimodal analgesia protocol for pediatric lower abdominal procedures as an alternative to caudal block.
Our findings indicate that the administration of preoperative ESPB or caudal block resulted in a prolonged time to first rescue analgesia compared to no block. Consistent with the findings of Luz et al. [Citation15], we observed a median analgesic duration of 5 h in patients who received caudal block. However, the administration of ESPB resulted in a nearly 2-fold longer duration of analgesia than caudal block. The observed difference in block duration between the two techniques may be attributed to several factors, including the site of spread, volume, and prolonged contact time between local anesthetics and sensitive fibers in ESPB [Citation16]. The spread of local anesthetics along the interfacial plane with relatively fewer vasculature results in a longer contact time, which may facilitate a more extensive sensory blockade for superior postoperative pain relief. Conversely, the greater vascularity of the caudal space leads to faster drug clearance [Citation17].
The exact mechanism of action and spread of local anesthetics in erector spinae plane block (ESPB) remains poorly understood or controversial [Citation8]. Initially, the proposed mechanism of action was local anesthetic spreading into the paravertebral space, where it could act on ventral and dorsal rami at several levels [Citation7]. Nevertheless, this has been challenged by recent anatomic and cadaveric studies [Citation18,Citation19]. Based on current anatomical knowledge and evidence, the most logical mechanism of ESPB involves the direct spread and diffusion of local anesthetic onto neural targets in the interfacial plane and neural foramina, thereby ensuring both somatic and visceral analgesia [Citation20,Citation21]. It is important to note several anatomic and sonographic differences between ESPB performed at the thoracic and lumbar vertebral levels. In the case of lumbar ESPB, local anesthetics are primarily deposited around the lumbar plexus and less frequently in the paravertebral space [Citation22]. During open inguinal hernia repair, the manipulation of fascial tissues and incisions extending from the pubic tubercle to the external ring were identified as the primary sources of somatic pain, as reported in previous studies [Citation23]. Our findings suggest that performing ESPB at the first lumbar (L1) vertebral level may involve the lumbosacral nerves and provide multilevel analgesia for inguinal hernia repair.
The analgesic efficacy of regional block was positively correlated with the concentration and volumes of local anesthetics [Citation24]. In specific clinical scenarios, administering local anesthetic agents for a caudal block may require dosages approaching the maximum allowable limit to achieve the desired dermatomal level. This practice, as demonstrated in this study with ropivacaine dosages of 2 mg kg−1, may increase the risk of local anesthetic systemic toxicity (LAST) in pediatric patients [Citation25]. To mitigate this risk, we employed a single injection of ESPB with a reduced dosage of ropivacaine (1 mg kg−1) in this study. This approach effectively limits the likelihood of LAST in the pediatric population. Furthermore, it is worth noting that caudal block, being a central neuraxial block, is associated with potentially severe complications (for example, dural puncture). The use of caudal block may be precluded due to contraindications such as coagulation disorders and neuraxial abnormalities, as noted in previous literature [Citation26].
Our study, however, had several limitations. First, sensory testing was not conducted to map the block area, as all blocks were performed under general anesthesia. In addition, the effect of the block on post-surgical sensation was not assessed to keep the patient blind to the group assignment. Second, our study refrained from implementing block care as a negative control group intervention, opting instead for blocks with 0.9% saline due to ethical considerations regarding the exposure of pediatric participants to an invasive technique. Third, morphine 25 μg kg−1 for rescue analgesia intravenously in the PACU or ibuprofen 10 mg kg−1 administered orally in the inpatient ward, as per standard practice at our institution. However, two different rescue analgesics could introduce bias for secondary outcomes. Last, it is essential to note that this study was conducted solely at a single center, encompassing a specific patient population and surgical procedure. Consequently, further evaluation is imperative to determine the generalizability of the findings.
Conclusion
In summary, a single injection of ESPB is an effective and safe technique for children undergoing unilateral open inguinal hernia repair, providing superior pain relief to caudal block. Therefore, as an alternative to caudal block, ESPB can be a part of a multimodal analgesia strategy for the pediatric population undergoing lower abdominal procedures, which may facilitate patient recovery, shorten discharge time, and reduce side effects. However, further studies are needed to determine the optimal volume and concentration of local anesthetics for ESPB.
Authors contributions
Jinsheng Guan, Junyu Li, and Zheng Zheng were involved in the conception and study design. Jinsheng Guan, Ying Yang, and Zongli Zheng collected and analyzed the data. Jinsheng Guan and Linwei Liu wrote the draft. Junyu Li and Zheng Zheng critically revised the manuscript. All authors approved the final manuscript to be published and agreed to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (20 KB)Acknowledgments
The authors thank Prof. Yusheng Yao (Fujian Provincial Key Laboratory of Critical Care Medicine, Fujian Provincial Hospital) for his generous support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data and materials supporting the results or analyses presented in this paper are available upon reasonable request.
Additional information
Funding
References
- Boric K, Dosenovic S, Jelicic Kadic A, et al. Interventions for postoperative pain in children: an overview of systematic reviews. Paediatr Anaesth. 2017;27(9):1–8. doi: 10.1111/pan.13203.
- Shum S, Lim J, Page T, et al. An audit of pain management following pediatric day surgery at British Columbia children’s hospital. Pain Res Manag. 2012;17(5):328–334. doi: 10.1155/2012/541751.
- Vittinghoff M, Lönnqvist PA, Mossetti V, et al. Postoperative pain management in children: guidance from the pain committee of the European society for pediatric anesthesiology (ESPA pain management ladder initiative). Paediatr Anaesth. 2018;28(6):493–506. doi: 10.1111/pan.13373.
- Genç Moralar D, Tok Cekmecelioglu B, Aslan M, et al. Effect of quadratus lumborum block on postoperative analgesic requirements in pediatric patients: a randomized controlled double-blinded study. Minerva Anestesiol. 2020;86(2):150–156. doi: 10.23736/S0375-9393.19.13361-5.
- Bryskin RB, Londergan B, Wheatley R, et al. Transversus abdominis plane block versus caudal epidural for lower abdominal surgery in children: a double-blinded randomized controlled trial. Anesth Analg. 2015;121(2):471–478. doi: 10.1213/ANE.0000000000000779.
- Relland LM, Tobias JD, Martin D, et al. Ultrasound-guided rectus sheath block, caudal analgesia, or surgical site infiltration for pediatric umbilical herniorrhaphy: a prospective, double-blinded, randomized comparison of three regional anesthetic techniques. J Pain Res. 2017;10:2629–2634. doi: 10.2147/JPR.S144259.
- Forero M, Adhikary SD, Lopez H, et al. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41(5):621–627. doi: 10.1097/AAP.0000000000000451.
- Saadawi M, Layera S, Aliste J, et al. Erector spinae plane block: a narrative review with systematic analysis of the evidence pertaining to clinical indications and alternative truncal blocks. J Clin Anesth. 2021;68:110063. doi: 10.1016/j.jclinane.2020.110063.
- Holland EL, Bosenberg AT. Early experience with erector spinae plane blocks in children. Paediatr Anaesth. 2020;30(2):96–107. doi: 10.1111/pan.13804.
- Hernandez MA, Palazzi L, Lapalma J, et al. Erector spinae plane block for surgery of the posterior thoracic wall in a pediatric patient. Reg Anesth Pain Med. 2018;43(2):217–219. doi: 10.1097/AAP.0000000000000716.
- Öksüz G, Arslan M, Urfalıoğlu A, et al. Comparison of quadratus lumborum and caudal block for postoperative analgesia in pediatric patients undergoing inguinal hernia repair and orchiopexy surgeries: a randomized controlled trial. Reg Anesth Pain Med. 2020;45(3):187–191. doi: 10.1136/rapm-2019-101027.
- Aksu C, Şen MC, Akay MA, et al. Erector spinae plane block vs quadratus lumborum block for pediatric lower abdominal surgery: a double blinded, prospective, and randomized trial. J Clin Anesth. 2019;57:24–28. doi: 10.1016/j.jclinane.2019.03.006.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. BMJ. 2010;340(1):c332–c332. doi: 10.1136/bmj.c332.
- Merkel SI, Voepel-Lewis T, Shayevitz JR, et al. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23(3):293–297.
- Luz G, Innerhofer P, Häussler B, et al. Comparison of ropivacaine 0.1% and 0.2% with bupivacaine 0.2% for single-shot caudal anesthesia in children. Paediatr Anaesth. 2000;10(5):499–504. doi: 10.1046/j.1460-9592.2000.00532.x.
- Fusco P, Cofini V, Petrucci E, et al. Unilateral paravertebral block compared with subarachnoid anesthesia for the management of postoperative pain syndrome after inguinal herniorrhaphy: a randomized controlled clinical trial. Pain. 2016;157(5):1105–1113. doi: 10.1097/j.pain.0000000000000487.
- Kodali VRK, Kandimalla A, Vakamudi M. Comparison of analgesic efficacy of ultrasound-guided transversus abdominus plane block and caudal block for inguinal hernia repair in pediatric population: a single-blinded, randomized controlled study. Anesth Essays Res. 2020;14(3):478–484. doi: 10.4103/aer.AER_77_20.
- Nielsen MV, Moriggl B, Hoermann R, et al. Are single-injection erector spinae plane block and multiple-injection costotransverse block equivalent to thoracic paravertebral block? Acta Anaesthesiol Scand. 2019;63(9):1231–1238. doi: 10.1111/aas.13424.
- Ivanusic J, Konishi Y, Barrington MJ. A cadaveric study investigating the mechanism of action of erector spinae blockade. Reg Anesth Pain Med. 2018;43(6):567–571. doi: 10.1097/AAP.0000000000000789.
- Chin KJ, El-Boghdadly K. Mechanisms of action of the erector spinae plane (ESP) block: a narrative review. Can J Anaesth. 2021;68(3):387–408. doi: 10.1007/s12630-020-01875-2.
- Schwartzmann A, Peng P, Maciel MA, et al. Magnetic resonance imaging study of local anesthetic spread in patients receiving an erector spinae plane block. Can J Anaesth. 2020;67(8):942–948. doi: 10.1007/s12630-020-01613-8.
- Kose HC, Kose SG, Thomas DT. Lumbar versus thoracic erector spinae plane block: similar nomenclature, different mechanism of action. J Clin Anesth. 2018;48:1. doi: 10.1016/j.jclinane.2018.03.026.
- Miller HJ. Inguinal hernia: mastering the anatomy. Surg Clin North Am. 2018;98(3):607–621. doi: 10.1016/j.suc.2018.02.005.
- Heydinger G, Tobias J, Veneziano G. Fundamentals and innovations in regional anesthesia for infants and children. Anesthesia. 2021;76(Suppl 1):74–88. doi: 10.1111/anae.15283.
- Suresh S, Ecoffey C, Bosenberg A, et al. The European Society of regional anesthesia and pain therapy/American society of regional anesthesia and pain medicine recommendations on local anesthetics and adjuvants dosage in pediatric regional anesthesia. Reg Anesth Pain Med. 2018;43(2):211–216. doi: 10.1097/AAP.0000000000000702.
- Ecoffey C, Bosenberg A, Lonnqvist PA, et al. Practice advisory on the prevention and management of complications of pediatric regional anesthesia. J Clin Anesth. 2022;79:110725. doi: 10.1016/j.jclinane.2022.110725.