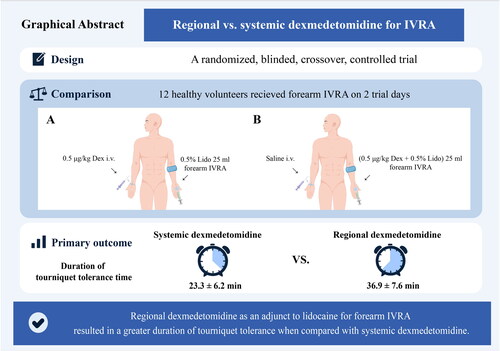
Abstract
Background
Dexmedetomidine enhances the quality and duration of lidocaine intravenous regional anaesthesia (IVRA). However, the two administration routes have not been directly compared regarding effects on tourniquet tolerance time with lidocaine IVRA. Additionally, it remains unclear whether the prolonged tourniquet tolerance stems from the direct peripheral action of dexmedetomidine or indirect systemic analgesic effects.
Methods
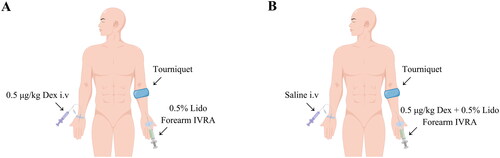
We conducted forearm IVRA in 12 healthy volunteers using a crossover design on two separate study days. One day, the systemic dexmedetomidine group received an intravenous infusion of 0.5 μg/kg dexmedetomidine (20 mL) in one arm, followed by 0.5% lidocaine (25 mL) forearm IVRA in the contralateral arm. On the other day, the regional dexmedetomidine group received an intravenous 0.9% saline infusion (20 mL) in one arm, followed by combined 0.5% lidocaine (25 mL) and 0.5 μg/kg dexmedetomidine forearm IVRA in the opposite arm. After a two-week washout period, participants crossed over to receive the alternate treatment. The primary outcome was tourniquet tolerance time, from initiating IVRA until the patient-reported tourniquet pain numerical rating scale exceeded three.
Results
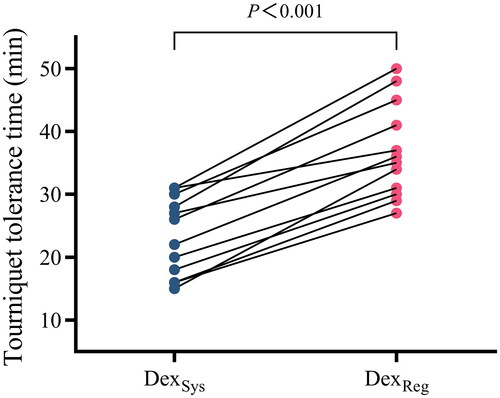
The tourniquet tolerance time was longer with regional versus systemic dexmedetomidine (36.9 ± 7.6 min vs 23.3 ± 6.2 min, respectively), with a 13.6 min mean difference (95% CI: 10.8 to 16.4 min, p < 0.001). Regional dexmedetomidine also hastened sensory onset and extended sensory recovery compared to systemic administration. Delayed sedation after tourniquet release occurred in 5 of 12 subjects receiving regional dexmedetomidine.
Conclusion
The addition of regional dexmedetomidine to lidocaine prolonged tourniquet tolerance time in forearm IVRA to a greater extent compared to systemic dexmedetomidine in healthy volunteers.
Trial registration
Chinese Clinical Trial Registry, ChiCTR2300067978.
KEY MESSAGES
The addition of regional dexmedetomidine prolongs tourniquet tolerance time with lidocaine forearm IVRA.
Regional dexmedetomidine accelerates sensory block onset time and extends sensory block recovery time when supplemented with lidocaine forearm IVRA.
Delayed sedative effects following tourniquet release were witnessed in some participants administered regional dexmedetomidine.
Introduction
Intravenous regional anaesthesia (IVRA) is a well-established and secure local-regional anaesthetic technique in minor surgical procedures involving the upper and lower extremities [Citation1]. It remains widely employed in emergency departments, outpatient settings, and high-risk individuals who are unsuitable candidates for general anaesthesia due to contraindications [Citation2]. IVRA offers numerous merits, such as a favourable risk-benefit ratio, cost-effectiveness, satisfactory muscle relaxation, and prompt onset and offset of effects [Citation3]. However, the effectiveness of IVRA is hindered by the occurrence of tourniquet pain and the swift dissipation of analgesia following tourniquet deflation [Citation4,Citation5]. Various adjuvant drugs (such as opioids, ketamine, and non-steroidal anti-inflammatory drugs) have been proposed to reduce tourniquet pain and improve the quality of anaesthesia during IVRA block in clinical practice [Citation6–8].
Dexmedetomidine, a highly selective potent α2 adrenoceptor agonist that produces sedative and analgesic effects, can also be used as an adjuvant for local anaesthetics in regional anaesthesia undergoing surgery [Citation9–11]. The existing clinical evidence indicates that the regional application of dexmedetomidine enhances the quality and duration of lidocaine IVRA [Citation12]. Furthermore, research has demonstrated that the systemic administration of dexmedetomidine can effectively alleviate tourniquet pain [Citation13]. However, the two administration routes regarding effects on tourniquet pain relief efficacy with lidocaine IVRA have not been directly compared. Additionally, it remains unclear whether the extended tourniquet tolerance time from the peripheral action of dexmedetomidine or indirect systemic analgesic effects [Citation14].
Therefore, we conducted a prospective, randomized, crossover study involving a group of volunteers to assess the efficacy of administering dexmedetomidine systemically versus regionally in lidocaine forearm IVRA. Our hypothesis posited that regional dexmedetomidine administration would lead to a prolonged duration of tourniquet tolerance compared to systemic dexmedetomidine administration in lidocaine forearm IVRA.
Materials and methods
Study design and participants
This was a prospective, randomized, double-blinded, crossover study in healthy volunteers. The trial was approved by the Institutional Review Board of Fujian Provincial Hospital (identifier: K2021-08-012/02, Primary investigator: Yusheng Yao), Fuzhou, China, on September 30, 2022. We registered the study at the Chinese Clinical Trials Registry (https://www.chictr.org.cn/showproj.html?proj=186061, ChiCTR2300067978) on February 02, 2023. Before enrollment, subjects were given information regarding the study’s nature, scope, procedures, and associated risks. Following the acquisition of written informed consent, 12 healthy volunteers, equally divided by sex, were recruited between February 03, 2023, and March 15, 2023. The study was conducted at Fujian Provincial Hospital, Fuzhou, China, adhering to the Declaration of Helsinki (revised in 2013), local related regulations, and the principles of Good Clinical Practice. No modifications were made to the methods or outcomes after the trial’s initiation. The manuscript was prepared under the Consolidated Standards of Reporting Trials (CONSORT) guidelines [Citation15].
The research recruited participants aged 18 to 45 with an American Society of Anesthesiologists (ASA) physical status I. Exclusion criteria included known hypersensitivity or allergy to the study medications (lidocaine and dexmedetomidine), pregnancy, physiological or lactation period, history of medication or alcohol abuse, body mass index ≥ 30 kg/m2, or inability to comprehend or comply with the study protocol.
Randomization and blinding
This two-period, two-sequence crossover trial used computer-generated randomization to allocate participants to an AB or BA sequence. In the AB sequence, participants received Intervention A (systemic administration of 0.5 μg/kg dexmedetomidine in 20 mL arm 1; 0.5% lidocaine 25 mL forearm IVRA arm 2) in period 1. After a two-week washout, they crossed to Intervention B (0.9% saline 20 mL arm 1; 0.5% lidocaine 25 mL + 0.5 μg/kg dexmedetomidine forearm IVRA arm 2) in period 2. The BA sequence received the interventions in reverse order (). The randomization list was sealed in consecutively numbered opaque envelopes by a research assistant not involved in block performance or outcome assessment. On the day of the study, a separate pharmacist opened the envelopes and identically prepared the study drugs. As a result, the participants, the investigator who conducted the forearm IVRA, and the separate investigator who collected the outcome measures were unaware of group allocation.
Procedures
Participants did not receive premedication before operating room admission. Standard anaesthetic monitoring was initiated upon entry, including electrocardiography, pulse oximetry (SpO2), and non-invasive blood pressure measurements. Following intravenous line placement, a single-cuff tourniquet was applied to one forearm. Next, the assigned 20 mL study solution was administered intravenously in the opposite arm at 4 mL/min over 15 min. The designated IVRA arm underwent exsanguination using an Esmarch bandage, followed by tourniquet inflation to 300 mmHg and Esmarch removal. After confirming the absence of pulse oximetry signals and radial artery occlusion ipsilateral to the tourniquet cuff, 25 mL of the IVRA solution was gradually injected over 3 min. The tourniquet cuff was deflated when the patient-reported tourniquet pain numerical rating scale (NRS) exceeded three. After cuff release, participants were monitored in the post-anesthesia care unit (PACU) for at least 2 h.
Outcome assessments
The primary outcome was tourniquet tolerance time, the interval from initiating IVRA until the patient-reported tourniquet pain NRS exceeded three. Secondary outcomes included sensory block onset/recovery time, depth of sedation, and adverse events. Specifically, sensory block onset was determined as the duration between finishing IVRA and achieving complete loss of pinprick sensation in the radial, ulnar, median, and musculocutaneous nerve distributions, assessed at 1-min intervals. Block failure was recorded if the complete sensory block was not attained. The sensory block recovery time was defined as the interval between complete tourniquet deflation and return of normal sensory function in the blocked arm (equivalent to the contralateral hand). Sensory function was assessed every minute after cuff release. Sedation levels were evaluated every 5 min using the 6-point Ramsay scale, ranging from 1 (anxious, agitated) to 6 (unresponsive) [Citation16]. Adverse events, such as bradycardia, hypoxemia, and local anaesthetic systemic toxicity (LAST), were documented throughout the observation period. Bradycardia was a heart rate (HR) under 50 beats/min. Hypotension was defined as a mean arterial pressure (MAP) less than 65 mmHg or 20% below baseline. Hypoxemia was considered oxygen saturation (SpO2) below 90%. Participants were closely monitored for potential local anaesthetic systemic toxicity (LAST) symptoms, including dizziness, tinnitus, perioral numbness, and metallic taste. An emergency contact number was provided for any medical issues arising within 24 h following IVRA.
Statistical analysis
A previous study reported a tourniquet tolerance time of approximately 20.0 ± 7.5 min for lidocaine forearm IVRA [Citation17]. In this study, we predefined a 50% difference in tourniquet tolerance time as a minimally important difference. A sample size of 9 achieves 94% power to detect a mean of paired differences of 10.0 min with an estimated standard deviation of differences of 7.5 min and with a significance level (alpha) of 0.05 using a two-sided paired t-test. To account for an anticipated 20% drop-out rate and facilitate grouping logistics, 12 volunteers were planned in the study.
Statistical analysis was performed using IBM SPSS Statistics 25.0 (IBM Inc., Armonk, NY, USA), and the normality of the continuously distributed data was assessed using the Shapiro–Wilk test and Q–Q plots. The data that followed a normal distribution were analyzed statistically using paired t-tests and are presented as the mean ± standard deviation (SD). Conversely, distributed data were analyzed using paired non-parametric tests and are presented as the median (interquartile range, IQR). Count data are presented as numbers (percentages, %) and were analyzed using Fisher’s exact test. The HR, MAP, and Ramsay sedation scores were compared using a two-way repeated measures analysis of variance and multiple post hoc comparisons with Bonferroni correction. The statistical significance was set at a two-sided P value < 0.05.
Results
From February 03, 2023, to March 15, 2023, 12 participants were randomly allocated into two sequences. The adapted Consolidated Standards of Reporting Trials (CONSORT) flow diagram is illustrated in , and provides an overview of the demographic characteristics of the participants.
Figure 2. Flow diagram of subjects’eligibility, randomization, and crossover. Abbreviations: IVRA: intravenous regional anaesthesia.
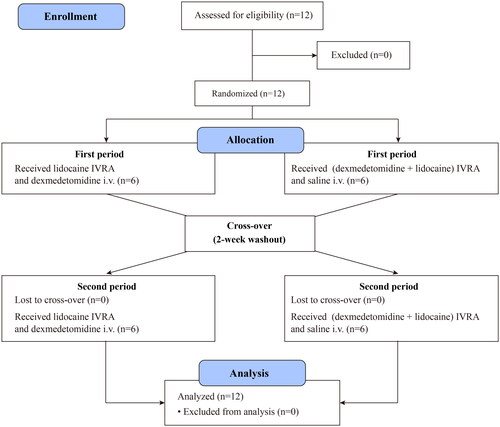
Table 1. Subject demographic data.
Primary outcome
The results indicate that the use of regional dexmedetomidine as an adjunct to lidocaine forearm IVRA led to a significantly prolonged duration of tourniquet tolerance time (36.9 ± 7.6 min vs. 23.3 ± 6.2 min) compared with systemic dexmedetomidine, with a mean difference of 13.6 min (95% confidence interval, 10.8 to 16.4 min) (p < 0.001, ).
Secondary outcomes
The results presented in and Supplementary Table 1 indicate that the application of regional dexmedetomidine during lidocaine forearm IVRA shortened the onset time of sensory block in the median area (2 [2–3] min vs. 4 [2.3–8] min, p = 0.018), ulnar area (1 [1–2] min vs. 2 [2–3.8] min, p = 0.016), radial area (2 [1–3] min vs. 4 [2–6.8] min, p = 0.013), and musculocutaneous area (2 [1.3–3] min vs. 4.5 [3–6] min, p = 0.005) when compared to systemic dexmedetomidine. Furthermore, regional dexmedetomidine was observed to prolong the recovery time of sensory block in the median area (6 [2.3–14] min vs. 2 [1–4.5] min, p = 0.028), ulnar area (17.5 [13–23.5] min vs. 6 [4.3–9] min, p = 0.002), radial area (13 [8–19.5] min vs. 2.5 [2–3.8] min, p = 0.003), and musculocutaneous area (4.5 [2.3–7.3] min vs. 2.5 [1.3–3] min, p = 0.015) in comparison to systemic dexmedetomidine.
Figure 4. Comparison of onset time and recovery time of the sensory block. Notes: Compared with systemic infusion, regional dexmedetomidine accelerated the onset of sensory block (A) and prolonged the recovery of sensory block (B). The data are presented as the median [interquartile range]. The P values were calculated using the paired Wilcoxon signed rank test. Abbreviations: DexSys: systemic dexmedetomidine; DexReg: regional dexmedetomidine.
![Figure 4. Comparison of onset time and recovery time of the sensory block. Notes: Compared with systemic infusion, regional dexmedetomidine accelerated the onset of sensory block (A) and prolonged the recovery of sensory block (B). The data are presented as the median [interquartile range]. The P values were calculated using the paired Wilcoxon signed rank test. Abbreviations: DexSys: systemic dexmedetomidine; DexReg: regional dexmedetomidine.](/cms/asset/6d49004f-2902-44b0-a411-f5b75960c042/iann_a_2300663_f0004_c.jpg)
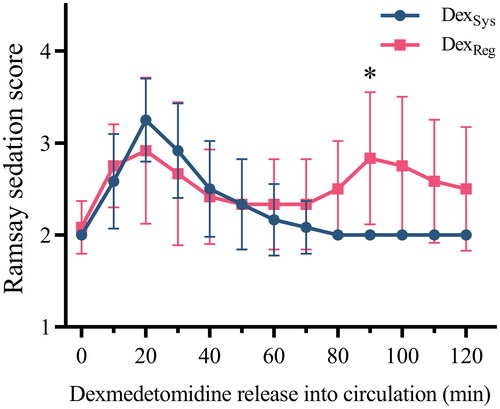
The Ramsay sedation scores obtained during the study period are depicted in . The most profound level of sedation was noted approximately 20 min after the systemic infusion of dexmedetomidine and 20 min after deflating the tourniquet with regional dexmedetomidine. Regional dexmedetomidine caused delayed sedation (Ramsay sedation score > 3) in 5 out of 12 subjects, manifesting approximately 90 min after tourniquet deflation.
Figure 5. Comparison of Ramsay sedation scores in two intravenous regional anesthesia (IVRA) schemes. The data are presented as the mean ± standard deviation and were analyzed using two-way repeated-measures ANOVA with Bonferroni’s multiple comparison tests at individual time points. Statistical significance between the two IVRA schemes is denoted by asterisks (*p < 0.05). Abbreviations: DexSys: systemic dexmedetomidine; DexReg: regional dexmedetomidine.
As depicted in Supplementary Figure 1, systemic administration of dexmedetomidine elicited significant decreases in HR and MAP, with nadirs of 14.0 ± 4.7% and 10.2 ± 6.4% below baseline, respectively. Analogously, regional application of dexmedetomidine induced declines in HR and MAP after tourniquet release, reaching maximal reductions of 10.7 ± 5.8% and 10.2 ± 4.5%, respectively. Notably, no bradycardia or hypotension was reported across either study arm.
Discussion
In this trial, we successfully demonstrated that administering regional dexmedetomidine in lidocaine IVRA led to a significantly longer duration of tourniquet tolerance than systemic dexmedetomidine. The mean difference of 13.6 min, corresponding to approximately 60% prolongation, suggests that regional dexmedetomidine may have a regional mechanism of action contributing to this effect. The localized application of the tourniquet during the IVRA procedure isolates the hand and forearm from systemic circulation. This controlled environment facilitates a more precise assessment of whether dexmedetomidine acts directly through a regional mechanism.
Given that tourniquet pain poses a significant obstacle in the clinical application of IVRA, strategies aimed at mitigating this pain are of utmost clinical importance. According to Chabel et al. [Citation18], various stimuli such as ischemia and compression have been found to activate sensory axons or peripheral receptors either directly beneath the tourniquet in the ischemic tissue or the ‘perischemic’ region at the proximal boundary of the tourniquet. Additionally, A and unmyelinated C fibres may play a partial role in tourniquet pain. However, the mechanism of action of adjunct dexmedetomidine has yet to be fully established. Despite the known central α2-mediated analgesic effects of dexmedetomidine, findings from an animal trial indicate that the effect of dexmedetomidine is primarily due to the blockade of the hyperpolarization-activated cyclic nucleotide-gated channels in peripheral regions rather than its α1- or α2-agonistic properties in central or peripheral areas [Citation19]. In the present study, regional dexmedetomidine accelerates the onset time of the sensory block, diminishes tourniquet pain, and prolongs the sensory block recovery time compared with systemic dexmedetomidine. This supports the earlier findings by Memis et al. [Citation20].
No concerning bradycardia or hypotension occurred in this study, though these are potential dexmedetomidine side effects via sympathetic inhibition and reduced norepinephrine release. The dosage and route seemingly avoided such unintended effects here. Upon tourniquet release enabling systemic distribution, dexmedetomidine may have exerted acute sedative effects centrally. Interestingly, delayed sedation arising approximately 90-min post-deflation occurred in 5 of 12 regional dexmedetomidine subjects, which has not been previously reported. We hypothesize that infiltrated dexmedetomidine undergoes sustained localized tissue release after the initial liberation of vascular contents. This differential release could yield gradual circulating accumulation, manifesting as intensifying delayed sedation. Though unconfirmed, the model provides a mechanistic theory reconciling the observed acute and prolonged sedative profiles. Further research into the mechanism is warranted. Clinically, extended post-procedural monitoring for at least 2 h is advisable when regional dexmedetomidine is used as a lidocaine IVRA adjuvant to detect potential delayed sedation.
The study has several limitations. First, a control group using lidocaine alone for forearm IVRA was omitted. Previous studies have shown that adding dexmedetomidine, whether administered regionally or systemically, was effective in reducing tourniquet pain and improving the quality of anaesthesia when used with lidocaine [Citation13,Citation21]. Thus, this study sought to investigate potential disparities in the impacts of the two dexmedetomidine conditions on tourniquet tolerance time in lidocaine forearm IVRA. Second, the administration of dexmedetomidine systemically resulted in alterations in sedation levels, which could undermine the study’s blinding. Third, the most effective dosage regimens for lidocaine forearm IVRA remain uncertain. Consequently, we chose to utilize a standardized dosage of lidocaine and dexmedetomidine based on our clinical experience and previous research [Citation13], further research is needed to address this issue.
Conclusion
Regional dexmedetomidine as an adjunct to lidocaine resulted in a greater duration of tourniquet tolerance in lidocaine forearm IVRA when compared with systemic dexmedetomidine in healthy volunteers.
Author contributions statement
Xincheng Liao: Conceptualization, Design, Investigation. Jie Lin: Conceptualization, Investigation, Writing-original draft. Xinru Shu: Investigation, Writing-original draft. Shisen Hong: Investigation, Data curation, Software. Yusheng Yao: Conceptualization, Design, Investigation, Funding acquisition, Supervision, Writing-review & editing. Hao Li: Conceptualization, Investigation, Resources, Validation. All authors read and gave final approval of the version to be published.
Supplemental Material
Download Zip (575.1 KB)Acknowledgments
This study was supported by the Natural Science Foundation of Fujian Province (No. 2021J01378) and the Medical Innovation Project of Fujian Province (No. 2022CXA007).
Disclosure statement
No potential conflict of interest was reported by the author(s). was created using Figdraw (www.figdraw.com).
Data availability statement
The data and materials supporting the results or analyses presented in this paper are available upon reasonable request.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Löser B, Petzoldt M, Löser A, et al. Intravenous regional anesthesia: a historical overview and clinical review. J Anesth Hist. 2019;5(3):1–8. doi: 10.1016/j.janh.2018.10.007.
- Beck S, Brunner-Parker A, Stamm R, et al. Periosteal block versus intravenous regional anesthesia for reduction of distal radius fractures: a randomized controlled trial. Acad Emerg Med. 2022;29(10):1213–1220. doi: 10.1111/acem.14555.
- Guay J. Adverse events associated with intravenous regional anesthesia (bier block): a systematic review of complications. J Clin Anesth. 2009;21(8):585–594. doi: 10.1016/j.jclinane.2009.01.015.
- Chiao FB, Chen J, Lesser JB, et al. Single-cuff forearm tourniquet in intravenous regional anesthesia results in less pain and fewer sedation requirements than upper arm tourniquet. Br J Anaesth. 2013;111(2):271–275. doi: 10.1093/bja/aet032.
- Turan A, White PF, Karamanlioglu B, et al. Premedication with gabapentin: the effect on tourniquet pain and quality of intravenous regional anesthesia. Anesth Analg. 2007;104(1):97–101. doi: 10.1213/01.ane.0000250408.56586.88.
- Abdel-Ghaffar HS, Kalefa MA, Imbaby AS. Efficacy of ketamine as an adjunct to lidocaine in intravenous regional anesthesia. Reg Anesth Pain Med. 2014;39(5):418–422. doi: 10.1097/AAP.0000000000000128.
- Bakri MH, Ismail EA, Abd-Elshafy SK. Analgesic effect of nalbuphine when added to intravenous regional anesthesia: a randomized control trial. Pain Physician. 2016;19(8):575–581.
- Sen S, Ugur B, Aydin ON, et al. The analgesic effect of lornoxicam when added to lidocaine for intravenous regional anesthesia. Br J Anaesth. 2006;97(3):408–413. doi: 10.1093/bja/ael170.
- Lee S. Dexmedetomidine: present and future directions. Korean J Anesthesiol. 2019;72(4):323–330. doi: 10.4097/kja.19259.
- Bao N, Shi K, Wu Y, et al. Dexmedetomidine prolongs the duration of local anesthetics when used as an adjuvant through both perineural and systemic mechanisms: a prospective randomized double-blinded trial. BMC Anesthesiol. 2022;22(1):176. doi: 10.1186/s12871-022-01716-3.
- Rao S, Rajan N. Dexmedetomidine as an adjunct for regional anesthetic nerve blocks. Curr Pain Headache Rep. 2021;25(2):8. doi: 10.1007/s11916-020-00926-z.
- Karmaniolou I, Staikou C, Surda P. The role of dexmedetomidine as an additive to intravenous regional anesthesia: a systematic review and meta-analysis. Balkan Med J. 2021;38(3):156–164. doi: 10.5152/balkanmedj.2021.20076.
- Mizrak A, Gul R, Erkutlu I, et al. Premedication with dexmedetomidine alone or together with 0.5% lidocaine for IVRA. J Surg Res. 2010;164(2):242–247. doi: 10.1016/j.jss.2009.03.005.
- Andersen JH, Grevstad U, Siegel H, et al. Does dexmedetomidine have a perineural mechanism of action when used as an adjuvant to ropivacaine?: a paired, blinded, randomized trial in healthy volunteers. Anesthesiology. 2017;126(1):66–73. doi: 10.1097/ALN.0000000000001429.
- Dwan K, Li T, Altman DG, et al. CONSORT 2010 statement: extension to randomized crossover trials. BMJ. 2019;366:l4378. doi: 10.1136/bmj.l4378.
- Arantzamendi M, Belar A, Payne S, et al. Clinical aspects of palliative sedation in prospective studies. A systematic review. J Pain Symptom Manage. 2021;61(4):831–844.e10. doi: 10.1016/j.jpainsymman.2020.09.022.
- Nijs K, Lismont A, De Wachter G, et al. The analgesic efficacy of forearm versus upper arm intravenous regional anesthesia (bier’s block): a randomized controlled non-inferiority trial. J Clin Anesth. 2021;73:110329. Oct doi: 10.1016/j.jclinane.2021.110329.
- Chabel C, Russell LC, Lee R. Tourniquet-induced limb ischemia: a neurophysiologic animal model. Anesthesiology. 1990;72(6):1038–1044. doi: 10.1097/00000542-199006000-00014.
- Zhou C, Ke B, Zhao Y, et al. Hyperpolarization-activated cyclic nucleotide-gated channels may contribute to regional anesthetic effects of lidocaine. Anesthesiology. 2015;122(3):606–618. doi: 10.1097/ALN.0000000000000557.
- Memiş D, Turan A, Karamanlioğlu B, et al. Adding dexmedetomidine to lidocaine for intravenous regional anesthesia. Anesth Analg. 2004;98(3):835–840, doi: 10.1213/01.ane.0000100680.77978.66.
- Dekoninck V, Hoydonckx Y, Van de Velde M, et al. The analgesic efficacy of intravenous regional anesthesia with a forearm versus conventional upper arm tourniquet: a systematic review. BMC Anesthesiol. 2018;18(1):86. doi: 10.1186/s12871-018-0550-4.