Abstract
Aim
This study aimed to identify the prevalence and distribution of high-risk human papillomavirus (HR-HPV) types among Kazakhstani women with abnormal cervical cytology.
Methods
A cross-sectional study was performed from May 2019 to June 2020. Cervical samples were collected from women in the different regions of Kazakhstan.
Results
A total of 316 patients’ samples were analysed for HR-HPV using real-time multiplex PCR. Cervical cytology abnormalities were reported according to the Bethesda classification. HPV detection by cytology showed a statistically significant association with HPV status and the number of HPV infection types (p < .05). Among women with abnormal cervical cytology, 62.4% were positive for HPV infection of those 79.4% had low-grade squamous intraepithelial lesions (LSIL), and 20.6% had high-grade squamous intraepithelial lesions (HSIL). Among patients with LSIL, 77.4% had HPV16 and 58.8% were infected with HPV18. Among patients with HSIL, 41.2% had HPV18 and 22.6% – HPV16.
Conclusions
There is a high prevalence of HR-HPV types among Kazakhstani women with abnormal cervical cytology. The most identified types were HPV16, 18, 31, 33 and 52. There is an emergency need to implement an HPV vaccination program to prevent cervical lesion development.
Introduction
During the recent decade, cervical cancer has been estimated to be the fourth most common cancer in women worldwide and the leading cause of cancer death in low- and middle-income countries (LMICs) [Citation1]. According to the GLOBACAN data, the absolute number of cervical cancer cases worldwide increased over time (from 471,000 in 2000 to 529,000 in 2008, and to 570,000 in 2018) [Citation1], and it continues to be a major public health issue with almost 0.6 million cases and 0.3 million deaths per year [Citation1,Citation2]. The estimated age-standardized incidence of cervical cancer differs between developed countries and LMICs [Citation1,Citation2].
Kazakhstan is a middle-income country, which shows a high incidence rate of cervical cancer in women of all ages that had risen significantly to 18.2 per 100,000 women for the past decade [Citation3–5]. As was reported by the local researchers, the crude rate of cervical cancer incidence increased from 16.3 ± 0.4 in 2009 to 19.5 ± 0.5 in 2018 [Citation6]. Cervical cancer ranks as the second leading cause of cancer and cancer-related death in Kazakhstani women with over 1700 new cervical cancer cases diagnosed annually [Citation3,Citation5,Citation7]. According to multiple resources, approximately 80–99% of all cervical cancer cases have been linked to human papillomavirus (HPV) infection [Citation2,Citation8,Citation9].
HPV is a small non-enveloped double-stranded DNA virus of the Papillomaviridae family [Citation7–10]. Among more than 200 HPV types, due to their strong correlation to anogenital cancers, 15 were identified as high-risk human papillomavirus (HR-HPV) − 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73 and 82 [Citation7–11]. HR-HPV types 16 and 18 are responsible for about 70% of all cervical cancer cases worldwide, while the other high-risk types (31, 33, 35, 45, 52 and 58) are responsible for 20% [Citation2,Citation8–11]. The natural progression of the disease is lengthy: it takes up to 20–30 years from the initial HPV infection until cervical cancer development through the stages of the premalignant cervical lesion [Citation10–15]. It gives a long time window, which should be used as an opportunity to make appropriate interventions to prevent cervical cancer.
The prevalence of HPV infection varies among countries in the world from 6% in Middle East countries to almost 50% in Africa and Oceania [Citation1,Citation2,Citation16]. While the HR-HPV epidemiology has been reported in the majority of countries worldwide, there is a lack of information about the prevalence of HR-HPV types in Kazakhstan. In a few studies [Citation17–20], it has been reported that prevalence among women attending gynaecological clinics varies from 26% to 43.6%. However, these reports were based on the analysis without linking them to cervical cytology results. Therefore, a large-scale investigation of the HPV infection prevalence, especially in patients with abnormal cytology, is required. Understanding HR-HPV types circulating among Kazakhstani women leading to premalignant cervical lesions, and potentially cervical cancer, could facilitate the planned implementation of the HPV vaccination program in 2024 [Citation15,Citation21–23]. This study aimed to identify the prevalence of HR-HPV among women with abnormal cervical cytology and to investigate the HR-HPV genotype distribution in Kazakhstan.
Methods
Study design and sample collection
A cross-sectional study was conducted between May 2019 and June 2020. The STROBE guideline for cross-sectional studies was followed. Women from five cities of central (Astana, capital city), southern (Almaty), western (Aktobe), northern (Pavlodar) and eastern (Oskemen) regions of Kazakhstan participated in this study. Women between the ages of 18 and 70 attending gynaecological clinics were invited to participate, using a convenience sampling method. Cervical specimens for HPV genotyping were collected as a part of cervical screening by the Papanicolaou test (Pap-test). Samples were collected using plastic cytobrush and placed into 1.5 mL tubes for further transportation and HPV genotyping. Samples were stored at −20 °C in the freezer until the DNA extraction process.
Laboratory methods
DNA extraction from the samples was performed using Wizard® Genomic DNA Purification Kit (Promega, Madison, WI) according to the manufacturer’s manual. The purity and quantity of the DNA were checked and recorded on Nanodrop 2000, Thermo Scientific (Waltham, MA). Purified DNA was stored in a −80 °C freezer until it was used for HPV DNA genotyping. HPV genotyping was performed by the real-time multiplex PCR method using the Vector Best’s RealBest DNA HPV HCR genotype quantitative assay kit according (Vector-Best, Novosibirsk, Russia) to manufacturer’s instructions. The kit can detect and differentiate 12 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59). The instrument used for real-time PCR was the CFX 96 Real-Time PCR, Bio-Rad Laboratories (Hercules, CA). For each PCR reaction, positive and negative controls were used. DNA concentration of samples was 3.75 ng/μL, resulting in 37.5 ng per well. The generated data were analysed in the software provided by the manufacturer and the positivity or negativity of the samples for HPV type was determined according to the manufacturer’s thresholds.
The Pap-test was performed on all participants included in the study. Gynaecologists followed standard procedures for cervical sampling: a cytobrush was introduced into the cervical canal and rotated five times (360°) to collect cells for cytology examination. Cytology samples were analysed using liquid-based cytology (LBC) following standard procedure. Smears were diagnosed and reported using the Bethesda system for cervical cytology [Citation24]. All women diagnosed with abnormal cervical cytology – low-grade squamous intraepithelial lesions (LSIL) and high-grade squamous intraepithelial lesions (HSIL) – were treated according to the national guideline on cervical lesions management.
Quality assurance
For quality assurance, all laboratory procedures were performed in the Nazarbayev University Medical cluster (NU Medicine) research laboratories following the study protocol (Supplementary Material 1). Special attention was drawn to the participants’ selection (based on inclusion/exclusion criteria), the protocol implementation, the utilization of an HPV test, and staff competence [Citation25]. HPV genotyping kits validated in the previous pilot study [Citation17] were utilized.
Ethical considerations
The study was approved by the Institutional Research Ethics Committee of Nazarbayev University on 23 April 2019 (IREC number: 146/4042019). All participants were informed of the risks, benefits, goals and methods of the study. Verbal consent was received from participants after they were informed about the volunteer and anonymous nature of the study.
Statistical analysis
Statistical analysis was performed using STATA version 16 (StataCorp LLC, College Station, TX). Descriptive statistics were calculated, including means, standard deviations, and absolute and relative frequencies, where applicable. To assess a relationship between a continuous variable and a categorical variable, a Student t-test or Wilcoxon rank-sum test was employed. For categorical independent variables, the Chi-square test or Fisher’s exact test was used. A p value of <.05 was considered a statistically significant finding.
Results
HPV prevalence and age distribution
In total, 316 women participated in the study (). The age ranged between 18 and 70 years, where the mean was 36.4 ± 10.96 years. Over a quarter (26.6%) of women were between 26 and 30 years old, and 18% of women were between 31 and 35 years old.
Table 1. Age distribution, HPV and CIN prevalence.
No cases of cervical cancer were identified among the study participants. Most participants had normal cytology results (negative for intraepithelial lesion or malignancy, NILM). Less than half of the participants (34.5%) had abnormal cytology results – LSIL and HSIL. The majority of the study participants (86.2%) had LSIL, 13.8% had HSIL based on the results from cervical cytology (). The most common HPV genotypes were: HPV16 (15.8%), HPV18 (8.2%), HPV31 (6.3%), HPV 51 (4.1%) and HPV52 (4.1%, ).
HPV prevalence by age
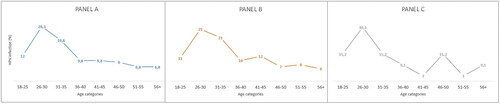
Among HPV-positive women (N = 133), women aged between 26 and 30 years had the highest prevalence of HPV (26.3%). The second highest prevalence of HPV was among women between 31 and 35 years old (19.6%). The lowest prevalence was among women aged between 51 and 55 years (6.8%) and women older than 56 years (6.8%) (, panel A). The highest prevalence of single HPV infection was among women aged between 26 and 30 years (25%) and among women aged between 31 and 35 years (21%) (, panel B). Among women with two or three HPV infections (N = 33), the highest prevalence was among women aged between 26 and 30 years (30.3%). Three age groups (18–25, 31–35, and 46–50) had a 15.2% prevalence of multiple HPV infections (, panel C).
Association of HR-HPV infection and abnormal cervical cytology
Among women with abnormal cervical cytology (N = 109), 62.4% were positive for HPV infection (). Approximately, 2/3 of women (79.4%) had LSIL and were HPV positive. More than 20% of women with HSIL cytology results were positive for HPV. HPV detection by cytology showed a statistically significant association with HPV status (p = .000) ().
Table 2. Association of HR-HPV infection and abnormal cervical cytology.
As shown in , among women with abnormal cytology and HPV infection (N = 68), 72.1% of the participants had a single infection. The majority of women with LSIL (87.8%) had a single HPV infection type, and only 26.3% had multiple HPV types. In contrast, most women with HSIL were infected with multiple HPV types (73.7%, p = .02).
Prevalence of HPV types stratified by the cervical cytology result
HPV16, HPV18, HPV31, HPV33 and HPV52 HR-HPV genotypes were found to be statistically significantly associated with the cytology result (p < .05, ). Among patients with LSIL, 77.4% had HPV16 and 58.8% were infected with HPV18. Among patients with HSIL, 41.2% had HPV18 and 22.6% – HPV16. Only two women were both HPV31 positive and HSIL status ().
Table 3. Prevalence of HPV types stratified by the cervical cytology result.
Discussion
Despite the World Health Organization’s and the local healthcare system’s efforts, the implementation of cervical cancer screening, and HPV vaccination programs [Citation12,Citation23], cervical cancer remains a serious health issue for clinical medicine and the public health sector worldwide [Citation1,Citation26]. Persistent HR-HPV infection has been ultimately linked to the development of cervical cancer [Citation9]. Studies report that HPV16 induces more than 50% of cervical cancers, HPV16 and 18 together lead to over 70% of cases, and the other known HR-HPV types contribute to around 25% of cases, making together 95% of cervical cancer cases associated with HR-HPVs [Citation26,Citation27]. Even though there is a national cervical cancer screening program implemented in Kazakhstan [Citation5,Citation28], cervical cancer incidence increased over the past decade in the country [Citation6]. Since the prevalence of HR-HPV types has never been investigated among Kazakhstani women with premalignant cervical lesions, the aim of this study was to identify the prevalence and distribution of HR-HPV types among women with abnormal cervical cytology.
In many studies, women’s age has been reported to have a significant impact on the HPV prevalence [Citation19,Citation20,Citation26,Citation29]. In this study, HR-HPV types were the most prevalent among reproductive-age women (18–35), and the prevalence decreased in older age groups, which is comparable with other similar studies [Citation19,Citation26,Citation30].
In the current investigation, abnormal cervical cytology was identified in one-third of the study participants, with a prevalence of HSIL of almost 14%. This indicator is lower than in a similar Kazakhstani study [Citation15], where HSIL was found in 19% of women. However, the study that was referred for comparison [Citation15], reported the results of a tertiary-care hospital with the expected higher rate of severe cases. Moreover, contrary to our research, the study by Imankulova et al. did not report results of the participants’ HPV status [Citation15].
The prevalence and distribution of HR-HPV types vary in different populations. In this study, 62.4% of women with abnormal cervical cytology were infected with either single or multiple HR-HPV types, which is higher than the prevalence among Turkish and Mongolian populations [Citation26,Citation30]. The results of the present study demonstrated that HPV16, HPV18, HPV31, HPV33 and HPV52 were the five most prevalent types in this study population. These findings are in line with similar research among Russian [Citation31], Turkish [Citation26,Citation32] and Mongolian [Citation30] populations where HPV16, HPV6, HPV45, HPV18, HPV53, HPV33 and HPV31 were the most prevalent types among women with abnormal cervical cytology.
In the present study, 27.9% of women with abnormal cervical cytology were found to be infected with multiple HR-HPV types, which increases the risk of premalignant cervical lesion development. This rate is lower than in the compared Turkish study where 35.9% of women were positive for multiple HPV types [Citation26]. Notably, in our investigation, most women with LSIL had a single HR-HPV type, while the majority of women with HSIL were infected with multiple HPV types. This finding definitively supports the association of HR-HPV with cervical malignancy as exposure to many types of HR-HPV resulted in HSIL.
Study strengths and limitations
To our knowledge, this is the first study in Kazakhstan investigating the HR-HPV prevalence among women with abnormal cervical cytology. Unlike the previous studies conducted in Kazakhstan [Citation7,Citation15], where only cervical lesion prevalence was reported, this study linked abnormal cervical cytology with the HPV status of the participants. The study findings are likely to be generalizable to women in Kazakhstan since participants from diverse regions of the country were included in the study. Furthermore, understanding the HR-HPV infection prevalence among women with premalignant cervical lesions will not only aid in selecting the appropriate HPV vaccine but also support the implementation of the HPV vaccination program. Another strength is the fact that this study utilizes a cross-sectional study design, which is appropriate for investigating the prevalence of HR-HPV and other HPV types. Nevertheless, several limitations should be taken into account, namely, a relatively small study sample was used that may contribute to a decreased precision of the estimates; the unavailability of the participants’ past medical history and parity history, and the absence of data on Pap-test results after appropriate management of cervical lesions. Besides, a convenience sampling method was used, which may introduce bias as opposed to random sampling. Further studies on the prevalence of HR-HPV and precancerous cervical lesions should include a larger random sample size to increase the data reliability. Moreover, it would be useful to investigate HR-HPV prevalence among patients with impaired immune response (e.g. HIV, diabetes mellitus, or prolonged corticosteroid treatment).
Conclusions
Cervical cancer is one of the preventable malignancies in the vast majority of women. Since the causal relationship between HPV infection and cervical cancer has been established, screening for HR-HPV is an important public health strategy in women with abnormal cervical cytology. Given the high prevalence of HR-HPV types among Kazakhstani women with abnormal cervical cytology and the distribution of HR-HPV types 16, 18, 31, 33, and 52, the implementation of an HPV vaccination program is of great importance. Considering the commonly identified HR-HPV types (16, 18, 31, 33, and 52), Gardasil-9 would be the most appropriate vaccine for immunization of Kazakhstani women. If implemented, vaccination could help to prevent the majority of LSIL and HSIL cases. Strict follow-up and appropriate guideline-based management are quite important for women with abnormal cytology.
Author contributions
GA and AA were involved in creating the study protocol; AM and YI were involved in the process of the samples collection. TI, AB and AA performed PCR sample analysis; AI, TI, AB, AG and NM performed statistical analysis and data interpretation; GA, AM, YI and AA involved in preliminary data design and assessment, and literature review. GA, AB and TI drafted the manuscript. AI and GA provided a critical revision and regular feedback of the manuscript. All authors contributed to the refinement of the study protocol. All authors have read and approved the final manuscript.
Ethical approval
The study was approved by the Institutional Research Ethics Committee of the Nazarbayev University on 23 April 2019 (IREC number: 146/4042019).
Supplemental Material
Download MS Word (24.6 KB)Acknowledgements
The authors acknowledge the Nazarbayev University School of Medicine for the support that enabled the completion of this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The study questionnaires and raw data are available from the project PI via email: [email protected]
Additional information
Funding
References
- Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):1–7. doi: 10.1016/S2214-109X(19)30482-6.
- Serrano B, Brotons M, Bosch FX, et al. Epidemiology and burden of HPV-related disease. Best Pract Res Clin Obstet Gynaecol. 2018;47:14–26. doi: 10.1016/j.bpobgyn.2017.08.006.
- Bruni L, Albero G, Serrano B, et al. ICO/IARC information centre on HPV and cancer (HPV information centre). Human papillomavirus and related diseases in Kazakhstan. Summary Report 17 June; 2019 [cited 2023 Jun 25].
- Aimagambetova G, Azizan A. Epidemiology of HPV infection and HPV-Related cancers in Kazakhstan: a review. Asian Pac J Cancer Prev. 2018;19(5):1175–1180. doi: 10.22034/APJCP.2018.19.5.1175.
- Aimagambetova G, Chan CK, Ukybassova T, et al. Cervical cancer screening and prevention in Kazakhstan and Central Asia. J Med Screen. 2021;28(1):48–50. doi: 10.1177/0969141320902482.
- Igissinov N, Igissinova G, Telmanova Z, et al. New trends of cervical cancer incidence in Kazakhstan. Asian Pac J Cancer Prev. 2021;22(4):1295–1304. doi: 10.31557/APJCP.2021.22.4.1295.
- Balmagambetova S, Gabutti G, Koyshybaev A, et al. Cervical screening in Western Kazakhstan: liquid-based cytology ‘cell scan’ versus azur-eosin staining. J Med Screen. 2020;27(2):90–95. doi: 10.1177/0969141319885409.
- de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670. doi: 10.1002/ijc.30716.
- Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–350. doi: 10.1038/nrc798.
- Paavonen J. Human papillomavirus infection and the development of cervical cancer and related genital neoplasias. Int J Infect Dis. 2007;11(Suppl. 2):S3–S9. doi: 10.1016/S1201-9712(07)60015-0.
- Clifford G, Franceschi S, Diaz M, et al. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;24(Suppl. 3):S3/26–34. doi: 10.1016/j.vaccine.2006.05.026.
- Akhatova A, Azizan A, Atageldiyeva K, et al. Prophylactic human papillomavirus vaccination: from the origin to the current state. Vaccines. 2022;10(11):1912. doi: 10.3390/vaccines10111912.
- McCredie MR, Sharples KJ, Paul C, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9(5):425–434. doi: 10.1016/S1470-2045(08)70103-7.
- Gravitt PE, Rositch AF, Silver MI, et al. A cohort effect of the sexual revolution may be masking an increase in human papillomavirus detection at menopause in the United States. J Infect Dis. 2013;207(2):272–280. doi: 10.1093/infdis/jis660.
- Imankulova B, Babi A, Issa T, et al. Prevalence of precancerous cervical lesions among nonvaccinated Kazakhstani women: the National Tertiary Care Hospital Screening Data (2018). Healthcare. 2023;11(2):235. doi: 10.3390/healthcare11020235.
- Vinodhini K, Shanmughapriya S, Das BC, et al. Prevalence and risk factors of HPV infection among women from various provinces of the world. Arch Gynecol Obstet. 2012;285(3):771–777. doi: 10.1007/s00404-011-2155-8.
- Niyazmetova L, Aimagambetova G, Stambekova N, et al. Application of molecular genotyping to determine prevalence of HPV strains in pap smears of Kazakhstan women. Int J Infect Dis. 2017;54:85–88. doi: 10.1016/j.ijid.2016.11.410.
- Bekmukhambetov Y, Balmagambetova S, Jarkenov T, et al. Distribution of high risk human papillomavirus types in Western Kazakhstan – retrospective analysis of PCR data. Asian Pac J Cancer Prev. 2016;17(5):2667–2672.
- Aimagambetova G, Babi A, Issanov A, et al. The distribution and prevalence of high-risk HPV genotypes other than HPV-16 and HPV-18 among women attending gynecologists’ offices in Kazakhstan. Biology. 2021;10(8):794. doi: 10.3390/biology10080794.
- Babi A, Issa T, Issanov A, et al. Prevalence of high-risk human papillomavirus infection among Kazakhstani women attending gynecological outpatient clinics. Int J Infect Dis. 2021;109:8–16. doi: 10.1016/j.ijid.2021.06.006.
- Aimagambetova G, Babi A, Issa T, et al. What factors are associated with attitudes towards HPV vaccination among Kazakhstani women? Exploratory analysis of cross-sectional survey data. Vaccines. 2022;10(5):824. doi: 10.3390/vaccines10050824.
- Babi A, Issa T, Issanov A, et al. Knowledge and attitudes of mothers toward HPV vaccination: a cross-sectional study in Kazakhstan. Womens Health. 2023;19:17455057231172355. doi: 10.1177/17455057231172355.
- Aimagambetova G, Azizan A. Human papillomavirus vaccination: past, present and future. Vaccines. 2022;10(9):1398. doi: 10.3390/vaccines10091398.
- Nayar R, Wilbur DC, editors. The Bethesda system for reporting cervical cytology. Springer; 2015. doi: 10.1007/978-3-319-11074-5
- Cuschieri K, Fellner MD, Arroyo Mühr LS, et al. Quality assurance in human papillomavirus testing for primary cervical screening. Int J Gynecol Cancer. 2023;33(5):802–811. doi: 10.1136/ijgc-2022-004197.
- Muderris T, Afsar I, Yıldız A, et al. HPV genotype distribution among women with normal and abnormal cervical cytology in Turkey. Rev Esp Quimioter. 2019;32(6):516–524.
- Wang X, Huang X, Zhang Y. Involvement of human papillomaviruses in cervical cancer. Front Microbiol. 2018;9:2896. doi: 10.3389/fmicb.2018.02896.
- Issa T, Babi A, Azizan A, et al. Factors associated with cervical cancer screening behaviour of women attending gynaecological clinics in Kazakhstan: a cross-sectional study. Womens Health. 2021;17:17455065211004135. doi: 10.1177/17455065211004135.
- Finan RR, Chemaitelly H, Racoubian E, et al. Genetic diversity of human papillomavirus (HPV) as specified by the detection method, gender, and year of sampling: a retrospective cross-sectional study. Arch Gynecol Obstet. 2023;307(5):1469–1479. doi: 10.1007/s00404-022-06907-4.
- Tsedenbal B, Yoshida T, Enkhbat B, et al. Human papillomavirus genotyping among women with cervical abnormalities in Ulaanbaatar, Mongolia. Int J Infect Dis. 2018;77:8–13. doi: 10.1016/j.ijid.2018.09.018.
- Shipitsyna E, Zolotoverkhaya E, Kuevda D, et al. Prevalence of high-risk human papillomavirus types and cervical squamous intraepithelial lesions in women over 30 years of age in St. Petersburg, Russia. Cancer Epidemiol. 2011;35(2):160–164. doi: 10.1016/j.canep.2010.08.010.
- Beyazit F, Sılan F, Gencer M, et al. The prevalence of human papillomavirus (HPV) genotypes detected by PCR in women with normal and abnormal cervico-vaginal cytology. Ginekol Pol. 2018;89(2):62–67. doi: 10.5603/GP.a2018.0011.