Abstract
Objective While studies have documented how metabolic dysfunction-associated steatotic liver disease (MASLD) can contribute to cardiovascular disease (CVD), whether MASLD is associated with myocardial infarction (MI) remains debateable. Herein, we systematically reviewed published articles and performed a meta-analysis to determine the relationship between MASLD and MI risk.
Methods PubMed, MEDLINE, Embase, Web of Science, CNKI, CBM, VIP, and WanFang databases were searched, and the DerSimonian Laird method was used to obtain hazard ratios (HRs) for binary variables to assess the correlation between MASLD and MI risk. Subgroup analyses for the study region, MASLD diagnosis, quality score, study design, and follow-up time were conducted simultaneously for the selected studies retrieved from the time of database establishment to March 2022. All study procedures were independently conducted by two investigators.
Results The final analysis included seven articles, including eight prospective and two retrospective cohort studies. The MI risk was higher among MASLD patients than among non-MASLD patients (HR = 1.26; 95% CI: 1.08-1.47, p = 0.003). The results of the subgroup analysis of the study region revealed an association of MASLD with MI risk among Americans and Asians, but not in Europeans. Subgroup analyses of MASLD diagnosis showed that ultrasonography and other (fatty liver index[FLI] and computed tomography [CT)]) diagnostic methods, but not international classification of disease (ICD), increased the risk of MI. Subgroup analysis of the study design demonstrated a stronger relationship between MASLD and MI in retrospective studies but not in prospective studies. Subgroup analysis based on the follow-up duration revealed the association of MASLD with MI risk in cases with < 3 years of follow-up but not with ≥3 years of follow-up.
Conclusion MASLD increases the risk of MI, independent of traditional risk factors.
Keywords:
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a frequently reported condition in >25% of adults worldwide, particularly in more than half of the patients with type 2 diabetes [Citation1,Citation2]. The global prevalence, as per a previous meta-analysis of 86 studies, was estimated to be 25.2% [Citation3]. MASLD is a common chronic liver metabolic disease, and obesity, hyperlipidaemia, and diabetes mellitus are considered risk factors. The worldwide incidence of MASLD has increased along with obesity and diabetes [Citation4].
MASLD has been a redefined term for non-alcoholic fatty liver disease (NAFLD) since June 2023 [Citation5]. MASLD is a multisystem disease that affects several organ systems and can gradually develop from simple liver steatosis to non-alcoholic steatohepatitis (NASH), cirrhosis, and liver failure. In addition to liver-related complications, people with MASLD are often diagnosed with cardiovascular disease (CVD) [Citation6–11]. The cause of MASLD is complex, involving a range of factors from genetics to lifestyle and energy balance. Metabolic dysfunction not only predisposes to liver pathology but also leads to a significantly higher CVD risk; both children and adults are at risk [Citation12,Citation13]. Growing evidence suggests that MASLD increases CVD risk, independent of traditional risk factors. A previous meta-analysis of 20 observational studies highlighted that MASLD increases the risk of stroke [Citation14], which is even more common than liver-related complications. Cardiovascular events (CVEs) are the leading causes of mortality among patients with MASLD, consistent with the high prevalence of cardiovascular risk factors among those afflicted with MASLD [Citation15,Citation16].
Myocardial infarction(MI) is a common cause of death and is associated with high mortality rates [Citation17]. Despite advancements in diagnosis and treatment, the incidence of myocardial infarction (MI) has gradually increased [Citation18]. Therefore, it is imperative to explore novel predictors of MI.
In addition, many recent studies have been conducted [Citation19,Citation20]. An independent committee of experts external to the nomenclature process made recommendations on the acronym and its diagnostic criteria; steatotic liver disease (SLD) was chosen as an overarching term to encompass various aetiologies of steatosis. The term steatohepatitis is an important pathophysiological concept that should be retained. Metabolic dysfunction-associated steatotic liver disease (MASLD) was chosen to replace NAFLD. Therefore, the following is described using the MASLD nomenclature:
While researchers have attempted to reveal the link between MASLD and MI risk, the results published thus far are controversial. Therefore, we conducted a systematic review and meta-analysis of the most updated cohort data.
Methods
This study protocol was registered in PROSPERO, and the study was conducted according to the PRISMA-P statement (CRD42022314085).
Literature search
Two investigators independently searched the PubMed, Medline, Embase, Web of Science, CNKI, CBM, VIP, and WanFang databases to complete a systematic literature search from the time of database establishment to March 2022. The search strategy was as follows: ‘heart infarct’ or ‘heart attack’ or ‘ST Elevation Myocardial Infarction’ or ‘Non-ST Elevated Myocardial Infarction’ or ‘Inferior Wall Myocardial Infarction’ or ‘Anterior Wall Myocardial Infarction’ or ‘Myocardial Infarction’ AND ‘non-alcoholic fatty liver disease’ or ‘NAFLD’ or ‘non-alcoholic steatohepatitis’ or ‘NASH.’ In addition, we scanned online databases, performed manual searches, and investigated the references in the included literature. The search was conducted without restrictions on the year and language.
Inclusion criteria
The following studies were included: (a) cohort studies, (b) original studies that evaluated the impact of MASLD on MI risk, (c) studies including odds ratios(ORs) or hazard ratios (HRs) with 95% confidence intervals(CIs) to calculate the risk, (d) studies that conducted MASLD diagnosis using serum biomarkers, ultrasonography, computed tomography (CT), or others, and (e) studies with binary research variables.
Exclusion criteria
The exclusion criteria were as follows: (1) animal research, review, meta-analysis, and comments; (2) duplicate studies; (3) studies irrelevant to the topic; (4) those unavailable in full-text; (5) studies with inconsistent research methods; (6) those with continuous research variables; and (7) studies with CVD/coronary heart disease (CHD)/major adverse cardiovascular event (MACE) outcome.
Data extraction and quality assessment
Literature screening and data extraction were performed by two investigators who read relevant studies. The original information included author, publication date, design, region, MASLD diagnosis, follow-up time, and outcome. Statistical analysis included multivariable-adjusted ORs for MI and the corresponding 95% CI. The Newcastle–Ottawa Quality Assessment Scale (NOS) was used by two independent authors to rate the quality of cohort studies based on the following criteria: selection (four items, up to four stars), exposure/outcome (three items, up to three stars), and comparability (one item, up to two stars) [Citation21]. The cohort study was evaluated using three modules with eight items. Specifically, it includes population selection, comparability, and exposure/outcome evaluation, and has a maximum score of 9.0, with 7.0–9.0 indicating high quality and 4.0–6.0 indicating medium quality [Citation22].
Statistical analysis
Stata 16.0 was used to perform the meta-analysis. The pooled values were adjusted for HR and 95% CI. HR was used to determine the association of MASLD with MI risk, and p < 0.05 indicated statistical significance. Statistical heterogeneity among studies was evaluated using I2 statistic and Q-test, where p < 0.10 indicated statistical significance. We chose a fixed-effects model (if I2<50%) or a random effects model. I2 values of 0–25%, 26–50%, 51–75%, and >75% indicated insignificant, low, moderate, and high heterogeneity, respectively [Citation23]. If necessary, the source of heterogeneity was determined through a subgroup analysis of the study region, MASLD diagnosis, quality score, study design, and follow-up duration. Funnel plots, Begg’s test, and Egger’s test were employed to determine publication bias.
Study selection
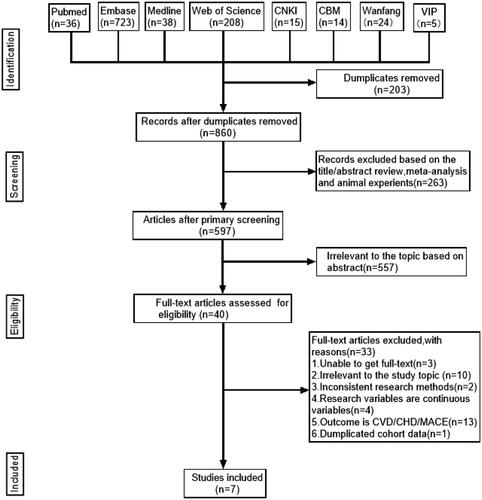
The initial search strategy yielded 1063 potentially relevant articles. In total, 203 publications were excluded as duplicates, 263 articles were excluded following review of titles and abstracts because of animal experiments and meta-analysis, and 557 articles were excluded because of irrelevance to the topic based on abstract. Overall, 33 articles were excluded because of (1) unavailability of full text, (2) irrelevance of the study topic, (3) inconsistency in research methods, (4) use of continuous variables, (5) CVD/CHD/MACE as outcome, and (6) duplicated cohort data. Therefore, seven articles qualified the study inclusion criteria. shows a schematic of the literature screening strategy employed in this study.
Study characteristics
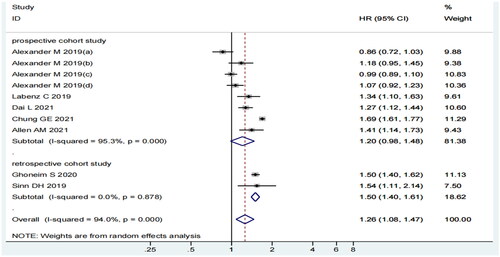
describes the characteristics of the seven articles (10 studies). All of these were cohort studies, two were retrospective, and eight were prospective studies. The sample sizes ranged from 19078 to 55099280. These studies used different methods to diagnose MASLD: two studies used ultrasonography, four used the International Classification of Diseases (ICD), and four employed others (including CT and fatty liver index [FLI] [Citation24]). These studies were conducted in different countries: five from Europe, two from America, and three from Asia. The NOS score deemed eight studies as ‘high quality’ and two as ‘low-quality.’ NOS scores ranged from 7 to 9. The follow-up duration among the selected studies ranged from 1 to 10.3 years.
Table 1. Characteristics of included studies.
MASLD and MI risk
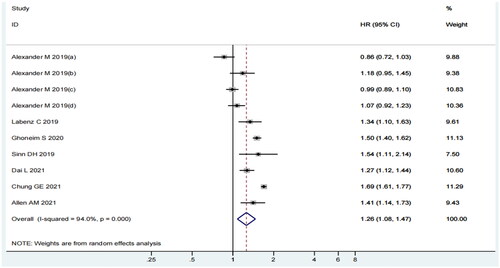
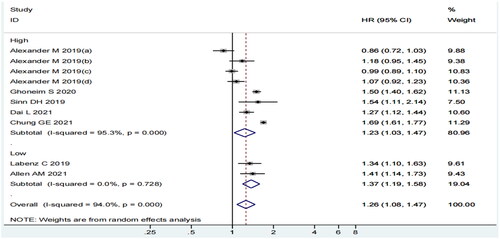
In the pooled analysis of seven articles (10 individual studies), the incidence of MI was higher among MASLD patients than among non-MASLD patients. The heterogeneity among the included studies was statistically significant (I2=94%, p = 0.000) (). Therefore, we selected a random-effects model and conducted subgroup analyses to determine the sources of heterogeneity. The random-effects summary estimate showed that the MASLD group had 1.26 times higher risk of MI than the control group (HR = 1.26; 95% CI 1.08–1.47; p = 0.003). Considering the statistical significance of the results, one might speculate that MASLD augments the risk of MI.
Subgroup of analysis
This meta-analysis revealed strong heterogeneity among the included studies. We conducted subgroup analyses of the study region, MASLD diagnosis, quality score, study design, and follow-up duration.
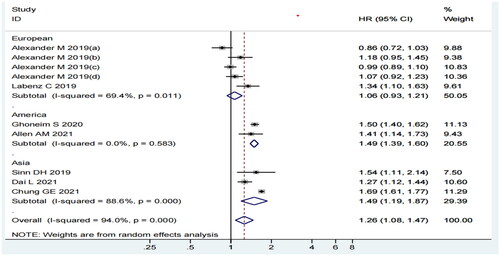
Considering the subgroup analysis of the study region, we observed that MASLD was related to the risk of MI among Americans (HR = 1.49, 95% CI: 1.39–1.60, p = 0.000, I2=0.0%) and Asians (HR = 1.49, 95% CI: 1.19–1.87, p = 0.001, I2=88.6%) but not in Europeans (HR = 1.06, 95% CI: 0.93–1.21, p = 0.343, I2=69.4%) (). Although the I2 statistic was 69.4% and 88.6% for Europeans and Asians, respectively, all the included studies had a CI value that overlapped with the 95% CI of our pooled estimate.
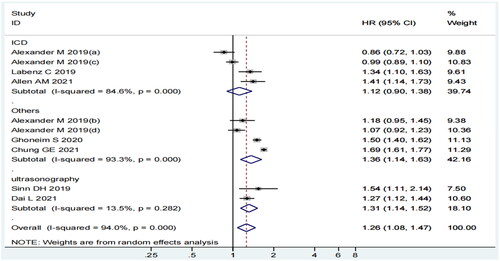
In the subgroup analysis of MASLD diagnosis, a positive relationship was evident in the subgroup of others (HR = 1.36, 95% CI: 1.14–1.63, p = 0.001, I2=93.3%) and ultrasonography (HR = 1.31, 95% CI: 1.14–1.52, p = 0.000, I2=13.5%), but not in the ICD subgroup (HR = 1.12, 95% CI: 0.90–1.38, p = 0.311, I2=84.6%) (). Although the I2 statistic in ICD and others was 84.6% and 93.3%, respectively, the CI of the included studies overlapped with the estimated 95% CI.
The subgroup analysis of quality score demonstrated a significant correlation between MASLD and MI risk in both high-quality (HR = 1.23, 95% CI: 1.03–1.47, p = 0.022, I2=95.3%) and low-quality (HR = 1.37, 95% CI: 1.19–1.58, p = 0.000, I2=0.0%) subgroups (). Although the I2 statistic was 95.3% in the high-quality subgroup, only two studies (25% of the weight in the analysis) showed a CI value that did not overlap with the 95% CI of our pooled estimate.
Considering the subgroup analysis of the study design, MASLD and MI risk were significantly correlated in the subgroup of retrospective cohort study (HR = 1.50, 95% CI: 1.40–1.61, p = 0.000, I2=0.0%) but not in the prospective cohort study subgroup (HR = 1.20, 95% CI: 0.98–1.48, p = 0.074, I2=95.3%) ().
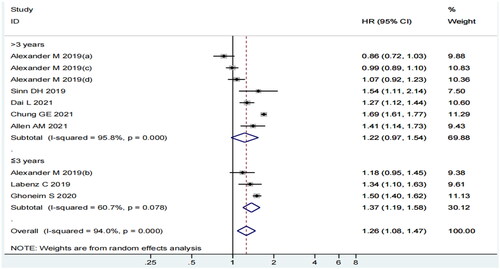
In the follow-up duration subgroup analysis, a significant correlation was observed in the ≤3 year follow-up subgroup (HR = 1.37, 95% CI: 1.19–1.58, p = 0.000, I2=60.7%) but not in the >3 year follow-up subgroup (HR = 1.22, 95% CI: 0.97–1.54, p = 0.085, I2=95.8%) (). Although the I2 statistic was 95.8%, the CI value of only one study did not overlap with the 95% CI of our pooled estimate.
Sensitivity analysis
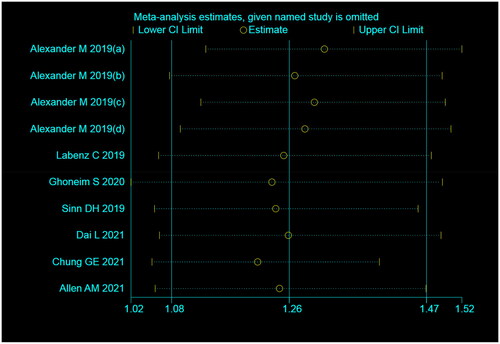
We performed a sensitivity analysis and excluded one study at a time to investigate the stability of the results. The difference between the point and interval estimates of the combined value of the effect was not significant. Overall, our sensitivity analysis results were consistent with those of our primary analysis after excluding literature. Thus, the results derived in this study were stable ().
Publication bias
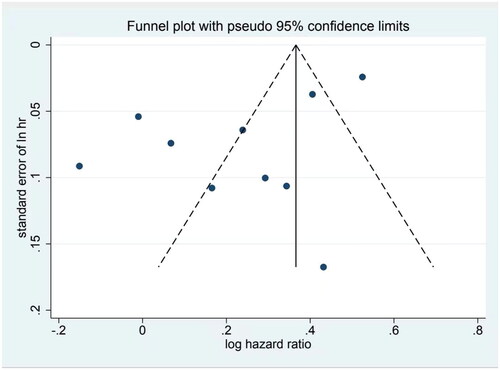
We tested the included studies for publication bias using Begg, Egger, and funnel plots. The results of the Begg’s test showed a Kendall’s score of 1 (z = 0.09, p = 1), which demonstrated no statistically significant difference. While Egger’s test result showed a statistically significant difference (t= −2.44, p = 0.04), the funnel plot revealed an asymmetrical scatter distribution of each study () with publication bias. As the included publications contained several super-large studies, and the sample size was relatively small for other studies, the funnel plot was expected to be more scattered and asymmetric.
Figure 9. Funnel plot of the literature publication bias on the association between MASLD and MI risk.
This systematic review and meta-analysis of seven articles revealed a correlation between MASLD and MI risk, with a more than one-fold increase in the combined outcomes studied (HR = 1.26, 95% CI: 1.08–1.47, p = 0.003). This association between the two entities was evident, even after adjusting for traditional risk factors.
Most published studies to date have reported the correlation between MASLD and CVD, but only a few studies have determined the correlation between MASLD and MI and have published controversial and inconsistent results. To the best of our knowledge, this is the first meta-analysis to shed light on MASLD as a contributing factor to the risk of MI. Our study has several strengths that offer a convincing conclusion. First, the comprehensive literature search performed followed strict inclusion/exclusion criteria, precise data extraction procedure, and rigorous quality assessment. Second, we performed subgroup analyses that helped us further explore and comprehensively understand the relationship between MASLD and MI risk.
Our study has a few limitations. First, we were unable to control for the variable tobacco use in some of the included studies because tobacco is an independent risk factor for MI [Citation25,Citation26]. Second, because we chose only observational studies, we were unable to establish a causal relationship. Third, we defined MASLD using ultrasonography, FLI, and ICD. The selection of literature is limited, including ultrasound, FLI, and ICD diagnostic methods. The difference in diagnostic criteria leads to a greater possibility of measurement bias, which affects the measured data [Citation27,Citation28]. However, ultrasonic detection of MASLD is highly accurate, and ICD is prone to coding errors [Citation29]. Fourth, MASLD is a dynamic state, not a static one, that can be alleviated by active intervention [Citation30]. Fifth, the selected literature confirmed the relationship between MASLD and myocardial infarction, but the factors influencing MASLD causing myocardial infarction are not uniform. The common risk factors of myocardial infarction include endotoxin and oxidative stress behaviour, and this study did not perform a single analysis of the influence of risk factors on this level. Further studies should confirm the trajectory of MASLD on the risk of MI. Finally, MASLD exhibits complex chronic pathophysiology, including genetic susceptibility and environmental insults, and the mechanisms underlying metabolic dysfunction are different. Although some reports suggest that NAFLD includes not only MASLD but also genetically associated fatty liver disease (GAMLD) or genetically associated steatotic liver disease (GASLD) [Citation31,Citation32], the prevailing view is that MASLD is a new definition of NAFLD, and genetic associations only underscore the relationship between mitochondrial dynamics and MASLD susceptibility [Citation33]. Therefore, whether MASLD is identical to NAFLD might affect the relationship between MASLD and MI, as the present study adopted studies on NAFLD for analyses.
The statistical heterogeneity in the overall results was high, as reflected from the I2 and Q-tests (I2=94%, p = 0.000), and could not be explained by sensitivity analysis. The results of our subgroup analysis suggested differences in the study regions, diagnostic methods of MASLD, study design, and follow-up duration as sources of heterogeneity.
According to evidence from published studies, the association between MASLD and MI is mixed. While published literature has shown a significant correlation between MASLD and the incidence of MI [Citation34–38], a recent large cohort study failed to confirm this finding [Citation39]. In this study, the overall HR decreased from 1.17 to 1.01 following the adjustment of traditional risk factors for CVD [Citation39]. This finding indicates that MASLD was not an independent factor in determining the risk of MI. We believe that the discrepancy in this finding can be attributed to important differences in the study region, study design, follow-up duration, and the MASLD diagnosis procedure. Our meta-analysis compensated for the lack of precision in previously published studies, an issue that was resolved through data pooling from all studies.
This systematic review sheds light on the potential association between MASLD and the risk of MI, as evident from their strong relationship. Although the mechanism underlying the effect of MASLD on MI risk is unclear, the potential explanation is as follows: the association might not be causal because it can mediate insulin resistance, endothelial dysfunction, systemic inflammatory tone, and ectopic fat deposition in other organs to promote atherosclerosis, all of which can contribute to the occurrence of MI [Citation40–46]. In addition, MASLD-mediated liver dysfunction can contribute to thrombotic vascular disease by negatively affecting biogenesis of lipoproteins, coagulation proteins, and inflammation-related factors [Citation47,Citation48].
There are large variations in the occurrence of MASLD among regions and ethnicities. The prevalence of MASLD between 2016 and 2018 was 24% among Americans, 23% among Europeans, and 33% among Asians [Citation1]. Therefore, region may act as a confounding factor in the estimation of this association. In the subgroup analysis of the region, the pooled results demonstrated the association of MASLD with MI risk among Americans and Asians but not in Europeans. Thus, MI risk assessment among Europeans with MASLD is important and warrants large-scale prospective studies.
We also performed a subgroup analysis based on MASLD diagnosis and found that MASLD diagnosed by ultrasonography and others (including FLI and CT) increased the risk of MI, but there was no significant association if ICD was employed for MASLD diagnosis. A possible explanation is as follows: First, while the accuracy of ultrasonography in diagnosing fatty liver is high, the technique is subject to measurement errors [Citation27,Citation28]. Second, ultrasonography examinations are affected by the subjective nature of the operator and might be limited by additional intraobserver and interobserver variability. Third, MASLD can alleviate by active interventions [Citation30]. Future studies should explore whether active MASLD intervention can reduce the incidence of MI.
Considering the study design, the results of the subgroup analysis demonstrated a stronger link between MASLD and MI in retrospective studies, but not in prospective studies. Notably, the definitions and diagnoses of MASLD and MI may change over time. Furthermore, patients with MASLD in retrospective studies were different from those without MASLD at baseline. Moreover, retrospective studies are prone to publication biases. Therefore, caution should be exercised when drawing conclusions.
In the subgroup analysis based on follow-up duration, the relationship between MASLD and the risk of MI was evident in the group with a follow-up duration of <3 years but not in those with ≥3 years of follow-up. The potential reasons underlying this observation are as follows: First, MASLD can be alleviated by active intervention during follow-up, where participants are advised to lose weight, improve their diet, and therefore alleviate MASLD. Second, long-term follow-up will lead to withdrawal bias. On the basis of these considerations, the results should be interpreted with caution.
Current evidence supports the correlation between MASLD and MI risk. These results are closely related to those observed in clinical practice. Therefore, it is necessary to actively identify and intervene in MASLD to reduce the incidence of MI.
Conclusion
In conclusion, MASLD increased the risk of MI, independent of traditional factors. Therefore, it is imperative to strictly monitor patients with MASLD to prevent MI. Future studies should investigate whether active MASLD intervention can reduce the incidence of MI.
Authors contribution
Qiong Lv designed the study. Huashan Zhao, Qiong Lv collated the data, carried out data analyses and produced the initial draft of the manuscript. Qiong Lv and Huashan Zhao contributed to drafting the manuscript. All authors have read and approved the final submitted manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s). The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical Approval
Not applicable.
Data availability statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):1–11. doi: 10.1002/hep.28431.
- Eskridge W, Cryer DR, Schattenberg JM, et al. Metabolic dysfunction-associated steatotic liver disease and metabolic dysfunction-associated steatohepatitis: the patient and physician perspective. J Clin Med. 2023;12(19):6216.doi: 10.3390/jcm12196216.
- Targher G, Byrne CD, Lonardo A, et al. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589–600. doi: 10.1016/j.jhep.2016.05.013.
- Yanai H, Adachi H, Hakoshima M, et al. Metabolic-dysfunction-associated steatotic liver disease-Its pathophysiology, association with atherosclerosis and cardiovascular disease, and treatments. Int J Mol Sci. 2023;24(20):15473. doi: 10.3390/ijms242015473.
- Lekakis V, Papatheodoridis GV. Natural history of metabolic dysfunction-associated steatotic liver disease. Eur J Intern Med. 2023. doi: 10.1016/j.ejim.2023.11.005.
- Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341–1350. doi: 10.1056/NEJMra0912063.
- Del Ben M, Baratta F, Polimeni L, et al. Non-alcoholic fatty liver disease and cardiovascular disease: epidemiological, clinical and pathophysiological evidences. Intern Emerg Med. 2012;7 Suppl 3: s 291–296. doi: 10.1007/s11739-012-0819-4.
- Lu H, Liu H, Hu F, et al. Independent association between nonalcoholic fatty liver disease and cardiovascular disease: a systematic review and meta-analysis. Int J Endocrinol. 2013;2013:124958–124957. doi: 10.1155/2013/124958.
- Bhatia LS, Curzen NP, Calder PC, et al. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J. 2012;33(10):1190–1200. doi: 10.1093/eurheartj/ehr453.
- Wu S, Wu F, Ding Y, et al. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: a systematic review and meta-analysis. Sci Rep. 2016;6(1):33386. doi: 10.1038/srep33386.
- Lee HH, Lee HA, Kim EJ, et al. Metabolic dysfunction-associated steatotic liver disease and risk of cardiovascular disease. Gut. 2023;:gutjnl-2023-331003. doi: 10.1136/gutjnl-2023-331003.
- Muzurović E, Peng CC-H, Belanger MJ, et al. Nonalcoholic fatty liver disease and cardiovascular disease: a review of shared cardiometabolic risk factors. Hypertension. 2022;79(7):1319–1326. doi: 10.1161/HYPERTENSIONAHA.122.17982.
- Muzurović E, Polyzos SA, Mikhailidis DP, et al. Non-alcoholic fatty liver disease in children. Curr Vasc Pharmacol. 2023;21(1):4–25. doi: 10.2174/1570161121666221118155136.
- Wang M, Zhou BG, Zhang Y, et al. Association between non-alcoholic fatty liver disease and risk of stroke: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:812030. doi: 10.3389/fcvm.2022.812030.
- Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65(5):1557–1565. doi: 10.1002/hep.29085.
- Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10(6):646–650. doi: 10.1016/j.cgh.2011.12.039.
- Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the european society of cardiology (ESC). Eur Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393.
- Han X, Bai L, Jeong MH, et al. Higher long-term mortality in patients with non-ST-Elevation myocardial infarction than ST-Elevation myocardial infarction after discharge. Yonsei Med J. 2021;62(5):400–408. doi: 10.3349/ymj.2021.62.5.400.
- Kokkorakis M, Boutari C, Katsiki N, et al. From non-alcoholic fatty liver disease (NAFLD) to steatotic liver disease (SLD): an ongoing journey towards refining the terminology for this prevalent metabolic condition and unmet clinical need. Metabolism. 2023;147:155664. doi: 10.1016/j.metabol.2023.155664.
- Méndez-Sánchez N, Bugianesi E, Gish RG, et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol Hepatol. 2022;7(5):388–390. doi: 10.1016/S2468-1253(22)00062-0.
- Li W, Ma D, Liu M, et al. Association between metabolic syndrome and risk of stroke: a meta-analysis of cohort studies. Cerebrovasc Dis. 2008;25(6):539–547. doi: 10.1159/000131672.
- Schuch FB, Vancampfort D, Firth J, et al. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am J Psychiatry. 2018;175(7):631–648. doi: 10.1176/appi.ajp.2018.17111194.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557.
- Chen X, Lin X, Chen LD, et al. Obstructive sleep apnea is associated with fatty liver index, the index of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2016;28(6):650–655. doi: 10.1097/MEG.0000000000000598.
- Lu Y, Wang Z, Zheng L. Association of smoking with coronary artery disease and myocardial infarction: a mendelian randomization study. Eur J Prev Cardiol. 2021;28(12):e11–e12. doi: 10.1177/2047487320907747.
- Teo KK, Ounpuu S, Hawken S, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368(9536):647–658. doi: 10.1016/S0140-6736(06)69249-0.
- Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54(3):1082–1090. doi: 10.1002/hep.24452.
- Lee DH. Imaging evaluation of non-alcoholic fatty liver disease: focused on quantification. Clin Mol Hepatol. 2017;23(4):290–301. doi: 10.3350/cmh.2017.0042.
- Labenz C, Huber Y, Michel M, et al. Impact of NAFLD on the incidence of cardiovascular diseases in a primary care population in Germany. Dig Dis Sci. 2020;65(7):2112–2119. doi: 10.1007/s10620-019-05986-9.
- Oh H, Jun DW, Saeed WK, et al. Non-alcoholic fatty liver diseases: update on the challenge of diagnosis and treatment. Clin Mol Hepatol. 2016;22(3):327–335. doi: 10.3350/cmh.2016.0049.
- Luukkonen PK, Qadri S, Ahlholm N, et al. Distinct contributions of metabolic dysfunction and genetic risk factors in the pathogenesis of non-alcoholic fatty liver disease. J Hepatol. 2022;76(3):526–535. doi: 10.1016/j.jhep.2021.10.013.
- Valenzuela-Vallejo L, Mantzoros CS. Time to transition from a negative nomenclature describing what NAFLD is not, to a novel, pathophysiology-based, umbrella classification of fatty liver disease (FLD). Metabolism. 2022;134:155246. doi: 10.1016/j.metabol.2022.155246.
- Shin S, Kim J, Lee JY, et al. Mitochondrial quality control: its role in metabolic dysfunction-associated steatotic liver disease (MASLD). J Obes Metab Syndr. 2023;32(4):289–302. doi: 10.7570/jomes23054.
- Ghoneim S, Dhorepatil A, Shah AR, et al. Non-alcoholic steatohepatitis and the risk of myocardial infarction: a population-based national study. World J Hepatol. 2020;12(7):378–388. doi: 10.4254/wjh.v12.i7.378.
- Sinn DH, Kang D, Chang Y, et al. Non-alcoholic fatty liver disease and the incidence of myocardial infarction: a cohort study. J Gastroenterol Hepatol. 2020;35(5):833–839. doi: 10.1111/jgh.14856.
- Xu J, Dai L, Zhang Y, et al. Severity of nonalcoholic fatty liver disease and risk of future ischemic stroke events. Stroke. 2021;52(1):103–110. doi: 10.1161/STROKEAHA.120.030433.
- Chung GE, Cho EJ, Yoo JJ, et al. Young adults with nonalcoholic fatty liver disease, defined using the fatty liver index, can be at increased risk of myocardial infarction or stroke. Diabetes Obes Metab. 2022;24(3):465–472. doi: 10.1111/dom.14597.
- Allen AM, Therneau TM, Mara KC, et al. Women with nonalcoholic fatty liver disease lose protection against cardiovascular disease: a longitudinal cohort study. Am J Gastroenterol. 2019;114(11):1764–1771. doi: 10.14309/ajg.0000000000000401.
- Alexander M, Loomis AK, van der Lei J, et al. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million european adults. BMJ. 2019;367:l5367. doi: 10.1136/bmj.l5367.
- Gaggini M, Morelli M, Buzzigoli E, et al. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5(5):1544–1560. doi: 10.3390/nu5051544.
- Harrison SA, Gawrieh S, Roberts K, et al. Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J Hepatol. 2021;75(2):284–291. doi: 10.1016/j.jhep.2021.02.034.
- Baratta F, Pastori D, Angelico F, et al. Nonalcoholic fatty liver disease and fibrosis associated with increased risk of cardiovascular events in a prospective study. Clin Gastroenterol Hepatol. 2020;18(10):2324–2331 e2324. doi: 10.1016/j.cgh.2019.12.026.
- Bugianesi E, Moscatiello S, Ciaravella MF, et al. Insulin resistance in nonalcoholic fatty liver disease. Curr Pharm Des. 2010;16(17):1941–1951. doi: 10.2174/138161210791208875.
- Byrne CD, Targher G. Ectopic fat, insulin resistance, and nonalcoholic fatty liver disease: implications for cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014;34(6):1155–1161. doi: 10.1161/ATVBAHA.114.303034.
- Targher G, Corey KE, Byrne CD. NAFLD, and cardiovascular and cardiac diseases: factors influencing risk, prediction and treatment. Diabetes Metab. 2021;47(2):101215. doi: 10.1016/j.diabet.2020.101215.
- Polyzos SA, Kechagias S, Tsochatzis EA. Review article: non-alcoholic fatty liver disease and cardiovascular diseases: associations and treatment considerations. Aliment Pharmacol Ther. 2021;54(8):1013–1025. doi: 10.1111/apt.16575.
- Dixon JL, Ginsberg HN. Hepatic synthesis of lipoproteins and apolipoproteins. Semin Liver Dis. 1992;12(4):364–372. doi: 10.1055/s-2008-1040406.
- Lisman T, Porte RJ. Pathogenesis, prevention, and management of bleeding and thrombosis in patients with liver diseases. Res Pract Thromb Haemost. 2017;1(2):150–161. doi: 10.1002/rth2.12028.