Abstract
Background
The effectiveness of nirmatrelvir–ritonavir has mainly been shown in non-hospitalized patients with mild-to-moderate coronavirus disease 2019 (COVID-19). The real-world effectiveness of nirmatrelvir-ritonavir urgently needs to be determined using representative in-hospital patients with COVID-19 during the Omicron wave of the pandemic.
Methods
We performed a multicentre, retrospective study in five Chinese PLA General Hospital medical centers in Beijing, China. Patients hospitalized with COVID-19 from 10 December 2022 to 20 February 2023 were eligible for inclusion. A 1:1 propensity score matching was performed between the nirmatrelvir-ritonavir group and the control group.
Results
1010 recipients of nirmatrelvir-ritonavir and 1010 matched controls were finally analyzed after matching. Compared with matched controls, the nirmatrelvir-ritonavir group had a lower incidence rate of all-cause death (4.6/1000 vs. 6.3/1000 person-days, p = 0.013) and a higher incidence rate of clinical improvement (47.6/1000 vs. 45.8/1000 person-days, p = 0.012). Nirmatrelvir-ritonavir was associated with a 22% lower all-cause mortality and a 14% higher incidence of clinical improvement. Initiation of nirmatrelvir-ritonavir within 5 days after symptom onset was associated with a 50% lower mortality and a 26% higher clinical improvement rate. By contrast, no significant associations were identified among patients receiving nirmatrelvir-ritonavir treatment more than 5 days after symptom onset. Nirmatrelvir-ritonavir was also associated with a 50% increase in survival days and a 12% decrease in days to clinical improvement.
Conclusion
Among hospitalized patients with COVID-19 during the Omicron wave in Beijing, China, the early initiation of nirmatrelvir-ritonavir was associated with clinical benefits of lowering mortality and improving clinical recovery.
Introduction
Since the beginning of the coronavirus disease 2019 (COVID-19) pandemic, the optimal antiviral treatment for reducing mortality of COVID-19 has been a hot research topic. Evidence from randomized controlled trial showed that nirmatrelvir-ritonavir within 5 days after symptom onset reduced COVID-19-related hospitalization or death in nonhospitalized, unvaccinated, high-risk patients [Citation1]. Cohort studies have also shown consistent benefits of nirmatrelvir-ritonavir in different types of non-hospitalized patients [Citation2–7]. Therefore, according to the guidelines provided by the World Health Organization (WHO), nirmatrelvir-ritonavir is highly recommended for non-severe COVID-19 patients who are at high risk. [Citation8].
Current studies rarely include patients hospitalized with COVID-19 for more than 5 days after symptom onset or required intensive respiratory support, due to considering the recommended indications indications of nirmatrelvir-ritonavir [Citation9,Citation10]. During the onset of the Omicron variant outbreak in China at the end of 2022, nirmatrelvir-ritonavir was widely used in hospitalized COVID-19 patients [Citation11,Citation12]. It was common to observe the use of nirmatrelvir-ritonavir beyond its recommended guidelines among hospitalized patients, including those who initiated treatment beyond the 5-day window after symptom onset and patients with severe COVID-19. Whether this empirical, off-label treatment of nirmatrelvir-ritonavir treatment is still unclear. Therefore, this retrospective cohort study aimed to examine the real-world effectiveness of nirmatrelvir-ritonavir in hospitalized patients with COVID-19, with additional interest in the clinical benefits among patients admitted for more than 5 days after symptom onset.
Methods
Study design and patients
This retrospective cohort study was conducted at five tertiary hospitals under the Chinese PLA General Hospital Group(First, Fourth, Fifth, Sixth, and Eighth medical centers), which comprise a large medicine system located in different districts of Beijing, China. During the study period, the predominant variants in Beijing were Omicron BF.7 and BA.5.2 [Citation13]. The Ethics Committee of PLA General Hospital approved all data analyses and exempted informed consent requirements because of the minimal risk of this retrospective cohort study (number: 309202302230712). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline [Citation14]. No compensation was offered for patients who participated in this study.
Patients were eligible for our study if they were admitted with a confirmed diagnosis of COVID-19 with positive reverse transcription-PCR or rapid antigen test results for SARS-CoV-2 infection for the first time between 10 December 2022 and 20 February 2023. Patients were excluded if they were younger than 18 years, admitted after 20 January 2023 (This was the date before the Lunar New Year and near the end of the COVID-19 pandemic in Beijing), discharged or died within 24 h after admission, received other antiviral medications or had antiviral medications cross-over with nirmatrelvir-ritonavir, or were pregnant.
Data collection
The data were derived from electronic medical records at five medical centres at the Chinese PLA General Hospital. Clinical researchers and engineers collaborated to build a database of inpatients with COVID-19 in these medical centres during the study period. Data including demographic information, vital signs, laboratory results, medical history, nursing records, diagnoses, and medical orders were collected. The pharmacy’s electronic records were used to verify the actual dosage of the prescribed order. Data on survival after discharge were obtained by telephone interviews, and the final observation deadline was 22 March 2023. Two clinical doctors verified the availability of the database. The data were accessible through a secure platform hosted by the hospital server within a private virtual network.
Covariates
Covariates included age, sex, body mass index (BMI), smoking status, time from symptom onset to admission, oxygen therapy or ventilator support, pre-existing comorbidities and pneumonia on radiology at baseline. The baseline was established on the day of admission. Obesity was defined as BMI > 28kg/m2. Comorbidities were categorized according to the Charlson comorbidity index (CCI), with values of 0, 1–3, and >3 [Citation15]. Immunocompromised status was defined as patients with malignant tumors, acute leukemia, lymphoma, autoimmune rheumatic conditions, and those receiving long-term immunosuppressive therapy after organ transplantation, based on references from the previous study [Citation7]. The baseline laboratory test results included the white blood cell count, haemoglobin, lymphocyte count, platelet count, albumin, alanine aminotransferase, estimated glomerular filtration rate (eGFR), C-reactive protein, and D-dimer on the day of baseline or within 48 h before and after baseline (if baseline values were missing). The World Health Organization (WHO) 8-point ordinal scale was assessed daily until 28 days of hospitalization or at discharge to evaluate the clinical severity. The range of this scale was from 3 (no oxygen required) to 8 (death) in our study, since scores 0–2 were used to evaluate outpatient patients. A score of 4 indicates the requirement for oxygen therapy. A score of 5 indicates the requirement of high-flow oxygen or non-invasive ventilation. A score of 6 corresponds to the need for mechanical ventilation, and a score of 7 represents ventilation with additional organ support [Citation16].
Treatment exposure
Hospitalized patients with COVID-19 who received nirmatrelvir-ritonavir at the five medical centres were defined as having treatment exposure. All centres had equal access to nirmatrelvir-ritonavir. In the control group, we selected hospitalized patients with COVID-19 who had not received any antiviral therapy during the same period as in the nirmatrelvir-ritonavir group. To mitigate potential immortal time bias, we excluded patients who had more than 2 days between admission and initiating nirmatrelvir-ritonavir treatment [Citation9]. During the study period, approximately half of the patients hospitalized for COVID-19 exceeded the best recommended time to start nirmatrelvir-ritonavir within 5 days of symptom onset. Based on the possible benefits of antiviral treatment, our hospital expert committee on COVID-19 treatment recommends using antiviral treatment for hospitalized patients with severe COVID-19 whose PCR tests are positive for SARS-CoV-2. This recommendation is based on the expert consensus from the Respiratory Society of the Chinese Medical Association and Treatment of COVID-19 in China [Citation12,Citation17].
Outcomes
Study outcomes included all-cause death and clinical improvement recorded during 28 days of hospitalization. Clinical improvement was defined as a decrease of 2 points on the WHO 8-point ordinal scale for clinical improvement within 28 days of hospitalization or at hospital discharge with no increase in score from the baseline score (whichever occurred first). The safety events included severe liver and kidney function impairment (defined as the new onset of an eGFR <30 ml/min/1.73 m2 or an eGFR <60 ml/min/1.73 m2 (mild impairment) and alanine aminotransferase concentrations >200 U/L during hospitalization) [Citation18].
Statistical analysis
The patients’ characteristics are shown as the mean ± standard deviation or the median with interquartile range for continuous variables and as number (%) for categorical variables. Differences in baseline characteristics were tested using Student’s t-test, the Wilcoxon rank test, or the chi-square test.
We performed a 1:1 propensity score matching to account for the observed imbalance in covariates between the nirmatrelvir-ritonavir and control groups. Binary logistic regression was applied to estimate the conditional probability of receiving nirmatrelvir-ritonavir after admission. The covariables used for matching included age, sex, time from symptom onset to admission, CCI, WHO 8-point ordinal scale at baseline, corticosteroid therapy, grade of GFR (cutoff value whether less than 30 ml/min/1.73 m2), medication at baseline, COVID-19 as the primary diagnosis rather than the secondary diagnosis, complications, and a positive result of COVID-19 nucleic acid in a retest during hospitalization, which was determined based on professional knowledge and previous reports [Citation9,Citation18]. The calliper matching algorithm without replacement was used, and a calliper value of 0.2 was applied for the matching procedure. The Love plot showing absolute standardized mean differences was used to evaluate the balance of covariates before and after propensity-score matching, with a threshold of 0.1 applied to determine imbalance [Citation19]. All analyses were conducted based on matched samples.
The primary analysis aimed to evaluate the associations between the treatment exposure and study outcomes, including all-cause death and clinical improvement recorded during 28 days of hospitalization. The time to study outcome was defined as the number of days from the date of admission to the occurrence of events, discharge date, or date of death (whichever occurred first). We calculated the cumulative incidence of the study outcomes using the Kaplan–Meier estimator, with the log-rank test applied for inference. We also calculated the crude incidence rate per 1000 person-days of outcomes. With regard to study outcomes, Cox proportional hazard regression was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) associated with the treatment exposure. We accounted for the heterogeneity between study hospitals by adding a frailty term to the Cox models [Citation20].
Several sensitivity analyses were also conducted. First, we performed a stratified analysis according to the duration from symptom onset to treatment initiation (≤5 days, 5–10 days, and >10 days). Second, we performed subgroup analyses according to selected baseline characteristics and applied the z-test to detect potential modifying factors [Citation21]. Third, we compared safety outcomes between the groups and used the Wald method to calculate the risk ratio. Fourth, we included a sensitivity analysis that considered drug interactions as part of the exclusion criteria to mitigate confounding. Drug contraindications to nirmatrelvir–ritonavir were included amiodarone, rifampicin, rifapentine, carbamazepine, St. John’s Wort, primidone, phenobarbital, or phenytoin used on admission [Citation9,Citation17]. Finally, in addition to modelling the hazard function of study outcomes and estimating the HR, we also compared the number of days to event occurrence for study outcomes between the groups. Accelerated failure time modelling was used, which can be interpreted as the time to an event using the time ratio (TR). Unlike the HR, a TR >1 implied that the treatment exposure was associated with a prolonged duration to outcomes (e.g. all-cause death) [Citation22]. Therefore, we were able to examine the hypothesis that nirmatrelvir-ritonavir is associated with an extended number of survival days within 28 days after hospital admission.
All statistical analyses were conducted using SAS software, version 9.4 (SAS Institute Inc.) and R language 3.6.2 (R Foundation, Vienna, Austria). A two-tailed alpha of 0.05 indicates a statistically significant level.
Role of the funding source
The funders were not involved in the study design, collection, analysis, or interpretation of data or report writing.
Results
Baseline characteristics
We identified 6218 consecutive hospitalized adults in the PLA healthcare system who were diagnosed with COVID-19 in the study period. 85% of these patients were older than 60 or had at least one chronic comorbidity. Among those who met the inclusion and exclusion criteria, 1023 received nirmatrelvir-ritonavir and 3147 were antiviral-free (Supplemental Figure 1). After propensity score matching, 1010 patients in the nirmatrelvir-ritonavir group and 1010 in the control group were included in the analysis. shows the baseline characteristics of patients in the nirmatrelvir-ritonavir group and control group before and after a 1:1 propensity score matching. Before matching, patients in the nirmatrelvir-ritonavir group were older, had a higher rate of requiring oxygen and respiratory support, and had a higher rate of corticosteroid use than those in the control group. Baseline covariates were balanced after matching, with a corresponding absolute standardized mean difference <0.1 (). In the nirmatrelvir-ritonavir group, the median time from symptom onset to initiating antiviral therapy was 7.0 days (95% CI: 4.0–11.0) (); in 62.3% of patients, the time from onset to admission exceeded 5 days; a total of 95.2% of patients completed the standard 5-day nirmatrelvir-ritonavir regimen.
Figure 1. Love plot assessing differences in baseline characteristics before and after 1:1 propensity-score matching, respectively.
Absolute standardized mean differences were applied for assessing covariate balance, with a threshold of > 0.1 used for determining imbalance.
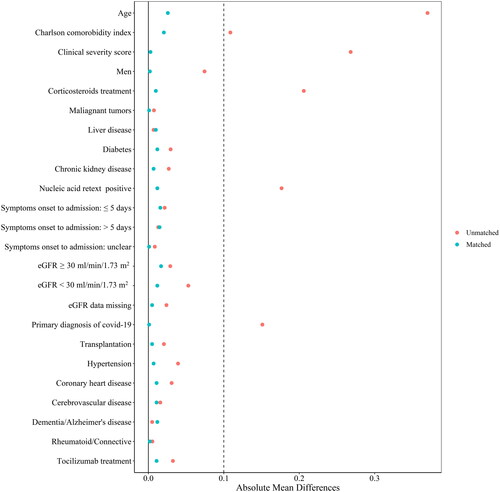
Table 1. Baseline characteristics of participants before and after propensity-score matching.
Associations between nirmatrelvir-ritonavir exposure and study outcomes
The 28-day all-cause mortality in the nirmatrelvir-ritonavir group was 12.0%, with a crude incidence of 4.6 (3.9–5.5)/1000 person-days (). The 28-day all-cause mortality in the control group was 15.7%, and the crude incidence was 6.3 (5.4–7.4)/1000 person-days. Nirmatrelvir-ritonavir was associated with a 22% lower risk of 28-day all-cause death (HR: 0.78, 95% CI: 0.61–0.98; ). The log-rank test also showed a significant difference in the cumulative risk of death at 28 days in the nirmatrelvir–ritonavir group (p = 0.013; ).
Figure 2. Cumulative incidence of study outcomes for nirmatrelvir-ritonavir recipients vs. matched controls.
Day 0 (baseline) represents the first day of admission to hospital. Kaplan-Merier estimator was used for estimating cumulative incidence, with Log-rank test applied for assessing differences between groups.
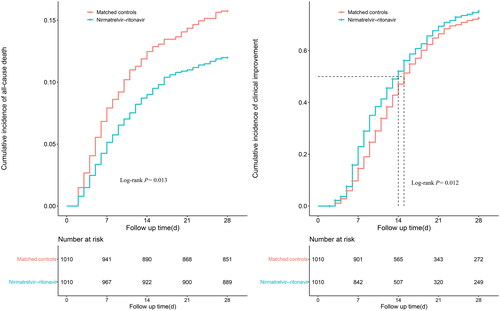
Table 2. Study outcomes in Nirmatrelvir-ritonavir group vs. matched controls.
As shown in , The clinical improvement rate in the nirmatrelvir-ritonavir group during the 28 days was 74.7%, and the median time to improvement was 14.0 (13.0–15.0) days. The clinical improvement rate in the control group was 71.1%, and the median time to clinical improvement was 15.0 (14.0–16.0) days. The nirmatrelvir-ritonavir group was associated with a 14% increase in 28-day clinical improvement compared with the control group (HR: 1.14, 95% CI: 1.02–1.27). The log-rank test showed a significant difference in the 28-day cumulative improvement rate (p = 0.012; ).
Safety analysis of nirmatrelvir-ritonavir exposure
As shown in the Supplemental Table 1,the incidence rate of liver impairment was not significantly different between the nirmatrelvir-ritonavir group (5.9%) and the control group (5.5%). There was no significant difference observed between the two groups in terms of the incidence rates of both moderate (nirmatrelvir-ritonavir 9.7% vs control 8.3%, p = 0.278) and severe kidney impairment (nirmatrelvir-ritonavir 6.1% vs control 4.7%, p = 0.141).
Sensitivity analyses
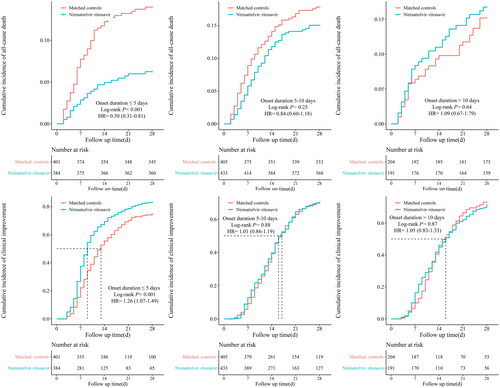
The primary analysis results were consistent with those in the subgroup of patients who initiated nirmatrelvir-ritonavir within 5 days from the onset of their symptoms. The early initiation of nirmatrelvir-ritonavir therapy was significantly associated with a reduced all-cause mortality at 28 days (HR: 0.50, 95% CI: 0.31–0.81), an increased clinical improvement rate (HR: 1.26, 95% CI: 1.07–1.49), and shorter duration to clinical improvement compared with controls (7.0 days, 95% CI: 4.0–10.0 vs 13 days, 95% CI: 4.0–9.0). In the subgroup of later initiation of nirmatrelvir-ritonavir treatment (i.e. at 5–10 days and >10 days after symptom onset), there was no difference in 28-day all-cause mortality, clinical improvement rate, or the time to clinical improvement compared with controls ().
Figure 3. Cumulative incidence of study outcomes for nirmatrelvir-ritonavir recipients vs. matched controls, further stratified by duration from symptoms onset to treatment initiation.
Day 0 (baseline) represents the first day of admission to hospital. Kaplan-Meier estimator was used for estimating cumulative incidence, with Log-rank test applied for assessing differences between groups. Cox proportional hazard regression was used for estimating hazard ratio (HR) and 95% confidence interval (CI).
Besides, we did not identify any significant modifying factors(including age, sex, respiratory support, comorbid conditions and so on) on the associations between nirmatrelvir-ritonavir treatment and outcomes (Supplemental figures 2 and 3). Additionally, as shown in Supplementary Table 2, after excluding hospitalized patients with drug contraindications to nirmatrelvir-ritonavir, we found a consistent association between nirmatrelvir-ritonavir therapy and clinical improvement in both primary and sensitivity analyses. Moreover, in patients requiring oxygen supplementation (WHO score 4), nirmatrelvir–ritonavir was associated with a superiority in reducing all-cause mortality compared to the controls (Supplemental figure 2). In patients with critically ill (WHO score greater than or equal to 5) there are no significant all-cause death events compared with controls (Supplementary Table 3). Finally, accelerated failure time modelling showed that nirmatrelvir-ritonavir was associated with a 50% increase in survival days (time ratio: 1.50; 95% CI: 1.10–2.03) and a 12% decrease in days to clinical improvement (time ratio: 0.88; 95% CI: 0.83–0.95) compared with matched controls (Supplemental Table 4).
Discussion
In this retrospective, multicentre, large-scale cohort study of hospitalized patients with COVID-19 during the Omicron wave in Beijing, we found that nirmatrelvir-ritonavir was significantly associated with reduced all-cause mortality. We also found a higher likelihood of achieving clinical improvement and a shorter time to clinical improvement in patients in the nirmatrelvir-ritonavir group, especially in those who received nirmatrelvir-ritonavir within 5 days after symptom onset. The benefits might be diminished when nirmatrelvir–ritonavir was prescribed after more than 5 days of symptom onset. To the best of our knowledge, this is the first multicentre, large sample-size, real-world study of the Omicron wave in Beijing that reported the effectiveness of nirmatrelvir-ritonavir treatment within and beyond recommended treatment 5 days after symptom onset.
A retrospective cohort study was conducted on COVID-19 hospitalized patients during the Omicron BA.2 wave in Hong Kong who did not require supplemental oxygen. It showed that nirmatrelvir-ritonavir was associated with significantly decreased risks of all-cause mortality (43%) and composite disease progression (66%). The present study was different from the previous study since we included patients who had severe comorbidities, required supplemental oxygen or mechanical ventilation or started nirmatrelvir-ritonavir treatment more than 5 days after symptom onset. The all-cause mortality rate in this study was higher than that in the previous study since increased risks of breakthrough infection and mortality in patients with COVID-19 who were old and had multiple comorbidities [Citation23–25]. We also found a significant benefit in survival time for the nirmatrelvir-ritonavir group. A prolonged in-hospital survival time could provide more opportunities for severe patients, which might explain lower mortality in the nirmatrelvir-ritonavir group. Moreover, The results of the sensitivity analysis support the WHO guideline recommendation to prescribe nirmatrelvir-ritonavir within 5 days of symptom onset. No significant clinical benefit was observed in patients who received nirmatrelvir-ritonavir later than 5 days after symptom onset. Delayed nirmatrelvir-ritonavir treatment is associated with delayed viral elimination, which might lead to persistent organ damage and death [Citation26].
In terms of the safety profile of nirmatrelvir-ritonavir, previous studies among outpatient and mild to moderate patients showed that nirmatrelvir-ritonavir mainly caused grade 1 and 2 adverse reactions, predominantly affecting the gastrointestinal system [Citation1,Citation2]. No significant increase in the occurrence of grade 3 or 4 adverse events. However, it is important to note that our study included patients with various underlying conditions, including critically ill patients, which represents a high-risk population for adverse reactions that were rarely explored in previous studies. We did not observe any significant differences between the nirmatrelvir-ritonavir group and the control group in terms of the occurrence of severe liver or kidney injury. Therefore, we support the use of nirmatrelvir-ritonavir in hospitalized patients, including those with critical illness or multiple comorbidity conditions. Notably, due to the retrospective nature of our study, we were unable to collect comprehensive adverse events data of the participants. In future studies, it would be valuable to monitor the pharmacokinetics of nirmatrelvir, particularly in critically ill patients which would provide important insights to guide the appropriate use of nirmatrelvir-ritonavir in this specific patient population.
The primary strength of this study is that it represents the first investigation into the effectiveness of off-label treatment with nirmatrelvir-ritonavir in hospitalized patients. Therefore, this study provides evidence regarding the real-world effectiveness of nirmatrelvir-ritonavir in hospitalized patients with COVID-19 who do not receive timely treatment or require more intensive respiratory support. Moreover, we assessed the association between nirmatrelvir-ritonavir and the prolonged time to death in this study using accelerated failure time modelling, which was not well investigated in previous studies.
There are several limitations to this study. First, although covariates such as disease severity, pre-existing conditions, and laboratory tests at baseline were well-balanced between the two groups, vaccination status was not adjusted due to the large proportion of missing values. With over 90% of China’s population fully vaccinated, adjusting for vaccinations likely had minimal effect on the outcome [Citation27]. Second, the onset time of symptoms might have been inaccurate because some COVID-19 symptoms were not obvious. Third, the heterogeneity in the hospitalized patient population might have influenced the interpretation of the results. By stratifying the data based on the levels of respiratory support, we performed subgroup analyses to examine the associations and outcomes within specific severity categories. In addition, the socioeconomic status of participants was not recorded. Consider that 90% of medication costs were covered by medical insurance of hospitalized patients with COVID-19 during the study period in Beijing. The socioeconomic status might not have affected the treatment strategy decision.
In summary, this multicentre retrospective cohort study shows that nirmatrelvir-ritonavir was associated with decreased 28-day all-cause mortality and a faster clinical recovery. Additionally, the early initialization of nirmatrelvir-ritonavir treatment within 5 days after symptom onset provides considerable benefits, whereas delayed administration might lack substantial benefits. Future prospective clinical studies on hospitalization of varying severities of COVID-19 should be conducted to obtain more precise definitions of populations who might benefit from nirmatrelvir-ritonavir treatment.
Author contributions
L. Xie conceived the study and obtained the funding. W. Xie and X. Yuan, J. Meng, and J. Cui, Z. Hai conceived and designed the study. Xiaobo Han and C. Li wrote the initial draft and conducted the statistical analyses. G. Mou, M. Zheng, Y. Liu, and K. Xiao participated in the planning and execution of this study. Zhongkuo Yu, Yinghan Shi, Xinjie Han and J. Xiong, R. Wang, K. Wang, X. Na, D. Zhao, and H. Cheng extracted and collected the data. W. Chen and X. Zhang accessed and verified the data. W. Xie, F. Xie, and W.Zhao revised the manuscript for important intellectual content, All co-authors had full access to the data, discussed the results and implications, commented on the manuscript at all stages, approved the final version, and agreed to be accountable for all aspects of the work.
Ethical approval
The Ethics Committee of Chinese PLA General Hospital, Beijing, China, approved all data analyses and exempted informed consent requirements on account of the minimal risk of this retrospective cohort study (number 309202302230712).
Supplemental Material
Download MS Word (578.6 KB)Acknowledgements
We thank Tiantian Zhang and Yue Wang, engineers from Shandong Future Network Research Institute, for their cooperation in the data extraction process.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data sharing statement
The data custodians (the Chinese PLA General Hospital) provided the underlying individual-patient data only for the study of this project. The data access was under the authorization of the confidentiality committees. The data and materials supporting the conclusions of the study are available from the corresponding author (E-mail: [email protected]) upon reasonable request.
Additional information
Funding
References
- Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386(15):1–10. doi: 10.1056/NEJMoa2118542.
- Arbel R, Wolff Sagy Y, Hoshen M, et al. Nirmatrelvir use and severe Covid-19 outcomes during the omicron surge. N Engl J Med. 2022;387(9):790–798. doi: 10.1056/NEJMoa2204919.
- Najjar-Debbiny R, Gronich N, Weber G, et al. Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients. Clin Infect Dis. 2023;76(3):e342–e349. doi: 10.1093/cid/ciac443.
- Salerno DM, Jennings DL, Lange NW, et al. Early clinical experience with nirmatrelvir/ritonavir for the treatment of COVID-19 in solid organ transplant recipients. Am J Transplant. 2022;22(8):2083–2088. doi: 10.1111/ajt.17027.
- Ganatra S, Dani SS, Ahmad J, et al. Oral nirmatrelvir and ritonavir in nonhospitalized vaccinated patients with coronavirus disease 2019. Clin Infect Dis. 2023;76(4):563–572. doi: 10.1093/cid/ciac673.
- Dryden-Peterson S, Kim A, Kim AY, et al. Nirmatrelvir plus ritonavir for early COVID-19 in a large U.S. health system: a population-based cohort study. Ann Intern Med. 2023;176(1):77–84. doi: 10.7326/M22-2141.
- Aggarwal NR, Molina KC, Beaty LE, et al. Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study. Lancet Infect Dis. 2023;3099(23):696–70. doi: 10.1016/S1473-3099(23)00011-7.
- World Health Organization. Therapeutics and COVID-19: living guideline. World Health Organization; [cited 2023 April 22]. Available from: https://files.magicapp.org/guideline/29b7d717-7bfd-415e-b642-cc70bf70ec1e/published_guideline_6141-10_0.pdf. Lancet Infect Dis. 2023;23(6):696–705, doi: 10.1016/S1473-3099(23)00011-7.
- Wong CKH, Au ICH, Lau KTK, et al. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalized patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: a retrospective cohort study. Lancet. 2022;400(10359):1213–1222. doi: 10.1016/S1473-3099(22)00507-2.
- Liu J, Pan X, Zhang S, et al. Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: a multicentre randomized controlled study. Lancet Reg Health West Pac. 2023;33:100694. doi: 10.1016/j.lanwpc.2023.100694.
- Deng G, Li D, Sun Y, et al. Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: a retrospective cohort study. J Med Virol. 2023;95(4):e28756. doi: 10.1002/jmv.28756.
- Chinese Thoracic Society, Chinese Association of Chest Physicians Critical Care Group. Expert consensus on the treatment of severe COVID-19 caused by Omicron variants. Zhonghua Jie He He Hu Xi Za Zhi. 2023;46(2):101–110. doi: 10.3760/cma.j.cn112147-20221230-00994.
- Gao Y, Luo Z, Ren S, et al. Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19. J Infect. 2023;86(6):e158–e160. doi: 10.1016/j.jinf.2023.03.023.
- Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X.
- Charlson ME, Carrozzino D, Guidi J, et al. Charlson comorbidity index: a critical review of clinimetric properties. Psychother Psychosom. 2022;91(1):8–35. doi: 10.1159/000521288.
- WHO. WHO R&D Blueprint novel Coronavirus COVID-19 Therapeutic Trial Synopsis; [cited 2023 Jan 9]. Available from: https://www.who.int/docs/default-source/blue-print/covid-19-therapeutic -trial-synopsis.pdf.
- General Office of the National Health Commission. Diagnosis and treatment protocol for COVID-19 in China (trial version 9); [cited 2022 Oct 20]. Available from: https://www.gov.cn/zhengce/zhengceku/2022-03/15/content_5679257.htm.
- Garibaldi BT, Wang K, Robinson ML, et al. Comparison of time to clinical improvement with vs without remdesivir treatment in hospitalized patients with COVID-19. JAMA Netw Open. 2021;4(3):e213071. doi: 10.1001/jamanetworkopen.2021.3071.
- Imai K, Ratkovic M. Covariate balancing propensity score. J R Stat Soc Series B Stat Methodol. 2014;76(1):243–263. doi: 10.1111/rssb.12027.
- Therneau TM, Grambsch PM, Pankratz VS. Penalized survival models and frailty. J Comput Graph Stat. 2003;12(1):156–175. doi: 10.1198/1061860031365.
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219–219. doi: 10.1136/bmj.326.7382.219.
- Jankowski JAZ, de Caestecker J, Love SB, et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomized factorial trial. Lancet. 2018;392(10145):400–408. doi: 10.1016/S0140-6736(18)31388-6.
- Agrawal U, Katikireddi SV, McCowan C, et al. COVID-19 hospital admissions and deaths after BNT162b2 and ChAdOx1 nCoV-19 vaccinations in 2.57 million people in Scotland (EAVE II): a prospective cohort study. Lancet Respir Med. 2021;9(12):1439–1449. doi: 10.1016/S2213-2600(21)00380-5.
- Agrawal U, Bedston S, McCowan C, et al. Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales. Lancet. 2022;400(10360):1305–1320. doi: 10.1016/S0140-6736(22)01656-7.
- Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4.
- Sun F, Lin Y, Wang X, et al. Paxlovid in patients who are immunocompromised and hospitalised with SARS-CoV-2 infection. Lancet Infect Dis. 2022;22(9):1279. doi: 10.1016/S1473-3099(22)00430-3.
- Chinese Center for Disease Control and Prevention. National report on novel coronavirus infection. 2023; [cited 2020 Jul 25]. Available from: https://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_13141/202301/t20230125_263519.html.

