Abstract
Background
The burden of carbapenem-resistant gram-negative bacteria (CRGNB) among solid organ transplant (SOT) recipients has not been systematically explored. Here, we discern the risk factors associated with CRGNB infection and colonization in SOT recipients.
Methods
This study included observational studies conducted among CRGNB-infected SOT patients, which reported risk factors associated with mortality, infection or colonization. Relevant records will be searched in PubMed, Embase and Web of Science for the period from the time of database construction to 1 March 2023.
Results
A total of 23 studies with 13,511 participants were included, enabling the assessment of 27 potential risk factors. The pooled prevalence of 1-year mortality among SOT recipients with CRGNB was 44.5%. Prolonged mechanical ventilation, combined transplantation, reoperation and pre-transplantation CRGNB colonization are salient contributors to the occurrence of CRGNB infections in SOT recipients. Renal replacement therapy, post-LT CRGNB colonization, pre-LT liver disease and model for end-stage liver disease score increased the risk of infection. Re-transplantation, carbapenem use before transplantation and ureteral stent utilization increaesd risk of CRGNB colonization.
Conclusion
Our study demonstrated that SOT recipients with CRGNB infections had a higher mortality risk. Invasive procedure may be the main factor contribute to CRGNB infection.
Introduction
The emergence of carbapenem-resistant gram-negative bacteria (CRGNB), including carbapenem-resistant Klebsiella pneumoniae (CRKP), carbapenem-resistant Acinetobacter baumannii (CRAB), carbapenem-resistant Pseudomonas aeruginosa (CRPA) and others, has become a global public health emergency with few therapeutic options, especially among solid organ transplant (SOT) recipients [Citation1]. The isolation of CRGNB has become a growing concern. SOT itself has also been independently associated with the development of carbapenem-resistant Enterobacteriaceae (CRE) acquisition [Citation2,Citation3]. However, to date, the relationship between risk factors and CRGNB in SOT recipients remains unclear due to the small number of scattered cases and inconsistent publications with varying quality across larger studies. Moreover, many studies have reported that the presence of CRGNB infection is associated with a high risk of death but without a relatively accurate mortality in SOT recipients [Citation4]. The objective of this systematic review and meta-analysis was to examine this relationship within the context of CRGNB endemicity and to clarify the risk factors for mortality, colonization and infection in SOT recipients.
Materials and methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation5]. The review protocol was prospectively registered with the National Institute for Health Research PROSPERO system (http://www.crd.york.ac.uk/prospero; Registration No. CRD42022306168).
Search strategy
A systematic electronic search of English-written articles was performed to identify all relevant studies published up to 1 March 2023, in PubMed, Embase and Web of Science. The following search terms were used: transplant* AND infection AND ‘carbapenem resistant’ OR ‘imipenem resistant’ OR ‘meropenem resistant’ OR ‘ertapenem resistant’ OR ‘doripenem resistant’. The references of the selected articles and relevant review articles were screened for potential misses.
Eligibility criteria
Eligible studies were case–control or cohort studies that investigated the association of CRGNB colonization, infection and mortality, with available data including hazard ratios (HRs), risk ratios (RRs), odds ratios (ORs) or death rates. Studies were excluded if they were (1) duplicated studies; (2) reviews, reports or meeting abstracts; (3) studies that did not distinguish between infection and colonization; (4) studies that did not provide sufficient data for 95% confidence intervals (CIs) and HRs, RRs or ORs; (5) studies that included only CRGNB cases in the absence of a comparison group comprised of SOT recipients without CRGNB and (6) studies from paediatric patients. When two or more studies from the same institution and the same author assessed the same risk factors, the study with the longest study period was selected for analyses [Citation6]. Both records were included if they assessed the different CRGNB [Citation7,Citation8]. Moreover, to control for any confounding effects in each study, only studies that performed multivariable analysis were included in the meta-analysis of risk factors.
Definition
Colonization was defined as the isolation of CRGNB from rectal swab from asymptomatic patients during admission. Collected rectal swab specimens were cultured and species identification was performed in the presence of CRGNB growth. Isolates identified as CRGNB were ascertained as CRGNB colonization. Positive surveillance cultures of clinical samples (including urine, respiratory samples and other skin cultures) in the absence of symptoms and signs of infection also were considered for colonization status. Rectal screening to identify CRGNB carriage was usually implemented before and after surgery as well as before and after transferred to intensive care unit (ICU). Recipients found to be colonized with CRGNB by observation performed during the hospital stay or at the time of transplantation were ascertained as CRGNB carriers at transplantation, and conversely those found to be colonized afterwards were ascertained as having acquired CRGNB carriage post-transplantation. Colonization or infection during ICU stay is the same. The infection was ascertained by integrating positive cultures of different samples with corresponding positive clinical manifestations. The occurrence of infection was defined as the presence of a bacterial pathogen in clinical samples (e.g. blood, respiratory secretions, ascites, pleural fluid, cerebrospinal fluid or surgical sites) in combination with clinical signs and symptoms of infection. We defined CRGNB as a strain of gram-negative bacteria resistant to at least one of the carbapenems with a minimum inhibitory concentration according to the standards of the Clinical and Laboratory Standards Institute (CLSI) or The European Committee on Antimicrobial Susceptibility Testing (EUCAST) [Citation9,Citation10]. The mortality was defined as deaths attributable to any cause.
Data extraction
Two researchers independently and blindly completed the literature screening, data extraction and quality evaluation. They browsed the title and abstract of the document to exclude irrelevant literature. They then extracted the data and documented the following details in standardized tables: first author, year and country, period, research type, study design, sample size, transplant type, infection type, identification method, risk factors, mortality and results of other events of interest. If the results differed, a third researcher would be consulted for judgement.
Statistical analysis and assessment of risk bias
For single-group data for mortality, pooled point mortality estimates regarding the relatively short observational period (30-day to 1-year mortality) along with 95%CIs for the included studies were calculated. Random-effects models were used to address expected heterogeneity.
When at least two studies analysed a potential risk factor, and the definition of such a factor was consistent across studies, we performed a meta-analysis of risk factors. Only the effect estimates (HRs, RRs or ORs) and 95%CIs of the multivariate analysis models were extracted from original studies. To estimate heterogeneity, P values for heterogeneity and I2 statistics were calculated. The random-effects model was used to combine the results when heterogeneity was present among the studies (I2 > 50% or P < 0.05). Otherwise, a fixed effects model was applied. Egger’s and Begg’s tests were used to assess publication bias statistically. For any variable presenting with large heterogeneity, a sensitivity analysis that excluded outlier studies was conducted to investigate the potential origin of heterogeneity. P < 0.05 was considered statistically significant for all included studies. The quality of each study was assessed using the Newcastle-Ottawa Quality Scale (NOS) [Citation7]. This scale assesses each study using three categories: (1) representativeness of the subjects, (2) comparability between the study groups and (3) ascertainment of the exposure or outcome of interest for case–control and cohort studies. Studies with a total score >6 and <4 were considered high and low quality, respectively. Statistical analyses were performed using the Stata software (version 15.0; Stata Corporation, College Station, TX, USA) and RevMan (Review Manager, version 4.3).
Result
Study selection
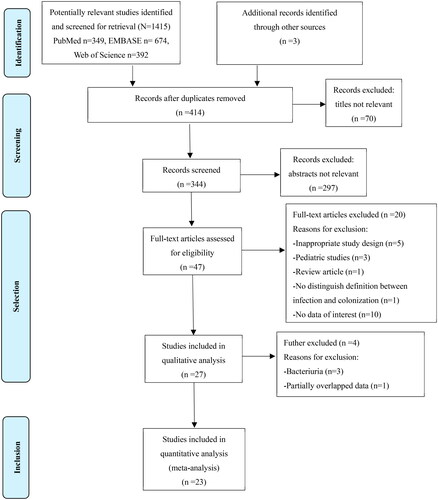
The flowchart of study selection is shown in . A total of 4556 records were retrieved from the electronic databases and reference lists of retrieved studies and relevant systematic reviews. After removing duplicate studies and a detailed evaluation of titles and abstracts, 47 studies were identified for full-text screening. A total of 27 studies were further screened in detail. Three studies of bacteriuria were excluded because they were difficult to classify as infection or colonization and lacked consistent sampling methods. Two studies conducted the same research on the same group of patients over a certain period, and one of them with a short total study time, was excluded. Ultimately, 23 studies met the inclusion criteria. Six studies were from the same author but comprised consecutive nonoverlapping cohorts [Citation6,Citation7,Citation11–14].
Study characteristics
presents the basic characteristics of the studies. In total, 13,511 participants were included in the analysis. The sample sizes of the studies ranged from 78 to 3005. These included studies were published from 2005 to 2020 in different countries, including China [Citation8,Citation15,Citation20,Citation29], Brazil [Citation6,Citation7,Citation11–14,Citation21,Citation24], Turkey [Citation16], Korea [Citation17,Citation18], Italy [Citation19,Citation26–28,Citation30] and the United States [Citation22,Citation23,Citation25]. Of the 23 studies, 6 were retrospective cohort studies, 5 were prospective cohort studies and 12 were case–control studies. Approximately half of the studies (n = 12, 52.2%) recruited liver transplantation (LT) recipients, seven studied kidney transplantation (KT; n = 7, 30.4%) and the rest (n = 4, 17.4%) from SOT (the SOT included heart, lung, liver, kidney, pancreas and combined organ transplants). CRKP was the most common isolate (n = 11, 47.8%). All studies were considered high-quality research after assessment via NOS. The outcomes of the NOS are shown in Supplementary Table S1.
Table 1. The characteristics of the 23 included studies.
Risk factors for mortality of SOT recipients with CRGNB infections
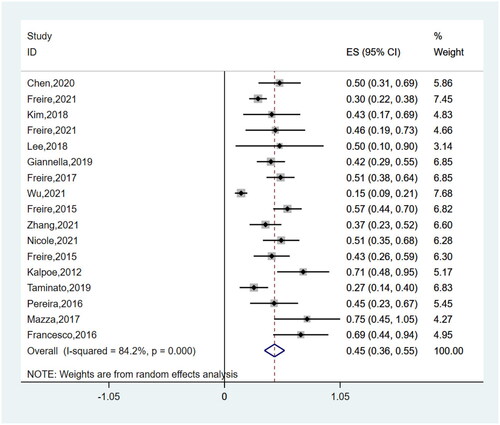
Post-transplantation CRGNB infections (OR 4.58, 95%CI 3.14–6.70) and reoperation (OR 2.19, 95%CI 1.61–2.98) were found to be significantly associated with mortality in SOT recipients (P < 0.00001). All the risk factors for mortality are summarized in Supplementary Table S2. The pooled mortality (1-year) of SOT recipients with CRGNB infections was 44.5% (95%CI 0.36–0.53%) in these studies (). I2 for a heterogeneity of 83.2% revealed high heterogeneity. The analysis included 722 participants from 18 studies. Interestingly, subgroup analyses concerning the date of pooled mortality assessment (Supplementary Figure S1) indicated the same mortality between 1-year and 180-day (44.2%, 95%CI 0.33–0.57%), and a small reduction in 90-day mortality (39.5%, 95%CI 0.24–0.55%). Moreover, subgroup analyses of 1-year mortality according type of transplantation showed LT recipients exhibit a higher mortality rate (49.6%, 95%CI 34.3–64.9%), in contrast to KT (30.3%, 95%CI 16.6–45.9%). Supplementary Figure S2illustrates forest plots for the risk factors of mortality. No significant differences were observed between subgroups (Supplementary Figure S3: subgroup analyses of risk factors for mortality).
Risk factors for CRGNB infections in SOT recipients
In the meta-analysis of the post-transplant occurrence of CRGNB infections, the pooled results demonstrated that these factors increase the risk of CRGNB infections, including prolonged mechanical ventilation (OR 2.99, 95%CI 1.93–4.63), combined transplantation (OR 3.46, 95%CI 2.12–5.65), re-operation (OR 2.09, 95%CI 1.42–3.09), pre-transplantation CRGNB colonization (OR 12.91, 95%CI 5.23–31.88) and the mean length of post-transplantation ICU stay (OR 1.13, 95%CI 1.11–1.15; ). However, cold ischemia time, age and delayed graft function were not significantly associated with CRGNB infections (OR 1.01, 95%CI, 0.99–1.04; OR 1.04, 95%CI, 1.00–1.08; OR 2.09, 95%CI 0.68–6.46). In addition, other variables for LT, such as renal replacement therapy (OR 3.05, 95%CI 2.08–4.50), post-LT CRGNB colonization (OR 8.58, 95%CI 4.08–18.04), pre-LT liver disease (OR 4.14, 95%CI 2.29–7.46), Model for end-stage liver disease (MELD) score (OR 1.04, 95%CI 1.02–1.06) and biliary complications (OR 4.92, 95%CI 2.16–11.23) also considerably increased infection risk (Supplementary Figure S4 illustrates the forest plots for risk factors of infection). Subgroup analyses showed differences in the type of organ and bacteria observed in pre-LT CRGNB acquisition (Supplementary Figure S5: subgroup analyses of risk factors for infection).
Table 2. Risk factors for CRGNB infections after SOT recipients.
Risk factors for CRGNB colonization in SOT recipients
Next, we investigated the risk factors for CRGNB colonization in SOT recipients (). The presence of re-transplantation (OR 10.43, 95%CI 3.45–31.33) and carbapenem use before transplantation (OR 3.78, 95%CI 1.28–11.14) were associated with a significantly increased risk of CRGNB colonization. However, hospital stay was not found to be significant (OR 4.79, 95%CI 0.69–33.44, P > 0.05). The data provided for three studies [Citation6,Citation11,Citation12] on KT noted a positive association between ureteral stent use and CRGNB colonization (P < 0.0001), and the pooled OR was 1.94 (95%CI 1.40–2.68). Age (OR 1.20, 95%CI 0.86–1.68) and low median lymphocyte count in the last 3 months (OR 0.43, 95%CI 0.02–10.95) were not found to be significant (P > 0.05; Supplementary Figure S6 illustrates the forest plots for risk factors of colonization). Nearly no significant differences were observed between subgroups (Supplementary Figure S7: subgroup analyses of risk factors for colonization).
Table 3. Risk factors for CRGNB colonization after SOT recipients.
Discussion
Considerable evidence confirms the detrimental impact of CRGNB infections on transplant recipients [Citation1], as well as the non-negligible role of CRGNB infections, with estimates of mortality rates up to 44.5% within one year, as the pre-eminent risk factor leading to death. Using this substantial SOT population, we identified the risk factors associated with both CRGNB colonization and infection. It is important to note that the relatively infrequent incidence of CRGNB infections has contributed to the paucity of studies assessing risk factors. To the best of our knowledge, this is the first systematic review and meta-analysis to identify risk factors for CRGNB burden in SOT recipients.
There are almost no definite data on mortality. In our study, the combined analysis involving mortality studies showed that the 1-year mortality rate of SOT recipients with CRGNB infection was 44.5%. The mortality rates ranged from 15% to 75% in each study. Among them, the study with the lowest mortality rate came from China [Citation8]. Chinese patients often leave the hospital before death, potentially resulting in low mortality rates [Citation31]. In contrast, in another study, up to 75% mortality might have been exaggerated due to the small number of positive cases [Citation26]. In the meantime, subgroup analyses of mortality showed that the mortality at 1-year and 180-day is almost consistent, which may indicate that the mortality after 180 days is no longer affected by CRGNB infections. Similar to a recent meta-analysis, Xu et al. reported a mortality rate of 43.13% among SOT patients infected with CRKP; however, their research did not explain different period of mortality [Citation32]. The outcomes of transplant patients are often complex, and despite subgroup analyses focusing on transplanted organs, the heterogeneity persists. Contrary to our findings, the study conducted by Lu et al. reveals an increase in mortality associated with KT compared to LT and lung transplantation [Citation33]. Nevertheless, this may illustrate, to some extent, the crucial role of CRGNB infection in mortality and outcomes.
Notably, some studies have been conducted not only in endemic transplant centres or hospitals with a certain risk of CRGNB infection, but also during periods when novel antimicrobial agents targeting the CRGNB were unavailable, which may have overestimated the actual incidence.
Whether pre- or post-transplantation, the presence of CRGNB colonization increases susceptibility to CRGNB infection. In addition, previous evidence has suggested that about half of the patients were colonized with CRKP prior to bloodstream infection [Citation34,Citation35]. Risk factors for post-transplantation infection are not just colonization, but other factors are crucial. Our study also found that SOT recipients with CRGNB infections had a longer post-transplantation ICU stay and were more likely to receive prolonged duration of mechanical ventilation. Transference to the ICU and protective mechanical ventilation were nothing to blame after the operation by convention. However, shortening the duration of ICU stay and minimizing the use of invasive mechanical ventilation could reduce the incidence of CRGNB infections. Solid organ transplantation and ICU stay are the most important conditions associated with a significant risk of CRE infections [Citation36–38].
Moreover, mechanical ventilation causes cyclic changes in cardiac load and puts extra pressure on the tracheal mucosa, thereby influencing physiological defence function [Citation39,Citation40]. Emma et al. observed that the composition of the respiratory microbiota deviated [Citation41]. These pathogenic bacteria, including the antibacterial drug-resistant bacteria, gradually dominate within 48–72 h subsequent to antibiotic administration [Citation42]. Similarly, combined transplantation, biliary complications, reoperation and renal replacement therapy are associated with a significant increase in CRGNB infections. These findings collectively underscore the role of invasive procedures and surgical interventions, which tend to evoke frequent barrier injuries, thereby compromising the body’s inherent resistance mechanisms and augmenting susceptibility to CRGNB exposure.
In addition, our research shows that cold ischemia time and delayed graft function have no significant correlations with CRGNB infections. In theory, extended cold ischemia time and delayed graft function could potentially affect recipient immune function under certain circumstances, thereby potentially increasing the risk of infection. However, there is currently a lack of direct evidence to establish a definite relationship between graft status and occurrence of infections.
In a word, our study suggests that more than the graft-related aspects, it is the invasive procedures, complex operations and ensuing complications that may be the main factors in precipitating CRGNB infections.
The propensity for CRGNB colonization in SOT patients parallels the risk observed in the ICU setting, including hospitalization duration and invasive procedural interventions. Dautzenberg et al. analysed 1077 patients in the ICU and found that patients colonized with CRE had, on average a 1.79 times higher risks of death, mainly attributed to prolonged hospital stay [Citation38].
Re-transplantation and ureteral stent use were associated with the highest risk of CRGNB colonization in our meta-analysis. In KT recipients, the augmented vulnerability to urinary tract infections and the utilization of ureteral stents after transplantation contribute to heightened colonization prospects. What makes it hard to explain is that age is a risk factor for colonization and infection, especially in KT recipients.
The use of antibiotics as a recognized risk factor merits further exploration of its potential effects on the timing and species of patient-acquired CRGNB. Regrettably, there is not enough data to perform an exhaustive analysis due to a lack of study. However, some valuable clues remain. Carbapenem use was the most common risk factor in the included studies. Prior use of piperacillin/tazobactam [Citation13] and ciprofloxacin use [Citation12] were considered independent risk factors, with antibiotic exposure occurring within the preceding 3 months. In a murine model of intestinal colonization, the timing of antibiotic use was critical for the effective colonization of CPE [Citation43]. Therefore, avoiding antibiotic misuse is significant for the management of CRGNB epidemic spread, while further research is required to elucidate the optimal timing of antibiotic administration.
We summarized the time to infection according to colonization status before or after transplantation, as well as the time from colonization acquisition pre-transplant and the risk of infection (Supplementary Tables S3–S4). Interestingly, we found that patients with colonization prior to transplantation were at a higher risk of infection following organ transplantation than those who acquired colonization during the post-transplantation period. This information was reported in a few studies and analysis was not possible, but this is useful information for planning preventive strategies.
Although some infection-prevention and control interventions were implemented on some included studies, no comparative data regarding the prevalence of CRGNB colonization. These strict contact precautions are convenient and acceptable, and we still believe measures able to prevent colonization are crucial to contain CRKP spread and to reduce the potential for post-transplantation infections [Citation44,Citation45].
The present meta-analysis only included studies of moderate to high quality and was conducted in a strict and comprehensive process. Despite the strict approach undertaken, this study is not without limitations, which merit acknowledgement and consideration. Firstly, nearly all the studies selected for analysis were observational, primarily originating from the CRGNB epidemic area, which could have potential impacts on the final outcomes. Secondly, it has some inherent weaknesses stemming from the amalgamation of studies displaying certain heterogeneity, such as divergent geographical locales, distinct time periods, variable original disease profiles and disparate follow-up durations. Thirdly, not all the included studies evaluated the same risk factors. Consequently, the pooling of data is derived from subsets of studies, and the analysis might omit a single risk factor due to data availability constraints. Moreover, subgroup analyses were performed based on the type of bacteria and transplanted organs. Notwithstanding the absence of overt dissimilarities in these subgroup analyses, the interpretation still necessitates cautious consideration owing to the potential residual confounding factors and the inherent limitations imposed by sample sizes. The intricate interplay of factors influencing CRGNB infections and their manifestations within subgroups further accentuates the complexity of this relationship. In any case, we also look forward to providing insights into risk factors, thereby contributing to a more comprehensive understanding of this critical healthcare concern. Overall, these limitations highlight the need for well-designed studies evaluating the impact of CRGNB in SOT recipients to provide informative insights for this population.
Conclusions
Our study identified risk factors among SOT recipients associated with CRGNB infection and colonization, and several identified factors resonate with medical interventions encountered within the ICU. It is our conviction that these risk signals contribute to a comprehensive understanding of CRGNB and have the potential to profoundly influence clinical decision-making processes, which would also help contain CRGNB spread and reduce the potential for post-transplantation infections by judicious antibiotic administration, subtractive unnecessary invasive procedure and preventive isolation strategies.
Authors contributions
SYG and XLH developed a review protocol with critical input from XLZ. SJT designed the search strategy in consultation with the QL. Title and abstract screening was conducted by two independent reviewers in TW and YL. A full-text review of the studies selected by title and abstract was conducted by YFZ and YDC. HQH made the final decisions with respect to study inclusion or exclusion. JH and HLQ performed the data extraction and risk of bias assessment of the included studies. SYG and XLH carried out all analyses and prepared the first draft of the manuscript, which was revised by all the authors until finalization.
Ethics approval and patient consent
Not applicable.
Compliance with Ethics guidelines
This article is based on previous studies and does not contain any new studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
| Abbreviations | ||
| CRGNB | = | carbapenem-resistant gram-negative bacteria |
| SOT | = | solid organ transplant |
| LT | = | liver transplantation |
| CRKP | = | carbapenem-resistant Klebsiella pneumoniae |
| CRAB | = | carbapenem-resistant Acinetobacter baumannii |
| CRPA | = | carbapenem-resistant Pseudomonas aeruginosa |
| CRE | = | carbapenem-resistant Enterobacteriaceae |
| PRISMA | = | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| HRs | = | hazard ratios |
| RRs | = | risk ratios |
| ORs | = | odds ratios |
| CIs | = | confidence intervals |
| CLSI | = | Clinical and Laboratory Standards Institute |
| EUCAST | = | The European Committee on Antimicrobial Susceptibility Testing |
| NOS | = | Newcastle-Ottawa Quality Scale |
| KT | = | kidney transplantation |
| ICU | = | intensive care unit |
| MELD | = | model for end-stage liver disease |
Supplemental Material
Download Zip (1.5 MB)Acknowledgement
Not applicable.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Additional information
Funding
References
- Giannella M, Bartoletti M, Conti M, et al. Carbapenemase-producing enterobacteriaceae in transplant patients. J Antimicrob Chemother. 2021;76(Suppl 1):1–11. doi: 10.1093/jac/dkaa495.
- Patel G, Perez F, Bonomo RA. Carbapenem-resistant enterobacteriaceae and Acinetobacter baumannii: assessing their impact on organ transplantation. Curr Opin Organ Transplant. 2010;15(6):676–682. doi: 10.1097/MOT.0b013e3283404373.
- Kassis-Chikhani N, Saliba F, Carbonne A, et al. Extended measures for controlling an outbreak of VIM-1 producing imipenem-resistant Klebsiella pneumoniae in a liver transplant centre in France, 2003–2004. Euro Surveill. 2010;15(46):19713.
- Tzouvelekis LS, Markogiannakis A, Psichogiou M, et al. Carbapenemases in Klebsiella pneumoniae and other enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25(4):682–707. doi: 10.1128/CMR.05035-11.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005.
- Freire MP, Carvalho LB, Reusing JO, et al. Carbapenem-resistant enterobacteriaceae among kidney transplant recipients – insights on the risk of acquisition and CRE infection. Infect Dis. 2021;53(6):430–439. doi: 10.1080/23744235.2021.1887511.
- Freire MP, Oshiro IC, Pierrotti LC, et al. Carbapenem-resistant enterobacteriaceae acquired before liver transplantation: impact on recipient outcomes. Transplantation. 2017;101(4):811–820. doi: 10.1097/TP.0000000000001620.
- Wu D, Chen C, Liu T, et al. Epidemiology, susceptibility, and risk factors associated with mortality in carbapenem-resistant gram-negative bacterial infections among abdominal solid organ transplant recipients: a retrospective cohort study. Infect Dis Ther. 2021;10(1):559–573. doi: 10.1007/s40121-021-00411-z.
- Cohen Stuart J, Leverstein-Van Hall MA, Dutch Working Party on the Detection of Highly Resistant Microorganisms. Guideline for phenotypic screening and confirmation of carbapenemases in enterobacteriaceae. Int J Antimicrob Agents. 2010;36(3):205–210. doi: 10.1016/j.ijantimicag.2010.05.014.
- Humphries R, Bobenchik AM, Hindler JA, et al. Overview of changes to the Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, M100, 31st edition. J Clin Microbiol. 2021;59(12):e0021321.
- Freire MP, de Oliveira Garcia D, Cury AP, et al. Outbreak of IMP-producing carbapenem-resistant Enterobacter gergoviae among kidney transplant recipients. J Antimicrob Chemother. 2016;71(9):2577–2585. doi: 10.1093/jac/dkw165.
- Freire MP, Camargo CH, Yamada AY, et al. Critical points and potential pitfalls of outbreak of IMP-1-producing carbapenem-resistant Pseudomonas aeruginosa among kidney transplant recipients: a case–control study. J Hosp Infect. 2021;115:83–92. doi: 10.1016/j.jhin.2021.05.006.
- Freire MP, Pierrotti LC, Oshiro IC, et al. Carbapenem-resistant Acinetobacter baumannii acquired before liver transplantation: impact on recipient outcomes. Liver Transpl. 2016;22(5):615–626. doi: 10.1002/lt.24389.
- Freire MP, Abdala E, Moura ML, et al. Risk factors and outcome of infections with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in kidney transplant recipients. Infection. 2015;43(3):315–323. doi: 10.1007/s15010-015-0743-4.
- Chen Y, Wang WL, Zhang W, et al. Risk factors and outcomes of carbapenem-resistant Enterobacteriaceae infection after liver transplantation: a retrospective study in a Chinese population. Infect Drug Resist. 2020;13:4039–4045. doi: 10.2147/IDR.S278084.
- Cinar G, Kalkan İA, Azap A, et al. Carbapenemase-producing bacterial infections in patients with liver transplant. Transplant Proc. 2019;51(7):2461–2465. doi: 10.1016/j.transproceed.2019.02.050.
- Kim YJ, Kim SI, Lee YD, et al. Carbapenem-resistant Acinetobacter baumannii bacteremia in liver transplant recipients. Transplant Proc. 2018;50(4):1132–1135. doi: 10.1016/j.transproceed.2018.01.043.
- Lee KH, Han SH, Yong D, et al. Acquisition of carbapenemase-producing Enterobacteriaceae in solid organ transplantation recipients. Transplant Proc. 2018;50(10):3748–3755. doi: 10.1016/j.transproceed.2018.01.058.
- Giannella M, Bartoletti M, Campoli C, et al. The impact of carbapenemase-producing enterobacteriaceae colonization on infection risk after liver transplantation: a prospective observational cohort study. Clin Microbiol Infect. 2019;25(12):1525–1531. doi: 10.1016/j.cmi.2019.04.014.
- Zhang F, Zhong J, Ding H, et al. Analysis of risk factors for carbapenem-resistant Klebsiella pneumoniae infection and its effect on the outcome of early infection after kidney transplantation. Front Cell Infect Microbiol. 2021;11:726282. doi: 10.3389/fcimb.2021.726282.
- Pagani N, Corcione S, Lupia T, et al. Carbapenemase-producing Klebsiella pneumoniae colonization and infection in solid organ transplant recipients: a single-center, retrospective study. Microorganisms. 2021;9(11):2272. doi: 10.3390/microorganisms9112272.
- Hong Nguyen M, Shields RK, Chen L, et al. Molecular epidemiology, natural history, and long-term outcomes of multidrug-resistant enterobacterales colonization and infections among solid organ transplant recipients. Clin Infect Dis. 2022;74(3):395–406.
- Kalpoe JS, Sonnenberg E, Factor SH, et al. Mortality associated with carbapenem-resistant Klebsiella pneumoniae infections in liver transplant recipients. Liver Transpl. 2012;18(4):468–474. doi: 10.1002/lt.23374.
- Taminato M, Fram D, Pereira RR, et al. Infection related to Klebsiella pneumoniae producing carbapenemase in renal transplant patients. Rev Bras Enferm. 2019;72(3):760–766. doi: 10.1590/0034-7167-2019-0009.
- Pereira MR, Scully BF, Pouch SM, et al. Risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae infections in liver transplant recipients. Liver Transpl. 2015;21(12):1511–1519. doi: 10.1002/lt.24207.
- Mazza E, Prosperi M, Panzeri MF, et al. Carbapenem-resistant Klebsiella pneumoniae infections early after liver transplantation: a single-center experience. Transplant Proc. 2017;49(4):677–681. doi: 10.1016/j.transproceed.2017.02.028.
- Giannella M, Bartoletti M, Morelli MC, et al. Risk factors for infection with carbapenem-resistant Klebsiella pneumoniae after liver transplantation: the importance of pre- and posttransplant colonization. Am J Transplant. 2015;15(6):1708–1715. doi: 10.1111/ajt.13136.
- Varotti G, Dodi F, Terulla A, et al. Impact of carbapenem-resistant Klebsiella pneumoniae (CR-KP) infections in kidney transplantation. Transpl Infect Dis. 2017;19(6):e12757. doi: 10.1111/tid.12757.
- Wu D, Chen C, Liu T, et al. Risk factors for acquisition of carbapenem-resistant Klebsiella pneumoniae and mortality among abdominal solid organ transplant recipients with K. pneumoniae infections. Med Sci Monit. 2020;26:e922996. doi: 10.12659/MSM.922996.
- Barchiesi F, Montalti R, Castelli P, et al. Carbapenem-resistant Klebsiella pneumoniae influences the outcome of early infections in liver transplant recipients. BMC Infect Dis. 2016;16(1):538. doi: 10.1186/s12879-016-1876-5.
- Weng L, Hu Y, Sun Z, et al. Place of death and phenomenon of going home to die in Chinese adults: a prospective cohort study. Lancet Reg Health West Pac. 2021;18:100301. doi: 10.1016/j.lanwpc.2021.100301.
- Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob. 2017;16(1):18. doi: 10.1186/s12941-017-0191-3.
- Lu J, Zhang A, Han L, et al. Clinical outcomes and risk factors for death following carbapenem-resistant Klebsiella pneumoniae infection in solid organ transplant recipients. Microbiol Spectr. 2023;11(1):e0475522. doi: 10.1128/spectrum.04755-22.
- Souli M, Galani I, Antoniadou A, et al. An outbreak of infection due to beta-lactamase Klebsiella pneumoniae carbapenemase 2-producing K. pneumoniae in a Greek University Hospital: molecular characterization, epidemiology, and outcomes. Clin Infect Dis. 2010;50(3):364–373. doi: 10.1086/649865.
- Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect. 2011;17(12):1798–1803. doi: 10.1111/j.1469-0691.2011.03514.x.
- Paño Pardo JR, Serrano Villar S, Ramos Ramos JC, et al. Infections caused by carbapenemase-producing enterobacteriaceae: risk factors, clinical features and prognosis. Enferm Infecc Microbiol Clin. 2014;32(Suppl 4):41–48. doi: 10.1016/S0213-005X(14)70173-9.
- Yan L, Sun J, Xu X, et al. Epidemiology and risk factors of rectal colonization of carbapenemase-producing enterobacteriaceae among high-risk patients from ICU and HSCT wards in a university hospital. Antimicrob Resist Infect Control. 2020;9(1):155.
- Dautzenberg MJ, Wekesa AN, Gniadkowski M, et al. The association between colonization with carbapenemase-producing enterobacteriaceae and overall ICU mortality: an observational cohort study. Crit Care Med. 2015;43(6):1170–1177. doi: 10.1097/CCM.0000000000001028.
- Lansdorp B, Hofhuizen C, van Lavieren M, et al. Mechanical ventilation-induced intrathoracic pressure distribution and heart-lung interactions. Crit Care Med. 2014;42(9):1983–1990. doi: 10.1097/CCM.0000000000000345.
- Zhang J, He Q, Du L, et al. Risk factor for lung infection in recipients after liver transplantation: a meta-analysis. Artif Organs. 2021;45(3):289–296. doi: 10.1111/aor.13826.
- de Koff EM, Man WH, van Houten MA, et al. The respiratory microbiota during and following mechanical ventilation for respiratory infections in children. Eur Respir J. 2021;57(4):2002652. doi: 10.1183/13993003.02652-2020.
- Otsuji K, Fukuda K, Ogawa M, et al. Dynamics of microbiota during mechanical ventilation in aspiration pneumonia. BMC Pulm Med. 2019;19(1):260. doi: 10.1186/s12890-019-1021-5.
- Le Guern R, Grandjean T, Bauduin M, et al. Impact of the timing of antibiotic administration on digestive colonization with carbapenemase-producing Enterobacteriaceae in a murine model. Antimicrob Agents Chemother. 2019;63(6):e00360-19. doi: 10.1128/AAC.00360-19.
- Li M, Wang X, Wang J, et al. Infection-prevention and control interventions to reduce colonisation and infection of intensive care unit-acquired carbapenem-resistant Klebsiella pneumoniae: a 4-year quasi-experimental before-and-after study. Antimicrob Resist Infect Control. 2019;8(1):8. doi: 10.1186/s13756-018-0453-7.
- Dai Y, Meng T, Wang X, et al. Validation and extrapolation of a multimodal infection prevention and control intervention on carbapenem-resistant Klebsiella pneumoniae in an epidemic region: a historical control quasi-experimental study. Front Med. 2021;8:692813. doi: 10.3389/fmed.2021.692813.