Abstract
Objectives
Tenecteplase, a modified variant of alteplase with greater fibrin specificity and longer plasma half-life, may have better efficacy and safety than alteplase in patients with acute ischemic stroke (AIS). We aimed to compare the benefits and risks of tenecteplase versus alteplase in the treatment of AIS.
Methods
Electronic databases were searched up to 10 February 2023 for randomized controlled trials evaluating the effect of tenecteplase versus alteplase in the treatment of AIS. The primary outcome was functional outcome at 90 days, and secondary outcomes including the symptomatic intracranial haemorrhage (SICH), and major neurological improvement. Subgroup analysis was performed based on the different dosage of tenecteplase.
Results
Ten studies with a total of 5123 patients were analysed in this meta-analysis. Overall, no significant difference between tenecteplase and alteplase was observed for functional outcome at 90 days (excellent: OR 1.08, 95%CI 0.93–1.26, I2 = 26%; good: OR 1.04, 95%CI 0.83–1.30, I2 = 56%; poor: OR 0.95, 95%CI 0.75–1.21, I2 = 31%), SICH (OR 1.12, 95%CI 0.79–1.59, I2 = 0%), and early major neurological improvement (OR 1.26, 95%CI 0.80–1.96, I2 = 65%). The subgroup analysis suggested that the 0.25 mg/kg dose of tenecteplase had potentially greater efficacy and lower symptomatic intracerebral haemorrhage risk compared with 0.25 mg/kg dose tenecteplase.
Conclusions
Among AIS patients, there was no significant difference on clinical outcomes between tenecteplase and alteplase. Subgroup analysis demonstrated that 0.25 mg/kg doses of tenecteplase were more beneficial than 0.4 mg/kg doses of tenecteplase. Further studies are required to identify the optimal dosage of tenecteplase.
Key Messages
Randomized controlled trials exploring comparative efficacy and safety of tenecteplase and alteplase have been yielding inconsistent results on various outcomes and merit the conduction of a meta-analysis to adequately answer these questions.
Analysis of evidence from randomized studies suggests that tenecteplase is as safe as alteplase for the treatment of acute ischemic stroke and tenecteplase is potentially associated with more favourable outcomes.
Tenecteplase at 0.25 mg/kg dose is more efficacious and at least as safe as alteplase for stroke thrombolysis.
Introduction
The acute ischemic stroke (AIS), defined as sudden neurologic dysfunction caused by focal brain ischemia with imaging evidence of acute infarction, is the most common and life-threatening acute cerebrovascular disease worldwide [Citation1]. Although the only available treatment options for AIS are intravenous alteplase and endovascular therapy [Citation2], growing evidence from clinical trials suggests that tenecteplase may be an effective treatment agent for the treatment of AIS compared to alteplase [Citation3,Citation4]. Tenecteplase is a target-modified variant tissue plasminogen activator [Citation5]. It offers a potential advance in acute thrombolysis for AIS with improved clot lysis, faster recanalization and lower bleeding risk compared with the current standard-of-care alteplase [Citation6]. In addition, compared with alteplase, tenecteplase is suitable for single bolus administration and has low cost [Citation7–9].
Although the use of intravenous tenecteplase for acute stroke treatment is still considered off-label, intravenous tenecteplase is increasingly being used for the treatment of AIS, particularly in countries where tenecteplase has a lower cost than alteplase [Citation7,Citation9]. Nevertheless, the effect of intravenous tenecteplase compared with alteplase for the treatment of AIS remains controversial. Several problems need to be solved, including the optimal dosage of tenecteplase and the efficacy and safety of the two drugs [Citation10,Citation11]. Recently, several stroke centres around the world have published their randomized controlled trials (RCTs) with the use of intravenous tenecteplase for AIS [Citation12–16]. Therefore, we decided to perform a meta-analysis of RCTs to evaluate the available evidence on the efficacy and safety of intravenous tenecteplase compared to intravenous alteplase for the treatment of patients with AIS.
Methods
Study selection
This meta-analysis was performed according to the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [Citation17]. The PRISMA checklist is shown in Supplementary Material 1. We preregistered our study protocol in Open Science Framework (https://osf.io/y7whf). Two authors (Jian Huang and Hui Zheng) searched the PubMed, Embase, Scopus and Cochrane Library for relevant studies in English up to 10 February 2023. The search algorithms included ‘stroke’, ‘tenecteplase’, ‘alteplase’ and ‘randomized’. The details of the search strategies are presented in Supplementary Material 2.
Inclusion criteria
Studies fulfilled the inclusion criteria were included:
Type of study: randomized trials;
Population: adult patients (≥18 years of age) with AIS and met standard criteria for intravenous thrombolysis;
Intervention: intravenous tenecteplase with a dose of 0.25 mg/kg, 0.4 mg/kg or other;
Comparison: intravenous alteplase with a standard dose;
Outcomes: the primary outcome of interest was functional outcome at 90 days, determined by the modified Rankin Scale (mRS), including excellent functional outcome (mRS 0–1, or no change from baseline), good functional outcome (mRS 0–2, or no change from baseline) and poor functional outcome (mRS 5–6). Secondary outcomes including the symptomatic intracranial haemorrhage (SICH), and major neurological improvement within 72 hours.
Data extraction and quality assessment
Two authors (Jian Huang and Hui Zheng) separately screened all retrieved studies, then extracted the relevant information (first author or study name, publication years, study design, population, intervention and control methods). Each clinical outcome of this meta-analysis was also extracted from each included study.
Two authors (Jian Huang and Xianfeng Zhu) adopted the Cochrane risk of bias tool [Citation18] to assess the methodological quality of including studies. Any disagreement between the two authors was resolved by a consensus after discussing with a third adjudicator (Xiaofeng Ping).
Statistical synthesis and analysis
Considering the expected clinical heterogeneity among the included trials, we used a random-effects model to compute the pooled odds ratio (OR) with 95% confidence interval (CI) for dichotomous outcomes. Findings were assessed within a framework of five non-inferiority margins established in the acute stroke literature for dichotomized mRS outcomes: −15%, −10%, −6.5%, −5% and −1.3% [Citation19]. The heterogeneity was assessed by the Higgins inconsistency (I2) statistics [Citation20]. Substantial heterogeneity was identified when I2 value > 30%. Publication bias was assessed by using the funnel plot and Egger’s regression test [Citation21].
A prespecified subgroup analysis was stratified by the dose of tenecteplase (0.1 mg/kg, 0.25 mg/kg, 0.4 mg/kg, according to the study by Haley et al. [Citation22]). Furthermore, we performed a random effects network meta-analysis on the frequentist method to further compare the efficacy and safety of alteplase and three dose of tenecteplase. The surface under the cumulative ranking (SUCRA) curve was used to rank the probabilities of alteplase and three doses of tenecteplase regarding the functional outcome at 90 days, SICH, and major neurological improvement, separately. A higher SUCRA score meant a higher ranking for efficacy and safety outcomes. Finally, we conducted sensitivity analyses by excluding each single study. All statistical analyses and assessments of bias risk were conducted by Review Manager Version 5.3, ‘meta’ and ‘gemtc’ package in R software (version 4.3.1) (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study characteristics
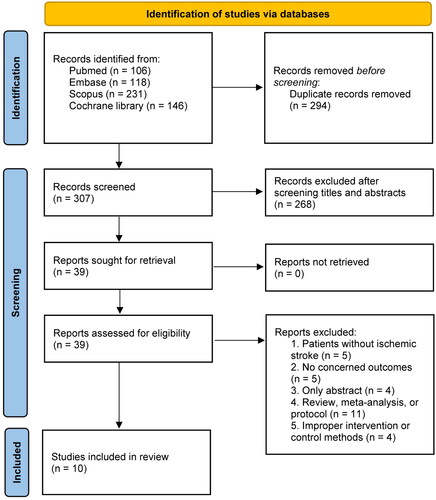
We identified 601 articles during the primary search, excluded 294 duplicated articles and 268 by screening the abstracts. Eventually, a total of 10 RCTs [Citation12–16,Citation22–26] were included in our study after retrieving 29 studies with various reasons (Supplementary Material 3 recorded the list of excluded studies). The literature screening flowchart is shown in .
presents the characteristics of included studies. A total of 5123 patients with AIS were included in the analysis, whereof 2677 patients received tenecteplase and 2446 patients received alteplase. The number of patients in each study ranged from a minimum of 75 up to 1577. Most of including studies had a non-inferiority study design, the non-inferiority margins ranged from −6.3% to −2.3%. The time window for thrombolysis ranged from 3 to 6 h from the onset of stroke across the included trials. Seven trials [Citation12,Citation13,Citation15,Citation16,Citation23–25] had a time window of 4.5 h, two [Citation14,Citation22] had a time window of 3 h, and Parsons et al. [Citation26] had a time window of 6 h. The 0.25 mg/kg of tenecteplase was studied in eight trials [Citation12,Citation14–16,Citation22–24,Citation26], 0.1 mg/kg of tenecteplase was studied in three trials [Citation14,Citation22,Citation26] and 0.4 mg/kg of tenecteplase was studied in three trials [Citation13,Citation22,Citation25]. The TRACE trial [Citation14] had a 0.32 mg/kg of tenecteplase arm as well but was not included in the meta-analysis. The 0.9 mg/kg of alteplase was used as a control in all included trials. Furthermore, the definition of SICH and major neurological improvement were varied among included studies (Supplementary Material 4).
Table 1. Characteristics of studies included in the meta-analysis.
Quality assessment
The results of risk of bias assessment () showed that nine trials were rated as high risk of bias since they were open-label studies. The TRACE trial [Citation14] did not provide the methods of allocation concealment, and the time window for thrombolysis in Parsons et al. [Citation26] was longer than other included trials.
Figure 2. Assessment of quality by the Cochrane risk of bias tool. Red denotes high risk, yellow unclear risk and green low risk.
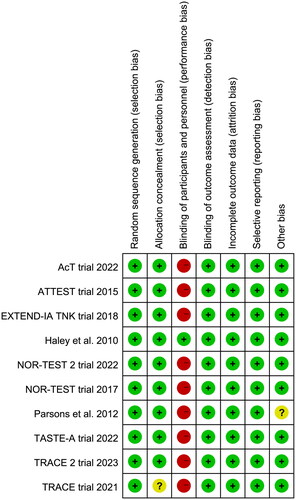
Table 2. Main findings and subgroup analysis.
In addition, the funnel plot and Egger’s test showed that there was no significant risk of publication bias (Supplementary Material 5).
Primary outcome
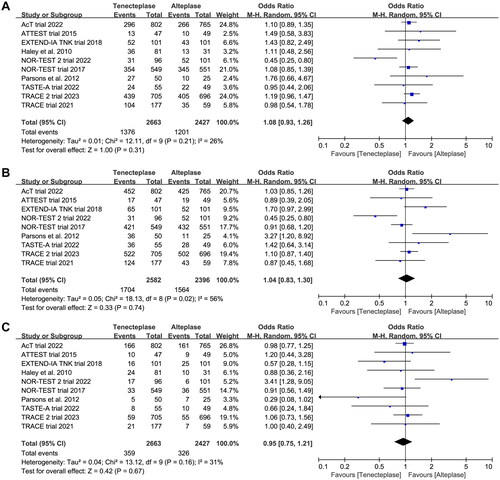
All included trials reported the mRS at 90 days. There was no significant difference between tenecteplase and alteplase in functional outcome at 90 days (, excellent: OR 1.08, 95%CI 0.93–1.26, I2 = 26%, ; good: OR 1.04, 95%CI 0.83–1.30, I2 = 56%, ; poor: OR 0.95, 95%CI 0.75–1.21, I2 = 31%, ). The pooled risk difference of functional outcome at 90 days was described as follows: excellent: 2% (95%CI −2% to 6%), good: 1% (95%CI −4% to 6%) and poor: 0% (95%CI −3% to 2%). The lower 95%CI bound fell within the non-inferiority margins of −15%, −10%, −6.5% and −5%, but crossed the most stringent non-inferiority margin of −1.3%.
Figure 3. Forest plot comparing the effect of tenecteplase versus alteplase on functional outcomes at 90-day: (A) excellent functional outcome, (B) good functional outcome and (C) poor functional outcome.
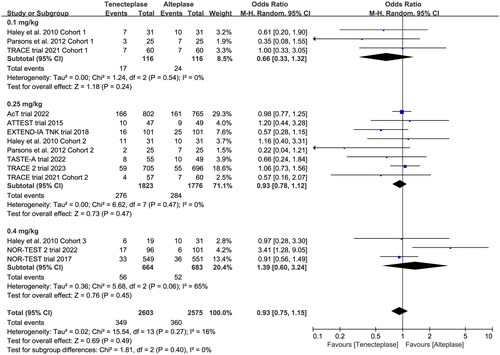
In the subgroup analysis (), we compared the effect of different dose of tenecteplase on the functional outcome at 90 days. The results indicated that the 0.25 mg/kg dose of tenecteplase was associated with greater rate of excellent functional outcome (OR 1.17, 95%CI 1.03–1.34, I2 = 0%, ). Though not statistically significant, compared with 0.4 mg/kg dose of tenecteplase, the point estimates of effect suggested that the 0.25 mg/kg dose of tenecteplase had potentially greater probability of good functional outcome (OR 1.20, 95%CI 0.95–1.53, I2 = 42%, ), and lower probability of poor functional outcome (OR 0.93, 95%CI 0.78–1.12, I2 = 0%, ). Furthermore, we performed a post-hoc subgroup analysis by the presence or not of a large vessel occlusion (LVO). The result indicated that among patients with LVO, the use of tenecteplase could result in significant improvement in excellent functional outcome at 90 days (OR 1.43, 95%CI 1.04–1.95, I2 = 0%, Supplementary Material 5).
Figure 4. Forest plot for the subgroup analysis stratified by the dose of tenecteplase on excellent functional outcome at 90 days.
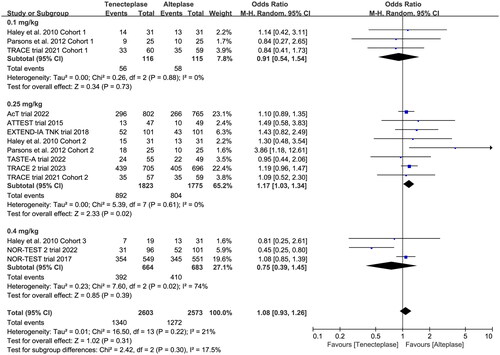
Figure 5. Forest plot for the subgroup analysis stratified by the dose of tenecteplase on good functional outcome at 90 days.
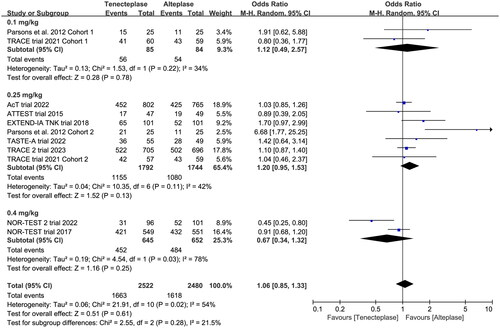
Figure 6. Forest plot for the subgroup analysis stratified by the dose of tenecteplase on poor functional outcome at 90 days.
The sensitivity analysis showed similar results to the overall analysis, indicating the good robustness (Supplementary Material 5).
The results of network meta-analysis are shown in Supplementary Material 6. Compared with alteplase, the 0.1 mg/kg, 0.25 mg/kg and 0.4 mg/kg doses of tenecteplase were not associated with greater rate of excellent, good or poor functional outcome. The ranking analysis results revealed that the hierarchy for efficacy in increasing excellent and good functional outcome was 0.25 mg/kg dose of tenecteplase > 0.1 mg/kg dose of tenecteplase > alteplase > 0.4 mg/kg dose of tenecteplase. The hierarchy for efficacy in increasing poor functional outcome was 0.4 mg/kg dose of tenecteplase > alteplase > 0.1 mg/kg dose of tenecteplase > 0.25 mg/kg dose of tenecteplase.
Secondary outcomes
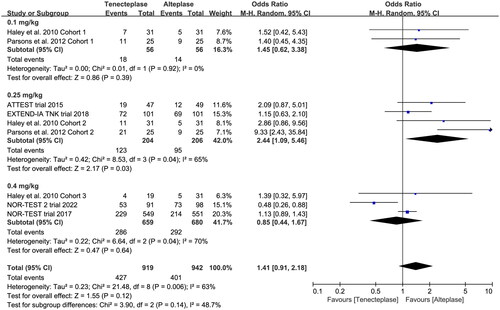
All included trials reported the incidence of SICH, and six trials reported the incidence of major neurological improvement within 72 h (). No treatment group differences in the outcomes of SICH (OR 1.12, 95%CI 0.79–1.59, I2 = 0%, ), and early major neurological improvement (OR 1.26, 95%CI 0.80–1.96, I2 = 65%, ) were detected. However, the subgroup analyses showed that the 0.4 mg/kg of tenecteplase was associated with higher risk of SICH (OR 1.72, 95%CI 0.89–3.29, I2 = 65%, ) than low-dose of tenecteplase (0.1 mg/kg: OR 0.81, 95%CI 0.23–2.87, I2 = 12%; 0.25 mg/kg: OR 1.01, 95%CI 0.68–1.52, I2 = 0%, ). Furthermore, the use of 0.25 mg/kg of tenecteplase was associated with better major neurological improvement (OR 2.44, 95%CI 1.09–5.46, I2 = 65%, ).
Figure 7. Forest plot comparing the effect of tenecteplase versus alteplase on (A) symptomatic intracranial haemorrhage and (B) major neurological improvement.
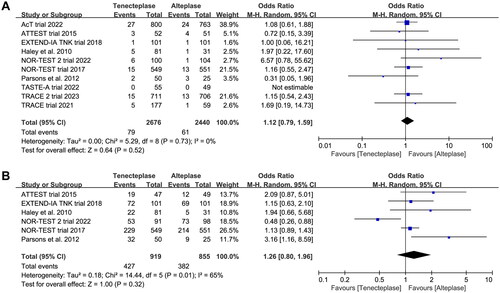
Figure 8. Forest plot for the subgroup analysis stratified by the dose of tenecteplase on symptomatic intracranial haemorrhage.
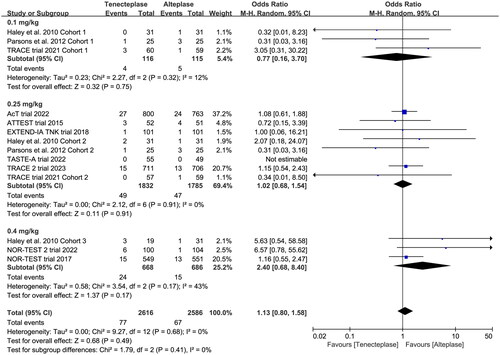
Figure 9. Forest plot for the subgroup analysis stratified by the dose of tenecteplase on major neurological improvement.
In addition, the sensitivity analysis showed similar results to the overall analysis, indicating the good robustness (Supplementary Material 5).
The results of network meta-analysis are shown in Supplementary Material 6. Compared with alteplase, the 0.1 mg/kg, 0.25 mg/kg and 0.4 mg/kg dose of tenecteplase were not associated with greater rate of SICH or major neurological improvement. The ranking analysis results revealed that the hierarchy for efficacy in increasing major neurological improvement was 0.25 mg/kg dose of tenecteplase > 0.1 mg/kg dose of tenecteplase > alteplase > 0.4 mg/kg dose of tenecteplase. The hierarchy for efficacy in increasing SICH was 0.4 mg/kg dose of tenecteplase > alteplase > 0.1 mg/kg dose of tenecteplase > 0.25 mg/kg dose of tenecteplase.
Discussion
In the meta-analysis, we analysed 10 RCTs with 5123 patients to compare the benefits and risks of tenecteplase versus alteplase for the treatment of AIS. The preliminary analysis showed that there were no significant differences on functional outcome at 90 days, SICH and early major neurological improvement between patients with AIS receiving tenecteplase or alteplase. However, the results of subgroup analyses indicated that the dose of 0.25 mg/kg of tenecteplase had greater probability of excellent functional outcome at 90 days, and better major neurological improvement when compared to the dose of 0.4 mg/kg of tenecteplase. Furthermore, the post hoc subgroup analysis further confirmed the beneficial effect of tenecteplase in patients with LVO.
To the best of knowledge, this study is the most up-to-date and comprehensive meta-analysis of randomized evidence to compare the value of tenecteplase versus alteplase in AIS treatment. A total of 10 RCTs with 5123 patients (2677 patients in the tenecteplase group and 2446 patients in the alteplase group) were finally analysed, including the latest RCT [Citation16] published in 2023. The results of our research are approximately consistent with the previous meta-analyses that the tenecteplase had similar efficacy and safety as alteplase [Citation27–32]. A previous meta-analysis which included six non-randomized studies suggested that patients treated with tenecteplase had higher recanalization rates and better 3-month good functional outcome than those receiving alteplase. Moreover, Ma et al. [Citation28] analysed eight RCTs and six non-randomized studies, indicated that there was no significant difference in the functional outcomes at 3-months between patients treated with tenecteplase or alteplase, but the use of tenecteplase might improve early neurological recovery. Recently, Kobeissi et al. [Citation32] performed the latest meta-analysis by enrolling nine RCTs, further demonstrated the safety and efficacy of tenecteplase for the treatment of AIS.
However, compared with previous meta-analyses, our study included the latest available RCT [Citation16], in which more than 1400 patients with AIS were included. Furthermore, the subgroup analysis found that patients receiving 0.25 mg/kg of tenecteplase were associated with higher rates of 90-day excellent outcome and early neurological improvement, whereas 0.40 mg/kg of tenecteplase had significantly higher rates of SICH.
In 2021, the European Stroke Organization (ESO) guidelines [Citation33] suggested that for patients with AIS within 4.5 h of stroke onset and not eligible for mechanical thrombectomy, the use of intravenous thrombolysis with alteplase was superior to tenecteplase. Among patients with LVO and AIS within 4.5 h of stroke onset, the guidelines [Citation33] recommended intravenous thrombolysis with 0.25 mg/kg of tenecteplase over alteplase. Nevertheless, for the treatment of AIS, the optimal dose of tenecteplase has not yet to be well defined at present [Citation34]. Most of previous studies focused on the dose range of tenecteplase from 0.1 to 0.4 mg/kg, and the commonly used dose was 0.25 mg/kg. In our meta-analysis, the subgroup analyses suggested that the tenecteplase at 0.25 mg/kg had better efficacy, including early neurologic improvement and better functional outcomes at 90-day. Recently, researchers conducted the first head-to-head RCT (EXTEND-IA TNK Part 2 trial [Citation11]) to directly compare the tenecteplase at a dose of 0.40 mg/kg with 0.25 mg/kg among patients with ischemic stroke. The results of the EXTEND-IA TNK Part 2 trial suggested that the use of 0.40 mg/kg of tenecteplase did not significantly improve cerebral reperfusion and neurological function outcome. The events of haemorrhages were instead significantly raised. Meanwhile, the recent NOR-TEST 2 trial [Citation13] reported an unexpected result that compared with standard dose of alteplase, the use of tenecteplase at a dose of 0.4 mg/kg yielded worse functional and safety outcomes for patients with moderate or severe ischemic stroke. Although one possible explanation for the discrepancy might be related to the imbalance in baseline characteristics (such as age, baseline mRS score, proportion of patients with LVO) between treatment groups, the adjusted analysis performed still showed worse functional and safety outcomes for patients receiving tenecteplase [Citation13]. In an individual patient data (IPD) meta-analysis [Citation35] of three RCTs, the pooled results indicated that the use of 0.25 mg/kg of tenecteplase was associated with better early neurological improvement and functional outcomes than other dosages. Moreover, the latest meta-analysis [Citation32] also concluded similar results. Therefore, existing evidence exists to support the use of tenecteplase at a dose of 0.25 mg/kg.
However, our study has several limitations. First of all, a considerable part of included trials included in our meta-analysis had a relatively small sample size (number of participants <100 per arm). The results may be affected by small-study effects and obtain greater beneficial treatment effects conclusion [Citation36]. Second, substantial heterogeneity was observed across the included studies, which limited the credibility of our conclusions. The potential source of heterogeneity may be the variation in treatment windows and dosage of tenecteplase. Third, the definition of SICH differed between included studies. Such minor differences might affect the frequency of bleeding rates across different studies. Furthermore, the results of subgroup analysis should be interpreted with caution because of the limited sample size in corresponding subgroups. Thus, the small number of trials and patients decreases the power of our evidence in the 0.1 and 0.4 mg/kg groups.
Conclusions
In this updated meta-analysis of 10 RCTs, we found that patients receiving tenecteplase had similar rates functional outcomes at 90-day when compared with alteplase. Furthermore, there were no differences between tenecteplase and alteplase for safety outcome such as symptomatic intracerebral haemorrhage. However, tenecteplase at a dose of 0.25 mg/kg was associated with greater odds of early major neurological improvement and higher rate of excellent functional outcome at 90 days. Further investigation of tenecteplase in AIS patients is warranted.
Author contributions
Jian Huang conceived the idea, performed the analysis, and drafted the initial draft writing of this paper. Hui Zheng and Xianfeng Zhu contributed to the collection and interpretation of data. Kai Zhang provided technical support and helped to draft the work. Xiaofeng Ping contributed to the revision of this paper, and the final approval of the version to be published. All authors contributed to the article and approved the submitted version.
Supplemental Material
Download Zip (2.3 MB)Acknowledgements
Registration: The protocol was preregistered in Open Science Framework (https://osf.io/y7whf).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The original contributions presented in the study are included in the article and Supplementary Material; further inquiries can be directed to the corresponding author.
Additional information
Funding
References
- Ospel JM, Holodinsky JK, Goyal M. Management of acute ischemic stroke due to large-vessel occlusion: JACC focus seminar. J Am Coll Cardiol. 2020;75(15):1–12. doi: 10.1016/j.jacc.2019.10.034.
- Coutts SB, Berge E, Campbell BC, et al. Tenecteplase for the treatment of acute ischemic stroke: a review of completed and ongoing randomized controlled trials. Int J Stroke. 2018;13(9):885–892. doi: 10.1177/1747493018790024.
- Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156–2162. doi: 10.1161/STROKEAHA.119.025080.
- Kheiri B, Osman M, Abdalla A, et al. Tenecteplase versus alteplase for management of acute ischemic stroke: a pairwise and network meta-analysis of randomized clinical trials. J Thromb Thrombolysis. 2018;46(4):440–450. doi: 10.1007/s11239-018-1721-3.
- Keyt BA, Paoni NF, Refino CJ, et al. A faster-acting and more potent form of tissue plasminogen activator. Proc Natl Acad Sci U S A. 1994;91(9):3670–3674. doi: 10.1073/pnas.91.9.3670.
- Benedict CR, Refino CJ, Keyt BA, et al. New variant of human tissue plasminogen activator (TPA) with enhanced efficacy and lower incidence of bleeding compared with recombinant human TPA. Circulation. 1995;92(10):3032–3040. doi: 10.1161/01.cir.92.10.3032.
- Nepal G, Kharel G, Ahamad ST, et al. Tenecteplase versus alteplase for the management of acute ischemic stroke in a low-income country-Nepal: cost, efficacy, and safety. Cureus. 2018;10(2):e2178. doi: 10.7759/cureus.2178.
- Tanswell P, Modi N, Combs D, et al. Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet. 2002;41(15):1229–1245. doi: 10.2165/00003088-200241150-00001.
- Gao L, Moodie M, Mitchell PJ, et al. Cost-effectiveness of tenecteplase before thrombectomy for ischemic stroke. Stroke. 2020;51(12):3681–3689. doi: 10.1161/STROKEAHA.120.029666.
- Coutts SB, Dubuc V, Mandzia J, et al. Tenecteplase-tissue-type plasminogen activator evaluation for minor ischemic stroke with proven occlusion. Stroke. 2015;46(3):769–774. doi: 10.1161/STROKEAHA.114.008504.
- Campbell BCV, Mitchell PJ, Churilov L, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND-IA TNK part 2 randomized clinical trial. JAMA. 2020;323(13):1257–1265. doi: 10.1001/jama.2020.1511.
- Bivard A, Zhao H, Churilov L, et al. Comparison of tenecteplase with alteplase for the early treatment of ischaemic stroke in the Melbourne Mobile Stroke Unit (TASTE-A): a phase 2, randomised, open-label trial. Lancet Neurol. 2022;21(6):520–527. doi: 10.1016/S1474-4422(22)00171-5.
- Kvistad CE, Næss H, Helleberg BH, et al. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, non-inferiority trial. Lancet Neurol. 2022;21(6):511–519. doi: 10.1016/S1474-4422(22)00124-7.
- Li S, Pan Y, Wang Z, et al. Safety and efficacy of tenecteplase versus alteplase in patients with acute ischaemic stroke (TRACE): a multicentre, randomised, open label, blinded-endpoint (PROBE) controlled phase II study. Stroke Vasc Neurol. 2022;7(1):47–53. doi: 10.1136/svn-2021-000978.
- Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet. 2022;400(10347):161–169. doi: 10.1016/S0140-6736(22)01054-6.
- Wang Y, Li S, Pan Y, et al. Tenecteplase versus alteplase in acute ischaemic cerebrovascular events (TRACE-2): a phase 3, multicentre, open-label, randomised controlled, non-inferiority trial. Lancet. 2023;401(10377):645–654. doi: 10.1016/S0140-6736(22)02600-9.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(2):d5928. doi: 10.1136/bmj.d5928.
- Lin CH, Saver JL, Ovbiagele B, et al. Endovascular thrombectomy without versus with intravenous thrombolysis in acute ischemic stroke: a non-inferiority meta-analysis of randomized clinical trials. J Neurointerv Surg. 2022;14(3):227–232. doi: 10.1136/neurintsurg-2021-017667.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557.
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629.
- Haley ECJr., Thompson JL, Grotta JC, et al. Phase IIB/III trial of tenecteplase in acute ischemic stroke: results of a prematurely terminated randomized clinical trial. Stroke. 2010;41(4):707–711. doi: 10.1161/STROKEAHA.109.572040.
- Campbell BCV, Mitchell PJ, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573–1582. doi: 10.1056/NEJMoa1716405.
- Huang X, Cheripelli BK, Lloyd SM, et al. Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): a phase 2, randomised, open-label, blinded endpoint study. Lancet Neurol. 2015;14(4):368–376. doi: 10.1016/S1474-4422(15)70017-7.
- Logallo N, Novotny V, Assmus J, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (nor-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017;16(10):781–788. doi: 10.1016/S1474-4422(17)30253-3.
- Parsons M, Spratt N, Bivard A, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. 2012;366(12):1099–1107. doi: 10.1056/NEJMoa1109842.
- Katsanos AH, Psychogios K, Turc G, et al. Off-label use of tenecteplase for the treatment of acute ischemic stroke: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(3):e224506. doi: 10.1001/jamanetworkopen.2022.4506.
- Ma P, Zhang Y, Chang L, et al. Tenecteplase vs. alteplase for the treatment of patients with acute ischemic stroke: a systematic review and meta-analysis. J Neurol. 2022;269(10):5262–5271. doi: 10.1007/s00415-022-11242-4.
- Oliveira M, Fidalgo M, Fontão L, et al. Tenecteplase for thrombolysis in stroke patients: systematic review with meta-analysis. Am J Emerg Med. 2021;42:31–37. doi: 10.1016/j.ajem.2020.12.026.
- Katsanos AH, Safouris A, Sarraj A, et al. Intravenous thrombolysis with tenecteplase in patients with large vessel occlusions: systematic review and meta-analysis. Stroke. 2021;52(1):308–312. doi: 10.1161/STROKEAHA.120.030220.
- Thelengana A, Radhakrishnan DM, Prasad M, et al. Tenecteplase versus alteplase in acute ischemic stroke: systematic review and meta-analysis. Acta Neurol Belg. 2019;119(3):359–367. doi: 10.1007/s13760-018-0933-9.
- Kobeissi H, Ghozy S, Turfe B, et al. Tenecteplase vs. alteplase for treatment of acute ischemic stroke: a systematic review and meta-analysis of randomized trials. Front Neurol. 2023;14:1102463. doi: 10.3389/fneur.2023.1102463.
- Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021;6(1):I–LXII. doi: 10.1177/2396987321989865.
- Nair R, Wagner AN, Buck BH. Advances in the management of acute ischemic stroke. Curr Opin Neurol. 2023;36(2):147–154. doi: 10.1097/WCO.0000000000001136.
- Huang X, MacIsaac R, Thompson JL, et al. Tenecteplase versus alteplase in stroke thrombolysis: an individual patient data meta-analysis of randomized controlled trials. Int J Stroke. 2016;11(5):534–543. doi: 10.1177/1747493016641112.
- Zhang Z, Xu X, Ni H. Small studies may overestimate the effect sizes in critical care meta-analyses: a meta-epidemiological study. Crit Care. 2013;17(1):R2. doi: 10.1186/cc11919.