Abstract
Objective
Atrial fibrillation (AF), the most common cardiac arrhythmia, presents significant health challenges, and the intricate connection between insomnia and AF has garnered substantial attention. This cohort study aims to investigate the relationship between insomnia and AF recurrences following radiofrequency ablation.
Materials and Methods
Data were retrieved from an electronic database of patients who underwent radiofrequency ablation for AF. The primary endpoint was AF recurrence. We utilized a multivariable Cox model, coupled with three propensity score methods, for analysis.
Results
Between January 1, 2017, and June 1, 2022, 541 patients who underwent radiofrequency ablation for AF were recorded in the database. After excluding 185 patients, the final cohort comprised 356 patients. Among them, 68 were afflicted by insomnia, while 288 were not. Over a median follow-up of 755 days, one patient died, and 130 (36.5%) experienced AF recurrence. Multivariate Cox regression analysis revealed that the insomnia group had a higher risk of AF recurrence compared to the non-insomnia group (HR: 1.83, 95% CI: 1.16–2.89). Further landmark analysis showed no significant difference in AF recurrence rates during the initial 1-year follow-up. However, beyond 1 year, the insomnia group demonstrated a significantly higher AF recurrence rate. As the number of insomnia symptoms increased, the risk of AF recurrence also rose significantly, indicating a dose-response relationship.
Conclusion
This study establishes a significant link between insomnia and long-term AF recurrence following radiofrequency ablation. It underscores the importance of identifying and addressing insomnia in patients with AF undergoing radiofrequency ablation.
Introduction
Atrial fibrillation (AF) is currently a highly prevalent cardiac arrhythmia in clinical settings [Citation1] and often leads to elevated risks of ischemic stroke [Citation2], dementia [Citation3], heart failure [Citation4], and overall mortality [Citation4]. Although catheter ablation is already established as a primary treatment for symptomatic AF [Citation5], the significant recurrence rate following this procedure remains a pressing clinical challenge that requires immediate attention [Citation6].
Insomnia, a prevalent sleep disorder characterized by difficulties in initiating or maintaining sleep, subsequently resulting in symptoms such as daytime sleepiness and fatigue [Citation7], has been reported to have an incidence ranging from 6% to 33% in various epidemiological studies, indicating a significant proportion of individuals affected by insomnia globally [Citation8].
A growing body of evidence indicates a high prevalence of insomnia among patients with AF and its association with an elevated risk of AF [Citation9–12], and patients frequently complain that insomnia can trigger AF episodes [Citation13]. These findings collectively suggest a potential association between AF and insomnia. Nevertheless, the relationship between insomnia and the recurrence of AF after ablation remains uncertain. Given the established connection between insomnia and an elevated risk of AF, we posit that insomnia may also serve as a risk factor for post-ablation recurrence of AF. To examine this hypothesis, we conducted this retrospective cohort study.
Methods
This study is conducted at the Cardiology Department of the Second Hospital of Hebei Medical University. The research protocol was reviewed by the Ethics Committee of the Second Hospital of Hebei Medical University (Ethical Approval Number: 2022-R636). The database is anonymous; therefore, the requirement for informed consent was waived.
Data source and research participants
The data in this study were obtained from an existing electronic database of AF catheter ablation in our department. Previous documentation provided comprehensive information regarding this database [Citation14]. In summary, the database encompasses a wide range of demographic information, clinical diagnoses, laboratory tests, echocardiographic findings, concomitant medications, procedure parameters, and follow-up data of patients who had undergone AF radiofrequency catheter ablation procedures at our cardiac center from January 1, 2017, onwards. Follow-up appointments in our outpatient electrophysiological (EP) clinic were initially scheduled at intervals of 3, 6, and 12 months during the first year and subsequently at 6-month intervals. Additionally, unscheduled visits were made available at any point in time, as deemed necessary.
Participants were consecutively enrolled in the study between January 1, 2017, and June 1, 2022, if they underwent their initial AF catheter ablation procedure at our department. Follow-up was conducted until December 1, 2022. Patients with missing data related to insomnia or follow-up were excluded from the analysis.
AF ablation procedures
Following the confirmation of thrombi disappearance through transesophageal echocardiography or left atrial pulmonary vein CT scans within a 48-h timeframe prior to the procedure, ablation therapy was administered. The procedure was conducted under local anesthesia and mild sedation, utilizing the CARTO3 system (Biosense Webster, Diamond Bar, CA, USA) for three-dimensional electroanatomic left atrial reconstruction. Radiofrequency ablation was executed using a 3.5 mm saline-irrigated catheter (TheromoCool SmartTouchTM, Biosense Webster, Diamond Bar, CA, USA). Pulmonary vein isolation (PVI) was successfully achieved in all patients, with verification of isolation effectiveness conducted using a decapolar circular catheter (LassoTM) or a multielectrode catheter with 2-6-2 mm electrode spacing (PentaRay, Biosense Webster, Diamond Bar, CA, USA). It is at the discretion of the operating surgeon to determine whether additional ablation (superior vena cava isolation, crista terminalis line, mitral isthmus line, anterior line, roof line, posterior wall BOX isolation, substrate modification, and other ablation strategies) should be performed following PVI. In all cases of linear ablation, the bidirectional block of the ablation line was confirmed using diverse pacing strategies. This verification process was repeated 30 min after the operation. Patients who did not have any contraindications were prescribed antiarrhythmic drugs (Class I/III) within 3 months of undergoing AF catheter ablation. Those who did not experience a recurrence of atrial arrhythmia after 3 months discontinued the use of antiarrhythmic medications.
Definition of insomnia
The surgical department staff, who were not directly involved in patient treatment or follow-up, administered the insomnia symptom questionnaire [Citation15] to patients prior to the procedure. Presented below is the insomnia symptom questionnaire: 1. In the past three months, did you face any difficulties when attempting to initiate sleep (difficulty initiating sleep)? 2. In the past three months, did you frequently experience awakenings during the night (difficulty maintaining sleep)? 3. In the past three months, did you find yourself waking up prematurely and struggling to resume sleep (early morning awakening)? 4. In the past three months, did you awaken in the morning feeling adequately rested (nonrestorative sleep)?
Each question had three options: ‘Most of the time’, ‘Sometimes’ and ‘Rarely or never’. If patients responded ‘Most of the time’ to questions 1, 2, and 3 or ‘Rarely or never’ to question 4, they were considered to have insomnia symptoms. Patients with one or more insomnia symptoms were classified into the insomnia group, while others were assigned to the noninsomnia group. We also further inquired about the following questions: In the past three months, how many hours did you sleep each day? (Encompassing all periods of sleep within a day).
Outcome events
Outcome events pertain to the recurrence of AF following catheter ablation, which was defined as the occurrence of AF, atrial flutter, or atrial tachycardia lasting more than 30 s after a 3-month blanking period following catheter ablation ascertained by ECG or 24-h ambulatory ECG monitoring.
Covariates assessed
The variables related to AF were extracted from the electronic database as covariates. Additional details about these covariates are accessible in online supplemental file 1.
Statistical analysis
Continuous variables following a normal distribution reported as the mean (SD) and skewed data reported as the median (IQR). Intergroup comparisons were conducted using either Student’s t test or the Mann–Whitney U test. Categorical variables were expressed as frequencies (%), and intergroup comparisons were carried out using the χ2 test or Fisher’s exact test.
Cox regression was employed to examine the association between insomnia and AF recurrence. The follow-up period ranged from the date of the procedure to the occurrence of outcome events, death, or December 1, 2022, whichever came first. Schoenfeld residual test was utilized to assess the proportional hazards assumption, and no violations of the proportional hazard assumptions were observed. Consistent with the STROBE guidelines [Citation16], the study presented both unadjusted and adjusted findings. Initially, all analyses were adjusted for demographic factors such as age and gender (Model 1), and then a stepwise bidirectional model selection was performed using the Akaike Information Criterion (AIC) (Model 2) [Citation17]. Additionally, a fully adjusted multivariate Cox regression model was employed, incorporating all covariates, including demographic information, clinical diagnoses, laboratory tests, echocardiographic findings, concomitant medications, and procedure parameters (Model 3). The estimation of AF recurrence curves was carried out using Kaplan–Meier methods, and comparisons were made using the log-rank test. Moreover, we performed landmark analysis to assess the impact of insomnia on AF recurrence during different follow-up periods [Citation18].
To minimize the impact of confounding factors, three propensity score methodologies were utilized for further analyses. First, inverse probability of treatment weighting (IPTW) based on propensity score (PS) was used to mitigate the influence of confounding variables (online supplemental file 2). Second, propensity score matching (PSM) was used, employing nonreplacement nearest neighbor matching with a matching ratio of 1:2 and a caliper value of 0.25. The balance of covariates between the post-matched insomnia and noninsomnia groups was assessed using the standardized mean difference (SMD) [Citation19]. Finally, we incorporated the propensity score as an additional covariate for further adjustment.
Furthermore, we investigated the association between insomnia and AF recurrence within various subgroups. In the subgroup analysis, the interaction test was conducted using the likelihood ratio test. (online supplemental file 3)
A multivariate imputation approach based on chained equations using random forest was utilized to fill in missing covariate values [Citation20]. Five complete datasets were generated through multiple imputations, and the results presented in this study were derived from the first dataset, with consistent findings observed across the remaining datasets.
Restricted cubic spline analysis was employed to investigate potential nonlinear relationships between sleep duration and the risk of AF recurrence [Citation21]. Likelihood ratio tests were conducted to compare models containing cubic spline terms to those with solely linear terms, aiming to evaluate the presence of nonlinearity [Citation22]. Three nodes were positioned at the 10th, 50th, and 90th percentiles of sleep duration. Furthermore, a multivariable Cox proportional hazards model was utilized to evaluate the association between sleep duration and AF recurrence.
Sensitivity analysis: To address unmeasured confounding, we further computed the E-value to assess the potential impact of unmeasured confounders on the observed association between insomnia and AF recurrence after radiofrequency ablation [Citation23]. The E-value quantified the strength of an unmeasured confounder that would be needed to nullify the observed relationship.
Statistical analysis was conducted using R software version 4.1.2. A two-tailed p < 0.05 was considered statistically significant.
Results
Characteristics of the study cohort
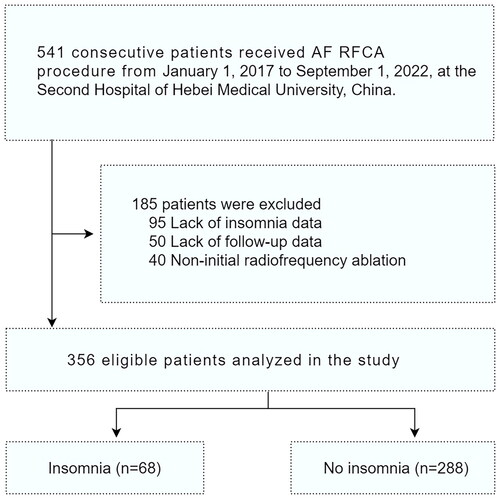
From January 1, 2017, to June 1, 2022, our database recorded 541 patients who underwent radiofrequency ablation for AF. We excluded 185 patients, including 95 with insufficient insomnia-related data, 50 with missing follow-up, and 40 who had undergone non-first radiofrequency ablation. The final cohort included 356 patients: 68 in the insomnia group and 288 in the noninsomnia group ().
Within our cohort, the prevalence of insomnia was 19.1%. Specifically, 49 patients (13.8%) had trouble initiating sleep, 34 (9.6%) had difficulty maintaining sleep, 41 (11.5%) experienced early morning awakenings, and 13 (3.7%) had nonrestorative sleep. Further analysis showed 22 patients (6.2%) with one insomnia symptom, 25 (7.0%) with two, 19 (5.3%) with three, and only 2 (0.6%) with four. The mean age was 62.44 years, with 44.4% female patients, paroxysmal AF was present in 64.6%. Further cohort details are in , and missing covariate information is in online supplemental file 4.
Table 1. Baseline characteristics of the included participants.
The odds ratios (ORs) and 95% confidence intervals (CIs) for all variables included in the propensity score logistic regression model are showed in online supplemental file 5. The c-statistic of the propensity score logistic regression model, which is 0.712, is available in online supplemental file 6. The propensity score distributions before and after PSM are presented in online supplemental file 7.
After PSM, successful pairing is achieved for 195 patients, with 66 in the insomnia group and 129 in the noninsomnia group. Differences in variables between the matched groups are minimized, as shown in online supplemental file 8. The depiction of the study population after PSM and IPTW is presented in .
Relationship between insomnia and AF recurrence
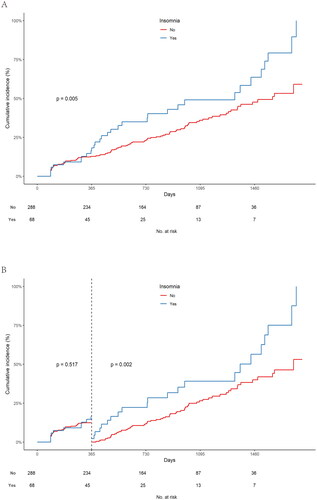
The median follow-up for the 356 patients was 755 days (max: 1780 days). During this period, one patient died, and 130 (36.5%) experienced AF recurrence. shows that the insomnia group had a significantly higher cumulative AF recurrence rate than the noninsomnia group (log-rank test, p = 0.005). Landmark analysis indicated no statistically significant difference in AF recurrence rates during the initial 1-year follow-up (log-rank test, p = 0.517). However, beyond 1 year, the insomnia group had a notably higher AF recurrence rate (log-rank test, p = 0.002) (). Kaplan–Meier curves based on PSM and IPTW supported these findings (online supplemental file 9 and online supplemental file 10).
Figure 2. Kaplan–Meier plot estimates of the rate of recurrence of atrial fibrillation according to insomnia group. A. Event rates of recurrence of atrial fibrillation in the insomnia and noninsomnia groups; B. Landmark analysis discriminating between events occurring prior to and subsequent to the one-year follow-up period.
To further elucidate the relationship between insomnia and AF recurrence following radiofrequency ablation, we utilized multivariable Cox regression analysis to control for potential confounding variables. In the initial unadjusted model, the insomnia group demonstrated a significantly higher likelihood of experiencing endpoint events than the noninsomnia group (HR: 1.77, 95% CI: 1.18–2.64). After adjusting for age and sex, the presence of insomnia remained linked to an increased risk of AF recurrence (HR: 1.73, 95% CI: 1.16–2.59). Consistent findings were obtained across various adjustment models and three propensity score methods, as shown in . Additionally, landmark analysis was conducted using the multivariable Cox regression model. Similar to the survival analysis, no significant association was observed between insomnia and AF recurrence rate within 1 year after radiofrequency ablation. However, when the follow-up duration exceeded 1 year, a noteworthy correlation between insomnia and the risk of AF recurrence became apparent (HR for follow-up within 1 year: 1.14, 95% CI: 0.54–2.41; HR for follow-up beyond 1 year: 2.21, 95% CI: 1.18–4.16) ( and online supplemental file 11). Similarly, multivariable Cox regression analysis based on other datasets derived from multiple imputation generated comparable outcomes (online supplemental file 12).
Table 2. Associations between insomnia and recurrence after radiofrequency catheter ablation for atrial fibrillation in the crude, multivariate and PS analyses.
Dose–response relationship between insomnia and AF recurrence
In comparison to individuals without insomnia symptoms, patients exhibiting one insomnia symptom demonstrated a noticeable upward trend in the likelihood of AF recurrence (HR: 1.39, 95% CI: 0.65–2.93). Patients with two insomnia symptoms displayed an HR of 1.99 (95% CI: 0.99–4.00), while those with three or four insomnia symptoms exhibited the highest risk of AF recurrence (HR: 2.25, 95% CI: 1.10–4.59). Furthermore, for each additional symptom of insomnia, the risk of AF recurrence increased by 34% (HR: 1.34, 95% CI: 1.09–1.64, p = 0.005) (). Individual analyses indicated that difficulty initiating sleep, difficulty maintaining sleep, early morning awakening, and nonrestorative sleep were all significantly correlated with a higher risk of AF recurrence, as presented in .
Table 3. Dose–response relationship between insomnia symptoms and events of atrial fibrillation recurrence.
Subgroup analysis
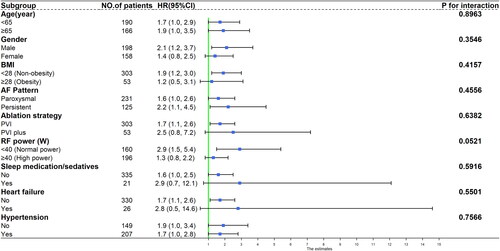
When considering various subgroups based on age (<65 and ≥65 years), sex (male and female), BMI (<28 and ≥28), AF type (paroxysmal and persistent), ablation strategy (PVI and PVI + additional ablation), ablation power (<40 W and ≥40 W), long-term use of sleep medication, and the presence of hypertension or heart failure, the association between insomnia and increased risk of AF recurrence did not attain statistical significance within specific subgroups due to limited sample sizes. However, a consistent trend of increased risk of AF recurrence was observed in relation to insomnia across the various subgroups (interaction test P values >0.05) ().
Relationship between sleep duration and AF recurrence
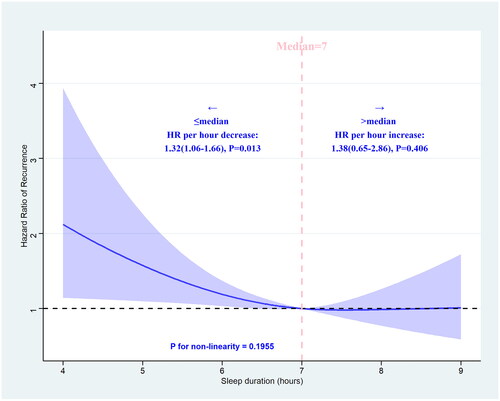
Restricted cubic splines were utilized to illustrate the correlation between sleep duration and the recurrence of AF. When sleep duration was equal to or less than the median sleep duration of 7 h, a decrease in sleep duration was found to be significantly associated with an increased risk of AF recurrence (HR: 1.32, 95% CI: 1.06–1.66, p = 0.013). Conversely, when sleep duration exceeded 7 h, no significant association was observed with an elevated risk of AF recurrence (HR: 1.38, 95% CI: 0.65–2.86, p = 0.406) (). Then, we incorporated sleep duration into a multivariate Cox regression model, considering it both as a continuous and categorical variable. The findings revealed a notable decrease in the likelihood of AF recurrence as sleep duration extended (HR: 0.86, 95% CI: 0.74–0.99) (online supplemental file 13).
Figure 4. Association between sleep duration and recurrence of atrial fibrillation after radiofrequency catheter ablation using a restricted cubic spline regression model.
The adjustment strategy is the same as Model 3 in . P value for the association between sleep duration and recurrence of AF based on restricted cubic spline regression model = 0.0444 (chi-square= 6.23, df = 2). The blue ribbon represents the 95% CI for the spline model. Reference lines for no association are indicated by the dashed black lines at an HR of 1.0.
Sensitivity analysis
The resulting E-value of 2.40 suggested that an unmeasured confounder with a correlation of at least 2.40 with both the risk of AF recurrence and the relative risk of insomnia would be necessary to account for the observed association between insomnia and increased risk of AF recurrence (online supplemental file 14). In the multivariable Cox regression model (Model 3), the only measured risk factor for post-ablation AF recurrence that surpassed the E-value was the relative hazard of long-standing persistent AF (online supplemental file 15). This implied that the correlation observed between insomnia and AF recurrence might be negated solely if there existed an unmeasured confounding factor that was more influential than long-standing persistent AF.
Discussion
In the cohort of patients who undergo primary radiofrequency ablation for AF, the following discoveries are derived: 1. Insomnia is primarily linked to a heightened risk of long-term AF recurrence after ablation, while no significant correlation is observed with short-term recurrence during the initial year; 2. Insomnia exhibits a dose-response relationship with AF recurrence, indicating that an increased number of insomnia symptoms is associated with a heightened probability of AF recurrence. 3. When the duration of sleep is equal to or less than 7 h, there is an increase in the risk of AF recurrence as sleep duration decreases. Conversely, if the duration of sleep exceeds 7 h, there is no significant correlation between sleep duration and the risk of AF recurrence.
Currently, there is a lack of relevant research on the connection between insomnia and AF recurrence after radiofrequency ablation. However, the association between insomnia and the occurrence of AF has been established. In a cross-sectional study, Han et al. examined a sample of 8371 Chinese individuals and discovered a potential correlation between insomnia and an elevated risk of AF [Citation9]. Similarly, Christensen et al. observed an association between insomnia and an increased likelihood of AF occurrence, independent of the influence of obstructive sleep apnea (OSA) [Citation12]. Chen et al.’s investigation further substantiated a robust connection between sleep disorders and the onset of AF, with insomnia exhibiting the most pronounced impact compared to other sleep disorders [Citation24]. Additional research has demonstrated a correlation between insomnia [Citation25] and excessive daytime sleepiness [Citation26] with an elevated susceptibility to AF, whereas the adoption of healthy sleep practices has been found to be associated with a diminished risk of AF [Citation27].
The association between insomnia and an augmented risk of AF can potentially be elucidated through various mechanisms. Studies have indicated that acute sleep deprivation is linked to heightened P-wave dispersion, QT interval dispersion, and intra/interatrial conduction delay, all of which contribute to the pathogenesis of AF [Citation28–30]. Insomnia may disrupt the autonomic balance between the sympathetic and parasympathetic nervous systems [Citation31], leading to significant electrophysiological heterogeneity in atrial tissue and ultimately contributing to the development of atrial arrhythmias. Insomnia is characterized as a condition characterized by elevated arousal disorder, which leads to an increase in activity within the hypothalamic–pituitary–adrenal axis and sympathetic nervous system. Ultimately, these physiological responses contribute to atrial remodeling, fibrosis, and a decrease in atrial myocardium [Citation32]. These mechanisms are likely to coexist and potentially interact synergistically, rather than being mutually exclusive, in inducing AF. The specific mechanisms underlying the increased susceptibility to AF recurrence following radiofrequency ablation in individuals with insomnia are not yet fully understood. However, we hypothesize that the aforementioned mechanisms may also play a role in the occurrence of post-ablation AF recurrence in individuals with insomnia. Our results signify that insomnia is linked an increased likelihood of long-term AF recurrence after ablation, while no significant correlation is observed with short-term recurrence during the initial year. This suggests that the mechanisms responsible for varying periods of recurrence following AF radiofrequency ablation may exhibit differences. It’s possible that the atrial remodeling and fibrosis caused by insomnia take some time to modify the atrial substrate in AF patients, increasing the likelihood of long-term recurrence.
Our analysis has revealed a notable correlation between insomnia and AF recurrence. However, the observational nature of the study restricts the capacity to establish definitive causal relationships and ascertain the direction of the observed correlation. Thus, it is conceivable that insomnia could potentially play a role in or be a consequence of AF recurrence. Nevertheless, the temporal sequence observed in our cohort study, with insomnia preceding AF recurrence following radiofrequency ablation, suggests a potential cause-effect relationship. Moreover, the aforementioned impacts of insomnia on atrial electrophysiological characteristics, the autonomic nervous system, atrial remodeling, and fibrosis offer a theoretical rationale for insomnia as a potential causal factor in the recurrence of atrial fibrillation post-radiofrequency ablation. Additionally, we utilize multivariable Cox regression analysis and three propensity score methods, contributing to a more accurate estimation of the causal effect between insomnia and AF recurrence.
The findings of this study indicate a positive correlation between the number of insomnia symptoms and AF recurrence. The existence of a dose–response relationship further strengthens the potential causal connection between insomnia and post-ablation AF recurrence. This observation may be attributed to the complex and potentially compensatory interrelationships among sleep behaviors. For instance, the presence of satisfying deep sleep may serve as a compensatory mechanism for difficulty initiating sleep. However, if this is accompanied by early awakening and poor sleep quality, these compensatory mechanisms may be lacking, leading to a significant increase in the risk of AF recurrence.
The relationship between sleep duration and the occurrence of AF remains a topic of debate. A previous cohort study conducted on a sample of 18,775 male physicians in the United States, with an average follow-up period of 6.9 years, revealed that deviations from normal sleep duration, both longer and shorter, were correlated with an elevated probability of AF [Citation33]. However, Christensen MA’s study did not identify sleep duration as a risk factor for AF incidence [Citation12]. In a meta-analysis encompassing seven cohort studies and three cross-sectional studies involving a total of 14,296,314 patients, no significant association was found between sleep duration and AF. Nevertheless, the analysis did suggest a potential connection between AF and frequent nocturnal awakenings [Citation11]. Morovatdar et al. conducted a meta-analysis involving 6 studies and 186323 participants, which indicated that both excessively short and long sleep durations are associated with AF. However, the risk appeared to be higher with shorter sleep duration [Citation34].
Currently, there is a lack of research examining the relationship between sleep duration and AF recurrence following radiofrequency ablation. The results of our study suggest that there is no significant change in the rate of post-ablation AF recurrence as sleep duration exceeds 7 h per day. However, a notable association was observed between sleeping less than 7 h per day and an increased risk of AF recurrence. This discovery implies that insufficient sleep could be more detrimental than excessive sleep when it comes to the recurrence of AF.
Our findings hold significant clinical implications. Current research results indicate a significant association between insomnia and future AF recurrence in patients undergoing AF radiofrequency ablation, suggesting that insomnia could serve as a potential therapeutic target for preventing and reducing post-ablation AF recurrence. Moreover, our study possesses noteworthy merit, as it is an inaugural cohort study to explore the correlation between insomnia and the recurrence of AF following radiofrequency ablation.
Limitations
This study also has several limitations. Initially, the present study relied on subjective sleep questionnaires to ascertain the manifestation of insomnia symptoms without employing polysomnography as an objective measure of sleep. However, polysomnography is not commonly employed for evaluating insomnia due to its inability to objectively quantify difficulties in sleep initiation, maintenance, and quality [Citation35]. Since we did not objectively measure insomnia, we had insufficient data regarding the prevalence of obstructive sleep apnea (OSA), a recognized risk factor for AF [Citation36]. Although direct adjustment for OSA was not feasible in our study, meticulous adjustments and stratified analysis were made for other patient characteristics, including age, hypertension, and BMI, which are positive associated with OSA. Hence, it is unlikely that OSA alone could explain the elevated risk of AF recurrence in the insomnia population.
Moreover, a potential correlation between insomnia and psychological disorders, particularly depression, exists. However, our database lacks data on depression, which could serve as a confounding variable in the heightened risk of AF recurrence linked to insomnia. Nevertheless, we conducted a sensitivity analysis employing the E-value to assess the potential influence of unmeasured confounders and determined that unaccounted factors, including depression, were improbable explanations for the association between insomnia and AF recurrence. Third, it should be noted that certain variables in our dataset were found to be missing. However, we took measures to address this issue by utilizing multiple imputation techniques to fill in the missing data, thereby reducing the potential biases. Additionally, it is important to acknowledge that the single-center design of our study may restrict the generalizability of our findings. Furthermore, considering the possibility of asymptomatic AF recurrence, the utilization of implantable recorders could enhance the detection rate of such recurrences. Consequently, it is plausible that misclassification in identifying AF recurrence may exist, and this potential misclassification of outcome events could introduce a bias toward null results, potentially underestimating the association between insomnia and AF recurrence risk. Therefore, the true association between insomnia and AF recurrence might be stronger than what we observed. Last, it is worth noting that the baseline insomnia status may undergo alterations during long-term follow-up. In this particular study, the evaluation of insomnia symptoms was conducted solely prior to the operation, thus impeding the examination of the potential influence of changes in insomnia severity over time.
Conclusions
In conclusion, this study establishes a significant link between insomnia and long-term AF recurrence following radiofrequency ablation. This underscores the critical importance of promptly identifying and comprehensively addressing insomnia in patients with AF undergoing radiofrequency ablation.
Authors contributions
JZ and XC acquired the data, and RL performed the statistical analyses, interpreted the data, and drafted and revised the manuscript. WC interpreted the data, designed the study, revised the manuscript for important intellectual content and approved the final version. All authors have read and approved the manuscript.
| Abbreviations | ||
| AAD | = | antiarrhythmic drugs |
| ACEI | = | angiotensin-converting enzyme inhibitors |
| AF | = | atrial fibrillation |
| AFL | = | atrial flutter |
| AIC | = | Akaike Information Criterion |
| ARB | = | angiotensin-receptor blocker |
| AT | = | atrial tachycardia |
| BMI | = | body mass index |
| CAD | = | coronary artery disease |
| CCB | = | calcium channel blocker |
| CI | = | confidence interval |
| CTI | = | cavotricuspid isthmus |
| HF | = | heart failure |
| HGB | = | hemoglobin |
| HR | = | hazard ratio |
| IPTW | = | inverse probability treatment weighting |
| LAD | = | left atrial diameter |
| LVEF | = | left ventricular ejection fraction |
| OR | = | odds ratio |
| IQR | = | interquartile range |
| PSM | = | propensity score matching |
| PVI | = | pulmonary vein isolation |
| RFCA | = | radiofrequency catheter ablation |
| SD | = | standard deviation |
| SMD | = | standardized mean difference |
| TC | = | total cholesterol |
Supplemental Material
Download MS Word (578.8 KB)Acknowledgements
The authors also thank Yi-chen Li for data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Availability of data and materials
The relevant raw data of this article will be made available upon request by the authors.
Additional information
Funding
References
- Bai Y, Wang YL, Shantsila A, et al. The global burden of atrial fibrillation and stroke: a systematic review of the clinical epidemiology of atrial fibrillation in Asia. Chest. 2017;152(4):1–12. doi:10.1016/j.chest.2017.03.048.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the framingham study. Stroke. 1991;22(8):983–988. doi:10.1161/01.str.22.8.983.
- Singh-Manoux A, Fayosse A, Sabia S, et al. Atrial fibrillation as a risk factor for cognitive decline and dementia. Eur Heart J. 2017;38(34):2612–2618. doi:10.1093/eurheartj/ehx208.
- Vermond RA, Geelhoed B, Verweij N, et al. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community-based study from The Netherlands. J Am Coll Cardiol. 2015;66(9):1000–1007. doi:10.1016/j.jacc.2015.06.1314.
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the european association for Cardio-Thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the european society of cardiology (ESC) developed with the special contribution of the european heart rhythm association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi:10.1093/eurheartj/ehaa612.
- Natale A, Reddy VY, Monir G, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol. 2014;64(7):647–656. doi:10.1016/j.jacc.2014.04.072.
- Buysse DJ. Insomnia. JAMA. 2013;309(7):706–716. doi:10.1001/jama.2013.193.
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi:10.1053/smrv.2002.0186.
- Han X, Yang Y, Chen Y, et al. Association between insomnia and atrial fibrillation in a chinese population: a cross-sectional study. Clin Cardiol. 2017;40(9):765–769. doi:10.1002/clc.22731.
- Lee HH, Chen YC, Chen JJ, et al. Insomnia and the risk of atrial fibrillation: a population-based cohort study. Acta Cardiol Sin. 2017;33(2):165–172.
- Chokesuwattanaskul R, Thongprayoon C, Sharma K, et al. Associations of sleep quality with incident atrial fibrillation: a meta-analysis. Intern Med J. 2018;48(8):964–972. doi:10.1111/imj.13764.
- Christensen MA, Dixit S, Dewland TA, et al. Sleep characteristics that predict atrial fibrillation. Heart Rhythm. 2018;15(9):1289–1295. doi:10.1016/j.hrthm.2018.05.008.
- Verrier RL, Josephson ME. Impact of sleep on arrhythmogenesis. Circ Arrhythm Electrophysiol. 2009;2(4):450–459. doi:10.1161/CIRCEP.109.867028.
- Li RB, Yang XH, Zhang JD, et al. The association between subclinical thyroid dysfunction and recurrence of atrial fibrillation after catheter ablation. Front Cardiovasc Med. 2022;9:902411. doi:10.3389/fcvm.2022.902411.
- Li Y, Zhang X, Winkelman JW, et al. Association between insomnia symptoms and mortality: a prospective study of U.S. men. Circulation. 2014;129(7):737–746. doi:10.1161/CIRCULATIONAHA.113.004500.
- Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. doi:10.1371/journal.pmed.0040297.
- Ho AS, Luu M, Barrios L, et al. Incidence and mortality risk spectrum across aggressive variants of papillary thyroid carcinoma. JAMA Oncol. 2020;6(5):706–713. doi:10.1001/jamaoncol.2019.6851.
- Maeng M, Tilsted HH, Jensen LO, et al. Differential clinical outcomes after 1 year versus 5 years in a randomised comparison of zotarolimus-eluting and sirolimus-eluting coronary stents (the SORT oUT III study): a multicentre, open-label, randomised superiority trial. Lancet. 2014;383(9934):2047–2056. doi:10.1016/S0140-6736(14)60405-0.
- Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387–398. doi:10.1016/s0895-4356(00)00321-8.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399. doi:10.1002/sim.4067.
- Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. doi:10.1002/sim.4780080504.
- Keum N, Bao Y, Smith-Warner SA, et al. Association of physical activity by type and intensity with digestive system cancer risk. JAMA Oncol. 2016;2(9):1146–1153. doi:10.1001/jamaoncol.2016.0740.
- Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602–603. doi:10.1001/jama.2018.21554.
- Chen CC, Lin CH, Yang TY, et al. Association between sleep disorder and atrial fibrillation: a nationwide population-based cohort study. Sleep Med. 2022;96:50–56. doi:10.1016/j.sleep.2022.05.002.
- Song Q, Liu X, Hu W, et al. Long sleep duration is an independent risk factor for incident atrial fibrillation in a chinese population: a prospective cohort study. Sci Rep. 2017;7(1):3679. doi:10.1038/s41598-017-04034-8.
- Full KM, Lutsey PL, Norby FL, et al. Association between excessive daytime sleepiness and measures of supraventricular arrhythmia burden: evidence from the atherosclerosis risk in communities (ARIC) study. Sleep Breath. 2020;24(3):1223–1227. doi:10.1007/s11325-020-02046-9.
- Li X, Zhou T, Ma H, et al. Healthy sleep patterns and risk of incident arrhythmias. J Am Coll Cardiol. 2021;78(12):1197–1207. doi:10.1016/j.jacc.2021.07.023.
- Esen Ö, Akçakoyun M, Açar G, et al. Acute sleep deprivation is associated with increased atrial electromechanical delay in healthy young adults. Pacing Clin Electrophysiol. 2011;34(12):1645–1651. doi:10.1111/j.1540-8159.2011.03186.x.
- Ozer O, Ozbala B, Sari I, et al. Acute sleep deprivation is associated with increased QT dispersion in healthy young adults. Pacing Clin Electrophysiol. 2008;31(8):979–984. doi:10.1111/j.1540-8159.2008.01125.x.
- Sari I, Davutoglu V, Ozbala B, et al. Acute sleep deprivation is associated with increased electrocardiographic P-wave dispersion in healthy young men and women. Pacing Clin Electrophysiol. 2008;31(4):438–442. doi:10.1111/j.1540-8159.2008.01013.x.
- Zhong X, Hilton HJ, Gates GJ, et al. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol (1985). 2005;98(6):2024–2032. doi:10.1152/japplphysiol.00620.2004.
- Mullington JM, Haack M, Toth M, et al. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51(4):294–302. doi:10.1016/j.pcad.2008.10.003.
- Khawaja O, Sarwar A, Albert CM, et al. Sleep duration and risk of atrial fibrillation (from the physicians’ health study). Am J Cardiol. 2013;111(4):547–551. doi:10.1016/j.amjcard.2012.10.038.
- Morovatdar N, Ebrahimi N, Rezaee R, et al. Sleep duration and risk of atrial fibrillation: a systematic review. J Atr Fibrillation. 2019;11(6):2132.
- Littner M, Hirshkowitz M, Kramer M, et al. Practice parameters for using polysomnography to evaluate insomnia: an update. Sleep. 2003;26(6):754–760. doi:10.1093/sleep/26.6.754.
- Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110(4):364–367. doi:10.1161/01.CIR.0000136587.68725.8E.