Abstract
Objective
To predict the incidence of postoperative ileus in bladder cancer patients after radical cystectomy.
Methods
We retrospectively analyzed the perioperative data of 452 bladder cancer patients who underwent radical cystectomy with urinary diversion at the Second Hospital of Tianjin Medical University between 2016 and 2021. Univariate and multivariate logistic regression were used to identify the risk factors for postoperative ileus. Finally, a nomogram model was established and verified based on the independent risk factors.
Results
Our study revealed that 96 patients (21.2%) developed postoperative ileus. Using multivariate logistic regression analysis, we found that the independent risk factors for postoperative ileus after radical cystectomy included age > 65.0 years, high or low body mass index, constipation, hypoalbuminemia, and operative time. We established a nomogram prediction model based on these independent risk factors. Validation by calibration curves, concordance index, and decision curve analysis showed a strong correlation between predicted and actual probabilities of occurrence.
Conclusion
Our nomogram prediction model provides surgeons with a simple tool to predict the incidence of postoperative ileus in bladder cancer patients undergoing radical cystectomy.
1. Introduction
Bladder cancer is the most common type of urological tumour and can be subclassified as non-muscle invasive bladder cancer (NMIBC) and muscle invasive bladder cancer (MIBC). Radical cystectomy (RC) with urinary diversion is the standard curative treatment for MIBC without distant metastasis [Citation1]. However, despite improvements in surgical technology and perioperative management, postoperative ileus (POI), a common postoperative gastrointestinal complication, is still an important clinical event after RC [Citation2]. Clinical manifestations of POI include abdominal pain, nausea, vomiting, intolerance of diet and delayed passage of flatus or stool, and can lead to increased duration of hospitalization, medical expenses, and risk of downstream adverse events [Citation3,Citation4]. A recent nationwide study showed that 26.0% of patients who underwent RC developed POI [Citation3]. Thus, in order to reduce the incidence of POI, it is essential to clarify the risk factors and identify the patients at greatest risk of POI. Although most of the previous studies on POI were based on gastrointestinal surgery, little is known about the risk factors for POI after RC and few predictive models have been established [Citation5,Citation6].
The nomogram model has been widely used in risk assessment and prognostic research for bladder cancer patients [Citation7,Citation8]. Instead of complex traditional regression models, nomograms can conduct convenient and accurate risk assessment for each patient by creating a user-friendly chart. This study aimed to investigate the perioperative risk factors for POI using large single-center data, then develop and validate a nomogram for patients who underwent RC.
2. Method
2.1. Study population
We retrospectively analyzed 452 bladder cancer patients who underwent RC with urinary diversion at the Second Hospital of Tianjin Medical University between January 2016 and December 2021. This study was approved by the hospital ethics committee and informed consent was obtained from each patient. Written consent was waived due to the retrospective nature of the study.
The inclusion criteria were as follows [Citation1]: bladder cancer clinical stage T1-T4a [Citation2]; no distant metastasis [Citation3]; aged ≥18.0 years; and [Citation4] scores ranging from I to III according to the American Society of Anaesthesiologists scale. The exclusion criteria were as follows [Citation1]: patients did not meet the inclusion criteria [Citation2]; palliative surgery; or [Citation3] preoperative ileus. In order to better verify this model, we randomly divided the entire study population into training and validation samples at a ratio of 3: 1 ().
Table 1. Baseline characteristics of the study population.
2.2. Outcome and variables
The outcome variable for the current study was POI occurring in the perioperative period. Despite its prevalence, the definition of POI varies among reports. Here, we defined POI as outlined in several highly cited papers [Citation9,Citation10]. Our definition included the following criteria [Citation1]: Delayed or impaired bowel function after surgery, as evidenced by the presence of any of the following on postoperative day 5 or later: absence of gas or stool; intolerance of an oral diet after ingestion; diminished or absent bowel sounds; abdominal distension or abdominal pain [Citation2]. the presence of multiple air-fluid levels that were confirmed radiologically. The occurrence of POI was independently diagnosed by two experienced surgeons.
The clinical and pathological data of valid patients were collected using the electronic medical records system. The recorded preoperative data included gender, age, body mass index (BMI), smoking, drinking, diabetes mellitus, hypertension, previous abdominal surgery, history of transurethral resection of bladder tumour (TURBt), intestinal tract reconstruction time, neoadjuvant chemotherapy, and preoperative levels of haemoglobin, albumin and creatinine. Relevant surgical data included different types of surgical approaches, methods of urinary diversion, estimated blood loss, blood transfusion, and pelvic lymph node dissection (PLND). Postoperative data included pathological TNM staging.
The surgical approaches used in our study included laparoscopic radical cystectomy (LRC) and robotic-assisted radical cystectomy (RARC). The most common methods of urinary diversion were ileal conduit, orthotopic neobladder and cutaneous ureterostomy. However, since cutaneous ureterostomy is the simplest form of urinary diversion normally associated with elderly and frail patients, its complication rate was significantly lower in patients compared with other methods [Citation11,Citation12]. Thus, to reduce selection bias, patients with cutaneous ureterostomy were not included in this study. The technology used for urinary diversion can be divided into intracorporeal and extracorporeal methods. Since a recent meta-analysis indicated that there were no significant differences between the two groups in POI [Citation13]. We uses the extracorporeal method for LRC and the intracorporeal for RARC.
Enhanced Recovery after Surgery (ERAS) is a perioperative method for managing surgical patients. Currently, the standardized ERAS protocol and the impact on radical cystectomy remains understudied [Citation14]. In our study, all patients were enrolled in an adapted ERAS protocol, which detailed items include patient education, optimization of bowel preparation, perioperative reduction of opioid use, prevention of intraoperative hypothermia, perioperative fluid management, early drinking and early ambulation.
2.3. Statistical analysis
Statistical analysis was performed using R version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria), GraphPad Prism 8.0 Software (GraphPad Software Inc., San Diego, CA, USA) and SPSS 26.0 software (SPSS Inc., Chicago, IL, USA). Continuous variables were described as mean ± standard deviation, and categorical variables were described as proportions. Student’s t-test and chi-square tests were used to confirm any statistical differences between continuous variables and categorical variables.
To identify the risk factors for POI, we first performed univariate logistic regression analysis. Variables that were significant with a p value < 0.20 were then included in a multivariate logistic regression analysis to screen for independent risk factors. Two- tailed P values < 0.05 were considered to be statistically significant. Forest plots were used to visualize the univariate and multivariate regression analyses data. Finally, the independent risk factors identified by multivariable logistic regression were used to build a nomogram model with R software.
Nomogram models were built and validated based on several recent studies [Citation15]. The predictive ability of the model was evaluated with a receiver operating characteristic curve (ROC), while the consistency was verified using a calibration curve. Decision curve analysis (DCA) was adopted to determine the clinical usefulness and net benefit of the nomogram.
3. Results
3.1. Clinical status
A total of 452 bladder cancer patients were included in this study with POI occurring in 96 (21.2%) patients. The mean age of the total patient population was 61.14 ± 7.01 years, and 82.1% patients were male. 295 (65.3%) patients underwent LRC, while 157 (34.7%) underwent RARC. 343 (75.9%) patients underwent ileal conduit and 109 (24.1%) patients underwent orthotopic neobladder after RC. The mean operation time was 4.75 ± 0.87 h, and the mean estimated blood loss was 370.58 ± 322.11 ml. Based on the pathological TNM staging system for bladder cancer, there were 30 (6.6%) stage T1, 288 (63.7%) stage T2, 102 (22.6%) stage T3 and 32 (7.1%) T4a patients.
3.2. Risk factors associated with POI
Univariate logistic regression analysis showed that gender (p = 0.563), smoking (p = 0.207), hypertension (p = 0.793), diabetes mellitus (p = 0.652), anaemia (p = 0.875), previous TURBt (p = 0.729), chemical therapy (p = 0.551), surgical approach (p = 0.547), estimated blood loss (p = 0.732) and pathological stage (p = 0.485) were not statistically significant factors, and therefore were not included in subsequent statistical analyses. Multivariate logistic regression analysis was performed on nine risk factors including age, BMI, constipation, renal inadequacy, previous abdominal surgery, hypoalbuminemia, urinary diversion, PLND, blood transfusion and operative time ( and ).
Table 2. Logistic regression assessing risk factors for POI.
In the multivariate logistic regression model, six independent risk factors for POI were identified, including age > 65.0 years (OR = 2.090, CI = 1.321–3.305, p = 0.002), low body mass index (OR = 3.021, CI = 1.760–5.186, p = 0.001), high body mass index (OR = 1.581, CI = 1.035–2.417, p = 0.034), constipation (OR = 1.843, CI = 1.101–3.088, p = 0.020), hypoalbuminemia (OR = 2.749 CI = 1.555–4.861, p = 0.001) and operative time (OR = 3.023, CI = 2.289–3.994, p = 0.001). The remaining factors did not show significant statistical significance ( and ).
Figure 1. The forest plots show the results of univariate (A) and multivariate (B) analyses. In the multivariate logistic regression model, six independent risk factors for POI were further screened out, including age older than 65.0 years (OR =2.090, CI = 1.321–3.305, p = 0.002), low body mass index (OR =3.021, CI = 1.760–5.186, p = 0.001), high body mass index (OR =1.581, CI = 1.035–2.417, p = 0.034), Constipation (OR =1.843, CI = 1.101–3.088, p = 0.020), Hypoalbuminemia (OR =2.749 CI = 1.555–4.861, p = 0.001) and Operative time (OR =3.023, CI = 2.289–3.994, p = 0.001).
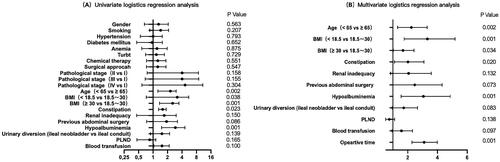
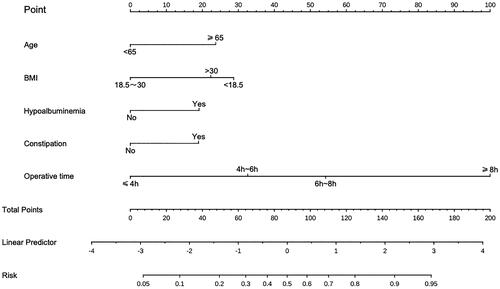
Figure 2. A nomogram model was established using independent risk factors screened out by multivariate regression analysis. The corresponding score for each factor is based on the condition of the patient, which can be determined by making a vertical line upwards (e.g. a patient with Hypoalbuminemia will receive 20 scores). Add all the scores to get the total score, then find the corresponding point on the total points axis and make a vertical line down to predict the risk of the POI after radical cystectomy.
3.3. Establishment and validation of the nomogram
A nomogram prediction model was established based on the six independent risk factors identified by multivariate logistic regression analysis (). The predictive factor’s weight was expressed by the length of the line segment. Each risk factor was assessed individually for each patient in accordance with the circumstances, and the numbers were then summed to provide a total score. The final total score was used to determine the estimated risk likelihood of postoperative POI for each patient. The nomogram demonstrated a correlation between a higher patient score and a higher probability of POI.
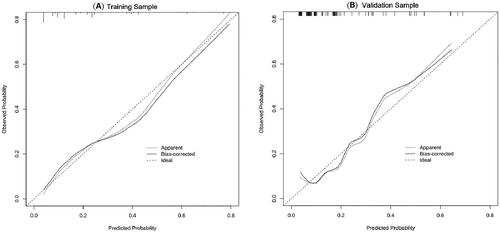
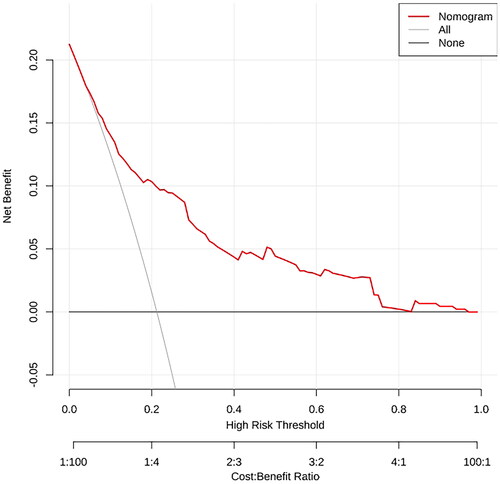
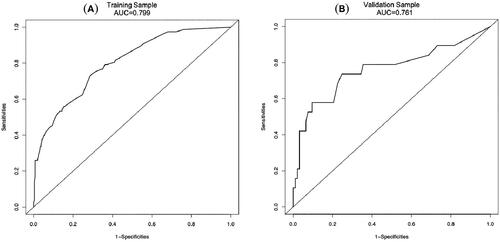
In order to determine the discrimination of the prediction model, ROC curves were drawn in the training and validation samples, and the AUC was computed. Our findings demonstrated that the model has a strong capacity for discrimination (). The AUCs for the training samples was 0.799, while for the validation samples it was 0.761. The calibration curves were also created to display the relationship between the expected and actual values. The training and validation samples both demonstrated a good correlation between the nomogram’s projected probability and the actual circumstance (). Besides, DCA curves were used to evaluate the clinical benefit of the nomogram model in predicting POI. When the threshold probabilities of the nomogram model were in the range of 0.05–0.90, the net benefit ratio was > 0, which suggested that the model had a better clinical value for predicting POI ().
Figure 3. The AUC of training sample (A) and validation sample (B) showed that the model had a high discrimination ability.
4. Discussion
POI is a common complication of RC with recently reported rates as high as 26.9% [Citation3]. The pathophysiology of POI is still under investigation, and complex interactions between neural reflexes, inflammation, fluid overload, electrolytes, and clinical anaesthesia are thought to be involved in the development of POI [Citation16,Citation17]. Current studies are limited, since they are not only based on a single RC surgical approach, but also lack the prediction model of POI in patients with RC. Since the development of POI can seriously affect the recovery of patients, urologists need a predictive model to assess patient risk and make appropriate interventions. Therefore, the purpose of this study was to identify independent risk factors for POI after various RC surgical approaches and establish a user-friendly predictive model.
Several studies have demonstrated that increased patient age is associated with a higher risk of POI after RC. Robert et al. retrospectively studied 283 patients who underwent RC with pelvic nodal dissection, and using multivariate logistic regression analysis showed that the independent risk factors included increasing age [Citation18]. One possible explanation may be the significant reductions in the number of intestinal myenteric neurons and the intrinsic and extrinsic innervation of the colorectum by visceral afferent and sympathetic motor neurons observed in advanced age patients. Furthermore, elderly patients tend to have comorbidities such as diabetes mellitus, malignant tumours, medications, and previous surgical procedures that can damage the gastrointestinal neuromuscular system [Citation19]. In addition, since basic metabolism slows down with increasing age, a longer period of body residue of anaesthetic drugs (such as opioids) may inhibit the recovery of bowel function [Citation20].
High BMI is related to the occurrence of POI. A recent national database found that increasing grades of obesity were independently associated with higher 30-day complication rates [Citation21]. Robert et al. reported that 30.3% of patients with class II-III obesity were found to have POI with a hazard ratio of 1.09 [Citation18], and proposed that obese patients may have less space for surgical manipulation leading to more obvious postoperative edoema with intestinal mucosal paralysis. In addition, a different study found that low BMI was still statistically significant [Citation22]. This may be because patients with low BMI generally do not tolerate RC and are often malnourished, which can lead to slow recovery of postoperative gastrointestinal function.
Chronic constipation is associated with a high risk of POI. A recent study found that constipation was strongly associated with worse bowel cleanliness [Citation23]. For patients with constipation, inadequate preoperative bowel cleansing may lead to excessive bowel preparation, which has been confirmed as a risk factor for developing POI by Xue et al. [Citation3]. In addition, despite the lack of robust evidence for their clinical utility, laxatives or prokinetic agents have been used in clinical practice for the treatment of POI with good effect. In a study conducted by Kim and colleagues, mosapride, commonly used as a treatment for constipation, was found to be effective in reducing the rate of POI in a guinea pig model [Citation24].
Hypoalbuminemia has also been associated with the occurrence of POI. Ornaghi et al. found that hypoalbuminemia significantly increased the rate of postoperative complications after RC [Citation25]. Dai et al. demonstrated that preoperative hypoalbuminemia prolonged the incidence of POI by 2.7-fold in patients undergoing colectomy [Citation26]. For patients undergoing RC, we propose that this is due to the effects of albumin on increasing plasma colloid osmotic pressure, reducing tissue edoema, as well as promoting tissue healing. When hypoalbuminemia is present, bowel edoema and delayed anastomotic healing compromise bowel function recovery.
Several studies have examined the relationship between operative time and POI. Haeuser et al. found that the operative time was associated with short-term RC complications [Citation27]. Furthermore, these studies indicated that surgeries lasting between 4.0 and 5.0 h had the lowest complication rates, which is consistent with our findings. Greenberg et al. found that 85 out of 261 patients developed POI after abdominal surgery, and that the median operation time for patients with and without POI were 313 min and 279 min, respectively [Citation28]. In the current study, regression analysis indicated that the operation time was an independent prognostic factor of POI after RC. We believe that an increased surgery time contributes to increased exposure of patients to higher doses of opioids. In addition, a long operation time normally causes liquid overload, acidosis and electrolyte disorders. All of these factors affect the recovery of the gastrointestinal tract, eventually leading to POI.
There are several limitations in our study. First, despite our efforts to expand the sample size, errors resulting from selection bias and recall bias may still occur. Therefore, multicentre studies with larger sample sizes are needed in follow-up studies. Second, due to the limitations of a single-center database, perioperative management of patients may differ from other institutions. Finally, we performed internal validation but did not complete external validation. Thus, future studies will use cohorts from other institutions to confirm our findings.
5. Conclusion
In conclusion, in the present study, we developed a prediction model for POI, which provides surgeons with a simple method that can be used to calculate the risk of POI after RC and may help surgeons to counsel and treat patients with potential risks. Further studies are required for validation.
Ethical approval and consent to publish
This study was approved by the research ethics committee of the Second Hospital of Tianjin Medical University (authorization number: KY2023K005). Its publication is also approved tacitly by the responsible authorities where the work was carried out. The authors have no relevant financial or non-financial interests to disclose.
Authors’ contributions
XYS and CL retrieved and analyzed all of the data in the study. CWZ and ZHZ revised the manuscript for important intellectual contents. XYS and CL designed, checked and supervise all study process. All authors contributed to the article and approved the submitted version.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The raw data of this study and more information can be found upon reasonable request from the author (SXY).
Additional information
Funding
References
- Lenis AT, Lec PM, Chamie K, et al. Bladder cancer: a review. Jama. 2020;324(19):1–9. doi: 10.1001/jama.2020.17598.
- Bochner BH, Dalbagni G, Sjoberg DD, et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: a randomized clinical trial. Eur Urol. 2015;67(6):1042–1050. doi: 10.1016/j.eururo.2014.11.043.
- Nutt M, Scaief S, Dynda D, et al. Ileus and small bowel obstruction after radical cystectomy for bladder cancer: analysis from the nationwide inpatient sample. Surg Oncol. 2018;27(3):341–345. doi: 10.1016/j.suronc.2018.05.019.
- Tevis SE, Carchman EH, Foley EF, et al. Postoperative ileus–more than just prolonged length of stay? J Gastrointest Surg. 2015;19(9):1684–1690. doi: 10.1007/s11605-015-2877-1.
- Xue X, Wang D, Ji Z, et al. Risk factors of postoperative ileus following laparoscopic radical cystectomy and developing a points-based risk assessment scale. Transl Androl Urol. 2021;10(6):2397–2409. doi: 10.21037/tau-21-112.
- Forbes CM, Chehroudi AC, Mannas M, et al. Defining postoperative ileus and associated risk factors in patients undergoing radical cystectomy with an enhanced recovery after surgery (ERAS) program. Can Urol Assoc J. 2021;15(2):33–39. doi: 10.5489/cuaj.6546.
- Zhang Y, Hong YK, Zhuang DW, et al. Bladder cancer survival nomogram: development and validation of a prediction tool, using the SEER and TCGA databases. Medicine . 2019;98(44):e17725.). doi: 10.1097/MD.0000000000017725.
- Wu S, Zheng J, Li Y, et al. A radiomics nomogram for the preoperative prediction of lymph node metastasis in bladder cancer. Clin Cancer Res. 2017;23(22):6904–6911. doi: 10.1158/1078-0432.CCR-17-1510.
- Ramirez JA, McIntosh AG, Strehlow R, et al. Definition, incidence, risk factors, and prevention of paralytic ileus following radical cystectomy: a systematic review. Eur Urol. 2013;64(4):588–597. doi: 10.1016/j.eururo.2012.11.051.
- Vather R, Trivedi S, Bissett I. Defining postoperative ileus: results of a systematic review and global survey. J Gastrointest Surg. 2013;17(5):962–972. doi: 10.1007/s11605-013-2148-y.
- Longo N, Imbimbo C, Fusco F, et al. Complications and quality of life in elderly patients with several comorbidities undergoing cutaneous ureterostomy with single stoma or ileal conduit after radical cystectomy. BJU Int. 2016;118(4):521–526. 6. doi: 10.1007/s11605-013-2148-y.
- Korkes F, Fernandes E, Gushiken FA, et al. Bricker ileal conduit vs. Cutaneous ureterostomy after radical cystectomy for bladder cancer: a systematic review. Int Braz j Urol. 2022;48(1):18–30. doi: 10.1111/bju.13462.
- Tanneru K, Jazayeri SB, Kumar J, et al. Intracorporeal versus extracorporeal urinary diversion following robot-assisted radical cystectomy: a meta-analysis, cumulative analysis, and systematic review. J Robot Surg. 2021;15(3):321–333. doi: 10.1590/S1677-5538.IBJU.2020.0892.
- Chenam A, Chan KG. Enhanced recovery after surgery for radical cystectomy. Cancer Treat Res. 2018;175:215–239. doi: 10.1007/978-3-319-93339-9_10.
- Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–1370. doi: 10.1200/JCO.2007.12.9791.
- Bragg D, El-Sharkawy AM, Psaltis E, et al. Postoperative ileus: recent developments in pathophysiology and management. Clin Nutr. 2015;34(3):367–376. doi: 10.1016/j.clnu.2015.01.016.
- de Boer HD, Detriche O, Forget P. Opioid-related side effects: postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best Pract Res Clin Anaesthesiol. 2017;31(4):499–504. doi: 10.1016/j.bpa.2017.07.002.
- Svatek RS, Fisher MB, Williams MB, et al. Age and body mass index are independent risk factors for the development of postoperative paralytic ileus after radical cystectomy. Urology. 2010;76(6):1419–1424. doi: 10.1016/j.urology.2010.02.053.
- Gidwaney NG, Bajpai M, Chokhavatia SS. Gastrointestinal dysmotility in the elderly. J Clin Gastroenterol. 2016;50(10):819–827. doi: 10.1097/MCG.0000000000000650.
- Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6–14. doi: 10.1046/j.1365-2125.2003.02007.x.
- Arora K, Hanson KT, Habermann EB, et al. Early complications and mortality following radical cystectomy: associations with malnutrition and obesity. Bladder Cancer. 2018;4(4):377–388. doi: 10.3233/BLC-180173.
- Meng YS, Su Y, Fan Y[, et al. Risk factors for the development of postoperative paralytic ileus after radical cystectomy: a report of 740 cases. ]. Beijing Da Xue Xue Bao Yi Xue Ban. 2015;47(4):628–633.
- Martel M, Ménard C, Restellini S, et al. Which patient-related factors determine optimal bowel preparation? Curr Treat Options Gastroenterol. 2018;16(4):406–416. doi: 10.1007/s11938-018-0208-9.
- Kim YM, Hussain Z, Lee YJ, et al. Altered intestinal permeability and drug repositioning in a post-operative ileus Guinea pig model. J Neurogastroenterol Motil. 2021;27(4):639–649. doi: 10.5056/jnm21018.
- Ornaghi PI, Afferi L, Antonelli A, et al. The impact of preoperative nutritional status on post-surgical complication and mortality rates in patients undergoing radical cystectomy for bladder cancer: a systematic review of the literature. World J Urol. 2021;39(4):1045–1081. doi: 10.1007/s00345-020-03291-z.
- Dai X, Ge X, Yang J, et al. Increased incidence of prolonged ileus after colectomy for inflammatory bowel diseases under ERAS protocol: a cohort analysis. J Surg Res. 2017;212:86–93. doi: 10.1016/j.jss.2016.12.031.
- Haeuser L, Marchese M, Noldus J, et al. Association between operative time and short-term radical cystectomy complications. Urol Int. 2023;107(3):273–279. doi: 10.1159/000522141.
- Greenberg AL, Kelly YM, McKay RE, et al. Risk factors and outcomes associated with postoperative ileus following ileostomy formation: a retrospective study. Perioper Med. 2021;10(1):55.). doi: 10.1186/s13741-021-00226-z.