Abstract
Background
Interstitial lung disease (ILD) is the most widespread and fatal pulmonary complication of rheumatoid arthritis (RA). Existing knowledge on the prevalence and risk factors of rheumatoid arthritis-associated interstitial lung disease (RA-ILD) is inconclusive. Therefore, we designed this review to address this gap.
Materials and Methods
To find relevant observational studies discussing the prevalence and/or risk factors of RA-ILD, EMBASE, Web of Science, PubMed, and the Cochrane Library were explored. The pooled odds ratios (ORs) / hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated with a fixed/ random effects model. While subgroup analysis, meta-regression analysis and sensitivity analysis were carried out to determine the sources of heterogeneity, the I2 statistic was utilized to assess between-studies heterogeneity. Funnel plots and Egger’s test were employed to assess publication bias. Following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines, our review was conducted.
Results
A total of 56 studies with 11,851 RA-ILD patients were included in this meta-analysis. The pooled prevalence of RA-ILD was 18.7% (95% CI 15.8–21.6) with significant heterogeneity (I2 = 96.4%). The prevalence of RA-ILD was found to be more likely as a result of several identified factors, including male sex (ORs = 1.92 95% CI 1.70–2.16), older age (WMDs = 6.89, 95% CI 3.10–10.67), having a smoking history (ORs =1.91, 95% CI 1.48–2.47), pulmonary comorbidities predicted (HRs = 2.08, 95% CI 1.89–2.30), longer RA duration (ORs = 1.03, 95% CI 1.01–1.05), older age of RA onset (WMDs =4.46, 95% CI 0.63–8.29), positive RF (HRs = 1.15, 95%CI 0.75-1.77; ORs = 2.11, 95%CI 1.65–2.68), positive ACPA (ORs = 2.11, 95%CI 1.65–2.68), higher ESR (ORs = 1.008, 95%CI 1.002–1.014), moderate and high DAS28 (≥3.2) (ORs = 1.87, 95%CI 1.36–2.58), rheumatoid nodules (ORs = 1.87, 95% CI 1.18-2.98), LEF use (ORs = 1.42, 95%CI 1.08–1.87) and steroid use (HRs= 1.70, 1.13–2.55). The use of biological agents was a protective factor (HRs = 0.77, 95% CI 0.69–0.87).
Conclusion(s)
The pooled prevalence of RA-ILD in our study was approximately 18.7%. Furthermore, we identified 13 risk factors for RA-ILD, including male sex, older age, having a smoking history, pulmonary comorbidities, older age of RA onset, longer RA duration, positive RF, positive ACPA, higher ESR, moderate and high DAS28 (≥3.2), rheumatoid nodules, LEF use and steroid use. Additionally, biological agents use was a protective factor.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease characterized by symmetrical polyarthritis. Although arthritis is the most frequently encountered manifestation, pulmonary manifestation is the most common extra-articular manifestation (EAM). Interstitial lung disease (ILD), among the pulmonary symptoms, has an enormous impact on morbidity and mortality, therefore it warrants special attention [Citation1]. In comparison to the general public, the prevalence of rheumatoid arthritis-related interstitial lung disease (RA-ILD) continues to rise annually. It causes 13% more deaths than expected, particularly among the advanced-age individuals [Citation2]. Since the clinical symptoms may not appear until the pulmonary involvement is well-established, a sizable portion of RA-ILD patients could remain asymptomatic at an early stage, impacting early diagnosis [Citation3,Citation4]. Nevertheless, once clinically obvious, ILD is linked to considerable mortality and hypofunction [Citation2,Citation5]. Therefore, to fully comprehend the underlying pathogenesis and enable earlier diagnosis and management, it is imperative to adequately identify the prevalence and risk factors of RA-ILD. This helps to prevent irreversible lung damage.
Across the published studies, the prevalence of RA-ILD varies significantly from 1.7% to 71.6% [Citation6–14]. This can be due to variations in the study populations and the diagnostic methods. Additionally, more studies reflect that some variables, such as being male, being older, and having a smoking history, are significant predictors of RA-ILD [Citation15–18]. However, the results are discordant (some factors like methotrexate (MTX) use are still controversial [Citation10,Citation19,Citation20]). Furthermore, no study has systematically summarized these risk factors and quantitatively assessed their correlation with RA-ILD. Thus, this meta-analysis was conducted to summarize the prevalence of RA-ILD and elucidate the risk factors for RA-ILD.
Methods
We conducted our study in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA 2020) [Citation21]. The protocol was pre-registered in PROSPERO (CRD42023388414).
Search strategy
Two reviewers conducted an independent search of EMBASE, Web of Science, PubMed, and the Cochrane Library through January 9, 2023. ‘Rheumatoid arthritis’, ‘interstitial lung disease’, ‘risk factor’, ‘prevalence’, and their related variants were the search terms. For a more comprehensive search, the Medical Subject Headings (MeSH) terms were paired with free texts. Potentially overlooked articles were also checked for in the reference lists of included research and pertinent reviews. Supplementary Appendix A1provides a detailed search strategy.
Study selection
The included studies were evaluated based on the following criteria: (1) studies related to the prevalence or/and risk factors of RA-ILD; (2) observational studies, including cohort, case-control, and cross-sectional studies (that recorded the prevalence); (3) RA-ILD patients (RA without ILD served as the control group). For prevalence calculations, the denominator was the number of RA who fulfilled diagnostic criteria. The numerator is the number of ILD after RA onset. Case definition for RA-ILD, patient with an ILD was defined as a patient with any abnormalities based on clinical data, radiologic evidence (HRCT, CT, CXRs, etc.), PFTs, and lung biopsies. Subclinical ILD, patients with ILD before or meantime to RA onset were excluded. There is no limit on observation time. Each study was evaluated by two independent reviewers (Hong-Fei Wang and Zhi-Yu Li) for eligibility. Each reviewer looks through the title and abstract, and then reviews the eligible full text sequentially. Letters, reviews, commentaries, abstracts, and editorials were not included. Only studies written in English were considered. If there were any overlaps in patients included in multiple studies, the one with a larger sample size was taken into consideration. Any uncertainties or discrepancies between the two reviewers were settled through consensus.
Data extraction and quality assessment
Two reviewers (Hong-Fei Wang and Yan-Yun Wang) extracted data alone and assessed its quality using predefined tables. Any disagreement was settled by discussion or consulting the third reviewer (Qiu-Shuang Li). The extracted data consisted of the following information: the first author’s name, region, publication year, study type, sample size, demographic features of subjects, classification criteria of RA-ILD, number of RA, number of RA-ILD patients, RA-ILD prevalence, risk factors and their effect estimates. The Newcastle Ottawa Quality Assessment Scale (NOS) was adopted for evaluating each cohort or case-control study’s quality [Citation22]. The Agency for Healthcare Research Quality (AHRQ) was utilized to estimate the quality of cross-sectional studies [Citation23]. Studies were categorized as low, moderate, or high quality based on the NOS or AHRQ scores. The NOS score range was 0-3, 4-6 and 7-9, while the AHRQ score range was 0–3, 4–7 and 8–11, respectively.
Statistical analysis
Stata version 17.0 (StataCorp, LLP, college station, TX, authorized by School of Public Health, Zhejiang Chinese Medicine University) was used for statistical analyses. Risk factors for RA-ILD in at least two included studies were weighed using the pooled Odds Ratios (ORs), hazard ratios (HRs), and weighted mean differences (WMDs) for dichotomous and continuous variables, respectively, and corresponding 95% confidence intervals (CIs). ORs and RRs were combined and pooled after the conversion: RR = OR ÷ (1 − p + (p × OR)) [Citation24], where p represents the prevalence of RA in the reference group.
Only estimates with the most comparable characteristics (i.e. set of confounders adjusted) were combined when multiple results were provided from a single article. To assess the between-studies heterogeneity and quantify the degree of inconsistency, the I2 value and Cochran’s Q statistic were employed. Significant heterogeneity was identified as I2 > 50% or p < 0.1 in the Q test [Citation25]. When there was significant heterogeneity, the fixed effects model was employed; otherwise, the random effects model was used [Citation26]. Subgroup analysis was applied to assess disparities in the prevalence of RA-ILD stratified by geographical region, ILD diagnostic modality, sample size, and study type. Furthermore, we conducted additional meta-regressions to investigate potential sources of heterogeneity. The independent variables included male-to-female ratio, mean RA duration, ILD diagnostic criteria, total sample size and geographical region were considered in the meta-regressions. Sensitivity analysis was performed by sequentially omitting individual studies. Given the availability of ten or more articles for meta-analysis, publication bias was assessed qualitatively and quantitatively with funnel plots and Egger’s linear regression respectively. Significant publication bias was shown by the asymmetric funnel plot or p < 0.05 [Citation27]. If there existed significant publication bias, subsequent sensitivity analysis using the trim-and-fill method was used to find the possible "missing studies" and further explore the effect of "missing studies" on the pooled effect estimate [Citation28,Citation29]. In instances where combining data was inappropriate owing to significant clinical or methodological variety or insufficient research, the results were reported qualitatively.
Results
Study selection
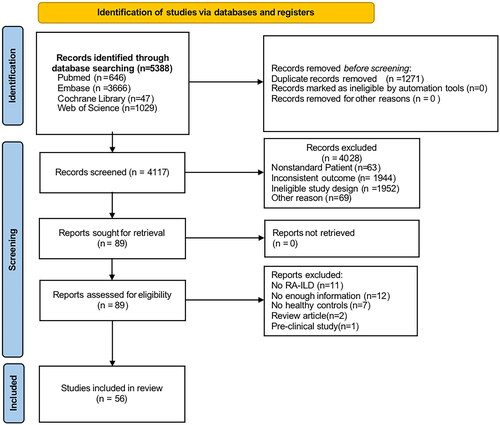
From EMBASE (n = 3666), Web of Science (n = 1029), PubMed (n = 646), and Cochrane Library (n = 47), a total of 5388 records were found. After the screening, 56 studies were included: 20 studies were selected for both prevalence and risk factors, 18 studies were selected solely for risk factors, and 18 studies were selected alone for prevalence ().
Study and subject characteristics
displays the characteristics of the included studies. The 56 studies consisted of 32 cross-sectional studies, 12 case-control studies, and 12 cohort studies. All were published between 1972 to 2022, and carried out in Europe (n = 13), Asia (n = 22), Africa (n = 2), America (n = 17), and Oceania (n = 1). The sample size varied from 18 to 373,940 RA patients. Most studies employed the 1987 American College of Rheumatology (ACR) classification criteria or the 2010 ACR/EULAR classification criteria for RA. Chest CT or high-resolution computed tomography (HRCT) findings, clinical symptoms, pulmonary function test (PFT) results, and database code were some of the criteria used to diagnose ILD. However, HRCT was the most used diagnostic criteria. All studies were classified as moderate to high quality. The NOS score ranges from 6 to 8 and the AHRQ score ranges from 5–10 (Supplementary Appendix A2).
Table 1. Baseline characteristics of included studies in the meta-analysis.
Prevalence of RA-ILD
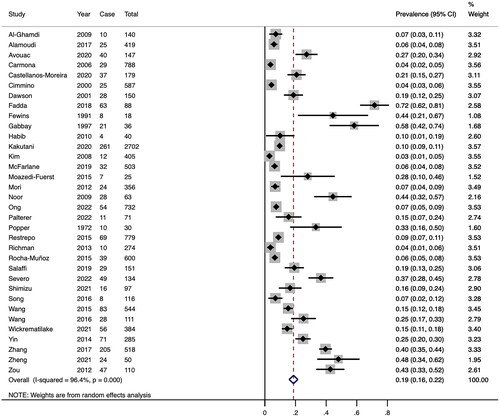
In the 34 studies included, a total of 11,632 RA patients were enrolled, among whom 1463 were diagnosed with RA-ILD. The prevalence of RA-ILD ranged from 2.96% to 71.60%. The pooled RA-ILD prevalence was 18.7% (95% CI 15.8–21.6) (). Between-studies heterogeneity was significant (I2 = 96.4%).
We carried out subgroups to explore the prevalence of RA-ILD. According to the region, the prevalence was lower in North America (7.6%) compared to Asia (17.8%), Europe (14.3%), and others. Furthermore, the prevalence identified only by HRCT (22.9%) was higher compared to other methods. In subgroup analysis based on sample size, RA-ILD prevalence was greater in sample sizes below 100 (36.7%) than in sample sizes above 100. Patients with RA duration longer than 10 years have a higher prevalence. displays the specific results of the subgroup analysis. The results of the meta-regression analyses revealed that sample size influenced the combined prevalence of RA-ILD significantly (Supplementary Appendix A3).
Table 2. Subgroup analysis for the prevalence of RA-ILD.
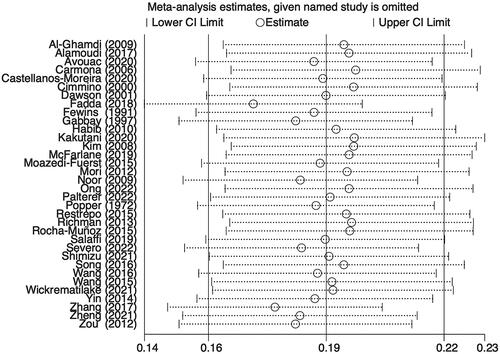
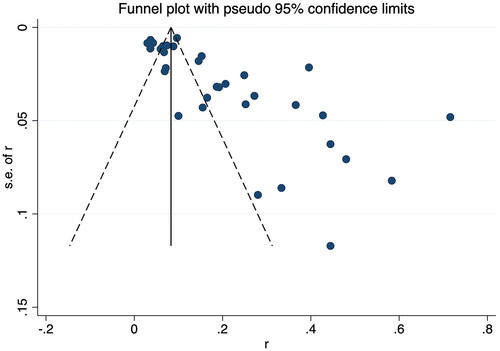
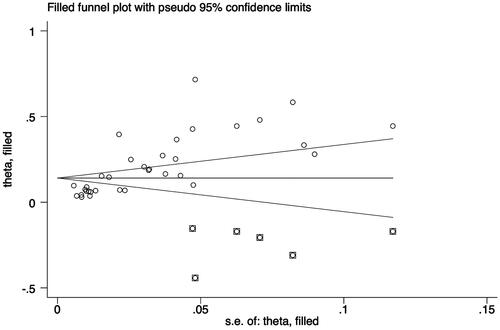
Additionally, a sensitivity analysis was carried out to discover the impact of individual-included studies on the overall aggregated estimate. The findings showed the overall pooled estimate was robust and credible since the pooled prevalence of RA-ILD corresponded to the overall pooled effect (). The presence of publication bias among studies was shown by an asymmetrical funnel plot and a significant value p < 0.001) in Egger’s test ().
Considering the significant publication bias, the trim-and-fill method was used to explore the effect of "missing studies" on the pooled prevalence of RA-ILD. The results showed that six studies were "missing" in the current meta-analysis. Interestingly, the new pooled estimate was less varied at 14.1% (95% CI 11.2–17.0), suggesting that the previous results were robust despite some publication bias ().
Factors associated with RA-ILD
Thirty-three studies reported the risk factors and measured effects [Citation1,Citation2,Citation10,Citation14–20,Citation30–52]. Data on these variables could only be pooled if they were collected at the time of initial RA-ILD diagnosis. Factors were categorized into three groups: sociodemographic factors include sex, age, ethnicity, education level, and exposure to air pollution; lifestyle factors include smoking history and body mass index (BMI); and clinical factors include age of RA onset, comorbidities, RA duration, anti-cyclic citrullinated peptide antibody (ACPA), rheumatoid factor (RF), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), surfactant protein D (SPD), Krebs von den Lungen-6 glycoprotein (KL-6), disease activity score with 28-joint counts (DAS28), erosion, rheumatoid nodules, functional capacity, the multidimensional Health Assessment Questionnaire (MDHAQ/HAQ), the clinical disease activity index (CDAI), lactate dehydrogenase (LDH), tumor markers, and methotrexate (MTX) usage, leflunomide (LEF) usage, biological agents usage, and steroid usage. summarizes the pooled results of all the factors related to RA-ILD prevalence. All forest plots are shown in Supplementary Appendix A4. No publication bias was observed in the funnel plot (Supplementary Appendix A5).
Table 3. Meta-analysis of risk factors for RA-ILD.
Sociodemographic factors
Among sociodemographic factors, male sex and older age were found to be the most significantly related to RA-ILD. With respect to gender, patients with RA-ILD were inclined to be male (ORs = 1.92, 95% CI 1.70–2.16; p < 0.05, Figure 5) [Citation1,Citation2,Citation10,Citation15–17,Citation19,Citation33,Citation35,Citation36,Citation38,Citation39,Citation43,Citation44,Citation47]. However, Ong et al. [Citation18] found that female sex was linked to increased prevalence. Moreover, a correlation between older age and greater frequency for RA-ILD was found (WMDs = 6.89, 95% CI 3.10–10.67; p < 0.05, Figure 6) [Citation1,Citation2,Citation14–16,Citation18,Citation30,Citation31,Citation39,Citation41,Citation42,Citation47–49,Citation52].
Lifestyle factors
Results from our study indicate that having a smoking history tends to be a higher prevalence (ORs = 1.91, 95% CI 1.48–2.47; p < 0.05, Figure 7) [Citation1,Citation15–18,Citation30,Citation31,Citation35–39,Citation41,Citation47,Citation52]. Samhouri et al. [Citation43] and Bongartz et al. [Citation10] examined the connection between having a smoking history and the prevalence of RA-ILD using the HRs. Samhouri et al. [Citation43] showed that smoking history is a significant risk factor (HR = 1.92, 95% CI 1.09–3.41), whereas no significant association was found by Bongartz et al. [Citation10] (HR = 1.6, 95%CI 0.87–2.94). In addition to smoking, the effect of obesity (BMI ≥ 30 kg/m2) varied across studies. Kronzer et al. [Citation37] revealed a moderately positive association (ORs= 2.42, 95%CI 1.11–5.24, p < 0.05) while Samhouri et al. [Citation43] and Salaffi et al. [Citation42] failed.
Clinical factors
Having pulmonary comorbidities, older age of RA onset, longer RA duration, positive RF, positive ACPA, higher ESR, moderate and high DAS28 (≥3.2), rheumatoid nodules, and use of LEF and steroids have a significant association with RA-ILD prevalence. Pulmonary comorbidities were associated with prevalence (HRs = 2.08, 95% CI 1.89–2.30; p < 0.05, Figure 8A) [Citation19,Citation51]. One study reporting OR also had the same conclusion (OR = 3.60, 95%CI 2.68–4.82) [Citation32]. No significant difference was observed between other comorbidities and prevalence (ORs =1.013, 95% CI 0.79–1.31; Figure 8B) [Citation18,Citation32,Citation35]. Furthermore, five studies showed that longer RA duration was correlated with higher RA-ILD prevalence (ORs = 1.03, 95%CI 1.01–1.05; p < 0.05, Figure 9) [Citation14,Citation15,Citation18,Citation45,Citation49]. Being older at the time of RA onset was associated with increased prevalence (WMDs= 4.46, 95% CI 0.63–8.29; p < 0.05, Figure 10)[Citation10,Citation16,1831,Citation36,Citation42,Citation51]. As is well-known, we can denote RF and ACPA in two distinct manners: quantitatively (titers) and qualitatively (positive/negative). High titers of RF/ACPA are biomarkers attached to the elevated RA-ILD prevalence [Citation40,Citation49]. Nevertheless, the combined quantitative result of RF/ACPA was omitted due to differences in the statistical methods and thresholds of RF/ACPA. Four studies reporting ORs revealed a positive ACPA status was more probable to be RA-ILD (ORs= 2.14, 95% CI 1.33–3.47; p < 0.05, Figure 11) [Citation17,Citation18,Citation47,Citation49]. In contrast, another study reported HR showed no association [Citation43]. Several studies reporting ORs showed a positive RF status and RA-ILD were significantly associated (ORs= 2.11, 95% CI 1.65–2.68; p < 0.05, Figure 12A) [Citation16,Citation17,Citation35,3839,Citation41,Citation45,Citation49]. However, studies reporting HRs indicated no significant correlation (HRs = 1.15, 95%CI 0.75–1.77, Figure 12B) [Citation36,Citation43]. Four studies reported higher ESR could significantly enhance the RA-ILD prevalence (ORs = 1.008, 95%CI 1.002–1.014; p < 0.05, Figure 13) [Citation35,Citation38, Citation41, Citation45]. Conversely, another study reporting HR found no significant correlation [Citation36]. Between CRP and RA-ILD prevalence, no significant difference was observed(ORs = 1.002, 95% CI 0.995–1.010; p = 0.59, Figure 14) [Citation38,Citation45,Citation47]. Five studies revealed a notable correlation between DAS28 and prevalence [Citation2,Citation16,Citation20,Citation31,Citation37], particularly moderate and high DAS28 [Citation16,Citation20,Citation37]. The pooled estimate indicated that moderate and high DAS28 (≥3.2) could be a notable risk factor for RA-ILD (ORs = 1.87, 95%CI 1.36–2.58; p < 0.05, Figure 15) [Citation16,Citation37]. Another study found similar results (HR = 2.08, 95%CI 1.06–4.05 and HR = 3.48, 95%CI 1.64–7.38) [Citation20]. In contrast, four studies did not reveal any link between DAS28 and prevalence [Citation15,Citation36,Citation38,Citation41]. In studies reporting ORs, rheumatoid nodules displayed a strong correlation(ORs = 1.87, 95%CI 1.18–2.98; p < 0.05, Figure 16A) [Citation35,Citation37]. In studies reporting HRs, rheumatoid nodules predicted no correlation (HRs = 1.34, 95%CI 0.86–2.07; p < 0.05, Figure 16B) [Citation10,Citation20,Citation36,Citation43]. The pooled estimate indicated no correlation between erosion and prevalence (HRs= 1.26, 95%CI 0.60–2.65, Figure 17) [Citation10,Citation20,Citation36].
Based on the medication used, no significant difference was found in MTX group (ORs =0.84, 95% CI 0.56–1.27, Figure 18B [Citation32,Citation34,Citation35,Citation37,Citation38,Citation46,Citation47]; HRs = 1.02, 95%CI 0.83–1.27, Figure 18A [Citation10,Citation19,Citation20,Citation33,Citation36,Citation43]). The use of LEF was reported a substantial correlation with prevalence (ORs = 1.42, 95%CI 1.08–1.87; p < 0.05, Figure 19)[Citation32,Citation38,Citation46,Citation47]. In studies reporting HRs, the use of steroids had a significant correlation with prevalence (HRs= 1.70, 95% CI 1.13–2.55; p < 0.05, Figure 20A) [Citation10,Citation19,Citation20,Citation43,Citation51]. Conversely, in studies reporting ORs, the use of steroids showed no correlation with RA-ILD prevalence (ORs = 1.54, 95%CI 0.92–2.59; Figure 20B)[Citation2,Citation37,3847]. Additionally, the pooled results manifested that biologic agents usage was an important protective factor (HRs = 0.77, 95% CI 0.69–0.87; p < 0.05, Figure 21A) [Citation19,Citation43,Citation51] which was contrary to studies reporting the ORs (ORs = 0.98, 95% CI 0.73–1.33; Figure 21B) [Citation32,Citation37,Citation38,Citation46].
Sensitivity analyses
The combined OR/HR values of each risk factor with significant heterogeneity (male sex, smoking history, older age of RA onset, ACPA, CRP, rheumatoid nodules, erosion, MTX use, biological agents use, and steroid use) remained consistent with the aforementioned results even after employing the leave-one-out sensitivity analysis test, indicating the results were stable. The study by Ong et al. [Citation18] was most likely responsible for the heterogeneity. The I2 value in the ACPA effect dropped significantly from 56.7% to 0% when we omitted this study. The absence of ACPA data in 11.6% of patients in this study could be the reason for this.
Discussion
This systematic review aims to scrutinize the available evidence on the prevalence and risk factors for RA-ILD in observational studies. Coinciding with a considerable rise in mortality of RA-ILD, the global prevalence is expected to rise and varies widely. Despite the identification of several risk factor, it’s still possible for ILD to develop without them. Therefore, a comprehensive search of evidence for risk factors for RA-ILD and thus constant surveillance of the progression of RA-ILD is essential for the prompt application of therapeutic strategies to improve prognosis.
The pooled prevalence of RA-ILD was 18.7% among the 34 included studies in this systematic review and meta-analysis, highlighting that ILD is a prevalent extra-articular manifestation of RA, as described in previous studies. The variations in prevalence can be ascribed to discrepancies in the study region, case definition (various RA classification criteria and ILD classification criteria), sample size, RA duration, and other factors.
The geographical location has significantly affected the prevalence of RA-ILD. Studies conducted in America (12.5%) reported significantly lower prevalence as compared to the other four continents. And prevalence in South America (36.6%) was higher than in North America (7.6%). Given the multi-ethnic populations in each region, it is likely that this variation is related to the local ethnic composition. Previous studies found that Indian ethnicity was more susceptible to developing RA-ILD [Citation18,Citation53] and African Americans developed ILD earlier than non-African Americans [Citation54], indicating the impact of genetic factors. Additionally, environmental factors (e.g. PM2.5 concentration and composition) and socioeconomic level differed greatly among diverse regions, which should be considered in any research assessing its impact on health [Citation32]. Variations in case definitions of RA-ILD also contributed to differences in prevalence. Since HRCT is the standard noninvasive method for RA-ILD diagnosis and follow-up [Citation55], the prevalence increased to 21.5% (95% CI 17.8–25.2) when we limited the analysis to studies conducting HRCT scans (n = 27). When we limited the analysis to studies only using HRCT scans (n = 16), the prevalence even increased to 22.7% (95%CI 17.5–27.9) when we only use HRCT. Although our meta-regression revealed that the diagnostic criteria was not related to the prevalence, it could be attributed to the fact that the majority of studies used HRCT and only a minority used other methods (n = 7). As certain studies relied on ICD-9-CM diagnoses on claims rather than utilizing comprehensive clinical information available in medical records, unsatisfactory classifications of RA-ILD status may have occurred. This highlights the need to develop a more reliable and consistent RA-ILD diagnostic method.
Due to the impact of diverse study designs and the interaction between variables, it is still obscure which factors may definitely influence the outcomes. Factors associated with the course of RA and other comorbidities could potentially impact the outcomes. Additionally, lung abnormalities associated with ILD and other subclinical involvement may affect the accuracy of research. We also found those employed a small sample size reported a higher prevalence. The small sample size may affect the reliability of the study. Therefore, longitudinal studies involving larger cohorts of RA are necessary to explore the prevalence of RA-ILD.
Out of all 32 factors, 18 factors were contained in our meta-analysis, while the rest were qualitatively analyzed. We identified 13 risk factors linked to RA-ILD: positive ACPA, positive RF, pulmonary comorbidities, male sex, smoking history, moderate and high DAS28 (≥3.2), rheumatoid nodules, steroid use, LEF use, longer RA duration, higher ESR (based on the pooled results, ORs/HRs > 1), and older age, older age of RA onset (based on WMDs). Moreover, biological agent use was found a protective factor. Most data included were multivariable-adjusted effect estimates (OR, RR, HR, and 95% CI). Knowledge of risk factors, which can be easily assessed in daily clinical practice, will help identify high-risk patients, thereby paving the way for strategies that can increase survival and alter the natural course before they become clinically severe.
Our study identified males [Citation10,Citation17,Citation18,Citation35], advanced age [Citation10,Citation56], late onset of the disease [Citation47] and smoking history [Citation57,Citation58] were inclined to be RA-ILD, which is also aligned with previous studies. Despite women being at a higher risk of developing RA, males tend to predominate in the development of RA-ILD. However, some studies suggest otherwise. Women tend to develop RA-ILD 3.4 times more often than men, according to Ong et al. [Citation18]. We hypothesize that this may be because women made up the majority of the study’s participant categories (RA patients with and without ILD), which has an impact on whether smoking is viewed as a risk factor. Intriguingly, Kronzer et al. [Citation37] observed that heavier smokers were more susceptible to acquiring ILD among patients with RA who smoked. This may be related to the fact that atelectasis or poor CT scans in obese individuals could lead to misclassification of RA-ILD. These discoveries have significant clinical ramifications. Since obesity and smoking are emerging modifiable risk factors for RA-ILD, clinicians can advise their patients on lifestyle modification, leading to a better health outcome. Regarding comorbidities, Curtis et al. [Citation19] reported that the risk of RA-ILD was elevated by other pulmonary comorbidities, such as chronic obstructive pulmonary disease (COPD) at baseline. This may be due to the induction of pulmonary inflammatory response by the underlying lung disease, leading to the persistence of lung injury thereby raising the risk of ILD. In recent years, other respiratory exposures like air pollution, have been suggested as potential contributors to the occurrence of RA-ILD. It’s well-established that pulmonary and systemic autoimmune diseases can be increased by exposure to PM2.5 [Citation59,Citation60]. Zhao et al. [Citation50] reported exposure to PM2.5 components, especially ammonium, enhances the potential of ILD in RA patients. Therefore, reducing air pollution emissions may have a positive impact on the public’s health. Significantly, some comorbidities (excluding respiratory) could be protective factors, possibly due to treatment differences. For example, the therapies for malignancy may have afforded immunosuppressive effects [Citation35].
When diagnosing RA, the seropositivity of RF/ACPA is favorable. However, the relationship between seropositivity for RF/ACPA and RA-ILD is still debatable. The significantly positive correlation between RF/ACPA positivity and RA-ILD was shown by Yin et al. [Citation49] and Kelly et al. [Citation17], while Chen et al. [Citation9]and Inui et al. [Citation61] were unable to find any connection. The inconsistency observed was likely due to study designs, particularly the heterogeneity within patient populations. Furthermore, our study found that moderate and high sustained RA activity, especially DAS28 (≥3.2), increases the risk of ILD prevalence. This suggested that systemic inflammation may be involved in the etiology of RA-ILD; thus, by lowering disease activity, we might also change the course of RA-ILD’s natural history. Although some studies have shown that longer RA duration, higher DAS28 scores, CRP, and ESR increase the likelihood of RA-ILD, others suggest otherwise. This may be due to the fact that some studies use continuous variables rather than stratified comparisons. Thus, we recommend the use of stratified data, which would be more clinically meaningful. For instance, rather than a prolonged RA progression, the onset of ILD may occur more frequently over the first 5 to 10 years [Citation47].
Concerns about a connection between RA treatment, such as disease-modifying antirheumatic drugs (DMARDs) usage, and the deterioration of RA-ILD have been highlighted in numerous recent studies [Citation62,Citation63]. Instead of establishing a causal effect, these studies raised RA-ILD patients’ uneasiness with utilizing these therapies. The question of whether MTX exposure, as a first-line medication for rheumatoid arthritis treatment, increased the risk of pulmonary damage was of greatest public health concern. In RA patients on MTX, Bongartz et al. [Citation10] observed a higher prevalence. However, most subsequent studies showed MTX had no relationship to the progression of RA-ILD, and in some cases, even could postpone the onset and lower the prevalence of RA-ILD [Citation37,Citation64]. Furthermore, MTX was preferred above other DMARDs for the treatment of inflammatory arthritis in patients with modest and stable airway or parenchymal lung disorders and moderate-to-high disease activity, according to the 2021 ACR treatment guideline [Citation65]. In our study, using biologic agents considerably lowered the risk of RA-ILD while using LEF dramatically raised the risk. According to a prior meta-analysis, using LEF did not increase respiratory adverse events [Citation62]. However, Roubille et al. [Citation63] highlighted that LEF and biologic agents may induce or worsen RA-ILD. These inconsistencies can be attributable to the difference in study designs as well as variations in medication duration and dosage of the included population.
Since therapies and other external factors can alter the natural development of RA-ILD by affecting the body’s immune response (e.g. treatments with DMARDs and biological agents may reduce the levels of ACPA, while smoking increase it [Citation66]), this implies that we should pay more attention to the interactions and mechanisms of action between the various pathogenic factors. Furthermore, the use of conventional synthetic antirheumatic drugs according to the severity of ILD and the disease activity of RA is of great significance in the treatment of RA-ILD.
Strengths and limitations
Our study possesses a lot of advantages. First of all, it’s the first systematic review and meta-analysis to analyze the prevalence of RA-ILD and its potential risk factors (32 factors in three domains), combining the outcome data of the studies with ORs and HRs thereafter, evaluating the consistency of the results in the two study types. The inconsistency in the results of ORs and HRs in rheumatoid nodules and the use of steroid and biologic agents can be attributed to variations in the study design, particularly significant heterogeneity within study populations and analytical methods. Second, all included data on association of interest were multivariable-adjusted effect estimates (OR, RR, HR, and 95% CI), which effectively control the influence of confounding factors (such as age, sex, etc.).
However, it is important to acknowledge several limitations within our study. First, the diagnostic criteria employed for our participants varied and may be affected by subclinical involvement. Despite the fact that research from five geographical regions was considered, some regions were underrepresented since there were fewer papers accessible. To account for heterogeneity, we chose to carry out subgroup and meta-regression analysis by geographical regions (or diagnostic modalities, etc.). Moreover, the heterogeneity measures (I2) revealed in our study are quite substantial (I2 >90%). Analogous to prior meta-analyses [Citation67,Citation68], a significant I2 is frequently produced by including large sample sizes. Based on observational research, heterogeneity remains an inherent limitation of meta-analyses. Second, the prevalence estimates were based on observational studies, which do not imply causality but only association. Finally, it’s crucial to keep in mind that some risk factors only had a sparsely populated body of research, which may affect the robustness of our findings.
Conclusion
This meta-analysis offers a thorough summary of the knowledge currently available regarding ILD among RA patients and the risk factors related to it. Our findings indicate that the onset of RA-ILD is related to environmental exposures, sociodemographic and clinical characterization factors such as PM2.5 and smoking, gender, disease duration, etc. It is imperative to comprehensively determine the risk factors for RA-ILD and appropriately manage any persistent or emerging RA-ILD. These findings have important public health implications as they provide valuable insights into the implementation of effective interventions to guide clinical prevention and treatment with the goal to reduce the risk of RA-ILD. Finally, given the reported significant heterogeneity further studies are needed to investigate the relevance of different risk and protective factors able to affect the RA-ILD prevalence.
Authors’ contributions
Hong-Fei Wang: Conceptualization, methodology, data collection and analysis, illustration, project administration, review writing and editing. Yan-Yun Wang: Methodology, data collection and analysis, review writing. Zhi-Yu Li: Investigation, review writing and editing, and supervision. Shan Liu and Pei-Jie He: Review writing and editing. Qiu-Shuang Li: Conceptualization, methodology, data analysis, project administration, review writing and editing, and supervision. All authors revised and approved the final manuscript.
Supplemental Material
Download MS Word (30.4 KB)Supplemental Material
Download MS Word (884.5 KB)Acknowledgments
We want to convey our appreciation to the reviewers for their valuable feedback that contributed to the improvement of our article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data supporting the findings of the present study can be accessed in this manuscript and its supplementary files.
Additional information
Funding
References
- Paulin F, Doyle TJ, Mercado JF, et al. Development of a risk indicator score for the identification of interstitial lung disease in patients with rheumatoid arthritis. Reumatol Clin. 2021;17(4):1–17. doi: 10.1016/j.reuma.2019.05.007.
- Restrepo JF, Del Rincón I, Battafarano DF, et al. Clinical and laboratory factors associated with interstitial lung disease in rheumatoid arthritis. Clin Rheumatol. 2015;34(9):1529–1536. doi: 10.1007/s10067-015-3025-8.
- Gochuico BR, Avila NA, Chow CK, et al. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med. 2008;168(2):159–166. doi: 10.1001/archinternmed.2007.59.
- Robles-Perez A, Luburich P, Rodriguez-Sanchon B, et al. Preclinical lung disease in early rheumatoid arthritis. Chron Respir Dis. 2016;13(1):75–81. doi: 10.1177/1479972315620746.
- Zamora-Legoff JA, Krause ML, Crowson CS, et al. Patterns of interstitial lung disease and mortality in rheumatoid arthriti. Rheumatology. 2017;56(10):1825. doi: 10.1093/rheumatology/kex299.
- Richman NC, Yazdany J, Graf J, et al. Extraarticular manifestations of rheumatoid arthritis in a multiethnic cohort of predominantly hispanic and Asian patients. Medicine. 2013;92(2):92–97. doi: 10.1097/MD.0b013e318289ce01.
- Carmona L, González-Alvaro I, Balsa A, et al. Rheumatoid arthritis in Spain: occurrence of extra-articular manifestations and estimates of disease severity. Ann Rheum Dis. 2003;62(9):897–900. doi: 10.1136/ard.62.9.897.
- Norton S, Koduri G, Nikiphorou E, et al. A study of baseline prevalence and cumulative incidence of comorbidity and extra-articular manifestations in RA and their impact on outcome. Rheumatology. 2013;52(1):99–110. doi: 10.1093/rheumatology/kes262.
- Chen J, Shi Y, Wang X, et al. Asymptomatic preclinical rheumatoid arthritis-associated interstitial lung disease. Clin Dev Immunol. 2013;2013:406927–406925. doi: 10.1155/2013/406927.
- Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62(6):1583–1591. doi: 10.1002/art.27405.
- Zhang Y, Li H, Wu N, et al. Retrospective study of the clinical characteristics and risk factors of rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol. 2017;36(4):817–823. doi: 10.1007/s10067-017-3561-5.
- Gabbay E, Tarala R, Will R, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med. 1997;156(2 P 1):528–535. doi: 10.1164/ajrccm.156.2.9609016.
- Bonilla Hernán MG, Gómez-Carrera L, Fernández-Velilla Peña M, et al. Prevalence and clinical characteristics of symptomatic diffuse interstitial lung disease in rheumatoid arthritis in a Spanish population. Rev Clin Esp. 2022;222(5):281–287. doi: 10.1016/j.rceng.2021.01.011.
- Fdda S, Khairy N, Fayed H, et al. Interstitial lung disease in Egyptian patients with rheumatoid arthritis: frequncy, pattern and correlation with clinical manifestations and anti-citrullinated peptide antibodies level. Egypt Rheumatol. 2018;40(3):155–160. doi: 10.1016/j.ejr.2017.10.006.
- Dhooria S, Babu V, Dhir V, et al. Factors associated with interstitial lung disease and the progressive fibrosing phenotype in rheumatoid arthritis–related interstitial lung disease. Medical J Armed Forces India. 2022. doi: 10.1016/j.mjafi.2022.08.004.
- Kakutani T, Hashimoto A, Tominaga A, et al. Related factors, increased mortality and causes of death in patients with rheumatoid arthritis-associated interstitial lung disease. Mod Rheumatol. 2020;30(3):458–464. doi: 10.1080/14397595.2019.1621462.
- Kelly CA, Saravanan V, Nisar M, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics–a large multicentre UK study. Rheumatology. 2014;53(9):1676–1682. doi: 10.1093/rheumatology/keu165.
- Ong SG, Ding HJ, Zuhanis AH, et al. Predictors and radiological characteristics of rheumatoid arthritis-associated interstitial lung disease in a multi-ethnic Malaysian cohort. Med J Malaysia. 2022;77(3):292–299.
- Curtis JR, Sarsour K, Napalkov P, et al. Incidence and complications of interstitial lung disease in users of tocilizumab, rituximab, abatacept and anti-tumor necrosis factor α agents, a retrospective cohort study. Arthritis Res Ther. 2015;17(1):319. doi: 10.1186/s13075-015-0835-7.
- Sparks JA, He X, Huang J, et al. Rheumatoid arthritis disease activity predicting incident clinically apparent rheumatoid arthritis–associated interstitial lung disease: a prospective cohort study. Arthritis Rheumatol. 2019;71(9):1472–1482. doi: 10.1002/art.40904.
- Page MJ, Mckenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
- Wells G, Shea B, O’connell J. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Health Research Institute Web site, 2014, 7.
- Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141.
- Viera AJ. Odds ratios and risk ratios: what’s the difference and why does it matter?. South Med J. 2008;101(7):730–734. doi: 10.1097/SMJ.0b013e31817a7ee4.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186.
- Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. doi: 10.1002/jrsm.12.
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629.
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x.
- Shi L, Lin L. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyse. Medicine. 2019;98(23):e15987. doi: 10.1097/MD.0000000000015987.
- Akiyama M, Kaneko Y, Yamaoka K, et al. Association of disease activity with acute exacerbation of interstitial lung disease during tocilizumab treatment in patients with rheumatoid arthritis: a retrospective, case-control study.Rheumatol Int. 2016;36(6):881–889. J doi: 10.1007/s00296-016-3478-3.
- Ben Tekaya A, Mokaddem S, Athimini S, et al. Risk factors for rheumatoid arthritis-associated interstitial lung disease: a retrospective study. Multidiscip Respir Med. 2022;17:877. doi: 10.4081/mrm.2022.877.
- Chen HH, Chao WC, Chen YH, et al. Air pollutants and development of interstitial lung disease in patients with autoimmune diseases. Ann Rheum Dis. 2020;79(SUPPL 1):865.1–865. doi: 10.1136/annrheumdis-2020-eular.204.
- Ibfelt EH, Jacobsen RK, Kopp TI, et al. Methotrexate and risk of interstitial lung disease and respiratory failure in rheumatoid arthritis: a nationwide population-based study. Rheumatology. 2021;60(1):346–352. doi: 10.1093/rheumatology/keaa327.
- Juge PA, Lee JS, Lau J, et al. Methotrexate and rheumatoid arthritis associated interstitial lung disease. Ann Rheum Dis. 2020;79(SUPPL 1):25.1–25. doi: 10.1136/annrheumdis-2020-eular.1678.
- Kiely P, Busby AD, Nikiphorou E, et al. Is incident rheumatoid arthritis interstitial lung disease associated with methotrexate treatment? Results from a multivariate analysis in the ERAS and ERAN inception cohorts. BMJ Open. 2019;9(5):e028466. doi: 10.1136/bmjopen-2018-028466.
- Koduri G, Norton S, Young A, et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology. 2010;49(8):1483–1489. doi: 10.1093/rheumatology/keq035.
- Kronzer VL, Huang W, Dellaripa PF, et al. Lifestyle and clinical risk factors for incident rheumatoid arthritis-associated interstitial lung disease. J Rheumatol. 2021;48(5):656–663. doi: 10.3899/jrheum.200863.
- Li L, Liu R, Zhang Y, et al. A retrospective study on the predictive implications of clinical characteristics and therapeutic management in patients with rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol. 2020;39(5):1457–1470. doi: 10.1007/s10067-019-04846-1.
- Mori S, Koga Y, Sugimoto M. Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir Med. 2012;106(11):1591–1599. doi: 10.1016/j.rmed.2012.07.006.
- Natalini JG, Baker JF, Singh N, et al. Autoantibody seropositivity and risk for interstitial lung disease in a prospective male-predominant rheumatoid arthritis cohort of US veterans. Ann Am Thorac Soc. 2021;18(4):598–605. doi: 10.1513/AnnalsATS.202006-590OC.
- Rocha-Muñoz AD, Ponce-Guarneros M, Gamez-Nava JI, et al. Anti-Cyclic citrullinated peptide antibodies and severity of interstitial lung disease in women with rheumatoid arthritis. J Immunol Res. 2015;2015:151626–151610. doi: 10.1155/2015/151626.
- Salaffi F, Carotti M, Di Carlo M, et al. High-resolution computed tomography of the lung in patients with rheumatoid arthritis: prevalence of interstitial lung disease involvement and determinants of abnormalities. Medicine. 2019;98(38):e17088. doi: 10.1097/MD.0000000000017088.
- Samhouri BF, Vassallo R, Achenbach SJ, et al. Incidence, risk factors, and mortality of clinical and subclinical rheumatoid arthritis–associated interstitial lung disease: a population-based cohort. Arthritis Care Res. 2022;74(12):2042–2049. doi: 10.1002/acr.24856.
- Severo CR, Chomiski C, Do Valle MB, et al. Assessment of risk factors in patients with rheumatoid arthritis-associated interstitial lung disease. J Bras Pneumol. 2022;48(6):e20220145. doi: 10.36416/1806-3756/e20220145.
- Shidara K, Hoshi D, Inoue E, et al. Incidence of and risk factors for interstitial pneumonia in patients with rheumatoid arthritis in a large Japanese observational cohort, IORRA. Mod Rheumatol. 2010;20(3):280–286. doi: 10.3109/s10165-010-0280-z.
- Suissa S, Hudson M, Ernst P. Leflunomide use and the risk of interstitial lung disease in rheumatoid arthritis. Arthritis Rheum. 2006;54(5):1435–1439. doi: 10.1002/art.21806.
- Wang JX, Du CG. A retrospective study of clinical characteristics of interstitial lung disease associated with rheumatoid arthritis in Chinese patients. Med Sci Monit. 2015;21:708–715. doi: 10.12659/MSM.890880.
- Wang T, Zheng XJ, Ji YL, et al. Tumour markers in rheumatoid arthritis-associated interstitial lung disease. Clin Exp Rheumatol. 2016;34(4):587–591.
- Yin Y, Liang D, Zhao L, et al. Anti-cyclic citrullinated peptide antibody is associated with interstitial lung disease in patients with rheumatoid arthritis. PLOS One. 2014;9(4):e92449. doi: 10.1371/journal.pone.0092449.
- Zhao N, Al-Aly Z, Zheng B, et al. Fine particulate matter components and interstitial lung disease in rheumatoid arthritis. Eur Respir J. 2022;60(1):2102149. doi: 10.1183/13993003.02149-2021.
- Zheng B, De Moura CS, Machado M, et al. Association between chronic obstructive pulmonary disease, smoking, and interstitial lung disease onset in rheumatoid arthritis. Clin Exp Rheumatol. 2022;40(7):1280–1284. doi: 10.55563/clinexprheumatol/i9au1r.
- Zheng M, Lou A, Zhang H, et al. Serum KL-6, CA19-9, CA125 and CEA are diagnostic biomarkers for rheumatoid arthritis-associated interstitial lung disease in the Chinese population. Rheumatol Ther. 2021;8(1):517–527. doi: 10.1007/s40744-021-00288-x.
- Mitha M, Ghammo H, Connolly C, et al. Rheumatoid arthritis associated interstitial lung disease in KwaZulu-Natal, South Africa: clinical profile and treatment outcomes. European Respiratory Journal. 2020; 56 (suppl 64): 804. doi: 10.1183/13993003.congress-2020.804.
- Adegunsoye A, Oldham JM, Bellam SK, et al. African-American race and mortality in interstitial lung disease: a multicentre propensity-matched analysis. Eur Respir J. 2018;51(6):1800255. doi: 10.1183/13993003.00255-2018.
- Biederer J, Schnabel A, Muhle C, et al. Correlation between HRCT findings, pulmonary function tests and bronchoalveolar lavage cytology in interstitial lung disease associated with rheumatoid arthritis. Eur Radiol. 2004;14(2):272–280. doi: 10.1007/s00330-003-2026-1.
- Suda T. Up-to-date information on rheumatoid arthritis-associated interstitial lung disease. Clin Med Insights Circ Respir Pulm Med. 2015;9(Suppl 1):155–162. doi: 10.4137/CCRPM.S23289.
- Gianfrancesco MA, Crowson CS. Where there’s smoke, there’s a joint: passive smoking and rheumatoid arthritis. Arthritis Rheumatol. 2021;73(12):2161–2162. doi: 10.1002/art.41940.
- Albano SA, Santana-Sahagun E, Weisman MH. Cigarette smoking and rheumatoid arthritis. Semin Arthritis Rheum. 2001;31(3):146–159. doi: 10.1053/sarh.2001.27719.
- Sunyer J. Urban air pollution and chronic obstructive pulmonary disease: a review. Eur Respir J. 2001;17(5):1024–1033. doi: 10.1183/09031936.01.17510240.
- Farhat SC, Silva CA, Orione MA, et al. Air pollution in autoimmune rheumatic diseases: a review. Autoimmun Rev. 2011;11(1):14–21. doi: 10.1016/j.autrev.2011.06.008.
- Inui N, Enomoto N, Suda T, et al. Anti-cyclic citrullinated peptide antibodies in lung diseases associated with rheumatoid arthritis. Clin Biochem. 2008;41(13):1074–1077. doi: 10.1016/j.clinbiochem.2008.06.014.
- Conway R, Low C, Coughlan RJ, et al. Leflunomide use and risk of lung disease in rheumatoid arthritis: a systematic literature review and metaanalysis of randomized controlled trials. J Rheumatol. 2016;43(5):855–860. doi: 10.3899/jrheum.150674.
- Roubille C, Haraoui B. Interstitial lung diseases induced or exacerbated by DMARDS and biologic agents in rheumatoid arthritis: a systematic literature review. Semin Arthritis Rheum. 2014;43(5):613–626. doi: 10.1016/j.semarthrit.2013.09.005.
- Juge PA, Lee JS, Lau J, et al. Methotrexate and rheumatoid arthritis associated interstitial lung disease. Eur Respir J. 2021;57(2):2000337. doi: 10.1183/13993003.00337-2020.
- Fraenkel L, Bathon JM, England BR, et al. 2021 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2021;73(7):924–939. doi: 10.1002/acr.24596.
- Alessandri C, Bombardieri M, Papa N, et al. Decrease of anti-cyclic citrullinated peptide antibodies and rheumatoid factor following anti-TNFalpha therapy (infliximab) in rheumatoid arthritis is associated with clinical improvement. Ann Rheum Dis. 2004;63(10):1218–1221. doi: 10.1136/ard.2003.014647.
- Kamiya H, Panlaqui OM. Systematic review and meta-analysis of the risk of rheumatoid arthritis-associated interstitial lung disease related to anti-cyclic citrullinated peptide (CCP) antibody. BMJ Open. 2021;11(3):e040465. doi: 10.1136/bmjopen-2020-040465.
- Xie S, Li S, Chen B, et al. Serum anti-citrullinated protein antibodies and rheumatoid factor increase the risk of rheumatoid arthritis-related interstitial lung disease: a meta-analysis. Clin Rheumatol. 2021;40(11):4533–4543. doi: 10.1007/s10067-021-05808-2.