?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
To compare the effectiveness, cost, and safety of four regimens recommended by the World Health Organization (WHO) for rifampicin resistance/multidrug-resistance tuberculosis (RR/MDR-TB) Treatment in Eastern China.
Methods
We performed a cohort study among patients with RR/MDR between 2020 and 2022 in Jiangsu Province. The treatment success rate, cost, and drug adverse reaction rate were compared.
Results
Between 2020 and 2022, 253 RR/MDR-TB patients were enrolled in the study. 37 (14.62%), 76 (30.04%), 74 (29.25%), and 66 (26.09%) patients had the short-term regimens, the new long-term oral regimens, the new long-term injectable regimens, and the traditional long-term regimens, respectively. The treatment success rate was the highest among patients treated with the short-term regimen (75.68%) and was the lowest among patients treated with the traditional long-term regimens (60.61%). The estimated mean cost per favorable outcome was 142.61 thousand Chinese Yuan (CNY), and the short-term regimens showed the lowest cost in the four regimes (88.51 thousand CNY vs. 174.24 thousand CNY, 144.00 thousand CNY, and 134.98 thousand CNY). Incremental cost-effectiveness ratios of the short-term regimens, the new long-term oral regimen, and the new long-term injectable regimens were −3083.04, 6040.09, and 819.68 CNY compared to the traditional long-term regimens.
Conclusions
For RR/MDR-TB patients in China who meet the criteria for short-term regimens, the short-term regimens were proven to be the most cost-effective of the four regimens recommended by WHO. For RR/MDR-TB patients in China who don’t meet the criteria for short-term regimens, the new long-term injectable regimens are more cost-effective than the remaining two regimens.
HIGHLIGHTS
This is the first study to evaluate the effectiveness, cost, and safety of four regimens recommended by the WHO for RR/MDR-TB treatment in China.
For RR/MDR-TB patients in China who meet the criteria for the short-term regimens, the short-term regimens were proven to be the most cost-effective of the four regimens recommended by WHO.
Introduction
Tuberculosis is a chronic disease caused by mycobacterium tuberculosis (Mtb) and has become a global public health problem, posing a threat to human health. In 2021, there were an estimated 450,000 incident cases of multidrug resistance tuberculosis (MDR-TB) or rifampicin resistance tuberculosis (RR-TB) worldwide [Citation1], which has turned into one of the difficulties in the control of TB epidemiology. China is one of the highest-burden countries in terms of number of RR/MDR-TB cases, with 33,000 estimated new RR/MDR-TB cases in 2021. Compared to general TB patients, people who developed RR/MDR-TB have poorer anti-tuberculosis treatment outcomes, higher treatment costs, and longer duration of the infectious period. In addition, RR/MDR-TB patients can cause more potential spreads due to the longer bacteria carrier time [Citation2].
The management of RR/MDR-TB demands extended treatment periods involving a combination of multiple second-line drugs. These drugs are generally less effective, more expensive, and more toxic compared to first-line drugs [Citation3]. In 2019, Jiangsu province tried to centrally purchase RR/MDR-TB second-line anti-tuberculosis drugs for the first time, and supply drugs freely for RR/MDR-TB patients to further achieve the containment of TB prevalence. The latest treatment guidelines for drug-resistant tuberculosis by the World Health Organization [Citation4–6] have proposed four RR/MDR-TB treatment regimens, including the traditional long-term regimens, the new short-term regimens, the new long-term oral regimens, and the new long-term regimens with injections. However, Adverse events are prevalent with current regimens for multidrug-resistant tuberculosis, and inadequate handling of these reactions can substantially influence adherence, heightening the risk of treatment interruptions or failure, and ultimately impacting the overall treatment outcome [Citation7,Citation8]. The effectiveness of these new regimens has been partially verified abroad [Citation9–11], whereas there were few related studies in China. In addition to the traditional long-term regimens, the effectiveness of the other three new treatment regimens remained unclear.
A study from South Africa has suggested that the direct disease burden of RR/MDR-TB patients included outpatient costs, hospitalization costs, community follow-up directly observed treatments (DOTS) costs, drug costs, test costs, strain transportation costs, and other death-related costs; and indirect costs included lost wages, nutrition costs, transportation costs, board and lodging costs, employment care costs and so on. Consequently, the direct economic burden of treating a drug-sensitive TB patient is 256.61 dollars, while the cost of treating an MDR-TB patient is 6771.92 dollars, nearly 30 times that of general TB patients [Citation12]. Long-term and expensive treatment for RR/MDR-TB patients has caused a huge economic burden both on individuals and society, therefore, it is necessary to analyze the treatment regimens’ cost-effectiveness.
This study aims to compare the treatment success rates of four RR/MDR-TB treatment regimens, and comprehensively evaluate their economic benefits and safety, to provide a theoretical basis for the selection of the most suitable RR/MDR-TB treatment regimens.
Methods
Study population
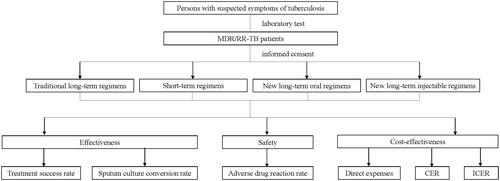
A prospective cohort was designed to continuously enroll RR/MDR-TB patients from 1 January 2021 to 31 December 2022 at 13 municipal designated hospitals in Jiangsu province. Regardless of whether they were patients undergoing initial treatment or those undergoing retreatment, individuals meeting the inclusion criteria would be included in the cohort. Inclusion criteria are (1) RR/MDR-TB patients; (2) patients aged over 15 years; (3) sputum is available for MTB-related examination; (4) patients agreed to participate in this project and sign the informed consent form. Exclusion criteria are (1) patients with current liver or kidney diseases (the examination indicator value is over two times the normal maximum value), metabolic diseases, autoimmune diseases, endocrine diseases, malignancies, etc.; (2) patients with abnormal hematological function, a history of long-term use of immunosuppressive drugs, or HIV/AIDS; (3) patients with a history of psychiatric or epilepsy; (4) pregnant or lactating women; (5) patients who do not agree to participate in this project and sign the informed consent form. The research flowchart is illustrated in .
Treatment regimens
According to the clinical characteristics, previous anti-TB treatment history, and the resistance of second-line anti-tuberculosis drugs, patients undergoing initial treatment or those undergoing retreatment would be assigned to different treatment regimen groups. The new short-term regimens: 4–6 Am-Mfx-Pto-Cfz-E-Z-HINH/5 Mfx-Cfz-E-Z; the new long-term oral regimens: 6Lfx (Mfx)-Lzd-Cfz-Cs-Z (E, Pto)/12–14 Lfx (Mfx)-Cfz-Cs-Z (E, Pto); the new long-term injectable regimens: 6Mfx (Lfx)-Lzd-Cfz (Cs)-Am-Pto-Z (E)/12 Mfx (Lfx)-Cfz (Cs)-Pto-Z (E); and the traditional long-term regimens(control group): 6 Cm (Am)-Lfx (Mfx)-Pto (PAS, E)-Cs (PAS, E)-Z/18 Lfx (Mfx)-Pto (PAS, E)-Cs (PAS, E)-Z. Patients diagnosed with multidrug-resistant tuberculosis are required to consume each dose under the direct supervision of a healthcare provider. Various methods of direct observation are employed, such as clinic visits, video calls, and electronic pillboxes. Notably, during the COVID-19 pandemic, patients primarily utilized WeChat video calls for medication supervision. Doctors would dispatch medication every one or two months.
Treatment outcomes
Enrolled patients will undergo monthly follow-ups during the intensive phase and bi-monthly follow-ups during the continuation phase. Follow-up assessments will include chest X-ray examinations, sputum smear tests, and culture examinations. Primary evaluation indicators will focus on the sputum negative conversion time and rates at the end of 2, 3, 4, 5, 6, and 12 months post-treatment initiation.
Patient symptoms and signs will be qualitatively assessed through questionnaire surveys to gauge improvement. The effectiveness of the four regimens will be evaluated at the end of the treatment course, considering the treatment success rate, sputum negative conversion time, and sputum negative conversion rates.
Adverse reaction
The number and types of adverse reactions among patients will be observed, including hepatic and biliary adverse reactions, such as jaundice, hepatitis, and abnormal liver function; dermatological adverse reactions, such as herpes, rash, and exfoliative dermatitis; allergic reactions, such as headache, fatigue, and fever; urinary system adverse reactions, such as elevated uric acid and decreased renal function; gastrointestinal adverse reactions, such as abdominal pain, nausea, vomiting, and loss of appetite; hematological adverse reactions; and other adverse reactions, such as blurred vision, tinnitus, and convulsion. At the end of the treatment course, the incidence rate of adverse reactions among several treatment regimens will be compared to assess whether these new regimens have severe adverse events and to assess their safety in comparison to the traditional long-term regimens. The Common Terminology Criteria for Adverse Events (CTCAE) was utilized to assess serious adverse events. It is structured into the following categories: (1) Grade 1: Mild; (2) Grade 2: Moderate; (3) Grade 3: Severe or medically significant but not immediately life-threatening; (4) Grade 4: Life-threatening consequences; urgent intervention indicated; (5) Grade 5: Death related to adverse event.
Micro-costing
We self-designed the questionnaire for the economic burden of RR/MDR-TB patients using a combination of open and closed questions, which includes two parts: a patient questionnaire and a primary caregiver questionnaire. The survey content is divided into three parts:
Patient basic information, including name, hospital ward, hospitalization number, admission date, past hospitalizations, origin place, family history, medication use, treatment regimens, etc.;
Patient questionnaire part, including age, sex, marital status, education level, occupation, personal monthly income, medical insurance form, quarterly outpatient visit frequency, time per visit, annual hospitalization frequency, time per hospitalization, medical costs, transportation and accommodation costs, work status, functional impairment, etc.;
Primary caregiver questionnaire part, including the relationship with the patient, sex, marital status, education level, occupation, family monthly income, costs and time spent caring for the patient, and caregiver employment costs.
Total costs consist of direct medical and direct non-medical costs. The direct medical costs include hospitalization fees, consultation fees, radiology fees, other imaging examination fees, laboratory testing fees, anti-tuberculosis drug fees, and other drug fees. The direct non-medical costs include transportation, meals, and other fees during hospitalization.
Data collection and analysis
In this study, investigators from tuberculosis designated hospitals collected relevant information through questionnaires for RR/MDR-TB patients directly. The questionnaires aimed to capture information about the patients’ socio-demographic characteristics, specifics of their diagnosis and treatment, and direct non-medical expenses incurred during the treatment period. After the completion of the treatment, the patients’ direct medical expenses were obtained from the hospital information system.
We will use the EpiData 3.1 software for double-entry of the data, and once inconsistencies are identified, the original questionnaire will be retrieved and checked by both data entry personnel simultaneously. The frequency of categorical variables was compared using chi-square test. Kruskal-Wallis rank sum test was used to compare the differences in expenses between groups. The cost of each patient will be calculated directly. In the economic analysis, we conducted a comparison of cost-effectiveness ratios (CER) among various treatment regimens, reflecting the cost associated with each unit of benefit or outcome achieved. Furthermore, we computed the incremental cost-effectiveness ratio (ICER), representing the average incremental cost linked to one additional unit of the intervention’s effect, for additional comparison. Generally, a lower CER or ICER is deemed more favorable. Statistical analyses will be performed using the SPSS 23.0 software (version 23.0, IBM Corporation, Armonk, NY, USA). The specific calculation formulas of CER and ICER are as follows:
Ethics approval and consent to participate
This study was reviewed and approved by the ethics committee of the Jiangsu Provincial Center for Disease Control and Prevention [Ethical Approval Number: JSJK2023-B022-01]. All eligible participants signed written informed consent.
Results
Patient characteristics
Between 2020 and 2022, 253 RR/MDR-TB patients were enrolled in the study. The mean age was 44.91 years old with an SD of 15.95. 180 (71.15%) were males. Nearly half (42.29%) of the people weighed more than 65 kg. Patients with urban employee basic medical insurance and urban resident medical insurance were similar and both took one-third. Two-thirds of individuals resided locally. More than half of the patients had a high school education. provides comprehensive information about the participants’ demographic background and initial clinical characteristics.
Table 1. Characteristics of patients eligible for a rifampicin resistance/multidrug resistance tuberculosis.
Overall outcomes
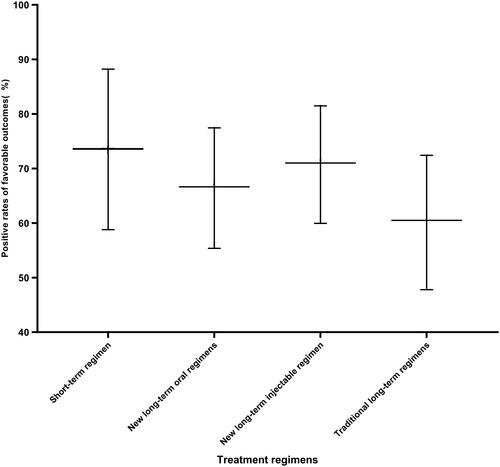
Of 253 patients, 37 (14.62%), 76 (30.04%), 74 (29.25%), and 66 (26.09%) had the short-term regimens, the new long-term oral regimens, the new long-term injectable regimens, and the traditional long-term regimens. 172 (67.98%) had favorable outcomes, 61 (24.12%) patients experienced treatment failure, 16 (6.32%) were lost to follow-up, and 4 (1.58%) died. The proportion with a favorable outcome was largest among patients treated with short-term regimens with 75.68% (95%CI: 58.80–88.23), and lowest in the traditional long-term regimens with 60.61% (95%CI: 47.81–72.42) having a favorable outcome. Success rates of long-term oral regimens and long-term injectable regimens, 67.11% (95%CI: 55.37–77.46) and 71.62% (95%CI: 59.95–81.50). however, there was no statistical significance among the four therapies (p = 0.372) ().
Sputum culture conversion
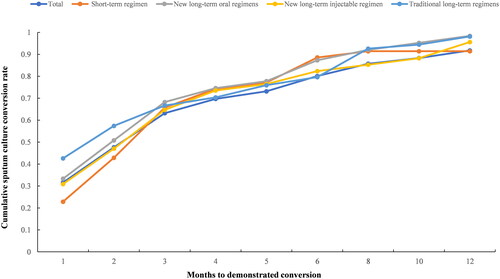
After excluding 34 patients who lacked the time of first culture conversion, the sputum culture conversion rates in the 1st, 2nd, 4th, 6th, and 12th months were 0.32, 0.48, 0.70, 0.80, and 0.92, respectively. The sputum culture conversion of short-term regimens was lower than the other three before 3rd month, however, after 4th, the cumulative conversion rate dramatic rise. Then by 12th month, the cumulative conversion remained relatively stable and consistent up to over 90% ().
Adverse drug reactions
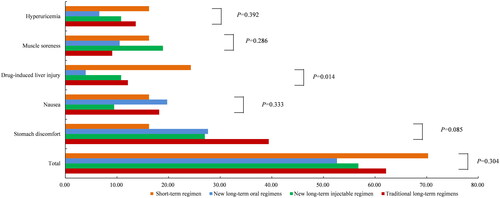
The majority of the patients (58.89%) experienced one or more adverse drug reactions during the course of the treatment, but no severe adverse occurred and none stopped treatments due to it definitively. The most common adverse reactions reported were stomach discomfort (28.85%), nausea (15.81%), muscle soreness (13.44%), drug-induced liver injury (11.07%), and hyperuricemia (11.07%). However, no participant experienced an adverse event reaching Grade 3 according to the Common Terminology Criteria for Adverse Events, which would pose a threat to life. Drug-induced liver injury was the most common reaction in the short-term regimens (24.32%), however, no one experienced a severe liver injury in the study. Different from the short-term regimens, commonly reported adverse reactions in the new long-term oral regimens, the new long-term injectable regimens, and the traditional long-term regimens were all stomach discomfort (27.63, 27.03, and 39.39%). There was no statistically significant difference in the incidence of adverse drug reactions among the different regimens (p = 0.304). The rate of adverse drug reactions varied depending on the treatment regimens, with the traditional long-term regimens showing a higher rate of drug-induced liver injury compared to others (p = 0.014) ().
Cost estimates
The average total annual cost of the four regimens was 142.61 thousand CNY, and after deducting medical insurance reimbursement, the cost was 37.02 thousand CNY. The average cost per patient of short-term regimens was much lower than the new long-term oral regimens, the new long-term injectable regimens, and the traditional long-term regimens (88.51 thousand CNY vs. 174.24 thousand CNY, 144.00, and 134.98 thousand CNY, p < 0.0001). The average annual out-of-pocket cost was 34.77 thousand CNY, 30.42 thousand CNY, 36.42 thousand CNY, and 46.54 thousand CNY (p = 0.028), however, after comparing pairwise, there was no statistically significant difference in cost between the short-term regimens and the other three regimens (data not shown) ().
Table 2. Cost estimates of economic burden of four regimens.
Cost-effectiveness
The estimated mean cost per favorable outcome was 145.61 thousand CNY, of which short-term regimens showed the lowest cost in the four regimes (91.09 thousand CNY vs. 181.51thousand CNY, 149.03 thousand CNY, and 133.45 thousand CNY). The CERs of these four regimes were listed in descending order from highest to lowest were the short-term regimens, the new long-term injectable regimens, the traditional long-term regimens, and long-term oral regimens (1169.58 vs. 2010.61 vs. 2226.95 vs. 2596.27 CNY). The ICER of the short-term regimens, the new long-term oral regimens, and the new long-term injectable regimens were −3083.04, 6040.09, and 819.68 CNY compared to the traditional long-term regimens ().
Table 3. The results of cost-effectiveness analysis of four regimens.
Discussion
To our knowledge, this is the first reported analysis in China of treatment outcomes and cost-effectiveness for RR/MDRTB patients using four different regimes WHO recommended [Citation6]. In this analysis, the original 9-month Bangladesh regimens were slightly modified: using amikacin(Am) and moxifloxacin (Mfx) instead of kanamycin (Km) and gatifloxacin (Gfx), respectively. In our study, the short-term regimens were proven to be the most cost-effective of the four regimens recommended by WHO. Considering the strict inclusion criteria of the short-term regimens, the new long-term oral regimens, the new long-term injectable regimens, and the traditional long-term regimens could serve as alternative replacement regimens. Besides, the new long-term injectable regimens are more cost-effective than the long-term oral regimens or the traditional long-term regimens.
RR/MDR-TB is a threat to tuberculosis control due to notorious for its toxicity, long duration, and poor effectiveness. Short-term and more effective regimens are urgently needed. A project offered by the Damien Foundation in Bangladesh over 27 million inhabitants provided annual treatment for about 24,000 patients with tuberculosis. The cure rate among these patients was up to 87.5% [Citation13], which provided us with new ideas and directions for the control of RR/MDR-TB. Subsequently, various regimens based on this scheme emerged. Van Deun et al. [Citation9] in Bangladesh in 2010, Aung et al. in Bangladesh in 2014 [Citation14], Piubello et al. [Citation15], in Niger in 2014, Kuaban et al. [Citation16], Cameroon in 2015, Trébucq et al. [Citation17], at nine countries in Africa in 2018, Yan et al. [Citation18] in China in 2018, and Du et al. [Citation19] in China in 2020 reported successful outcome rates 87.80, 84.50, 89.20, 89.30, 72.40, 70.50, and 68.70%. Our results demonstrated that the favorable outcome for treating RR/MDR-TB patients who were initiated with shorter regimens was 75.68%. Our results were consistent with the study by Trébucq et al. [Citation17], in 9 countries in Africa, Yan et al. [Citation18] in China in 2018, and, in which the short-term regimens resulted in a favorable rate of ∼70% treating RR/MDR-TB patients, whereas the rate was much lower than that in Bangladesh [Citation14], Niger [Citation15], and Cameroon [Citation16]. The superior treatment success rate of the Bangladesh regimens (short-term regimens) in treating RR/MDR-TB patients may be attributed to several potential factors. On the one hand, all patients were enrolled between 2020 and 2022, when there was a COVID-19 outbreak in China, resulting in adverse effects on the treatment [Citation20]. Patients might be quarantined at home, which would decrease the effectiveness by comparison, the treatment in other places was through directly observed treatment. On the other hand, Fluoroquinolones (FQs) continue to be the fundamental component of regimens for treating MDR-TB, as they exhibit high effectiveness and sterilizing power [Citation21]. In comparison with FQs in the Bangladesh regimens using Gfx, we used Mfx instead. According to a recent meta-analysis, there was a significant association between the use of Mfx and a higher rate of treatment failure in comparison to Gfx [Citation22]. This suggests that GFX may be a more appropriate choice among FQs for enhancing the treatment outcomes of RR/MDR-TB.
A noteworthy discovery was that patients with the short-term regimens had a lower rate of sputum-culture conversion n at the beginning until 3rd month, then higher compared to the control group. It was not quite consistent with previous research, which showed that the Clofazimine(CFZ)-containing treatment group had significantly quicker culture conversion compared to the control group [Citation19,Citation23]. The reason was unclear. We speculated that it might be due to the delayed antimicrobial activity against Mycobacterium tuberculosis of Mfx in vivo like CFZ [Citation24]. We supported the lower conversion rate of short-term regimens for RR/MDR-TB, suggesting changing the therapies depending on the results of 3–4th sputum culture conversion. The treatment of drug-resistant tuberculosis demands a prolonged and intricate therapeutic approach, often accompanied by a range of adverse drug reactions associated with second-line medications. Coping with these reactions necessitates additional effort, time, and financial resources from patients, typically impacting their quality of life, diminishing adherence, and contributing to suboptimal treatment outcomes [Citation25]. In this study, there was no significant difference in the incidence of adverse reactions among the four regimens. Existing research has demonstrated that a shorter treatment duration is associated with a decreased likelihood of adverse drug reactions, lower treatment-related costs, and enhanced adherence [Citation26]. Despite the higher incidence of drug-induced liver injury observed in the short-term regimen compared to other regimens in this study, it was easy to be controlled [Citation27,Citation28]. Additionally, the cost-effectiveness of the short-course regimen was significantly superior to that of other regimens. Appropriate liver-protecting medication is necessary when using the short-term regimens.
Treatment of RR/MDR-TB is a financial change. The average cost of antimicrobial combination regimens recommended by the 2016 WHO guidelines was €52,144 [Citation29]. In our study, the short-term regimens showed the lowest cost compared to other regimens, however, after medical insurance reimbursement the cost of the four regimens was comparable. It was hard to compare the cost to the Bangladesh regimens as the diagnosis, hospitalization, and all medications were provided free of charge to the patients with the cost around 225 Euros [Citation9]. The RR/MDR-TB in Europe per QALY was €30,000 to €50,000 commonly applied in European healthcare systems. A study conducted in Germany showed that compared to the cost of background regimens alone, which was €60,962, the total discounted costs per patient were €85,575 for bedaquiline plus background regimens, €81,079 for delamanid plus background regimens, and €80,460 for linezolid plus background regimens [Citation30]. A recent study revealed the complete treatment expenses, which also included outpatient monitoring was €73,551.56 per patient, which was also much higher than ours [Citation29]. Patients with tuberculosis were always poor, lower treatment costs would help improve patients’ adherence to treatment and thus increase the cure rate. As a developing country, China has relatively scarce medical resources. The use of new regimens will be beneficial in saving a large number of funds while ensuring the effectiveness of treatment, which is most in line with economic efficiency evaluation.
The effectiveness, safety, and cost of drug-resistant TB treatment regimens play a crucial role in patient adherence. In this study, while the short-term regimen did not exhibit a significant difference compared to other regimens in terms of the incidence of adverse events and successful treatment, it proved to be the most cost-effective option. The adoption of these new regimens has the potential to further alleviate the economic burden, enhance patient adherence, and ensure effectiveness, thereby advancing the treatment and management of drug-resistant tuberculosis. Our study also had several limitations. Firstly, the sample size for the short-term regimen was limited due to stringent inclusion criteria, potentially impacting the validity and generalizability of the findings. It is essential to acknowledge that more data on short-term regimens will be necessary in future research to enhance the robustness of the study. Secondly, due to the outbreak of COVID-19, we could not obtain all the cost and sputum culture conversion information, which would be underestimated. We tried our best to have this information through the hospital HIS system and asked the patients to reduce bias. Thirdly, we did not collect information on the severity of RR/MDR-TB, which could affect the cost of various treatment regimens.
Authors contributions
Pengcheng Gu, Peng Lu, and Hui Ding conceived the study, analyzed the data, and drafted the manuscript. Limei Zhu and Yongfa Chen conceived the study and revised the manuscript. Qiao Liu, Hui Ding, and Xiaoyan Ding implemented the field investigation. All authors read and approved the final manuscript.
Acknowledgements
The authors thank all investigators from the Jiangsu Provincial Center for Disease Control and Prevention.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- WHO. Global tuberculosis report 2022. Geneva: WHO; 2022.
- Resch SC, Salomon JA, Murray M, et al. Cost-effectiveness of treating multidrug-resistant tuberculosis. PLOS Med. 2006;3(7):1. doi: 10.1371/journal.pmed.0030241.
- Lu P, Liu Q, Martinez L, et al. Time to sputum culture conversion and treatment outcome of patients with multidrug-resistant tuberculosis: a prospective cohort study from urban China. Eur Respir J. 2017;49(3):1601558. doi: 10.1183/13993003.01558-2016.
- Falzon D, Schünemann HJ, Harausz E, et al. World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur Respir J. 2017;49(3):1602308. doi: 10.1183/13993003.02308-2016.
- World Health Organization. WHO treatment guidelines for multidrug-and rifampicin-resistant tuberculosis. 2018 update. Geneva: World Health Organization; 2018.
- World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: World Health Organization; 2019.
- Lan Z, Ahmad N, Baghaei P, et al. Drug-associated adverse events in the treatment of multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir Med. 2020;8(4):383–11. doi: 10.1016/S2213-2600(20)30047-3.
- Prasad R, Singh A, Gupta N. Adverse drug reactions in tuberculosis and management. Indian J Tuberc. 2019;66(4):520–532. doi: 10.1016/j.ijtb.2019.11.005.
- Van Deun A, Maug AKJ, Salim MAH, et al. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182(5):684–692. doi: 10.1164/rccm.201001-0077OC.
- Nunn AJ, Phillips PPJ, Meredith SK, et al. A trial of a shorter regimen for rifampin-resistant tuberculosis. N Engl J Med. 2019;380(13):1201–1213. doi: 10.1056/NEJMoa1811867.
- Abidi S, Achar J, Assao Neino MM, et al. Standardised shorter regimens versus individualised longer regimens for multidrug-resistant TB. Eur Respir J. 2019;55(3):1901467. doi: 10.1183/13993003.01467-2019.
- Pooran A, Pieterson E, Davids M, et al. What is the cost of diagnosis and management of drug resistant tuberculosis in South Africa? PLOS One. 2013;8(1):e54587. doi: 10.1371/journal.pone.0054587.
- Van Deun A, et al. Drug resistance monitoring: combined rates may be the best indicator of programme performance. Int J Tuberc Lung Dis. 2004;8(1):23–30.
- Aung KJM, Van Deun A, Declercq E, et al. Successful ‘9-month Bangladesh regimen’ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis. 2014;18(10):1180–1187. doi: 10.5588/ijtld.14.0100.
- Piubello A, Harouna SH, Souleymane MB, et al. High cure rate with standardised short-course multidrug-resistant tuberculosis treatment in Niger: no relapses. Int J Tuberc Lung Dis. 2014;18(10):1188–1194. doi: 10.5588/ijtld.13.0075.
- Kuaban C, Noeske J, Rieder HL, et al. High effectiveness of a 12-month regimen for MDR-TB patients in Cameroon. Int J Tuberc Lung Dis. 2015;19(5):517–524. doi: 10.5588/ijtld.14.0535.
- Trébucq A, Schwoebel V, Kashongwe Z, et al. Treatment outcome with a short multidrug-resistant tuberculosis regimen in nine African countries. Int J Tuberc Lung Dis. 2018;22(1):17–25. doi: 10.5588/ijtld.17.0498.
- Yan L, Kan X, Zhu L, et al. Short-course regimen for subsequent treatment of pulmonary tuberculosis: a prospective, randomized, controlled multicenter clinical trial in China. Clin Ther. 2018;40(3):440–449. doi: 10.1016/j.clinthera.2018.01.013.
- Du Y, Qiu C, Chen X, et al. Treatment outcome of a shorter regimen containing clofazimine for multidrug-resistant tuberculosis: a randomized control trial in China. Clin Infect Dis. 2020;71(4):1047–1054. doi: 10.1093/cid/ciz915.
- Liu Q, Lu P, Shen Y, et al. Collateral impact of the coronavirus disease 2019 (COVID-19) pandemic on tuberculosis control in Jiangsu province, China. Clin Infect Dis. 2021;73(3):542–544. doi: 10.1093/cid/ciaa1289.
- Migliori GB, Lange C, Girardi E, et al. Multicenter Italian study on resistance to anti-tuberculosis drugs/tuberculosis network in Europe trials study group. Fluoroquinolones: are they essential to treat multidrug-resistant tuberculosis. Eur Respir J. 2008;31(4):904–905. doi: 10.1183/09031936.00159807.
- Khan FA, HamidSalim MA, du Cros P, et al. Effectiveness and safety of standardised shorter regimens for multidrug-resistant tuberculosis: individual patient data and aggregate data meta-analyses. Eur Respir J. 2017;50(1):1–13.
- Tang S, Yao L, Hao X, et al. Clofazimine for the treatment of multidrug-resistant tuberculosis: prospective, multicenter, randomized controlled study in China. Clin Infect Dis. 2015;60(9):1361–1367. doi: 10.1093/cid/civ027.
- Ammerman NC, Swanson RV, Tapley A, et al. Clofazimine has delayed antimicrobial activity against Mycobacterium tuberculosis both in vitro and in vivo. J Antimicrob Chemother. 2016;72(2):455–461. doi: 10.1093/jac/dkw417.
- Ausi Y, Santoso P, Sunjaya DK, et al. Between curing and torturing: burden of adverse reaction in drug-resistant tuberculosis therapy. Patient Prefer Adherence. 2021;15:2597–2607. doi: 10.2147/PPA.S333111.
- Batte C, Namusobya MS, Kirabo R, et al. Prevalence and factors associated with non-adherence to multi-drug resistant tuberculosis (MDR-TB) treatment at Mulago National Referral Hospital, Kampala, Uganda. Afr Health Sci. 2021;21(1):238–247. doi: 10.4314/ahs.v21i1.31.
- Shin S, Pasechnikov AD, Gelmanovaet IY, et al. Adverse reactions among patients being treated for MDR-TB in Tomsk, Russia. Int J Tuberc Lung Dis. 2007;11(12):1314–1320.
- Sagwa E, Mantel-Teeuwisse AK, Ruswa N, et al. The burden of adverse events during treatment of drug-resistant tuberculosis in Namibia. South Med Rev. 2012;5(1):6–13.
- Diel R, Sotgiu G, Andres S, et al. Cost of multidrug resistant tuberculosis in Germany—an update. Int J Infect Dis. 2021;103:102–109. doi: 10.1016/j.ijid.2020.10.084.
- Wirth D, Dass R, Hettle R. Cost-effectiveness of adding novel or group 5 interventions to a background regimen for the treatment of multidrug-resistant tuberculosis in Germany. BMC Health Serv Res. 2017;17(1):182. doi: 10.1186/s12913-017-2118-2.
Appendix A
Table A1. Characteristics of patients eligible for a programme of treating drug-resistant pulmonary tuberculosis.
Table A2. The diagnosis and treatment information of patients with drug-resistant pulmonary tuberculosis.