Abstract
The multisystem disease Lyme borreliosis is the most frequent tick‐transmitted disease in the northern hemisphere. In Europe Lyme borreliosis is most frequent in Central Europe and Scandinavia (up to 155 cases per 100,000 individuals) and is caused by the species, B. burgdorferi sensu stricto, B. afzelii and B. garinii. The recently detected genospecies A14S may also play a role in skin manifestations. Microbiological diagnosis in European patients must consider the heterogeneity of borreliae for development of diagnostic tools. According to guidelines of the USA and Germany, serological diagnosis should follow the principle of a two‐step procedure (enzyme‐linked immunosorbent assay (ELISA) as first step, if reactive; followed by immunoblot). The sensitivity and standardization of immunoblots has been considerably enhanced by use of recombinant antigens (p100, p58, p41i, VlsE, OspC, DbpA) including those expressed primarily in vivo (VlsE and DbpA) instead of whole cell lysates. VlsE is the most sensitive antigen for IgG antibody detection, OspC for IgM antibody detection. At present, detection rates for serum antibodies are 20%–50% in stage I, 70%–90% in stage II, and nearly 100% in stage III Lyme disease. Detection of the etiological agent by culture or polymerase chain reaction (PCR) should be confined to specific indications and specialized laboratories. Recommended specimens are skin biopsy specimens, cerebrospinal fluid (CSF) and synovial fluid. The best results are obtained from skin biopsies with culture or PCR (50%–70%) and synovial tissue or fluid (50%–70% with PCR). CSF yields positive results in only 10%–30% of patients except when the duration of symptoms is shorter than 2 weeks (50% sensitivity). Methods which are not recommended or adequately documented for diagnosis are antigen tests on body fluids, PCR of urine, and lymphocyte transformation tests.
Introduction
Lyme borreliosis is a multisystem disease involving many organs such as the skin, the nervous system, the joints and the heart Citation1–3. The disease is caused by Borrelia burgdorferi s.l. detected in the early eighties by Burgdorfer et al. in the American tick vector Ixodes scapularisCitation4. Due to the diversity of clinical symptoms, Lyme disease is often considered in differential diagnosis. Laboratory tests for diagnosis of Lyme borreliosis are thus in high demand, and are among the most frequently requested tests in microbiological laboratories.
Frequency of Lyme borreliosis
Lyme borreliosis is the most frequent tick‐borne disease in the northern hemisphere including North America and Eurasia. Lyme disease is not a reportable disease in most European countries in contrast to the United States. However, in studies performed in Scandinavia and in Slovenia, disease incidence was assessed as up to 155 per 100,000 inhabitants Citation1,Citation5,6. Lyme borreliosis occurs with similar frequencies in women and men with the exception of acrodermatitis chronica atrophicans (ACA) which is more frequent in women Citation7–9. Early neuroborreliosis cases showed a bimodal age distribution with a lower frequency in the age range of 20 to 29 years Citation7 whereas ACA occurs primarily in older patients Citation7,8.
Causative agents
In Europe Lyme borreliosis is caused by at least three species: B. burgdorferi sensu stricto, B. afzelii and B. garinii. In contrast, B. burgdorferi sensu stricto is the only human‐pathogenic species in the United States () Citation10. The three human‐pathogenic species comprise at least 7 OspA‐serotypes in Europe () Citation11. Skin isolates primarily belong to B. afzelii (OspA‐type 2), especially those from patients with ACA, a chronic skin disease not present in America Citation11–13. Isolates from CSF and ticks are heterogeneous with a predominance of B. gariniiCitation11,Citation14–16. Sequence analysis of polymerase chain reaction (PCR) ospA amplicons from synovial fluid of Lyme arthritis patients revealed considerable species‐ and OspA heterogeneity Citation17,18 (), whereas some other studies found a prevalence of B. burgdorferi s.s. Citation19–21. The most frequent genomic groups in Europe, B. afzelii and B. garinii occur across the continent and the islands, whereas the third frequent group B. burgdorferi s.s. has only rarely been isolated in Eastern Europe (for a survey see Citation22). Strains may be very heterogeneous even within small areas Citation14,Citation23–26. On the other side a focal prevalence of certain species or subtypes was also observed Citation24,Citation27. Mixed infections have been repeatedly observed in ixodid ticks (for a survey see Citation22) and sometimes also in specimens from patients Citation16,Citation18,Citation28,29.
Table I. Geographical distribution of B. burgdorferi sensu lato species and tick vectors
Figure 1. Distribution of species ofBorrelia burgdorferi sensu lato as well as of OspA types in European isolates from ticks, CSF, skin and synovial fluid specimens [17;18;101]. Clinical data for the skin specimens are known in 46 patients: 30 cases with erythema migrans (of which there were 1, 26, 1 and 2 cases infected with OspA‐types 1, 2, 4 and 6 respectively; 16 cases with acrodermatitis chronica atrophicans (ACA) (of which there were 1 and 15 cases infected with OspA‐types 1 and 2 respectively). B. burgdorferi s.l. speciation from synovial fluid samples is based on ospA PCR results. Culture isolates from this tissue are too few to estimate species distribution. is modified from figures 5 and 6 in reference Citation102.
![Figure 1. Distribution of species ofBorrelia burgdorferi sensu lato as well as of OspA types in European isolates from ticks, CSF, skin and synovial fluid specimens [17;18;101]. Clinical data for the skin specimens are known in 46 patients: 30 cases with erythema migrans (of which there were 1, 26, 1 and 2 cases infected with OspA‐types 1, 2, 4 and 6 respectively; 16 cases with acrodermatitis chronica atrophicans (ACA) (of which there were 1 and 15 cases infected with OspA‐types 1 and 2 respectively). B. burgdorferi s.l. speciation from synovial fluid samples is based on ospA PCR results. Culture isolates from this tissue are too few to estimate species distribution. Figure 1 is modified from figures 5 and 6 in reference Citation102.](/cms/asset/0efee03e-0f1d-48c2-84eb-b068f34882b0/iann_a_143176_f0001_b.jpg)
Vectors and reservoirs
Borrelia burgdorferi s.l. is transmitted by hard ticks (genus Ixodes). The larvae and nymphs feed primarily on small rodents whereas adult ticks feed on a variety of larger animals. The feeding period of Ixodes species ticks is rather long (several days to over a week) and contributes to their geographic dispersal along with the movement of the host. Birds, particularly migratory seabirds, can transport the ticks (I. uriae) over very long distances and thus distribute borreliae (especially B. garinii) worldwide Citation30. There appears to be an association between certain Borrelia burgdorferi s.l. species and certain vertebrate hosts: B. afzelii and small rodents and B. garinii and birds, possibly due to different serum‐sensitivities of the borreliae Citation31,32. Complement‐resistant Borreliae bind complement regulators and prevent the formation of toxic activation products which kill the borreliae Citation33. In unfed ticks B. burgdorferi s.l. lives in the midgut. During the blood meal on humans or mice molecular changes (e.g. switch from OspA to OspC expression) are induced in the borreliae that lead to their migration to the salivary glands Citation34,35. The migration process takes >36 h in I. scapularisCitation36. In I. ricinus nymphs, however, spirochete migration within the tick and transmission to the mammalian host has been observed with ticks feeding for a few as 17h Citation37. Infection rates of larvae are usually very low (ca 1%). Borrelial infection is mostly acquired by feeding on infected reservoir hosts leading to much higher infection rates in nymphs and adults. In a study from southern Germany prevalence of borreliae increased from 1% in larvae to 10% in nymphs and 20% in adult ticks Citation7.
Key messages
Epidemiology and etiological agent. Lyme borreliosis is the most frequent tick‐borne disease in the northern hemisphere. The etiological agent of this multisystem disease is B. burgdorferi sensu lato which comprises at least three pathogenic species (B. burgdorferi sensu stricto, B. afzelii and B. garinii) and the recently detected genospecies A14S.
Antibody detection. Serology is the most commonly used diagnostic tool. Sensitivity of antibody detection is 20%–50% in stage I, 70%–90% in stage II, and nearly 100% in stage III. Currently, a two‐step approach is recommended (ELISA as first step, if reactive, followed by immunoblot). The detection of highly immunogenic, primarily in vivo‐produced, proteins provided new diagnostic tools. Recombinant proteins as VlsE, OspC, DbpA and BBK32 were successfully used as ELISA antigens. A recombinant immunoblot (with p100, p58, p41i, VlsE, OspC, and DbpA as antigens including several homologues of these proteins) was more sensitive than the whole‐cell sonicate immunoblot and is easier to standardize. Use of recombinant VlsE, DbpA and BBK32 as antigens for ELISA and immunoblot increased especially IgG antibody detection in early disease (erythema migrans (EM) and acute neuroborreliosis).
Culture and polymerase chain reaction (PCR). Detection of borreliae using PCR or culture is confined to specific indications and specialized laboratories. The best results are obtained from skin biopsies with culture or PCR (50%–70%) and synovial tissue or fluid (50%–70% with PCR). CSF yields positive results in only 10%–30% of patients with the exception when duration of symptoms is shorter than 2 weeks (50% sensitivity).
Implications of heterogeneity of borreliae for diagnosis
The heterogeneity of the causative strains () is a challenge for the microbiological diagnosis of Lyme borreliosis in Europe and must be kept in mind for development of diagnostic tools such as PCR primers and diagnostic antigens. For example, ospA PCR has been widely used. Here it is important to be sure that not only representatives of the three species are detected, but also the different ospA‐types of the heterogeneous B. garinii group Citation14. In addition, PCR should detect B. valaisiana and the recently detected new genospecies A14S Citation24 since B. valaisiana and genotype A14S might also be pathogenic for humans, as suggested by positive PCR results or cultures obtained from skin biopsy specimens in a few studies Citation38,39. Recently A14S‐like organisms have been found in four patients with erythema migrans from Germany confirming the pathogenic potential of this new genospecies (Fingerle and Wilske, unpublished results).
Most of the proteins relevant for serodiagnosis are heterogeneous. Interspecies amino acid sequence identities are for example only 40%–44% for DbpA and 54%–68% for OspC for representative strains of B. burgdorferi sensu stricto, B. afzelii, and B. gariniiCitation40. However, highly heterogeneous proteins sometimes have conserved immunogenic epitopes (e.g. the C6 peptide of VlsE and the pepC10 peptide derived from OspC) Citation41–43.
Microbiological diagnosis of Lyme borreliosis
Except in cases with the pathognomic clinical manifestation erythema migrans the diagnosis of Lyme borreliosis usually requires confirmation by means of a microbiological diagnostic assay. Antibody detection methods mainly are used for this purpose, whereas detection of the causative agent by culture isolation and nucleic acid techniques is confined to special situations. Since the present review is limited in space the reader who wants to go deeper into this field is referred to more comprehensive reviews Citation44,45.
Specimens for the microbiological diagnosis
For culture and PCR, skin biopsy samples are the most promising specimens (). In general poor results are obtained from body fluids with the exception of PCR from synovial fluid. Examination of urine is not recommended (see last section). Examination of ticks should be performed only for epidemiological or other scientific studies. Ticks removed from patients should not be examined in order to decide antibiotic prophylaxis Citation46,47. For antibody determination, serum or CSF can be investigated. CSF examination should always be done together with serum antibody analysis (determination of the CSF/serum antibody index).
Table II. Specimen types used for the diagnosis of Lyme borreliosis.
Direct detection methods
Culture. B. burgdorferi can be cultivated in modified Kellýs medium Citation44,Citation48,49. This, however, is a very time‐consuming method (generation time of B. burgdorferi is about 7–20 h) characterized by low sensitivity, especially in body fluids Citation50–53 (). Only under special conditions (3 specimens of 3 ml plasma cultured in a 70 mL medium for 12 weeks) have positive cultures been derived from about 50% of patients with erythema migrans Citation54.
Culturing may be of help in individual cases if the clinical picture suggests Lyme borreliosis despite a negative antibody assay (seronegative Lyme borreliosis), e.g. in atypical erythema migrans, suspected acute neuroborreliosis without detection of intrathecal antibodies or in the case of suspected Lyme borreliosis in patients with immune deficiencies.
Table III. Sensitivity of direct pathogen detection methods in Lyme borreliosis.
Polymerase chain reaction (PCR)
For DNA amplification under experimental conditions various target sequences have been used, e.g. from plasmid‐borne genes such as ospA and ospB, or chromosomal genes such as the genes for the flagellar protein or p66, or from gene segments of the 16S rRNA or the 5S/23S rRNA intergenic spacer region (for surveys see Citation55,56). Borrelia PCR should allow diagnosis of the Borrelia species, i.e. the medical report should contain information as to which of the species pathogenic for humans has been found.
Sensitivity of culture and PCR
gives a survey about sensitivity of direct detection methods in clinical specimens from patients with Lyme borreliosis. Borreliae are detected with much more difficulty from body fluids than from tissue specimens Citation19,Citation50,51. Culture and PCR have the highest detection rates (50%–70%) in skin biopsies from patients with erythema migrans or ACA Citation15,Citation53,Citation57,58. In contrast, borreliae are detected by PCR or culture in the CSF of only 10%–30% of patients with acute neuroborreliosis Citation14,Citation51,Citation59. CSF isolates are more frequently obtained from patients with short duration of disease than from patients with disease of long duration Citation51. Accordingly CSF‐PCR is positive in up to 50% of patients with disease duration of less than 2 weeks compared with only 13% patients in whom the illness duration was greater than 2 weeks Citation60. Borreliae are detected by PCR in 50%–70% of the synovial fluids of Lyme arthritis patients but culture is rarely successful Citation17,18,Citation61. The best PCR results are obtained from synovial tissue, not fluid Citation19.
Antibody detection
It is generally accepted that serological examination should follow the principles of a two‐step approach Citation44,Citation46,Citation62,63: 1) A serological screening assay, and 2) in the event of a positive or equivocal result a confirmatory assay. A sensitive ELISA is recommended, which – in case it is reactive – should be confirmed by the immunoblot. The new development of highly sensitive and specific ELISAs based on recombinant antigens or synthetic peptides raises the question whether two ELISAs could be the two‐step procedure. However the two ELISA results might have a common error source that would be difficult to discover. Therefore immunoblotting has merits because the error sources are at least partly different and there is possibility to see background binding artifacts. In addition immunoblotting allows analysis of the antibody pattern against different borrelial proteins possibly associated with different stages of the disease. New technologies (as the Luminex bead system) may provide the possibility of quantitative multiple parameter analysis combining features of ELISA and immunoblot.
ELISA
The ELISA tests used for screening should be at least second generation tests Citation46, which have been improved with respect to cross reactivity with other bacteria (e.g. extract antigen with previous Reiter treponeme adsorption) Citation64 or purified intact flagella as antigen Citation65 or third generation tests using specific and sensitive recombinant antigens or synthetic peptides. Strains used as antigen source should express OspC the immunodominant antigen of the IgM response and DbpA an immunodominant antigen of the IgG response Citation46. Specific recombinant antigens (i.e. VlsE, DbpA, BBK23, and OspC) or synthetic peptides (i.e. the pepC10 peptide derived from OspC and the C6 peptide derived from VlsE) have been successfully used Citation42,43,Citation66–70. The most sensitive antigens were for IgM antibody detection OspC (or the pepC10 peptide) and for IgG antibody detection VlsE (or the C6 peptide) respectively. Since VlsE is not present in relevant amounts in cultivated borreliae, sensitivity of IgG ELISAs based on whole cell lysates or detergent extract antigens might be increased with recombinant VlsE or C6 peptide. ELISAs using the C6 peptide, VlsE enriched antigens or VlsE alone are commercially available.
Immunoblot
As a confirmatory assay the immunoblot should have high specificity (at least 95%). If a whole cell lysate is used as antigen, diagnostic bands must be defined by monoclonal antibodies or other reliable identification. In case of recombinant antigens, identification of diagnostic bands is much easier. For the whole cell lysate blot, strains expressing immunodominant variable antigens (OspC, DbpA) in culture should be used Citation46.
The immunoblot criteria recommended by the Centers of Disease Control (CDC) for use in the United States cannot be used for Europe Citation71–73. The immune response of European patients is restricted to a narrower spectrum of Borrelia proteins, compared with that shown by American patients Citation74. Hauser et al. demonstrated in two studies (first serum panel from Germany, second serum panel from various European countries) that strain‐specific interpretation rules must be defined Citation71,72. Interpretation criteria for the immunoblot recommended by the German Society for Hygiene and Microbiology (DGHM) are published in the ‘MiQ 12 Lyme‐Borreliose’ Citation46 which is available in English via internet (http://www.dghm.org/red/index.html?cname = MIQ).
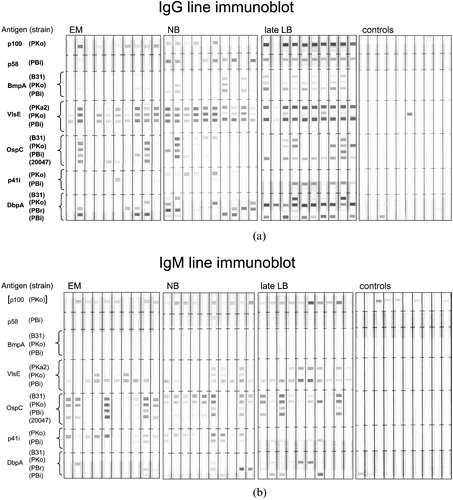
Patients with early manifestations of acute neuroborreliosis have an immune response restricted to only a few proteins. Patients with late disease such as ACA or arthritis have IgG antibodies to a broad spectrum of antigens (). Recombinant antigens for the immunoblot have several advantages compared to whole cell lysate antigens (specific antigens can be selected, homologous antigens derived from different strains can be combined, and antigens primarily expressed in vivo can be used Citation75,76. Commercial recombinant antigen immunoblots are better standardized than the conventional ones. If a broad panel of recombinant antigens (including the recently described VlsE) is used the recombinant blot is at least as sensitive as the conventional one. An in‐house recombinant IgG immunoblot could be significantly improved by addition of recombinant VlsE and an additional DbpA homologue Citation75. Using the line blot technique which allows detection of antibodies against antigens with identical molecular weight (i.e. homologues of the same borrelial protein) () the recombinant IgG immunoblot became even significantly more sensitive than the conventional IgG sonicate immunoblot (i.e. 91.7% versus 68.8% in patients with early neuroborreliosis for the detection of IgG antibodies) Citation77. In both immunoblots the criterion for a positive test was reactivity of at least two different proteins. Using this criterion none of the control sera shown in was reactive (reactivity of controls was restricted to maximal one protein). In this study the immunodominant protein of the IgM response was OspC followed by VlsE (). For the IgG response VlsE was the immunodominant antigen in all stages (80%–100%) whereas OspC is reactive only in 20 to 50% ( and ). Other proteins have low reactivities in early manifestations compared to those in late manifestations. This is especially apparent for p58 where reactivities increase from 7% (stage I) to 54% (stage II) and to 95% in stage III. The combination of different homologues from one protein was especially efficient in case of DbpA. Out of 50 patients with acute neuroborreliosis 39 (78.0%) were positive with at least one of the 4 DbpAs, but only 6 (12%) with B. burgdorferi s.s. DbpA, 17 (34%) with B. afzelii DbpA and 32 (64.0%) with at least one of the 2 B. garinii DbpAs. Thus a panel of DbpAs representing the four major DbpA groups of B. burgdorferi s.s., B. afzelii and B. garinii appears to provide optimal detection rates Citation78. The prevalence of reactivity with B. garinii DbpA is in agreement with the fact that B. garinii is prevalent in patients with neuroborreliosis. Future investigations will show whether quantitative multi‐antigen assays i.e. such as those based on the Luminex bead technology can give reliable information about the stage of the disease and the causative Borrelia species or type. Such assays might also help to discriminate between present and past infections or to control success of antibiotic therapy which is not possible with the presently available tests.
Figure 2. Whole cell immunoblot from patients with early neuroborreliosis(stage II) and acrodermatitis (stage III). Note, the antigen used is B. afzelii strain PKo and the sera are from European patients. is modified from Figure 5 of reference Citation71.
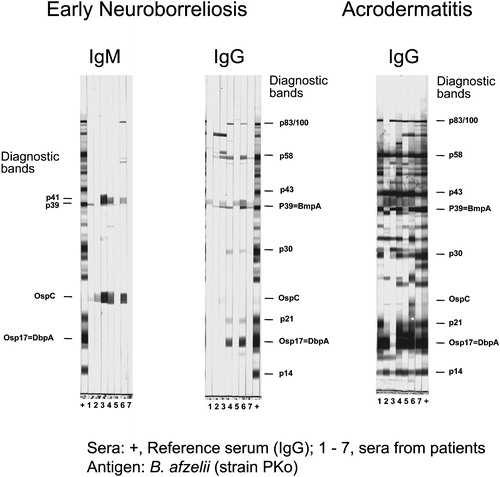
Figure 3. Recombinant line immunoblot.(a) Representative IgG blots and (b) IgM blots of patient and control sera. Strains belong to the following species: B31 and PKa2 to B. burgdorferi s.s., PKo to B. afzelii, PBr to B. garinii OspA‐type 3, PBi to B. garinii OspA‐type 4, and 20047 to B. garinii unknown OspA‐type. Sera were obtained from patients with erythema migrans (EM), early neuroborreliosis (NB), Acrodermatitis chronica atrophicans or Lyme arthritis (late LB), and controls. a and b are modified from and of reference Citation77. The Borrelia protein encoding genes used correspond to the B31 sequence database as follows: p100, BB0744; p58, BB0329, bmpA, BB0383; ospC, BBB19; flaB (p41) BB668; dbpA, BBA24, the vlsE gene is not in the database.
Determination of the CSF/serum index
Methods taking into account potential dysfunction of the blood‐CSF barrier are suitable for the detection of intrathecal antibody production Citation79–81. Determination of the CSF/serum index should be performed if neuroborreliosis is considered, since a positive CSF/serum index confirms involvement of the nervous system. It may be positive in some cases when serum antibody tests are negative or equivocal, especially if the patient's illness has been of short duration Citation46. Depending on the time elapsed since the first manifestation of neurological symptoms, the IgG CSF/serum index is positive for 80%–90% of patients (8–41 days after onset of the disease) up to 100% of patients (>41 days after onset) Citation81. Detection of intrathecally produced IgM antibodies shows a high degree of sensitivity in neuroborreliosis with short duration of symptoms, especially in children Citation81,82. However false positive IgM reativity has been observed in some cases with viral meningitis that are difficult to distinguish from specific borrelial antibodies without the presence of IgG class antibodies Citation82. CSF/serum index determination is especially important for diagnosis of chronic neuroborreliosis. A positive IgG CSF/serum index is essential for the diagnosis of chronic borreliosis of the central nervous system (see EUCALB case definitions Citation83) whereas chronic peripheral polyneuropathy is usually negative for intrathecal antibody production Citation84.
Serological findings in various stages of the disease
Interpretation of serological test results must always be done in context with clinical data (). Here case definitions are helpful Citation46,Citation83. In stage I (erythema migrans) only 20%–50% of the patients are seropositive for IgM and/or IgG antibodies Citation85,86. IgM antibodies usually prevail. An exception might be the immune response against some primarily in vivo expressed antigens. In American patients with erythema migrans IgG responses against VlsE are observed earlier than IgM responses Citation66. In European patients with erythema migrans an early IgG response to VlsE was observed in 20 of 23 (87%) culture‐confirmed erythema migrans cases Citation42. BBK32 is another antigen with considerable IgG reactivity (73%) in EM patients Citation87. In stage II (acute neuroborreliosis) seropositivity (IgM and/or IgG antibodies) increases to 70%–90% Citation59,Citation65. Also here IgG antibody detection could be improved using recombinant VlsE or DbpA as antigens in the ELISA or the immunoblot Citation69,Citation75,Citation77. In principle, patients with early manifestations may be seronegative especially in case of short duration of symptoms. Then serological follow up is recommended and in case of neurological symptoms the CSF/serum index should be determined. Six weeks or more after onset of symptoms, 100% of the patients with stage II neuroborreliosis were seropositive Citation65. In cases with late disease (stage III, ACA and arthritis) IgG antibodies were detectable in all patients tested Citation64,Citation86. A negative IgG test argues against late Lyme borreliosis. Thus a positive IgM test without a positive IgG test is not diagnostic for late disease manifestations Citation46. Since serological findings vary considerably and antibodies may persist for a long time in successfully treated individuals, serological follow up is not suitable for therapy control. Recently the C6 peptide ELISA has been recommended from American authors for therapy control Citation88, however data were not convincing in a study from Europe Citation89. The presence of specific antibodies does not prove the presence of disease, a positive antibody test may also be due to clinical or subclinical infections in the past. The more non‐specific the symptoms, the lower is the predictive value of a positive serological test. Seropositivity in the normal healthy population varies with age and increased outdoor activities.
Table IV. Sensitivity of antibody detection methods in the diagnosis of Lyme disease.
Table V. IgG reactivity of recombinant Borrelia proteins in the line immunoblot in early erythema migrans (EM) and acute neuroborreliosis (NB) and late manifestations acrodermatitis chronica atrophicans (ACA) or arthritis (AT). The table is based on data from reference Citation77.
Methods which are not recommended or adequately documented for diagnosis
Recently, various methods have been used in commercially oriented laboratories which are not sufficiently evaluated for diagnostic purposes. Among them are the antigen tests in body fluids, PCR of urine, and lymphocyte transformation tests Citation90. The T‐lymphocyte proliferation assays have been used in various scientific studies performed with blood from Lyme borreliosis patients to investigate the T‐cell response to Borrelia antigens Citation91–93. However T‐lymphocyte proliferation assays cannot be recommended as diagnostic tests due to their cumbersome nature and concerns about their specificity and standardization Citation40,Citation90,Citation94. Antigen detection tests have been used for the detection of borrelial antigen in body fluids from patients with Lyme borreliosis including CSF and urine Citation95,96. However, the validity of this technique is controversial and its use for diagnosis is no longer recommended for microbiological diagnosis Citation97. PCR from urine is unreliable too Citation98, borrelia DNA has been detected also from healthy seropositive individuals Citation99.
References
- Stanek G., Strle F. Lyme borreliosis. Lancet 2003; 362: 1639–47
- Steere A. C. Lyme disease. N Engl J Med 1989; 321: 586–96
- Pfister H. W., Wilske B., Weber K. Lyme borreliosis: basic science and clinical aspects. Lancet 1994; 343: 1013–6
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease‐a tick‐borne spirochetosis?. Science 1982; 216: 1317–9
- Berglund J., Eitrem R., Ornstein K., Lindberg A., Ringer A., Elmrud H., et al. An epidemiologic study of Lyme disease in southern Sweden. N Engl J Med 1995; 333: 1319–27
- Strle F. Lyme borreliosis in Slovenia. Zentralbl Bakteriol 1999; 289: 643–52
- Wilske B., Steinhuber R., Bergmeister H., Fingerle V., Schierz G., Preac‐Mursic V., et al. [Lyme borreliosis in South Germany. Epidemiologic data on the incidence of cases and on the epidemiology of ticks (Ixodes ricinus) carrying Borrelia burgdorferi]. Dtsch Med Wochenschr 1987; 112: 1730–6
- Asbrink E., Hovmark A., Hederstedt B. The spirochetal etiology of acrodermatitis chronica atrophicans Herxheimer. Acta Derm Venereol 1984; 64: 506–12
- Schmidt R., Kabatzki J., Hartung S., Ackermann R. [Erythema migrans borreliosis in the Federal Republic of Germany. Epidemiology and clinical aspects]. Dtsch Med Wochenschr 1985; 110: 1803–7
- Wang G., van Dam A. P., Schwartz I., Dankert J. Molecular typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiological, and clinical implications. Clin Microbiol Rev 1999; 12: 633–53
- Wilske B., Preac‐Mursic V., Göbel U. B., Graf B., Jauris S., Soutschek E., et al. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J Clin Microbiol 1993; 31: 340–50
- Canica M. M., Nato F., du M. L., Mazie J. C., Baranton G., Postic D. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand J Infect Dis 1993; 25: 441–8
- Ohlenbusch A., Matuschka F. R., Richter D., Christen H. J., Thomssen R., Spielman A., et al. Etiology of the acrodermatitis chronica atrophicans lesion in Lyme disease. J Infect Dis 1996; 174: 421–3
- Eiffert H., Ohlenbusch A., Christen H. J., Thomssen R., Spielman A., Matuschka F. R. Nondifferentiation between Lyme disease spirochetes from vector ticks and human cerebrospinal fluid. J Infect Dis 1995; 171: 476–9
- van Dam A. P., Kuiper H., Vos K., Widjojokusumo A., de Jongh B. M., Spanjaard L., et al. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis 1993; 17: 708–17
- Wilske B., Busch U., Eiffert H., Fingerle V., Pfister H. W., Rössler D., et al. Diversity of OspA and OspC among cerebrospinal fluid isolates of Borrelia burgdorferi sensu lato from patients with neuroborreliosis in Germany. Med Microbiol Immunol (Berl) 1996; 184: 195–201
- Eiffert H., Karsten A., Thomssen R., Christen H. J. Characterization of Borrelia burgdorferi strains in Lyme arthritis. Scand J Infect Dis 1998; 30: 265–8
- Vasiliu V., Herzer P., Rössler D., Lehnert G., Wilske B. Heterogeneity of Borrelia burgdorferi sensu lato demonstrated by an ospA‐type‐specific PCR in synovial fluid from patients with Lyme arthritis. Med Microbiol Immunol (Berl) 1998; 187: 97–102
- Jaulhac B., Chary‐Valckenaere I., Sibilia J., Javier R. M., Piemont Y., Kuntz J. L., et al. Detection of Borrelia burgdorferi by DNA amplification in synovial tissue samples from patients with Lyme arthritis. Arthritis Rheum 1996; 39: 736–45
- Jaulhac B., Heller R., Limbach F. X., Hansmann Y., Lipsker D., Monteil H., et al. Direct molecular typing of Borrelia burgdorferi sensu lato species in synovial samples from patients with lyme arthritis. J Clin Microbiol 2000; 38: 1895–900
- Lunemann J. D., Zarmas S., Priem S., Franz J., Zschenderlein R., Aberer E., et al. Rapid typing of Borrelia burgdorferi sensu lato species in specimens from patients with different manifestations of Lyme borreliosis. J Clin Microbiol 2001; 39: 1130–3
- Hubalek Z., Halouzka J. Distribution of Borrelia burgdorferi sensu lato genomic groups in Europe, a review. Eur J Epidemiol 1997; 13: 951–7
- Gern L., Hu C. M., Kocianova E., Vyrostekova V., Rehacek J. Genetic diversity of Borrelia burgdorferi sensu lato isolates obtained from Ixodes ricinus ticks collected in Slovakia. Eur J Epidemiol 1999; 15: 665–9
- Michel H., Wilske B., Hettche G., Goettner G., Heimerl C., Reischl U., et al. An ospA‐polymerase chain reaction/restriction fragment length polymorphism‐based method for sensitive detection and reliable differentiation of all European Borrelia burgdorferi sensu lato species and OspA types. Med Microbiol Immunol (Berl) 2003; 193: 219–26
- Rauter C., Oehme R., Diterich I., Engele M., Hartung T. Distribution of clinically relevant Borrelia genospecies in ticks assessed by a novel, single‐run, real‐time PCR. J Clin Microbiol 2002; 40: 36–43
- Rijpkema S., Golubic D., Molkenboer M., Verbeek‐de Kruif N., Schellekens J. Identification of four genomic groups of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in a Lyme borreliosis endemic region of northern Croatia. Exp Appl Acarol 1996; 20: 23–30
- Peter O., Bretz A. G., Bee D. Occurrence of different genospecies of Borrelia burgdorferi sensu lato in ixodid ticks of Valais, Switzerland. Eur J Epidemiol 1995; 11: 463–7
- Demaerschalck I., Ben Messaoud A., De Kesel M., Hoyois B., Lobet Y., Hoet P., et al. Simultaneous presence of different Borrelia burgdorferi genospecies in biological fluids of Lyme disease patients. J Clin Microbiol 1995; 33: 602–8
- Ruzic‐Sabljic E., Arnez M., Logar M., Maraspin V., Lotric‐Furlan S., Cimperman J., et al. Comparison of Borrelia burgdorferi Sensu Lato Strains Isolated from Specimens Obtained Simultaneously from Two Different Sites of Infection in Individual Patients. J Clin Microbiol 2005; 43: 2194–200
- Olsen B., Duffy D. C., Jaenson T. G., Gylfe A., Bonnedahl J., Bergstrom S. Transhemispheric exchange of Lyme disease spirochetes by seabirds. J Clin Microbiol 1995; 33: 3270–4
- Humair P., Gern L. The wild hidden face of Lyme borreliosis in Europe. Microbes Infect 2000; 2: 915–22
- Kurtenbach K., Sewell H. S., Ogden N. H., Randolph S. E., Nuttall P. A. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect Immun 1998; 66: 1248–51
- Kraiczy P., Skerka C., Kirschfink M., Zipfel P. F., Brade V. Mechanism of complement resistance of pathogenic Borrelia burgdorferi isolates. Int Immunopharmacol 2001; 1: 393–401
- Fingerle V., Liegl G., Munderloh U., Wilske B. Expression of outer surface proteins A and C of Borrelia burgdorferi in Ixodes ricinus ticks removed from humans. Med Microbiol Immunol (Berl) 1998; 187: 121–6
- Schwan T. G., Piesman J., Golde W. T., Dolan M. C., Rosa P. A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A 1995; 92: 2909–13
- de Silva A. M., Fikrig E. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am J Trop Med Hyg 1995; 53: 397–404
- Kahl O., Janetzki‐Mittmann C., Gray J. S., Jonas R., Stein J., de Boer R. Risk of infection with Borrelia burgdorferi sensu lato for a host in relation to the duration of nymphal Ixodes ricinus feeding and the method of tick removal. Zentralbl Bakteriol 1998; 287: 41–52
- Rijpkema S. G., Tazelaar D. J., Molkenboer M. J., Noordhoek G. T., Plantinga G., Schouls L. M., et al. Detection of Borrelia afzelii, Borrelia burgdorferi sensu stricto, Borrelia garinii and group VS116 by PCR in skin biopsies of patients with erythema migrans and acrodermatitis chronica atrophicans. Clin Microbiol Infect 1997; 3: 109–16
- Wang G., van Dam A. P., Dankert J. Phenotypic and genetic characterization of a novel Borrelia burgdorferi sensu lato isolate from a patient with lyme borreliosis. J Clin Microbiol 1999; 37: 3025–8
- Wilske B. Diagnosis of lyme borreliosis in europe. Vector Borne Zoonotic Dis 2003; 3: 215–27
- Liang F. T., Philipp M. T. Analysis of antibody response to invariable regions of VlsE, the variable surface antigen of Borrelia burgdorferi. Infect Immun 1999; 67: 6702–6
- Liang F. T., Aberer E., Cinco M., Gern L., Hu C. M., Lobet Y. N., et al. Antigenic conservation of an immunodominant invariable region of the VlsE lipoprotein among European pathogenic genospecies of Borrelia burgdorferi SL. J Infect Dis 2000; 182: 1455–62
- Mathiesen M. J., Christiansen M., Hansen K., Holm A., Asbrink E., Theisen M. Peptide‐based OspC enzyme‐linked immunosorbent assay for serodiagnosis of Lyme borreliosis. J Clin Microbiol 1998; 36: 3474–9
- Wilske B., Schriefer M. Borrelia. Manual of Clinical Microbiology., P. R Murray, E. J Baron, J. H Jorgensen, M. A Pfaller, R. H Yolken, editors. ASM Press, Washington, D.C. 2003; 8th edition Volume 1: p. 937–54, In
- Aguero‐Rosenfeld M. E., Wang G., Schwartz I., Wormser G. P. Diagnosis of lyme borreliosis. Clin Microbiol Rev 2005; 18: 484–509
- Wilske B., Zöller L., Brade V., Eiffert H., Göbel U. B., Stanek G., . MIQ 12, Lyme‐Borreliose. Qualitätsstandards in der mikrobiologisch‐ infektiologischen Diagnostik., H Mauch, R Lütticken, editors, et al. Urban & Fischer Verlag, MunichGermany 2000; p. 1–59, In
- Kaiser R. [Prevention of early summer meningoencephalitis and Lyme borreliosis before and after tick bites]. Dtsch Med Wochenschr 1998; 123: 847–53
- Preac‐Mursic V., Wilske B., Reinhardt S. Culture of Borrelia burgdorferi on six solid media. Eur J Clin Microbiol Infect Dis 1991; 10: 1076–9
- Preac‐Mursic V., Wilske B., Schierz G. European Borrelia burgdorferi isolated from humans and ticks culture conditions and antibiotic susceptibility. Zentralbl Bakteriol Mikrobiol Hyg [A] 1986; 263: 112–8
- Arnez M., Ruzic‐Sabljic E., Ahcan J., Radsel‐Medvescek A., Pleterski‐Rigler D., Strle F. Isolation of Borrelia burgdorferi sensu lato from blood of children with solitary erythema migrans. Pediatr Infect Dis J 2001; 20: 251–5
- Karlsson M., Hovind‐Hougen K., Svenungsson B., Stiernstedt G. Cultivation and characterization of spirochetes from cerebrospinal fluid of patients with Lyme borreliosis. J Clin Microbiol 1990; 28: 473–9
- Strle F. Principles of the diagnosis and antibiotic treatment of Lyme borreliosis. Wien Klin Wochenschr 1999; 111: 911–5
- Zore A., Ruzic‐Sabljic E., Maraspin V., Cimperman J., Lotric‐Furlan S., Pikelj A., et al. Sensitivity of culture and polymerase chain reaction for the etiologic diagnosis of erythema migrans. Wien Klin Wochenschr 2002; 114: 606–9
- Wormser G. P., Bittker S., Cooper D., Nowakowski J., Nadelman R. B., Pavia C. Yield of large‐volume blood cultures in patients with early Lyme disease. J Infect Dis 2001; 184: 1070–2
- Schmidt B. L. PCR in laboratory diagnosis of human Borrelia burgdorferi infections. Clin Microbiol Rev 1997; 10: 185–201
- Aguero‐Rosenfeld M. E., Wang G., Schwartz I., Wormser G. P. Diagnosis of lyme borreliosis. Clin Microbiol Rev 2005; 18: 484–509
- Asbrink E., Olsson I. Clinical manifestations of erythema chronicum migrans Afzelius in 161 patients. A comparison with Lyme disease. Acta Derm Venereol 1985; 65: 43–52
- von Stedingk L. V., Olsson I., Hanson H. S., Asbrink E., Hovmark A. Polymerase chain reaction for detection of Borrelia burgdorferi DNA in skin lesions of early and late Lyme borreliosis. Eur J Clin Microbiol Infect Dis 1995; 14: 1–5
- Wilske B., Preac‐Mursic V. Microbiological diagnosis of Lyme borreliosis. Aspects of Lyme Borreliosis., K Weber, W Burgdorfer, editors. Springer Verlag, Berlin, Heidelberg, Germany and New YorkUSA 1993; p. 267–300, In
- Lebech A. M., Hansen K., Brandrup F., Clemmensen O., Halkier‐Sorensen L. Diagnostic value of PCR for detection of Borrelia burgdorferi DNA in clinical specimens from patients with erythema migrans and Lyme neuroborreliosis. Mol Diagn 2000; 5: 139–50
- Nocton J. J., Dressler F., Rutledge B. J., Rys P. N., Persing D. H., Steere A. C. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 1994; 330: 229–34
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease., MMWR Morb Mortal Wkly Rep. 44. 590
- Johnson B. J., Robbins K. E., Bailey R. E., Cao B. L., Sviat S. L., Craven R. B., et al. Serodiagnosis of Lyme disease: accuracy of a two‐step approach using a flagella‐based ELISA and immunoblotting. J Infect Dis 1996; 174: 346–53
- Wilske B., Fingerle V., Herzer P., Hofmann A., Lehnert G., Peters H., et al. Recombinant immunoblot in the serodiagnosis of Lyme borreliosis. Comparison with indirect immunofluorescence and enzyme‐linked immunosorbent assay. Med Microbiol Immunol (Berl) 1993; 182: 255–70
- Hansen K., Hindersson P., Pedersen N. S. Measurement of antibodies to the Borrelia burgdorferi flagellum improves serodiagnosis in Lyme disease. J Clin Microbiol 1988; 26: 338–46
- Bacon R. M., Biggerstaff B. J., Schriefer M. E., Gilmore R. D., Jr., Philipp M. T., Steere A. C., et al. Serodiagnosis of Lyme disease by kinetic enzyme‐linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2‐tiered testing using whole‐cell lysates. J Infect Dis 2003; 187: 1187–99
- Lawrenz M. B., Hardham J. M., Owens R. T., Nowakowski J., Steere A. C., Wormser G. P., et al. Human antibody responses to VlsE antigenic variation protein of Borrelia burgdorferi. J Clin Microbiol 1999; 37: 3997–4004
- Liang F. T., Steere A. C., Marques A. R., Johnson B. J., Miller J. N., Philipp M. T. Sensitive and specific serodiagnosis of Lyme disease by enzyme‐linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi vlsE. J Clin Microbiol 1999; 37: 3990–6
- Panelius J., Lahdenne P., Saxen H., Carlsson S. A., Heikkila T., Peltomaa M., et al. Diagnosis of Lyme neuroborreliosis with antibodies to recombinant proteins DbpA, BBK32, and OspC, and VlsE IR6 peptide. J Neurol 2003; 250: 1318–27
- Heikkila T., Seppala I., Saxen H., Panelius J., Yrjanainen H., Lahdenne P. Species‐specific serodiagnosis of Lyme arthritis and neuroborreliosis due to Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii by using decorin binding protein A. J Clin Microbiol 2002; 40: 453–60
- Hauser U., Lehnert G., Lobentanzer R., Wilske B. Interpretation criteria for standardized Western blots for three European species of Borrelia burgdorferi sensu lato. J Clin Microbiol 1997; 35: 1433–44
- Hauser U., Lehnert G., Wilske B. Diagnostic value of proteins of three Borrelia species (Borrelia burgdorferi sensu lato) and implications for development and use of recombinant antigens for serodiagnosis of Lyme borreliosis in Europe. Clin Diagn Lab Immunol 1998; 5: 456–62
- Robertson J., Guy E., Andrews N., Wilske B., Anda P., Granström M., et al. A European multicenter study of immunoblotting in serodiagnosis of Lyme borreliosis. J Clin Microbiol 2000; 38: 2097–102
- Dressler F., Ackermann R., Steere A. C. Antibody responses to the three genomic groups of Borrelia burgdorferi in European Lyme borreliosis. J Infect Dis 1994; 169: 313–8
- Schulte‐Spechtel U., Lehnert G., Liegl G., Fingerle V., Heimerl C., Johnson B. J., et al. Significant improvement of the recombinant Borrelia‐specific immunoglobulin G immunoblot test by addition of VlsE and a DbpA homologue derived from Borrelia garinii for diagnosis of early neuroborreliosis. J Clin Microbiol 2003; 41: 1299–303
- Wilske B., Habermann C., Fingerle V., Hillenbrand B., Jauris‐Heipke S., Lehnert G., et al. An improved recombinant IgG immunoblot for serodiagnosis of Lyme borreliosis. Med Microbiol Immunol (Berl) 1999; 188: 139–44
- Goettner G., Schulte‐Spechtel U., Hillermann R., Liegl G., Wilske B., Fingerle V. Improvement of Lyme borreliosis serodiagnosis by a newly developed recombinant immunoglobulin G (IgG) and IgM line immunoblot assay and addition of VlsE and DbpA homologues. J Clin Microbiol 2005; 43: 3602–9
- Schulte‐Spechtel U., Fingerle V., Goettner G., Rogge S., Wilske B. Molecular analysis of decorin binding protein A (DbpA) reveals five major groups among European Borrelia burgdorferi sensu lato strains with impact for the development of serological assays and indicates lateral gene transfer of the dbpA gene. Int J Med Microbiol 2005, In press
- Wilske B., Schierz G., Preac‐Mursic V., von Busch K., Kuhbeck R., Pfister H. W., et al. Intrathecal production of specific antibodies against Borrelia burgdorferi in patients with lymphocytic meningoradiculitis (Bannwarth's syndrome). J Infect Dis 1986; 153: 304–14
- Hansen K., Cruz M., Link H. Oligoclonal Borrelia burgdorferi‐specific IgG antibodies in cerebrospinal fluid in Lyme neuroborreliosis. J Infect Dis 1990; 161: 1194–202
- Hansen K., Lebech A. M. Lyme neuroborreliosis: a new sensitive diagnostic assay for intrathecal synthesis of Borrelia burgdorferi‐specific immunoglobulin G, A, and M. Ann Neurol 1991; 30: 197–205
- Christen H. J., Hanefeld F., Eiffert H., Thomssen R. Epidemiology and clinical manifestations of Lyme borreliosis in childhood. A prospective multicentre study with special regard to neuroborreliosis. Acta Paediatr Suppl 1993; 386: 1–75
- Stanek G., O'Connell S., Cimmino M., Aberer E., Kristoferitsch W., Granstrom M., et al. European Union Concerted Action on Risk Assessment in Lyme Borreliosis: clinical case definitions for Lyme borreliosis. Wien Klin Wochenschr 1996; 108: 741–7
- Kristoferitsch W. Neurologic manifestations in Lyme borreliosis. Clin Dermatol 1993; 11: 393–400
- Asbrink E., Hovmark A., Hederstedt B. Serologic studies of erythema chronicum migrans Afzelius and acrodermatitis chronica atrophicans with indirect immunofluorescence and enzyme‐linked immunosorbent assays. Acta Derm Venereol 1985; 65: 509–14
- Hansen K., Asbrink E. Serodiagnosis of erythema migrans and acrodermatitis chronica atrophicans by the Borrelia burgdorferi flagellum enzyme‐linked immunosorbent assay. J Clin Microbiol 1989; 27: 545–51
- Lahdenne P., Panelius J., Saxen H., Heikkila T., Sillanpaa H., Peltomaa M., et al. Improved serodiagnosis of erythema migrans using novel recombinant borrelial BBK32 antigens. J Med Microbiol 2003; 52: 563–7
- Philipp M. T., Wormser G. P., Marques A. R., Bittker S., Martin D. S., Nowakowski J., et al. A decline in c6 antibody titer occurs in successfully treated patients with culture‐confirmed early localized or early disseminated Lyme borreliosis. Clin Diagn Lab Immunol 2005; 12: 1069–74
- Peltomaa M., McHugh G., Steere A. C. Persistence of the antibody response to the VlsE sixth invariant region (IR6) peptide of Borrelia burgdorferi after successful antibiotic treatment of Lyme disease. J Infect Dis 2003; 187: 1178–86
- Department of Health and Human Services CfDCaP. Caution regarding testing for Lyme disease., MMWR Morb Mortal Wkly Rep. 54. 125
- Buechner S. A., Lautenschlager S., Itin P., Bircher A., Erb P. Lymphoproliferative responses to Borrelia burgdorferi in patients with erythema migrans, acrodermatitis chronica atrophicans, lymphadenosis benigna cutis, and morphea. Arch Dermatol 1995; 131: 673–7
- Dattwyler R. J., Volkman D. J., Luft B. J., Halperin J. J., Thomas J., Golightly M. G. Seronegative Lyme disease. Dissociation of specific T‐ and B‐lymphocyte responses to Borrelia burgdorferi. N Engl J Med 1988; 319: 1441–6
- Kalish R. S., Wood J. A., Golde W., Bernard R., Davis L. E., Grimson R. C., et al. Human T lymphocyte response to Borrelia burgdorferi infection: no correlation between human leukocyte function antigen type 1 peptide response and clinical status. J Infect Dis 2003; 187: 102–8
- Horowitz H. W., Pavia C. S., Bittker S., Forseter G., Cooper D., Nadelman R. B., et al. Sustained cellular immune responses to Borrelia burgdorferi: lack of correlation with clinical presentation and serology. Clin Diagn Lab Immunol 1994; 1: 373–8
- Coyle P. K., Schutzer S. E., Belman A. L., Krupp L. B., Dheng Z. Cerebrospinal fluid immunologic parameters in neurologic Lyme disease. Lyme disease: Molecular and immunologic approaches., S. E Schutzer, editor. Cold Spring Harbor Laboratory Press, New YorkUSA 1992; p. 31–44, In
- Hyde F. W., Johnson R. C., White T. J., Shelburne C. E. Detection of antigens in urine of mice and humans infected with Borrelia burgdorferi, etiologic agent of Lyme disease. J Clin Microbiol 1989; 27: 58–61
- Klempner M. S., Schmid C. H., Hu L., Steere A. C., Johnson G., McCloud B., et al. Intralaboratory reliability of serologic and urine testing for Lyme disease. Am J Med 2001; 110: 217–9
- Brettschneider S., Bruckbauer H., Klugbauer N., Hofmann H. Diagnostic value of PCR for detection of Borrelia burgdorferi in skin biopsy and urine samples from patients with skin borreliosis. J Clin Microbiol 1998; 36: 2658–65
- Karch H., Huppertz H. I., Bohme M., Schmidt H., Wiebecke D., Schwarzkopf A. Demonstration of Borrelia burgdorferi DNA in urine samples from healthy humans whose sera contain B. burgdorferi‐specific antibodies. J Clin Microbiol 1994; 32: 2312–4
- Richter D., Schlee D. B., Allgower R., Matuschka F. R. Relationships of a novel Lyme disease spirochete, Borrelia spielmani sp. nov., with its hosts in Central Europe. Appl Environ Microbiol 2004; 70: 6414–9
- Wilske B., Busch U., Fingerle V., Jauris‐Heipke S., Preac M., V., Rössler D., et al. Immunological and molecular variability of OspA and OspC. Implications for Borrelia vaccine development. Infection 1996; 24: 208–12
- Wilske B. Microbiological diagnosis in Lyme borreliosis. Int J Med Microbiol 2002; 291((Suppl 33))114–9
