Abstract
The association of metabolic disorders with liver disease is receiving increasing attention in the gastroenterological community. Cohort studies have shown that advanced liver disease may stem from metabolic disorders, via fatty liver, non‐alcoholic steatohepatitis, cryptogenic cirrhosis, and eventually hepatocellular carcinoma. In both obesity and diabetes, deaths from cirrhosis are higher than expected, mainly in subjects with no or moderate alcohol consumption, but high rates of fatty liver disease have been associated with all features of the metabolic syndrome. Also the risk of hepatocellular carcinoma is higher than normal, being dependent on body mass index (BMI) in obesity, and independent of age, BMI, gender and race in diabetes. Finally, metabolic liver disease may interact with hepatitis C virus infection, increasing the risk of steatosis and liver disease progression, as well as reducing the chances of an effective antiviral treatment. There is evidence that treatments aimed at reducing insulin resistance are also effective in improving liver histology. Although cardiovascular disease remains the major cause of increased morbidity and excess mortality in metabolic disorders, the risk of progressive liver disease should no longer be underestimated, being a threat to millions of people at risk in the present epidemics of obesity and diabetes, and therapeutic strategies need to be tested.
| Abbreviations | ||
| ALF | = | acute liver failure |
| ALT | = | alanine aminotransferase |
| AR | = | attributable risk |
| AST | = | aspartate aminotransferase |
| BMI | = | body mass index |
| CC | = | cryptogenic cirrhosis |
| CI | = | confidence interval |
| DM | = | diabetes mellitus |
| FFA | = | free fatty acids |
| GGT | = | gamma‐glutamyltransferase |
| HBV | = | hepatitis B virus |
| HCC | = | hepatocellular carcinoma |
| HCV | = | hepatitis C virus |
| HR | = | hazard ratio |
| ICD‐9 | = | international classification of diseases (9th edition) |
| IRS‐1 | = | insulin receptor substrate‐1 |
| MS | = | metabolic syndrome |
| NAFLD | = | non‐alcoholic fatty liver disease |
| NASH | = | non‐alcoholic steatohepatitis |
| OR | = | odds ratio |
| PI‐3K | = | phosphatidyl inosytol‐3 kinase |
| PPAR | = | peroxisome‐proliferator activated receptor |
| RR | = | relative risk |
| SIR | = | standardized incidence ratio |
| SMR | = | standardized mortality rate |
| T2DM | = | type 2 diabetes mellitus |
| TNF | = | tumor necrosis factor |
Introduction
The association of liver disease with metabolic disorders is receiving increased attention Citation1. Hepatic steatosis is a common feature in obesity and type 2 diabetes mellitus (T2DM), but its potential significance as a cause of advanced liver disease has long been underestimated. More recently, the identification of non‐alcoholic fatty liver disease (NAFLD) as a clinical entity Citation2 and cohort studies suggesting a potential link between pure fatty liver, non‐alcoholic steatohepatitis (NASH), cryptogenic cirrhosis Citation3, and eventually hepatocellular carcinoma (HCC) Citation4 have suggested that metabolic disorders per se may cause advanced liver failure.
Although cardiovascular risk remains the main cause accounting for increased morbidity and excess mortality in obesity and T2DM, many studies are in keeping with the hypothesis that liver failure might also be a threat to the millions of people at risk, whose number is further increasing due to the epidemics of obesity and T2DM in western countries Citation5. Metabolic disorders also interact with viral infections in the pathogenesis of liver failure, particularly in areas where hepatitis C (HCV) virus is endemic.
The aim of the present report is to review the existing evidence that advanced liver disease may develop in patients with metabolic disorders, and to review the pathogenic and therapeutic relevance of the association of metabolic liver disease with viral infection.
Search strategy and selection criteria
We searched MEDLINE to May 2005, using different combinations of the following key‐words: obesity, diabetes, survival, cirrhosis, liver disease, hepatocellular carcinoma, hepatitis B, hepatitis C, fatty liver, and related terms. We also searched the bibliographies of most recent articles for relevant references. Because of the large number of articles identified and limitations for quoting references, the final decision on what to include was based on personal judgment, with preferences for the most recent articles.
Key messages
Fatty liver, commonly observed in metabolic disorders, may progress to advanced liver disease, via non‐alcoholic steatohepatitis, cryptogenic cirrhosis, and eventually hepatocellular carcinoma.
The prevalence of liver disease, death rates for cirrhosis and the risk of hepatocellular carcinoma are higher than expected in subjects with features of the metabolic syndrome, particularly in obesity and diabetes, and metabolic liver disease may also interact with hepatitis C virus infection, favoring disease progression and reducing the chances of an effective antiviral treatment.
Although cardiovascular disease remains the major cause of increased morbidity and excess mortality in metabolic disorders, the risk of progressive liver disease should no longer be underestimated, being a threat to millions of people at risk in the present epidemics of obesity and diabetes.
Association of liver disease with diabetes, obesity and the metabolic syndrome
Fatty liver is the marker of metabolic liver disease, with or without raised aminotransferase levels, namely alanine aminotransferase (ALT). Although an association between the severity of liver disease and raised aminotransferases has not been unequivocally demonstrated Citation6, Citation7, raised enzymes are the most common reason for patients' concern and extensive diagnostic workup. Several epidemiological data have convincingly associated the whole spectrum of NAFLD with the metabolic syndrome (MS) Citation6, Citation8–10, and with its individual components as suggested by Adult Treatment Panel III proposal Citation11. There is now evidence that fatty liver and insulin resistance may be early predictors of metabolic disorders, even in non‐obese, non‐diabetic subjects, particularly in the normal‐weight population Citation12.
Diabetes
The relationship of liver disease to T2DM is difficult to evaluate. End‐stage liver disease can cause glucose abnormalities and overt diabetes (the so‐called ‘hepatogenous diabetes’) Citation13, being a source of bias in prevalence studies. Old studies, based on liver biopsy, were also biased by the selection of cases with clinically suspected liver disease Citation14. Limiting the analysis to fatty liver, the estimated prevalence of diabetes ranges from 21% to 78% in recent studies, the difference being largely dependent upon diagnostic criteria Citation15–17. Ultrasonography is not sensitive enough to detect all cases with fatty liver infiltration, the detection limit being around 25%–30% of hepatocytes with fatty droplets Citation18, Citation19. No systematic studies are available on the prevalence of diabetes in subjects with more severe histological alterations (necroinflammation, fibrosis), potentially progressive to advanced liver disease, which would require the extensive use of liver biopsy.
Surprisingly, very few cohort studies have been published on the prevalence of clinically‐detectable liver disease or raised liver enzymes in subjects with T2DM. The overall incidence of elevated aminotransferase levels in T2DM treated with oral antidiabetic agents is reported in the order of 50/10,000 person‐years, but the criteria of ascertainment were questionable, and mild liver enzyme elevation was not considered Citation20. In the records of the Department of Veteran Affairs, the incidence of chronic non‐alcoholic liver disease was doubled among patients with diabetes Citation21. In turn, elevated ALT Citation22, Citation23 or GGT Citation24 were prospectively associated with the development of type 2 diabetes in the general population, and the predictive role of ALT was maintained after adjustment for insulin resistance Citation25.
Obesity
More data are available in obesity. Ultrasound studies show evidence of hepatic steatosis in 76% of cases not drinking alcohol in toxic amounts Citation26, and that liver histology is compatible with NAFLD of variable severity in over 80% of morbidly obese subjects Citation27, Citation28, where bariatric surgery provides an easy access to liver biopsy. Among 551 patients prospectively examined, steatosis was found in 86%, fibrosis in 74%, mild inflammation or NASH in 24%, and unexpected cirrhosis in 2% Citation27. In overweight subjects with abnormal aminotransferase levels but without overt liver disease, steatosis ⩾10% was observed in 79% of cases, fibrosis outside the portal tracts in 30%, necroinflammation in 81% Citation29. The prevalence of fatty degeneration is progressively higher in relation to glucose control (from normoglycemia, to impaired glucose tolerance, to T2DM) Citation30, Citation31.
A role of obesity in high aminotransferase cases has been repeatedly demonstrated. High levels of alanine and aspartate aminotransferases (ALT and AST), as well as raised gamma‐glutamyltranspeptidase (GGT) activity, are frequently observed, in association with body mass index (BMI) and raised insulin levels Citation32, Citation33, also in apparently healthy obese subjects with fatty liver at ultrasonography Citation34, the prevalence being higher in males and in relation to raised BMI. In a cohort study, Vozarova et al. Citation23 showed that raised liver enzymes were associated with body fat and insulin resistance, measured by the clamp technique. Prati et al. Citation35 confirmed that ALT levels are significantly associated with BMI in healthy individuals; their data suggested revising the upper limits of normal ALT to improve the sensitivity for identifying liver disease. In the general population, Stranges et al. Citation36 demonstrated an independent role of central adiposity in predicting increased levels of aminotransferases and GGT, a possible expression of an unrecognized hepatic disease.
Hyperlipidemia
In hyperlipidemic patients admitted to an urban hospital‐based clinic for the evaluation and management of hyperlipidemia, fatty liver was detected at ultrasounds in 50%, the prevalence being higher in subjects with severe hypertriglyceridemia and mixed hyperlipidemia Citation37. Hyperglycemia was an independent predictor of fatty liver, confirming the role of multiple metabolic disorders in the pathogenesis of steatosis.
Hypertension
In hypertension, the prevalence of ultrasonographically‐detected steatosis is more than doubled when compared to subjects with normal blood pressure Citation38. An association of hypertension with insulin resistance has long been documented Citation39. The higher prevalence of fatty liver in non‐obese hypertensive patients with normal liver enzymes was related to increased insulin resistance and body weight Citation38.
Metabolic syndrome
In a recent cross‐sectional analysis of 3,405 South Korean adults Citation40, an association between ALT and the metabolic syndrome was found in both sexes, independently of age, BMI, smoking, alcohol drinking, and sedentary life style. The association was maintained even in the range below the current upper limits of ALT. Similarly, in 799 obese Italian patients entering a weight‐reducing program, raised ALT levels were demonstrated in 21.0% of cases, with a lower prevalence of raised AST (8.6%) and GGT (13.7%). The median value of ALT increased slightly, but significantly, with obesity class (P for trend: = 0.001). Hyperglycemia (⩾110 mg/dl) and hypertriglyceridemia (⩾150 mg/dl) were the features of the metabolic syndrome most commonly associated with raised liver enzymes Citation41. In logistic regression analysis, after correction for age, gender, BMI and features of the metabolic syndrome, insulin resistance (homeostasis model assessment) maintained a highly predictive value for raised ALT, AST and GGT, suggesting that raised liver enzyme levels, indicative of subclinical liver disease, may be part of the insulin‐resistance (metabolic) syndrome.
As previously outlined, the presence of multiple metabolic disorders is strictly associated with a higher prevalence of liver disease. Both in subjects cared for in liver units Citation6 and in patients observed in metabolic/diabetes units Citation42, the association of obesity with diabetes and/or altered lipid metabolism leads to a multiplicative effect on the final prevalence of MS, and significantly increases the risk of more severe stages of liver disease Citation43–45.
Association between diabetes and viral liver disease
Diabetes in HCV
The prevalence of abnormal glucose regulation and diabetes is significantly elevated in HCV infected patients, after correction for the degree of liver cell failure. At population level (Third National Health and Nutrition Examination Survey), in persons older than 20 years of age, the presence of diabetes was over three times more prevalent in subjects with HCV infection than in those without HCV Citation46. In American‐Indian women, increasing age, obesity, and positive HCV status were each independently related to the diagnosis of diabetes Citation47. Older age, obesity, severe liver fibrosis and family history of diabetes help identify HCV patients at risk for development of T2DM Citation48. A threefold increase in the prevalence of glucose abnormalities was observed in HCV positive patients with chronic hepatitis in comparison with HCV negative subjects (32% versus 12%; P = 0.0003) Citation49. In contrast, among patients with cirrhosis the difference was not statistically significant, although the prevalence of both diabetes and impaired fasting glucose (110–125 mg/dL) were more prevalent in HCV positive patients (40% versus 36% in HCV negative). This suggests that the connection between HCV infection and diabetes starts at early stages of hepatic disease.
Diabetogenic effect of HCV
The mechanism accounting for the pro‐diabetogenic effect of HCV is under investigation. In liver biopsies of HCV‐infected subjects, defects in upstream insulin signaling pathways have been demonstrated at the level of insulin receptor substrate‐1 (IRS‐1) tyrosine phosphorylation, IRS‐1/p85 phosphatidylinositol 3‐kinase (PI3‐kinase) and IRS‐1‐associated PI3‐kinase enzymatic activity. They were not present in HCV negative biopsies, and might contribute to insulin resistance, which leads to progression to type 2 diabetes mellitus in patients with HCV infection Citation50. In a mouse model transgenic for the HCV core gene, plasma glucose levels during a glucose tolerance test were moderately higher than in control mice, in association with insulin resistance. The levels of tumor necrosis factor‐α were also elevated, as commonly observed in human infection. The administration of an anti‐tumor necrosis factor‐α antibody restored insulin sensitivity, providing a direct experimental evidence for the contribution of HCV to the development T2DM Citation51.
Virus‐induced steatosis
The association of steatosis with HCV infection is larger than expected by chance. In patients with untreated chronic hepatitis C, steatosis is significantly associated with BMI (P < 0.0001) Citation52, namely with visceral adiposity Citation53, but is particularly common in genotype 3a infection Citation54. Steatosis also correlates with viral load Citation55; it disappears in subjects with sustained response to interferon and reoccurs after liver transplantation in subjects with HCV‐genotype 3, whereas it does not change in patients with HCV genotype 1, irrespective of response Citation56, giving support for a causal association between genotype 3 infection and fat accumulation. The pathogenesis of HCV‐related steatosis remains poorly understood. Serfaty et al. Citation57 found that cholesterol levels are lower in HCV‐infected patients compared to a reference male population, as well as to patients with chronic hepatitis B or with non‐alcoholic fatty liver. Hypobetalipoproteinemia is corrected by HCV eradication, suggesting a direct involvement of HCV in both hypobetalipoproteinemia and steatosis. Apoprotein AII and HCV core colocalize in human HCV‐infected liver biopsies, and the hepatic overexpression of HCV core protein interferes with the hepatic assembly and secretion of triglyceride‐rich very‐low‐density lipoproteins in a transgenic murine model Citation58.
Metabolic disorders and HCV‐related disease severity
Altered glucose regulation is significantly associated with insulin resistance and with the staging of liver fibrosis Citation48. In a large series of patients with chronic HCV‐related hepatitis, age at infection, duration of infection, serum glucose and daily alcohol intake but not BMI were independently associated with significant fibrosis Citation59. Patients with high serum glucose had features of the metabolic syndrome, including a higher prevalence of steatosis, as well as faster fibrosis progression rates, suggesting that hyperglycemia is an independent co‐factor of fibrosis, with a higher pro‐fibrogenic impact than overweight Citation59. Finally, a BMI ⩾30 kg/m2 is a negative predictor of sustained response to interferon treatment Citation60. The reason(s) might be lower maximal interferon concentrations in obese patients, as well as a weaker biologic response to exogenous interferon‐α Citation60, Citation61.
Liver disease and survival in metabolic patients
Whatever the origin of liver disease, the key question is whether liver disease may harm patients with metabolic disorders. These patients remain at very high risk of cardiovascular diseases, but evidence is emerging suggesting that liver disease needs to be tackled as well. The study of the natural history of NAFLD recently suggested that the presence of diabetes and high BMI are significantly associated with higher rates of liver fibrosis progression, also in the absence of raised aminotransferases Citation62. Metabolic liver disease is slowly progressive Citation63, and liver‐related morbidity and mortality are expected to occur only in aged cohorts.
Liver‐related mortality and cirrhosis
The prognostic studies on liver disease‐related mortality in metabolic disorders are reported in , Citation64–70. In a registry‐based cohort study, Jepsen et al. Citation68 studied overall and cause‐specific standardized mortality ratios in Danish subjects discharged from hospital with a diagnosis of fatty liver. During follow‐up, mortality was increased 2.6‐fold (95% CI 2.4–2.9) in patients with non‐alcoholic or unspecified fatty liver, independently of diabetes and after censoring patients upon diagnosis of liver cirrhosis. However, survival estimates in patients with pure fatty liver, without inflammation, were not different from those observed in the general Danish population Citation70, suggesting the need for additional factors favoring disease progression. In T2DM patients observed in the Verona cohort study, the standardized mortality ratio for cirrhosis during a 5‐year follow‐up was remarkably high (2.5; 95% CI, 2.0–3.2) Citation65, the ratio being even higher than that reported for cardiovascular disease. Less clear data had previously been reported in a Japanese cohort with a low prevalence of diabetes Citation64. Contrary to what is commonly believed, obesity is frequently associated with cirrhosis, even in subjects without significant alcohol intake. In a multicenter Italian study of 1402 patients with cirrhosis 29% of females and 18% of males appeared to be overnourished Citation71. In obesity‐related cryptogenic cirrhosis, severe liver disease was as frequent as in HCV‐related cirrhosis, and survival was even lower than in age‐ and sex‐matched cirrhosis of viral origin Citation72. These data were not confirmed in a U S series of NASH patients, where prognosis was either similar or less severe than in HCV‐cirrhosis Citation73. In a long‐term follow‐up of the NHANES I cohort, cirrhosis‐related deaths or hospitalizations were more common in obese persons (adjusted hazard ratio, 1.7; 95% CI, 1.0–3.0), after exclusion of subjects with evidence of cirrhosis at entry or during the first 5 years of follow‐up Citation69. The association was particularly strong among obese persons who did not consume alcohol (4.1; 1.4–11.4). In the presence of cirrhosis, diabetes is a risk factor for mortality Citation13, the larger mortality rate being related to an increased risk of hepatocellular failure, heralded by low platelet counts and high bilirubin Citation73, not to diabetes or diabetes‐related complications. In subjects followed for NAFLD, overall mortality and mortality related to liver disease (relative risk, 22.8; P = 0.003) were both more common in subjects with diabetes Citation74. Obesity Citation67 and T2DM Citation66 also increase the risk of acute liver failure in a few cases. On the basis of the clinical and histological findings, Caldwell and Hespenheide Citation67 suggested that these patients had undiagnosed NASH with silent progression to cirrhosis followed by subacute liver failure.
Table I. Liver disease‐related mortality associated with metabolic disorders.
Hepatocellular carcinoma (HCC)
The prognostic studies on progression to HCC and HCC‐related mortality in metabolic disorders are reported in , Citation64, Citation75–89. In a recent mortality study of the American Cancer Society, the risk of death from HCC during 16 years of follow‐up was increased both in males (P for trend <0.001) and in females (P < 0.04) with increasing BMI class Citation83. These data expand previous reports linking obesity to HCC Citation75, Citation90.
Table II. Progression to hepatocellular carcinoma and HCC‐related mortality in metabolic disorders.
An association of T2DM with HCC was first established by comparison with the prevalence of diabetes observed in subjects with other tumors Citation91. It has been confirmed in several recent reports Citation21, Citation77, Citation80, Citation86, Citation92. In a case‐control study, El‐Serag et al. Citation81 found that diabetes increases the risk of HCC in the presence of hepatitis B, hepatitis C, or alcoholic cirrhosis, whereas in chronic HCV‐related hepatitis, steatosis per se was a risk for HCC Citation93. The temporal relationship between diabetes and HCC is important, due to the high prevalence of diabetes in cirrhosis. In a large cohort of subjects admitted to Veteran Administration hospitals Citation87, the incidence of HCC was over twice as high among patients with diabetes, and was higher in subjects with a longer follow‐up. Diabetes was a risk factor independent of age, BMI, gender and race, with a hazard ratio of 2.16. A high BMI further increases the risk of HCC. In the Verona cohort, where 70% of cases were overweight or obese, the hazard ratio of HCC was 1.86 (95% CI, 1.43–2.38) Citation84. The majority of these studies have been carried out on referred cohorts, and are subject to referral biases. Only recently have population‐based studies become available. In a Korean cohort with low average BMI (23.2 kg/m2, only approximately 25% overweight or obese), the age‐adjusted incidence rate of HCC increased progressively with increasing fasting glucose and BMI, reaching the top in men with diabetes (hazard ratio, 1.66; 95% CI, 1.53–1.79) Citation88. In this population with high HBV carrier state, neither NAFLD nor HBV were demonstrated as confounders, suggesting a primary role of hyperglycemia. In a large U S Medicare study in subjects aged 65 years or over, the risk of HCC was increased 2 to 3 times in the presence of diabetes, after adjustment for HBV, HCV, alcohol and hemocromatosis, and did not change in analyses restricted to subjects with diabetes detected between two and three years prior to HCC diagnosis Citation89. Thus diabetes may be considered an additional risk factor for HCC, when other major risk factors are ruled out.
Finally, the association of NAFLD with HCC is well known, and is probably mediated by cryptogenic cirrhosis Citation4, Citation72, Citation85, Citation93–95. Decreased surveillance Citation96 might be implicated in the poor risk of subjects with NAFLD‐related HCC, compared with HCC occurring in cirrhosis of different etiology Citation72.
Proposed mechanism(s) for liver disease and disease progression
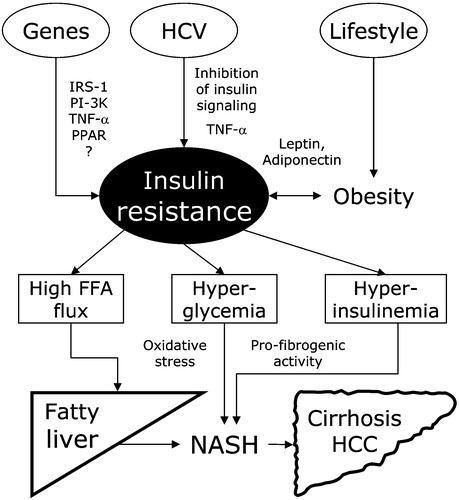
The mechanism(s) implicated in fatty liver have been outlined in several recent reviews Citation16, Citation97–99; they have insulin resistance as the key factor (). The family clustering of diseases strongly supports a genetic inheritance of decreased insulin sensitivity, but the search for genes has so far been disappointing. Polygenic transmission is very likely in metabolic disorders, and determining the genetics of insulin resistance will not be easy Citation100, Citation101. There are hundreds of gene interactions involved in fat storage Citation102 and in insulin action Citation103, and abnormalities in at least 23 genes have been detected in NAFLD Citation103. Further studies should also identify the site of insulin resistance (liver versus adipose tissue and skeletal muscle). Lifestyle maintains the primary role in fatty liver, via excessive food intake and low physical exercise, promoting obesity and further decreasing insulin sensitivity. Finally, the contribution of HCV in selected populations should not be forgotten. Disease progression and carcinogenesis may stem from hyperglycemia, either generating oxidative stress Citation104–106 or facilitating septal fibrosis Citation59. Also hyperinsulinemia and increased insulin‐like growth factor‐1 Citation107–109 have been associated with fibrogenesis and the generation of hepatic hyperplasia, through the up‐regulation of connective‐tissue growth factor in stellate cells demonstrated in human liver biopsies and in in vitro experiments Citation108. Finally, obesity per se may increase oxidative stress, causing a dysregulation of adipocytokines promoting the development of MS Citation110.
Figure 1 Interaction between genes, virus and lifestyle in the initiation and progression of metabolic liver disease. Insulin resistance is the core of the mechanism. Genes, through various and largely undefined polymorphisms, may cause or favor insulin resistance. Hepatitis‐C virus, either by its viral genome, or via increased TNF‐α production, may interfere with insulin signaling. Finally lifestyle, through obesity, adipokines and increased release of TNF‐α, produces or enhances insulin resistance. Increased FFA flux promotes steatosis (possibly aggravated by HCV‐dependent interference with the hepatic assembly and secretion of triglyceride‐rich very‐low‐density lipoproteins); hyperglycemia induces oxidative stress, hyperinsulinemia favors fibrosis and hepatic hyperplasia, both leading from fatty liver to non‐alcoholic steatohepatitis, fibrosis, cirrhosis and eventually to hepatocellular carcinoma. FFA = free fatty acids; HCV = hepatitis C virus; TNF = tumor necrosis factor; NASH = non‐alcoholic steatohepatitis; HCC = hepatocellular carcinoma; IRS‐1 = insulin receptor substrate‐1; PI‐3K = phosphatidyl inosytol‐3 kinase; PPAR = peroxisome‐proliferator activated receptor.
Treatment of liver disease in metabolic patients
The liver disease of subjects cared for in metabolic/diabetic units is by no means less severe than that observed in hepatology units Citation42. Although the burden of cardiovascular disease largely outweighs that of liver failure, progressive liver disease is expected to reduce cardiovascular risk, by reducing several diabetes‐related risk factors (arterial pressure, hyperlipidemia, platelets and clotting factors) Citation111. As a consequence, these subjects may ultimately be at risk because of their liver disease, and treatment may be worthwhile.
NAFLD treatment
Several review articles are available on NAFLD treatment Citation112. Therapeutic options range from vitamins, antioxidants and cytoprotective agents to lipid‐lowering drugs, phlebotomy and insulin‐sensitizers Citation112, Citation113. In all NAFLD subjects, weight‐reducing programs and life‐style interventions remain the first line of treatment () Citation114–120. They were reported as effective in reducing aminotransferase levels and improving liver histology in the short‐term Citation116, Citation120, but the results were not long‐lasting. Similar approaches are being considered as background treatment in HCV‐positive patients with obesity and/or T2DM before and during anti‐viral therapy.
Table III. Clinical studies on treatment of non‐alcoholic fatty liver disease with lifestyle/weight loss modifications and insulin‐sensitizers, published in extension in peer‐reviewed journals.
Insulin‐sensitizing agents have proved effective as well Citation121–127, independently of glucose levels, with a single exception Citation126. Data on short‐term treatment Citation121, Citation124, Citation128 have now been expanded to one year with both metformin Citation127 and glitazones Citation122, Citation123; aminotransferase levels return within normal values, steatosis at histology is markedly reduced, apparently more with the use of glitazones Citation129, and also necroinflammation and fibrosis improve, but aminotransferase tends to return to pre‐treatment levels after stopping treatment Citation121, Citation124. We need long‐term, placebo‐controlled trials to validate therapeutic interventions on lifestyle and pharmacological treatment against hard outcomes (disease progression to cirrhosis and ultimately death).
Conclusions
Epidemiological studies suggest that 20% to 30% of adults in U S and other Western countries have excess fat accumulation in the liver, that about 10% of these individuals meet current diagnostic criteria for NASH, and that one third of them progress to fibrosis and cryptogenic cirrhosis Citation113. These estimates may become even higher in the next few years, considering the rapid spread of obesity and T2DM in the general population Citation5, thus increasing the global burden of theoretically‐preventable metabolic diseases in Western countries Citation130.
Prevention and treatment strategies of metabolic liver disease are largely based on the same approaches used to tackle the metabolic syndrome, but we all know how difficult it is to implement lifestyle changes Citation131. Cognitive approaches to the treatment of patients with the metabolic syndrome need to include liver disease as an additional complication, preventable by a healthy lifestyle. More importantly, physicians need to consider the importance of surveillance for detection and early treatment of complications. The potential for disease prevention and health gain from tackling major known risk factors is substantial in metabolic disorders Citation132, and also the risk of progressive liver disease should no longer be underestimated in these subjects.
Conflict of interests
The authors declare that they have no conflict of interests in relation to this review.
References
- Tolman K. G., Fonseca V., Tan M. H., Dalpiaz A. Narrative review: hepatobiliary disease in type 2 diabetes mellitus. Ann Intern Med 2004; 141: 946–56
- Ludwig J., Viaggiano T. R., McGill D. B., Oh B. J. Nonalcoholic steatohepatitis: Mayo Clinic experience with an hitherto unnamed disease. Mayo Clin Proc 1980; 55: 434–8
- Falck‐Ytter Y., Younossi Z. M., Marchesini G., McCullough A. J. Clinical features and natural history of nonalcoholic steatosis syndromes. Semin Liver Dis 2001; 21: 17–26
- Bugianesi E., Leone N., Vanni E., Marchesini G., Brunello F., Carucci P., et al. Expanding the natural history of nonalcoholic steatohepatitis: From cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002; 123: 134–40
- Mokdad A. H., Ford E. S., Bowman B. A., Dietz W. H., Vinicor F., Bales V. S., et al. Prevalence of obesity, diabetes, and obesity‐related health risk factors, 2001. JAMA 2003; 289: 76–9
- Marchesini G., Bugianesi E., Forlani G., Cerrelli F., Lenzi M., Manini R., et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003; 37: 917–23
- Mofrad P., Contos M. J., Haque M., Sargeant C., Fisher R. A., Luketic V. A., et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003; 37: 1286–92
- Marchesini G., Brizi M., Morselli‐Labate A. M., Bianchi G., Bugianesi E., McCullough A. J., et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med 1999; 107: 450–5
- Cortez‐Pinto H., Camilo M. E., Baptista A., De Oliveira A. G., De Moura M. C. Non‐alcoholic fatty liver: another feature of the metabolic syndrome?. Clin Nutr 1999; 18: 353–8
- Chitturi S., Abeygunasekera S., Farrell G. C., Holmes‐Walker J., Hui J., Fung C., et al. NASH and insulin resistance: insulin secretion and specific association with the insulin resistance syndrome. Hepatology 2002; 35: 373–9
- Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–97
- Kim H. J., Lee K. E., Kim D. J., Kim S. K., Ahn C. W., Lim S. K., et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med 2004; 164: 2169–75
- Bianchi G., Marchesini G., Zoli M., Bugianesi E., Fabbri A., Pisi E. Prognostic significance of diabetes in patients with cirrhosis. Hepatology 1994; 20: 119–25
- Creutzfeldt W., Frerichs H., Sickinger K. Liver diseases and diabetes mellitus. Prog Liver Dis 1970; 3: 371–407
- Kumar K. S., Malet P. F. Nonalcoholic steatohepatitis. Mayo Clin Proc 2000; 75: 733–9
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002; 346: 1221–31
- Clark J. M., Brancati F. L., Diehl A. M. Nonalcoholic fatty liver disease. Gastroenterology 2002; 122: 1649–57
- Saverymuttu S. H., Joseph A. E., Maxwell J. D. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. BMJ 1986; 292: 13–5
- Ricci C., Longo R., Gioulis E., Bosco M., Pollesello P., Masutti F., et al. Noninvasive in vivo quantitative assessment of fat content in human liver. J Hepatol 1997; 27: 108–13
- Jick S. S., Stender M., Myers M. W. Frequency of liver disease in type 2 diabetic patients treated with oral antidiabetic agents. Diabetes Care 1999; 22: 2067–71
- El‐Serag H. B., Tran T., Everhart J. E. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004; 126: 460–8
- Ohlson L. O., Larsson B., Bjorntorp P., Eriksson H., Svardsudd K., Welin L., et al. Risk factors for type 2 (non‐insulin‐dependent) diabetes mellitus. Thirteen and one‐half years of follow‐up of the participants in a study of Swedish men born in 1913. Diabetologia 1988; 31: 798–805
- Vozarova B., Stefan N., Lindsay R. S., Saremi A., Pratley R. E., Bogardus C., et al. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 2002; 51: 1889–95
- Perry I. J., Wannamethee S. G., Shaper A. G. Prospective study of serum gamma‐glutamyltransferase and risk of NIDDM. Diabetes Care 1998; 21: 732–7
- Hanley A. J., Williams K., Festa A., Wagenknecht L. E., D'Agostino R. B, Jr., Kempf J., et al. Elevations in markers of liver injury and risk of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes 2004; 53: 2623–32
- Bellentani S., Saccoccio G., Masutti F., Croce L. S., Brandi G., Sasso F., et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med 2000; 132: 112–7
- Marceau P., Biron S., Hould F. S., Marceau S., Simard S., Thung S. N., et al. Liver pathology and the metabolic syndrome X in severe obesity. J Clin Endocrinol Metab 1999; 84: 1513–7
- Dixon J. B., Bhathal P. S., O'Brien P. E. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology 2001; 121: 91–100
- Ratziu V., Giral P., Charlotte F., Bruckert E., Thibault V., Theodorou I., et al. Liver fibrosis in overweight patients. Gastroenterology 2000; 118: 1117–23
- Silverman J. F., Pories W. J., Caro J. F. Liver pathology in diabetes mellitus and morbid obesity. Clinical, pathological, and biochemical considerations. Pathol Annu 1989; 24: 275–302
- Silverman J. F., O'Brien K. F., Long S., Leggett N., Khazanie P. G., Pories W. J., et al. Liver pathology in morbidly obese patients with and without diabetes. Am J Gastroenterol 1990; 85: 1349–55
- Clark J. M., Brancati F. L., Diehl A. M. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 2003; 98: 960–7
- Ruhl C. E., Everhart J. E. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology 2003; 124: 71–9
- Hsiao T. J., Chen J. C., Wang J. D. Insulin resistance and ferritin as major determinants of nonalcoholic fatty liver disease in apparently healthy obese patients. Int J Obes Relat Metab Disord 2004; 28: 167–72
- Prati D., Taioli E., Zanella A., Della Torre E., Butelli S., Del Vecchio E., et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002; 137: 1–10
- Stranges S., Dorn J. M., Muti P., Freudenheim J. L., Farinaro E., Russell M., et al. Body fat distribution, relative weight, and liver enzyme levels: A population‐based study. Hepatology 2004; 39: 754–63
- Assy N., Kaita K., Mymin D., Levy C., Rosser B., Minuk G. Fatty infiltration of liver in hyperlipidemic patients. Dig Dis Sci 2000; 45: 1929–34
- Donati G., Stagni B., Piscaglia F., Venturoli N., Morselli‐Labate A. M., Rasciti L., et al. Increased prevalence of fatty liver in arterial hypertensive patients. Role of insulin resistance. Gut 2004; 53: 1020–3
- Ferrannini E., Buzzigoli G., Bonadonna R., Giorico M. A., Oleggini M., Graziadei L., et al. Insulin resistance in essential hypertension. N Engl J Med 1987; 317: 350–7
- Jeong S. K., Nam H. S., Rhee J. A., Shin J. H., Kim J. M., Cho K. H. Metabolic syndrome and ALT: a community study in adult Koreans. Int J Obes Relat Metab Disord 2004; 28: 1033–8
- Marchesini G., Avagnina S., Barantani E. G., Ciccarone A. M., Corica F., Dall'Aglio E., et al. Aminotransferase and gamma‐glutamyltranspeptidase levels in obesity are associated with insulin resistance and the metabolic syndrome. J Endocrinol Invest 2005; 28: 333–9
- Marchesini G., Bugianesi E., Forlani G., Marzocchi R., Zannoni C., Vanni E., et al. Non‐alcoholic steatohepatitis in patients cared in metabolic units. Diabetes Res Clin Pract 2004; 63: 143–51
- Bugianesi E., Manzini P., D'Antico S., Vanni E., Longo F., Leone N., et al. Relative contribution of iron burden, HFE mutations and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology 2004; 39: 179–87
- Papadia F. S., Marinari G. M., Camerini G., Murelli F., Carlini F., Stabilini C., et al. Liver damage in severely obese patients: a clinical‐biochemical‐morphologic study on 1,000 liver biopsies. Obes Surg 2004; 14: 952–8
- Angulo P., Keach J. C., Batts K. P., Lindor K. D. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999; 30: 1356–62
- Mehta S. H., Brancati F. L., Sulkowski M. S., Strathdee S. A., Szklo M., Thomas D. L. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Hepatology 2001; 33: 1554
- Wilson C. Hepatitis C infection and type 2 diabetes in American‐Indian women. Diabetes Care 2004; 27: 2116–9
- Petit J. M., Bour J. B., Galland‐Jos C., Minello A., Verges B., Guiguet M., et al. Risk factors for diabetes mellitus and early insulin resistance in chronic hepatitis C. J Hepatol 2001; 35: 279–83
- Lecube A., Hernandez C., Genesca J., Esteban J. I., Jardi R., Simo R. High prevalence of glucose abnormalities in patients with hepatitis C virus infection: a multivariate analysis considering the liver injury. Diabetes Care 2004; 27: 1171–5
- Aytug S., Reich D., Sapiro L. E., Bernstein D., Begum N. Impaired IRS‐1/PI3‐kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology 2003; 38: 1384–92
- Shintani Y., Fujie H., Miyoshi H., Tsutsumi T., Tsukamoto K., Kimura S., et al. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology 2004; 126: 840–8
- Hourigan L. F., Macdonald G. A., Purdie D., Whitehall V. H., Shorthouse C., Clouston A., et al. Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology 1999; 29: 1215–9
- Adinolfi L. E., Gambardella M., Andreana A., Tripodi M. F., Utili R., Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology 2001; 33: 1358–64
- Monto A., Alonzo J., Watson J. J., Grunfeld C., Wright T. L. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology 2002; 36: 729–36
- Rubbia‐Brandt L., Giostra E., Mentha G., Quadri R., Negro F. Expression of liver steatosis in hepatitis C virus infection and pattern of response to alpha‐interferon. J Hepatol 2001; 35: 307
- Kumar D., Farrell G. C., Fung C., George J. Hepatitis C virus genotype 3 is cytopathic to hepatocytes: Reversal of hepatic steatosis after sustained therapeutic response. Hepatology 2002; 36: 1266–72
- Serfaty L., Andreani T., Giral P., Carbonell N., Chazouilleres O., Poupon R. Hepatitis C virus induced hypobetalipoproteinemia: a possible mechanism for steatosis in chronic hepatitis C. J Hepatol 2001; 34: 428–34
- Perlemuter G., Sabile A., Letteron P., Vona G., Topilco A., Chretien Y., et al. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral‐related steatosis. FASEB J 2002; 16: 185–94
- Ratziu V., Munteanu M., Charlotte F., Bonyhay L., Poynard T. Fibrogenic impact of high serum glucose in chronic hepatitis C. J Hepatol 2003; 39: 1049–55
- Bressler B. L., Guindi M., Tomlinson G., Heathcote J. High body mass index is an independent risk factor for nonresponse to antiviral treatment in chronic hepatitis C. Hepatology 2003; 38: 639–44
- Lam N. P., Pitrak D., Speralakis R., Lau A. H., Wiley T. E., Layden T. J. Effect of obesity on pharmacokinetics and biologic effect of interferon‐alpha in hepatitis C. Dig Dis Sci 1997; 42: 178–85
- Adams L. A., Sanderson S., Lindor K. D., Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 2005; 42: 132–8
- Poynard T., Mathurin P., Lai C. L., Guyader D., Poupon R., Tainturier M. H., et al. A comparison of fibrosis progression in chronic liver diseases. J Hepatol 2003; 38: 257–65
- Sasaki A. Mortality and causes of death in patients with diabetes mellitus in Japan. Diabetes Res Clin Pract 1994; 24
- de Marco R., Locatelli F., Zoppini G., Verlato G., Bonora E., Muggeo M. Cause‐specific mortality in type 2 diabetes. The Verona Diabetes Study. Diabetes Care 1999; 22: 756–61
- El‐Serag H. B., Everhart J. E. Diabetes increases the risk of acute hepatic failure. Gastroenterology 2002; 122: 1822–8
- Caldwell S. H., Hespenheide E. E. Subacute liver failure in obese women. Am J Gastroenterol 2002; 97: 2058–62
- Jepsen P., Vilstrup H., Mellemkjaer L., Thulstrup A. M., Olsen J. H., Baron J., et al. Prognosis of patients with a diagnosis of fatty liver ‐ a registry‐based cohort study. Hepatogastroenterology 2003; 50: 2101–4
- Ioannou G. N., Weiss N. S., Kowdley K. V., Dominitz J. A. Is obesity a risk factor for cirrhosis‐related death or hospitalization? a population‐based cohort study. Gastroenterology 2003; 125: 1053–9
- Dam‐Larsen S., Franzmann M., Andersen I. B., Christoffersen P., Jensen L. B., Sorensen T. I., et al. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut 2004; 53: 750–5
- Italian Multicentre Cooperative Project on Nutrition in Liver Cirrhosis. Nutritional status in cirrhosis. J Hepatol 1994; 21: 317–25
- Ratziu V., Bonyhay L., Di Martino V., Charlotte F., Cavallaro L., Sayegh‐Tainturier M. H., et al. Survival, liver failure, and hepatocellular carcinoma in obesity‐related cryptogenic cirrhosis. Hepatology 2002; 35: 1485–93
- Hui J. M., Kench J. G., Chitturi S., Sud A., Farrell G. C., Byth K., et al. Long‐term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology 2003; 38: 420–7
- Younossi Z. M., Gramlich T., Matteoni C. A., Boparai N., McCullough A. J. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol 2004; 2: 262–5
- Moller H., Mellemgaard A., Lindvig K., Olsen J. H. Obesity and cancer risk: a Danish record‐linkage study. Eur J Cancer 1994; 30A: 344–50
- Adami H. O., Chow W. H., Nyren O., Berne C., Linet M. S., Ekbom A., et al. Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst 1996; 88: 1472–7
- La Vecchia C., Negri E., Decarli A., Franceschi S. Diabetes mellitus and the risk of primary liver cancer. Int J Cancer 1997; 73: 204–7
- Wideroff L., Gridley G., Mellemkjaer L., Chow W. H., Linet M., Keehn S., et al. Cancer incidence in a population‐based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst 1997; 89: 1360–5
- Braga C., La Vecchia C., Negri E., Franceschi S. Attributable risks for hepatocellular carcinoma in northern Italy. Eur J Cancer 1997; 33: 629–34
- Lagiou P., Kuper H., Stuver S. O., Tzonou A., Trichopoulos D., Adami H. O. Role of diabetes mellitus in the etiology of hepatocellular carcinoma. J Natl Cancer Inst 2000; 92: 1096–9
- El‐Serag H. B., Richardson P. A., Everhart J. E. The role of diabetes in hepatocellular carcinoma: a case‐control study among United States Veterans. Am J Gastroenterol 2001; 96: 2462–7
- Nair S., Mason A., Eason J., Loss G., Perrillo R. P. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis?. Hepatology 2002; 36: 150–5
- Calle E. E., Rodriguez C., Walker‐Thurmond K., Thun M. J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003; 348: 1625–38
- Verlato G., Zoppini G., Bonora E., Muggeo M. Mortality from site‐specific malignancies in type 2 diabetic patients from Verona. Diabetes Care 2003; 26: 1047–51
- Sorensen H. T., Mellemkjaer L., Jepsen P., Thulstrup A. M., Baron J., Olsen J. H., et al. Risk of cancer in patients hospitalized with fatty liver: a Danish cohort study. J Clin Gastroenterol 2003; 36: 356–9
- Regimbeau J. M., Colombat M., Mognol P., Durand F., Abdalla E., Degott C., et al. Obesity and diabetes as a risk factor for hepatocellular carcinoma. Liver Transpl 2004; 10: S69–73
- Coughlin S. S., Calle E. E., Teras L. R., Petrelli J., Thun M. J. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol 2004; 159: 1160–7
- Jee S. H., Ohrr H., Sull J. W., Yun J. E., Ji M., Samet J. M. Fasting serum glucose level and cancer risk in Korean men and women. JAMA 2005; 293: 194–202
- Davila J. A., Morgan R. O., Shaib Y., McGlynn K. A., El‐Serag H. B. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut 2005; 54: 533–9
- Wolk A., Gridley G., Svensson M., Nyren O., McLaughlin J. K., Fraumeni J. F., et al. A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control 2001; 12: 13–21
- Lawson D. H., Gray J. M., McKillop C., Clarke J., Lee F. D., Patrick R. S. Diabetes mellitus and primary hepatocellular carcinoma. QJM 1986; 61: 945–55
- Huo T. I., Lui W. Y., Huang Y. H., Chau G. Y., Wu J. C., Lee P. C., et al. Diabetes mellitus is a risk factor for hepatic decompensation in patients with hepatocellular carcinoma undergoing resection: a longitudinal study. Am J Gastroenterol 2003; 98: 2293–8
- Ohata K., Hamasaki K., Toriyama K., Matsumoto K., Saeki A., Yanagi K., et al. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer 2003; 97: 3036–43
- Yang S., Lin H. Z., Hwang J., Chacko V. P., Diehl A. M. Hepatic hyperplasia in noncirrhotic fatty livers: is obesity‐related hepatic steatosis a premalignant condition?. Cancer Res 2001; 61: 5016–23
- Zen Y., Katayanagi K., Tsuneyama K., Harada K., Araki I., Nakanuma Y. Hepatocellular carcinoma arising in non‐alcoholic steatohepatitis. Pathol Int 2001; 51: 127–31
- Marrero J. A., Fontana R. J., Su G. L., Conjeevaram H. S., Emick D. M., Lok A. S. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology 2002; 36: 1349–54
- Medina J., Fernandez‐Salazar L. I., Garcia‐Buey L., Moreno‐Otero R. Approach to the pathogenesis and treatment of nonalcoholic steatohepatitis. Diabetes Care 2004; 27: 2057–66
- Bugianesi E., Zannoni C., Vanni E., Marzocchi R., Marchesini G. Non‐alcoholic fatty liver and insulin resistance: a cause‐effect relationship?. Dig Liver Dis 2004; 36: 165–73
- Browning J. D., Horton J. D. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 2004; 114: 147–52
- Groop L. Genetics of the metabolic syndrome. Br J Nutr 2000; 83( Suppl 1)S39–48
- Rich S. S., Concannon P. Challenges and strategies for investigating the genetic complexity of common human diseases. Diabetes 2002; 51( Suppl 3)S288–94
- Ashrafi K., Chang F. Y., Watts J. L., Fraser A. G., Kamath R. S., Ahringer J., et al. Genome‐wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 2003; 421: 268–72
- Sreekumar R., Rosado B., Rasmussen D., Charlton M. Hepatic gene expression in histologically progressive nonalcoholic steatohepatitis. Hepatology 2003; 38: 244–51
- Marfella R., Quagliaro L., Nappo F., Ceriello A., Giugliano D. Acute hyperglycemia induces an oxidative stress in healthy subjects. J Clin Invest 2001; 108: 635–6
- Esposito K., Nappo F., Marfella R., Giugliano G., Giugliano F., Ciotola M., et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 2002; 106: 2067–72
- Guha M., Bai W., Nadler J. L., Natarajan R. Molecular mechanisms of tumor necrosis factor alpha gene expression in monocytic cells via hyperglycemia‐induced oxidant stress‐dependent and ‐independent pathways. J Biol Chem 2000; 275: 17728–39
- Svegliati‐Baroni G., Ridolfi F., Di Sario A., Casini A., Marucci L., Gaggiotti G., et al. Insulin and insulin‐like growth factor‐1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology 1999; 29: 1743–51
- Paradis V., Perlemuter G., Bonvoust F., Dargere D., Parfait B., Vidaud M., et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology 2001; 34: 738–44
- Hickman I. J., Powell E. E., Prins J. B., Clouston A. D., Ash S., Purdie D. M., et al. In overweight patients with chronic hepatitis C, circulating insulin is associated with hepatic fibrosis: implications for therapy. J Hepatol 2003; 39: 1042–8
- Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004; 114: 1752–61
- Marchesini G., Ronchi M., Forlani G., Bugianesi E., Bianchi G., Fabbri A., et al. Cardiovascular disease in cirrhosis–a point‐prevalence study in relation to glucose tolerance. Am J Gastroenterol 1999; 94: 655–62
- Bugianesi E., Marzocchi R., Villanova N., Marchesini G. Non‐alcoholic fatty liver disease/non‐alcoholic steatohepatitis (NAFLD/NASH): treatment. Best Pract Res Clin Gastroenterol 2004; 18: 1105–16
- Neuschwander‐Tetri B. A., Caldwell S. H. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology 2003; 37: 1202–19
- Eriksson S., Eriksson K. F., Bondesson L. Nonalcoholic steatohepatitis in obesity: a reversible condition. Acta Med Scand 1986; 220: 83–8
- Palmer M., Schaffner F. Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology 1990; 99: 1408–13
- Ueno T., Sugawara H., Sujaku K., Hashimoto O., Tsuji R., Tamaki S., et al. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol 1997; 27: 103–7
- Franzese A., Vajro P., Argenziano A., Puzziello A., Iannucci M. P., Saviano M. C., et al. Liver involvement in obese children. Ultrasonography and liver enzyme levels at diagnosis and during follow‐up in an Italian population. Dig Dis Sci 1997; 42: 1428–32
- Knobler H., Schattner A., Zhornicki T., Malnick S. D., Keter D., Sokolovskaya N., et al. Fatty liver–an additional and treatable feature of the insulin resistance syndrome. QJM 1999; 92: 73–9
- Kugelmas M., Hill D. B., Vivian B., Marsano L., McClain C. J. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology 2003; 38: 413–9
- Hickman I. E., Jonsson J. R., Prins J. B., Ash S., Purdie D. M., Clouston A. D., et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut 2004; 53: 413–9
- Caldwell S. H., Hespenheide E. E., Redick J. A., Iezzoni J. C., Battle E. H., Sheppard B. L. A pilot study of a thiazolidinedione, troglitazone, in nonalcoholic steatohepatitis. Am J Gastroenterol 2001; 96: 519–25
- Neuschwander‐Tetri B. A., Brunt E. M., Wehmeier K. R., Oliver D., Bacon B. R. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR‐gamma ligand rosiglitazone. Hepatology 2003; 38: 1008–17
- Promrat K., Lutchman G., Uwaifo G. I., Freedman R. J., Soza A., Heller T., et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology 2004; 39: 188–96
- Marchesini G., Brizi M., Bianchi G., Tomassetti S., Zoli M., Melchionda N. Metformin in non‐alcoholic steatohepatitis. Lancet 2001; 358: 893–4
- Uygun A., Kadayifci A., Isik A. T., Ozgurtas T., Deveci S., Tuzun A., et al. Metformin in the treatment of patients with non‐alcoholic steatohepatitis. Aliment Pharmacol Ther 2004; 19: 537–44
- Nair S., Diehl A. M., Wiseman M., Farr G. H, Jr., Perrillo R. P. Metformin in the treatment of non‐alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther 2004; 20: 23–8
- Bugianesi E., Gentilcore E., Manini R., Natale S., Vanni E., Villanova N., et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol 2005; 100: 1082–90
- Neuschwander‐Tetri B. A., Brunt E. M., Wehmeier K. R., Sponseller C. A., Hampton K., Bacon B. R. Interim results of a pilot study demonstrating the early effects of the PPAR‐gamma ligand rosiglitazone on insulin sensitivity, aminotransferases, hepatic steatosis and body weight in patients with non‐alcoholic steatohepatitis. J Hepatol 2003; 38: 434–40
- Tiikkainen M., Hakkinen A. M., Korsheninnikova E., Nyman T., Makimattila S., Yki‐Jarvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes 2004; 53: 2169–76
- Ezzati M., Lopez A. D., Rodgers A., Vander Hoorn S., Murray C. J. Selected major risk factors and global and regional burden of disease. Lancet 2002; 360: 1347–60
- Marchesini G., Trovati M. Type 2 diabetes and the Naaman syndrome. Diabetes Care 2003; 26: 3195
- Ezzati M., Hoorn S. V., Rodgers A., Lopez A. D., Mathers C. D., Murray C. J. Estimates of global and regional potential health gains from reducing multiple major risk factors. Lancet 2003; 362: 271–80