Abstract
Background, Aims. Whether diabetes mellitus affects the prognosis of stroke patients, and whether admission hyperglycemia influences prognosis similarly in diabetic as in non‐diabetic patients is assessed controversially. The aims of the study were: 1) to compare the course of diabetic and non‐diabetic acute stroke patients, and 2) to assess the influence of admission serum glucose levels on case fatality.
Methods. In 57 Austrian medical departments the hospital course of consecutive stroke patients was documented prospectively between June 1999 and October 2000.
Results. Two hundred and ninety‐six (30%) of 992 patients had a history of diabetes mellitus. Intracerebral hemorrhage was more frequent in non‐diabetic patients than diabetic (13% versus 5%, P = 0.0001). Coronary heart disease was more frequent in diabetic than in non‐diabetic patients (35% versus 24%, P = 0.0003). The case fatality was 18% among non‐diabetic and 16% among diabetic patients (P = 0.3559). Among patients who were discharged alive, the Barthel Index increased from 50 to 90 in non‐diabetic and from 45 to 75 in diabetic patients (P = 0.0403). In non‐diabetic patients, admission serum glucose>9.2 mmol/L was associated with a more than 4‐fold increase in case fatality, compared with patients with serum glucose<5.7 mmol/L (P<0.0001).
Conclusions. Diabetic stroke patients need special care since they tend to have a poorer recovery than non‐diabetic patients. Admission hyperglycemia in non‐diabetic acute stroke patients predicts a poor prognosis.
| Abbreviations | ||
| AF | = | atrial fibrillation |
| BI | = | Barthel Index |
| CAD | = | coronary artery disease |
| Hyp | = | arterial hypertension |
| ICH | = | intracerebral hemorrhage |
| NIHSS | = | National Institute of Health Stroke Scale |
| RS | = | Rankin scale |
Introduction
Diabetes mellitus is a well‐established independent risk factor for stroke Citation1–4. Stroke in diabetic patients has been found to be associated with high case fatality in a long‐term study Citation5.Whether diabetes mellitus affects the short‐term prognosis of acute stroke patients is controversial Citation5–12. A poor outcome in acute stroke has been linked to admission hyperglycemia Citation13, Citation14. Whether this effect is equally important in diabetic as in non‐diabetic patients is controversial Citation6, Citation13.Thus, the aims of the present study were 1) to compare the in‐hospital course in acute stroke patients with and without diabetes mellitus, and 2) to assess the influence of admission serum glucose levels on case fatality depending on presence or absence of diabetes mellitus.
Key messages
Diabetic stroke patients need special care since they tend to have a poorer recovery than non‐diabetic patients.
Admission hyperglycemia in non‐diabetic acute stroke patients predicts a poor prognosis.
Patients and methods
The Austrian Stroke registry is a prospective multicenter study Citation15. Medical departments all over Austria participated in the trial documenting the in‐hospital course of consecutive patients with acute stroke from June 1999 to October 2000. All consecutive patients with acute stroke were documented and evaluated. Excluded were patients with transient ischemic attacks, cerebral tumor, sub‐ or epidural hemorrhage or other conditions imitating ischemic stroke. Additionally, patients were excluded if they were transferred to other departments within 3 days after admission or if the onset of stroke was >3 days before admission. There was not any common protocol on how to manage diabetic patients in the acute phase of stroke. Using specific forms the following data were recorded by the treating physicians:
Baseline characteristics: Age, sex, weight, height, blood‐pressure, heart‐rate, random serum glucose level at hospital admission, hematocrit, cholesterol, fibrinogen and electrocardiogram within 24 hours of admission. Glycated hemoglobin was not recorded.
Vascular risk factors and co‐morbid conditions: Coronary heart disease, other heart disease (dilated or hypertrophic cardiomyopathy, valvular heart disease, congenital heart disease), previous stroke, hypertension, obstructive pulmonary disease, current smoking, malignancy and dementia. Diabetes was assessed as present in patients with a history of diabetes or treatment with antidiabetic drugs.
Neurological findings: Type and location of the stroke were assessed. On admission and at discharge the Barthel Index (BI) scoring from 0 to 100 points Citation16, the Rankin Scale (RS) Citation17 and the National Institute of Health Stroke Scale (NIHSS) Citation18 were applied.
Stroke‐related neurological complications: Cerebral edema (as seen on cerebral computed tomography or magnetic resonance imaging), hydrocephalus, recurrent stroke, symptomatic intracerebral bleeding and seizures.
Medical complications: Pneumonia (fever, leukocytosis, infiltrate on chest X‐ray), urinary tract infection (leukocytosis, positive findings on urine culture), sepsis (fever, leukocytosis, positive findings on blood culture, organ involvement), deep vein thrombosis (demonstrated by venography or ultrasound), pulmonary embolism (demonstrated by helical computed tomography), pulmonary edema (clinical signs, pulmonary congestion on chest X‐ray) and extracerebral bleeding.
Treatment during hospitalization and at discharge: Parenteral fluid, parenteral nutrition, antibiotics, antipyretics, insulin, antihypertensives, heparin, acetylsalicylic acid or antiplatelet drugs, thrombolysis, neurosurgical therapy and transfer to an intensive care unit. The medical therapy at discharge was registered. Dietary treatment only for diabetic patients was not registered.
Statistical analyses were performed by using the statistical software package SYSTAT version 10 (SPSS Inc, Chicago Ill. USA). Continuous data were expressed as median values and quartiles. Non‐continuous data were expressed as percent. In univariate descriptive analysis the Kruskal‐Wallis‐test and the Pearson chi‐square test were used. During univariate testing a total of 125 tests have been calculated and so the level of significance was chosen to be α = 0.0004 according to the Bonferroni correction. All tests were two‐sided.
Stepwise multivariable logistic regression modeling was used to assess the prognostic significance of predictor variables for case fatality. Continuous variables were plotted against the outcome. In order to satisfy the assumption of linearity they were transformed to binary variables. For virtually all variables a clear threshold could be identified which indicated an increased case fatality. For age, this was >75 years, for mean arterial blood pressure<80 mmHg, for heart rate>100 bpm, for blood glucose>7 mmol/L, for hemoglobin<7.1 mmol/L, for serum cholesterol<4 mmol/L, for serum creatinine>125 µmol/L and for fibrinogen>11 µmol/L. The BI showed an increased risk for the value zero at admission versus all higher values. For the RS a value of 5 at admission had a higher risk than all lower values. The distribution of the NIHSS suggested 3 categories: patients with values above 21 had a moderate risk while those who were comatose had the highest risk. The coding for binary variables followed the ‘partial method’ using ‘0’ for the absence and ‘1’ for the presence of the condition in question. In analogy to that, the binary variables derived from the continuous data were coded with ‘0’ for the absence and ‘1’ for the presence of the criteria shown above. The NIHSS was coded with ‘0’ for values up to 21, with ‘1’ for values above 21 and with ‘2’ for the comatose patients. Sex was coded with ‘1’ for male and ‘2’ for female gender. In order to check for ‘influential observations’ the variable centre was forced into the model and declared as a categorical variable with ‘dummy’ coding. The following independent variables were retained for the analysis: centre, sex, age groups, intracerebral bleeding, localization: brainstem, BI, RS, NIHSS, previous stroke, coronary artery disease, hypertension, diabetes, atrial fibrillation, obstructive pulmonary disease, malignancy, mean arterial pressure, heart rate, glucose, hemoglobin and creatinine. A forward stepwise selection of the variables was chosen for the multivariable logistic regression. The probability for entry of a variable was set at 0.05 and for the removal of a variable at 0.10.
Results
The Austrian Stroke Registry recruited 1100 patients with acute stroke in 57 hospital departments. Excluded were patients who were transferred to other departments within 3 days after admission (n = 55) or in whom the onset of stroke was >3 days before admission (n = 39), or whose data forms were incomplete (n = 14). Therefore, 992 patients remained for evaluation. These 992 patients were hospitalized for a median of 14 days (range 1–92 days). Two hundred and ninety‐six patients (30%) of the 992 patients had a history of diabetes mellitus. Cerebral computed tomography was carried out in 98% of diabetic and 95% of non‐diabetic patients.
Baseline characteristics (Table).
Diabetic patients had more ischemic strokes and less intracerebral hemorrhages than non‐diabetics. There were no differences between the groups with respect to the localization of stroke or to severity as estimated by neurological scores such as the BI, RS or NIHSS.
Table I. Baseline characteristics of 992 diabetic and non‐diabetic patients with acute stroke
As expected, admission serum glucose levels were higher in patients with diabetes. All other laboratory parameters were similar in the two groups. The frequency of coronary heart disease was higher in diabetic than in non‐diabetic patients; for hypertension the level of statistical significance was not quite reached. In the subgroup of patients with hypertension, stroke was due to intracerebral hemorrhage in 5% of the diabetics and in 14% of the non‐diabetic patients (P = 0.0001).
Stroke‐related neurological and medical complications (Table).
No differences were found in the incidence of neurological complications between diabetic and non‐diabetic patients. Among the medical complications, urinary tract infection occurred somewhat more frequently in diabetic than in non‐diabetic patients.
Table II. Complications in diabetic and non‐diabetic patients with acute stroke
Treatment during hospitalization and at discharge (Table).
As expected, diabetic patients received insulin more frequently than non‐diabetics. The higher prescription rate of antibiotics – consistent with the slightly higher incidence of infections – and antihypertensive drugs did not quite reach the level of statistical significance. At discharge from hospital, patients with diabetes were prescribed more antidiabetics and antihypertensive drugs, in particular angiotensin‐converting‐enzyme (ACE) inhibitors and diuretics, reflecting the higher cardiovascular co‐morbidity or the higher prescription rate of ACE‐inhibitors in diabetic patients. In the 4 non‐diabetic patients (1%) who were discharged with antidiabetic therapy, diabetes mellitus was diagnosed during hospitalization.
Table III. Treatment in diabetic and non‐diabetic patients with acute stroke
Outcome:
The in‐hospital case fatality did not differ between groups and was 18% among the non‐diabetic and 16% among the diabetic patients (P = 0.3559). For multivariable analysis a total of 88 patients with missing values of any of the variables chosen had to be excluded, leaving 904 patients. Case fatality was 17% in this population, showing no change from the original group of 992 patients. Multivariable logistic regression analysis identified the following characteristics as significant predictors of case fatality: BI of zero on admission (odds ratio 5.30, 95% CI 3.10–9.08, P<0.0001), NIHSS>21 or comatose on admission (odds ratio 3.13, 95% CI 2.26–4.32, P<0.0001), age>75 years (odds ratio 3.15, 95% CI 1.85–5.37, P<0.0001), heart rate>100/min (odds ratio 2.15, 95% CI 1.26–3.66, P = 0.0049), obstructive pulmonary disease (odds ratio 2.58, 95% CI 1.03–6.48, P = 0.0442) and creatinine>125 µmol/L (odds ratio 1.84, 95% CI 1.00–3.37, P = 0.0479).
Among patients who were discharged alive, the median of the BI increased from 50 to 90 in non‐diabetic and from 45 to 75 in diabetic patients (P = 0.0403). The median of the RS score decreased from 4 to 3 in diabetic as well as non‐diabetic patients. The proportion of patients in RS score 0–1, however, was higher in non‐diabetic patients than diabetic (14% versus 7%, P = 0.004). The median of the NIHSS decreased from 5 to 2 in diabetic as well as non‐diabetic patients.
Hyperglycemia at admission:
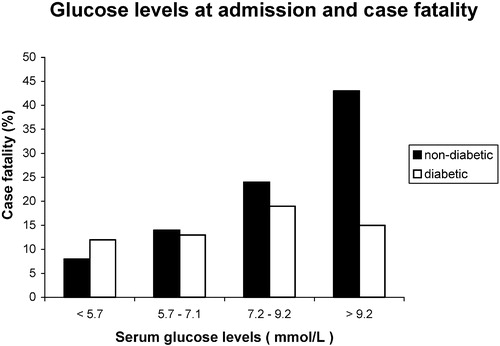
Median admission serum glucose level was 7.1 mmol/L (Q1: 5.7 mmol/L, Q3: 9.2 mmol/L). Patients with serum glucose levels above the median more often had diabetes mellitus (49% versus 12%, P<0.0001), and a lower BI (25 versus 45, P<0.0001) at admission. Admission serum glucose levels were lowest (6.7 mmol/L) in alert patients, 7.9 mmol/L in benumbed, 7.7mmol/L in obtunded and highest (8.6 mmol/L) in unresponsive patients (P<0.0001). Case fatality increased with increasing levels of serum glucose (). This increase was more pronounced in non‐diabetic than in diabetic patients. In non‐diabetic patients, admission serum glucose>9.2 mmol/L was associated with a more than 4‐fold crude increased case fatality, compared to patients with serum glucose<5.7 mmol/L (P<0.0001). When controlled for level of consciousness, there was a trend of a higher case fatality in alert patients with increasing serum glucose levels (P = 0.0115).
Discussion
This study shows that the in‐hospital case fatality of stroke did not differ between diabetic and non‐diabetic patients. There was a tendency, however, of a poorer recovery in diabetic than in non‐diabetic patients as reflected by a higher disability (BI) at discharge. Admission serum glucose levels were higher in patients who died during hospitalization than in patients who were discharged alive. In non‐diabetic patients admission serum glucose levels>9.2 mmol/L were associated with a more than 4‐fold increased case fatality, compared with patients with serum glucose levels<5.7 mmol/L.
Diabetic patients, particularly women, have a high risk of stroke Citation1–4. Whether diabetes influences the prognosis after stroke is controversial (). Diabetes has been shown to increase the short‐term Citation6, Citation8–11 and long‐term Citation5 case fatality after stroke. In two further studies Citation7, Citation12 however, the 3‐month case fatality did not differ between diabetic and non‐diabetic patients. These findings are similar to the results of the present study, which found no differences in the case fatality between diabetic and non‐diabetic patients. The controversy about diabetes and stroke case fatality may be due to differences in the age of the studied patients, in the prevalence of diabetes and co‐morbid conditions, in the duration of follow‐up and whether only patients with ischemic strokes or also patients with intracerebral hemorrhage were included (). The prevalence of 30% diabetes in the present study is much higher than the 15%–22% reported previously Citation5, Citation6, Citation8–10, Citation12, Citation19 and may reflect that the study included patients admitted only to medical and not to neurological departments. During the same period, the prevalence of diabetes in acute stroke patients admitted to neurologic stroke units was 24% Citation20.
Table IV. Prevalence of diabetes, co‐morbid conditions and case fatality in studies of acute stroke patients
Differences in the recovery of stroke between diabetic and non‐diabetic patients were detected in the present study and in previous studies. Neurological outcome was worse Citation11, speed of recovery was slower Citation7, Citation10 and recovery of motor function was poorer Citation12 in diabetic compared to non‐diabetic patients. This may be explained by the higher prevalence of coronary heart disease, peripheral arterial occlusive disease, neuropathy, retinopathy, nephropathy and pre‐stroke disability in diabetic compared to non‐diabetic patients.
Diabetic patients had less intracerebral hemorrhages than non‐diabetic. Although diabetes has been identified as a risk factor for cerebral hemorrhages by epidemiologic studies Citation21, the relatively lower proportion of cerebral hemorrhages among diabetic compared to non‐diabetic patients has been shown in previous reports Citation6, Citation8, Citation10, Citation12, Citation19. This phenomenon may due to vessel wall abnormalities such as thickened capillary basement membranes of small cerebral vessels or endothelial proliferation Citation22–24. These vascular changes might prevent hemorrhages.
Our findings confirm previously described associations between acute hyperglycemia in stroke patients and a high short‐term case fatality and poor functional recovery Citation7, Citation10, Citation13, Citation14, Citation25–28. The reasons for the increased case fatality in patients with admission hyperglycemia are not completely understood, but there are strong indications that hyperglycemia may be directly toxic to the ischemic brain since it leads to intracellular acidosis Citation29. These neurotoxic effects may be particularly important in the ischemic penumbra, the region of brain tissue surrounding the core of infarcted tissue where neurons are injured but still viable Citation25. Furthermore, hyperglycemic patients are relatively deficient in insulin and not likely to receive insulin, especially when they are non‐diabetic. Possibly, it is still assumed erroneously that glucose is ‘good for the brain’. Insulin deficiency leads to both reduced peripheral uptake of glucose and increasing circulating free fatty acids. On the other hand, non‐diabetic patients who develop hyperglycemia in acute stroke are likely to have undiagnosed diabetes mellitus. Citation8, Citation10, Citation11, Citation14. In the present study at least 1% of the non‐diabetic patients were diagnosed as having diabetes mellitus during hospitalization since they were discharged with antidiabetic therapy (). Patients with hyperglycemia or undiagnosed diabetes mellitus have a higher risk of vascular disease than patients with normal glucose levels Citation30, which could contribute to the higher case fatality from cardiovascular diseases. Furthermore, hyperglycemia may be a marker of the extent of ischemic damage in patients with stroke due to release of higher levels of stress hormones like cortisol or norepinephrine.
Whereas the association between hyperglycemia and poor outcome is a consistent finding in non‐diabetic stroke patients, this association has not been found consistently in diabetic patients, as in the present study Citation6, Citation10, Citation13. Possible explanations for these discrepancies are the low number of diabetic patients and the fact that diabetic patients are more likely to receive therapy for hyperglycemia. Glucose‐lowering therapy will reduce the amount of glucose available to enter into the brain and thereby might reduce cerebral acidosis. In animal studies it has been shown that administration of insulin reduces the size of the infarct and improves prognosis after stroke Citation29, Citation31. Whether in humans glucose lowering at the time of stroke can improve outcome, remains to be elucidated Citation32.
Limitations of the study are the lack of long‐term follow‐up data, that serum glucose was only registered once at admission, that no data about the quality of serum glucose management during hospitalization were recorded and possibly selection bias of the reported patients.
We conclude that diabetic stroke patients need special care since they tend to have a poorer recovery than non‐diabetic patients. Admission hyperglycemia, especially in non‐diabetic stroke patients is an indicator of an increased case fatality.
Acknowledgement
The study was supported by the Working Group for Cerebrovascular Diseases of the Austrian Cardiologic Society.
References
- Kannel W. B., McGee D. L. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care 1979; 2: 120–6
- Tuomilehto J., Rastenyte D., Jousilahti P., Sarti C., Vartiainen E. Diabetes mellitus as a risk factor for death from stroke. Prospective study of the middle‐aged Finnish population. Stroke 1996; 27: 210–5
- Haheim L. L., Holme I., Hjermann I., Leren P. Nonfasting serum glucose and the risk of fatal stroke in diabetic and nondiabetic subjects. 18‐year follow‐up of the Oslo Study. Stroke 1995; 26: 774–7
- Hart C. L., Hole D. J., Smith G. D. Risk factors and 20‐year stroke mortality in men and women in the Renfrew/Paisley study in Scotland. Stroke 1999; 30: 1999–2007
- Olsson T., Viitanen M., Asplund K., Eriksson S., Hägg E. Prognosis after stroke in diabetic patients. A controlled prospective study. Diabetologia 1990; 33: 244–9
- Lithner F., Asplund K., Eriksson S., Hägg E., Strand T., Wester P. O. Clinical characteristics in diabetic stroke patients. Diabet Metab 1988; 14: 15–9
- Woo J., Lam C. W. K., Kay R., Wong A. H. Y., Teoh R., Nicholls M. G. The influence of hyperglycemia and diabetes mellitus on immediate and 3‐month morbidity and mortality after acute stroke. Arch Neurol 1990; 47: 1174–7
- Kiers L., Davis S. M., Larkins R., Hopper J., Tress B., Rossiter S. C., et al. Stroke topography and outcome in relation to hyperglycaemia and diabetes. J Neurol Neurosurg Psychiatry 1992; 55: 263–70
- Toni D., Sacchetti M. L., Argentino C., Gentile M., Cavalletti C., Frontoni M., et al. Does hyperglycaemia play a role on the outcome of acute ischaemic stroke patients?. J Neurol 1992; 239: 382–6
- Jorgensen H. S., Nakayama H., Raaschou H. O., Olsen T. S. Stroke in patients with diabetes. The Copenhagen Stroke Study. Stroke 1994; 25: 1977–84
- Topic E., Pavlicek I., Brinar V., Korsic M. Glycosylated haemoglobin in clarification of the origin of hyperglycaemia in acute cerebrovascular accident. Diabet Med 1989; 6: 12–5
- Megherbi S. E., Milan C., Minier D., Couvreur G., Osseby G. V., Tilling, , for the European BIOMED Study of Stroke Care Group, et al. Association between diabetes and stroke subtype on survival and functional outcome 3 months after stroke. Data from the European BIOMED Stroke Project. Stroke 2003; 34: 688–94
- Capes S. E., Hunt D., Malmberg K., Pathak P., Gerstein H. C. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients. A systematic overview. Stroke 2001; 32: 2426–32
- Williams L. S., Rotich J., Qi R., Fineberg N., Espay A., Bruno A., et al. Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology 2002; 59: 67–71
- Slany J., Lenzhofer R., Kaiser R., Exner I., Schmid W., Hügel H., , für die Arbeitsgemeinschaft internistisches Insultregister, et al. Insultbehandlung an internen Abteilungen. Eine österreichische Multizenterstudie. Dtsch Med Wochenschr 2002; 127: 1575–80
- Mahoney F. I., Barthel D. W. Functional evaluation: The Barthel Index. Maryland State Med J 1965; 14: 61–5
- van Swieten J. C., Koudstaal P. J., Visser M. C., Schouten H. J. A., van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–7
- Brott T., Adams H. P Jr., Olinger C. P., Marler J. R., Barsan W. G., Biller J., et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989; 20: 864–70
- Karapanayiotides T. h., Piechowski‐Jozwiak B., van Melle G., Bogousslavsky J., Devuyst G. Stroke patterns, etiology, and prognosis in patients with diabetes mellitus. Neurology 2004; 62: 1558–62
- Steiner M. M., Brainin M. Austrian Stroke Registry for Acute Stroke Units. The quality of acute stroke units on a nation‐wide level: the Austrian Stroke Registry for acute stroke units. Eur J Neurol 2003; 10: 353–60
- Rodriguez B. L., D'Agostino R., Abbott R. D., Kagan A., Burchfiel C. M., Yano K., et al. Risk of hospitalised stroke in men enrolled in the Honolulu Heart Program and the Framingham Study. A comparison of incidence and risk factor effects. Stroke 2002; 33: 230–7
- Alex M., Baron E. K., Goldenberg S., Blumenthal H. T. An autopsy study of cerebrovascular accident in diabetes mellitus. Circulation 1962; 25: 663–73
- McCuskey P. A., McCuskey R. S. In vivo and electron microscopic study of the development of cerebral diabetic microangiography. Microcirc Endothelium Lymphatics 1984; 1: 221–44
- Powell H. C., Rosoff J., Myers R. R. Microangiopathy in human diabetic neuropathy. Acta Neuropathol (Berl) 1985; 68: 295–305
- Alvarez‐Sabin J., Molina C. A., Montaner J., Arenillas J. F., Huertas R., Ribo M., et al. Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator‐treated patients. Stroke 2003; 34: 1235–41
- Baird T. A., Parsons M. W., Phanh T., Butcher K. S., Desmond P. M., Tress B. M., et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke 2003; 34: 2208–14
- Bruno A., Biller J., Adams H. P Jr., Clarke W. R., Woolson R. F., Williams L. S., , for the Trial of ORG in Acute Stroke Treatment (TOAST) Investigators, et al. Acute blood glucose level and outcome from ischemic stroke. Neurology 1999; 52: 280–4
- Weir C. J., Murray G. D., Dyker A. G., Lees K. R. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long term follow up study. BMJ 1997; 314: 1303–6
- Anderson R. E., Tan W. K., Martin H. S., Meyer F. B. Effects of glucose and PaO2 modulation on cortical intracellular acidosis, NADH redox state, and infarction in the ischemic penumbra. Stroke 1999; 30: 160–70
- Coutinho M., Wang Y., Gerstein H. C., Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95 783 individuals followed for 12.4 years. Diabetes Care 1999; 22: 233–40
- Hamilton M. G., Tranmer B. I., Auer R. N. Insulin reduction of cerebral infarction due to transient focal ischemia. J Neurosurg 1995; 82: 262–8
- Scott J. F., Robinson G. M., French J. M., O'Connell J. E., Alberti K. G. M. M., Gray C. S. Glucose potassium insulin infusions in the treatment of acute stroke patients with mild to moderate hyperglycemia. The Glucose Insulin in Stroke Trial (GIST). Stroke 1999; 30: 793–9