Abstract
Phytoestrogens are plant‐derived hormone‐like diphenolic compounds of dietary origin that are present at high levels in plasma of subjects living in areas with low atherosclerosis and cancer incidence. The term phytoestrogen is commonly applied to the soy isoflavones genistein, daidzein and glycitein. As outlined in a previous review article in this journal by Adlercreutz and Mazur Citation, these compounds are weakly estrogenic and appear to influence the cardiovascular system, the production, metabolism and biological activity of sex‐hormones, as well as malignant cell proliferation, differentiation and angiogenesis. Recently skepticism has developed concerning the true potential of phytoestrogens to beneficially modify these processes. A critical analysis of the early findings from supplementing the diet with soy protein has failed to confirm phytoestrogens as the responsible agent for beneficial cardiovascular effects, be it by way of lipid reduction, vasodilation or lipoprotein oxidation. Furthermore, contrasting data have been reported on the potential of phytoestrogens to prevent hormone‐dependent cancers (e.g. breast and prostate) and to successfully treat post‐menopausal complaints, an indication for which they are widely used. These potentially negative findings have led health authorities in several countries to suggest maximum daily intake levels for phytoestrogens. There is now growing interest in the use of soy products containing low levels of phytoestrogens and in research on other phytoestrogen free legumes such as lupin.
Introduction
Phytoestrogen is a general term given to a large number of plant‐derived estrogen‐like compounds found in legumes, seeds, fruits and vegetables. The attitude of the scientific community towards these compounds has generally been positive: for example a 1997 review article in Annals of Medicine Citation1 discussed a large number of findings suggesting potentially beneficial effects of phytoestrogen consumption, in particular against arterial disease and cancer. The first ever literature report on the effects of phytoestrogen intake, however, described a potentially toxic activity, related to multiple fertility problems in sheep grazing on pastures based on red clover Citation2. This plant contains high amounts of two phytoestrogens, formononetin and biochanin A. Subsequent studies showed that these and other phytoestrogens interact with the estrogen receptors ERα or ERβ, due to their structural similarity to 17β‐estradiol Citation3.
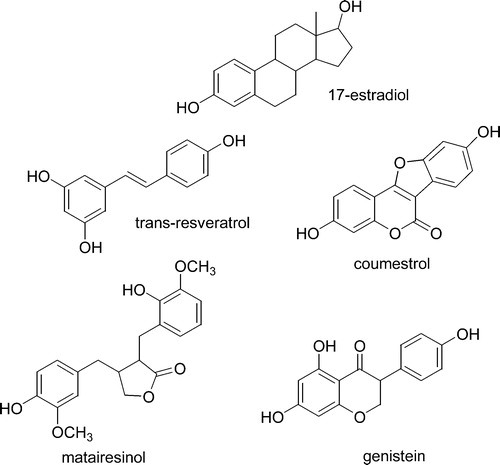
Currently, four groups of phenolic compounds are classified as phytoestrogens Citation3: the isoflavones, stilbenes, coumestans, and lignans (). The main stilbene is resveratrol, found primarily in grapes and peanuts Citation3. Although there are two isomers, cis and trans, only the latter shows estrogenic activity. Resveratrol is biosynthesized only in grape skin; therefore white wines contain only traces, whereas higher levels of resveratrol are found in red wines, fermented with skins Citation4, Citation5. Only a few coumestans are characterized by estrogenic activity, the most important being coumestrol, whose main dietary source is legumes, although it has also been reported in other vegetables, such as Brussels sprouts and spinach Citation6. ‘Lignans’ is a general term for a large family of compounds. One important example, matairesinol, is a non‐estrogenic dimer, converted by gut microflora to enterolactone, which is estrogenic and easily absorbed Citation7. The main dietary source of lignans is flax‐seed, but they are present also in whole wheat flour, fruits, and tea Citation3. Isoflavones are a subclass of the flavonoids, which are found almost exclusively in soybeans and in a few other legumes including red clover. The main isoflavones are genistein, daidzein and glycitein, which in soybean and soy‐based foods exist as 7O‐glucosides Citation3. As isoflavones are the most widely investigated phytoestrogens, unless specifically indicated, throughout this review the term ‘phytoestrogens’ will refer specifically to this class of compounds.
Figure 1 Chemical structures of major phytoestrogens compared to 17β‐estradiol. Genistein and the chemically related isoflavones daidzein and glycitein are the most widely used compounds. Genistein, daidzein and glycitein are generally labelled as “phytoestrogens” and they are thus the major topic of the present review article.
Key messages
Critical analysis of clinical findings from studies on soy‐treated subjects has failed to identify phytoestrogens as responsible agents for cardiovascular benefit, anti‐cancer properties, and beneficial effects on post‐menopausal complaints.
Proteins from soy seem to provide essentially all of the clinical benefit, at least for cardiovascular indications.
Regulations in some nations restrict use of soy phytoestrogens to relatively low daily intakes.
Phytoestrogens: Metabolism and intake
Phytoestrogens are primarily ingested in the form of their glycosides, genistin, daidzin and glycitin. De‐conjugation occurs in the gastrointestinal tract through the activity of microflora to produce the free aglycones genistein, daidzein and glycitein, which are readily absorbed, re‐conjugated in the liver and then undergo enterohepatic circulation Citation8. It is however the minor fraction of free and sulphated forms that are considered biologically active Citation9. Excretion occurs primarily through the urine (conjugated forms) and feces (unconjugated forms).
Differences in individual gastrointestinal metabolism may be an important factor determining the efficacy of these compounds for disease risk reduction. Apart from deconjugation, they can also be further metabolized within the intestine by resident microflora. For example, in around a third of the population (‘good equol producers’) daidzein is readily converted via dehydroequol to equol, leading to high levels of equol detectable in the urine Citation10–12. This conversion appears to depend on an individual's endogenous gastrointestinal microflora, which may in turn be modulated by habitual diet Citation11. All laboratory rats can however convert daidzein to equol Citation13 and this may help explain some of the discrepancies in the effects of phytoestrogens in rats compared to humans. It has been proposed that the ability to produce equol may be associated with specific health benefits Citation13 (see the next two sections).
The phytoestrogen intake in the average East and Southeast Asian diet is estimated to be around 20–50 mg/day Citation14, Citation15, whereas in Western countries due to limited soy product consumption, it is much lower. Intakes ranging from 0.15 mg/d to >3 mg/d have been reported in the United States Citation16. Data regarding European countries are very scarce: typical values range from 0.63 to 1.00 mg/d in men and from 0.49 to 0.66 mg/d in women Citation17, Citation18.
Growing knowledge of the physiological effects of phytoestrogens has gone hand in hand with a rapid growth in the use of soy in Western diets. The growth of soy consumption was further catalyzed by the United States Food and Drug Administration (US FDA) decision to allow labeling of soy protein rich foods with the indication that 25 g/day can help reduce cardiovascular disease risk Citation19.
As a result of the wide popularity of soy in general, phytoestrogens, as one of the key soy components, have also gained a reputation for being beneficial to health. This hypothesis arose from a number of observations, ranging from the recognition that phytoestrogens have estrogen‐like activity in some animal models, to the low prevalence of cardiovascular disease and hormone‐dependent cancers of the breast, endometrium and prostate in Asia, where phytoestrogen intake is relatively high. In addition, a large number of studies have demonstrated the hypocholesterolemic properties of soy proteins Citation20, frequently containing isoflavones. These findings have wrongfully led to the conclusion that phytoestrogens are the active beneficial agents within soy protein.
Potential cardiovascular benefits
A number of epidemiological observations have supported a protective role of phytoestrogens in modulating cardiovascular disease (CVD) risk markers. Consumption of soy products has been associated with reduced serum cholesterolCitation21 and lignans have also shown a moderately protective effect on triglyceridemia Citation22. The US FDA decision to allow promotion of soy protein for heart health was based on a meta‐analysis indicating a significant total and low density lipoprotein (LDL) cholesterol reduction following an average soy protein intake of 47 g/day Citation23. However, studies reporting significant cholesterol reduction within the meta‐analysis (that were essentially the basis for the approval of the health claim by the US FDA) were on relatively severe cases of hypercholesterolemia and were mainly carried out in Italy and Switzerland using soy proteins with very low isoflavone levels Citation24, Citation25. A possible role of phytoestrogens in cholesterol reduction was however indicated by clinical studies in which they were removed from the soy protein preparations hot ethanol extraction Citation26. This rather extreme chemical treatment resulted in a dramatic reduction of the hypocholesterolemic properties of the phytoestrogen‐free soy protein in animal models of hypercholesterolemia Citation27. However, in a study on ovariectomized adult female cynomolgous monkeys, a phytoestrogen rich ethanol extract of soybean did not exert any lipid lowering effect Citation28. This finding and the fact that ethanol treatment removes bioactive compounds other than phytoestrogens and may denature the protein, tends to invalidate any conclusion on the hypocholesterolemic activity of phytoestrogen based on the ethanol extraction studies.
Little support for the role of phytoestrogens in modulating cholesterolemia has been gained from human randomized placebo‐controlled studies using isolated phytoestrogens in tablet/capsule form or soy protein with varying levels of phytoestrogens Citation29–31. These studies have generally failed to show an effect of phytoestrogens on cholesterolemia independent from that of soy protein.
Recently, a comparison of different commercial soy protein isolates performed by some of the authors of this review using proteomic techniques indicated that soy protein isolates, used in clinical studies in the US, showed extensive proteolysis Citation32: this was even more significant in samples submitted to ethanol extraction. The damage of the protein structure may possibly be related to the loss of the hypocholesterolemic activity, a fact that has certainly been underestimated in available literature. In fact, a soy protein product, in which the isoflavones had been removed by mild column chromatography, proved to maintain its hypocholesterolemic properties Citation33, thus providing evidence that specific components of the proteins are most likely responsible for the cholesterol lowering properties of soybeans Citation34.
At a mechanistic level, low density lipoprotein receptor (LDL‐R) stimulation is believed to be the route by which soy protein preparations elicit their hypocholesterolemic activity Citation35–38. There have been a number of in vivo investigations showing this same effect suggesting that peptides resulting from soy protein digestion may enter the circulation and exert beneficial effects on cholesterol metabolism in the liver. An early study at the University of Milan Citation39 showed an 8‐fold increase in LDL receptor activity in isolated lymphomonocytes from severely hypercholesterolemic patients treated with soy proteins. A similar finding, but with lesser increase of LDL receptors was reported by Baum et al. Citation40 in post‐menopausal women with less dramatic elevations of LDL cholesterolemia. In rats the purified 7S α' component of soy protein has been found to elicit a 10‐fold higher hypocholesterolemic activity than that exerted by a synthetic hypocholesterolemic drug Citation41. The major soy phytoestrogen genistein, however, at concentrations of up 1 mg/ml, failed to demonstrate any LDL‐R stimulatory activity in studies on HepG2 cells Citation38.
A recent consensus paper Citation42 indicates that both soy protein and isoflavones may be needed for the maximal cholesterol lowering effect of soy, also recommending a diet low in saturated fat and cholesterol to promote heart health. It is however difficult at present to identify possible mechanisms whereby phytoestrogens may exert any additional effects to those exerted by soy protein. It has been considered that by binding to estrogen receptors, phytoestrogens may stimulate LDL receptors. By using phytoestrogen concentrations elevated far beyond physiological levels, Borradaile et al. Citation43 show reduced secretion of apolipoprotein B and increased LDL receptor activity in liver cells: the clinical relevance of these findings is however questionable.
Soy phytoestrogens do exert some vasodilatory activity in special conditions. Acute intravenous administration of genistein or daidzein was evaluated in healthy humans of both sexes Citation44. Genistein was infused for 6 minutes at concentrations of between 10 and 300 nmol/min. At the two highest doses a significantly increased forearm arterial flow was observed in both sexes. Similar effects were exerted by equimolar amounts of 17β‐estradiol. Both genistein and 17β‐estradiol effects were antagonized by a nitric oxide (NO) synthase antagonist. The plasma genistein concentrations after i.v. administration were, however, 8–10‐fold higher than those observed after oral intake of high phytoestrogen soy proteins. In a randomized double blind trial, genistein intake (54 mg/day) significantly increased flow‐mediated endothelium dependent vasodilation in post‐menopausal women Citation45 with a significant increase in serum nitrates after 6 months of treatment along with a 50% reduction of plasma endothelin‐1. The discovery of this NO dependent mechanism was supported by a one‐year investigation on post‐menopausal women where genistein (54 mg/day) was compared to standard hormone replacement therapy Citation46. Flow‐mediated dilatation significantly increased from 3.9% to 7% supporting a potential protective role of genistein in conditions of altered vasomotility. It should, however, be pointed out that another study in a similar series of women given purified phytoestrogens failed to confirm these findings and again did not report any plasma lipid changes Citation47. Similarly, a recent randomized controlled trial of phytoestrogen‐containing soy protein versus casein in 202 post‐menopausal women found no beneficial effect of the soy diet on vascular function Citation48.
There is no clinical evidence currently available on the effects of isoflavones on atherosclerosis, as assessed by the standard clinical procedures of carotid intima‐media thickness, or coronary angiography. A report in diabetic and non‐diabetic monkeys has however reported a significantly reduced delivery of lipoproteins into monkey arteries after a phytoestrogen rich soy diet Citation49: no effort was however made to assess the separate contributions of proteins and phytoestrogens.
Another beneficial mechanism attributed to phytoestrogens is their potential antioxidant activity. The consumption of soy proteins compared to casein does lead to a significant decrease in arterial lipid peroxidation, a putative mechanism of atherosclerosis development/progression. In vitro and in vivo studies investigating the antioxidant effects of phytoestrogens Citation50 concluded that they act as antioxidants directly or indirectly by enhancing the anti‐oxidant enzyme activities of catalase, superoxide dismutase, glutathione peroxidase and glutathione reductase. Arterial lipid peroxidation levels were ∼17% lower in post‐menopausal non‐human primates fed a soy protein isolate containing phytoestrogens, compared to animals fed casein and lactalbumin as the protein sources Citation51. Tikkanen et al. Citation52 examined the effects of feeding soy protein containing 60 mg phytoestrogens/day on copper‐induced LDL oxidation in six healthy volunteers. Two weeks of soy consumption significantly prolonged the LDL oxidation lag time by ∼ 20 min.
More recently the effects on in vivo lipid peroxidation and resistance of LDL to in vitro oxidation in men and post‐menopausal women fed a textured soy protein diet with either high or low phytoestrogen content were reported. The phytoestrogen‐rich diet induced significantly lower levels of the F2 isoprostane biomarker of lipid peroxidation, 8‐epi‐prostaglandin F2α (−19.5%) and, in addition the lag time for copper‐mediated LDL oxidation was significantly prolonged Citation53. However, in a study using phytoestrogens in pill form (86 mg/day) Citation54, there was no evidence of reduced LDL oxidation. This latter finding suggests a direct antioxidant activity of soy proteins per se, an hypothesis also supported by the findings of Castiglioni et al. Citation55 that showed a powerful hypolipidemic and anti‐atheromatous activity of an essentially phytoestrogen free soy protein diet in rabbits. Moreover, a very recent study in mildly hypercholesterolemic individuals found very little effect of soy protein or phytoestrogens on plasma antioxidant capacity or biomarkers of oxidative stress Citation56. Similar inconclusive findings were also observed in a large Dutch study (Dutch Prospect‐EPIC cohort) on 16,165 women from 49 to 70 years of age that carefully evaluated phytoestrogen intake. In this study phytoestrogens were not associated with decreased CVD risk. However when stratifying for ‘ever’ versus ‘never’ smokers, CVD risk did decrease with increasing intake of lignans in ‘ever’ smokers Citation57.
Recently, some evidence has arisen that the metabolism of genistein to equol that occurs in some humans may be related to a reduced cardiovascular disease risk Citation13. In a crossover dietary intervention study of 26 mildly hypercholesterolemic and/or hypertensive volunteers, Meyer et al. Citation10 found no difference in the effect on serum lipids of a three‐week consumption of a phytoestrogen‐containing soy protein compared to dairy protein. However when the data of the 8 good equol producers were analysed separately, significantly lower levels of total cholesterol, LDL‐cholesterol, triglycerides and lipoprotein(a) were found after the soy protein diet. In addition, equol reportedly is a better antioxidant than daidzein ex vivoCitation58 and unlike daidzein, dehydroequol has demonstrated vasodilatory properties in humans Citation59. In the study by Kreijkamp‐Kaspers et al. Citation48, beneficial changes (though not statistically significant) in blood pressure and endothelial function after a phytoestrogen‐containing soy‐supplemented diet were observed only in the sub‐set of equol producing subjects.
A summary of the randomized clinical studies investigating the effect of isolated phytoestrogens or low versus high phytoestrogen‐containing soy on risk factors for cardiovascular disease is given in . At present, there appears little evidence for a major contribution to cardiovascular health of soy components other than the protein itself. Recent experimental findings indicate that some peptide fractions from soy protein given orally may in fact reduce cholesterolemia in animals to a greater extent even than lipid lowering drugs Citation41 and appropriately genetically modified soy proteins can reduce blood pressure in animals at doses achievable in man Citation60. In addition, naturally phytoestrogen‐free proteins from other legumes, e.g. lupin, lower cholesterolemia to a similar extent as soy proteins Citation61.
Table I. Summary of randomized clinical studies investigating the effect of isolated phytoestrogens or low versus high phytoestrogen‐containing soy on risk factors for cardiovascular disease.
Hormone dependent tumors
The endocrine effect of phytoestrogens was suggested as a potential mechanism to explain the epidemiological observation that Asian women have a significantly lower rate of breast cancer (BC) than Western women. The soy phytoestrogen genistein is known to bind rather weakly to the classical estrogen receptor (ERα), but binds with much higher affinity to ERβ Citation62 and may, therefore, display more potent effects in tissues with a higher ERβ expression such as the arteries. After endothelial denudation Citation63, expression of both the ERα and ERβ are both increased, but ERβ is over‐expressed to a much greater extent (∼30‐times more), versus the case of the uterus. Treatment of rats with various doses (0–2.5 mg/kg) of either 17β‐estradiol or genistein resulted in protection against cell proliferation, confirming a selective inhibition by genistein of the migration and proliferation of vascular smooth muscle cells Citation64, Citation65.
A number of data have indicated an inverse association between BC risk and phytoestrogen intake Citation66, both in relation to consumption and urinary excretion. Further, Asian women who move to the US and adopt a Western diet lose the lower risk of BC Citation67. Lignans have also received attention for their contribution to reduced BC risk Citation68. However, epidemiological studies have not provided consistent results. There was no relationship between soy food consumption and BC risk in a case‐control study in Chinese women Citation69 or in a more recent prospective study, in Japanese women Citation70. In this last study, however, consumption of miso soup several times per week was associated with a reduced BC risk. BC risk was not affected by phytoestrogen intake in a case‐control study involving multi‐ethnic American women Citation71 and similar inconclusive findings were reported in post‐menopausal Dutch women Citation72.
Lowered BC risk may be related to the ability of some humans to metabolize daidzein to equol, independent of level of isoflavone intake. Urinary excretion of equol (used as a surrogate of intake) was found to be inversely proportional to breast cancer risk in a case controlled study Citation73. Equol excretors also exhibited hormonal profiles more indicative of lower breast cancer risk than non‐excretors Citation12. This possible cancer protective effect of equol could be related to differences in the estrogenic properties of equol compared to its parent molecule Citation74, Citation75.
In the light of these contradictory findings, it has been hypothesized that phytoestrogens may protect against BC by a mechanism independent of their activity on estrogen receptors. They can, in fact, inhibit enzymes involved in the synthesis of steroid hormones, including aromatase and 17 β‐hydroxysteroid dehydrogenase Citation76. In addition, genistein has the capacity to inhibit tyrosine kinases, DNA topoisomerase, and angiogenesis Citation77. One last confusing issue is that of age of exposure. Lamartiniere et al. Citation78 demonstrated that the maximal protection against chemically induced BC in adult rats is obtained from exposure to genistein pre‐pubertally and again in adulthood. Therefore, possible use of phytoestrogens for BC protection could be complicated by the need to expose very young individuals where other safety issues (described below) may be present. Further, caution has been recently been suggested when using phytoestrogens in women treated with tamoxifen, since low intakes of phytoestrogen may antagonize the therapeutic effect of this estrogen antagonist Citation79.
Contradictory findings have also been reported for the potential role of phytoestrogens in protection against prostate cancer (PC). There appears to be a lower rate of PC in Asian men relative to Western men Citation80 and this, like BC risk in women, is associated with a higher intake of phytoestrogens. Elevated phytoestrogen levels can be detected in blood, urine and prostatic fluids of Asian compared to Western men Citation8, and an increased risk of developing PC occurs when Asian men are exposed to a Western diet Citation81. However, epidemiological studies relating intake of phytoestrogen‐rich soy and PC risk have not been consistent. Prospective studies in Japanese‐Hawaiian men and California's Seventh Day Adventists indicated a reduced PC risk following consumption of tofu or soy milk Citation82 and two US case‐control studies found a significant inverse relationship between soy food consumption and PC risk Citation83. However, two case‐control and one prospective study in Asian countries could not find an association between soy consumption and PC risk Citation84–86. Further, there has been no evidence that soy consumption may reduce prostate specific antigen (PSA) levels Citation87, Citation88, although an international study focusing specifically on lignan‐rich flaxseed in patients awaiting prostate surgery did find some reduction in testosterone, free androgen index and tumor proliferation index, but again with no change in PSA Citation89. There remains the possibility that PC protection may be related to mechanisms such as inhibition of 5α‐reductase, responsible for converting testosterone to dihydrotestosterone, or other enzymes regulating steroid hormone biosynthesis. Nevertheless the evidence of a protective effect of phytoestrogens on PC is far from proven.
Results of studies investigating the activity of phytoestrogens on other types of cancer have been even less conclusive. There is an apparent relationship between consumption of soy foods and gastric cancer; this appears to depend on whether or not the soy food is fermented Citation90. Fermented soy foods may possibly increase the risk of stomach cancer because of their high content of N‐nitroso compounds or other unidentified pro‐carcinogenic components Citation90. Soy intake and colon cancer have been found to be inversely related, but some data on miso also indicated an increased risk of rectal cancer Citation91, Citation92. Finally consumption of soy products has been linked to a decreased risk of endometrial cancer in a case‐control study performed in Hawaii Citation93.
Osteoporosis and bone health
Reducing the risk of osteoporosis is among the most relevant health topics in post‐menopausal women, with estrogen production being a major contributing factor. Hormone replacement therapy (HRT) can ameliorate the loss of estrogen in the menopause and provide clear benefit against osteoporosis Citation94, and a synthetic phytoestrogen, ipriflavone, was found to reduce bone loss in post‐menopausal women Citation95. There is substantial interest in alternative therapies for osteoporosis risk reduction, particularly those of dietary origin. A bone‐preserving effect of phytoestrogens has been supported by a number of epidemiological studies, based on observations of a significantly lower risk of fractures among Asian women compared to Caucasian women Citation96. Observational studies have also indicated that soy intake in pre‐ and post‐menopausal women is significantly associated with improved bone mineral density (BMD) Citation97, Citation98. In a Japanese study, in 478 post‐menopausal women, there was evidence of significantly different BMD adjusted to post‐menopausal years in the group with highest versus lowest daily intake of phytoestrogens (P<0.01) Citation99. These findings were not however supported by a 10‐year follow‐up study of post‐menopausal women in the Netherlands where no association was found between bone changes and the excretion of phytoestrogens Citation100.
While observational studies have provided some evidence of the osteoporosis‐protective role of phytoestrogens, controlled investigations have provided inconsistent results. Potter et al. Citation101 investigated supplementation with two different doses of phytoestrogens for 6 months on the effect on BMD. This double‐blind study noted significant increases (P<0.005) in both bone mineral content and density in lumbar spine (but not elsewhere), in the high dose phytoestrogens group (90 mg/day) versus controls. In a controlled study, also on pre‐ and post‐menopausal women, soy protein isolates containing different amounts of phytoestrogens (from 8 to 130 mg/day) failed to induce significant changes in the markers of bone turnover, though a possible reduction of osteocalcin, insulin‐like growth factor (IGF)‐1 and IGFB‐3, following high‐dose phytoestrogens was seen in the post‐menopausal group Citation102. Therefore this study did not support any useful effect of phytoestrogens on bone turnover. The conclusion of these earlier studies was that post‐menopausal women should not be advised to replace HRT with phytoestrogens in order to improve bone health Citation103.
More recently some beneficial effects of phytoestrogens have been noted when evaluating lumbar spine BMD and bone mineral content (BMC) by dual‐energy X‐ray absorptiometry. In the study by Alekel et al. Citation104, women were treated with a phytoestrogen‐rich soy (SOY+, 80.4 mg aglycones/day) a low phytoestrogen soy (SOY−, 4.4 mg/day) or a whey protein control. Both SOY+ and SOY− groups experienced no loss of BMD and BMC in the lumbar spine, whereas a loss in BMD and BMC occurred in the whey protein control group (−1.28% and −1.73% respectively, both P<0.005). Regression analysis identified significant positive effects of SPI+ on BMD (+5.6%) and BMC (+10.1%). A 2‐year study on the effects of a soy milk analogue (SOY+, 76 mg/day phytoestrogens) compared to transdermal progesterone (TPD+), the combination of the two or a placebo control was carried out on post‐menopausal Danish women with established osteoporosis or at least 3 risk factors Citation105. BMD and BMC were measured in the lumbar spine and hip using X‐ray absorptiometry. Confirming the study by Alekel et al. Citation104, increases in the percentage changes in lumbar spine BMD and BMC after 2 years were observed in the SOY+ group (+1.1% and +2.0% respectively) and the TDP+ group (+1.1% and +0.4% respectively). A significant bone loss occurred in the group on no treatment (−4.2% and −4.3% respectively) though also in the combined treatment group (SOY+/TDP+) (−2.8% and −2.4% respectively). This study therefore provides evidence that two glasses of soy milk a day, containing 76 mg/phytoestrogens, may prevent lumbar spine bone loss with similar efficacy to transdermal progesterone. However, the negative effect of the combination of the two treatments is difficult to explain. Further, the authors did not evaluate a classical HRT but just a progesterone addition. Somewhat similar findings were also reported by Chen et al. Citation106 and in abstract form by Vitolins et al. again in a 2 year study Citation107. In contrast, two recent, large randomized trials on post‐menopausal women showed no clear activity of phytoestrogens on bone changes Citation108, Citation109.
A very recent study on calcium metabolism carried out in 15 post‐menopausal women using metabolic balance and kinetic modeling Citation110 did not confirm earlier conclusions that phytoestrogens have at best a calcium sparing effect Citation8 and, as reported in one study Citation111, an apparent stimulatory effect on osteoblastic activity. This randomized crossover investigation of three, one‐month controlled dietary interventions was carried out Citation110: soy protein with phytoestrogens (SOY+, 82 mg/day), soy protein without phytoestrogens, (SOY−) and a casein/whey protein enriched diet. There was evidence of a lower urinary calcium excretion (P<0.01) with consumption of either soy based diet (SOY+ and SOY−) i.e. 85 and 80 mg/day, compared to the control diet (121 mg/day). However, fractional calcium absorption was unaffected by the treatments and overall no effects on bone deposition, resorption and calcium retention were seen, indicating no overall effect of phytoestrogens on calcium metabolism.
A summary of randomized clinical studies investigating the effect of phytoestrogens on osteoporosis and bone metabolism markers is given in . The reported effects of phytoestrogens on osteoporosis appear mixed, some studies showing mild benefit others no effect. The majority of trials have not, however, strongly supported clear beneficial effects Citation112, Citation113.
Table II. Summary of randomized clinical studies investigating the effect of phytoestrogens on osteoporosis and bone metabolism markers.
Post‐menopausal complaints
Observational studies have indicated that post‐menopausal vasomotor symptoms (e.g. hot flushes and/or night sweats) in Japanese women are nearly 10‐fold lower than in US or other Western women. This reduction of symptoms has been related to the 100‐fold higher excretion of phytoestrogens in the urine of Japanese women compared to their Western counterparts Citation114, Citation115.
HRT in post‐menopausal women is generally associated with a reduction in the number and severity of vasomotor symptoms and a potentially beneficial effect against cognitive decline was described by some authors Citation116 but not recently confirmed Citation117. Only a relatively small percentage of post‐menopausal women take HRT (12% to 21% of US women in 2000) and this percentage has recently declined after the negative results of randomized studies evaluating cardiovascular effects of HRT Citation118.
Clinical studies on the effects of phytoestrogens on vasomotor symptoms of menopause have provided mixed results. Modest reductions in the frequency and severity of hot flushes was reported in a number of studies. In an open study of soy flour versus wheat flour, both diets reduced hot flushes (by 40% and 25% respectively) and menopausal symptom scores Citation119. A few studies using higher doses of phytoestrogens (50–80 mg/day) in women with a high incidence of vasomotor symptoms at baseline (4–7 symptoms/day) have shown mildly beneficial effects on self‐reported frequency and severity of vasomotor symptoms Citation120, Citation121. In the largest of these studies, a double blind, parallel, multicenter, placebo controlled trial, 51 post‐menopausal women took 60 g of soy protein isolate (with 76 mg/d of phytoestrogens) and 53 patients took 60 g of placebo (casein) for 12 weeks. Patients taking soy had a 45% reduction in daily hot flushes versus 30% obtained with the placebo (P<0.01) Citation122. However, in a cross‐over double blind study involving 177 pre‐ and post‐menopausal women with hot flushes and a history of mammary carcinoma, soy‐derived capsules rich in phytoestrogens at a dose of 150 mg phytoestrogens/day for 4 weeks did not result in any significant symptom change compared to the placebo Citation123. Similar inconclusive findings were reported by other groups on women with breast cancer Citation124, Citation125. Decreases in the number and severity of hot flushes in smaller studies on post‐menopausal women treated with soy containing phytoestrogens have been described but, overall, results have been inconsistent. Some studies have reported a significant reduction of hot flushes Citation126–130, whereas others reported no significant effects, secondary to an apparent placebo activity Citation131, Citation132.
A summary of randomized clinical studies investigating the effect of phytoestrogens on menopausal vasomotor symptoms is given in . There appears some evidence of a possible role of phytoestrogens in the management of menopausal hot flushes, but this is clouded by a large placebo effect and specific effects due to soy phytoestrogens appear modest in degree Citation133. A consensus opinion of the Northern American Menopause Society only recommended that menopausal women consume whole foods that contain phytoestrogens (especially for the cardiovascular benefits). It also suggested a level of caution to be observed in making these recommendations Citation134.
Table III. Summary of randomized clinical studies investigating the effect of phytoestrogens on menopausal vasomotor symptoms.
Potential toxic effects of phytoestrogens
Any consideration of the potential protective effects of phytoestrogens needs to be weighed against their potential toxicity Citation113. It has been reported that the major soy phytoestrogen genistein, at concentrations about 10‐fold higher than that found in humans after an average daily intake of soy products Citation135, leads to a clear mutagenic effect in human cells Citation136. The relevance of this finding becomes clear when it is considered that regulations on drug safety state that a drug is rated as a ‘mutagen’ when mutagenic effects occur at levels below 10‐fold of those found in plasma.
In addition to the potential of genistein to exert a clastogenic activity in human chromosomes, this compound has also been associated with an inhibition of purified topoisomerase II activity, which may result in DNA strand breakage and arrested cell growth in human leukemia and gastric cancer cell lines Citation137. This kind of activity is potentially also exerted at the thymic level thus reducing cell‐dependent immunity Citation138. There have been somewhat controversial data reported on the possibility that consumption of soy in infancy may lead to an increased risk of allergy development, possibly because of a prevalence of the Th2 activity in the periphery, resulting in immunological alterations in adult life, requiring a more frequent intake of antihistamine medications Citation139.
Two recent publications have suggested some other potentially important side effects of soy phytoestrogens on infant development. In one case, a meta‐analysis of soy intake suggested a thyrotoxic activity, possibly resulting in thyroid alterations in young individuals Citation140. While these findings were not clearly supported by evidence of an increased intake of thyromimetic drugs, they still called for caution. More recently, a study in piglets, comparing soy infant formulas with those from cow's milk, showed a 50% lower number of proliferating cells in the intestine after soy intake, possibly resulting in intestinal immaturity Citation141. Newborn piglets appear to be a good model for human infants and concentrations of genistein in the piglets' blood were similar to those of babies fed soy formulas that contain relatively high concentrations of genistein (32 to 46 mg/L of reconstituted formula) Citation142. In fact, when fed these soy formulas, babies are exposed to 6–11‐fold higher levels of phytoestrogens per kg body weight Citation135 than women receiving phytoestrogens for menopausal complaints.
The possibility of high levels of dietary phytoestrogen intake in infants has led health authorities in several countries to recommend maximum levels of daily intake Citation143. Countries such as UK, Australia, Canada, Ireland, New Zealand, and Switzerland, have recommended the preferential use of infant formulas based on cow's milk, when breast feeding is not possible, and that only women advised by their doctor should continue to use soy infant formulas. A very short time ago, French authorities also issued a detailed public statement cautioning against the indiscriminate use of phytoestrogens for any major therapeutic indication: they indicated a maximal daily intake of 1 mg/kg of body weight and suggested the use of infant formulas based on soybean with less than 1 mg/L of phytoestrogens Citation144.
Phytoestrogens are also available as dietary supplements that may contain more than 100 mg per tablet. Recent data, indicative of a small but significant endometrial hyperplasia in women receiving 150 mg daily of phytoestrogens for 5 years, questioned the safety of these treatments Citation145. Due to the potential toxicity of phytoestrogens at very high intakes and their increasing use by women for menopausal complaints, the Italian Health Authorities have advised the public to maintain daily intake of phytoestrogens as dietary supplements below 80 mg Citation146, which again represents a maximal daily intake of about 1 mg/kg of body weight.
In light of the Italian recommendation, a recent study has considered the phytoestrogen content of Italian soy food products and the daily intakes for some specific classes of consumers. Total phytoestrogen contents of soy foods were in the range of 21–803 µg/g dry weight (dw) and were particularly high in soy‐based imitation dairy and meat products Citation147. The phytoestrogen content of gluten‐free products was surprisingly high, being in the range of 77–220 µg/g dw, due to the inclusion of soy protein for technological purposes. Infant formulas contained 121–427 µg/g dw: these levels may possibly lead to daily intakes of about 2–3 mg/kg body weight. These intakes are far higher than the maximal daily intakes of 1 mg/kg established by the Italian and French Health Authorities Citation144, Citation146.
Conclusions
Based on available data, the major therapeutic activity of soy appears to reside exclusively in the proteins, whereas the role of phytoestrogens appears to be minimal. Yet even now it is somewhat perplexing to note the frequent confusion between phytoestrogens and proteins as the major hypocholesterolemic components of soy based diets, even in highly reputed journals Citation148.
Phytoestrogens have yet to be proven as beneficial to health and their potential negative effects may not be acceptable in the absence of any clear benefit. Substantial effort and financial support has been given to studies aiming at elucidating the beneficial activities and mechanisms of action of phytoestrogens. For example the European Commission has financed several projects that have cost around 12 million euros to European citizens (for information see the CORDIS database at http://ica.cordis.lu/search/). Unfortunately these studies have produced very little published material in support of the health benefits of phytoestrogens.
At present soy still appears to have great potential for treatment in a variety of conditions and the US FDA claim for CVD protection remains well supported. However, it is clear that the benefit of soy intake resides essentially in the protein itself, whereas phytoestrogens provide little if any additional benefit. From a practical point of view, promotion of the cultivation of soybean varieties with low phytoestrogen contents for manufacturing human food products is warranted. In addition the efficacy of using other legumes Citation149 characterized by much lower phytoestrogen contents, such as peas, beans, chickpeas or lupin Citation61, Citation150, Citation151, as sources of food ingredients and nutraceuticals for the prevention of cardiovascular disease is worthy of further investigation. The phytoestrogen tale may be essentially over.
References
- Adlercreutz H., Mazur H. Phyto‐oestrogens and Western Diseases. Ann Med 1997; 29: 95–120
- Bennetts H. W., Underwood E. J., Shier F. L. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust Vet J 1946; 22: 2–12
- Cornwell T., Cohick W., Raskin I. Dietary phytoestrogens and health. Phytochemistry 2004; 65: 995–1016
- Lamikanra O., Grimm C. C., Rodin J. B., Inyang I. D. Hydroxylated stilbenes in selected american wines. J Agric Food Chem 1996; 44: 1111–5
- Fremont L. Biological effects of resveratrol. Life Sci 2000; 66: 663–73
- Franke A. A., Custer L. J., Cerna C. M., Narala K. K. Quantitation of phytoestrogens in legumes by HPLC. J Agric Food Chem 1994; 42: 1905–13
- Setchell K. D., Lawson A. M., Borriello S. P., Harkness R., Gordon H., Morgan D. M. Lignan formation in man – microbial involvement and possible roles in relation to cancer. Lancet 1981; 2: 4–7
- Duncan A. M., Phipps W. R., Kurzer M. S. Phyto‐oestrogens. Best Practice Res Clin Endocrinol Metab 2003; 17: 253–71
- Munro I. C., Harwood M., Hlywka J. J., Stephen A. M., Doull J., Flamm W. G. Soy isoflavones: A safety review. Nutr Rev 2003; 61: 1–33
- Meyer B. J., Larkin T. A., Owen A. J., Astheimer L. B., Tapsell L. C., Howe P. R. C. Limited lipid‐lowering effects of regular consumption of whole soybean foods. Ann Nutr Metab 2004; 48: 67–78
- Rowland I. R., Wiseman H., Sanders T. A. B., Adlercreutz H., Bowey E. A. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer 2000; 36: 27–32
- Duncan A. M., Merz‐Demlow B. E., Xu X., Phipps W. R., Kurzer M. S. Premenopausal equol excretors show plasma hormone profiles associated with lowered risk of breast cancer. Cancer Epidemiol Biomarkers Prev 2000; 9: 581–6
- Setchell K. D. R., Brown N. M., Lydeking‐Olsen E. The clinical importance of the metabolite equol – a clue to the effectiveness of soy and its isoflavones. J Nutr 2002; 132: 3577–84
- Nagata C., Kabuto M., Kurisu Y., Shimizu H. Decreased serum estradiol concentration associated with high dietary intake of soy products in premenopausal Japanese women. Nutr Cancer 1997; 29: 228–33
- Chen Z., Zheng W., Custer L. J., Dai Q., Shu X. O., Jin F. Usual dietary consumption of soy foods and its correlation with the excretion rate of isoflavonoids in overnight urine samples among Chinese women in Shanghai. Nutr Cancer 1999; 33: 82–7
- Horn‐Ross P. L., John E. M., Lee M., Stewart S. L., Koo J., Sakoda L. C. Phytoestrogen consumption and breast cancer risk in a multiethnic population: the Bay Area Breast Cancer Study. Am J Epidemiol 2001; 154: 434–41
- Kiely M., Faughnan M., Wähälä K., Brandts H., Mulligan A. Phyto‐oestrogen levels in foods: the design and construction of the VENUS database. Br J Nutr 2003; 89: 808–13
- Van Erp‐Baart M. A. J., Brants H. A. M., Kiely M. Isoflavone intake in four different European countries: the VENUS approach. Br J Nutr 2003; 89: 25–30
- FDA Food labeling health claims. soybean protein and coronary heart disease. Food and Drug Administration. Final rule. Federal Register 1999; 64: 57699–733
- Sirtori C. R., Lovati M. R. Soy proteins and cardiovascular disease. Curr Atheroscl Rep 2001; 3: 47–53
- Sirtori C. R., Pazzucconi F., Colombo L., Battistin P., Bondioli A., Descheemaeker K. Double‐blind study of the addition of high‐protein soya milk v. cow's milk to the diet of patients with severe hypercholesterolaemia and resistance to or intolerance of statins. Br J Nutr 1999; 82: 91–6
- de Kleijn M. J., van der Schouw Y. T., Wilson P. W., Grobbee D. E., Jacques P. F. Dietary intake of phytoestrogens is associated with a favorable metabolic cardiovascular risk profile in post‐menopausal U.S. women: the Framingham study. J Nutr 2002; 132: 276–82
- Anderson J. W., Johnstone B. M., Cook‐Newell M. E. Meta‐analysis of the effects of soy protein intake on serum lipids. N Engl J Med 1995; 333: 276–82
- Sirtori C. R., Agradi E., Conti F., Mantero O., Gatti E. Soybean‐protein diet in the treatment of type II hyperlipoproteinaemia. Lancet 1977; i: 275–7
- Descovich G. C., Ceredi C., Gaddi A., Benassi M. S., Mannino G., Colombo L. Multicentre study of soybean protein diet for outpatient hyper‐cholesterolaemic patients. Lancet 1980; ii: 709–12
- Crouse 3rd J. R., Morgan T., Terry J. G., Ellis J., Vitolins M., Burke G. L. A randomized trial comparing the effect of casein with that of soy protein containing varying amounts of isoflavones on plasma concentrations of lipids and lipoproteins. Arch Intern Med 1999; 159: 2070–6
- Anthony S., Clarkson T. B., Bullock B. C., Wagner J. D. Soy protein versus soy phytoestrogens in the prevention of diet‐induced coronary artery atherosclerosis of male cynomolgus monkeys. Arterioscler Thromb Vasc Biol 1997; 17: 2524–31
- Greaves K. A., Parks J. S., Williams J. K., Wagner J. D. Intact dietary soy protein, but not adding an isoflavone‐rich soy extract to casein, improves plasma lipids in ovariectomized cynomolgus monkeys. J Nutr 1999; 129: 1585–92
- Nestel P. J., Yamashita T., Sasahara T., Pomeroy S., Dart A., Komesaroff P. Soy isoflavones improve systemic arterial compliance but not plasma lipids in menopausal and perimenopausal women. Arterioscler Thromb Vasc Biol 1997; 17: 3392–8
- Hodgson J. M., Puddey I. B., Beilin L. J., Mori T. A., Croft K. D. Supplementation with isoflavonoid phytoestrogens does not alter serum lipid concentrations: a randomized controlled trial in humans. J Nutr 1998; 128: 728–32
- Dewell A., Hollenbeck C. B., Bruce B. The effects of soy‐derived phytoestrogens on serum lipids and lipoproteins in moderately hypercholesterolemic postmenopausal women. J Clin Endocrinol Metab 2002; 87: 118–21
- Gianazza E., Eberini I., Arnoldi A., Wait R., Sirtori C. R. A proteomic investigation of isolated soy proteins with variable effects in experimental and clinical studies. J Nutr 2003; 133: 9–14
- Fukui K., Tachibana N., Wanezaki S., Tsuzaki S., Takamatsu K., Yamamoto T. Isoflavone‐free soy protein prepared by column chromatography reduces plasma cholesterol in rats. J Agr Food Chem 2002; 50: 5717–21
- Anderson J. W. Diet first, then medication for hypercholesterolemia. JAMA 2003; 290: 531–3
- Lovati M. R., Manzoni C., Corsini A., Granata A., Frattini R., Fumagalli R. Low density lipoprotein receptor activity is modulated by soybean globulins in cell culture. J Nutr 1992; 122: 1971–8
- Lovati M. R., Manzoni C., Gianazza E., Sirtori C. R. Soybean protein products as regulators of liver low‐density lipoprotein receptors. I. Identification of active β‐conglycinin subunits. J Agric Food Chem 1998; 46: 2474–80
- Manzoni C., Lovati M. R., Gianazza E., Sirtori C. R. Soybean protein products as regulators of liver low‐density lipoprotein receptors. II. α‐α' rich commercial soy concentrate and α' deficient mutant differently affect low‐density lipoprotein receptor activation. J Agric Food Chem 1998; 46: 2481–4
- Lovati M. R., Manzoni C., Gianazza E., Arnoldi A., Kurowska E., Carroll K. K. Soy protein peptides regulate cholesterol homeostasis in Hep G2 cells. J Nutr 2000; 130: 2543–9
- Lovati M. R., Manzoni C., Canavesi A., Sirtori M., Vaccarino V., Marchi M. Soybean protein diet increases low density lipoprotein receptor activity in mononuclear cells from hypercholesterolemic patients. J Clin Invest 1987; 80: 1498–502
- Baum J. A., Teng H., Erdman J. W. J., Weigel R. M., Klein B. P., Persky V. W. Long‐term intake of soy protein improves blood lipid profiles and increases mononuclear cell low‐density‐lipoprotein receptor messenger RNA in hypercholesterolemic, postmenopausal women. Am J Clin Nutr 1998; 68: 545–51
- Duranti M., Lovati M. R., Dani V., Barbiroli A., Scarafoni A., Castiglioni S. The alpha' subunit from soybean 7S globulin lowers plasma lipids and upregulates liver beta‐VLDL receptors in rats fed a hypercholesterolemic diet. J Nutr 2004; 134: 1334–9
- Erdman J. W. Soy protein and cardiovascular disease ‐ A statement for healthcare professionals from the Nutrition Committee of the AHA. Circulation 2000; 102: 2555–9
- Borradaile N. M., de Dreu L. E., Wilcox L. J., Edwards J. Y., Huff M. W. Soya phytoestrogens, genistein and daidzein, decrease apolipoprotein B secretion from HepG2 cells through multiple mechanisms. Biochem J 2002; 366: 531–9
- Walker H. A., Dean T. S., Sanders T. A. B., Jackson G., Ritter J. M., Chowienczyk P. J. The phytoestrogen genistein produces acute nitric oxide‐dependent dilation of human forearm vasculature with similar potency to 17β‐estradiol. Circulation 2001; 103: 258–62
- Squadrito F., Altavilla D., Morabito N., Crisafulli A., D'Anna R., Corrado F. The effect of the phytoestrogen genistein on plasma nitric oxide concentrations, endothelin‐1 levels and endothelium dependent vasodilation in postmenopausal women. Atherosclerosis 2002; 163: 339–47
- Squadrito F., Altavilla D., Crisafulli A., Saitta A., Cucinotta D., Morabito N. Effect of genistein on endothelial function in postmenopausal women: a randomized, double‐blind, controlled study. Am J Med 2003; 114: 470–6
- Simons L. A., van Konigsmark M., Simons J., Celermajer D. S. Phytoestrogens do not influence lipoprotein levels or endothelial function in healthy, postmenopausal women. Am J Cardiol 2000; 85: 1297–301
- Kreijkamp‐Kaspers S., Kok L., Bots M. L., Grobbee D. E., Lampe J. W., van der Schouw Y. T. Randomized controlled trial of the effects of soy protein containing isoflavones on vascular function in postmenopausal women. Am J Clin Nutr 2005; 81: 189–95
- Wagner J. D., Zhang L., Greaves K. A., Shadoan M. K., Schwenke D. C. Soy protein reduces the arterial low‐density lipoprotein (LDL) concentration and delivery of LDL cholesterol to the arteries of diabetic and nondiabetic male cynomolgus monkeys. Metabolism 2000; 49: 1188–96
- Kurzer M. S., Xu X. Dietary phytoestrogens. Annu Rev Nutr 1997; 17: 353–81
- Wagner J. D., Cefalu W. T., Anthony M. S., Litwak K. N., Zhang L., Clarkson T. B. Dietary soy protein and estrogen replacement therapy improve cardiovascular risk factors and decrease aortic cholesteryl ester content in ovariectomized cynomolgus monkeys. Metabolism 1997; 46: 698–705
- Tikkanen M. J., Wahala K., Ojala S., Vihma V., Adlercreutz H. Effect of soybean phytoestrogen intake on low density lipoprotein oxidation resistance. Proc Natl Acad Sci U S A 1998; 95: 3106–10
- Wiseman H., O'Reilly J. D., Adlercreutz H., Mallet A. I., Bowey E. A., Rowland I. R. Isoflavone phytoestrogens consumed in soy decrease F2‐isoprostane concentrations and increase resistance of low‐density lipoprotein to oxidation in humans. Am J Clin Nutr 2000; 72: 395–400
- Samman S., Lyons Wall P. M., Chan G. S., Smith S. J., Petocz P. The effect of supplementation with isoflavones on plasma lipids and oxidizability of low density lipoprotein in premenopausal women. Atherosclerosis 1999; 147: 277–83
- Castiglioni S., Manzoni C., D'Uva A., Spiezie R., Monteggia E., Chiesa G. Soy proteins reduce progression of a focal lesion and lipoprotein oxidability in rabbits fed a cholesterol‐rich diet. Atherosclerosis 2003; 171: 163–70
- Vega‐Lopez S., Yeum K‐J., Lecker J. L., Ausman L. M., Johnson E. J., Devaray S. Plasma antioxidant capacity in response to diets high in soy or animal protein with or without isoflavones. Am J Clin Nutr 2005; 81: 43–9
- van der Schouw Y. T., Kreikamp‐Kaspers S., Peeters P. H. M., Keinan‐Boker L., Rimm E. B., Grobbee D. E. Prospective study on usual dietary phytoestrogen intake and cardiovascular disease risk in Western women. Circulation 2005; 111: 465–71
- Arora A., Nair M. G., Strasburg G. M. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch Biochem Biophys 1998; 356: 133–41
- Chin‐Dusting J. P. F., Boak L., Husband A., Nestel P. J. The isoflavone metabolite dehydroequol produced vasodilation in human resistance arteries via a nitric‐oxide dependent mechanism. Atherosclerosis 2004; 176: 45–8
- Matoba N., Doyama N., Yamada Y., Maruyama N., Utsumi S., Yoshikawa M. Design and production of genetically modified soybean protein with anti‐hypertensive activity by incorporating potent analogue of ovokinin(2–7). FEBS Lett 2001; 497: 50–4
- Sirtori C. R., Lovati M. R., Manzoni C., Castiglioni S., Duranti M., Magni C. Proteins of white lupin seed, a naturally isoflavone‐poor legume, reduce cholesterolemia in rats and increase LDL receptor activity in HepG2 cells. J Nutr 2004; 134: 18–23
- Kuiper G. G., Lemmen J. G., Carlsson B., Corton J. C., Safe S. H., van der Saag P. T. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor ß. Endocrinology 1998; 139: 4252–63
- Mäkelä S., Savolainen H., Aavik E., Myllarniemi M., Strauss L., Taskinen E. Differentiation between vasculoprotective and uterotrophic effects of ligands with different binding affinities to estrogen receptors alpha and beta. Proc Natl Acad Sci U S A 1999; 96: 7077–82
- Shimokado K., Yokota T., Umezawa K., Sasaguri T., Ogata J. Protein tyrosine kinase inhibitors inhibit chemotaxis of vascular smooth muscle cells. Arterioscler Thromb 1994; 14: 973–81
- Shimokado K., Umezawa K., Ogata J. Tyrosine kinase inhibitors inhibit multiple steps of the cell cycle of vascular smooth muscle cells. Exp Cell Res 1995; 220: 266–73
- Parkin D. M. Cancers of the breast, endometrium and ovary: geographic correlations. Eur J Cancer Clin Oncol 1989; 25: 1917–25
- Ziegler R. G., Hoover R. N., Pike M. C., Hildesheim A., Nomura A. M., West DW. Migration patterns and breast cancer risk in Asian‐American women. J Nat Cancer Inst 1993; 85: 1819–27
- Adlercreutz H., Fotsis T., Heikkinen R., Dwyer J. T., Woods M., Goldin B. R. Excretion of the lignans enterolactone and enterodiol and of equol in omnivorous and vegetarian post‐menopausal women and in women with breast cancer. Lancet 1982; ii: 1295–9
- Yuan J. M., Wang Q. S., Ross R. K., Henderson B. E. Yu MC. Diet and breast cancer in Shanghai and Tianjin, China. Br J Cancer 1995; 71: 1353–8
- Key T. J., Sharp G. B., Appleby P. N., Beral V., Goodman M. T., Soda M. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. Br J Cancer 1999; 81: 1248–56
- Horn‐Ross P. L., John E. M., Lee M., Stewart S. L., Koo J., Sakoda L. C. Phytoestrogen consumption and breast cancer risk in a multiethnic population: the Bay Area Breast Cancer Study. Am J Epidemiol 2001; 154: 434–41
- den Tonkelaar I., Keinan‐Boker L., Veer P. V., Arts C. J., Adlercreutz H., Thijssen J. H. Urinary phytoestrogens and postmenopausal breast cancer risk. Cancer Epidemiology, Biomarkers and Prevention 2001; 10: 223–8
- Ingram D., Sanders K., Kolybaba M., Lopez D. Case‐control study of phyto‐estrogens and breast cancer. Lancet 1997; 350: 990–4
- Markiewicz L., Garey J., Adlercreutz H., Gurpide E. In vitro bioassays of non‐steroidal phytoestrogens. J Steroid Biochem 1993; 45: 399–405
- Sathyamoorthy N., Wang T. T. Y. Differential effects of dietary phyto‐oestrogens daidzein and equol on human breast cancer MCF‐7 cells. Eur J Cancer 1997; 33: 2384–9
- Krazeisen A., Breitling R., Moller G., Adamski J. Phytoestrogens inhibit human 17beta‐hydroxysteroid dehydrogenase type 5. Mol Cell Endocrinol 2001; 171: 151–62
- Adlercreutz H. Phyto‐oestrogens and cancer. Lancet Oncology 2002; 3: 364–73
- Lamartiniere C. A., Cotroneo M. S., Fritz W. A. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr 2002; 132: 552S–8
- Liu B., Edgerton S., Yang X., Kim A., Ordonez‐Ercan D., Mason T., Alvarez K. Low‐dose dietary phytoestrogen abrogates tamoxifen‐associated mammary tumor prevention. Cancer Res 2005; 65: 879–86
- Pienta K. J., Goodson J. A., Esper P. S. Epidemiology of prostate cancer: molecular and environmental clues. Urology 1996; 48: 676–83
- Shimizu H., Ross R. K., Bernstein L., Yatani R., Henderson B. E., Mack T. M. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer 1991; 63: 963–6
- Jacobsen B. K., Knutsen S. F., Fraser G. E. Does high soy milk intake reduce prostate cancer incidence? The Adventist Health Study (United States). Cancer Causes Control 1998; 9: 553–7
- Kolonel L. N., Hankin J. H., Whittemore A. S., Wu A. H., Gallagher R. P., Wilkens L. R. Vegetables, fruits, legumes and prostate cancer: a multiethnic case‐control study. Cancer Epidemiol Biomarkers Prev 2000; 9: 795–804
- Lee M. M., Wang R. T., Hsing A. W., Wang T. Spitz M. Case‐control study of diet and prostate cancer in China. Cancer Causes Control 1998; 9: 545–52
- Oishi K., Okada K., Yoshida O., Yamabe H., Ohno Y., Hayes R. B., Schroeder F. H. A case‐control study of prostatic cancer with reference to dietary habits. Prostate 1988; 12: 179–90
- Hirayama T. Epidemiology of prostate cancer with special reference to the role of diet. National Cancer Institute Monograph 1979; 53: 149–55
- Urban D., Irwin W., Kirk M., Markiewicz M. A., Myers R., Smith M. The effect of isolated soy protein on plasma biomarkers in elderly men with elevated serum prostate specific antigen. J Urol 2001; 165: 294–300
- Adams K. F., Chen C., Newton K. M., Potter J. D., Lampe J. W. Soy isoflavones do not modulate prostate‐specific antigen concentrations in older men in a randomized controlled trial. Cancer Epidemiol Biomarkers Prev 2004; 13: 644–8
- Demark‐Wahnefried W., Price D. T., Polascik T. J., Robertson C. N., Anderson E. E., Paulson D. F. Pilot study of dietary fat restriction and flaxseed supplementation in men with prostate cancer before surgery: exploring the effects on hormonal levels, prostate‐specific antigen, and histopathologic features. Urology 2001; 58: 47–52
- Wu A. H., Yang D., Pike M. C. A meta‐analysis of soyfoods and risk of stomach cancer: the problem of potential confounders. Cancer Epidemiol Biomarkers Prev 2000; 9: 1051–8
- McKeown‐Eyssen G. E., Bright‐See E. Dietary factors in colon cancer: international relationships. Nutr Cancer 1984; 6: 160–70
- Tajima K., Tominaga S. Dietary habits and gastro‐intestinal cancers: a comparative case‐control study of stomach and large intestinal cancers in Nagoya, Japan. Jpn J Cancer Res 1985; 76: 705–16
- Goodman M. T., Wilkens L. R., Hankin J. H., Lyu L. C., Wu A. H., Kolonel L. N. Association of soy and fiber consumption with the risk of endometrial cancer. Am J Epidemiology 1997; 146: 294–306
- Nelson H. D., Humphrey L. L., Nygren P., Fanelli M., Venturelli E., Cantatore F. Postmenopausal hormone replacement therapy: scientific review. JAMA 2002; 288: 872–1
- Gennari C., Agnusdei D., Crepaldi G., Isaia G., Mazzuoli G., Ortolani S. Effect of ipriflavone – a synthetic derivative of natural isoflavones – on bone mass loss in the early years after menopause. Menopause 1998; 5: 9–15
- Lauderdale D. S., Jacobsen S. J., Furner S. E., Levy P. S., Brody J. A., Goldberg J. Hip fracture incidence among elderly Asian‐American populations. Am J Epidemiol 1997; 146: 502–9
- Tsuchida K., Mizushima S., Toba M., Soda K. Dietary soybeans intake and bone mineral density among 995 middle‐aged women in Yokohama. J Epidemiol 1999; 9: 14–9
- Kritz‐Silverstein D., Goodman‐Gruen D. L. Usual dietary isoflavone intake, bone mineral density, and bone metabolism in post‐menopausal women. J Womens Health Gend Based Med 2002; 11: 69–78
- Somekawa Y., Chiguchi M., Ishibashi T., Aso T. Soy intake related to menopausal symptoms, serum lipids, and bone mineral density in postmenopausal Japanese women. Obstet Gynecol 2001; 97: 109–15
- Kardinaal A. F., Morton M. S., Bruggemann Rotgans I. E., van Beresteijn E. C. Phyto‐oestrogen excretion and rate of bone loss in postmenopausal women. Eur J Clin Nutr 1998; 52: 850–5
- Potter S. M., Baum J. A., Teng H. Soy protein and isoflavones: their effects on blood lipids and bone density in post‐menopausal women. Am J Clin Nutr 1998; 68: 1375S–9
- Wangen K. E., Duncan A. M., Merz‐Demlow B. E., Xu X., Marcus R., Phipps W. R. Effects of soy isoflavones on markers of bone turnover in premenopausal and postmenopausal women. J Clin Endocrinol Metab 2000; 85: 3043–8
- Burke G. L., Vitolins M. Z., Bland D. Soybean isoflavones as an alternative to traditional hormone replacement therapy: are we there yet?. J Nutr 2000; 130: 664S–5
- Alekel D. L., Germain A. S., Peterson C. T., Hanson K. B., Stewart J. W., Toda T. Isoflavone‐rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am J Clin Nutr 2000; 72: 844–52
- Lydeking‐Olsen E., Beck‐Jensen J‐E., Setchell K. D. R., Holm‐Jensen T. Soymilk or progesterone for prevention of bone loss. A 2 year randomized, placebo‐controlled trial. Eur J Nutr 2004; 43: 246–57
- Chen Y. M., Ho S. C., Lam S. S., Ho S. S., Woo J. L. Beneficial effect of soy isoflavones on bone mineral content was modified by years since menopause, body weight, and calcium intake: a double‐blind, randomized, controlled trial. Menopause 2004; 11: 246–54
- Vitolins M., Anthony M., Lenschik L., Bland D. r., Burke G. L. Does soy protein and its isoflavones prevent bone loss in peri‐ and post‐menopausal women? Results of a Two‐Year Randomized Clinical Trial. J Nutr 2002; 582S, (Abstr)
- Kreikamp‐Kaspers S., Kok L., Grobbee D. E., de Haan E. H. F., Aleman A., Lampe J. W., van der Schouw Y. T. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women. JAMA 2004; 292: 65–74
- Arjmandi B. H., Lucas E. A., Khalil D. A., Devareddy L., Smith B. J., McDonald J. One year soy protein supplementation has positive effects on bone formation markers but not bone density in postmenopausal women. Nutr J 2005; 4: 8
- Spence L. A., Lipscomb E. R., Cadogan J., Martin B., Wastney M. E., Peacocock M. The effect of soy protein and soy isoflavones on calcium metabolism in postmenopausal women: a randomized crossover study. Am J Clin Nutr 2005; 81: 916–22
- Chiechi L., Secreto G., D'Amore M., Fanelli M., Venturelli E., Cantatore F. Efficacy of a soy rich diet in preventing post‐menopausal osteoporosis: the Menfis randomized trial. Maturitas 2002; 42: 295–300
- Glazier M. G., Bowman M. A. A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Arch Intern Med 2001; 161: 1161–72
- Sirtori C. R. Risks of benefits of soy phytoestrogens in cardiovascular diseases, cancer, climacteric symptoms and osteoporosis. Drug Safety 2001; 24: 665–82
- Adlercreutz H., Hamalainen E., Gorbach S., Goldin B. Dietary phyto‐oestrogens and the menopause in Japan. Lancet 1992; 339: 1233
- Nagata C., Takatsuka N., Kawakami N., Shimizu H. Soy product intake and hot flushes in Japanese women: results from a community‐based prospective study. Am J Epidemiol 2001; 153: 790–3
- Yaffe K., Grady D., Pressman A., Cummings S. Serum estrogen levels, cognitive performance, and risk of cognitive decline in older community women. J Am Geriatr Soc 1998; 46: 816–21
- Espeland M. A., Rapp S. R., Shumaker S. A., Brunner R., Manson J. E., Sherwin B. B. Women's Health Initiative Memory Study. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA 2004; 291: 2959–68
- Hersh A. L., Stefanick M. L., Stafford R. S. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA 2004; 291: 47–53
- Murkies A. L., Lombard C., Strauss B. J. G., Wilcox G., Burger H. G., Morton M. S. Dietary flour supplementation decreases post‐menopausal hot flushes: effect of soy and wheat. Maturitas 1995; 21: 189–95
- Washburn S., Burke G. L., Morgan T., Anthony M. Effect of soy protein supplementation on serum lipoproteins, blood pressure, and menopausal symptoms in perimenopausal women. Menopause 1999; 6: 7–13
- Duncan A. M., Underhill K. E., Xu X., Lavalleur J., Phipps W. R., Kurzer M. S. Modest hormonal effects of soy isoflavones in postmenopausal women. J Clin Endocrinol Metab 1999; 84: 3479–84
- Albertazzi P., Pansini F., Bonaccorsi G., Zanotti L., Forini E., De Aloysio D. The effect of dietary soy supplementation on hot flushes. Obstet Gynecol 1998; 91: 6–11
- Quella S. K., Loprinzi C. L., Barton D. L., Knost J. A., Sloan J. A., LaVasseur B. I. Evaluation of soy phytoestrogens for the treatment of hot flashes in breast cancer survivors: A North Central Cancer Treatment Group Trial. J Clin Oncol 2000; 18: 1068–74
- Van Patten C. L., Olivotto I. A., Chambers G. K., Gelmon K. A., Hislop T. G., Templeton E. Effect of soy phytoestrogens on hot flashes in postmenopausal women with breast cancer: a randomized, controlled clinical trial. J Clin Oncol 2002; 20: 1449–55
- MacGregor C. A., Canney P. A., Patterson G., McDonald R., Paul J. A randomised double‐blind controlled trial of oral soy supplements versus placebo for treatment of menopausal symptoms in patients with early breast cancer. Eur J Cancer 2005; 41: 708–14
- Washburn S., Burke G. L., Morgan T., Anthony M. Effect of soy protein supplementation on serum lipoproteins, blood pressure, and menopausal symptoms in perimenopausal women. Menopause 1999; 6: 7–13
- Faure E. D., Chantre P., Mares P. Effects of a standardized soy extract on hot flushes: a multicenter, double‐blind, randomized, placebo‐controlled study. Menopause 2002; 9: 329–34
- Han K. K., Soares J. M, Jr., Haidar M. A., de Lima G. R., Baracat E. C. Benefits of soy isoflavone therapeutic regimen on menopausal symptoms. Obstet Gynecol 2002; 99: 389–94
- Colacurci N., Zarcone R., Borrelli A., De Franciscis P., Fortunato N., Cirillo M. Effects of soy isoflavones on menopausal neurovegetative symptoms. Minerva Ginecologica 2004; 56: 407–12
- Petri Nahas E., Nahas Neto J., De Luca L., Traiman P., Pontes A., Dalben I. Benefits of soy germ isoflavones in postmenopausal women with contraindication for conventional hormone replacement therapy. Maturitas 2004; 48: 372–80
- Baber R. J., Templeman C., Morton T., Kelly G. E. West L. Randomized placebo‐controlled trial of an isoflavone supplement and menopausal symptoms in women. Climacteric 1999; 2: 85–92
- Knight D. C., Howes J. B., Eden J. A. The effect of Promensil, an isoflavone extract, on menopausal symptoms. Climacteric 1999; 2: 79–84
- Kronenberg F., Fugh‐Berman A. Complimentary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. Ann Intern Med 2002; 137: 805–13
- Anonymous. The role of isoflavones in menopausal health‐consensus opinion of The North American Menopause Society. Menopause 2000; 7: 215–29
- Setchell K. D., Zimmer‐Nechemias L., Cai J., Heubi J. E. Exposure of infants to phyto‐oestrogens from soy‐based infant formula. Lancet 1997; 350: 23–7
- Kulling S. E., Rosenberg B., Jacobs E., Metzler M. The phytoestrogens coumoestrol and genistein induce structural chromosomal aberrations in cultured human peripheral blood lynphocytes. Arch Toxicol 1999; 73: 50–4
- Yellayi S., Naaz A., Szewcczykowski M., Sato T., Woods J. A., Chang J. The phytoestrogen genistein induces thymic and immmune changes: a human health concern?. Proc Natl Acad Sci U S A 2002; 99: 7616–21
- Sakabe K., Okuma M., Karaki S., Matsuura S., Yoshida T., Aikawa H. Inhibitory effect of natural and environmental estrogens on thymic hormone production in thymus epithelial cell culture. Int J Immunopharmacol 1999; 21: 861–8
- Strom B. L., Schinnar R., Ziegler E. E., Barnhart K. T., Sammel M. D., Macones G. A. Exposure to soy‐based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA 2001; 286: 807–14
- Doerge D. R., Sheehan D. M. Goitrogenic and estrogenic activity of soy isoflavones. Environ Health Perspect 2002; 110: 349–53
- Chen A. C., Berhow M. A., Tappenden K. A., Donovan S. M. Genistein inhibits intestinal cell proliferation in piglets. Pediatr Res 2005; 57: 192–200
- Chen A., Rogan W. J. Isoflavones in soy infant formula: a review of evidence for endocrine and other activity in infants. Annu Rev Nutr 2004; 24: 33–54
- Committee on Toxicity of Chemicals in Food Consumer Products the Environment. (1996) Annual report of the Committees on Toxicity, Mutagenicity and Carcinogenicity. HMSO, London 1996; 20–2
- Agence Française de sécurité sanitaire des aliments. Présentation du rapport sur "Sécurité et bénéfices des phyto‐estrogènes apportés par l'alimentation Recommandations
- Arici A., Bukulmez O. Phyto‐oestrogens and the endometrium. Lancet 2004; 364: 2081–2
- Gazzetta Ufficiale Italiana 18/7/2002, number 188, annex 2
- Morandi S., D'Agostina A., Ferrario F., Arnoldi A. Isoflavone content of Italian soy food products and daily intakes of some specific classes of consumers. Eur Food Res Technol 2005; 221: 84–91
- Kreikamp‐Kaspers, Kok L., Bots M. L., Grobbee D. E., van der Schouw Y. T. Dietary phytoestrogens and plasma lipids in Dutch postmenopausal women; a cross‐sectional study. Atherosclerosis 2005; 178: 95–100
- Arnoldi A. Grain legumes as a source of food ingredients for the prevention of cardiovascular disease. Functional Foods, Diet, Cardiovascular Disease and Diabetes, A Arnoldi. Woodhead Publications Ltd, CambridgeUK 2004; 422–47
- Hall R. S., Johnson S. K., Baxter A. L., Ball M. J. Lupin kernel fibre‐enriched foods beneficially modify serum lipids in men. Eur J Clin Nutr 2005; 59: 325–33
- Martins J. M., Riottot M., Abreu M. C., Viegas‐Crespo A. M., Lanca M. J., Almeida J. A. Cholesterol‐lowering effects of dietary blue lupin seeds (Lupinus angustifolius L.) in intact and ileo‐rectal anastomosed growing pigs fed cholesterol‐rich diets. J Lipid Res 2005; 46: 1539–47
