Abstract
Percutaneous coronary intervention (PCI) has become the most important revascularization method in the treatment of coronary artery disease. The major problem in PCI has been renarrowing of the dilated vessel after the procedure (restenosis). The best results in the prevention of restenosis have been obtained by covering the stent with drugs that inhibit cellular growth, thus limiting excessive scar formation inside of the stent. With drug‐eluting stents, restenosis has been reduced to one‐tenth compared with balloon angioplasty and to one‐fourth compared to bare metal stents. Due to drug‐eluting stents, PCI is an alternative to bypass surgery. However, restenosis will remain a challenge due to the increased number of procedures and more difficult disease treated with PCI.
| Abbreviations | ||
| ACC/AHA | = | American College of Cardiology/American Heart Association |
| ACE | = | angiotensin‐converting enzyme |
| AT II | = | angiotensin II |
| bFGF | = | basic fibroblast growth factor |
| CABG | = | coronary artery bypass gratting |
| CRP | = | C‐reactive protein |
| EC | = | endothelial cell |
| IGF | = | insulin‐like growth factor |
| MAC‐1 | = | a member of beta2‐integrin family of cellular adhesion molecules |
| MACE | = | major adverse clinical event |
| MAPK | = | mitogen‐activated protein kinases |
| NO | = | nitric oxide |
| PCI | = | percutaneous coronary intervention |
| PDGF | = | platelet‐derived growth factor |
| QTc | = | corrected QT‐interval |
| SMC | = | smooth muscle cell |
| TGFβ | = | transforming growth factor beta |
| TLR | = | target lesion revascularization |
| TVR | = | target vessel revascularization |
| VEGF | = | vascular endothelial growth factor |
Introduction
Percutaneous coronary intervention (PCI) is nowadays the most important method of revascularization in the treatment of coronary artery disease Citation1. Development of PCI techniques and improvement of the equipment used have led to technical success in over 95% of cases Citation2. The most important problem associated with PCI has been reocclusion of the dilated segment after the procedure (restenosis) Citation3. A true breakthrough in the prevention of restenosis was finding that development of neointimal hyperplasia can be hindered with drugs that inhibit cell division. Drugs can be attached to the surface of the stent, from which the drug is slowly released, exerting a local effect on the vessel wall. There are already thousands of patients in randomized trials Citation4,5. These have shown that drug‐eluting stents reduce incidence of restenosis to one‐fourth compared with bare metal stents.
Table I. Summary of major clinical trials on the efficacy of drug‐eluting stents. Target lesion revascularization (TLR) rate is used as the adverse clinical endpoint.
Definition of restenosis
After PCI the dilated segment can occlude again. This renarrowing is called restenosis. Restenosis can be defined in several ways. The concept of clinical restenosis means recurrence of the patient's symptoms. Clinical restenosis often leads to repeat angiography and revascularization. The terms target lesion revascularization (TLR) and target vessel revascularization (TVR) are also used. TLR and TVR refer to repeat revascularization procedure because of stenosis in the target lesion or the target vessel, respectively. The term major adverse clinical event (MACE) usually means the combined endpoint of the above‐mentioned procedures plus death, infarction and stroke. However, the definition of MACE can be variable in different studies, so care must be taken when comparing results.
Another concept is angiographic restenosis. This means restenosis that constricts the dilated segment to less than 50% of the reference segment. Repeat angiography is needed to reveal this. In a large meta‐analysis, half of the patients with angiographic restenosis developed clinical (symptomatic) restenosis Citation6.
After PCI (balloon‐only angioplasty or bare metal stenting) the coronary artery wall usually reaches its final state after 6–9 months. To find angiographic restenosis control angiography is usually done 6–9 months after the procedure. Clinical restenosis occurs usually 9–12 months after PCI. Symptoms developing later than this are usually due to newly formed lesions. In the era of drug‐eluting stents, angiographic and clinical restenosis may be detected in longer time intervals than those characteristic for balloon‐only angioplasty of bare metal stenting.
Pathophysiology of restenosis
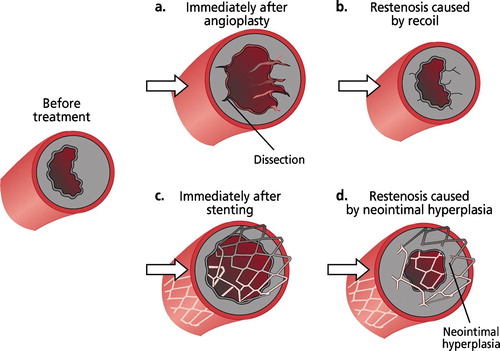
PCI is a violent procedure in which randomly dispersing lesions (dissection, ) occur in all the vessel wall layers (intima, media, adventitia). This damage is most important in the innermost layer, the intima, and in the muscle layer, the media.
Restenotic process can be divided to several components. According to a widely accepted view the components are: 1) local thrombosis, 2) recoil and remodeling of treated vessel and 3) neointimal proliferation.
Acute and subacute thrombosis and inflammation
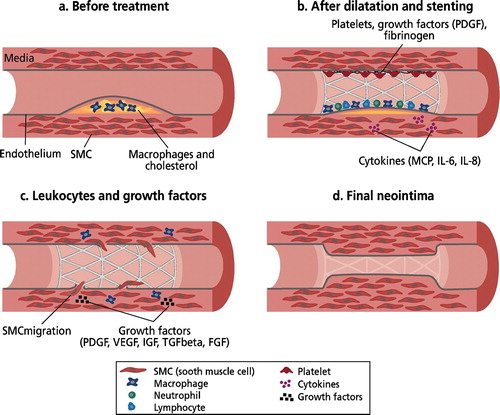
The most immediate result of PCI is disruption of endothelium. Up to 90% of endothelium in the dilated segment is disrupted (denuded) Citation7. Immediately after endothelial damage thrombocytes gather at the lesion site, activate and adhere to the collagen revealed with aid of von Willebrandt factor launching the coagulation cascade and as result triggering fibrin accumulation leading to thrombus formation. Thrombus plays an essential role in adhesion of inflammatory cells, local increase in concentration of growth factors and other cytokines and subsequently neointimal cells (). Thrombin secreted by thrombocytes strongly attracts neutrophil cells and a smooth muscle cell proliferating factor Citation8. Thrombosis thus plays a role in restenosis Citation9 in spite of the fact that antithrombotic treatment has not had significant effect on clinical restenosis. The most important mediator of the restenosis cascade is the recruitment of leukocytes at the injury site Citation10.
Figure 2. Development of neointimal hyperplasia.(a): Macrophages and cholesterol in the atherosclerotic plaque. (b): Rupture of the vessel wall results in activation of the thrombosis cascade, inflammation. Foreign body reaction caused by the stent results in inflammation and local accumulation of growth factors. (c): The smooth muscle cells (SMC) activate, migrate inside the stent and proliferate. (d): The process fades as soon as the endothelium covers the neointima. Modified from Welt and Rogers Citation12.
Recoil and remodeling
The concept recoil is understood as re‐contraction of the dilated vessel wall. Immediately after dilation the vessel shrinks back to similar measurements as those before the procedure (). Remodeling (negative remodeling) refers to gradual attenuation of the vessel diameter during days to months following balloon dilation Citation9. In both phenomena the medial layer is thought to shrink and scar so that the end result is worse than expected. Both recoil and remodeling can be avoided with stent implantation (). The radial force of contemporary stents is sufficient to hinder practically all shrinking of the vessel. Thus the only restenosis process is neointimal hyperplasia growing inside of the stent ().
Neointimal hyperplasia
The accumulating thrombocytes, inflammation and stent‐induced foreign body reaction in the stented vessel wall stimulate growth factor secretion leading to neointimal hyperplasia Citation11. The most important growth factors are PDGF (platelet‐derived growth factor) produced by thrombocytes, bFGF (basic fibroblast growth factor), TGFβ (transforming growth factor beta), IGF (insulin‐like growth factor), VEGF (vascular endothelial growth factor) and AT II (angiotensin II) Citation12 ().
Cells accumulating into newly formed neointima are thought to originate from smooth muscle cells (SMCs) of the medial layer, even though the role of medial fibroblasts and adventitial cells has been recognized in recent years Citation13. Cells released from the medial (and possibly also from adventitial) layer migrate to the damaged site over the inner surface of the stent and proliferate (). Activated and proliferated SMCs secrete several cytokines that can promote this process through a positive feedback mechanism Citation14. It is important to note that this process is usually strongest in those areas where the original plaque resided; this reflects the importance of the inflammatory response to the development of neointima.
Simultaneously with the growth of neointima a number of beneficial phenomena can be observed. These include re‐growth of the endothelial cell layer over the dilation site and the stent, and invasion of cells between stent struts and from both ends of the stent to the damaged zone. When the stent and adjacent areas of the vessel wall are again covered with endothelium the described neointimal cascade fades due to nitric oxide (NO) and heparin secreted by endothelial cells (ECs) (). NO and heparin have a direct inhibiting action on the growth of SMCs Citation15. Controlled growth of endothelial cells is thus needed. The sooner this process is completed, the smaller the developing neointimal mass will be. Endothelization takes time, however. In animal models the delay to complete healing has been approximately 20–50 days, in humans probably as long as 3 months Citation16,17.
It has been shown in animal studies that neointimal cell content decreases during first year after healing of the lesion. Neointimal cells are replaced by fibrous tissue proteins—collagen, elastin and glycoproteins. Chronically the neointima becomes a fibrotic mass that no longer maintains the restenosis process (). From clinical experience it is known that the dilation result after stenting is permanent after 6–9 months.
Factors related to restenosis
Many factors related to restenosis have been identified. These can be divided into three groups: patient‐related, procedure‐related and lesion‐related factors.
Patient‐related factors that increase the risk of restenosis are diabetes, multivessel disease, hypertension and unstable angina pectoris. Among these, diabetes is by far the most important factor Citation18.
There are several pathological mechanisms to enhance the restenotic process in diabetics. The neointimal response in diabetic patients is pronounced. The inflammatory response to stenting is also enhanced in diabetic patients. Trombocytes in diabetic patients are bigger and express more glycoprotein IIb/IIIa receptors than in their non‐diabetic counterparts. In addition, the hemostatic cascade is enhanced in diabetics Citation19. Increased tendency towards restenosis in diabetic patients has been shown in both bare metal stents and drug‐eluting stents, Citation2,Citation18,Citation20.
Procedure‐related factors that predict in‐stent restenosis are the final diameter of the stented segment and the total length of the implanted stents. The larger the lumen after the procedure, the lower the risk of restenosis. Conversely, the smaller the diseased vessel, the higher the risk of restenosis Citation21. Stent length (also in relation to lesion length) has been shown to be an independent risk factor for restenosis Citation22.
The angiographic characteristics of the treated lesion are also predictors of restenosis. A small diameter (minimum lumen diameter), a long stenosis, atherosclerosis upstream and downstream of the stenosis, total occlusion, bifurcational lesion and ostial lesion are significant predictors of restenosis Citation6. The risk of restenosis is highest in a diabetic patient with small coronary arteries (diameter<2.5–2.7 mm).
Other factors investigated for restenotic effect include pre‐ and post‐procedural C‐reactive protein (CRP), serum homocysteine level and associated vitamin therapy, allergies to stent constituent metals and several genetic polymorphisms. Baseline levels of CRP do not seem to predict restenosis Citation23,24. However, there are reports that the change of CRP in response to stenting could be prognostic for developing restenosis Citation25,26. Homocysteine was suspected of being predictive for restenosis, but several recent reports have disproved this. There is even strong evidence that attempts to lower homocysteine levels with folate therapy may lead to adverse outcomes Citation27.
Prevention of restenosis
Prevention of restenosis is very important, because after treatment of restenosis the risk of development of re‐restenosis is very high, circa 50%. In order to prevent restenosis, the interest has moved from targeting platelets and blood clotting to inhibition of smooth muscle cell proliferation and migration, and regulation of extracellular and inflammatory mediators of cell cycle.
Oral and other systemic drugs
Several drugs have been tested in the prevention of restenosis. Over 60 clinical trials were undertaken before the stent era, and 16 during the stent era. Most of the drugs turned out to be ineffective in the prevention of restenosis. Only eight of them can be considered as positive. ACE inhibitors, calcium channel blockers, heparin, tranilast, statins and folate have been shown to be of no benefit Citation27–Citation29. Tranilast is an anthranilic acid derivate which has been shown to prevent upregulation of TGFβ isoforms and TGF receptors. In experimental studies it has reduced the accumulation of leukocytes and collagen as a result of stent injury Citation30. PRESTO was a large multicenter study comprising more that 11,000 patients in which tranilast was used to prevent restenosis after PCI. However, tranilast showed no benefits with regard to clinical and angiographical endpoints during a 9‐month follow‐up Citation31.
Cilostazol is a phosphoesterase II inhibitor with an additional inhibitory effect on MAC‐1 receptors that participate in the adhesion of leukocytes. In the CREST study cilostazol resulted in a reduction of angiographic restenosis from 34.6% to 20.9% during a follow‐up of 6 months in 705 patients. Particularly, restenosis of small coronary arteries in patients with non‐insulin‐treated diabetes decreased by 50% in the cilostazol arm. On the contrary, in patients with insulin‐treated diabetes and in patients with larger coronary arteries cilostazol was of no benefit Citation32. The study population of CREST study was small, however, and the results need confirmation from larger trials.
Troglitazone is a novel insulin sensitizer which has been shown to inhibit proliferation of smooth muscle cells Citation33,34. Troglitazone was reported to reduce restenosis by 50% in diabetic patients with small coronary arteries Citation35.
Probucol is a potent antioxidant that is well tolerated and with only minor side effects. However, it can prolong the QTc interval and therefore can be proarrhythmic. In the MVP study, probucol reduced restenosis after PCI. In addition, in the CART‐1 study probucol and its more stable derivate AGI‐1067 reduced restenosis during the 6‐month follow‐up. AGI‐1067 did not prolong the QTc interval. In addition, in the AGI‐1067 group the reference segment was larger after the follow‐up than in the control group suggesting that AGI‐1067 may have direct effect on atherosclerosis Citation36.
In the ORBIT study open labeled oral sirolimus (2 or 5 mg daily) turned out to be ineffective in the prevention of restenosis. In addition, during the 30 days of therapy in the 5‐mg group, every third patient had to stop the study because of side effects Citation37. The OSIRIS study investigated the effect of oral sirolimus in the prevention of further restenosis in patients who already had restenosis Citation38. Sirolimus was given from day 2 before to day 7 after the PCI. Restenosis, as judged by quantitative coronary angiography, was lower in sirolimus‐treated patients than in the control group. Also reinterventions tended to be lower among sirolimus‐treated patients, but the difference did not reach statistical significance.
In summary, the results of systemic medication in the prevention of restenosis after PCI have been disappointing. Thus, the topic of interest has changed from systemic drug treatment to local drug therapy (see drug‐eluting stents).
Stents
Stents are used to support the vessel wall after balloon dilatation. In addition, the diameter of the vessel lumen is larger after stenting than after balloon dilatation (acute gain). Furthermore, stents prevent restenosis caused by recoil and dissection. On the other hand, the damage of the vessel wall produced by the PCI procedure is larger with stenting than with balloon dilatation. In addition, the metal compound of the stent is a strong stimulus for neointimal hyperplasia, which can, if exaggerated, narrow the vessel lumen and result in (total) occlusion of the vessel.
The development of restenosis has decreased markedly with the use of stents. The risk of angiographic restenosis after PCI without stents in single‐vessel disease is 30%–40% and the risk of clinical restenosis is 20%–30% Citation28–30. With stents, the risk of clinical restenosis has been reduced to circa 10% (STRESS, BENESTENT I, BENESTENT II, STARS) Citation39–42. In multivessel disease the risk of restenosis after PCI without stents is as high as 50%–60% (EAST, GABI, CABRI, RITA, BARI) Citation43–47. The use of stents reduces the risk of restenosis to 20%–30% in multivessel disease during a follow‐up of 3 years (SoS, ARTS) Citation48,49. However, in spite of stents, the need for reintervention is still higher after PCI than after coronary bypass surgery. In the ARTS and SoS trials the need for reintervention was 7% after surgery. It has to be noted, however, that superiority of bare metal stents over balloon‐only angioplasty has not been proved in small vessels (diameter<2.5 mm) Citation50.
Brachytherapy
Intracoronary brachytherapy was the first clinically effective method to prevent restenosis. It inhibits the proliferation of the smooth muscle cells of the vessel wall and reduces neointimal proliferation. Beta‐ and gamma radiations are currently used in clinical practice. The effect of beta‐radiation extends 2–3 mm and gamma radiation extends circa 10 mm from the radiation source.
On the cellular level, radiation inhibits neointimal proliferation but the main effect is the dose‐rated reproduction sterilization of cells that may proliferate in response to injury.
The effect of radiation depends on the temporal and spatial dose of radiation. Small doses (circa 3–5 Gy) induce neointimal proliferation. A medium dose (5–15 Gy) has shown to be most effective in the prevention of neointimal proliferation. A higher dose (>15 Gy) has resulted in necrotic cell death and delayed healing of the vessel wall resulting in the risk of subacute thrombosis and aneurysms of the treated vessel Citation51. Endothelial cells are more resistant to the effects of radiation than other layers of the vessel wall. After the endothelial layer has been destroyed as a result of balloon dilatation, the effect of radiation becomes exaggerated in the medial and adventitial layers Citation7.
Radiation is not used as a first‐line therapy to prevent restenosis. However it has been used in the treatment of in‐stent restenosis. The stenosed stent is redilated, and thereafter the stent is treated with radiation. With radiation the risk of re‐restenosis can be reduced by 50% compared with balloon dilatation only. The drawbacks of radiation are high costs, need for radiation protection and risk to the patient and hospital personnel. In addition, delayed healing of the endothelial layer constitutes a potential risk of late thrombosis Citation52.
Drug‐eluting stents
The concept behind drug‐eluting stents is to cover the stent with a drug that is liberated slowly around the stent to prevent excessive neointimal growth and thereby restenosis. The drug (or an enzyme, a protein growth factor, a DNA sequence or gene construct) must fulfill several criteria: 1) The drug needs to be liberated from the stent in a predictable manner during a long period of time (15–60 days); 2) The drug or the carrier vehicle must not be toxic (a toxic effect results in cell death and can prolong the inflammatory reaction); 3) the drug must not prevent the normal healing process of the vessel wall.
Currently there are five drug‐eluting stents on the market, a sirolimus‐covered Cypher® (Cordis) stent, a paclitaxel‐covered Taxus® (Boston Scientific) stent, an ABT‐578‐covered Endeavor® (Medtronic) stent, a tacrolimus‐covered Janus® (Sorin) stent and paclitaxel‐covered Axxion (Biosensors International) stent. In clinical testing there are several other stents, including sirolimus‐derivative biolimus A9‐covered Nobori® (Terumo) stent, everolimus with stable polymer XIENCE V® (Guidant), pimecrolimus‐eluting stent (Avantec) and MAPK‐inhibitor trapidil Destiny® (Icon) stent. Among other compounds that have been tested are actinomycin D, dexamethasone, heparin, and taxol derivative QP2 or 7‐hexanoyl‐taxol.
Other stent materials and coverings
Titanium oxide‐covered stents have been tested in restenosis prevention. In a small clinical trial a significant reduction of neointima was seen Citation53. Carbon (Carbostent®, Sorin Biomedica), silicon carbide (Lekton®, Biotronik) and phosphoryl choline stents (BiodivYsio®, Biocompatibles) are available. The idea behind these stents is to minimize the foreign body reaction and subsequent neointimal formation. There have also been degradable stents. Stents made of magnesium alloy (AMS, Biotronik) and different polylactides have been designed, but their usefulness in clinical practice remains to be proved.
Gene therapy
Gene therapy has been subject to intensive research. Theoretically, genetic intervention in the fundamental processes of restenosis without toxic systemic side effects would be an ideal solution. Clinical results have been modest. In the first randomized, placebo‐controlled gene therapy trial aimed at prevention of restenosis (Kuopio Angioplasty Trial, KAT), adenoviral VEGF transfection was performed with a Dispatch® perfusion catheter as adjunctive therapy to coronary stenting Citation54. In KAT no difference in the restenosis rate in the treatment arm versus placebo was seen. It has to be noted, however, that the overall incidence of restenosis in this trial was much lower than usually seen in this kind of trial, with only 6% angiographic restenosis at 6 months (15%–40% in most stent trials).
Controlled, randomized studies with drug‐eluting stents
Sirolimus
The first reports on the effects of drug‐eluting stents were based on sirolimus‐eluting stents. The First‐In‐Man (FIM) pilot study recruited 30 patients who were treated with the Cypher® stent. During a follow‐up of 8 months, no clinical restenosis was reported Citation55. In the same study, only one patient needed a reintervention during a 2‐year follow‐up Citation56.
The real effect of drug‐eluting stents was proven by larger, randomized studies in which drug‐eluting stents were compared with otherwise identical stents, but without a drug coating. In the RAVEL study, a sirolimus‐coated stent (Cypher®, Cordis) was compared with an uncoated bare metal stent (Velocity®, Cordis) in 238 patients with single‐vessel disease. During a follow‐up of 1 year, none of the patients treated with a drug‐eluting stent underwent reintervention, whereas 22.9% of patients treated with a bare metal stent required reintervention Citation57. Recently the 4‐year results of the RAVEL study reported that target lesion revascularization was required in 5.9% of patients treated with drug‐eluting stents and in 25.2% of patients treated with bare metal stents Citation58.
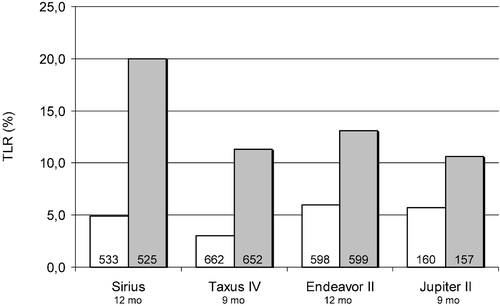
The RAVEL study was followed by the larger SIRIUS program, which was carried out in the USA, Canada and Europe. In the USA, 1058 patients with single‐vessel disease were randomized to drug‐eluting stent (n = 533) and bare metal stent groups (n = 525). After 1‐year follow‐up the reintervention rate was 4.9% in the sirolimus group and 20% in the bare metal stent group () Citation4. Sirolimus stents also markedly decreased the risk of reintervention in high‐risk groups (smaller vessels, long lesions, diabetic patients) by 70%–80% Citation59.
Paclitaxel
The second drug used in drug‐eluting stents, paclitaxel, has also been shown to be effective in reducing the incidence of in‐stent restenosis after PCI. The effect of paclitaxel has been studied in the TAXUS study program. The first results were reported by the TAXUS I study. Sixty‐one patients were randomized into two groups. The first group was treated with a paclitaxel‐coated stent (n = 31), and the other group with the same kind of stent without a paclitaxel coating (n = 30). During a follow‐up of 1 year, reintervention was needed in 3% of the drug‐eluting stent group and 10% in the bare metal stent group Citation60.
The Taxus I study was followed by the larger TAXUS IV study, where 1314 patients with single‐vessel disease were randomized to paclitaxel‐coated drug‐eluting stent treatment (n = 662) or bare metal stent treatment (n = 652). The reintervention rate was significantly lower in the drug‐eluting stent group (3.0%) than in the bare metal stent group (11.3%) during the 9‐month follow‐up Citation5. The reduction of reinterventions was 73% (). The 3‐year combined follow‐up of TAXUS II and TAXUS IV studies shows persistent benefit with target lesion revascularization of 9.4% in the Taxus group and 19.9% in the control group Citation61. The TAXUS VI study enrolled 446 patients with complicated lesions; 55.6% were ACC/AHA type C lesions, and average stenosis length was 20.6 mm. At 9 months, the primary endpoint of target vessel revascularization was 9.1% in the Taxus group and 19.4% in controls. Significantly, there were only four stent thromboses in the total population despite a mean stent‐covered length of 33.4 mm Citation62.
ABT‐578
ABT‐578 has been studied in the ENDEAVOR trials. ENDEAVOR I was a nonrandomized trial in which 100 patients with stable angina pectoris were treated with ABT‐578‐eluting stent (Endeavor®, Medtronic). After a follow‐up of 12 months, the target lesion revascularization rate was reported to be as low as 1% Citation63.
The ENDEAVOR II trial was a randomized trial in which 1197 patients were treated with an ABT‐578‐coated stent (n = 598) or a corresponding bare metal stent (Driver®, Medtronic) (n = 599). During the 12‐month follow‐up the target lesion revascularization rate in the drug‐eluting stent group (6.0%) was significantly lower (54%) than in the bare‐metal stent group (13.1%) Citation64 ().
Tacrolimus
The effect of tacrolimus has been studied in the JUPITER II trial. The JUPITER II trial compared tacrolimus‐eluting Janus® carbostent (Sorin) (n = 160) with Technic® carbostent (Sorin) (n = 157) in the prevention of restenosis. The study reported a non‐significant (46%) reduction in the need of reinterventions in the drug‐eluting stent patients (5.7% versus 10.6%) Citation65 ().
Everolimus
Everolimus is another sirolimus derivative. Its effect on stent restenosis is tested in the SPIRIT FIRST and FUTURE‐trial series. FUTURE I enrolled only 42 patients with de novo lesions randomized in a 2:1 ratio to everolimus and bare metal stent groups. Everolimus stents had significantly lower in‐stent late lumen loss at 6‐month angiographic follow‐up Citation66.
Drug‐eluting stents without polymer coating
There has been some concern about inflammatory properties of stent‐coating polymers Citation67. New polymer‐free stent designs have emerged, and first reports have been published. Hausleiter et al. tested on‐site coating with sirolimus solutions and found a significantly lower rate of restenosis compared with bare metal stents Citation68. This model was further tested in the ISAR‐TEST trial. 450 patients were randomized to 2% sirolimus solution on‐site coating and commercial paclitaxel stent (Taxus®) groups. On‐site coating was found to be non‐inferior to Taxus®‐stent Citation69. In the ELUTES trial, paclitaxel was applied directly to the surface of the stent in de novo lesions, and these were compared with bare metal stents alone. At 6 months binary restenosis decreased from 20.6% to 3.2% in highest‐dose groups Citation70.
Comparisons between sirolimus‐eluting and paclitaxel‐eluting stents
Clinical efficacy of sirolimus‐ and paclitaxel‐eluting stents has been similar in randomized controlled trials. To date, there are already several head‐to‐head comparisons of the two stent systems. In a small TAXi‐trial Goy et al. found no clinical advantage of one stent over the other Citation71. In the SIRTAX‐trial, 1012 patients undergoing percutaneous coronary intervention were randomized to sirolimus‐stent and paclitaxel‐stent groups. Sirolimus‐treated patients had lower rate of MACE during follow‐up. The difference was mainly due to lower need of target lesion revascularization (4.8% versus 8.3%). Notably, the cumulative incidence of endpoints rose markedly in the paclitaxel‐stent group 4–5 months after implantation Citation72. The REALITY trial compared sirolimus‐eluting Cypher® and paclitaxel‐eluting Taxus® in native lesions. There was no difference in the reintervention rates (5.0% versus 5.4%) between the two groups. However, there was a significant difference in angiographic parameters in favor of sirolimus. Also there were more subacute thrombosis events in the paclitaxel group, but significance was dependent on one single patient Citation73.
ISAR‐DIABETES compared sirolimus and paclitaxel in diabetic patients. Angiographic results at 9 months were better in the sirolimus group (in‐segment restenosis 6.9% versus 16.5%), but this did not translate to significant difference in target vessel revascularization Citation74.
Another high‐risk group, patients with coronary in‐stent restenosis, was studied in the ISAR‐DESIRE trial. In this open‐label study 300 patients were randomly assigned to receive sirolimus‐stent, paclitaxel‐stent or PCI without stents Citation75. The need of target lesion revascularization was lower in the sirolimus group (8.0%) than in the paclitaxel group (19.0%) and the bare metal stent group (33.0%). Both drug‐eluting stents were superior compared to PCI without stents.
As a conclusion, there seems to be a slight advantage of sirolimus‐coated stents over paclitaxel when difficult or high‐risk lesions are treated. However, the difference between the two cannot be seen in low‐risk lesions or weakly powered studies. Larger studies are needed to fully address this question Citation76.
Drug‐eluting stents in high‐risk patients
Diabetes
Diabetes is associated with a high risk of complications and reinterventions after revascularization procedures. This has been true also in the era of drug‐eluting stents; the relative risk of restenosis is 30%–40% higher in diabetic that in non‐diabetic patients. In patients with diabetes the high rate of restenosis has been reduced markedly with drug‐eluting stents. This is very important, because a high proportion, 20%–30%, of patients undergoing PCI have diabetes. Moreover, the number of diabetic patients is rapidly increasing worldwide.
In the TAXUS IV study, 318 of 1314 patients were diabetic, 105 of whom required insulin. Paclitaxel‐eluting stents reduced the 12‐month rate of revascularization by 65% compared with bare‐metal stents (7.4% versus 20.9%) Citation20. The 9‐month results of the DIABETES study, comparing sirolimus stents and bare metal stents in 160 patients with diabetes, reported that the reintervention rate in the drug‐eluting stent group (7.3%) was significantly lower than in the bare metal stent group (31.3%) Citation77.
Currently there are two large, randomized studies (CARDIA and FREEDOM) underway in which PCI with stenting is compared with coronary bypass surgery in diabetic patients with multivessel disease.
Small vessels and long stenoses
The length of the stenosis and the small diameter of the vessel are significant predictors of restenosis and reintervention (). The effect of drug‐eluting stents in the treatment of small vessels has been studied in the SES‐SMART and TAXUS V studies. In the SES‐SMART‐study the need for reintervention in small vessels (diameter⩽2.75 mm) was significantly lower in patients treated with a sirolimus‐coated stent than in those treated with an otherwise identical bare metal stent (7.0% versus 21.1%) Citation78. In the TAXUS V study the need of reintervention was also lower in patients receiving a paclitaxel eluting stent than in patients treated with a bare metal stent (10.4% versus 21.5%) Citation79. The issue of small vessels was addressed also in the E‐SIRIUS and C‐SIRIUS studies. In the E‐SIRIUS study the reintervention rates in drug‐eluting stent and bare metal stent groups were 4.0% and 20.0%, respectively Citation80. In the C‐SIRIUS study the corresponding reintervention figures were 4.0% and 18.0%, respectively Citation81.
Figure 4. Need of reintervention after percutaneous coronary intervention with respect to the length of the stent and diameter of the stent. Drug‐eluting stent (left panel) and bare metal stent (right panel). Modified from Stone, TCT 2004 Citation61.
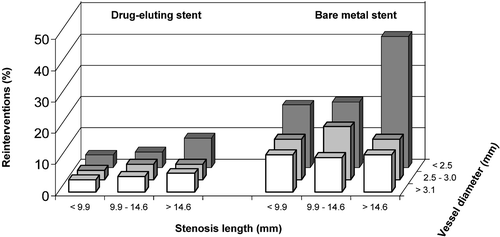
The treatment of long lesions has been studied in the TAXUS V and VI studies. In the TAXUS V study the reintervention rate in patients treated with multiple overlapping stents was in favor of paclitaxel‐eluting stents (12.6% versus 28.2%) Citation79. In the TAXUS VI study, reintervention at lesion site was needed in 6.8% of patients randomized to the drug‐eluting stent group and 18.9% of patients in the bare metal stent group Citation62.
In light of these studies it is evident that drug‐eluting stents have markedly reduced the risk of restenosis associated with small vessels and long lesions ().
In‐stent restenosis
In‐stent restenosis means restenosis that develops inside a stent. Usually, a redilatation of the stenosed stent results in excellent immediate results. However, the risk of re‐restenosis is very high, about 50%. The ISAR‐DESIRE study compared drug‐eluting stents (Cypher® and Taxus®) and PCI without stents in the treatment of in‐stent restenosis Citation82. One hundred patients were randomized to each group. Drug‐eluting stents turned out to be more effective than balloon dilatation‐only in the prevention of re‐restenosis. Clinical restenosis in the sirolimus‐eluting stent group, paclitaxel‐eluting stent group and PCI without stents group were 8%, 19% and 33%, respectively.
Drug‐eluting stents are compared with radiation therapy in the treatment of in‐stent restenosis. In the SISR trial sirolimus‐eluting stents and intravascular brachytherapy are compared in the treatment of in‐stent restenosis Citation83. During a 9‐month follow‐up the target lesion revascularization rate was significantly lower in the stent‐treated patients than in patients treated with brachytherapy (8.5% versus 19.2%). Correspondingly, in the TAXUS V ISR study a paclitaxel‐eluting stent and radiation therapy are compared in the treatment of in‐stent restenosis. The results of this trial are still pending.
Radke et al. treated 25 patients with in‐stent restenosis with a paclitaxel‐coated stent (Archieve®, Cook) and compared these with 25 patients derived from the hospital PCI registry who had been treated with radiation therapy (beta radiation) Citation84. The patients were matched with respect to the length and diameter of the vessel. During a follow‐up of 6 months, an angiographic restenosis developed in 20% treated with drug‐eluting stents and in 16% of patients treated with radiation therapy. Correspondingly, the need for reintervention due to re‐restenosis was 8% and 24% in the stent and radiation‐treated patients, respectively. The differences did not reach statistical significance, but they suggest that drug‐eluting stents are as effective as radiation in the treatments of in‐stent restenosis. Thus, although controlled randomized studies are not available, drug‐eluting stents have replaced radiation therapy in the treatment of in‐stent restenosis.
Multivessel disease
Controlled studies about drug‐eluting stents in multivessel disease do not exist. In an ongoing SYNTAX‐study patients with a left main disease or a multivessel disease are randomized to either PCI with paclitaxel‐eluting stents or coronary bypass surgery. While controlled studies are pending, nonrandomized studies and registry studies have provided valuable information about the benefits of drug‐eluting stents in multivessel disease.
The ARTS II study was an open study in which 607 patients with multivessel disease were treated with drug‐eluting stents Citation85. Patients were compared with the ARTS I study in which 600 patients with multivessel disease were treated with bare metal stents and 605 patients with coronary bypass surgery. After a 1‐year follow‐up, 7.4% of patients treated with drug‐eluting stents and 3.7% treated with bypass surgery underwent a new revascularization. Notably the combined rate of death, cerebrovascular events and myocardial infarction was 8.1% in the stent group and 13.1% in the CABG group Citation85. In the single‐center RESEARCH registry the center decided to use only drug‐eluting stents in all patients undergoing PCI. A total of 508 patients treated with drug‐eluting stents during the 6‐month drug‐eluting stent era were compared with 450 patients undergoing PCI during the 6‐month period immediately before the conversion to drug‐eluting stents. During a follow‐up of 12 months the need for reintervention was significantly lower in the drug‐eluting stent‐treated patients (3.7%) than in the bare metal stent‐treated patients (10.9%) Citation2.
Percutaneous coronary intervention during the drug‐eluting stent era
Already results from the bare metal stent era showed that PCI and coronary bypass surgery are equal with regard to prognosis Citation48,49. On the other hand, patients treated with PCI needed more reinterventions than patients treated with coronary bypass surgery.
The evidence on the effects of drug‐eluting stents in the prevention of restenosis is based on studies done in single‐vessel disease. Based on those studies, nonrandomized studies and registry studies, drug‐eluting stents have been used increasingly in situations in which randomized controlled evidence does not exist. Drug‐eluting stents are used currently instead of bare metal stents in situations that are known to be associated with a high risk of restenosis, or in situations where restenosis is not acceptable, such as in the treatment of diabetic patients, long stenoses, small vessels and multivessel disease. PCI is not the primary therapy for left main disease or bifurcation disease. However, if for some reason these patients are treated with PCI, drug‐eluting stents should be used Citation86.
Open questions
Although drug‐eluting stents have been shown to be very effective, the restenosis problem has not yet been solved. The use of PCI with stenting for revascularization has increased and will increase further in the future. It will be used in more complicated cases, and many of the patients currently treated with coronary bypass surgery will be treated with PCI. In addition, diabetes, an important determinant of restenosis, is increasing worldwide. All these factors indicate that restenosis will remain an important challenge also in the future. Important issues that need to be addressed are randomized controlled trials comparing PCI using drug‐eluting stents with coronary bypass surgery in patients with multivessel disease, bifurcational lesions, left main disease and vein graft disease.
Restenosis takes place within 6–9 months after the PCI procedure when bare metal stents are used. After that, the restenosis process slows down and finally stops. Is this true also for drug‐eluting stents? Drug‐eluting stents have been available for no longer than a few years. Thus, the long‐term effects of drug‐eluting stents are not known. In controlled studies, the longest follow‐up time has been 4 years. In those studies, the benefit compared with bare metal stents has persisted, or even increased with time.
In addition to the prevention of neointimal formation, drug‐eluting stents result also in delayed endothelization of the stent struts. A concern has arisen that this could lead to potential for late stent thrombosis with drug‐eluting stents Citation86. Indeed, there is some evidence that the discontinuation of antiplatelet therapy after implantation of drug‐eluting stents increases the risk of stent thrombosis Citation87. This has led to active discussion about the use of drug‐eluting stents in patients with risk of bleeding, such as patients with oral anticoagulants or a history of hemorrhage. In addition, the duration of antiplatelet therapy, particularly clopidogrel, has become a topic of growing interest.
PCI is increasingly used in the treatment of acute ST‐elevation infarcts. In acute infarction, a fresh thrombus is often found at the site of acute plaque rupture. In addition, thrombolytic therapy is associated with activation of the hemostatic process. There are no studies on the use of drug‐eluting stents in the setting of acute myocardial infarction.
The mechanisms of neointimal proliferation have been under very active research during recent years. It is possible that the best means to prevent restenosis is to combine many tools. For example, the combination of drug‐eluting stents and oral medication may be one potential option. This is an especially challenging option for diabetic patients. In addition, the development of competition between different manufacturers of drug‐eluting stents will bring new perspectives. The price of drug‐eluting stents will decrease in the near future. In addition, new drugs and constructs for stent covering will be available. As a result of better stent platforms and better tools to prevent restenosis, PCI will be used instead of coronary bypass surgery in increasingly complex cases.
References
- Silber S., Albertsson P., Aviles F. F., Camici P. G., Colombo A., Hamm C., et al. Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Eur Heart J 2005; 26: 804
- Lemos P. A., Serruys P. W., van Domburg R. T., Saia F., Arampatzis C. A., Hoye A., et al. Unrestricted utilization of sirolimus‐eluting stents compared with conventional bare stent implantation in the “real world”: the Rapamycin‐Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) registry. Circulation 2004; 109: 190–5
- Greenberg D., Bakhai A., Cohen D. J. Can we afford to eliminate restenosis? Can we afford not to?. J Am Coll Cardiol 2004; 43: 513–8
- Moses J. W., Leon M. B., Popma J. J., Fitzgerald P., Holmes D. R., O'Shaughnessy C., et al. Sirolimus‐eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003; 349: 1315–23
- Stone G. W., Ellis S. G., Cox D. A., Hermiller J., O'Shaughnessy C., Mann J. T., et al. A polymer‐based, paclitaxel‐eluting stent in patients with coronary artery disease. N Engl J Med 2004; 350: 221–31
- Cutlip D. E., Chauhan M. S., Baim D. S., Ho K. K. L., Popma J. J., Carrozza J. P., et al. Clinical restenosis after coronary stenting: perspectives from multicenter clinical trials. J Am Coll Cardiol 2002; 40: 2082–9
- Sindermann J. R., Verin V., Hopewell J. W., Rodemann H. P., Hendry J. H. Biological aspects of radiation and drug‐eluting stents for the prevention of restenosis. Cardiovasc Res 2004; 63: 22–30
- Donners M. M., Daemen M. J., Cleutjens K. B., Heeneman S. Inflammation and restenosis: implications for therapy. Ann Med 2003; 35: 523–31
- Schwartz R. S. Pathophysiology of restenosis: interaction of thrombosis, hyperplasia, and/or remodeling. Am J Cardiol 1998; 81: 14E–17E
- Costa M. A., Simon D. I. Molecular basis of restenosis and drug‐eluting stents. Circulation 2005; 111: 2257
- Farb A., Sangiorgi G., Carter A. J., Walley V. M., Edwards W. D., Schwartz R. S., et al. Pathology of acute and chronic coronary stenting in humans. Circulation 1999; 99: 44–52
- Welt F. G. P., Rogers C. Inflammation and Restenosis in the Stent Era. Arterioscler Thromb Vasc Biol 2002; 22: 1769–76
- Sartore S., Chiavegato A., Faggin E., Franch R., Puato M., Ausoni S., et al. Contribution of Adventitial Fibroblasts to Neointima Formation and Vascular Remodeling: From Innocent Bystander to Active Participant. Circ Res 2001; 89: 1111–21
- Libby P., Schwartz D., Brogi E., Tanaka H., Clinton S. K. A cascade model for restenosis. A special case of atherosclerosis progression. Circulation 1992; 86: III47–III52
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest 1983; 49: 327–33
- Grewe P. H., Deneke T., Machraoui A., Barmeyer J., Muller K. M. Acute and chronic tissue response to coronary stent implantation: pathologic findings in human specimen. J Am Coll Cardiol 2000; 35: 157–63
- Losordo D. W., Isner J. M., Diaz‐Sandoval L. J. Endothelial Recovery: The Next Target in Restenosis Prevention. Circulation 2003; 107: 2635–7
- Cutlip D. E., Chhabra A. G., Baim D. S., Chauhan M. S., Marulkar S., Massaro J., et al. Beyond restenosis. Five‐year clinical outcomes from second‐generation coronary stent trials. Circulation 2004; 110: 1226
- Karha J., Bhatt D. L. Percutaneous coronary intervention in diabetics. Rev Endocr Metab Disord 2004; 5: 277–85
- Hermiller J. B., Raizner A., Cannon L., Gurbel P. A., Kutcher M. A., Wong S. C., et al. Outcomes with the polymer‐based paclitaxel‐eluting TAXUS stent in patients with diabetes mellitus. The TAXUS‐IV trial. J Am Coll Cardiol 2005; 45: 1172
- Kastrati A., Schulen H., Schomig A. Stenting for small coronary vessels: a contestable winner (Editorial Comment). J Am Col Cardiol 2001; 38: 1604–7
- Mauri L., O'Malley A. J., Cutlip D. E., Ho K. K., Popma J. J., Chauhan M. S., et al. Effects of Stent Length and Lesion Length on Coronary Restenosis. Am J Cardiol 2004; 93: 1340
- Gomma A. H., Hirschfield G. M., Gallimore J. R., Jr., Lowe G. D., Pepys M. B., Fox K. M. Preprocedural inflammatory markers do not predict restenosis after successful coronary stenting. Am Heart J 2004; 147: 1071–7
- Rittersma S. Z., de Winter R. J., Koch K. T., Schotborgh C. E., Bax M., Heyde G. S., et al. Preprocedural C‐reactive protein is not associated with angiographic restenosis or target lesion revascularization after coronary artery stent placement. Clin Chem 2004; 50: 1589–96
- Karaca I., Aydin K., Yavuzkir M., Ilkay E., Akbulut M., Isik A., et al. Predictive value of C‐reactive protein in patients with unstable angina pectoris undergoing coronary artery stent implantation. J Int Med Res 2005; 33: 389–96
- Dibra A., Mehilli J., Braun S., Hadamitzky M., Baum H., Dirschinger J., et al. Inflammatory response after intervention assessed by serial C‐reactive protein measurements correlates with restenosis in patients treated with coronary stenting. Am Heart J 2005; 150: 344–50
- Lange H., Suryapranata H., De Luca G., Borner C., Dille J., Kallmayer K., et al. Folate therapy and in‐stent restenosis after coronary stenting. N Engl J Med 2004; 350: 2673–81
- Mody V. H., Durairaj A., Mehra A. O. Pharmacological approaches to prevent restenosis. Restenosis: A Guide to Therapy., D. P Faxon, editor. Martin Durnitz, LondonUK 2001; p. 97–112, In
- Kuchulakanti P., Waksman R. Therapeutic potential of oral antiproliferative agents in the prevention of coronary restenosis. Drugs 2004; 64: 2379–88
- Ward M. R., Agrotis A., Kanellakis P., Hall J., Jennings G., Bobik A. Tranilast Prevents Activation of Transforming Growth Factor‐{beta} System, Leukocyte Accumulation, and Neointimal Growth in Porcine Coronary Arteries After Stenting. Arterioscler Thromb Vasc Biol 2002; 22: 940–8
- Holmes D. R., Jr., Savage M., Lablanche J. M., Grip L., Serruys P. W., Fitzgerald P., et al. Results of Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) Trial. Circulation 2002; 106: 1243–50
- Douglas J. S., Holmes D. R., Kereiakis D. J., Grines C. L., Block E., Ghazzal Z. M., et al. Coronary stent restenosis in patients treated with cilostazol (CREST). Circulation 2005; 112: 2826–32
- Law R. E., Meehan W. P., Xi X. P., Graf K., Wuthrich D. A., Coats W., et al. Troglitazone inhibits vascular smooth muscle cell growth and intimal hyperplasia. J Clin Invest 1996; 98: 1897–905
- Bruemmer D., Law R. E. Thiazolidinedione regulation of smooth muscle cell proliferation. Am J Med 2003; 115((Suppl 8A))87S–92S
- Takagi T., Yamamuro A., Tamita K., Yamabe K., Katayama M., Morioka S., et al. Impact of troglitazone on coronary stent implantation using small stents in patients with type 2 diabetes mellitus. Am J Cardiol 2002; 89: 318–22
- Tardif J. C., Gregoire J., Schwartz L., Title L., Laramee L., Reeves F., et al. Effects of AGI‐1067 and probucol after percutaneous coronary interventions. Circulation 2003; 107: 552–8
- Waksman R., Ajani A. E., Pichard A. D., Torguson R., Pinnow E., Canos D., et al. Oral rapamycin to inhibit restenosis after stenting of de novo coronary lesions: the Oral Rapamune to Inhibit Restenosis (ORBIT) study. J Am Coll Cardiol 2004; 44: 1386–92
- Hausleiter J., Kastrati A., Mehilli J., Vogeser M., Zohlnhofer D., Schuhlen H., et al. OSIRIS Investigators. Randomized, double‐blind, placebo‐controlled trial of oral sirolimus for restenosis prevention in patients with in‐stent restenosis: the Oral Sirolimus to Inhibit Recurrent In‐stent Stenosis (OSIRIS) trial. Circulation 2004; 110: 790–5
- Fischman D. L., Leon M. B., Baim D. S., Schatz R. A., Savage M. P., Penn I., et al. A randomized comparison of coronary‐stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med 1994; 331: 496–501
- Serruys P. W., de Jaegere P., Kiemeneij F., Macaya C., Rutsch W., Heyndrickx G., et al. A comparison of balloon‐expandable‐stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med 1994; 331: 489–95
- Serruys P. W., van Hout B., Bonnier H., Legrand V., Garcia E., Macaya C., et al. Randomised comparison of implantation of heparin‐coated stents with balloon angioplasty in selected patients with coronary artery disease (Benestent II). Lancet 1998; 352: 673–81
- Leon M. B., Baim D. S., Popma J. J., Gordon P. C., Cutlip D. E., Ho K. K., et al. A clinical trial comparing three antithrombotic‐drug regimens after coronary‐artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998; 339: 1665–71
- King S. B., III., Lembo N. J., Weintraub W. S., Kosinski A. S., Barnhart H. X., Kutner M. H., et al. A randomized trial comparing coronary angioplasty with coronary bypass surgery. Emory Angioplasty versus Surgery Trial (EAST). N Engl J Med 1994; 331: 1044–50
- Hamm C. W., Reimers J., Ischinger T., Rupprecht H. J., Berger J., Bleifeld W. A randomized study of coronary angioplasty compared with bypass surgery in patients with symptomatic multivessel coronary disease. German Angioplasty Bypass Surgery Investigation (GABI). N Engl J Med 1994; 331: 1037–43
- CABRI Trial Participants. First‐year results of CABRI (Coronary Angioplasty versus Bypass Revascularisation Investigation). Lancet 1995; 346: 1179–84
- Henderson R. A., Pocock S. J., Sharp S. J., Nanchahal K., Sculpher M. J., Buxton M. J., et al. Long‐term results of RITA‐1 trial: clinical and cost comparisons of coronary angioplasty and coronary‐artery bypass grafting. Randomised Intervention Treatment of Angina. Lancet 1998; 352: 1419–25
- BARI Investigators. Five‐year clinical and functional outcome comparing bypass surgery and angioplasty in patients with multivessel coronary disease. A multicenter randomized trial. Writing Group for the Bypass Angioplasty Revascularization Investigation (BARI) Investigators. JAMA 1997; 277: 715–21
- SoS Investigators. Coronary artery bypass surgery versus percutaneous coronary intervention with stent implantation in patients with multivessel coronary artery disease (the Stent or Surgery trial): a randomised controlled trial. Lancet 2002; 360: 965–70
- Legrand V. M., Serruys P. W., Unger F., van Hout B. A., Vrolix M. C., Fransen G. M., et al. Three‐year outcome after coronary stenting versus bypass surgery for the treatment of multivessel disease. Circulation 2004; 109: 1114–20
- Kastrati A., Schomig A., Dirschinger J., Mehilli J., Dotzer F., von Welser N., et al. Randomized trial comparing stenting with balloon angioplasty in small vessels in patients with symptomatic coronary artery disease. ISAR‐SMART Study Investigators. Intracoronary Stenting or Angioplasty for Restenosis Reduction in Small Arteries. Circulation 2000; 102: 2593–8
- Salame M. Y., Verheye S., Mulkey S. P., Choronos N. A. F., King SB I. I. I., Crocker I. R., et al. The Effect of Endovascular Irradiation on Platelet Recruitment at Sites of Balloon Angioplasty in Pig Coronary Arteries. Circulation 2000; 101: 1087–90
- Virmani R., Farb A., Kolodgie F. D. Histopathologic alterations after endovascular radiation and antiproliferative stents: similarities and differences. Herz 2002; 27: 1–6
- Windecker S., Simon R., Klauss V., Eberli F. R., Roffi M., Pedrazzini G., et al. Randomized comparison of a titanium‐nitriode‐oxide‐coated stent with a stainless steel stent for coronary revascularization. The TiNOX Trial. Circulation 2005; 111: 2617–22
- Hedman M., Hartikainen J., Syvänne M., Stjernvall J., Hedman A., Kivelä A., et al. Safety and feasibility of catheter‐based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in‐stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT). Circulation 2003; 107: 2677–83
- Sousa J. E., Costa M. A., Abizaid A., Abizaid A. S., Feres F., Pinto I. M., et al. Lack of neointimal proliferation after implantation of sirolimus‐coated stents in human coronary arteries: a quantitative coronary angiography and three‐dimensional intravascular ultrasound study. Circulation 2001; 103: 192–5
- Sousa J. E., Costa M. A., Sousa A. G., Abizaid A. C., Seixas A. C., Abizaid A. S., et al. Two‐year angiographic and intravascular ultrasound follow‐up after implantation of sirolimus‐eluting stents in human coronary arteries. Circulation 2003; 107: 381–3
- Morice M. C., Serruys P. W., Sousa J. E., Fajadet J., Ban H. E., Perin M., et al. A randomized comparison of a sirolimus‐eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002; 346: 1773–80
- Sousa J. E., Morice M. C., P. W., et al. A randomised, double‐blind study with the Sirolimus‐eluting BxVelocity balloon expandable stent in the treatment of patients with de novo native coronary artery lesions. 2005, EuroPCR
- Holmes D. R., Jr., Leon M. B., Moses J. W., Popma J. J., Cutlip D., Fitzgerald P. J., et al. Analysis of 1‐year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus‐eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation 2004; 109: 634–40
- Grube E., Silber S., Hauptmann K. E., Mueller R., Buellesfeld L., Gerckens U., et al. TAXUS I: Six‐ and Twelve‐Month Results From a Randomized, Double‐Blind Trial on a Slow‐Release Paclitaxel‐Eluting Stent for De Novo Coronary Lesions. Circulation 2003; 107: 38–42
- Stone G. W. Paclitaxel‐eluting stents: Update 2005. 2005, TCT
- Dawkins K. D., Grube E., Guagliumi G., Banning A. P., Zmudka K., Colombo A., et al. TAXUS VI Investigators. Clinical efficacy of polymer‐based paclitaxel‐eluting stents in the treatment of complex, long coronary artery lesions from a multicenter, randomized trial: support for the use of drug‐eluting stents in contemporary clinical practice. Circulation 2005; 112: 3306–13
- Meredith I. T. Endeavor I. 2005, ESC
- Fajadet W., Wijns, Kuntz R. A Randomized trial to evaluate the safety and efficacy of the Medtronic AVE ABT‐578 eluting driver coronary stent in de novo native coronary artery lesion. 12 month follow‐up. 2005, ESC
- Morice M. ‐C. Jupiter II: Double blind randomised comparison of Janus eluting stent with the Technic Carbostent. 2005, ESC
- Costa R. A., Lansky A. J., Mintz G. S., Mehran R., Tsuchiya Y., Negoita M., et al. Angiographic results of the first human experience with everolimus‐eluting stents for the treatment of coronary lesions (the FUTURE I trial). Am J Cardiol 2005; 95: 113–6
- Virmani R., Farb A., Guagliumi G., Kolodgie F. D. Drug‐eluting stents: caution and concerns for long‐term outcome. Coron Artery Dis 2004; 15: 313–20
- Hausleiter J., Kastrati A., Wessely R., Dibra A., Mehilli J., Schratzenstaller T., , for the investigators of the individualizable durg‐eluting Stent System to Abrogate Restenosis Project, et al. Prevention of restenosis by a novel drug‐eluting stent system with a dose‐adjustable, polymer‐free, on‐site stent coating. Eur Heart J 2005; 26: 1475–81
- Mehilli J., Kastrati A., Wessely R., Dibra A., Hausleiter J., Jaschke B., et al. Intracoronary Stenting and Angiographic Restenosis–Test Equivalence Between 2 Drug‐Eluting Stents (ISAR‐TEST) Trial Investigators. Randomized trial of a nonpolymer‐based rapamycin‐eluting stent versus a polymer‐based paclitaxel‐eluting stent for the reduction of late lumen loss. Circulation 2006; 113: 273–9
- Gershlick A., De Scheerder I., Chevalier B., Stephens‐Lloyd A., Camenzind E., Vrints C., et al. Inhibition of restenosis with a paclitaxel‐eluting, polymer‐free coronary stent: the European evaLUation of pacliTaxel Eluting Stent (ELUTES) trial. Circulation 2004; 109: 487–93
- Goy J. J., Stauffer J. C., Siegenthaler M., Benoit A., Seydoux C. A prospective randomized comparison between paclitaxel and sirolimus stents in the real world of interventional cardiology: the TAXi trial. J Am Coll Cardiol 2005; 45: 308–11
- Windecker S., Remondino A., Eberli F. R., Juni P., Raber L., Wenaweser P., et al. Sirolimus‐eluting and paclitaxel‐eluting stents for coronary revascularization. N Engl J Med 2005; 353: 653–62
- Morice M. ‐C. A prospective, randomised, multicenter comparison of the Cypher® sirolimus‐eluting and the Taxus® paclitaxel‐eluting stent systems. 2005, ACC
- Dibra A., Kastrati A., Mehilli J., Pache J., Schuhlen H., von Beckerath N., , ISAR‐DIABETES Study Investigators, et al. Paclitaxel‐eluting or sirolimus‐eluting stents to prevent restenosis in diabetic patients. N Engl J Med 2005; 353: 663–70
- Kastrati A., Mehilli J., von Beckerath N., Dibra A., Hausleiter J., Pache J., , ISAR‐DESIRE Study Investigators, et al. Sirolimus‐eluting stent or paclitaxel‐eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in‐stent restenosis: a randomized controlled trial. JAMA 2005; 293: 165–71
- Moliterno D. J. Healing Achilles‐sirolimus versus paclitaxel. N Engl J Med 2005; 353: 724–7
- Sabate M., Jimenez‐Quevedo, Angiolillo D., Gomez‐Hospital J. A., Alfonso F., Hernandez‐Antolin R., et al. Randomized comparison of sirolimus‐eluting stent versus standard stent for percutaneous coronary revascularization in diabetic patients: the diabetes and sirolimus‐eluting stent (DIABETES) trial. Circulation 2005; 112: 2175–83
- Ardissino D., Cavallini C., Bramucci E., Indolfi C., Marzocchi A., Manari A., et al. Sirolimus‐Eluting vs Uncoated Stents for Prevention of Restenosis in Small Coronary Arteries: A Randomized Trial. JAMA 2004; 292: 2727–34
- Stone G. W., Ellis S. G., Cannon L., Mann J. T., Greenberg J. D., Spriggs D., et al. Comparison of a polymer‐based paclitaxel‐eluting stent with a bare metal stent in patients with complex coronary artery disease. JAMA 2005; 294: 1215–23
- Schofer J., Schluter M., Gershlick A. H., Wijns W., Garcia E., Schampaert E., et al. Sirolimus‐eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double‐blind, randomised controlled trial (E‐SIRIUS). Lancet 2003; 362: 1093–9
- Schampaert E., Cohen E. A., Schluter M., Reeves F., Traboulsi M., Title L. M., et al. The Canadian study of the sirolimus‐eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C‐SIRIUS). J Am Coll Cardiol 2004; 43: 1110–5
- Kastrati A., Dibra A., Eberle S., Mehilli J., Suárez de Lezo J., Goy J. ‐J., et al. Sirolimus‐Eluting Stents vs Paclitaxel‐Eluting Stents in Patients With Coronary Artery Disease. Meta‐analysis of Randomized Trials. JAMA 2005; 294: 819–25
- Holmes D. R., Popma J., Kuntz R., Fitzgerald P. J., Teirstein P. S., Satler L., et al. The SISR Trial. A multicenter, randomized, study of the Sirolimus‐eluting Bx Velocity® stent vs intravascular brachytherapy in the treatment of patients with in‐stent restenotic coronary artery lesions. 2005, TCT
- Radke P. W., Kobella S., Kaiser A., Franke A., Schubert D., Grube E., et al. Treatment of in‐stent restenosis using a paclitaxel‐eluting stent: acute results and long‐term follow‐up of a matched‐pair comparison with intracoronary beta‐radiation therapy. Eur Heart J 2004; 25: 920–5
- Serruys P. W., Lemos P. A., van Hout B. A. Arterial Revascularisation Therapies Study part II Steering Committee and Investigators. Sirolimus eluting stent implantation for patients with multivessel disease: rationale for the Arterial Revascularisation Therapies Study part II (ARTS II). Heart 2004; 90: 995–8
- Valgimigli M., van Mieghem C. A. G., Ong A. T. L., Aoki J., Rodriguez Granillo G. A., McFadden E. P., et al. Short‐ and long term clinical outcome after drug‐eluting stent implantation for the percutaneous treatment of left main coronary artery disease. Insights from the rapamycin‐eluting and Taxus stent evaluated at Rotterdam cardiology hospital registries (RESEARCH and T‐SEARCH). Circulation 2005; 111: 1383–89
- Iakovou I., Schmidt T., Bonizzoni E., Ge L., Sangiorgi G. M., Stankovic G., et al. Incidence, Predictors, and Outcome of Thrombosis After Successful Implantation of Drug‐Eluting Stents. JAMA 2005; 2126–30, 293