Abstract
Many bacterial toxins kill animal cells by adenosine diphosphate (ADP)‐ribosylating intracellular target proteins. Mammalian cells express toxin‐related cell surface ADP‐ribosyltransferases (ARTs) that transfer ADP‐ribose from nicotinamide adenine dinucleotide (NAD) onto arginine residues of other membrane proteins. The association of these glycosylphosphatidylinositol (GPI)‐anchored ectoenzymes with glycolipid rafts focuses them onto components of the signal transduction machinery. Exposing murine T cells to NAD, the ART substrate, induces a cascade of reactions that culminates in cell death by apoptosis. This mechanism, dubbed ‘NAD‐induced cell death’ or NICD, is initiated when ART2 ADP‐ribosylates the cytolytic P2X7 purinergic receptor, inducing formation of a cation channel, opening of a nonselective pore, shedding of CD62L from the cell surface, exposure of phosphatidylserine on the outer leaflet of the plasma membrane, breakdown of the mitochondrial membrane potential, and DNA‐fragmentation. The ART substrate NAD is produced in large amounts inside the cell and can be released from damaged cells during inflammation and tissue injury. In the extracellular environment, the signaling function of NAD is terminated by NAD‐degrading ectoenzymes such as CD38. We propose that ART2‐catalyzed ADP‐ribosylation of P2X7 represents the paradigm of a regulatory mechanism by which ART‐expressing cells can sense and respond to the release of NAD from damaged cells.
ADP‐ribosylation is a posttranslational protein modification used by bacterial toxins and mammalian ectoenzymes to alter the function of target proteins
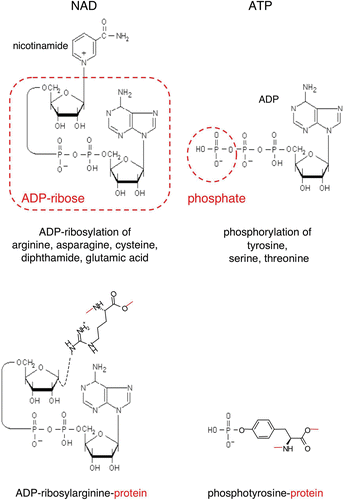
Adenosine diphosphate (ADP)‐ribosylation is an enzyme‐catalyzed posttranslational protein modification in which ADP‐ribosyltransferases (ARTs) transfer the ADP‐ribose moiety from nicotinamide adenine dinucleotide (NAD) onto specific amino acid residues in target proteins while nicotinamide is released Citation1–6. Like protein kinase‐catalyzed protein phosphorylation, ADP‐ribosylation often activates or blocks the function of the modified target protein. Note that ADP‐ribosylation attaches a much bulkier group onto the target protein than protein phosphorylation (). Both protein modifications are reversible. Protein phosphatases restore protein function by removing the phosphate group from the target protein; protein‐ADP‐ribosylhydrolases (ARHs) restore protein function by removing the ADP‐ribosyl group from the target protein Citation7.
Figure 1. Schematic diagrams of the ADP‐ribose and phosphate moieties transferred from NAD and ATP onto amino acid side chains during protein‐ADP‐ribosylation and protein‐phosphorylation. ADP‐ribosyltransferases (ARTs) transfer the ADP‐ribose moiety from NAD onto specific amino acid side chains in target proteins (e.g. arginine, asparagine, cysteine, diphthamide or glutamate) while nicotinamide is released. Protein kinases transfer the terminal phosphate of ATP onto specific amino acid side chains (e.g. tyrosine, serine, threonine) in target proteins, while ADP is released. Note that ADP‐ribosylation results in the covalent attachment of a much bulkier group to the target protein than phosphorylation. (ADP = adenosine diphosphate; ATP = adenosine triphosphate; NAD = nicotinamide adenine dinucleotide)
ADP‐ribosylation was originally discovered as the mechanism by which diphtheria toxin (DT) blocks protein synthesis in intoxicated cells, i.e. by ADP‐ribosylating diphthamide, an unusual modified amino acid found only in elongation factor 2 Citation8. Cholera, pertussis, and a large number of other potent bacterial toxins were subsequently shown to carry ADP‐ribosyltransferase activity Citation1. Most known ARTs from the bacterial world are secretory proteins and, typically, are encoded by mobile genetic elements such as phages, plasmids or pathogenicity islands. Most known bacterial ADP‐ribosylating enzymes are involved in host pathogen interactions. Typically the pathogen‐expressed ADP‐ribosyltransferase exerts its cytotoxic effects upon translocation into a host cell and subsequent ADP‐ribosylation of host cell protein(s). For example, the C3 exoenzymes of Clostridium botulinum and Staphylococcus aureus disrupt the cytoskeleton of mammalian cells by ADP‐ribosylating small guanosine triphosphate (GTP)‐binding proteins of the rho family on asparagine 41 Citation9,10. The clostridial C2 and iota toxins, VIP2 of Bacillus cereus and SpvB of Salmonella entericae disrupt the cytoskeleton by ADP‐ribosylating actin on arginine 177, thereby blocking the cell's capacity to polymerize this protein Citation11–13. Following translocation into the cytosol of host cells via a type III secretion system, ExoS of Pseudomonas aeruginosa intoxicates cells by ADP‐ribosylating arginine residues on multiple cellular proteins including Ras, a signal transducing oncogenic protein Citation14,15.
Toxin‐related ADP‐ribosyltransferases have also been found in mammals (). Interestingly, the family of mammalian ADP‐ribosyltransferases appears to be split into at least two distinct branches: mono‐ADP‐ribosylating ectoenzymes (designated mARTs or ARTs) and the poly‐ADP‐ribosylating intracellular enzymes (pARTs or PARPs) Citation16–20. Structurally, the mono‐ADP‐ribosylating ectoenzymes show closer resemblance to a large subfamily of bacterial toxins (including ExoS, C2, C3, VIP2, and SpvB) than to the pARTs. Conversely, the pARTs show closer structural similarity to a small subfamily of bacterial toxins (including diphtheria toxin and pseudomonas exotoxin A) than to the ARTs Citation20. ADP‐ribosyltransferases show a remarkable flexibility of amino acid sequences Citation16,17. The protein structure database contains the folds of 18 ADP‐ribosyltransferases, including two vertebrate poly‐ADP‐ribosyltransferases and one vertebrate mono‐ADP‐ribosyltransferase. The 3D structures of these ADP‐ribosylating enzymes contain a common core of five anti‐parallel β strands, yet only a single amino acid residue, the catalytic glutamic acid residue at the beginning of the fifth β strand, is strictly conserved in all of these structures Citation16,Citation20,21. Three ART‐related folds lacking this glutamate have been found as a structural component of larger bacterial toxins (Bacillus cereus VIP2, Clostridium perfringens iota toxin, Bacillus anthracis lethal toxin) Citation11,Citation22,23. These ART domains evidently lack ADP‐ribosyltransferase activity and may have acquired a new function, e.g. as a protein‐protein interaction domain. VIP2 and iota toxin actually contain two ART‐domains—one active, the other inactive. Sequence analyses suggest that some members of the eukaryotic ART family (e.g. ART3 and ART4) similarly lack the conserved glutamate residue Citation18.
Figure 2. ADP‐ribosylation by bacterial and mammalian ADP‐ribosyltransferases. A: Cholera toxin is a secreted AB5 toxin. The pentameric B subunit binds to the host cell receptor–raft‐associated ganglioside GM1. Following endocytosis, the catalytic A subunit is translocated retrograde through the endoplasmic reticulum (ER) membrane to the cytosol where it ADP‐ribosylates the alpha subunit of heterotrimeric G proteins on an arginine residue. B: Mammalian extracellular mono‐ADP‐ribosyltransferases (ARTs 1–5) most closely resemble the C3/VIP2 family of bacterial toxins. The preferential site of expression is indicated above each ART. ART1 and ART2 ADP‐ribosylate other membrane proteins (mp) or secretory proteins (sp) on arginine residues. Mammalian intracellular poly‐ADP‐ribosyltransferases (pARTs 1–17) bear closer resemblance to diphtheria toxin (DT) and pseudomonas exotoxin A (ETA) than to other bacterial or vertebrate mono‐ADP‐ribosyltransferases. PARP‐1, for example, ADP‐ribosylates nuclear proteins (np) on glutamic acid residues and also catalyzes the extension and branching of poly‐ADP‐ribose polymers on the target protein. (ADP = adenosine diphosphate; NAD = nicotinamide adenine dinucleotide)
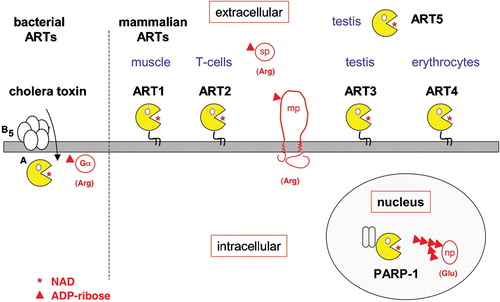
The pART family encompasses 17 members in the human and 16 members in the mouse (the mouse lacks the gene for pART7) Citation20, while the mART family encompasses 4 members in the human (ART1, ART3, ART4, and ART5) and 6 members in the mouse (ART1, ART2.1, ART2.2, ART3, ART4, and ART5) Citation18. PARP1 and other pARTs are multidomain intracellular proteins, in which the catalytic ART domain is fused to a variety of protein‐ and nucleic acid‐binding domains. In contrast, mARTs are relatively small (200–250 amino acids long) extracellular proteins composed of an isolated catalytic domain, which may be fused via the C‐terminal amino acid to a glycosylphosphatidylinositol (GPI)‐membrane anchor. Intriguingly, the mouse expresses two functional copies of ART2 (following a gene duplication event), whereas the human does not express ART2 owing to a functional inactivation of the ART2 gene by premature stop codons Citation24,25. Mouse ART2.1 and ART2.2 are coexpressed on T cells and show similar target specificities, yet ART2.1 is active only in the presence of reducing agents while ART2.2 appears to be constitutively active Citation25,26. The requirement for reducing agents can be ascribed to an additional pair of cysteine residues present in ART2.1 which is absent from ART2.2 and all other known ARTs Citation18. On transfected cells, human ART1 ADP‐ribosylates a similar profile of target proteins as mouse ART2 and it is conceivable that ART1 may have adopted the function of ART2 on leukocytes Citation27. Human ART1 was recently assigned the CD number CD296; it is expressed by activated granulocytes as well as by skeletal muscle, heart, and epithelial cells Citation28–31. ART3 is prominently expressed in testis, muscle, brain and in the embryo Citation18,Citation32. ART4 has been identified as the carrier of the Dombrock blood group alloantigens and was recently assigned the CD number CD297 Citation33. ART4 is expressed prominently by erythrocytes and at lower levels also on monocytes and splenic macrophages. ART5 shows a similar expression profile as ART3. ART5 lacks a C‐terminal GPI‐anchor and is a secreted protein; in some cells, e.g. the murine YAC‐1 lymphoma cell line, ART5 may be associated noncovalently to other membrane proteins Citation34,35. Recombinant human ART5 exhibits promiscuous arginine‐specific ART activity, mouse ART5 shows strong NADase activity, but increased ART activity following auto‐ADP‐ribosylation Citation18,Citation36. No enzyme activity has yet been demonstrated for ART3 or ART4.
Two distinct families of de‐ADP‐ribosylating enzymes have been described: the ARH family encompasses three family members in humans and other mammals (designated ARH1–ARH3), while the PARG family contains only a single member in known mammals Citation18,Citation37,38. ARH1 has been shown to de‐ADP‐ribosylate proteins carrying ADP‐ribose on arginine residues, ARH3 and PARG both de‐ADP‐ribosylate proteins carrying chains of ADP‐ribose groups attached to glutamate Citation37,Citation39.
Key messages
ADP‐ribosyltransferases (ARTs) are toxin‐related ectoenzymes on mammalian cells which adenosine diphosphate (ADP)‐ribosylate other cell surface and secretory proteins.
Nicotinamide adenine dinucleotide (NAD), the ART substrate, is released from damaged cells as an extracellular signaling molecule whose activity is terminated by the CD38 ecto‐NAD glycohydrolase.
On T cells, the association with lipid rafts focuses ART2 onto specific target proteins, including the cytolytic P2X7 purinoceptor.
P2X7 is activated by ADP‐ribosylation, causing exposure of phosphatidylserine, shedding of the CD62L L‐selectin, membrane permeabilization, and cell death.
Cytotoxic activity of murine ADP‐ribosyltransferase ART2 results from activation of the cytolytic P2X7 purinoceptor by ADP‐ribosylation
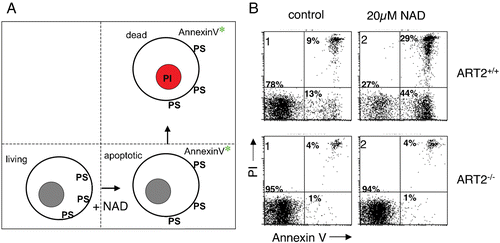
Given the cytotoxic effects of many bacterial ADP‐ribosyltransferases, it was not farfetched to suspect a cytotoxic activity also for some mammalian ARTs. This suspicion was nurtured by the finding that treatment of ART‐expressing lymphocytes with NAD exerted suppressive and in some instances cytotoxic effects Citation40,41. Following exposure to NAD, murine T cells exhibited classic signs of apoptosis, including exposure of phosphatidylserine (PS) on the outer leaflet of the plasma membrane, staining of DNA by propidium iodide (PI), and DNA‐fragmentation Citation41–43. PS exposure in response to treatment of cells with NAD can be monitored conveniently by flow cytometry using a fluorochrome conjugate of the PS‐binding protein annexin‐V (). Binding of propidium iodide to DNA occurs in dead cells and causes a characteristic shift in fluorescence, which can similarly be monitored by flow cytometry. Two crucial findings demonstrated that ART2 is a necessary mediator of these effects, dubbed NICD (NAD‐induced cell death). Firstly, cells from ART2 knockout mice proved resistant to NAD‐induced PS exposure and PI uptake Citation44. (ART2KO mice are healthy and fertile but show enhanced resistance to autoimmune disease—see below). Secondly, pre‐incubation of cells with ART2‐specific antibodies, which blocked ART2‐catalyzed ADP‐ribosylation of cell surface proteins, also effectively protected cells from NAD‐induced PS exposure and PI staining Citation42.
Figure 3. NAD‐induced cell death (NICD). A: Exposure of murine T cells to the ART substrate NAD induces exposure of phosphatidylserine (PS) on the outer leaflet of the cell membrane, which can be detected with a fluorochrome‐conjugate of the PS‐binding protein annexin‐V. NAD‐treatment induces a cascade of reactions culminating in cell death, as evidenced by irreversible staining of cells by the DNA staining dye propidium iodide (PI). NAD‐induced cell death (NICD) has features of both apoptosis and necrosis, including caspase activation, breakdown of the mitochondrial membrane potential, DNA fragmentation, and loss of plasma membrane integrity. B: Fluorescence‐activated cell sorter (FACS) profiles of murine T cells from wild type (ART2+/+) and ART2‐deficient mice (ART2 ‐/‐) stained with fluorochrome‐conjugated annexin‐V and propidium iodide following a 60‐minute incubation in the absence (control) or presence of ecto‐NAD. ART2‐/‐ T cells are resistant to NAD‐induced cell death. Moreover, treatment of ART2‐expressing cells with ART2‐specific antisera blocks cell surface protein ADP‐ribosylation and NICD (not shown), confirming the center stage role of ART2‐catalyzed ADP‐ribosylation of cell membrane proteins in NICD. ‘Spontaneous’ annexin‐V and PI staining of wild type T cells in the control sample is probably caused by NAD released from cells before or during T cell preparation. (NAD = nicotinamide adenine dinucleotide; ART = ADP‐ribosyltransferase; ADP = adenosine diphosphate)
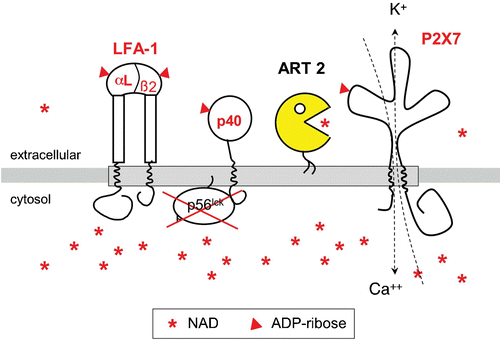
NICD in many aspects resembles cell death induced by activation of the cytolytic purinoceptor P2X7 by high concentrations of its soluble ligand, adenosine triphosphate (ATP) Citation45,46. Indeed, we discovered that P2X7 itself is a target of ART2‐catalyzed ADP‐ribosylation and that ADP‐ribosylation results in activation of P2X7 Citation42 (). P2X7 is one of seven members of the P2X family of purine‐gated cation channels Citation47,48. P2X7 is composed of 595 amino acids, contains two transmembrane domains, a short N‐terminal and a long‐C‐terminal cytosolic domain, and a 287 amino acid long extracellular domain containing the ATP‐binding site and five conserved disulfide bonds. Upon chronic activation by high concentrations of ATP and certain ATP analogues, P2X7 mediates the formation of large nonselective pores in the plasma membrane that permit the passage of molecules of <800 Da, including some DNA‐staining dyes.
Figure 4. GPI‐anchored ART2 ADP‐ribosylates the cytolytic P2X7 purinoceptor and several other key players of signal transduction on the murine T cell surface. Exposing murine T cells to the ART substrate NAD results in ADP‐ribosylation of several cell surface proteins, including the cytolytic P2X7 purinoceptor, both chains of the integrin LFA‐1, and a 40‐kd protein that is associated with the lck‐tyrosine kinase. ADP‐ribosylation activates P2X7 to from a nonselective cation channel causing influx of calcium and efflux of potassium ions. Its GPI‐anchor ensures that ART2 is associated with specialized, glycosphingolipid enriched membrane microdomains called rafts (indicated by the shaded bar). Raft association focuses the promiscuous enzyme activity of ART2 onto resident raft proteins and proteins that transiently associate with rafts. NAD is much more abundant in the cytosolic than in the extracellular compartment. (GPI = glycosylphosphatidylinositol; ART = ADP‐ribosyltransferase; ADP = adenosine diphosphate)
Dose response analyses revealed that much lower doses of extracellular NAD than ATP suffice to activate P2X7, provided that ART2 is coexpressed on the same cell as P2X7 Citation42,43. Moreover, we discovered that certain mouse strains carry a natural variant of P2X7 in the cytosolic tail (P451L), rendering T cells from these strains relatively resistant to both NICD and ATP‐induced cell death Citation42,Citation49. Furthermore, cells could be protected from NICD by pre‐incubation with ART2‐specific or P2X7‐specific antibodies Citation42. Together, these results strongly suggest that ADP‐ribosylation activates P2X7, presumably by providing ADP‐ribose as a covalently bound ligand. We consider it most likely that the ADP‐ribose moiety attached to P2X7 itself provides the ligand, although it cannot formally be excluded that the ligand can be displayed by other neighboring ADP‐ribosylated proteins. It is tempting to speculate that the ADP‐ribose moiety attached to P2X7 activates the receptor to which it is attached, akin to the carrot that is hung on a stick in front of a donkey. Alternatively, the ADP‐ribose moiety attached to one P2X7 molecule might be recognized by the neighboring P2X7 molecule, thereby stabilizing the trimerization of P2X7 that is required for channel formation, akin to a ring of elephants linked via trunks and tails. In any case, elucidation of the arginine residue in P2X7 that is ADP‐ribosylated by ART2 and of the 3‐D structure of P2X7 will shed more light on this issue.
ART2 is released from activated T cells as an active soluble enzyme following cleavage of its juxtamembrane stalk by an endogenous cell‐associated metalloprotease Citation50. Shedding of ART2 renders activated T cells resistant to NICD. Similarly, cleavage of its GPI‐anchor, either by bacterial phosphatidylinositol‐specific phospholipase C or by endogenous secretory phosphatidylinositol‐specific phospholipase D, could release a soluble form of ART2 into the extracellular environment. It is not known whether soluble ART2 retains its cytotoxic activity and/or whether ART2, akin to its bacterial ART cousins, can translocate into cells to ADP‐ribosylate intracellular targets Citation5,Citation51. Interestingly, incubation of T cells with soluble bacterial ExoS has also been shown to induce T cell apoptosis Citation52. Of all known bacterial ARTs, ExoS exhibits the highest degree of amino acid sequence similarity to ART2 Citation15,Citation18. Both ExoS and ART2 show a rather promiscuous ART activity and are capable of ADP‐ribosylating many different substrates. It will be interesting to determine whether ExoS can also ADP‐ribosylate P2X7 and/or other targets of ART2 on the extracellular face of the plasma membrane and whether this may account for its apoptosis‐inducing activity.
Association with lipid rafts focuses promiscuous ART2 onto specific targets
Lipid rafts, also known as GEMs (glycosphingolipid enriched microdomains) or DIGs (detergent‐insoluble, glycosphingolipid‐enriched membrane domains) are plasma membrane microdomains enriched in gangliosides and cholesterol which form liquid‐ordered domains Citation53,54. Lipid rafts are postulated to segregate molecules in the plasma membrane and to regulate signaling through the spatial coordination of intermolecular interactions. Lipid rafts are characterized by insolubility in nonionic detergents and low buoyancy in sucrose density gradients. The modification of proteins with saturated acyl groups can result in their localization within lipid rafts. Thus these microdomains are enriched in many signaling molecules such as the lck protein kinase, the adaptor protein LAT, heterotrimeric G proteins, and GPI‐anchored proteins Citation55. In addition, the T cell receptor (TCR) and other transmembrane receptors including the interleukin (IL)‐2‐receptor and the integrin LFA‐1 are recruited to or stabilized in lipid rafts Citation56–58. Activation of signaling molecules in lipid rafts is thought to facilitate signaling through the TCR and other immune receptors Citation59.
Considering that ART2 and other members of the ART family carry a GPI‐anchor Citation18,Citation60, we hypothesized that the GPI‐anchor mediates lateral segregation of ART2 in rafts, and that this might be a mechanism to regulate the activity and specificity of ART2. In order to test these hypotheses, we adopted an experimental approach that had been successfully used in previous studies addressing the lateral segregation of cell surface enzymes in membrane microdomains Citation61,62, i.e. we replaced the native GPI‐anchor of ART2 with the transmembrane and cytosolic domains of CD8 and established lymphoma transfectants expressing either ART2 with its native GPI‐anchor (ART2‐GPI) or ART2 with the transmembrane anchor of CD8 (ART2‐Tm) Citation63. In this system, GPI‐anchored but not transmembrane‐anchored ART2 associated with lipid rafts. Under conditions of limiting substrate concentrations ART2‐GPI exhibited a more than ten‐fold higher activity than ART2‐Tm. On intact cells ART2‐GPI ADP‐ribosylated a limited number of distinct target proteins, including P2X7 and LFA‐1. Disruption of lipid rafts by methyl‐β‐cyclodextrin (MCD) or membrane solubilization by Triton X‐100 (TX‐100) dramatically increased the spectrum of target proteins modified by ART2. Strikingly, when relieved from the restraint of the cell membrane, both ART2‐GPI and ART2‐Tm showed dramatically enhanced, highly promiscuous enzyme activities and ADP‐ribosylated many different proteins. These findings imply that membrane association restricts the accessibility of ART2 to its targets. Moreover, the lateral distribution within the cell membrane—orchestrated by its membrane anchor—further restricts the access of ART2 toward its targets. On the basis of these observations we proposed that raft association may provide a geographic isolation, permitting native GPI‐anchored ART2 to efficiently ADP‐ribosylate specific targets that constitutively or inducibly associate with lipid rafts ().
Availability of extracellular NAD as substrate for ART2 is limited by the CD38 NAD glycohydrolase
The type II transmembrane protein CD38 is a potent ecto‐NAD glycohydrolase (ecto‐NADase) Citation64,65. CD38 is expressed by lymphocytes (especially B cells), macrophages, endothelial cells, dendritic cells, pancreatic islet cells and several other cell types. A soluble form of CD38, presumably generated by proteolytic cleavage of its juxtamembrane stalk, has been found in serum and other extracellular fluids. CD38‐deficient mice are healthy and show normal development of the major lymphocyte subsets, but show impaired humoral immune responses, neutrophil chemotaxis and dendritic cell trafficking Citation66–68. CD38 is the predominant NADase in most murine tissues, including spleen and liver, and, accordingly, tissues from CD38 ‐/‐ mice show a dramatically reduced NADase activity versus those of wild type mice Citation68.
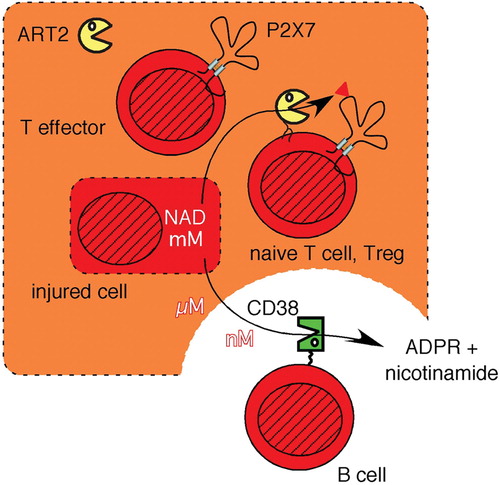
The fate of extracellular signaling molecules (ligands, transmitters) is determined by the rate of their release, metabolism, reuptake by cells, and/or renal excretion. For example, the concentration of ATP and the duration of signaling via ATP in the extracellular compartment are controlled by CD39 and related ecto‐nucleotidases which hydrolyze ATP to ADP and/or adenosine monophosphate (AMP) Citation69. Using an immunoassay for monitoring ADP‐ribosylation of cell surface proteins on living cells by flow cytometry Citation27 and primary lymphocytes from CD38‐/‐ and ART2‐/‐ mice, we demonstrated that the ecto‐NADase CD38 similarly controls the signaling function of NAD by limiting the availability of NAD as a substrate for ART‐catalyzed ADP‐ribosylation of cell surface proteins Citation70. Exogenously added etheno‐NAD and 32P‐NAD were readily catabolized by CD38‐expressing splenocytes but not by CD38 ‐/‐ cells, implying that local concentrations of NAD may vary dramatically in vivo, depending on the presence of CD38‐expressing cells and/or soluble CD38. Given the strikingly different patterns of expression of CD38 and ART2, areas rich in B cells which express very high levels of CD38 but not ART2 can be predicted to be essentially ‘ecto‐NAD‐free zones’ since extracellular NAD will be rapidly degraded by CD38. In contrast, local levels of ecto‐NAD may be sustained longer and at higher levels in areas rich in T cells, most of which express ART2 but little if any CD38 (). It is important to note that NAD may be released from cells also by nonlytic mechanisms, for example through gap‐junction hemichannels Citation71.
Figure 5. The signaling function of extracellular NAD is relayed by ART2 into ADP‐ribosylated cell surface proteins and is terminated by the hydrolysis of NAD through CD38. NAD concentrations within a cell lie in the mM range (indicated by dark shading), but are 100–10,000 times lower in extracellular tissue fluids. NAD can be released from cells by lytic and nonlytic mechanisms. The extracellular signaling functions of NAD are terminated by its hydrolysis through the CD38 NAD‐glycohydrolase to ADP‐ribose (ADPR) and nicotinamide. The local levels of extracellular NAD are high in the vicinity of damaged cells (light shading) and low in the vicinity of CD38 expressing cells, e.g. B cells. Extracellular NAD levels are relayed by ART2 into ADP‐ribosylated membrane and/or secretory proteins. Naive T cells and regulatory T cells (Tregs) are particularly sensitive to NICD, whereas activated effector T cells shed ART2, resulting in a strongly reduced responsiveness of activated T cells to NAD. (NAD = nicotinamide adenine dinucleotide; ART = ADP‐ribosyltransferase; ADP = adenosine diphosphate; NICD = NAD‐induced cell death)
Insights from knockout models
The knockout technology has provided fascinating opportunities to probe the function of individual genes in the context of the immune system of the mouse Citation72. Strikingly, targeted disruption of individual genes has often yielded mice that do not show any apparent phenotype at all. Moreover, the ‘genetic background’ can strongly influence a phenotype Citation73. For example, when challenged with immunizations or infections, BALBc and C57BL/6 mice, the two most commonly used mouse strains in the field of immunology, develop distinct responses: BALBc mice tend toward T helper 2 (Th2)‐biased allergic responses while C57BL/6 mice tend toward inflammatory Th1‐biased responses. Interestingly, these two strains also happen to harbor allelic differences in ART2 and P2X7: C57BL/6 mice carry a defective ART2.1 allele but express high levels of ART2.2, while BALBc mice express low levels of both ART2.1 and ART2.2 Citation74,75. Further, a point mutation in the cytosolic domain of the C57BL/6 P2X7 allele severely impairs its activation by ATP and NAD in transfected cells and in the majority of peripheral T cells Citation49. Remarkably, two minor but important cell populations retain exquisite sensitivity to ART2‐catalyzed activation of P2X7 in C57BL/6 mice: 1) CD4+/CD25+ regulatory T cells that suppress antigen‐specific immune responses and provide protection against autoimmune disease Citation76; and 2) NKT cells of the liver that play an important role in tolerance induction Citation77.
Knockout (KO) mice have been generated for all key players of NICD: P2X7, ART2.1 and ART2.2 (as single and double knockouts), and CD38 Citation44,Citation68,Citation78. In all cases, the KO mice are fertile and healthy with normal development of major lymphocyte subsets. Recognizable phenotypes became apparent in these mice only after immunizations, infections or upon backcrossing onto an autoimmune susceptible genetic background. ART2KO and P2X7KO mice on the C57BL/6 background exhibit normal humoral and cellular immune responses to protein antigens but show increased resistance to autoimmune hepatitis induced by toxic doses of the lectin concanavalin A Citation77. P2X7KO mice also show increased resistance to a second experimentally induced autoimmune disease, collagen‐induced arthritis Citation79. CD38KO mice on the C57BL/6 background show reduced humoral immune responses to protein antigens and enhanced sensitivity to infections with Streptococcus pneumoniaeCitation67,68. Moreover, transfer of the disrupted CD38 allele into the background of autoimmune diabetes‐prone NOD (nonobese diabetic) mice markedly accelerated diabetes onset, whereas transfer of the disrupted ART2.1/ART2.2 complex had no effect Citation80. However, the accelerated diabetes onset mediated by CD38 deficiency required ART2, as demonstrated by combining the two deficiencies Citation80.
Within the context of NICD, ablation of CD38 results in enhanced and sustained levels of extracellular NAD and, therefore, enhanced ADP‐ribosylation of cell surface proteins on ART‐expressing cells Citation70. Ablation of ART2, on the other hand, abolishes the capacity of T cells to ADP‐ribosylate P2X7 and other cell surface proteins. This prevents NAD‐induced but not ATP‐induced activation of P2X7 Citation42. Ablation of P2X7, finally, abolishes the capacity of ATP and NAD to mediate cytotoxicity via P2X7 itself but retains ATP‐induced responses via other purinoceptors and NAD‐induced ADP‐ribosylation of other cell surface proteins. Caution is warranted when translating these in vitro findings to complex in vivo situations since CD38, ART2 and P2X7 all play multifaceted, only partially interconnected functions: ART2 modifies other cell surface and secreted proteins in addition to P2X7, including CD38 Citation81. P2X7 plays a key role also in the secretion of inflammatory cytokines by macrophages Citation82. And CD38 not only deprives ART2 of its substrate, NAD, but also generates NAD‐metabolites with potent signaling functions Citation83 and acts as a receptor for membrane proteins on other cells Citation84.
Fine‐tuning of immune responses by nucleotides released from lysed cells
It has been proposed that nucleotides released from cells may have immunomodulatory functions Citation85. Our results are consistent with this hypothesis and support the notion that NAD functions as an extracellular signaling molecule. We propose that ART2 acts as a sensor of ecto‐NAD levels, translating the local concentration of ecto‐NAD into corresponding levels of ADP‐ribosylated cell surface proteins. The duration and intensity of this ART‐relayed signal are determined by the local levels of CD38 on the cell surface or in the extracellular medium. In this scenario, the NAD‐metabolizing ectoenzymes CD38 and ART2 together convey information about the presence of the signaling molecule ecto‐NAD to lymphocytes and thereby may help to fine‐tune meaningful immune responses.
The sensitivity to NAD‐signaling changes considerably during T cell development Citation86. Naive T cells express ART2 and P2X7, but not CD38 and thus are very sensitive to NAD‐induced cell death. NKT cells and regulatory T cells may express even higher levels of P2X7 and may be particularly prone to NICD Citation76,Citation86. Antigen‐activated T cells in turn shed ART2, downregulate P2X7 and upregulate CD38 Citation42, Citation50. Early in the immune response, NAD released at inflammatory sites and possibly transported to draining lymph nodes would promote proliferation of antigen‐specific T cells at the expense of naive and regulatory T cells. Thereby, NICD may contribute to protection from autoimmunity, by providing a mechanism to prevent the proliferation of naive, potentially auto‐reactive bystander cells. However, NICD may be a double‐edged sword: once auto‐reactive T cells have become established, NICD may tip the balance in favor of auto‐reactive effector versus regulatory T cells.
Perspectives for therapeutic applications of ARTs and ART‐inhibitors
Even though their physiological functions are still not fully solved, bacterial and mammalian ARTs pose attractive targets for therapeutic intervention Citation2,Citation5,Citation21. If inhibitors could be developed that specifically block bacterial but not mammalian ARTs, these might be used for alleviating the symptoms caused by toxin‐mediated ADP‐ribosylation of human target proteins, e.g. the severe diarrhea caused by cholera toxin and Escherichia coli heat labile toxins, or the whooping cough caused by pertussis toxin. Specific inhibitors and/or activators of mammalian ecto‐ARTs would be useful also for experimental and therapeutic settings in mammalian cells and organisms Citation5. However, since ARTs exhibit rather distinct expression patterns in rodents and humans, care has to be taken when translating findings from murine models to the human. Finally, it might be possible to utilize protein‐engineering technology to alter the target specificity of ARTs Citation21. Indeed, in an elegant proof of principle, Barbieri and co‐workers recently showed that it was possible to transplant the target specificities between ExoS and ExoT, two closely related ARTs from Pseudomonas aeruginosa, by grafting of two surface loops Citation87. With a better understanding of the structural principles governing target specificity of ARTs, it may even be possible to direct ARTs onto novel targets, e.g. in order to inactivate proteins or even other molecules by ADP‐ribosylation Citation5,Citation21,Citation87.
Conclusions
A number of well‐known cytotoxic bacterial toxins ADP‐ribosylate and thereby inactivate target proteins in their animal hosts. Toxin‐related ARTs have been found in mammals and birds. The 3D‐structures of rat ART2, Bacillus cereus VIP2, and C3 exoenzymes of Clostridium botulinum and Staphylococcus aureus show a close structural relationship of these enzymes and confirm that these mammalian and bacterial ARTs are derived from a common ancestor. C3 and VIP2 translocate across the plasma membrane to reach cytosolic target proteins. ART2, in contrast, resides on the extracellular leaflet of the T cell plasma membrane as a GPI‐anchored protein. Association with lipid rafts focuses the promiscuous ADP‐ribosylating activity of ART2 onto specific targets. Treatment of T cells with NAD, the substrate for ART2, suppresses T cell functions and induces T cell death following ART2 catalyzed ADP‐ribosylation and activation of the P2X7 purinoceptor to form nonselective ion channels and to induce the formation of large membrane pores. The extracellular signaling function of NAD is restricted by the NAD‐glycohydrolase CD38. ARTs pose attractive new targets for experimental and therapeutic manipulations of immune cell functions.
Acknowledgements
This work was supported by DFG (Deutsche Forschungs gemeinschaft) grant No310/6‐1 to FKN and FH; SA was a recipient of stipends from the Fonds pour la Recherche Medicale and the DFG; and PB and CK received stipends from the Werner Otto Stiftung.
References
- Aktories K., Just I. Bacterial Protein Toxins. Springer Verlag, Berlin 2000
- Corda D., Di Girolamo M. Functional aspects of protein mono‐ADP‐ribosylation. Embo J 2003; 22: 1953–8
- Haag F., Koch‐Nolte F. ADP‐Ribosylation in Animal Tissues: Structure, Function and Biology of Mono(ADP‐Ribosyl)transferases and Related Enzymes,. Plenum Press, New York 1997; vol. 419
- Moss J., Vaughan M. ADP‐ribosylating toxins and G proteins: Insights into signal transduction. American Society for Microbiology, Washington DC 1990
- Seman M., Adriouch S., Haag F., Koch‐Nolte F. Ecto‐ADP‐ribosyltransferases (ARTs): emerging actors in cell communication and signaling. Curr Med Chem 2004; 11: 857–72
- Salmi M., Jalkanen S. Cell‐surface enzymes in control of leukocyte trafficking. Nat Rev Immunol 2005; 5: 760–71
- Moss J., Zolkiewska A., Okazaki I. ADP‐ribosylarginine hydrolases and ADP‐ribosyltransferases. Partners in ADP‐ribosylation cycles. Adv Exp Med Biol 1997; 419: 25–33
- Collier R. J. Understanding the mode of action of diphtheria toxin: a perspective on progress during the 20th century. Toxicon 2001; 39: 1793–803
- Wilde C., Aktories K. The Rho‐ADP‐ribosylating C3 exoenzyme from Clostridium botulinum and related C3‐like transferases. Toxicon 2001; 39: 1647–60
- Wilde C., Chhatwal G. S., Aktories K. C3stau, a new member of the family of C3‐like ADP‐ribosyltransferases. Trends Microbiol 2002; 10: 5–7
- Han S., Craig J. A., Putnam C. D., Carozzi N. B., Tainer J. A. Evolution and mechanism from structures of an ADP‐ribosylating toxin and NAD complex. Nat Struct Biol 1999; 6: 932–6
- Richard J. F., Petit L., Gibert M., Marvaud J. C., Bouchaud C., Popoff M. R. Bacterial toxins modifying the actin cytoskeleton. Int Microbiol 1999; 2: 185–94
- Otto H., Tezcan‐Merdol D., Girisch R., Haag F., Rhen M., Koch‐Nolte F. The spvB gene‐product of the salmonella enterica virulence plasmid is a mono(ADP‐ribosyl)transferase. Mol Microbiol 2000; 37: 1106–15
- Kaufman M. R., Jia J., Zeng L., Ha U., Chow M., Jin S. Pseudomonas aeruginosa mediated apoptosis requires the ADP‐ribosylating activity of exoS. Microbiology 2000; 146((Pt 10))2531–41
- Barbieri J. T. Pseudomonas aeruginosa exoenzyme S, a bifunctional type‐III secreted cytotoxin. Int J Med Microbiol 2000; 290: 381–7
- Domenighini M., Rappuoli R. Three conserved consensus sequences identify the NAD‐binding site of ADP‐ribosylating enzymes, expressed by eukaryotes, bacteria and T‐even bacteriophages. Mol Microbiol 1996; 21: 667–74
- Bazan J. F., Koch N. F. Sequence and structural links between distant ADP‐ribosyltransferase families. Adv Exp Med Biol 1997; 419: 99–107
- Glowacki G., Braren R., Firner K., Nissen M., Kuhl M., Reche P., et al. The family of toxin‐related ecto‐ADP‐ribosyltransferases in humans and the mouse. Protein Sci 2002; 11: 1657–70
- Ame J. C., Spenlehauer C., de Murcia G. The PARP superfamily. Bioessays 2004; 26: 882–93
- Otto H., Reche P., Bazan F., Dittmar K., Haag F., Koch‐Nolte F. In silico characterization of the family of PARP‐like poly(ADP‐ribosyl)transferases (pARTs). BMC Genomics 2005; 6: 139
- Koch‐Nolte F., Reche P., Haag F., Bazan F. ADP‐ribosyltransferases: plastic tools for inactivating protein and small molecular weight targets. J Biotech 2001; 92: 81–7
- Pannifer A. D., Wong T. Y., Schwarzenbacher R., Renatus M., Petosa C., Bienkowska J., et al. Crystal structure of the anthrax lethal factor. Nature 2002; 414: 229–33
- Tsuge H., Nagahama M., Nishimura H., Hisatsune J., Sakaguchi Y., Itogawa Y., et al. Crystal structure and site‐directed mutagenesis of enzymatic components from Clostridium perfringens iota‐toxin. J Mol Biol 2003; 325: 471–83
- Haag F., Koch‐Nolte F., Kuhl M., Lorenzen S., Thiele H. G. Premature stop codons inactivate the RT6 genes of the human and chimpanzee species. J Mol Biol 1994; 243: 537–46
- Koch‐Nolte F., Petersen D., Balasubramanian S., Haag F., Kahlke D., Willer T., et al. Mouse T cell membrane proteins Rt6‐1 and Rt6‐2 are arginine/protein mono(ADPribosyl)transferases and share secondary structure motifs with ADP‐ribosylating bacterial toxins. J Biol Chem 1996; 271: 7686–93
- Hara N., Badruzzaman M., Sugae T., Shimoyama M., Tsuchiya M. Mouse Rt6.1 is a thiol‐dependent arginine‐specific ADP‐ ribosyltransferase. Eur J Biochem 1999; 259: 289–94
- Krebs C., Koestner W., Nissen M., Welge V., Parusel I., Malavasi F., et al. Flow cytometric and immunoblot assays for cell surface ADP‐ribosylation using a monoclonal antibody specific for ethenoadenosine. Anal Biochem 2003; 314: 108–15
- Yadollahi‐Farsani M., Kefalas P., Saxty B. A., MacDermot J. Polymorphic forms of human ADP‐ribosyltransferase‐1 differences in their catalytic activities revealed by labeling of membrane‐associated substrates. Eur J Biochem 1999; 262: 342–8
- Zolkiewska A., Moss J. Integrin alpha 7 as substrate for a glycosylphosphatidylinositol‐anchored ADP‐ribosyltransferase on the surface of skeletal muscle cells. J Biol Chem 1993; 268: 25273–6
- Zhao Z., Gruszczynska‐Biegala J., Zolkiewska A. ADP‐ribosylation of integrin alpha7 modulates the binding of integrin alpha7beta1 to laminin. Biochem J 2005; 385((Pt 1))309–17
- Koch‐Nolte F., Glowacki G., Bannas P., Braasch F., Dubberke G., Ortolan E., et al. Use of genetic immunization to raise antibodies recognizing toxin‐related cell surface ADP‐ribosyltransferases in native conformation. Cell Immunol 2005; 236: 66–71
- Levy I., Wu Y. Q., Roeckel N., Bulle F., Pawlak A., Siegrist S., et al. Human testis specifically expresses a homologue of the rodent T lymphocytes RT6 mRNA. Febs Lett 1996; 382: 276–80
- Parusel I., Kahl S., Braasch F., Glowacki G., Halverson G. R., Reid M. E., et al. A panel of monoclonal antibodies recognizing GPI‐anchored ADP‐ribosyltransferase ART4, the carrier of the Dombrock blood group antigens. Cell Immunol 2005; 236: 59–65
- Okazaki I. J., Kim H. J., Moss J. Cloning and characterization of a novel membrane‐associated lymphocyte NAD:arginine ADP‐ribosyltransferase. J Biol Chem 1996; 271: 22052–7
- Glowacki G., Braren R., Cetkovic‐Cvrlje M., Leiter E. H., Haag F., Koch‐Nolte F. Structure, chromosomal localization, and expression of the gene for mouse ecto‐mono(ADP‐ribosyl)transferase ART5. Gene 2001; 275: 267–77
- Weng B., Thompson W. C., Kim H. J., Levine R. L., Moss J. Modification of the ADP‐ribosyltransferase and NAD glycohydrolase activities of a mammalian transferase (ADP‐ribosyltransferase 5) by auto‐ADP‐ribosylation. J Biol Chem 1999; 274: 31797–803
- Oka S., Kato J., Moss J. Identification and characterization of a mammalian 39‐kDa poly(ADP‐ribose) glycohydrolase. J Biol Chem 2006; 281: 705–13
- Lin W., Ame J. C., Aboul‐Ela N., Jacobson E. L., Jacobson M. K. Isolation and characterization of the cDNA encoding bovine poly(ADP‐ribose) glycohydrolase. J Biol Chem 1997; 272: 11895–901
- Patel C. N., Koh D. W., Jacobson M. K., Oliveira M. A. Identification of three critical acidic residues of poly(ADP‐ribose) glycohydrolase involved in catalysis: determining the PARG catalytic domain. Biochem J 2005; 388((Pt 2))493–500
- Adriouch S., Ohlrogge W., Haag F., Koch‐Nolte F., Seman M. Rapid induction of naive T cell apoptosis by ecto‐nicotinamide adenine dinucleotide: requirement for mono(ADP‐ribosyl)transferase 2 and a downstream effector. J Immunol 2001; 167: 196–203
- Liu Z. X., Azhipa O., Okamoto S., Govindarajan S., Dennert G. Extracellular nicotinamide adenine dinucleotide induces T cell apoptosis in vivo and in vitro. J Immunol 2001; 167: 4942–7
- Seman M., Adriouch S., Scheuplein F., Krebs C., Freese D., Glowacki G., et al. NAD‐induced T cell death: ADP‐ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity 2003; 19: 571–82
- Scheuplein F., Adriouch S., Glowacki G., Haag F., Seman M., Koch‐Nolte F. Triggering of T‐cell apoptosis by toxin‐related ecto‐ADP‐ribosyltransferase ART2. Ann N Y Acad Sci 2003; 1010: 296–9
- Ohlrogge W., Haag F., Lohler J., Seman M., Littman D. R., Killeen N., et al. Generation and characterization of ecto‐ADP‐ribosyltransferase ART2.1/ART2.2‐deficient mice. Mol Cell Biol 2002; 22: 7535–42
- Zanovello P., Bronte V., Rosato A., Pizzo P., Di Virgilio F. Responses of mouse lymphocytes to extracellular ATP. II. Extracellular ATP causes cell type‐dependent lysis and DNA fragmentation. J Immunol 1990; 145: 1545–50
- Di Virgilio F., Chiozzi P., Ferrari D., Falzoni S., Sanz J. M., Morelli A., et al. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood 2001; 97: 587–600
- Surprenant A., Rassendren F., Kawashima E., North R. A., Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 1996; 272: 735–8
- North R. A., Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol 2000; 40: 563–80
- Adriouch S., Dox C., Welge V., Seman M., Koch‐Nolte F., Haag F. Cutting Edge: A Natural P451L Mutation in the Cytoplasmic Domain Impairs the Function of the Mouse P2X7 Receptor. J Immunol 2002; 169: 4108–12
- Kahl S., Nissen M., Girisch R., Duffy T., Leiter E. H., Haag F., et al. Metalloprotease‐mediated shedding of enzymatically active mouse ecto‐ADP‐ribosyltransferase ART2.2 upon T cell activation. J Immunol 2000; 165: 4463–9
- Koch‐Nolte F., Haag F., Kastelein R., Bazan F. Uncovered: the family relationship of a T‐cell‐membrane protein and bacterial toxins. Immunol Today 1996; 17: 402–5
- Bruno T. F., Woods D. E., Mody C. H. Exoenzyme S from Pseudomonas aeruginosa induces apoptosis in T lymphocytes. J Leukoc Biol 2000; 67: 808–16
- Simons K., Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 2000; 1: 31–9
- Pike L. J. Lipid rafts: heterogeneity on the high seas. Biochem J 2004; 378((Pt 2))281–92
- Horejsi V. The roles of membrane microdomains (rafts) in T cell activation. Immunol Rev 2003; 191: 148–64
- Janes P. W., Ley S. C., Magee A. I. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol 1999; 147: 447–61
- Marmor M. D., Julius M. Role for lipid rafts in regulating interleukin‐2 receptor signaling. Blood 2001; 98: 1489–97
- Marwali M. R., Rey‐Ladino J., Dreolini L., Shaw D., Takei F. Membrane cholesterol regulates LFA‐1 function and lipid raft heterogeneity. Blood 2003; 102: 215–22
- Bromley S. K., Burack W. R., Johnson K. G., Somersalo K., Sims T. N., Sumen C., et al. The immunological synapse. Annu Rev Immunol 2001; 19: 375–96
- Okazaki I. J., Kim H. J., McElvaney N. G., Lesma E., Moss J. Molecular characterization of a glycosylphosphatidylinositol‐linked ADP‐ ribosyltransferase from lymphocytes. Blood 1996; 88: 915–21
- Parkin E. T., Tan F., Skidgel R. A., Turner A. J., Hooper N. M. The ectodomain shedding of angiotensin‐converting enzyme is independent of its localisation in lipid rafts. J Cell Sci 2003; 116((Pt 15))3079–87
- Cordy J. M., Hussain I., Dingwall C., Hooper N. M., Turner A. J. Exclusively targeting beta‐secretase to lipid rafts by GPI‐anchor addition up‐regulates beta‐site processing of the amyloid precursor protein. Proc Natl Acad Sci U S A 2003; 100: 11735–40
- Bannas P., Adriouch S., Kahl S., Braasch F., Haag F., Koch‐Nolte F. Activity and specificity of toxin‐related mouse T cell ecto‐ADP‐ribosyltransferase ART2.2 depends on its association with lipid rafts. Blood 2005; 105: 3663–70
- Lund F. E., Cockayne D. A., Randall T. D., Solvason N., Schuber F., Howard M. C. CD38: a new paradigm in lymphocyte activation and signal transduction. Immunol Rev 1998; 161: 79–93
- Schuber F., Lund F. E. Structure and enzymology of ADP‐ribosyl cyclases: conserved enzymes that produce multiple calcium mobilizing metabolites. Curr Mol Med 2004; 4: 249–61
- Partida‐Sanchez S., Goodrich S., Kusser K., Oppenheimer N., Randall T. D., Lund F. E. Regulation of dendritic cell trafficking by the ADP‐ribosyl cyclase CD38: impact on the development of humoral immunity. Immunity 2004; 20: 279–91
- Partida‐Sanchez S., Cockayne D. A., Monard S., Jacobson E. L., Oppenheimer N., Garvy B., et al. Cyclic ADP‐ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat Med 2001; 7: 1209–16
- Cockayne D. A., Muchamuel T., Grimaldi J. C., Muller‐Steffner H., Randall T. D., Lund F. E., et al. Mice deficient for the ecto‐nicotinamide adenine dinucleotide glycohydrolase CD38 exhibit altered humoral immune responses. Blood 1998; 92: 1324–33
- Zimmermann H., Braun N. Ecto‐nucleotidases—molecular structures, catalytic properties, and functional roles in the nervous system. Prog Brain Res 1999; 120: 371–85
- Krebs C., Adriouch S., Braasch F., Koestner W., Leiter E. H., Seman M., et al. CD38 controls ADP‐ribosyltransferase‐2‐catalyzed ADP‐ribosylation of T cell surface proteins. J Immunol 2005; 174: 3298–305
- Bruzzone S., Guida L., Zocchi E., Franco L., De Flora A. Connexin 43 hemi channels mediate Ca2+‐regulated transmembrane NAD+ fluxes in intact cells. FASEB J 2001; 15: 10–2
- Mak T. W., Penninger J. M., Ohashi P. S. Knockout mice: a paradigm shift in modern immunology. Nat Rev Immunol 2001; 1: 11–9
- Hickman‐Davis J. M. Implications of mouse genotype for phenotype. News Physiol Sci 2001; 16: 19–22
- Kanaitsuka T., Bortell R., Stevens L. A., Moss J., Sardinha D., Rajan T. V., et al. Expression in BALB/c and C57BL/6 mice of Rt6‐1 and Rt6‐2 ADP‐ ribosyltransferases that differ in enzymatic activity: C57BL/6 Rt6‐1 is a natural transferase knockout. J Immunol 1997; 159: 2741–9
- Koch‐Nolte F., Duffy T., Nissen M., Kahl S., Ablamunits V., Leiter E. H., et al. A new monoclonal antibody detects a developmentally regulated mouse ecto ADP‐ribosyltransferase on T cells: subset distribution, inbred strain variation, and modulation upon T cell activation. J Immunol 1999; 163: 6014–22
- Aswad F., Kawamura H., Dennert G. High sensitivity of CD4+CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J Immunol 2005; 175: 3075–83
- Kawamura H., Aswad F., Minagawa M., Govindarajan S., Dennert G. P2X7 Receptors Regulate NKT Cells in Autoimmune Hepatitis. J Immunol 2006; 176: 2152–60
- Solle M., Labasi J., Perregaux D. G., Stam E., Petrushova N., Koller B. H., et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem 2001; 276: 125–32
- Labasi J. M., Petrushova N., Donovan C., McCurdy S., Lira P., Payette M. M., et al. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol 2002; 168: 6436–45
- Chen J., Chen Y‐G., Reifsnyder P. C., Schott W. H., Lee C‐H., Osborne M., et al. Targeted disruption of CD38 accelerates autoimmune diabetes in NOD/Lt mice by enhancing autoimmunity in an ART2‐dependent fashion. J Immunol 2006; 176, in press
- Han M. K., Cho Y. S., Kim Y. S., Yim C. Y., Kim U. H. Interaction of two classes of ADP‐ribose transfer reactions in immune signaling. J Biol Chem 2000; 275: 20799–805
- Verhoef P. A., Estacion M., Schilling W., Dubyak G. R. P2X7 receptor‐dependent blebbing and the activation of Rho‐effector kinases, caspases, and IL‐1 beta release. J Immunol 2003; 170: 5728–38
- Guse A. H. Regulation of calcium signaling by the second messenger cyclic adenosine diphosphoribose (cADPR). Curr Mol Med 2004; 4: 239–48
- Deaglio S., Vaisitti T., Bergui L., Bonello L., Horenstein A. L., Tamagnone L., et al. CD38 and CD100 lead a network of surface receptors relaying positive signals for B‐CLL growth and survival. Blood 2005; 105: 3042–50
- la Sala A., Ferrari D., Di Virgilio F., Idzko M., Norgauer J., Girolomoni G. Alerting and tuning the immune response by extracellular nucleotides. J Leukoc Biol 2003; 73: 339–43
- Haag F., Freese D., Scheuplein F., Ohlrogge W., Adriouch S., Seman M., et al. T Cells of Different Developmental Stages Differ in Sensitivity to Apoptosis Induced by Extracellular NAD. Dev Immunol 2002; 9: 197–202
- Sun J., Maresso A. W., Kim J. J., Barbieri J. T. How bacterial ADP‐ribosylating toxins recognize substrates. Nat Struct Mol Biol 2004; 11: 868–76
