Abstract
The molecular circadian clock entrains biological rhythms to a 24‐hour schedule. Aspects of cardiovascular physiology and, indeed, the incidence of myocardial infarction and stroke are also subject to diurnal variation. The use of rodent models of disrupted clock function has begun to elucidate the role of the molecular clock in the pathophysiology of cardiovascular and metabolic disease.
Yet taught by time, my heart has learned to glow for other's good and melt at other's woe.
Homer.
| Abbreviations | ||
| ACTH | = | adrenocorticotrophic hormone |
| Epi | = | epinephrine |
| GR | = | glucocorticoid receptor |
| HPA | = | hypothalamic‐pituitary‐adrenal |
| KO | = | knockout |
| MAP | = | mean arterial pressure |
| NO | = | nitric oxide |
| NSAID | = | non‐steroidal anti‐inflammatory drug |
| PAI | = | plasminogen activator inhibitor |
| PAS | = | Per Arnt Sim |
| SUMO | = | small ubiquitin‐like modifier |
| tPA | = | tissue plasminogen activator |
Introduction
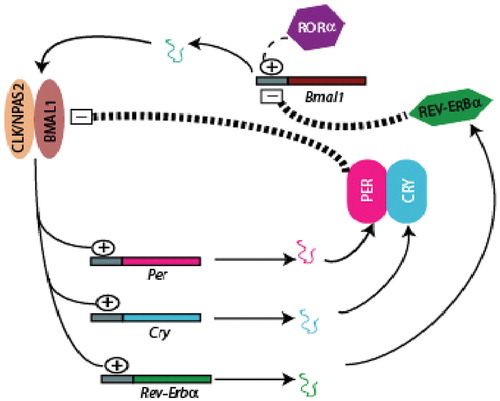
Twenty‐four‐hour changes in light and temperature present a formidable challenge to biological systems. Organisms have evolved to adapt to this changing environment with molecular oscillators that allow the tracking of time. These oscillators are termed collectively the ‘molecular clock’; they drive and sustain circadian (approximately one‐day) rhythms in physiology and behavior. Distinct from circadian rhythms, others of varied periodicity also pertain Citation1, although how they interact with circadian biology and how they are regulated in mammals is poorly understood. While interest in circadian variability in physiological function is long‐standing, the field was largely descriptive until Konopa and Benzer Citation2 identified a chromosomal region controlling the period of eclosion time in Drosophila in 1971. Since that time, a series of molecular components of the clock have been identified. In mammals, the basic helix loop helix (bHLH) transcription factors BMAL1, CLOCK and NPAS2 act as activators, while the PAS proteins PERCitation1‐3 and CRYCitation1‐2 act as repressors. Circadian gene expression exhibits peaks and troughs within a 24‐hour cycle, resulting from a complex interplay of transcription and translational feedback loops. In a highly simplified model (Figure ), CLOCK:BMAL1 or NPAS2:BMAL1 heterodimers bind to E‐box consensus sequences in the Per and Cry genes. Their translated products can, by feedback inhibition, autoregulate their own expression. A lag time then occurs until nuclear PER/CRY protein levels fall, allowing the heterodimeric transcription factors to initiate transcription, thus restarting the cycle approximately every 24 hours. Circadian oscillations of BMAL1, and perhaps also NPAS2, are driven by the two orphan nuclear receptors, RORα and REV‐ERBα, both of which are transcribed by BMAL1 and act as positive and negative regulators, respectively. Therefore, through transcriptional/translational interlocking feedback loops, all components of the oscillator listed above (except CLOCK) cycle. This, in turn, regulates a number of output genes. More comprehensive reviews on the molecular oscillator are available Citation3, Citation4.
Figure 1 The clockwork mechanism. The molecular circadian model is comprised of interlocking transcriptional and translational feedback loops. CLOCK:BMAL1 or NPAS2:BMAL1 heterodimerize at the beginning of the light cycle and activate gene transcription of perCitation1‐3, cryCitation1‐2 and rev‐erbα. PER/CRY complex can feedback on transcription factors and inhibit their expression—once nuclear levels of PER/CRY drop, the cycle is restarted. Oscillations of bmal1 are generated by opposing actions of REV‐ERBα and RORα, which inhibits or activates transcription. (Adapted with permission from Lakin‐Thomas Citation8.)
Posttranslational modifications act on clock proteins to control finely circadian rhythms. Clock proteins undergo robust circadian changes in phosphorylation, which affects transcriptional activity, stability and cellular localization Citation5. SUMOylation of BMAL1 affects its rhythmic expression Citation6 and dimerization of BMAL1 and CLOCK causes phosphorylation and directs nuclear accumulation of the complex Citation7. Interestingly, the central tenet of the clockwork model—the dependence of rhythmic transcription of core clock genes—has been called into question in many nonmammalian organisms, indicating that rhythmic mRNA may not be necessary for oscillating protein levels Citation8. These studies suggest that rhythmic protein phosphorylation is perhaps more fundamental to generating output oscillations in protein levels.
Familial advanced sleep phase syndrome is a rare Mendelian inherited human syndrome of clock dysfunction, which is characterized by early sleep times and early morning awakening. A mutation in a phosphorylation site within the casein kinase I binding domain of PER2 or the casein kinase I delta gene, both cause a shortening of period length and give rise to the disease. This was the first human circadian rhythm variant to be well characterized. Evidence also links polymorphisms in clock genes with psychiatric disorders such as seasonal affective disorder (SAD) Citation9 and alcohol consumption Citation10.
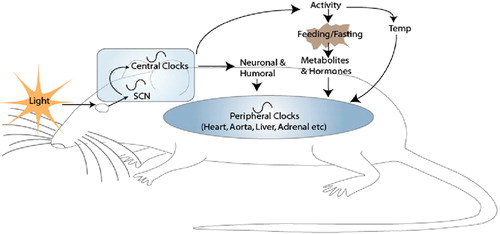
Surgical ablation of the suprachiasmatic nucleus (SCN), a remarkable paired nuclear structure in the hypothalamus, ablates all hormonal and activity rhythms Citation11. In mammals, the SCN is perceived to be the ‘master clock’, entraining tissue clocks in the periphery and, adjusted by photic input via the retinohypothalamic tract, maintaining rhythms on a 24‐hour cycle (Figure ). The SCN can signal to the medial cortex around the SCN, the hypothalamic‐pituitary‐adrenal (HPA) axis, pineal and adrenal gland and to peripheral sites through multisynaptic autonomic connections Citation12. Peripheral clocks receive entraining signals from the SCN in the form of neural and/or humoral signals which they then might modulate to amplify or dampen a central signal according to local demands. Until recently, peripheral clocks were thought to rely solely on SCN‐driven signals; however, peripheral tissues explanted and maintained in culture demonstrate continued oscillations of per2 for up to 20 days, and SCN ablation does not abolish this circadian oscillation Citation13. Thus, the periphery has the potential to function autonomously, perhaps with periodic phase adjustment from the SCN.
Figure 2 Central and peripheral oscillators. The retinohypothalamic tract relays photic information from the eye to the suprachiasmatic nucleus(SCN). The SCN alone or via central signaling generates a number of oscillatory neuronal and humoral signals (e.g. melatonin, catecholamines, glucocorticoids, vasopressin) which entrain peripheral clocks. Peripheral clocks can function independently, but require SCN driven signals to coordinate the appropriate phase of gene expression. Feeding and temperature, gated by SCN‐driven locomotor activity, can entrain peripheral oscillators. Restricted feeding of rodents uncouples oscillations in peripheral tissues from the SCN.
Glucocorticoids may function as hormonal entraining signals; however, there is likely redundancy amongst such signals, as circadian phase in peripheral tissues remains unaffected both in glucocorticoid receptor (GR) deficiency in hepatocytes and in kidneys and livers of adrenalectomized mice Citation14.
We identified the role of retinoic acid in resetting the phase of circadian expression in the vascular clock Citation15. Feeding is the most dominant of the entraining signals identified to date but does not affect the SCN Citation16. This has been observed in rodents, where daytime restricted feeding inverts circadian phase in peripheral tissues, but circadian phase in the SCN remains unaffected. It is believed that the SCN indirectly controls feeding through regulation of activity (Figure ); however, the mechanisms are still unclear. Restricted feeding induces circadian gene expression in the dorsomedial hypothalamic nucleus (DMH) Citation17 and ablation of this area, but not the SCN, blocks food entrainable circadian rhythms Citation18. The DMH may signal to orexin‐producing neurons, neuropeptides involved in the regulation of sleep and feeding behavior. These studies begin to suggest that the DMH may integrate the SCN with food anticipatory behavior, feeding and peripheral signaling.
Diurnal variation in cardiovascular function and dysfunction
Many common cardiovascular events, such as sudden cardiac death, myocardial infarction, unstable angina, ventricular tachycardia and ischemic and hemorrhagic stroke, are subject to diurnal variation, peaking in the early morning hours. For example, the risk of myocardial infarction increases by 40% Citation19, of sudden cardiac death by 29% Citation19 and of the onset of a stroke by 49% for the period 0600–1200 hrs when compared with 24 hour averages Citation20. These cardiovascular events also display seasonal variations Citation21 (circannual rhythms), which correlate with higher plasma fibrinogen levels in the colder winter months. Disruption of normal circadian rhythms via nighttime shift work or prolonged air travel may also increase the risk of cardiovascular events Citation22 and, in the former case, the emergence of biomarkers of the metabolic syndrome Citation23.
Key messages
The molecular clock, composed of a number of transcriptional and translational feedback loops, drives circadian (24‐hour) gene expression and physiology.
In addition to the suprachiasmatic nucleus in the brain, peripheral clocks exist which may function to both modulate a central signal and to adjust to local demands.
Cardiovascular events display a diurnal variation with increased number of events in the early morning hours; this may correlate with oscillations in gene expression, circulating hormones, blood pressure and the ability to respond to environmental stress.
The circadian transcriptome
Roughly 5%–10% of the transcriptome in each tissue is under circadian control Citation24. This exhibits some tissue specificity Citation25, but may still underestimate the true impact of the molecular clock on tissue function. Thus, genes are assigned to be oscillators based on their satisfying a bioinformatic paradigm that assesses the sinusoidal variability of gene expression over time. Depending on the stringency of the criteria for assignment, true oscillators may be missed. A further level of complexity is suggested by recent studies of the circadian proteome Citation26. The oscillations of genes and their corresponding proteins were often dissociated. Analysis of gene expression in mouse aorta (and perivascular fat) identified as oscillators, genes which segregated into discrete functional cassettes: vascular integrity and the response to injury, glucose and lipid metabolism and adipocyte maturation—all elements of the metabolic syndrome Citation27. In accordance with cyclical variation of transcripts involved in glucose homeostasis, we observe that the response to insulin‐induced hypoglycemia and gluconeogenesis is impaired with clock dysfunction. Interestingly, high‐fat feeding in wild type animals amplifies the insulin and glucose response but clock mutation reverts the phenotype to that observed with regular feed Citation28. Clock mutant mice were protected from diet‐induced obesity which involved dysregulation of dietary fat digestion and absorption Citation29, and BMAL1 deletion retarded adipocyte differentiation Citation30. However, a subsequent report characterized Clock mutant mice as having a metabolic syndrome phenotype Citation31. Although differences in findings maybe attributed to different mouse strains and experimental procedures, collectively, these studies implicate the molecular clock in some aspects of energy control and metabolism.
Neurohumoral influences on the vascular clock
The reasons for the morning increase in cardiovascular events have been long discussed. They include both a ‘clock‐independent’, but time‐dependent variation in exposure to environmental stress and an exaggeration of ‘clock‐dependent’ oscillation in vasoactive hormones, several of which vary in their circulating levels according to the time of day.
Glucocorticoids, of which cortisol in humans Citation32 and corticosterone in rodents Citation28 are the most potent, have robust diurnal rhythms, reaching a peak in plasma from 0400–0800 hrs and a nadir at 2300–2400 hrs; there is a particular increase upon awakening. The cortisol rhythm only partially adapts to the night‐oriented work schedule in shift workers Citation33. Increases in cortisol in the morning hours could affect vascular smooth muscle cell (VSMC) tone by potentiating the arterial contractile sensitivity to catecholamines, such as norepinephrine (NE) and epinephrine (Epi), which themselves subserve a diurnal rhythm Citation34 and vascular resistance through glucocorticoid receptors. Indeed, levels of the glucocorticoid receptor are also known to vary over the 24‐hr cycle both in tissues and leukocytes Citation35. Glucocorticoids can suppress the induced production of vasodilators, such as prostacyclin and nitric oxide (NO) in the endothelium Citation36. However, glucocorticoids are also known to suppress inflammation and regulate vascular permeability through control on tight junction proteins.
The diurnal variation in cortisol and that of the awakening response may be regulated differentially. The cortisol response upon awakening in humans with hippocampal damage is abolished, whereas the remainder of the diurnal cycle is unaffected Citation37. This suggests the existence of separate mechanisms to control the steroid response to an asynchronous cue— such as environmental stress—and their diurnal variation. In animal models, there is molecular evidence consistent with such discordance. For example, the corticosterone responses to both insulin‐induced hypoglycemia Citation28 and restraint Citation38 are retained in BMAL1 knockout (KO) animals, even though these animals display deficiencies in glucose homeostasis and adrenergic activation, respectively, in response to these manipulations Citation28, Citation38. Indeed, light activates the adrenal gland and causes glucocorticoid release via an adrenocorticotrophic hormone (ACTH)‐independent route Citation39. Therefore regulation of corticosterone may be regulated through SCN‐dependent and independent pathways, and may vary according to stimulus and time of day.
Similarly, both plasma NE and Epi are responsive both to time of day and environmental stress, in both humans Citation40 and mice Citation41, issues of potential relevance to the incidence of cardiovascular events. Acutely, catecholamines might contribute to vasoconstriction, endothelial dysfunction and platelet activation in such patients. More chronic elevation of basal levels and/or amplification of diurnal variation may influence atherogenesis, the response to vascular injury and cardiac pump function Citation42, Citation43. Animal models suggest that the positive components of the molecular clock can affect expression of key rate‐limiting enzymes in catecholamine generation such as tyrosine hydroxylaseCitation44, comt, pnmt and maoBCitation38. Furthermore, we have found that adrenergic signaling can affect clock gene expression in vitro: however, loss of both Epi and NE by deletion of dopamine‐beta hydroxylase does not affect peripheral rhythms in vivo (Reilly D, FitzGerald GA, unpublished observation). However, plasma dopamine is elevated in these mice and may substitute as an entraining agonist and regulate circadian function in the periphery Citation45. It is important to note that other neurohumoral factors, such as those involved in the renin‐angiotensin pathway, vasopressin and atrial natriuretic hormone all describe circadian variation and may also influence the timing of cardiovascular events. The impact of selective approaches to disruption of the molecular clock on these systems remains to be defined.
Diurnal variation in blood pressure
Circadian variations in blood pressure and heart rate are the most intensely studied circadian rhythms in cardiovascular physiology. A variety of descriptive approaches to phenotypic segregation have been employed. These include quantitation of the rise in blood pressure from its nadir to its maximum, ‘the morning blood pressure surge’ (MBPS), and the presence or absence of a significant decline of blood pressure during sleep, ‘dipping’.
Human blood pressure begins to increase before awakening around 0400 hrs, with highest values around mid morning and a trough around midnight. There is a sharp increase in blood pressure upon arousal from sleep and assumption of the upright posture. Activation of the sympathetic nervous system is an important mediator of the morning surge. A study on patients with varying degrees of progressive autonomic failure (affecting sympathetic and parasympathetic function) due to familial amyloid polyneuropathy displayed a progressive blunting of the circadian blood pressure (BP) rhythm with disease progression Citation46. The MBPS in community‐dwelling Japanese subjects is an independent predictor of stroke in hypertensive patients independently of their 24‐hour blood pressure levels and is associated with cardiac hypertrophy even when adjusted for physical activity Citation47, Citation48. Another study found that MBPS correlated more strongly with indices of target organ damage than did the morning surge of systolic blood pressure or daytime blood pressure variability Citation49. Therefore it appears that the morning surge in BP, irrespective of presence or absence of hypertension, relates directly to the incidence and outcomes of cardiovascular events.
Most normotensive individuals have a greater that 10% reduction in nighttime systolic blood pressure when compared with mean daytime values—‘dipping’. However, some patients do not exhibit this nocturnal dip and are at increased risk of developing hypertension Citation50, Citation51 and consequent end organ damage Citation52. For example, the absence of dipping and the absolute nighttime diastolic blood pressure were both strong predictors of heart failure (CHF) in a long‐term follow‐up study of elderly men Citation53.
The molecular clock and pressor control
Although always assumed, evidence to support the direct role of the molecular clock in blood pressure regulation has been limited. The recent use of telemetry systems suitable for small animals has facilitated such investigations. This system allows for recording of systolic BP, diastolic BP, heart rate, pulse pressure and activity in unrestrained animals. Both mean blood pressure and its variability over time are affected differentially by disruption of the positive (BMAL1, CLOCK and NPAS2) Citation38 or negative components (CRY1 and CRY2) of the oscillator Citation54. Indeed, the absolute level of blood pressure and the baroreflex response to hypotension are differentially affected by deletion of BMAL1 or NPAS2 disruption on the one hand, versus deletion of both CRY1 and CRY2 on the other Citation54. Another study revealed that disruption of the baroreflex in rats results in loss of circadian variation in mean arterial pressure (MAP) Citation55. We have previously reported that the baroreflex response, along with blood pressure, is subject to diurnal variation in humans Citation56.
Circadian fluctuations in fibrinolytic efficiency
Platelet aggregation and resultant thrombus formation subsequent to plaque rupture is fundamental to vascular occlusions, and resultant infarction and ischemia. Platelets are anucleate cells with a very limited capacity for de novo protein synthesis Citation57 and therefore are unlikely to contain endogenous oscillators. Although there have been reports of diurnal variation in platelet aggregability ex vivoCitation58, the relationship of these observations (prone as they are to artifact ex vivo, attributable to diurnal variation in extracellular volume in vivo) to actual platelet activation in vivo is unknown. However, factors external to the platelet—such as plasminogen activator inhibitor (PAI)‐1 and tissue plasminogen activator (tPA), which do indeed oscillate—may influence platelet activation in vivo. Although this issue has not been addressed rigorously, there are some suggestions of clinical relevance. For example, platelets become more aggregable by conventional agonists in vitro when they are loaded with cholesterol. Circulating cholesterol, platelet number and thromboxane generation in vivo peak in late afternoon Citation59 and may contribute to the smaller secondary peak in cardiovascular events that occurs at this time Citation60.
Pai‐1 gene expression is predominantly regulated by the molecular clock. The Pai‐1 gene contains two consensus E‐boxes in its promoter, and is driven by the endothelial specific circadian heterodimer BMAL2 Citation61 (also known as CLIF):CLOCK, and also BMAL1:CLOCK Citation62. Circadian expression of pai‐1 was severely blunted in hearts of Clock mutant mice Citation63. Interestingly, the efficacy of thrombolytic therapy is decreased, coincident with peak levels of pai‐1 in the early morning hours Citation64.
Diurnal variation and the response to stress
While circadian rhythms in many organisms may subserve a dominant role in physiology, their main function in mammals may be to condition the response to environmental stimuli. Genotoxic stress, induced by chemotherapy, radiation and anticancer drugs, is conditioned by time of therapy and is configured on the functional status of the CLOCK:BMAL1 heterodimer Citation65. Both mice Citation66 and humans Citation67 show a diurnal variation in sensitivity to chemotherapeutic agents. Interestingly, both the adrenergic and pressor responses to the stress of standardized restraint depend on timing. Superimposition of the stress on the nadir of the blood pressure rhythm results in the maximal absolute pressor response. However, deletion of BMAL1 revealed an unexpected role for the clock in the stress response, which was reduced dramatically, irrespective of timing Citation38. These data raise the possibility that the clock may influence the temporal incidence of clinical cardiovascular events, not only by regulating the magnitude of the early morning rise in blood pressure and other factors as discussed, but also the extent of the pressor response to environmental stress, which itself is conditioned by timing.
Chronotherapeutics
The aim of chronotherapy is to utilize rhythms of disease determinants and try to synchronize concentration and dosing of medications to increase their efficacy and safety. The clinical use of chronotherapy is attracting more attention as the role of the clock in regulating both pharmacodynamics and pharmacokinetics is increasingly appreciated. For example, P450 isozymes and transporters are amongst the most striking oscillatory transcripts in liver Citation68. Pharmacodynamic effects of non‐steroidal anti‐inflammatory drugs (NSAIDs) Citation69, aspirin Citation70, and anticancer drugs Citation67 are well established to be influenced by time of dosing. Surprisingly, while these effects have, in some cases, been long appreciated Citation71, this has had minimal impact on practical therapeutics. Perhaps the refinement of our understanding of the diverse and pleiotropic impacts of the clock on physiology and disease afforded by rodent models will lead to a renewed evaluation of its role in therapy and the design of appropriate clinical trials to asses its importance.
Conclusions
Molecular deletion and mutation of transcription factors involved in the molecular clock have already revealed unexpected roles for this mechanism in discrete aspects of physiology—hemodynamics, coagulation, glucose and lipid metabolism, inflammation and aging—which impact on cardiovascular disease. The appreciation of the existence of distinct molecular clocks, which have the capacity for entrained and autonomous functions Citation72, in endothelium, vascular smooth muscle cells, perivascular fat and cardiomyocytes, suggests a complex interrelationship by which oscillations in cardiovascular function might be determined. Added to this, the clock may influence in a fundamental and similarly diverse way, drug response. Presently, we understand little of how central and peripheral clock function is integrated, how the distinct clocks in adjacent cardiovascular cells interact, and whether genetic variation in clock genes condition a predisposition to cardiovascular disease. However, the emergence of increasingly diverse rodent models of clock function and an emerging interest in clock genetics Citation73 suggest that the time is ripe for this field to impact on translational medicine.
References
- Schibler U., Naef F. Cellular oscillators: rhythmic gene expression and metabolism. Curr Opin Cell Biol 2005; 17: 223–9
- Konopka R. J., Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A 1971; 68: 2112–6
- Hirayama J., Sassone‐Corsi P. Structural and functional features of transcription factors controlling the circadian clock. Curr Opin Genet Dev 2005; 15: 548–56
- Bell‐Pedersen D., Cassone V. M., Earnest D. J., Golden S. S., Hardin P. E., Thomas T. L., et al. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet 2005; 6: 544–56
- Lee C., Etchegaray J. P., Cagampang F. R., Loudon A. S., Reppert S. M. Posttranslational mechanisms regulate the Mammalian circadian clock. Cell 2001; 107: 855–67
- Cardone L., Hirayama J., Giordano F., Tamaru T., Palvimo J. J., Sassone‐Corsi P. Circadian clock control by SUMOylation of BMAL1. Science 2005; 309: 1390–4
- Kondratov R. V., Chernov M. V., Kondratova A. A., Gorbacheva V. Y., Gudkov A. V., Antoch M. P. BMAL1‐dependent circadian oscillation of nuclear CLOCK: posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes Dev 2003; 17: 1921–32
- Lakin‐Thomas P. L. Transcriptional feedback oscillators: maybe, maybe not. J Biol Rhythms 2006; 21: 83–92
- Johansson C., Willeit M., Smedh C., Ekholm J., Paunio T., Kieseppa T., et al. Circadian clock‐related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology 2003; 28: 734–9
- Spanagel R., Pendyala G., Abarca C., Zghoul T., Sanchis‐Segura C., Magnone M. C., et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med 2005; 11: 35–42
- Weaver D. R. The suprachiasmatic nucleus: a 25‐year retrospective. J Biol Rhythms 1998; 13: 100–12
- Bartness T. J., Song C. K., Demas G. E. SCN efferents to peripheral tissues: implications for biological rhythms. J Biol Rhythms 2001; 16: 196–204
- Yoo S. H., Yamazaki S., Lowrey P. L., Shimomura K., Ko C. H., Buhr E. D., et al. PERIOD2::LUCIFERASE real‐time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A 2004; 101: 5339–46
- Le Minh N., Damiola F., Tronche F., Schutz G., Schibler U. Glucocorticoid hormones inhibit food‐induced phase‐shifting of peripheral circadian oscillators. EMBO J 2001; 20: 7128–36
- McNamara P., Seo S. B., Rudic R. D., Sehgal A., Chakravarti D., FitzGerald G. A. Regulation of CLOCK and MOP4 by nucear hormone receptors in the vasculature: A humoral mechanism to reset a peripheral clock. Cell 2001; 105: 877–889
- Damiola F., Le Minh N., Preitner N., Kornmann B., Fleury‐Olela F., Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 2000; 14: 2950–61
- Mieda M., Williams S. C., Richardson J. A., Tanaka K., Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food‐entrainable circadian pacemaker. Proc Natl Acad Sci U S A 2006; 103: 12150–5
- Gooley J. J., Schomer A., Saper C. B. The dorsomedial hypothalamic nucleus is critical for the expression of food‐entrainable circadian rhythms. Nat Neurosci 2006; 9: 398–407
- Cohen M. C., Rohtla K. M., Lavery C. E., Muller J. E., Mittleman M. A. Meta‐analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol 1997; 79: 1512–6
- Elliott W. J. Circadian variation in the timing of stroke onset: a meta‐analysis. Stroke 1998; 29: 992–6
- Manfredini R., Boari B., Smolensky M. H., Salmi R., Gallerani M., Guerzoni F., et al. Seasonal variation in onset of myocardial infarction—a 7‐year single‐center study in Italy. Chronobiol Int 2005; 22: 1121–35
- Tuchsen F., Hannerz H., Burr H. A 12 year prospective study of circulatory disease among Danish shift workers. Occup Environ Med 2006; 63: 451–5
- Karlsson B., Knutsson A., Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med 2001; 58: 747–52
- Albrecht U., Eichele G. The mammalian circadian clock. Curr Opin Genet Dev 2003; 13: 271–7
- Duffield G. E. DNA microarray analyses of circadian timing: the genomic basis of biological time. J Neuroendocrinol 2003; 15: 991–1002
- Reddy A. B., Karp N. A., Maywood E. S., Sage E. A., Deery M., O'Neill J. S., et al. Circadian orchestration of the hepatic proteome. Curr Biol 2006; 16: 1107–15
- Rudic R. D., McNamara P., Reilly D., Grosser T., Curtis A. M., Price T. S., et al. Bioinformatic analysis of circadian gene oscillation in mouse aorta. Circulation 2005; 112: 2716–24
- Rudic R. D., McNamara P., Curtis A. M., Boston R. C., Panda S., Hogenesch J. B., et al. BMAL1 and CLOCK, Two Essential Components of the Circadian Clock, Are Involved in Glucose Homeostasis. PLoS Biol 2004; 2: e377
- Oishi K., Atsumi G., Sugiyama S., Kodomari I., Kasamatsu M., Machida K., et al. Disrupted fat absorption attenuates obesity induced by a high‐fat diet in Clock mutant mice. FEBS Lett 2006; 580: 127–30
- Shimba S., Ishii N., Ohta Y., Ohno T., Watabe Y., Hayashi M., et al. Brain and muscle Arnt‐like protein‐1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci U S A 2005; 102: 12071–6
- Turek F. W., Joshu C., Kohsaka A., Lin E., Ivanova G., McDearmon E., et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005; 308: 1043–5
- Edwards S., Clow A., Evans P., Hucklebridge F. Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci 2001; 68: 2093–103
- Hennig J., Kieferdorf P., Moritz C., Huwe S., Netter P. Changes in cortisol secretion during shiftwork: implications for tolerance to shiftwork?. Ergonomics 1998; 41: 610–21
- Sauerbier I., von Mayersbach H. Circadian variation of catecholamines in human blood. Horm Metab Res 1977; 9: 529–30
- Xu R. B., Liu Z. M., Zhao Y. A study on the circadian rhythm of glucocorticoid receptor. Neuroendocrinology 1991; 53 Suppl 1: 31–6
- Yang S., Zhang L. Glucocorticoids and vascular reactivity. Curr Vasc Pharmacol 2004; 2: 1–12
- Buchanan T. W., Kern S., Allen J. S., Tranel D., Kirschbaum C. Circadian regulation of cortisol after hippocampal damage in humans. Biol Psychiatry 2004; 56: 651–6
- Curtis A. M., Price T. S., Reilly D., Kapoor S., FitzGerald G. A. Circadian variation of blood pressure and the vascular response to asynchronous stress. Manuscript submitted.
- Ishida A., Mutoh T., Ueyama T., Bando H., Masubuchi S., Nakahara D., et al. Light activates the adrenal gland: Timing of gene expression and glucocorticoid release. Cell Metab 2005; 2: 297–307
- Wisser H., Breuer H. Circadian changes of clinical chemical and endocrinological parameters. J Clin Chem Clin Biochem 1981; 19: 323–37
- Fu L., Patel M. S., Bradley A., Wagner E. F., Karsenty G. The molecular clock mediates leptin‐regulated bone formation. Cell 2005; 122: 803–15
- Zhang H., Faber J. E. Trophic effect of norepinephrine on arterial intima‐media and adventitia is augmented by injury and mediated by different alpha1‐adrenoceptor subtypes. Circ Res 2001; 89: 815–22
- Julius S. Corcoran Lecture. Sympathetic hyperactivity and coronary risk in hypertension. Hypertension 1993; 21((Pt 2))886–93
- McClung C. A., Sidiropoulou K., Vitaterna M., Takahashi J. S., White F. J., Cooper D. C., et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A 2005; 102: 9377–81
- Yujnovsky I., Hirayama J., Doi M., Borrelli E., Sassone‐Corsi P. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK:BMAL1. Proc Natl Acad Sci U S A 2006; 103: 6386–91
- Carvalho M. J., van Den Meiracker A. H., Boomsma F., Lima M., Freitas J., Veld A. J., et al. Diurnal blood pressure variation in progressive autonomic failure. Hypertension 2000; 35: 892–7
- Kario K., Pickering T. G., Umeda Y., Hoshide S., Hoshide Y., Morinari M., et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation 2003; 107: 1401–6
- Kaneda R., Kario K., Hoshide S., Umeda Y., Hoshide Y., Shimada K. Morning blood pressure hyper‐reactivity is an independent predictor for hypertensive cardiac hypertrophy in a community‐dwelling population. Am J Hypertens 2005; 18((Pt 1))1528–33
- Polonia J., Amado P., Barbosa L., Nazare J., Silva J. A., Bertoquini S., et al. Morning rise, morning surge and daytime variability of blood pressure and cardiovascular target organ damage. A cross‐sectional study in 743 subjects. Rev Port Cardiol 2005; 24: 65–78
- Verdecchia P., Porcellati C., Schillaci G., Borgioni C., Ciucci A., Battistelli M., et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension 1994; 24: 793–801
- Verdecchia P., Schillaci G., Borgioni C., Ciucci A., Pede S., Porcellati C. Ambulatory pulse pressure: a potent predictor of total cardiovascular risk in hypertension. Hypertension 1998; 32: 983–8
- Verdecchia P., Schillaci G., Guerrieri M., Gatteschi C., Benemio G., Boldrini F., et al. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation 1990; 81: 528–36
- Ingelsson E., Bjorklund‐Bodegard K., Lind L., Arnlov J., Sundstrom J. Diurnal blood pressure pattern and risk of congestive heart failure. JAMA 2006; 295: 2859–66
- Masuki S., Todo T., Nakano Y., Okamura H., Nose H. Reduced {alpha}‐adrenoceptor responsiveness and enhanced baroreflex sensitivity in Cry‐deficient mice lacking a biological clock. J Physiol 2005; 566((Pt 1))213–24
- Makino M., Hayashi H., Takezawa H., Hirai M., Saito H., Ebihara S. Circadian rhythms of cardiovascular functions are modulated by the baroreflex and the autonomic nervous system in the rat. Circulation 1997; 96: 1667–74
- Hossmann V., FitzGerald G. A., Dollery C. T. Circadian rhythm of baroreflex reactivity and adrenergic vascular response. Cardiovasc Res 1980; 14: 125–9
- Weyrich A. S., Zimmerman G. A. Evaluating the relevance of the platelet transcriptome. Blood 2003; 102: 1550–1
- Dalby M. C., Davidson S. J., Burman J. F., Davies S. W. Diurnal variation in platelet aggregation iwth the PFA‐100 platelet function analyser. Platelets 2000; 11: 320–4
- Bremner W. F., Sothern R. B., Kanabrocki E. L., Ryan M., McCormick J. B., Dawson S., et al. Relation between circadian patterns in levels of circulating lipoprotein(a), fibrinogen, platelets, and related lipid variables in men. Am Heart J 2000; 139((Pt 1))164–73
- Peters R. W. Circadian patterns and triggers of sudden cardiac death. Cardiol Clin 1996; 14: 185–94
- Maemura K., de la Monte S. M., Chin M. T., Layne M. D., Hsieh C. M., Yet S. F., et al. CLIF, a novel cycle‐like factor, regulates the circadian oscillation of plasminogen activator inhibitor‐1 gene expression. J Biol Chem 2000; 275: 36847–51
- Schoenhard J. A., Smith L. H., Painter C. A., Eren M., Johnson C. H., Vaughan D. E. Regulation of the PAI‐1 promoter by circadian clock components: differential activation by BMAL1 and BMAL2. J Mol Cell Cardiol 2003; 35: 473–81
- Oishi K., Ohkura N., Amagai N., Ishida N. Involvement of circadian clock gene Clock in diabetes‐induced circadian augmentation of plasminogen activator inhibitor‐1 (PAI‐1) expression in the mouse heart. FEBS Lett 2005; 579: 3555–9
- Kono T., Morita H., Nishina T., Fujita M., Hirota Y., Kawamura K., et al. Circadian variations of onset of acute myocardial infarction and efficacy of thrombolytic therapy. J Am Coll Cardiol 1996; 27: 774–8
- Gorbacheva V. Y., Kondratov R. V., Zhang R., Cherukuri S., Gudkov A. V., Takahashi J. S., et al. Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the CLOCK/BMAL1 transactivation complex. Proc Natl Acad Sci U S A 2005; 102: 3407–12
- Haus E. Chronobiology of the mammalian response to ionizing radiation. Potential applications in oncology. Chronobiol Int 2002; 19: 77–100
- Lis C. G., Grutsch J. F., Wood P., You M., Rich I., Hrushesky W. J. Circadian timing in cancer treatment: the biological foundation for an integrative approach. Integr Cancer Ther 2003; 2: 105–11
- Panda S., Antoch M. P., Miller B. H., Su A. I., Schook A. B., Straume M., et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 2002; 109: 307–20
- Labrecque G., Bureau J. P., Reinberg A. E. Biological rhythms in the inflammatory response and in the effects of non‐steroidal anti‐inflammatory drugs. Pharmacol Ther 1995; 66: 285–300
- Hermida R. C., Ayala D. E., Calvo C., Lopez J. E. Aspirin administered at bedtime, but not on awakening, has an effect on ambulatory blood pressure in hypertensive patients. J Am Coll Cardiol 2005; 46: 975–83
- Lemmer B., Labrecque G. Chronopharmacology and chronotherapeutics: definitions and concepts. Chronobiol Int 1987; 4: 319–29
- Young M. E. The circadian clock within the heart: potential influence on myocardial gene expression, metabolism, and function. Am J Physiol Heart Circ Physiol 2006; 290: H1–16
- Woon P. Y., Curtis A. M., Kaisaki P. J., Argoud K., Wallace K. J., Bihoreau M. T., et al. Genomic organization of the rat Clock gene and sequence analysis in inbred rat strains. Genomics 2006; 87: 208–17
