Abstract
In the current review we summarize the available data concerning the gastric hormone ghrelin and its receptor. Ghrelin stimulates short‐term food intake and long‐term body weight regulation via its adipogenic and diabetogenic effects. Ghrelin stimulates gastric emptying, and these effects could be explored from a therapeutic point of view. Ghrelin levels change profoundly in anorexia, in states of insulin resistance, in obesity, and after bariatric surgery, suggesting that this is an important hormone in body weight regulation.
Overview of appetite regulation and the gut‐brain axis
An ideal system to regulate energy balance needs to have many different strengths. It must be able to manage both short‐term (i.e. meal‐to‐meal and day‐to‐day) and long‐term (year‐on‐year) energy demands. It needs to maintain body weight within fairly tight limits, since extremes of weight in either direction can result in disabling health problems. It must be prepared to deal with both increases and decreases in food availability and requirements for energy expenditure. And, due to its importance in terms of survival, it needs to be robust, with fall‐back mechanisms and safety guards. It is therefore no great surprise that researchers have found the control of energy balance and appetite to be both complicated and multi‐faceted, and therefore the clinical applications of this new‐found knowledge are proving difficult to determine and even harder to put into practice.
There are numerous peptides involved in the regulation of energy homeostasis, some of which are produced centrally and others peripherally in the gastrointestinal tract, with some produced at both locations. These peptides have come to be known as members of the ‘gut‐brain axis’.
Interestingly, so far only one peripheral hormone has been discovered that stimulates appetite, ghrelin, while many others inhibit appetite. The anorectic peripheral peptides, including leptin, insulin, pancreatic polypeptide (PP), peptide YY (PYY3‐36), cholecystokinin, oxyntomodulin, and glucagon‐like peptide‐1 (GLP‐1) all signal satiety. This may appear unusual, in a system that is supposedly geared towards maintaining weight and preparing for future times of scarcity, but it seems that in spite of this, the human energy homeostatic system really is predisposed towards gaining and maintaining weight Citation1. And in some cases at least, the relative decrease in or absence of a circulating peripheral signal is a much more potent stimulus for weight gain than its presence is in inducing weight loss Citation2. An excellent overview Citation3, and several detailed discussions are now available Citation1,2,Citation4–7 about this topic. In this review we will focus on ghrelin, the peripheral hunger hormone.
What is ghrelin?
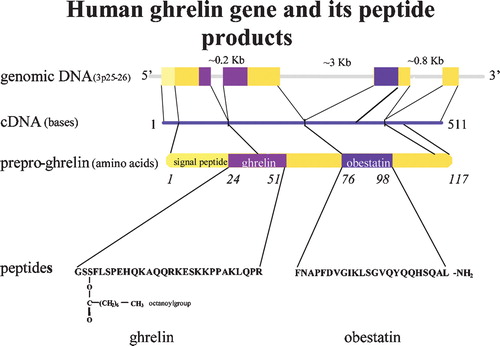
Ghrelin is a peptide hormone, and the majority of circulating ghrelin is released from a subpopulation of the neuroendocrine cells (ghrelin cells) of the stomach mucosa Citation8,9. It was first discovered in 1999, as part of a study to identify the previously unknown endogenous ligand for the GHS‐R (growth hormone secretagogue receptor), a receptor that is known to be stimulated by synthetic growth hormone‐releasing compounds Citation8. Ghrelin is a 28‐amino‐acid protein with an n‐octanoylated serine residue that is formed after post‐translational processing from the 117‐amino‐acid peptide preproghrelin Citation8 (). It is also synthesized in small amounts in all of the human tissues studied, including several areas of the brain (hypothalamus, hippocampus, and cortex) Citation8,Citation10, and the pituitary gland, the small intestine, adrenal gland, pancreas, etc. Citation9,Citation11.
Recent research revealed the discovery of a second hormone derived from the preproghrelin peptide Citation12 (). This 23‐amino‐acid peptide was named obestatin and is expressed in the stomach.
Key messages
Ghrelin is a brain‐gut peptide but has very wide tissue distribution, and data suggest that it has both endocrine and paracrine effects in various organs.
Ghrelin increases appetite, has diabetogenic and lipogenic effects, and has an important role in weight regulation.
Ghrelin has a positive effect on growth hormone regulation.
Emerging data of its wide‐ranging effects on immune function, cognition, gonadal axis regulation, bone metabolism, gastrointestinal motility, and cardiovascular system suggest that it is a truly multifunctional hormone.
Clinical applications of agonist or antagonist compounds, however, are not prominent.
The ghrelin receptor
The only receptor so far identified for ghrelin is the GHS‐R Citation8,Citation13,14—a G protein‐linked receptor that is expressed in the hypothalamus, in all three components of the dorsal vagal complex, including the area postrema, the nucleus of the solitary tract, the dorsal motor nucleus of the vagus and parasympathetic preganglionic neurons, Citation15, as well as in numerous other tissues, including the stomach, intestine, kidney, pancreas, adipose tissues, heart, and lung Citation8,Citation16. There are two splice variants—GHS‐R type 1a, which is the receptor to which ghrelin binds and through which it exerts its effects on growth hormone release, and GHS‐R type 1b, which is a truncated form and seems to be pharmacologically inactive Citation16.
Effects of ghrelin
Ghrelin was identified via its growth hormone‐releasing effect, but numerous other effects have been described in the last few years. Here we concentrate on those effects related to appetite and weight regulation, while ghrelin's other effects are summarized in and have been detailed elsewhere Citation17,18.
Table I. A summary of the effects of ghrelin (original reports or reviews with further details are indicated in brackets).
Short‐term energy balance and meal initiation
Ghrelin may play a role in meal initiation—it is known that giving exogenous ghrelin makes animals eat, even when they normally would not Citation22,Citation43, and it was found that levels of endogenous ghrelin rise immediately before eating and fall again within the next hour Citation44. In addition, ghrelin levels go up in rats that are starved, but are quickly reduced if those same, starved rats are re‐fed Citation22, while anti‐ghrelin immunoglobulin causes a reduction in food intake Citation45. There is thus some evidence to show that ghrelin may be important in the regulation of appetite and short‐term energy balance. However, there is also evidence to the contrary. Cummings et al. looked at the relationship between endogenous ghrelin levels and meal initiation in humans and concluded that it was not yet possible to be certain that pre‐prandial ghrelin surges actually do act to initiate eating, and that in fact ghrelin levels may rise as a consequence of the expectation of food Citation44. In rats, data from the comparison of freely feeding and meal‐feeding animals shows that both anticipation and feeding/fasting status influence pre‐ and postprandial plasma ghrelin levels, suggesting a role for ghrelin in the regulation of anticipatory processes involved in food intake and nutrient deposition Citation46. Species differences are also present, since a recent paper reported that ghrelin administration in sheep had no effect on food intake, and may be more important for the regulation of postprandial GH levels Citation47. Another recently recognized aspect of ghrelin's feeding effect is that in growth hormone receptor‐deficient mice ghrelin cannot induce food intake compared to wild‐type controls Citation48. Further studies are needed to fully understand this effect.
In summary, it is not yet clear whether ghrelin levels rise because the body needs food, and this rise then increases food intake; or whether levels rise in anticipation of food intake; or possibly the rise in ghrelin levels happens to coincide with meal times, but it is not directly related to hunger or meal initiation at all, and may, for example, be more concerned with the other short‐term functions of ghrelin, such as choosing metabolic substrates and promoting fat storage Citation49.
Long‐term energy balance
Ghrelin seems to be part of the body's mechanism for controlling the long‐term energy balance. It is an anabolic hormone that in addition to increasing food intake Citation50, reduces the use of fat as a metabolic fuel, while at the same time promoting fat storage Citation22. The biochemical explanation for this effect is mentioned later in this review. Endogenous ghrelin levels are negatively correlated with body weight and percentage body fat Citation51, and it is thought that this may be physiologically relevant both in times of starvation and food shortage, when the elevated ghrelin levels cause the body to be more energy‐efficient (i.e. to eat more and store more nutrients as fat) Citation22 and conversely in times of plenty, when weight is being gained, low ghrelin levels (i.e. the relative absence of ghrelin) will cause one to eat less and to store less fat Citation49.
Thus ghrelin appears to be involved in both sides of the energy balance equation; in energy gain, by its active presence, which makes people hungry and their bodies more energy‐efficient; and in energy loss, since ghrelin levels go down during times of caloric excess, which prevents further energy gain and makes the body less energy‐efficient.
Other gastrointestinal effects
Ghrelin has been found to stimulate gastric acid secretion Citation52 and to have effects on gastrointestinal motility. It increases gastric motility, accelerates gastric emptying and the passage of food through the small intestine (but not the colon), and induces migrating motility complexes in the duodenum Citation52–54. These effects are mediated by its actions on the myenteric neurons and the vagus nerve, and also through central effects Citation53. It has previously been assumed that results from animal (usually rodent) models can be directly applied to humans; however, there is some potentially conflicting evidence that may suggest this is not the case. Earlier this year Ohno et al. Citation55 found that ghrelin did not stimulate gastrointestinal motility nor accelerate gastric emptying in dogs, in either the fed or fasted state. Preliminary research to identify the kinetic effects of ghrelin in humans has found that at pharmacological doses it does stimulate gastric motility, and also causes a prolonged increase in gastric tone Citation56. It is possible that hunger pangs (‘tummy rumblings’) that may be experienced during short‐term fasting are due to the increased gastric motility induced by the high ghrelin levels. Whether or not ghrelin has a relevant physiological role in gastrointestinal motility has yet to be determined. However, the potential to exploit this pharmacologically and therapeutically is obviously of great interest (see later).
Models of ghrelin or ghrelin receptor deficiency
Research into the functional importance of ghrelin has looked at animals that had either a genetic or an acquired deficiency of ghrelin (); i.e. ghrelin‐null mice Citation49,Citation57, and mice that had received a synthetic oligonucleotide that neutralized its effects during adulthood Citation60. The first study of genetic deletion of ghrelin in mice looked at the size, weight, and histology of different organs, blood biochemistry, bone mineral density and reproductive functions, as well as body weight, food intake, percentage fat mass, and development of diet‐induced obesity. The study found that the genetic deletion of ghrelin prior to birth had no significant effect on any of these parameters, thus showing that both the metabolic and non‐metabolic effects of ghrelin deficiency can be compensated for Citation57. A possible explanation was provided that in these animals obestatin, with its opposite effects on food intake, has also been knocked out. However, another study showed that while animals that were born with a genetic absence of ghrelin did not appear to have major problems with appetite, digestion, feeding, eating behaviour or maintenance of body weight: on high‐fat diet there was a reduction in weight gain and increased use of fat as fuel as compared to wild‐type littermates Citation49,Citation58,Citation67. An acquired ghrelin deficiency resulted in diet‐induced obese mice eating less and storing less fat, and ultimately having a lower body weight and body fat mass as compared to control or vehicle groups Citation60, while pigs also showed reduced food intake and weight loss after ghrelin immunoneutralization Citation61. It was concluded that prenatal ablation of the ghrelin gene produced more subtle phenotypic effects as a consequence of the plasticity within the system—i.e. because other metabolic pathways had been able to develop in a way that enabled them to compensate for its loss. However, this compensation was not possible when the effects of ghrelin were neutralized in adult life Citation60. This suggests that ghrelin is an important player in the field of energy homeostasis and appetite regulation, as part of a bigger network of regulatory molecules, which can only compensate for its loss if this occurs early in development. Similar discrepancies between embryogenic and adult studies have been shown with other peptides as well Citation68–71. The majority of these studies concur with the findings of Sun Citation57, Wortley Citation49, and Shearman Citation60, by demonstrating that the knockout of a molecule has a much greater effect in adult life than in an embryo or neonate, since its loss can only be compensated during early development. Interestingly the leptin‐ghrelin double knockout model shows obesity and hyperphagia but improved insulin release (via reduction of levels of the uncoupling protein 2 (UCP2)) and insulin sensitivity, suggesting an important role for ghrelin in β‐cell physiology Citation64.
Table II. Transgenic and knockout models for ghrelin and GHS‐R.
The recent discovery of obestatin Citation12, which appears to have directly opposing (anorexigenic) effects to the orexigenic actions of ghrelin itself, is very interesting, and could provide an alternative or additional explanation for the surprisingly normal phenotype of ghrelin knockout mice, in spite of the impressive effects of giving pharmacological doses of exogenous ghrelin. The deletion of the preproghrelin gene would presumably cause the elimination of both hormones, thus removing their opposite effects Citation72.
Sun Citation13 and Zigman Citation14 both examined the effects of creating GHS‐R‐null mice. In keeping with the assumption that the lack of phenotypic differences in ghrelin‐null mice occurs because these mice also lose the anorexigenic obestatin, it could be supposed that if researchers were to remove the ghrelin receptor instead, there should be a noticeable reduction in ghrelin's effects. Sun's findings Citation13 seem to dispute this, but the longer study by Zigman Citation14 showed that the knockout mice did not accumulate as much weight as the controls. It is thought that this is because the GHS‐R‐null mice ate less, were less efficient at storing the fat they had eaten, and utilized fat in preference to other metabolic fuels Citation14. They therefore concluded that in the absence of the GHS‐R mice are resistant to the development of diet‐induced obesity. In keeping with this, a study into the effects of antagonizing the GHS‐R receptor (in adult mice) found that this decreased feeding in both lean and obese mice, lowered body weight gain, and reduced the rate of gastric emptying Citation65.
Genetic variations in ghrelin and GHS‐R gene in humans
Several studies have investigated genetic alterations in the ghrelin and GHS‐R genes, focusing on obesity, diabetic traits and stature. As expected, the published results show a wide range of genetic variations, suggesting that the impact of ghrelin and its receptor at the genetic level in height and weight determination is complex, and further studies are necessary.
Obesity
The first report on single nucleotide polymorphisms (SNPs) in the ghrelin gene documented that among obese females, 6.3% of the studied cohort were heterozygotes for the Arg51Gln SNP. These subjects have a lower weight than those with the wild‐type (Arg51Arg) variant Citation73. The Leu72Met polymorphism (found in 12.5% of hetero‐ and 3.1% of homozygotes among obese subjects; and in 12.5% of heterozygotes among controls) was associated with a trend to earlier self‐reported onset of obesity (15.6±7.9 versus 20.5±10.5 years, P = 0.09) in this study. Another group Citation74 could not show any association between these two SNPs and a third SNP, Gln90Leu, with features of obesity. Only one study showed correlation between Arg51Gln and lower ghrelin levels Citation75, but this was not confirmed by another study Citation76. A large population study (3004 subjects) of three cohorts reported an association between Leu72Met and obesity, with homozygote or heterozygote 72Met being related to having the lowest body mass index (BMI), and to having the lowest fat mass/less visceral fat Citation75. We have identified a correlation between the ghrelin Leu72Met heterozygote variant and early‐onset obesity (before the age of 11), higher ZBMI (z‐score of BMI, standardized BMI) and reduced insulin secretion during an oral glucose tolerance test (OGTT) among French Caucasian children Citation77. An Italian group observed a similar significant association between this same polymorphism (Leu72Met) and early‐onset obesity among children Citation78. This association, however, could not be reproduced in a group of Korean children Citation79. A Danish study did not find any association between Leu72Met variant and metabolic syndrome or related quantitative traits in the DanMONICA cohort (MONItoring of trends and determinants in CArdiovascular diseases) of 2413 subjects Citation80. The Leu72Met variant was connected with the metabolic syndrome (increased fasting glucose, low levels of high‐density lipoprotein (HDL) and high levels of plasma triglycerides) in the Old Order Amish Citation81. Vartiainen et al. found a trend towards an association between the A‐501C (rs26802) variant in the ghrelin gene promoter region and BMI and waist circumference: allele A carriers (homozygotes and heterozygotes) had higher BMIs and larger waist circumferences compared to allele CC homozygotes? Citation82. Furthermore, the Leu72Met variant was correlated with a positive family history for obesity and with infants born with ‘large for gestational age’ status Citation76. Interestingly, a duplication of chromosome 3p25.3p26.2, a locus including the GHRL gene, was found in a 9‐year‐old boy with early‐onset obesity (at the age of 4 years), developmental delay, and delayed speech. These are features similar to those of Prader‐Willi syndrome, but without the classical chromosome 15q11‐q13 abnormalities. This suggests that this gene may cause obesity Citation83.
Genetic linkage and the association between obesity and GHS‐R SNPs and haplotypes within the GHS‐R gene region (locus 3q26.2) was detected by Baessler et al. Citation84. No association could be demonstrated between obesity and several GHS‐R polymorphisms in young subjects (age range 11.7±3.1 year and 25.6±3.8 year) Citation85, although one rare mutation, 611C/A (Ala204Glu, SNP002901685), was detected in one obese subject.
Anorexia/bulimia
Ghrelin SNPs have not been found to correlate with anorexia or bulimia status Citation86,87. However, GHS‐R polymorphism C171T (rs495225) and ghrelin variants Leu72Met and rs2075356 (individually and as a haplotype) were correlated with bulimia and binge‐eating, respectively, in Japanese cohorts (including in non‐obese subjects) Citation88, Citation89. Interestingly, the Leu72Met ghrelin polymorphism was correlated with higher hunger scores in the Old Order Amish Citation81.
Type 2 diabetes
We showed that the ghrelin Leu72Met variant heterozygote status correlated with reduced insulin secretion during an OGTT among French Caucasian children Citation77. This was supported by the fact that subjects with the Leu72Leu genotype were found to be at lower risk for the development of type 2 diabetes Citation90. Pöykkö et al. concluded that the ghrelin Arg51Gln variant is a risk factor for type 2 diabetes in middle‐aged subjects Citation91. Larsen et al. did not find any association between several ghrelin gene polymorphisms and juvenile‐onset obesity or type 2 diabetes among Danish Caucasians Citation92, which was also the case in a Korean population with several ghrelin polymorphisms Citation93. Vartiainen reported a correlation between GHS‐R 171C/T and 477G/A variants and the values of the area under the insulin curve during an OGTT Citation94. Ukkola et al. reported a significant correlation between Leu72Met and lower serum creatinine levels in type 2 diabetic patients Citation95. Although a Korean group found that the Leu72Met variant was not associated with type 2 diabetes mellitus, they found that carriers of this variant had lower serum creatinine levels than non‐carriers, and that among subjects with diabetic nephropathy, carriage of the Leu72Met variant correlated with a reduced risk of developing renal dysfunction. The authors concluded that carriage of this heterozygote variant may have a role to play in protecting against/predicting the risk of developing renal dysfunction in patients with diabetic nephropathy Citation96,97.
Lipid metabolism
The Leu72Met variant was reported to correlate with lower plasma lipoprotein‐a levels in patients with type 2 diabetes Citation95, and with low HDL and high triglyceride levels (and the metabolic syndrome) in the Old Order Amish Citation81. In a study of Korean subjects, a polymorphism in the ghrelin promoter region (‐1062G/C) was found to correlate with lower HDL cholesterol levels among non‐diabetic subjects as compared to diabetic subjects Citation93. Vartiainen reported a correlation between GHS‐R 477G/A variants and plasma low‐density lipoprotein (LDL) and very‐low‐density lipoprotein (VLDL) levels in a Finish cohort (the OPERA study) Citation94.
Hypertension
A large population study of three cohorts reported an association between the Leu72Met variant and the lowest prevalence of hypertension in obese female subjects Citation75. The ghrelin Arg51Gln variant appeared to represent a risk factor for hypertension in another population study of middle‐aged subjects Citation91. Recently, a four‐SNP haplotype of ghrelin (‐604G/A, ‐501A/C, Leu72Met and Gln90Leu) has been associated with lower systolic and diastolic blood pressures among the Finnish Diabetes Prevention Study cohort and seems to have a protective effect, while the Arg51Gln did not show any correlation Citation90. A cohort from the German general population in the MONICA Study showed an association between two of the commonest five‐SNP haplotypes (rs509035, rs572169, rs519384, rs512692, rs863441) of the GHS‐R gene and left ventricular hypertrophy (LVH): one of these, a ‘non‐susceptibility’ haplotype, was more frequently found among individuals without LVH, while the other ‘at risk’ haplotype, as well as two individual SNPs (rs509035 and rs572169), was significantly associated with LVH and related parameters Citation98.
Stature
Evidence for linkage of the 3p26 locus, the locus for ghrelin gene, with height (logarithm of the odds (LOD) score 3.17) was shown in patients with type 2 diabetes from the Warren 2 UK cohort Citation99. The ghrelin Arg51Gln polymorphism was found to correlate with serum insulin‐like growth factor (IGF)‐I levels in subjects with type 2 diabetes Citation100, while neither the Leu72Met variant nor GHS‐R SNPs were correlated with serum IGF‐I levels Citation76,Citation94. Wang et al. discovered two rare variants within GHS‐R: 611C/A (Ala204Glu, SNP002901685) was found in an obese child, and 837C/A (Phe279Leu, SNP002901686), in a normal short child Citation85. Furthermore, the Ala204Glu (SNP002901685) mutation, characterized by loss of constitutive activity of the GHS‐R, has been found to segregate with short stature within two unrelated families Citation101.
Although several studies have been carried out in this field, a number of them have so far only been conducted on a small number of patients, and large‐scale studies are necessary to confirm these findings.
Structural variants of ghrelin
Ghrelin has a unique fatty acid chain on its N‐terminal end. However, the majority of circulating ghrelin is actually lacking this acyl group and is known as des‐acyl or des‐octanoyl ghrelin. Small amounts of other ghrelin variants with slightly longer fatty acid chain can be detected, but their biological activity seems to be similar to ghrelin itself Citation102. Various studies have looked at the functions of des‐acyl ghrelin, and whether or not these are the same as those of acylated ghrelin Citation24,Citation62,Citation103–116. In terms of food intake three of them showed that either centrally or peripherally administered des‐acyl ghrelin reduces food intake and delays gastric emptying Citation106,107,Citation114, while one showed that centrally administered des‐acyl ghrelin stimulates food intake, but peripherally administered des‐acyl ghrelin has no effect Citation108. A human study showed no effect of des‐acyl ghrelin on appetite Citation117. Several studies described proliferative Citation103,Citation109–111 or anti‐apoptotic effects of des‐acyl ghrelin Citation112, but it is shown to be anti‐proliferative in breast cancer cell lines Citation113. Des‐acyl ghrelin is known to exert its effects through a different pathway to acylated ghrelin—ghrelin binds to and acts through the GHS‐R, and the acylated residue is vital for this to occur. By contrast, des‐acyl ghrelin continues to function in GHS‐R‐deficient mice possibly via an orexin‐dependent pathway Citation108. Although the results of these studies are inconclusive, it is possible that des‐acyl ghrelin forms another component of the appetite‐regulatory system.
The effects of obestatin were studied after intraperitoneal and intracerebroventricular administration into adult mice. Obestatin induced suppression of food intake and of body weight gain, as compared to control or ghrelin (which induced increases in both of these variables) Citation12. It was also found to have effects on gastrointestinal motility—suppressing gastric emptying and reducing jejunal contraction. Obestatin was found to bind to the previously orphan G protein‐coupled receptor GPR39, a receptor that is expressed in many tissues, including the stomach, intestine and hypothalamus Citation12. This is consistent with the possibility that obestatin is part of the appetite regulatory system, and it is therefore thought that obestatin is a novel anorexigenic hormone. The concept of the same propeptide coding for hormones with opposite actions is unique, and further data is necessary to confirm these findings.
How ghrelin exerts its effects
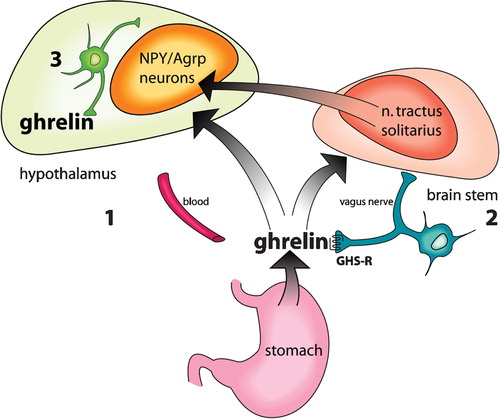
There are several studies regarding the mechanism of the central effects of ghrelin while hardly any look at the mechanisms in the periphery. Circulating peripheral ghrelin is released from the gastrointestinal (GI) tract, and is believed to provide input to the brain through three mechanisms (): 1) directly, via the blood stream, to enter the anterior pituitary gland and other areas of the brain not protected by the blood‐brain barrier (BBB) Citation118; 2) directly, by crossing the BBB via a saturable transport system Citation119; and 3) indirectly, via the vagus nerve Citation120. It acts in the arcuate nucleus (the ARC), a part of the brain highly important in the regulation of feeding and appetite Citation43,Citation118. It also causes neuronal activity in the paraventricular nucleus, the dorsomedial hypothalamus and the lateral hypothalamus, and in the nucleus of solitary tract and the area postrema in the brain‐stem Citation121. GHS receptors are found in the ARC on neurons that release NPY (neuropeptide Y) and AgRP (agouti protein related peptide; both highly potent stimulators of weight gain) Citation122, where ghrelin acts to increase the output from these neurons Citation22,Citation43. GHS‐R can be found on presynaptic nerve endings, directly influencing neurotransmitter release. It was found that ghrelin acts here to promote appetite in two ways—directly, by depolarizing the orexigenic NPY/AgRP neurons, and indirectly, by increasing the tonic inhibition exerted by the NPY/AgRP neurons over the anorexigenic proopiomelanocortin/cocain‐amphetamine related transcript (POMC/CART) neurons. Both of these ultimately enhance appetite Citation123.
Figure 2. Ghrelin reaches the hypothalamus via three different pathwaysCitation18.
Various studies have been done to see what happens if either NPY or AgRP, or both, are removed from the ghrelin signalling pathway, by studying various types of knockout mice. It has been shown that removing either or both of these peptides in an embryo or neonate does little to affect appetite or weight gain—all the mice have a grossly normal phenotype and normal feeding behaviours Citation68,Citation124–126, and the AgRP−/− and NPY−/− mice were still susceptible to the effects of ghrelin Citation124, although some of the double knockout mice were not. However, loss of either or both of these sets of neurons in adult animals tends to cause a significant reduction in body weight and appetite Citation68–71. This is consistent with the findings from other knockout studies, and again highlights the plasticity in the system in early development, and its importance for survival.
Ghrelin has been shown to stimulate the metabolic regulatory enzyme adenosine monophosphate‐induced protein kinase (AMPK) in the hypothalamus and this leads to increased food intake Citation127,128. However, we have also shown that ghrelin can influence AMPK activity in peripheral tissues as well. It stimulates myocardial AMPK and inhibits liver and adipose AMPK activity. These data are in accordance to the known ghrelin effects in these tissues and the physiological consequences of AMPK stimulation or inhibition of tissue‐specific metabolism Citation128.
Surprising results were found with the GHS‐R antagonist BIM‐28163. When examining the effects of this molecule Citation129.130, it was found to be a full antagonist on intracellular calcium increase and growth hormone release. However, its actions on food intake and weight gain were also examined, and it was found that BIM‐28163 had an agonist, orexigenic and weight‐increasing effect. When studied in more depth, the results of this study imply that the metabolic effects of ghrelin may be mediated through a different receptor to the GHS‐R, and that therefore 1) the GHS‐R is not the only receptor on which ghrelin acts, and 2) drugs to tackle anorexia and obesity may be better directed at this novel, alternative receptor. These results are supported by another similar study Citation131. The existence of alternative receptors has been suggested by various studies using des‐acyl ghrelin as mentioned above. It is possible that the short‐ and long‐term metabolic functions of ghrelin need to be considered separately, but in any case further work needs to be done to test these hypotheses.
Regulation of ghrelin levels
Although the functions and effects of ghrelin have been well documented, the mechanisms that regulate its release are less well understood. It is known that levels rise before meals and fall after eating Citation44, and that there is a negative correlation between ghrelin levels and body mass index (BMI) Citation51. Different bodies of evidence have looked at the effects on ghrelin release of leptin Citation123,Citation132–134, insulin and glucose levels Citation135–140, somatostatin Citation141–143, glucagon Citation144–146 cephalic control Citation135,Citation147,148, feedback within the GI tract Citation149, and the vagus nerve and parasympathetic nervous system Citation120,Citation150–155. Some of the results of the research on this question are quite controversial.
Leptin and ghrelin are known to have opposite effects on appetite and the activation of NPY/AgRP neurons, and react in opposite ways to weight gain Citation123,Citation132,Citation134. Several studies are investigating the relationship between ghrelin and leptin, but a definite answer has not been established yet. A human study could not detect any changes in ghrelin levels in response to several days of treatment with physiological or pharmacological doses of exogenous leptin Citation134.
Insulin has a temporal relationship with postprandial ghrelin concentrations: insulin levels rise as ghrelin levels fall. Various studies have been done to try to determine whether or not there is also a functional relationship, and whether or not glucose levels are a factor in this equation. Three of the studies concluded that insulin does induce the postprandial fall in ghrelin levels Citation137,138,Citation140, and one of them showed that not only is insulin able to induce this fall, but also that it is in fact a prerequisite for it Citation137. One study examined whether it was truly the change in insulin levels, or merely the associated change in glucose levels that led to this effect, and concluded that changing insulin levels alone were sufficient to cause the postprandial drop, but that hyperglycaemia may have an additional effect Citation138. Other research contradicts this by showing that the control of ghrelin release is mediated by the presence or absence of nutrients within the GI tract and is not affected by insulin levels Citation136.
Glucagon is suggested to reduce ghrelin levels by a pituitary‐dependent mechanism Citation145,146. Free fatty acids also reduce ghrelin levels in humans Citation156.
Natalucci et al. considered the possibility that ghrelin is under cephalic control, at least in the fasting state. They found that ghrelin levels maintain their circadian pattern of release in fasting subjects Citation135—that is, they rise at customary meal‐times, when food is anticipated, whether or not it is actually later received. This suggests that pre‐prandial ghrelin secretion is a sort of conditioned physiological reflex. Interestingly, if the subjects did not get anything to eat, the ghrelin levels fell, without a change in their blood glucose or insulin levels. This concurs with the results of a similar experiment in sheep Citation148. Another study in sheep showed that the pattern of anticipatory pre‐prandial ghrelin surges observed in the fasting state can be altered by changing the feeding schedules to which the animals become habituated Citation147, thus adding further weight in support of this theory. At first this may seem to directly contradict the observed effects of postprandial insulin on ghrelin, but it is possible that insulin exerts its effect in the fed state, while cephalic mechanisms affect ghrelin release in the fasting state (including pre‐prandially).
The reduction in ghrelin levels after a meal depends on an as‐yet‐unidentified postgastric factor (i.e. on an event that only occurs once the nutrients have passed out of the stomach and down the GI tract) Citation149. One study also questioned whether or not insulin was responsible for this postgastric control, and concluded that it was not, but that cholecystokinin (CCK) or GIP (glucose‐dependent insulinotropic polypeptide) might be Citation157. It has also been suggested that PYY may have a role to play in the reduction of ghrelin levels after food intake Citation158.
Various studies have looked at the relationship between ghrelin and the vagus nerve, by using either surgical or pharmacological means to block the vagal output. The results of the different studies are mixed, but the general conclusion seems to be that 1) after vagotomy there is loss of the ability to respond to the fasted state with an increase in ghrelin levels (150,151,153,154), and 2) after vagotomy there is loss of the ability to respond to high doses of exogenous ghrelin with an increase in food intake Citation155. By contrast, enhancing the vagal output with pyridostigmine increases circulating ghrelin levels Citation150. Given that it has been shown that ghrelin input to the brain is mediated at least in part by the afferent fibres of the vagus nerve Citation120, these results suggest that not only are circulating ghrelin levels under cholinergic control, but that both the afferent and the efferent vagal pathways are involved. It is possible that the vagus nerve is a route by which the cephalic control of ghrelin levels (as discussed above) is mediated. One paper suggested that the vagus exerts its effects on ghrelin through tonic inhibitory control, which is reduced in the fasting state so that ghrelin levels rise before meals Citation152. However, since studies of vagotomized animals and human subjects show that complete removal of vagal output does not cause a rebound rise in ghrelin levels, it would seem that this is unlikely.
Further to this, Williams et al. Citation151 established that an intact vagus nerve is important for the long‐term control of ghrelin secretion (such as in response to food deprivation), but that it is not necessary for the control of baseline levels, or for postprandial reductions.
Ghrelin levels are known to be affected by food intake and disease states. They are elevated in patients with illness‐induced cachexia Citation159,160 and anorexia nervosa Citation161, and are reduced in the obese Citation51. The exact causes of this are unknown—they may represent adaptive responses to try to increase/decrease food intake as required, or may be the result of a relative resistance/hypersensitivity to ghrelin in these states. By contrast, it has been observed that prolonged fasting in healthy subjects (for periods of up to 44 days) causes ghrelin levels to fall rather than rise Citation162–164. Somatostatin suppresses ghrelin levels in both healthy Citation165 and obese subjects Citation166, and the difference in ghrelin levels between healthy prolonged fasting individuals (low ghrelin), and those with disease‐induced anorexia (high ghrelin) may be because somatostatin levels are elevated during prolonged fasting Citation141,Citation143, but reduced in anorectic disease states Citation142. The relationship between somatostatin, ghrelin, and obesity remains to be clearly defined.
Clinical applications of ghrelin
Anorexia and cachexia
A debilitating anorexia‐cachexia syndrome accompanies many disease states, such as cancer, severe cardiac and pulmonary disease, chronic renal failure, and HIV/AIDS. Cachexia is the main cause of death in more than 20% of patients with cancer Citation167. Malnutrition in combination with co‐morbid disease is associated with a high mortality rate in patients with chronic renal failure Citation168, and is an important determinant of mortality in patients with AIDS Citation169, chronic heart failure, and chronic obstructive pulmonary disease (COPD) Citation170. These are just some examples of the consequences of unintentional weight loss in disease, and the gravity of the statistics highlights the need to find effective forms of treatment for patients suffering from illness‐induced anorexia. Recently research has begun to look at whether or not ghrelin would be one such form of suitable treatment.
A randomized controlled clinical trial Citation171 looked at how appetite is affected by giving exogenous ghrelin to patients with cancer‐associated anorexia. They found that food intake at a single meal was increased by an average of 31%, and also that the patients perceived the food tasted better. Another study showed that in tumour‐bearing mice, exogenous ghrelin administration increased food intake, body weight, and whole body fat, but only at high doses (40 µg/day) Citation172. It has also been found that giving ghrelin to malnourished patients with chronic renal failure caused a rapid increase in food intake, and that this was maintained over the following 24 hours, albeit to a lesser degree Citation173.
A series of studies on cardiopulmonary disease‐associated cachexia found that the administration of ghrelin caused a reversal of the cachexia in both rat and human patients with chronic heart failure, as shown by the increase in food intake, body weight, and muscle strength Citation174,175. In addition, ghrelin treatment of cachectic patients with COPD also caused an increase in food intake, body weight, BMI and muscle strength Citation176.
Anorexia nervosa
In addition to the research on the effects of ghrelin on organic disease‐induced anorexia, other studies have looked at the effects of exogenous ghrelin administration in patients with anorexia nervosa (AN). Unlike in other diseases in which anorexia and cachexia are prominent features, psychiatric illness is the principal cause of weight loss in AN Citation177. Circulating ghrelin levels in patients with AN are significantly elevated outside of the normal range as compared to constitutionally thin (but not BMI‐matched) controls, and fall back to within the normal range upon weight gain Citation161. However, this also applies to many other causes of low BMI Citation178 and may reflect only the normal physiological ghrelin response to an extreme of body weight. In addition, research has found that the immediate proportional reduction in postprandial ghrelin levels is not significantly different in patients with anorexia nervosa as compared to controls, or in patients with anorexia nervosa at various stages of weight gain Citation179. It was found that when comparing patients with anorexia nervosa to constitutionally thin but healthy controls, the ghrelin infusions did not have a significant effect on appetite Citation177. This suggests a certain level of ghrelin resistance in anorexia nervosa which could be the result of down‐regulation of GHS‐R expression due to chronic high levels of circulating ghrelin. Ghrelin may be more useful in the management of AN as part of a multidisciplinary approach.
Disorders of gastrointestinal motility
The effects of ghrelin on disorders of gastrointestinal motility have been studied, and ghrelin has been found to reverse postoperative Citation180,181, septic Citation182, and morphine‐induced Citation181 gastric ileus, and to enhance gastric emptying in patients with idiopathic Citation56 and diabetic Citation183 gastroparesis. Chemotherapy‐induced delayed gastric emptying, early satiety, anorexia, nausea, and vomiting, described collectively as the cancer‐associated dyspepsia syndrome, have been shown to improve after ghrelin injection Citation184.
Research has now begun on developing ghrelin analogues. Recently two papers Citation181,Citation185 have compared the effects of ghrelin agonists on gastric motility with the effects of ghrelin itself, and have found them to be equally efficacious.
Ghrelin and obesity
Circulating ghrelin levels are negatively correlated with percentage body fat and BMI Citation51, and are therefore lower in obese subjects than in normal body weight controls. In obesity, the postprandial reductions in plasma ghrelin levels are smaller than those of normal‐weight subjects Citation186; however, the baseline fasting ghrelin levels are also lower Citation51, so it may be that the proportionate reduction is similar. It is thought that ghrelin is linked to excessive food intake in two ways. Firstly, the lesser postprandial reduction in ghrelin levels may directly increase the length of time for which the subject feels hungry, and secondly, as a consequence of the elevated ghrelin levels, the speed of gastric emptying may not be reduced, and the resulting feeling of satiety not elicited. Without these feelings of satiety, obese individuals eat more than they need, and thus gain weight.
In addition to this, although ghrelin levels are low in obesity, they rise with weight loss. This could explain why is it exceptionally difficult to maintain weight loss after dieting Citation45. Furthermore, ghrelin regulates the body's choice of metabolic substrates Citation22,Citation49. In obesity, it is thought that the lower ghrelin levels may drive an increase in the use of fats as a metabolic fuel Citation49. However, as weight is lost, higher ghrelin levels reduce the use of fat as a metabolic fuel, so that more fat is conserved and stored Citation22. And so it can be seen that ghrelin strongly opposes the maintenance of weight loss.
Ghrelin is linked to obesity in two ways: firstly, through its short‐term effects on hunger and meal initiation (resulting in excessive food intake, as described above); and secondly, through its long‐term control of energy balance. In essence, ghrelin makes people eat more than they need to, it makes them store excess calories as fat, and it makes it very difficult to maintain diet‐induced weight loss. And in view of this, there is both undeniable opportunity and obligation to try to manipulate ghrelin physiology for the purposes of inducing long‐lasting weight reduction.
Shearman et al. Citation60 tested the effects of giving a synthetic oligonucleotide (Spiegelmer) that binds to, and therefore neutralizes the effects of, ghrelin, to mice with diet‐induced obesity (DIO). The results of their study showed that this promoted fat and weight loss in DIO mice, and that it reduced food intake, fat storage, and body fat mass, as compared to controls. Citation60. Shearman and colleagues observed tachyphylaxis with their continuous infusion regime after only a few days. Another study showed that antagonism of the GHS receptor reduced feeding in both lean and DIO mice, and also that peripheral administration of this antagonist reduced the gastric emptying rate, which may have contributed to the observed reduction in feeding Citation65.
The studies are promising, but not perfect. But while none of these methods are likely to enter the realms of clinical practice in the near future, they do at least prove that it is possible to disrupt the ghrelin appetite/energy balance regulatory pathways in order to induce weight loss, and are thus extremely encouraging.
The recent discovery of obestatin provides exciting new prospects for the management of obesity. If the relationship between these two hormones is as close and straightforward as it would initially appear, the discovery may eventually result in the development of drugs that have a paired mechanism of action to reduce body weight—i.e. that are both ghrelin antagonists (to reduce food intake, fat storage, etc.) and obestatin agonists (to promote its anorexigenic, weight‐losing effects) Citation72. Drugs that exploit this knowledge could become potent anti‐orexigens.
Another potential application of the kinetic effects of ghrelin comes from studies showing that gastric emptying is faster in clinically obese individuals as compared to controls Citation54,Citation187. This may be because in obesity ghrelin concentrations are not reduced in the postprandial state as much as they are in normal subjects Citation186. This may contribute to the increased food intake that occurs with obesity Citation54, and it is therefore possible that a ghrelin antagonist may be used to slow down the gastric emptying, so that individuals feel satisfied more quickly, and thus eat less Citation54. However, other drugs reducing gastric emptying are not used in obesity therapy.
Ghrelin and diabetes mellitus
It has been found that fasting ghrelin concentrations are lower in people who have type 2 diabetes mellitus (T2D) than in people who do not have diabetes, even after adjusting for BMI Citation188, and that plasma ghrelin levels are inversely correlated with fasting insulin concentrations and with insulin resistance Citation188–191, even in obese children and adolescents with insulin insensitivity Citation192,193. It has also been shown that the decrease in circulating ghrelin is proportionate to the degree of insulin insensitivity. These observations would seem to suggest that ghrelin and insulin sensitivity are linked. Numerous studies have been carried out to try to establish the nature of this link between insulin and ghrelin in diabetes, much of it attempting to establish whether or not circulating ghrelin levels are dependent on the levels of circulating insulin. To date, however, there does not seem to be conclusive evidence either way. It has been shown that in people who have diabetes mellitus, changes in circulating insulin concentrations cause inverse changes in ghrelin levels, independent of the effects of insulin on glucose Citation138, and even allowing for the effects of insulin resistance Citation194. Also, rodent models of type 1 diabetes mellitus (T1D) have shown that the associated elevated ghrelin levels are reduced when insulin treatment is begun Citation195,196. In addition, it has been proven that the normal postprandial reduction in ghrelin levels in people with T1D is dependent on the presence of basal circulating insulin levels Citation137,Citation197. On the other side of the debate, there is also evidence to the contrary. Briatore et al. showed that ghrelin levels decreased during the first 10 minutes of the postprandial period in subjects with T2D, even though it is known that in this group of patients insulin does not respond to glucose intake straight away. Thus this initial reduction in postprandial ghrelin levels was not mediated by insulin. This may suggest that during this period the reduction in ghrelin levels is a response to blood glucose instead Citation198. In addition to this, research involving children with newly diagnosed T1D has found two cases where the low insulin levels have not induced a rise in ghrelin levels Citation197,Citation199, and one of these found that ghrelin levels were not reduced after food intake either Citation197.
One particular study to note is one that found that ghrelin levels are not reduced in lean diabetic subjects, although they are in obese subjects. Since insulin concentrations and sensitivity were not reported, this may or may not cast doubt on the idea that insulin resistance causes ghrelin levels to rise; however, it does highlight the fact that insulin secretion may not be the only factor in play here Citation200. In addition, work is also being carried out to try to establish the significance of the relationship between ghrelin and insulin in diabetes.
There is a growing body of evidence to suggest that in diabetes, as in non‐diabetic subjects, ghrelin functions in the control of energy balance and body weight. As discussed above, it has been shown that in T2D, ghrelin levels are negatively correlated with BMI Citation200. It has been suggested that the hyperinsulinaemia/insulin resistance seen in obese, non‐diabetic subjects may be part of a feedback mechanism by which body weight is regulated. Since there appears not to be a direct relationship between BMI and ghrelin levels, it has been suggested that the link between rising body weight and lowered ghrelin levels (and thus a reduction in the drive to eat) is mediated by insulin: as body weight rises, insulin levels rise, and ghrelin levels fall Citation189. Although these results are not specific to diabetic patients with insulin resistance, they may help to explain the changes seen in ghrelin levels related to insulin resistance, which is frequently seen in type 2 diabetes. Recent research has tried to further clarify the link between ghrelin levels and body fat, and has established that ghrelin levels are negatively correlated much more strongly with visceral fat accumulation than subcutaneous fat Citation191,192.
By contrast to patients with type 2 diabetes, those with uncontrolled type 1 diabetes characteristically present with very low levels of circulating insulin and high levels of ghrelin Citation195,196. Studies of rodent models of type 1 diabetes mellitus (DM) have shown that these animals present with hyperphagia and reduced body weight Citation195,196, and that both the clinical features and the elevated ghrelin levels are reduced when insulin treatment is begun. It is thought that the elevated ghrelin levels may be indicative of the negative state of energy balance with which T1D presents, and that they may be responsible for the hyperphagia, since this can be partially reversed by the administration of a ghrelin antagonist Citation196. Conversely, other work has shown that at the point of diagnosis of T1D, plasma ghrelin levels were much lower than in controls Citation197,Citation199. Of the two studies supporting this, one found that ghrelin responses to food intake were also abnormal prior to insulin therapy (reductions in ghrelin levels were absent), but that ghrelin levels and responses to food intake had normalized after insulin therapy was begun Citation197; while the other found that they remained low during the 4‐month period after starting insulin therapy, and concluded that this may be a control mechanism to protect against the damaging effects of hypoglycaemia Citation199.
Prader‐Willi syndrome
Prader‐Willi syndrome (PWS) is a relatively common human genetic obesity syndrome, and the only known cause of obesity in which ghrelin levels are elevated (2–3 times higher than those of normal obese controls) Citation166. The cause of the elevated ghrelin levels is not yet known, but it may be the result of abnormal innervation of the stomach by the vagus nerve, abnormal sympathetic tone (due to abnormalities of the hypothalamus and/or brain‐stem Citation201), or may be due to the relatively low insulin levels and insulin sensitivity retained by the PWS patients, as compared to obese non‐PWS controls Citation201,202. It had originally been suggested that the elevated ghrelin levels are responsible for the characteristic hyperphagia and morbid obesity experienced by these patients Citation203,204, so agents to reduce ghrelin levels would in theory have a therapeutic effect. However, in a small study, pharmacological reduction of ghrelin levels with octreotide to within the normal range had no immediate effect on food intake Citation166.
Bariatric surgery
It is well documented that diet‐induced weight loss causes a rebound elevation in plasma ghrelin levels, and it is thought that this is one of the reasons why it is so hard for dieters to maintain their new, lower body weight. By contrast, gastric bypass surgery is associated with weight reduction that is sustained on a long‐term basis. Gastric bypass surgery was shown to be associated with markedly reduced ghrelin levels in the postoperative period Citation45, and this paper suggested that this was one mechanism through which the prolonged weight loss was maintained. However, many further studies into the effects of postoperative weight loss on ghrelin levels disputed the findings of this original report. A review Citation205 of 18 prospective and cross‐sectional studies summarized the results so far as being ambiguous and inconsistent—with insufficient evidence to support a firm conclusion of any sort about the relationship between ghrelin levels and bariatric surgery.
Papers published since this review only furthered this point, with one study showing that the ghrelin levels increased after surgery, four studies showing that they were reduced, four showing that they did not change, and two showing that the effects depended on the procedure performed (). Some papers show biphasic changes in ghrelin levels depending on the length of follow‐up; with one showing that ghrelin levels rise initially after laparoscopic adjustable gastric bypass, but then decrease after the first 12 months Citation206, while another study found that they decrease initially with a rise after 3–12 months Citation207.
Table III. Summary of the findings of studies published between January 2005 and July 2006 with reference to the subject of bariatric surgery and its effects on ghrelin levels. (For previous studies, refer to Citation205).
Conclusion
There is currently much on‐going research into the wide‐spread actions of ghrelin, not only into its metabolic effects, which have been considered here, but also with regards to various others, including the cardiovascular, endocrine, and immunological.
Ghrelin is believed to have various metabolic functions. It is thought to be involved in both short‐term and long‐term regulation of energy balance, control of appetite and meal initiation, selection of metabolic substrates, stimulation of gastric acid secretion and regulation of GI tract motility. It produces these effects in the arcuate nucleus, both directly, through activating the AgRP/NPY neurons, and also indirectly, through inhibiting the POMC/CART neurons. Its effects are then mediated by further downstream processing.
The mechanisms controlling ghrelin release are still being studied—leptin, insulin, and somatostatin may play a role. Postgastric feedback seems to be required. There may be an element of cephalic control, involving a conditioned reflex. It seems likely that the vagus nerve is also important, maybe in particular with regards to the long‐term control of ghrelin secretion and the response to starvation.
Newer areas of research include the recent discovery of obestatin, a second hormone derived from the ghrelin gene with apparently opposing actions to ghrelin itself. In addition, studies have shown potential functions for des‐acyl ghrelin, the major circulating type of ghrelin molecule. Other work has suggested the presence of at least one so‐far‐undiscovered ghrelin receptor.
The clinical applications of a hormone that induces hunger and has other effects on the GI system are numerous. Preliminary trials have shown that it may prove extremely valuable in the management of illness‐induced cachexia, and also in the management of disorders of gut motility. In addition, ghrelin antagonists may in future have a powerful role to play in the medical management of obesity.
References
- Schwartz M. W., Woods S. C., Seeley R. J., Barsh G. S., Baskin D. G., Leibel R. L. Is the energy homeostasis system inherently biased toward weight gain?. Diabetes 2003; 52: 232–8
- Zigman J. M., Elmquist J. K. Minireview: From anorexia to obesity—the yin and yang of body weight control. Endocrinology 2003; 144: 3749–56
- Park A. J., Bloom S. R. Neuroendocrine control of food intake. Curr Opin Gastroenterol 2005; 21: 228–33
- Korner J., Leibel R. L. To eat or not to eat—how the gut talks to the brain. N Eng J Med 2003; 349: 926–8
- Small C. J., Bloom S. R. Gut hormones and the control of appetite. Trends Endocrinol Metab 2004; 15: 259–63
- Marx J. Cellular warriors at the battle of the bulge. Science 2003; 299: 846–9
- Gura T. Obesity drug pipeline not so fat. Science 2003; 299: 849–52
- Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. Ghrelin is a growth‐hormone‐releasing acylated peptide from stomach. Nature 1999; 402: 656–60
- Date Y., Kojima M., Hosoda H., Sawaguchi A., Mondal M. S., Suganuma T., et al. Ghrelin, a novel growth hormone‐releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000; 141: 4255–61
- Hou Z., Miao Y., Gao L., Pan H., Zhu S. Ghrelin‐containing neuron in cerebral cortex and hypothalamus linked with the DVC of brainstem in rat. Regul Pept 2006; 134: 126–31
- Gnanapavan S., Kola B., Bustin S. A., Morris D. G., McGee P., Fairclough P., et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS‐R, in humans. J Clin Endocrinol Metab 2002; 87: 2988–91
- Zhang J. V., Ren P. G., Avsian‐Kretchmer O., Luo C. W., Rauch R., Klein C., et al. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake. Science 2005; 310: 996–9
- Sun Y., Wang P., Zheng H., Smith R. G. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA 2004; 101: 4679–84
- Zigman J. M., Nakano Y., Coppari R., Balthasar N., Marcus J. N., Lee C. E., et al. Mice lacking ghrelin receptors resist the development of diet‐induced obesity. J Clin Invest 2005; 115: 3564–72
- Zigman J. M., Jones J. E., Lee C. E., Saper C. B., Elmquist J. K. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 2006; 494: 528–48
- Kojima M., Kangawa K. Ghrelin: structure and function. Physiol Rev 2005; 85: 495–522
- Ghigo E., Broglio F., Arvat E., Maccario M., Papotti M., Muccioli G. Ghrelin: more than a natural GH secretagogue and/or an orexigenic factor. Clin Endocrinol (Oxf) 2005; 62: 1–17
- Korbonits M., Goldstone A. P., Gueorguiev M., Grossman A. B. Ghrelin—a hormone with multiple functions. Front Neuroendocrinol 2004; 25: 27–68
- Tena‐Sempere M. Exploring the role of ghrelin as novel regulator of gonadal function. Growth Horm IGF Res 2005; 15: 83–8
- Hosoda H., Kojima M., Kangawa K. Biological, physiological, and pharmacological aspects of ghrelin. J Pharmacol Sci 2006; 100: 398–410
- Arvat E., Maccario M., Di Vito L., Broglio F., Benso A., Gottero C., et al. Endocrine activities of ghrelin, a natural GH secretagogue, in humans: comparison and interactions with hexarelin, a non natural peptidyl GHS, and GH‐releasing hormone. J Clin Endocrinol Metab 2001; 86: 1169–74
- Tschöp M., Smiley D. L., Heiman M. L. Ghrelin induces adiposity in rodents. Nature 2000; 407: 908–13
- Tebbe J. J., Mronga S., Tebbe C. G., Ortmann E., Arnold R., Schafer M. K. Ghrelin‐induced stimulation of colonic propulsion is dependent on hypothalamic neuropeptide Y1‐ and corticotrophin‐releasing factor 1 receptor activation. J Neuroendocrinol 2005; 17: 570–6
- Gauna C., Delhanty P. J., Hofland L. J., Janssen J. A., Broglio F., Ross R. J., et al. Ghrelin stimulates, whereas des‐octanoyl ghrelin inhibits, glucose output by primary hepatocytes. J Clin Endocrinol Metab 2005; 90: 1055–60
- Muccioli G., Pons N., Ghe C., Catapano F., Granata R., Ghigo E. Ghrelin and des‐acyl ghrelin both inhibit isoproterenol‐induced lipolysis in rat adipocytes via a non‐type 1a growth hormone secretagogue receptor. Eur J Pharmacol 2004; 498: 27–35
- Cao J. M., Ong H., Chen C. Effects of ghrelin and synthetic GH secretagogues on the cardiovascular system. Trends Endocrinol Metab 2006; 17: 13–18
- Henriques‐Coelho T., Roncon‐Albuquerque J. R., Lourenco A. P., Baptista M. J., Oliveira S. M., Brandao‐Nogueira A., et al. Ghrelin reverses molecular, structural and hemodynamic alterations of the right ventricle in pulmonary hypertension. Rev Port Cardiol 2006; 25: 55–63
- Tesauro M., Schinzari F., Iantorno M., Rizza S., Melina D., Lauro D., et al. Ghrelin improves endothelial function in patients with metabolic syndrome. Circulation 2005; 112: 2986–92
- Rudd J. A., Ngan M. P., Wai M. K., King A. G., Witherington J., Andrews P. L., et al. Anti‐emetic activity of ghrelin in ferrets exposed to the cytotoxic anti‐cancer agent cisplatin. Neurosci Lett 2006; 392: 79–83
- Brunetti L., Recinella L., Orlando G., Michelotto B., Di Nisio C., Vacca M. Effects of ghrelin and amylin on dopamine, norepinephrine and serotonin release in the hypothalamus. Eur J Pharmacol 2002; 454: 189–92
- Dixit V. D., Taub D. D. Ghrelin and immunity: A young player in an old field. Exp Gerontol 2005; 40: 900–10
- Yada T., Kaiya H., Mutoh K., Azuma T., Hyodo S., Kangawa K. Ghrelin stimulates phagocytosis and superoxide production in fish leukocytes. J Endocrinol 2006; 189: 57–65
- Takeda R., Nishimatsu H., Suzuki E., Satonaka H., Nagata D., Oba S., et al. Ghrelin improves renal function in mice with ischemic acute renal failure. J Am Soc Nephrol 2006; 17: 113–21
- Konturek P. C., Brzozowski T., Pajdo R., Nikiforuk A., Kwiecien S., Harsch I., et al. Ghrelin‐a new gastroprotective factor in gastric mucosa. J Physiol Pharmacol 2004; 55: 325–36
- Granado M., Priego T., Martin A. I., Villanua M. A., Lopez‐Calderon A. Anti‐inflammatory effect of the ghrelin agonist growth hormone‐releasing peptide‐2 (GHRP‐2) in arthritic rats. Am J Physiol Endocrinol Metab 2005; 288: E486–92
- Wu R., Dong W., Zhou M., Cui X., Hank S. H., Wang P. Ghrelin improves tissue perfusion in severe sepsis via downregulation of endothelin‐1. Cardiovasc Res 2005; 68: 318–26
- Fukushima N., Hanada R., Teranishi H., Fukue Y., Tachibana T., Ishikawa H., et al. Ghrelin directly regulates bone formation. J Bone Miner Res 2005; 20: 790–8
- Caminos J. E., Gualillo O., Lago F., Otero M., Blanco M., Gallego R., et al. The endogenous growth hormone secretagogue (ghrelin) is synthesized and secreted by chondrocytes. Endocrinology 2005; 146: 1285–92
- Matsuda K., Miura T., Kaiya H., Maruyama K., Uchiyama M., Kangawa K., et al. Stimulatory effect of n‐octanoylated ghrelin on locomotor activity in the goldfish, Carassius auratus. Peptides 2006; 27: 1335–40
- Jaszberenyi M., Bujdoso E., Bagosi Z., Telegdy G. Mediation of the behavioral, endocrine and thermoregulatory actions of ghrelin. Horm Behav 2006; 50: 266–73
- Tang‐Christensen M., Vrang N., Ortmann S., Bidlingmaier M., Horvath T. L., Tschop M. Central Administration of Ghrelin and Agouti‐Related Protein (83‐132) Increases Food Intake and Decreases Spontaneous Locomotor Activity in Rats. Endocrinology 2004; 145: 4645–52
- Diano S., Farr S. A., Benoit S. C., McNay E. C., da Silva I., Horvath B., et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci 2006; 9: 381–8
- Nakazato M., Murakami N., Date Y., Kojima M., Matsuo H., Kangawa K., et al. A role for ghrelin in the central regulation of feeding. Nature 2001; 409: 194–8
- Cummings D. E., Purnell J. Q., Frayo R. S., Schmidova K., Wisse B. E., Weigle D. S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001; 50: 1714–19
- Cummings D. E., Weigle D. S., Frayo R. S., Breen P. A., Ma M. K., Dellinger E. P., et al. Plasma ghrelin levels after diet‐induced weight loss or gastric bypass surgery. N Eng J Med 2002; 346: 1623–30
- Drazen D. L., Vahl T. P., D'Alessio D. A., Seeley R. J., Woods S. C. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology 2006; 147: 23–30
- Iqbal J., Kurose Y., Canny B., Clarke I. J. Effects of central infusion of ghrelin on food intake and plasma levels of growth hormone, luteinizing hormone, prolactin, and cortisol secretion in sheep. Endocrinology 2006; 147: 510–19
- Egecioglu E., Bjursell M., Ljungberg A., Dickson S. L., Kopchick J. J., Bergstrom G., et al. Growth hormone receptor deficiency results in blunted ghrelin feeding response, obesity, and hypolipidemia in mice. Am J Physiol Endocrinol Metab 2006; 290: E317–25
- Wortley K. E., Anderson K. D., Garcia K., Murray J. D., Malinova L., Liu R., et al. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci USA 2004; 101: 8227–32
- Wren A. M., Small C. J., Ward H. L., Murphy K. G., Dakin C. L., Taheri S., et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 2000; 141: 4325–8
- Tschöp M., Weyer C., Tataranni P. A., Devanarayan V., Ravussin E., Heiman M. L. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001; 50: 707–09
- Masuda Y., Tanaka T., Inomata N., Ohnuma N., Tanaka S., Itoh Z., et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun 2000; 276: 905–08
- Peeters T. L. Central and peripheral mechanisms by which ghrelin regulates gut motility. J Physiol Pharmacol 2003; 54 Suppl 4: 95–103
- Inui A., Asakawa A., Bowers C. Y., Mantovani G., Laviano A., Meguid M. M., et al. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J 2004; 18: 439–56
- Ohno T., Kamiyama Y., Aihara R., Nakabayashi T., Mochiki E., Asao T., et al. Ghrelin does not stimulate gastrointestinal motility and gastric emptying: an experimental study of conscious dogs. Neurogastroenterol Motil 2006; 18: 129–35
- Tack J., Depoortere I., Bisschops R., Verbeke K., Janssens J., Peeters T. Influence of ghrelin on gastric emptying and meal‐related symptoms in idiopathic gastroparesis. Aliment Pharmacol Ther 2005; 22: 847–53
- Sun Y., Ahmed S., Smith R. G. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol 2003; 23: 7973–81
- Wortley K. E., Del Rincon J. P., Murray J. D., Garcia K., Iida K., Thorner M. O., et al. Absence of ghrelin protects against early‐onset obesity. J Clin Invest 2005; 115: 3573–8
- De Smet B., Depoortere I., Moechars D., Swennen Q., Moreaux B., Cryns K., et al. Energy homeostasis and gastric emptying in ghrelin knockout mice. J Pharmacol Exp Ther 2006; 316: 431–9
- Shearman L. P., Wang S. P., Helmling S., Stribling D. S., Mazur P., Ge L., et al. Ghrelin neutralization by a ribonucleic acid‐SPM ameliorates obesity in diet‐induced obese mice. Endocrinology 2006; 147: 1517–26
- Vizcarra J. A., Kirby J. D., Kim S. K., Galyean M. L. Active immunization against ghrelin decreases weight gain and alters plasma concentrations of growth hormone in growing pigs. Domest Anim Endocrinol 2006 Jun 8, [Epub ahead of print]
- Ariyasu H., Takaya K., Iwakura H., Hosoda H., Akamizu T., Arai Y., et al. Transgenic mice overexpressing des‐acyl ghrelin show small phenotype. Endocrinology 2005; 146: 355–64
- Wei W., Qi X., Reed J., Ceci J., Wang H. Q., Wang G., et al. Effect of chronic hyperghrelinemia on ingestive action of ghrelin. Amer J Physiol Regul Integr Comp Physiol 2006; 290: R803–08
- Sun Y., Asnicar M., Saha P. K., Chan L., Smith R. G. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab 2006; 3: 379–86
- Asakawa A., Inui A., Kaga T., Katsuura G., Fujimiya M., Fujino M. A., et al. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut 2003; 52: 947–52
- Shuto Y., Shibasaki T., Otagiri A., Kuriyama H., Ohata H., Tamura H., et al. Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J Clin Invest 2002; 109: 1429–36
- Wortley K. E., Anderson K., Garcia K., Murray J., Malinova L., Liu R., et al. Deletion of ghrelin reveals no effect on food intake, but a primary role in energy balance. Obes Res 2004; 12: 170
- Luquet S., Perez F. A., Hnasko T. S., Palmiter R. D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 2005; 310: 683–5
- Gropp E., Shanabrough M., Borok E., Xu A. W., Janoschek R., Buch T., et al. Agouti‐related peptide‐expressing neurons are mandatory for feeding. Nat Neurosci 2005; 8: 1289–91
- Ste M. L., Luquet S., Cole T. B., Palmiter R. D. Modulation of neuropeptide Y expression in adult mice does not affect feeding. Proc Natl Acad Sci USA 2005; 102: 18632–7
- Bewick G. A., Gardiner J. V., Dhillo W. S., Kent A. S., White N. E., Webster Z., et al. Postembryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. FASEB J 2005; 19: 1680–2
- Nogueiras R., Tschop M. Biomedicine. Separation of conjoined hormones yields appetite rivals. Science 2005; 310: 985–6
- Ukkola O., Ravussin E., Jacobson P., Snyder E. E., Chagnon M., Sjostrom L., et al. Mutations in the preproghrelin/ghrelin gene associated with obesity in humans. J Clin Endocrinol Metab 2001; 86: 3996–9
- Hinney A., Hoch A., Geller F., Schafer H., Siegfried W., Goldschmidt H., et al. Ghrelin gene: identification of missense variants and a frameshift mutation in extremely obese children and adolescents and healthy normal weight students. J Clin Endocrinol Metab 2002; 87: 2716–19
- Ukkola O., Ravussin E., Jacobson P., Perusse L., Rankinen T., Tschöp M., et al. Role of ghrelin polymorphisms in obesity based on three different studies. Obes Res 2002; 10: 782–91
- Vivenza D., Rapa A., Castellino N., Bellone S., Petri A., Vacca G., et al. Ghrelin gene polymorphisms and ghrelin, insulin, IGF‐I, leptin and anthropometric data in children and adolescents. Eur J Endocrinol 2004; 151: 127–33
- Korbonits M., Gueorguiev M., O'Grady E., Lecoeur C., Swan D. C., Mein C. A., et al. A variation in the ghrelin gene increases weight and decreases insulin secretion in tall, obese children. J Clin Endocrinol Metab 2002; 87: 4005–08
- Miraglia D. G., Santoro N., Cirillo G., Raimondo P., Grandone A., D'Aniello A., et al. Molecular screening of the ghrelin gene in Italian obese children: the Leu72Met variant is associated with an earlier onset of obesity. Int J Obes Relat Metab Disord 2004; 28: 447–50
- Jo D. S., Kim S. L., Kim S. Y., Hwang P. H., Lee K. H., Lee D. Y. Preproghrelin Leu72Met polymorphism in obese Korean children. J Pediatr Endocrinol Metab 2005; 18: 1083–6
- Bing C., Ambye L., Fenger M., Jorgensen T., Borch‐Johnsen K., Madsbad S., et al. Large‐scale studies of the Leu72Met polymorphism of the ghrelin gene in relation to the metabolic syndrome and associated quantitative traits. Diabet Med 2005; 22: 1157–60
- Steinle N. I., Pollin T. I., O'Connell J. R., Mitchell B. D., Shuldiner A. R. Variants in the ghrelin gene are associated with metabolic syndrome in the Old Order Amish. J Clin Endocrinol Metab 2005; 90: 6672–7
- Vartiainen J., Kesaniemi Y. A., Ukkola O. Sequencing analysis of ghrelin gene 5′ flanking region: relations between the sequence variants, fasting plasma total ghrelin concentrations, and body mass index. Metabolism 2006; 55: 1420–5
- Bittel D. C., Kibiryeva N., Dasouki M., Knoll J. H., Butler M. G. A 9‐year‐old male with a duplication of chromosome 3p25.3p26.2: Clinical report and gene expression analysis. Am J Med Genet A 2006; 140: 573–9
- Baessler A., Hasinoff M. J., Fischer M., Reinhard W., Sonnenberg G. E., Olivier M., et al. Genetic linkage and association of the growth hormone secretagogue receptor (ghrelin receptor) gene in human obesity. Diabetes 2005; 54: 259–67
- Wang H. J., Geller F., Dempfle A., Schauble N., Friedel S., Lichtner P., et al. Ghrelin receptor gene: identification of several sequence variants in extremely obese children and adolescents, healthy normal‐weight and underweight students, and children with short normal stature. J Clin Endocrinol Metab 2004; 89: 157–62
- Cellini E., Nacmias B., Brecelj‐Anderluh M., Badia‐Casanovas A., Bellodi L., Boni C., et al. Case‐control and combined family trios analysis of three polymorphisms in the ghrelin gene in European patients with anorexia and bulimia nervosa. Psychiatr Genet 2006; 16: 51–2
- Monteleone P., Tortorella A., Castaldo E., Di Filippo C., Maj M. No association of the Arg51Gln and Leu72Met polymorphisms of the ghrelin gene with anorexia nervosa or bulimia nervosa. Neurosci Lett 2006; 398: 325–7
- Miyasaka K., Hosoya H., Sekime A., Ohta M., Amono H., Matsushita S., et al. Association of ghrelin receptor gene polymorphism with bulimia nervosa in a Japanese population. J Neural Transm 2006; 113: 1279–85
- Ando T., Komaki G., Naruo T., Okabe K., Takii M., Kawai K., et al. Possible role of preproghrelin gene polymorphisms in susceptibility to bulimia nervosa. Am J Med Genet B Neuropsychiatr Genet 2006; 141: 929–34
- Mager U., Kolehmainen M., Lindstrom J., Eriksson J. G., Valle T. T., Hamalainen H., et al. Association between ghrelin gene variations and blood pressure in subjects with impaired glucose tolerance. Am J Hypertens 2006; 19: 920–6
- Pöykkö S. M., Ukkola O., Kauma H., Savolainen M. J., Kesäniemi Y. A. Ghrelin Arg51Gln mutation is a risk factor for Type 2 diabetes and hypertension in a random sample of middle‐aged subjects. Diabetologia 2003; 46: 455–8
- Larsen L. H., Gjesing A. P., Sorensen T. I., Hamid Y. H., Echwald S. M., Toubro S., et al. Mutation analysis of the preproghrelin gene: no association with obesity and type 2 diabetes. Clin Biochem 2005; 38: 420–4
- Choi H. J., Cho Y. M., Moon M. K., Choi H. H., Shin H. D., Jang H. C., et al. Polymorphisms in the Ghrelin Gene Are Associated with Serum High‐Density Lipoprotein Cholesterol Level and not with Type 2 Diabetes Mellitus in Koreans. J Clin Endocrinol Metab 2006; 91: 4657–63
- Vartiainen J., Poykko S. M., Raisanen T., Kesaniemi Y. A., Ukkola O. Sequencing analysis of the ghrelin receptor (growth hormone secretagogue receptor type 1a) gene. Eur J Endocrinol 2004; 150: 457–63
- Ukkola O., Kesäniemi Y. A. Preproghrelin Leu72Met polymorphism in patients with type 2 diabetes mellitus. J Intern Med 2003; 254: 391–4
- Kim S. Y., Jo D. S., Hwang P. H., Park J. H., Park S. K., Yi H. K., et al. Preproghrelin Leu72Met polymorphism is not associated with type 2 diabetes mellitus. Metabolism 2006; 55: 366–70
- Lee D. Y., Kim S. Y., Jo D. S., Hwang P. H., Kang K. P., Lee S., et al. Preproghrelin Leu72Met polymorphism predicts a lower rate of developing renal dysfunction in type 2 diabetic nephropathy. Eur J Endocrinol 2006; 155: 187–90
- Baessler A., Kwitek A. E., Fischer M., Koehler M., Reinhard W., Erdmann J., et al. Association of the Ghrelin receptor gene region with left ventricular hypertrophy in the general population: results of the MONICA/KORA Augsburg Echocardiographic Substudy. Hypertension 2006; 47: 920–7
- Wiltshire S., Frayling T. M., Hattersley A. T., Hitman G. A., Walker M., Levy J. C., et al. Evidence for linkage of stature to chromosome 3p26 in a large U.K. family data set ascertained for type 2 diabetes. Am J Hum Genet 2002; 70: 543–6
- Pöykkö S. M., Ukkola O., Kauma H., Kellokoski E., Horkko S., Kesaniemi Y. A. The negative association between plasma ghrelin and IGF‐I is modified by obesity, insulin resistance and type 2 diabetes. Diabetologia 2005; 48: 309–16
- Pantel J., Legendre M., Cabrol S., Hilal L., Hajaji Y., Morisset S., et al. Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. J Clin Invest 2006; 116: 760–8
- Hosoda H., Kojima M., Matsuo H., Kangawa K. Purification and characterization of rat des‐Gln14‐Ghrelin, a second endogenous ligand for the growth hormone secretagogue receptor. J Biol Chem 2000; 275: 21995–2000
- Delhanty P. J., van der Eerden B. C., van der Velde M., Gauna C., Pols H. A., Jahr H., et al. Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen‐activated protein kinase (MAPK)/phosphoinositide 3‐kinase (PI3K) pathways in the absence of GHS‐R1a. J Endocrinol 2006; 188: 37–47
- Broglio F., Gottero C., Prodam F., Gauna C., Muccioli G., Papotti M., et al. Non‐acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J Clin Endocrinol Metab 2004; 89: 3062–5
- Gauna C., Meyler F. M., Janssen J. A., Delhanty P. J., Abribat T., van Koetsveld P., et al. Administration of acylated ghrelin reduces insulin sensitivity, whereas the combination of acylated plus unacylated ghrelin strongly improves insulin sensitivity. J Clin Endocrinol Metab 2004; 89: 5035–42
- Chen C. Y., Chao Y., Chang F. Y., Chien E. J., Lee S. D., Doong M. L. Intracisternal des‐acyl ghrelin inhibits food intake and non‐nutrient gastric emptying in conscious rats. Int J Mol Med 2005; 16: 695–9
- Chen C. Y., Inui A., Asakawa A., Fujino K., Kato I., Chen C. C., et al. Des‐acyl Ghrelin Acts by CRF Type 2 Receptors to Disrupt Fasted Stomach Motility in Conscious Rats. Gastroenterology 2005; 129: 8–25
- Toshinai K., Yamaguchi H., Sun Y., Smith R. G., Yamanaka A., Sakurai T., et al. Des‐acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology 2006; 147: 2306–14
- Nanzer A. M., Khalaf S., Mozid A. M., Fowkes R. C., Patel M. V., Burrin J. M., et al. Ghrelin exerts a proliferative effect on a rat pituitary somatotroph cell line via the mitogen‐activated protein kinase pathway. Eur J Endocrinol 2004; 151: 233–40
- Thompson N. M., Gill D. A., Davies R., Loveridge N., Houston P. A., Robinson I. C., et al. Ghrelin and des‐octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of GHS‐R1a. Endocrinology 2004; 145: 232–42
- Bedendi I., Alloatti G., Marcantoni A., Malan D., Catapano F., Ghe C., et al. Cardiac effects of ghrelin and its endogenous derivatives des‐octanoyl ghrelin and des‐Gln(14)‐ghrelin. Eur J Pharmacol 2003; 476: 87–95
- Baldanzi G., Filigheddu N., Cutrupi S., Catapano F., Bonissoni S., Fubini A., et al. Ghrelin and des‐acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3‐kinase/AKT. J Cell Biol 2002; 159: 1029–37
- Cassoni P., Papotti M., Ghe C., Catapano F., Sapino A., Graziani A., et al. Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J Clin Endocrinol Metab 2001; 86: 1738–45
- Asakawa A., Inui A., Fujimiya M., Sakamaki R., Shinfuku N., Ueta Y., et al. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut 2005; 54: 18–24
- Kleinz M. J., Maguire J. J., Skepper J. N., Davenport A. P. Functional and immunocytochemical evidence for a role of ghrelin and des‐octanoyl ghrelin in the regulation of vascular tone in man. Cardiovasc Res 2006; 69: 227–35
- Heijboer A. C., van den Hoek A. M., Parlevliet E. T., Havekes L. M., Romijn J. A., Pijl H., et al. Ghrelin differentially affects hepatic and peripheral insulin sensitivity in mice. Diabetologia 2006; 49: 732–8
- Neary N. M., Druce M. R., Small C. J., Bloom S. R. Acylated ghrelin stimulates food intake in the fed and fasted states but desacylated ghrelin has no effect. Gut 2006; 55: 135
- Horvath T. L., Diano S., Sotonyi P., Heiman M., Tschöp M. Minireview: ghrelin and the regulation of energy balance—a hypothalamic perspective. Endocrinology 2001; 142: 4163–9
- Banks W. A., Tschöp M., Robinson S. M., Heiman M. L. Extent and direction of ghrelin transport across the blood‐brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther 2002; 302: 822–7
- Date Y., Murakami N., Toshinai K., Matsukura S., Niijima A., Matsuo H., et al. The role of the gastric afferent vagal nerve in ghrelin‐induced feeding and growth hormone secretion in rats. Gastroenterology 2002; 123: 1120–8
- Lawrence C. B., Snape A. C., Baudoin F. M., Luckman S. M. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology 2002; 143: 155–62
- Dickson S. L., Luckman S. M. Induction of c‐fos messenger ribonucleic acid in neuropeptide Y and growth hormone (GH)‐releasing factor neurones in the rat arcuate nucleus following systemic injection of the GH secretagogue, GH‐releasing peptide‐6. Endocrinology 1997; 138: 771–7
- Riediger T., Traebert M., Schmid H. A., Scheel C., Lutz T. A., Scharrer E. Site‐specific effects of ghrelin on the neuronal activity in the hypothalamic arcuate nucleus. Neurosci Lett 2003; 341: 151–5
- Chen H. Y., Trumbauer M. E., Chen A. S., Weingarth D. T., Adams J. R., Frazier E. G., et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y (NPY) and agouti‐related protein (AgRP). Endocrinology 2004; 145: 2607–12
- Tschöp M., Statnick M. A., Suter T. M., Heiman M. L. GH‐Releasing peptide‐2 increases fat mass in mice lacking NPY: indication for a crucial mediating role of hypothalamic agouti‐related protein. Endocrinology 2002; 143: 558–68
- Qian S., Chen H., Weingarth D., Trumbauer M. E., Novi D. E., Guan X., et al. Neither agouti‐related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Cell Biol 2002; 22: 5027–35
- Andersson U., Filipsson K., Abbott C. R., Woods A., Smith K., Bloom S. R., et al. AMP‐activated protein kinase plays a role in the control of food intake. J Biol Chem 2004; 279: 12005–08
- Kola B., Hubina E., Tucci S. A., Kirkham T. C., Garcia E. A., Mitchell S. E., et al. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP‐activated protein kinase. J Biol Chem 2005; 280: 25196–201
- Halem H. A., Taylor J. E., Dong J. Z., Shen Y., Datta R., Abizaid A., et al. Novel analogs of ghrelin: physiological and clinical implications. Eur J Endocrinol 2004; 151 Suppl 2: S071–5
- Halem H. A., Taylor J. E., Dong J. Z., Shen Y., Datta R., Abizaid A., et al. A novel growth hormone secretagogue‐1a receptor antagonist that blocks ghrelin‐induced growth hormone secretion but induces increased body weight gain. Neuroendocrinol 2005; 81: 339–49
- Zizzari P., Halem H., Taylor J., Dong J. Z., Datta R., Culler M. D., et al. Endogenous ghrelin regulates episodic growth hormone (GH) secretion by amplifying GH Pulse amplitude: evidence from antagonism of the GH secretagogue‐R1a receptor. Endocrinology 2005; 146: 3836–42
- Shintani M., Ogawa Y., Ebihara K., Aizawa‐Abe M., Miyanaga F., Takaya K., et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes 2001; 50: 227–32
- Ueno N., Dube M. G., Inui A., Kalra P. S., Kalra S. P. Leptin modulates orexigenic effects of ghrelin, attenuates adiponectin and insulin levels, and selectively the dark‐phase feeding as revealed by central leptin gene therapy. Endocrinology 2004; 145: 4176–84
- Chan J. L., Bullen J., Lee J. H., Yiannakouris N., Mantzoros C. S. Ghrelin levels are not regulated by recombinant leptin administration and/or three days of fasting in healthy subjects. J Clin Endocrinol Metab 2004; 89: 335–43
- Natalucci G., Riedl S., Gleiss A., Zidek T., Frisch H. Spontaneous 24‐h ghrelin secretion pattern in fasting subjects: maintenance of a meal‐related pattern. Eur J Endocrinol 2005; 152: 845–50
- Caixas A., Bashore C., Nash W., Pi‐Sunyer F., Laferrere B. Insulin, unlike food intake, does not suppress ghrelin in human subjects. J Clin Endocrinol Metab 2002; 87: 1902–06
- Murdolo G., Lucidi P., Di Loreto C., Parlanti N., De Cicco A., Fatone C., et al. Insulin is Required for Prandial Ghrelin Suppression in Humans. Diabetes 2003; 52: 2923–7
- Flanagan D. E., Evans M. L., Monsod T. P., Rife F., Heptulla R. A., Tamborlane W. V., et al. The influence of insulin on circulating ghrelin. Am J Physiol Endocrinol Metab 2003; 284: E313–16
- Nakagawa E., Nagaya N., Okumura H., Enomoto M., Oya H., Ono F., et al. Hyperglycaemia suppresses the secretion of ghrelin, a novel growth‐ hormone‐releasing peptide: responses to the intravenous and oral administration of glucose. Clin Sci (Lond) 2002; 103: 325–8
- Saad M. F., Bernaba B., Hwu C. M., Jinagouda S., Fahmi S., Kogosov E., et al. Insulin regulates plasma ghrelin concentration. J Clin Endocrinol Metab 2002; 87: 3997–4000
- Coiro V., Volpi R., Capretti L., Caffarri G., Colla R., Chiodera P. Effects of pyridostigmine and naloxone on the abnormal TSH response to TRH during starvation in humans. J Investig Med 1999; 47: 227–31
- Stoving R. K., Andersen M., Flyvbjerg A., Frystyk J., Hangaard J., Vinten J., et al. Indirect evidence for decreased hypothalamic somatostatinergic tone in anorexia nervosa. Clin Endocrinol (Oxf) 2002; 56: 391–6
- Thuesen B., Schaffalitzky de Muckadell O. B., Holst J. J., Bahnsen M. The relationship of secretin and somatostatin levels in plasma to glucose administration and acid secretion during fasting. Am J Gastroenterol 1987; 82: 723–6
- Hirsh D., Heinrichs C., Leenders B., Wong A. C., Cummings D. E., Chanoine J. P. Ghrelin Is Suppressed by Glucagon and Does Not Mediate Glucagon‐Related Growth Hormone Release. Horm Res 2005; 63: 111–18
- Arafat M. A., Otto B., Rochlitz H., Tschop M., Bahr V., Mohlig M., et al. Glucagon inhibits ghrelin secretion in humans. Eur J Endocrinol 2005; 153: 397–402
- Arafat A. M., Perschel F. H., Otto B., Weickert M. O., Rochlitz H., Schofl C., et al. Glucagon suppression of ghrelin secretion is exerted at hypothalamus‐pituitary level. J Clin Endocrinol Metab 2006; 91: 3528–33
- Sugino T., Yamaura J., Yamagishi M., Ogura A., Hayashi R., Kurose Y., et al. A transient surge of ghrelin secretion before feeding is modified by different feeding regimens in sheep. Biochem Biophys Res Commun 2002; 298: 785–8
- Sugino T., Hasegawa Y., Kikkawa Y., Yamaura J., Yamagishi M., Kurose Y., et al. A transient ghrelin surge occurs just before feeding in a scheduled meal‐fed sheep. Biochem Biophys Res Commun 2002; 295: 255–60
- Williams D. L., Cummings D. E., Grill H. J., Kaplan J. M. Meal‐related ghrelin suppression requires postgastric feedback. Endocrinology 2003; 144: 2765–7
- Broglio F., Gottero C., van Koetsveld P., Prodam F., Destefanis S., Benso A., et al. Acetylcholine regulates ghrelin secretion in humans. J Clin Endocrinol Metab 2004; 89: 2429–33
- Williams D. L., Grill H. J., Cummings D. E., Kaplan J. M. Vagotomy dissociates short‐ and long‐term controls of circulating ghrelin. Endocrinology 2003; 144: 5184–7
- Lee H. M., Wang G., Englander E. W., Kojima M., Greeley J. G., Jr. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology 2002; 143: 185–90
- Asakawa A., Inui A., Kaga T., Yuzuriha H., Nagata T., Ueno N., et al. Ghrelin is an appetite‐stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 2001; 120: 337–45
- Maier C., Schaller G., Buranyi B., Nowotny P., Geyer G., Wolzt M., et al. The cholinergic system controls ghrelin release and ghrelin‐induced growth hormone release in humans. J Clin Endocrinol Metab 2004; 89: 4729–33
- Le Roux C. W., Neary N. M., Halsey T. J., Small C. J., Martinez‐Isla A. M., Ghatei M. A., et al. Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J Clin Endocrinol Metab 2005; 90: 4521–4
- Gormsen L. C., Gjedsted J., Gjedde S., Vestergaard E. T., Christiansen J. S., Jorgensen J. O., et al. Free fatty acids decrease circulating ghrelin concentrations in humans. Eur J Endocrinol 2006; 154: 667–73
- Blom W. A., Lluch A., Vinoy S., Stafleu A., van den Berg R., Holst J. J., et al. Effects of gastric emptying on the postprandial ghrelin response. Am J Physiol Endocrinol Metab 2006; 290: E389–95
- Batterham R. L., Cohen M. A., Ellis S. M., Le Roux C. W., Withers D. J., Frost G. S., et al. Inhibition of food intake in obese subjects by peptide YY3‐36. N Eng J Med 2003; 349: 941–8
- Nagaya N., Uematsu M., Kojima M., Date Y., Nakazato M., Okumura H., et al. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation 2001; 104: 2034–8
- Shimizu Y., Nagaya N., Isobe T., Imazu M., Okumura H., Hosoda H., et al. Increased plasma ghrelin level in lung cancer cachexia. Clin Cancer Res 2003; 9: 774–8
- Otto B., Cuntz U., Fruehauf E., Wawarta R., Folwaczny C., Riepl R. L., et al. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol 2001; 145: 669–73
- Espelund U., Hansen T. K., Hojlund K., Beck‐Nielsen H., Clausen J. T., Hansen B. S., et al. Fasting unmasks a strong inverse association between ghrelin and cortisol in serum: studies in obese and normal‐weight subjects. J Clin Endocrinol Metab 2005; 90: 741–6
- Hansen T. K., Dall R., Hosoda H., Kojima M., Kangawa K., Christiansen J. S., et al. Weight loss increases circulating levels of ghrelin in human obesity. Clin Endocrinol (Oxf) 2002; 56: 203–06
- Korbonits M., Blaine D., Elia M., Powell‐Tuck J. Refeeding Blaine: studies following a 44 day fast. N Eng J Med 2005; 353: 2307–08
- Broglio F., Koetsveld P. P., Benso A., Gottero C., Prodam F., Papotti M., et al. Ghrelin secretion is inhibited by either somatostatin or cortistatin in humans. J Clin Endocrinol Metab 2002; 87: 4829–32
- Tan T. M., Vanderpump M., Khoo B., Patterson M., Ghatei M. A., Goldstone A. P. Somatostatin infusion lowers plasma ghrelin without reducing appetite in adults with Prader‐Willi syndrome. J Clin Endocrinol Metab 2004; 89: 4162–5
- Inui A. Cancer anorexia‐cachexia syndrome: current issues in research and management. CA Cancer J Clin 2002; 52: 72–91
- Chung S. H., Lindholm B., Lee H. B. Is malnutrition an independent predictor of mortality in peritoneal dialysis patients?. Nephrol Dial Transplant 2003; 18: 2134–40
- Tang A. M., Forrester J., Spiegelman D., Knox T. A., Tchetgen E., Gorbach S. L. Weight loss and survival in HIV‐positive patients in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2002; 31: 230–6
- Nagaya N., Kojima M., Kangawa K. Ghrelin, a novel growth hormone‐releasing peptide, in the treatment of cardiopulmonary‐associated cachexia. Intern Med 2006; 45: 127–34
- Neary N. M., Small C. J., Wren A. M., Lee J. L., Druce M. R., Palmieri C., et al. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo‐controlled trial. J Clin Endocrinol Metab 2004; 89: 2832–6
- Wang W., Andersson M., Iresjo B. M., Lonnroth C., Lundholm K. Effects of ghrelin on anorexia in tumor‐bearing mice with eicosanoid‐related cachexia. Int J Oncol 2006; 28: 1393–400
- Wynne K., Giannitsopoulou K., Small C. J., Patterson M., Frost G., Ghatei M. A., et al. Subcutaneous ghrelin enhances acute food intake in malnourished patients who receive maintenance peritoneal dialysis: a randomized, placebo‐controlled trial. J Am Soc Nephrol 2005; 16: 2111–18
- Nagaya N., Moriya J., Yasumura Y., Uematsu M., Ono F., Shimizu W., et al. Effects of Ghrelin Administration on Left Ventricular Function, Exercise Capacity, and Muscle Wasting in Patients With Chronic Heart Failure. Circulation 2004; 110: 3674–9
- Nagaya N., Uematsu M., Kojima M., Ikeda Y., Yoshihara F., Shimizu W., et al. Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation 2001; 104: 1430–5
- Nagaya N., Itoh T., Murakami S., Oya H., Uematsu M., Miyatake K., et al. Treatment of Cachexia With Ghrelin in Patients With COPD. Chest 2005; 128: 1187–93
- Miljic D., Pekic S., Djurovic M., Doknic M., Milic N., Casanueva F., et al. Ghrelin has partial or no effect on appetite, growth hormone (GH), prolactin and cortisol release in patients with anorexia nervosa. J Clin Endocrinol Metab 2006; 91: 1491–5
- Cummings D. E., Shannon M. H. Ghrelin and gastric bypass: is there a hormonal contribution to surgical weight loss?. J Clin Endocrinol Metab 2003; 88: 2999–3002
- Otto B., Tschop M., Fruhauf E., Heldwein W., Fichter M., Otto C., et al. Postprandial ghrelin release in anorectic patients before and after weight gain. Psychoneuroendocrinology 2005; 30: 577–81
- Trudel L., Tomasetto C., Rio M. C., Bouin M., Plourde V., Eberling P., et al. Ghrelin/motilin‐related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol Gastrointest Liver Physiol 2002; 282: G948–52
- Poitras P., Polvino W. J., Rocheleau B. Gastrokinetic effect of ghrelin analog RC‐1139 in the rat Effect on post‐operative and on morphine induced ileus. Peptides 2005; 26: 1598–601
- De Winter B. Y., De Man J. G., Seerden T. C., Depoortere I., Herman A. G., Peeters T. L., et al. Effect of ghrelin and growth hormone‐releasing peptide 6 on septic ileus in mice. Neurogastroenterol Motil 2004; 16: 439–46
- Murray C. D., Martin N. M., Patterson M., Taylor S., Ghatei M. A., Kamm M. A., et al. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double‐blind, placebo‐controlled, crossover study. Gut 2005; 54: 1693–8
- Liu Y. L., Malik N. M., Sanger G. J., Andrews P. L. Ghrelin alleviates cancer chemotherapy‐associated dyspepsia in rodents. Cancer Chemother Pharmacol 2006; 58: 326–33
- Kitazawa T., De Smet B., Verbeke K., Depoortere I., Peeters T. Gastric motor effects of peptide and non‐peptide ghrelin agonists in mice in vivo and in vitro. Gut 2005; 54: 1078–84
- Le Roux C. W., Patterson M., Vincent R. P., Hunt C., Ghatei M. A., Bloom S. R. Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal‐weight but not obese subjects. J Clin Endocrinol Metab 2005; 90: 1068–71
- Valera Mora M. E., Scarfone A., Valenza V., Calvani M., Greco A. V., Gasbarrini G., et al. Ghrelin does not influence gastric emptying in obese subjects. Obes Res 2005; 13: 739–44
- Pöykkö S. M., Kellokoski E., Horkko S., Kauma H., Kesaniemi Y. A., Ukkola O. Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes 2003; 52: 2546–53
- McLaughlin T., Abbasi F., Lamendola C., Frayo R. S., Cummings D. E. Plasma ghrelin concentrations are decreased in insulin‐resistant obese adults relative to equally obese insulin‐sensitive controls. J Clin Endocrinol Metab 2004; 89: 1630–5
- Purnell J. Q., Weigle D. S., Breen P. A., Cummings D. E. Ghrelin levels correlate with insulin levels, insulin resistance, and high‐density lipoprotein cholesterol, but not with gender, menopausal status, or cortisol levels in humans. J Clin Endocrinol Metab 2003; 88: 5747–52
- Goldstone A. P., Thomas E. L., Brynes A. E., Castroman G., Edwards R., Ghatei M. A., et al. Elevated fasting plasma ghrelin in Prader‐Willi syndrome adults is not solely explained by their reduced visceral adiposity and insulin resistance. J Clin Endocrinol Metab 2004; 89: 1718–26
- Ikezaki A., Hosoda H., Ito K., Iwama S., Miura N., Matsuoka H., et al. Fasting plasma ghrelin levels are negatively correlated with insulin resistance and PAI‐1, but not with leptin, in obese children and adolescents. Diabetes 2002; 51: 3408–11
- Bacha F., Arslanian S. A. Ghrelin suppression in overweight children: a manifestation of insulin resistance?. J Clin Endocrinol Metab 2005; 90: 2725–30
- Anderwald C., Brabant G., Bernroider E., Horn R., Brehm A., Waldhausl W., et al. Insulin‐dependent modulation of plasma ghrelin and leptin concentrations is less pronounced in type 2 diabetic patients. Diabetes 2003; 52: 1792–8
- Masaoka T., Suzuki H., Hosoda H., Ota T., Minegishi Y., Nagata H., et al. Enhanced plasma ghrelin levels in rats with streptozotocin‐induced diabetes. FEBS Lett 2003; 541: 64–8
- Ishii S., Kamegai J., Tamura H., Shimizu T., Sugihara H., Oikawa S. Role of ghrelin in streptozotocin‐induced diabetic hyperphagia. Endocrinology 2002; 143: 4934–7
- Holdstock C., Ludvigsson J., Karlsson F. A. Abnormal ghrelin secretion in new onset childhood Type 1 diabetes. Diabetologia 2004; 47: 150–1
- Briatore L., Andraghetti G., Cordera R. Acute plasma glucose increase, but not early insulin response, regulates plasma ghrelin. Eur J Endocrinol 2003; 149: 403–06
- Soriano‐Guillen L., Barrios V., Lechuga‐Sancho A., Chowen J. A., Argente J. Response of circulating ghrelin levels to insulin therapy in children with newly diagnosed type 1 diabetes mellitus. Pediatr Res 2004; 55: 830–5
- Shiiya T., Nakazato M., Mizuta M., Date Y., Mondal M. S., Tanaka M., et al. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab 2002; 87: 240–4
- Goldstone A. P. Prader‐Willi syndrome: advances in genetics, pathophysiology and treatment. Trends Endocrinol Metab 2004; 15: 12–20
- Goldstone A. P., Patterson M., Kalingag N., Ghatei M. A., Brynes A. E., Bloom S. R., et al. Fasting and postprandial hyperghrelinemia in Prader‐Willi syndrome is partially explained by hypoinsulinemia, and is not due to peptide YY3‐36 deficiency or seen in hypothalamic obesity due to craniopharyngioma. J Clin Endocrinol Metab 2005; 90: 2681–90
- Cummings D. E., Clement K., Purnell J. Q., Vaisse C., Foster K. E., Frayo R. S., et al. Elevated plasma ghrelin levels in Prader‐Willi syndrome. Nat Med 2002; 8: 643–4
- DelParigi A., Tschöp M., Heiman M. L., Salbe A. D., Vozarova B., Sell S. M., et al. High circulating ghrelin: a potential cause for hyperphagia and obesity in Prader‐Willi syndrome. J Clin Endocrinol Metab 2002; 87: 5461–4
- Aylwin S. J. Gastrointestinal surgery and gut hormones. Current Opinion In Endocrinology and Diabetes 2005; 12: 89–98
- Mariani L. M., Fusco A., Turriziani M., Veneziani A., Marini M. A., de Lorenzo A., et al. Transient increase of plasma ghrelin after laparoscopic adjustable gastric banding in morbid obesity. Horm Metab Res 2005; 37: 242–5
- Stratis C., Alexandrides T., Vagenas K., Kalfarentzos F. Ghrelin and peptide YY levels after a variant of biliopancreatic diversion with Roux‐en‐Y gastric bypass versus after colectomy: a prospective comparative study. Obes Surg 2006; 16: 752–8
- Langer F. B., Reza Hoda M. A., Bohdjalian A., Felberbauer F. X., Zacherl J., Wenzl E., et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg 2005; 15: 1024–9
- Foschi D., Corsi F., Rizzi A., Asti E., Carsenzuola V., Vago T., et al. Vertical banded gastroplasty modifies plasma ghrelin secretion in obese patients. Obes Surg 2005; 15: 1129–32
- Suzuki S., Ramos E. J., Goncalves C. G., Chen C., Meguid M. M. Changes in GI hormones and their effect on gastric emptying and transit times after Roux‐en‐Y gastric bypass in rat model. Surgery 2005; 138: 283–90
- Stylopoulos N., Davis P., Pettit J. D., Rattner D. W., Kaplan L. M. Changes in serum ghrelin predict weight loss after Roux‐en‐Y gastric bypass in rats. Surg Endosc 2005; 19: 942–6
- Lin E., Gletsu N., Fugate K., McClusky D., Gu L. H., Zhu J. L., et al. The effects of gastric surgery on systemic ghrelin levels in the morbidly obese. Arch Surg 2004; 139: 780–4
- Borg C. M., Le Roux C. W., Ghatei M. A., Bloom S. R., Patel A. G., Aylwin S. J. Progressive rise in gut hormone levels after Roux‐en‐Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg 2006; 93: 210–15
- Le Roux C. W., Aylwin S. J., Batterham R. L., Borg C. M., Coyle F., Prasad V., et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 2006; 243: 108–14
- Korner J., Bessler M., Cirilo L. J., Conwell I. M., Daud A., Restuccia N. L., et al. Effects of Roux‐en‐Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab 2005; 90: 359–65
- Ram E., Vishne T., Diker D., Gal‐Ad I., Maayan R., Lerner I., et al. Impact of gastric banding on plasma ghrelin, growth hormone, cortisol, DHEA and DHEA‐S levels. Obes Surg 2005; 15: 1118–23