Abstract
The prevalence of immunoglobulin E (IgE)‐mediated allergic diseases has been increasing for the last four decades. In this review determinants for an increased IgE synthesis are discussed on both an epidemiological and on an immunological level with special emphasis on the differentiation of the B cell to an IgE‐producing plasma cell. Factors that favor an IgE immune response are low antigen doses and immunization via mucous membranes, but it is highly likely that other environmental factors besides exposure to the allergenic sources play a role. Important factors in the formation of the Thelper type 2 (Th2) T cell subset are the actions of thymic stromal lymphopoietin (TSLP) on dendritic cells and the OX40 ligand on CD4+ T cells. In order for a B lymphocyte to switch to IgE production it needs two signals provided by a Th2 cell in the form of the cytokines interleukin (IL‐) 4/IL‐13 and ligation of the CD40. In spite of a half‐life of only a few days, there is evidence that the IgE response may last for years even without allergen stimulation. This is likely to be caused by long‐lived IgE‐producing plasma cells, and such cells may be difficult to target therapeutically thus emphasizing the need for more knowledge on preventable causes of IgE‐ and allergy development.
Introduction
The immunoglobulin (IgE)‐mediated allergic reaction is one of the fastest and potentially most vigorous reactions mounted by the immune system, where life‐threatening asthma or fatal reactions due to anaphylaxis may occur only 5–10 minutes after administration of an allergen. Luckily, only few of the many allergic patients will ever experience generalized reactions involving the vascular system, whereas symptoms from the mucous membranes of the lungs (asthma), upper airways/eyes (hay fever), and the digestive tract (food allergy) are much more common.
While the last three to four decades of the previous millennium exhibited a dramatic increase in the prevalence of allergic diseases in the westernized world, it now seems that a similar increase is becoming manifest in the fast developing countries such as India and China Citation1. Even in the Arctic region, the development is clearly visible, with a doubling of sensitization rates in only 11 years from 1987 to 1998 Citation2.
In the present paper we shall focus on the sensitization process, i.e. the afferent part of the IgE immune response, where specific IgE antibodies are produced and distributed throughout the body, where they bind to high‐affinity receptors on the effector cells: mast cells and basophil granulocytes. An overview of the entire pathogenesis is presented in Figure .
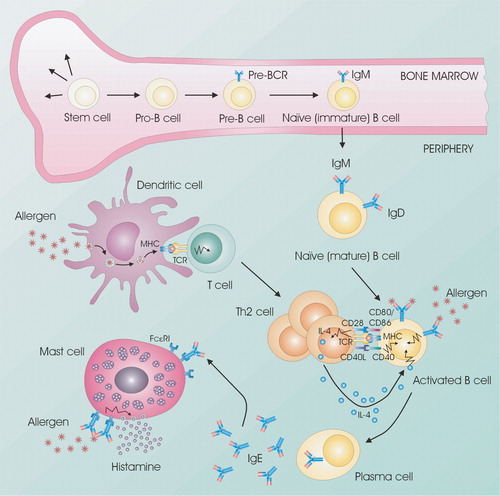
Figure 1 Cellular interactions are important in allergic reactions. B cells develop from stem cells in the bone marrow. Naïve B cells that exit the bone marrow will travel to the lymph nodes for activation. Allergens are taken up by antigen‐presenting cells (e.g. dendritic cells) and presented to T cells via MHC class II, which become activated and proliferate into Th2 cells. When B cells encounter their specific antigen, it is presented on the surface of the B cell via MHC class II. The subsequent stimulation of specific Th2 cells leads to the production of IL‐4 and the upregulation of expression of CD40L by T cells. CD40 stimulation of allergen‐specific B cells upregulates the expression of the costimulatory molecules CD80 and CD86, which allows for more efficient T cell expression of CD40L and enhanced stimulation of B cells through the induction of IL‐4. CD40‐mediated stimulation of B cells synergizes with IL‐4 receptor signals to enhance rearrangement of the IgE genomic locus and production of IgE antibodies. Allergen‐specific IgE binds to the high‐affinity receptor for IgE (FcϵRI) on mast cells. Allergen exposure induces cross‐linking of receptor‐bound IgE with subsequent mast cell degranulation and the release of proinflammatory molecules such as histamine. Notes: BCR: B‐cell receptor; FcϵRI: High affinity IgE receptor; MHC: Major histocompatibility complex; TCR: T‐cell receptor.
Natural history of the IgE response
Phylogeny
IgE is found in most mammals and marsupials, and while it is difficult to see how allergic diseases might be of any evolutionary benefit, it is believed that the IgE system plays an important role in the defense against multicellular parasites, such as helminths Citation3.
Ontogeny
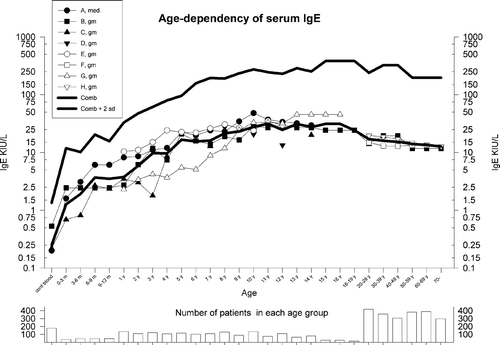
IgE may be synthesized early in the uterine life and can be detected in cord blood Citation4, Citation5. The mean normal level in newborns is considered to be about 0.3–0.5 KIU/L (0.8–1.2 ng/mL), and this level gradually increases to 10–40 KIU/L (25–100 ng/mL) culminating in adolescence (Figure ). In large population studies men often demonstrate higher levels than women, and most but not all studies find that the IgE level decreases with ages above 20, but smoking habits may be a confounder in some studies.
Figure 2 The combined results of various studies on age‐related reference values for serum IgE. Only studies using nonatopic populations have been included, but smoking habits and gender differences have not been taken into account. A weighted geometric mean (gm) for the individual gm and gm +2× SD have been calculated (SD based on A–E), and are shown with thick lines as ‘combined’. If other age intervals were used by the authors the total number of subjects in a group were evenly distributed in the appropriate age groups. In the lower panel is shown the number of patients in each age group. A total of 4104 subjects were used for the calculations. A: Citation153; B: Citation4; C: Citation154; D: Citation155; E: Citation156; F: Citation157; G: Citation158; H: Citation159.
Metabolism and distribution
Since IgE was early demonstrated to have a short serum half‐life of about 2.5 days Citation6, a high level of serum IgE has been considered synonymous with a high IgE production or synthesis. It is important to realize, however, that high levels of IgE lead to a lower relative catabolism, with half‐life increasing from 1.8 to 5.8 days when moving from normal IgE levels of 0.07 to 35 µg/mL (30 to 14,500 KIU/L) Citation7. Thus patients with severe atopic diseases and hyper‐IgE syndrome demonstrated only 40% and 30% of the synthetic rate that would have been expected from their high levels of serum IgE Citation7. It has been estimated that in normals about 67% of all IgE is extravascular as compared to 48% and 36% in the two above‐mentioned patient groups Citation7. A more recent study of the metabolism in patients receiving plasma, where specific IgE in donor plasma was used as marker, found a half‐life of 1.13 days, and a specific basophil sensitization already 3 h after transfusion Citation8. The clinical implications of these findings—supporting the historical report of postsurgery asthma mediated by transfusion of blood from a horse‐allergic donor Citation9—are that passively sensitizing a patient is indeed a possibility. The half‐life of IgE when bound to its high‐affinity receptor on mast cells in the skin has been estimated to be about 8–14 days Citation7.
IgE response
In spite of the short half‐life of the IgE molecule Citation6, it is the general impression that the IgE response is long lasting. From food and insect venom allergy, where exposure to the offending allergen can be controlled, it has been demonstrated that the level of specific IgE may be reduced to about 50% 4–6 years after the last allergen exposure Citation10, Citation11. As discussed below, this apparent ongoing IgE synthesis in the absence of allergen stimulation may be explained by long‐lived IgE‐producing plasma cells, but other possibilities are molecular mimicry by cross‐reacting antigens or long‐time deposition of allergen in follicular dendritic cells.
Key messages
Three steps are crucial to mount an IgE immune response: 1) differentiation of dendritic cells that 2) promote the formation of allergen‐specific Thelper type 2 lymphocyte which 3) subsequently induce the differentiation of B lymphocytes to IgE isotype switch. The IgE switched B cell undergoes transformation to an IgE‐producing plasma cell, and it is likely that some of these may live and produce IgE for several years, thereby maintaining the individual's status as allergically sensitized.
Cellular and molecular events in switch to IgE production
IgE is produced by B lymphocytes and plasma cells, but in order to become an IgE‐producing plasma cell the naive B cell needs a number of molecular signals, most importantly provided by a Thelper lymphocyte with a specialized cytokine secretion profile—the so‐called Th2 type (Th2) which can produce the cytokines necessary for stimulation of the B cell to IgE class switch (interleukin (IL‐) 4, IL‐13), for stimulation and recruitment of eosinophils (IL‐5, IL‐9, granulocyte/macrophage‐colony stimulating factor (GM‐CSF) and for other inflammatory tissue reactions (IL‐9, IL‐13). Undifferentiated Th cells on the other hand also need signals to develop into the Th2 phenotype, and it is likely that specialized dendritic cells are responsible for this process. Thus three steps are crucial to mount an IgE immune response: 1) differentiation of dendritic cells to ‘DC2’ promoting 2) the formation of allergen‐specific Th2 cells which subsequently 3) induce the differentiation of B lymphocytes to IgE isotype switch and transformation to an IgE‐producing plasma cell. The molecular background of these processes is discussed in more detail in the second part of this paper.
Clinical and epidemiological studies of IgE‐modifying factors
Genetics
Atopic diseases have a strong genetic trait, but multiple genes are involved, each probably playing a minor role. The readers are referred to Ober and Hoffjan Citation12 for a review of the current status.
Relation to disease
Specific IgE is increased in many allergic diseases like rhinitis, asthma, and food, drug, and insect venom allergies. It is important to emphasize, however, that the presence of specific IgE is not equal to disease, but only indicates that sensitization has occurred Citation13, Citation14. Many patients with atopic dermatitis tend to have high levels (5–25 µg/mL) of total IgE, often exceeding those of inhalation allergic patients. Patients with helminthic infestations may have extremely high levels of IgE, exceeding the normal level by a factor of 100 or more Citation15–17. Conditions such as certain states of acquired immunodeficiency syndrome Citation18 and recent surgery Citation19, Citation20 have been reported to lead to increased IgE synthesis. An increased level of serum IgE has been found in alcoholics after periods with large consumption of alcohol Citation21. The latter finding, which was corrected for smoking habits, interestingly showed a decline in serum IgE in heavy drinkers after withdrawal of alcohol.
Hygiene
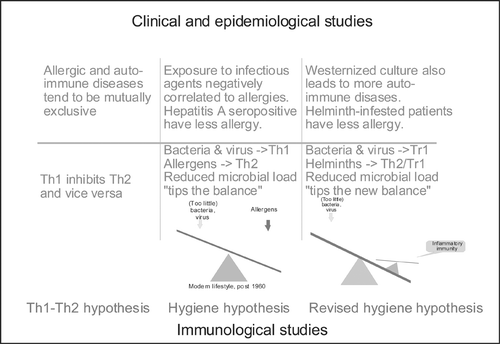
The last 40 years of increasing prevalence of atopic diseases in the westernized societies coinciding with the reduction of severe infections early in life has led to speculations on a causal relationship, and many different theories have been advanced under the originally coined term, the hygiene hypothesisCitation22. Evidence has been presented that hepatitis A seropositivity as a surrogate marker of poor orofecal hygiene was linked to lower prevalence of sensitization Citation23. Later studies seem to focus the issue on food‐borne and orofecal infections rather than on viral infections of the respiratory system Citation24. Likewise, a reduced risk of sensitization and the prevalence of allergic diseases have been linked to growing up on a farm Citation25 or living an anthroposophic lifestyle Citation26. More recently, an inverse relationship has been established between IgE‐allergic manifestations and parasitic infestations which also involves the IgE system Citation27. These data were initially interpreted as a Th1‐driven downregulation of a Th2 immune response in individuals that were affected by infections, the assumption being that a reduced infectious pressure would allow the Th2 part of the immune system to flourish. An important argument speaks against this, however: diseases with an assumed Th1 etiology, such as autoimmune diabetes, rheumatoid arthritis, and inflammatory bowel disease, are also on the rise in the same societies that experience increases in allergy prevalence. Moreover, Th1‐defective patients do not have increased allergies, and the data on coexistence of allergic and autoimmune diseases in the same individuals are conflicting Citation28–30. These arguments in combination with the lessons learned from the allergy‐parasite dichotomy have led to proposals of a common environment‐driven skewing in the regulatory (Treg) versus the inflammatory (Th1 and Th2) T cells Citation31, Citation32. It should be emphasized that the ‘hygiene hypothesis’ is still only a hypothesis, and attempts to make all observations fit into a common scheme have not been entirely successful. It may be more productive to discuss the many disparate studies in relation to whether they support/challenge the hypothesis on a clinical/epidemiological versus an immunological level (Figure ). Moreover, findings such as a reduced frequency of allergy diseases in patients with autoimmune diseases may easily be explained by genetic determinants, so a Th1‐Th2 dichotomy may exist without being influenced by hygienic factors. As discussed below, an additional number of Th cell subtypes has now been suggested, which may lead to a major revision of our thoughts on environment‐driven Th cell responses.
Figure 3 Breakdown of evidence for the hygiene hypothesis. It is suggested that evidence is divided into clinical/epidemiological versus immunological. Furthermore, studies of allergy versus autoimmunity can be studied (and results explained) independently of hygienic influences (‘Th1‐Th2 hypothesis’). Studies involving hygienic factors should make clear whether they are based on a Th1‐Th2 dichotomy (‘hygiene hypothesis’) or the concept of T cell regulation and tolerance induction (‘the revised hygiene hypothesis’) by means of regulatory T cells (Tr1/Th3/Treg).
Relation between specific and total IgE in serum
The fact that close to 100% of the population have detectable IgE protein, but only 20%–30% detectable specific IgE, raises the question as to the role of ‘nonspecific’ IgE. We and others have tried to determine the fraction of specific IgE in the serum of monoallergic patients, and specific IgE accounts for at most 20%–50% of the total IgE Citation33–36. It is likely that the remainder of the IgE has some of the same specificities as other immunoglobulin classes, and that a few, random B cell clones have undergone an IgE isotype switch. In conditions like atopic dermatitis and hyper‐IgE syndrome (Job's syndrome) it appears that a polyclonal activation has occurred, and that only a minor part of the total IgE can be accounted for by known allergen specificities.
Antigen exposure
Investigations of the relationships between exposure to antigens and levels of specific or total IgE are numerous, and only a few examples will be mentioned. In general, there seems to be correlation between exposure to inhalation allergens and sensitization, but much less clear correlations to actual disease have been found Citation37. An aspect that has failed to draw much attention is the antigens to which we are exposed but to which we never or rarely develop an IgE response. One example is the immunizations with diphtheria, tetanus, or polio vaccines, where sensitizations are reported very infrequently Citation38 when purified preparations are used. This supports the notion that the IgE immune response is mostly initiated by small doses of antigen adsorbed via mucosal surfaces. Regarding specific IgE, a dose‐response relationship has been demonstrated between exposure for cat or house dust mite allergen and the frequency of sensitization Citation39. In occupational settings with special allergenic proteins such as enzymes or plant proteins, specific IgE has been used as a criterion for work‐related sensitization Citation40, Citation41.
Adjuvants
While the effect of outdoor air pollution, including that caused by traffic, is controversial Citation37, it is clear from a large number of animal studies that diesel exhaust particles have an adjuvant effect in inducing an IgE response. This was confirmed in the human system Citation42, when diesel exhaust particles in combination with a neoantigen induced an IgE response to the antigen. Moreover, the nonlinear dose‐response relationship discussed above was confirmed, since an optimal dose of 10 microgram was established, whereas dosages 100 and 10,000 times higher were less optimal in producing sensitization Citation43. It is not known what causes the adjuvant activity in diesel exhaust particles, but it is interesting that lipid substances with an apparent Th2‐inducing effect have also been isolated from pollen Citation44, whereas lipid substances with long‐chained acyl groups isolated from parasite (Schistosoma mansoni) eggs seem to have a Th2‐ and/or Treg‐inducing activity Citation45. The molecular and cellular background for these findings are further discussed below; suffice here to mention that the common hydrophobic theme in these substances is in concordance with the proposition that hydrophobicity itself is a common biological activator of the innate immune system Citation46.
Dendritic cells and CD4+ T cell differentiation
A seminal work from 1986 Citation47 suggested that the phenotypes of the Th cells could be separated into two forms, the Th1 cell secreting the cytokines IFN‐γ and IL‐2, whereas the Th2 cell secretes IL‐4, IL‐5 and IL‐13. It soon became clear that primary cells from humans expressed a less clear dichotomy than the one observed for murine T cell clones, but the Th1‐Th2 paradigm showed very productive for segregating the many observations on immunopathology with IgE‐related diseases, such as allergy and parasitic infestations, being the prime example of a Th2 disease. In later years several observations have emerged which rocks the paradigm, the most prominent being various forms of regulatory T cells (Treg, Th3, Tr1) secreting mainly TGF‐β and/or IL‐10 Citation48 and the recently described T cells secreting IL‐17A and F, named Th17 cells Citation49. At this point in time a new consensus has not yet crystallized and many observations are conflicting or noncoherent. It falls beyond the scope of this paper to give an overall review of Th cell differentiation, but we have daringly tried to combine some of the data into a framework (Figure ), which is likely to be revised in the coming years.
Figure 4 Tentative model of the differentiation of Thelper(CD4+) subsets. The Thelper phenotypes Th17 (producing IL‐17), Th1 (IFN‐γ), Th2 (IL‐4, IL‐5, and IL‐13), and various regulatory Th cells (IL‐10 and/or TGF‐β) are induced by dendritic cells secreting (IL‐6+TGF‐β), IL‐12, IL‐4, and (TGF‐β and/or IL‐10). Recent results suggest that Th1, Th2, and Tr1 can be subdifferentiated into IL‐10 or TNF‐α producers via the OX40‐OX40 ligand system. It is not known whether Th17 cells can be subdifferentiated this way. Notes: APC: Antigen presenting cell; DC: Dendritic cell; Dex+Vit D3: Dexamethasone plus vitamin D3; Foxp3: Forkhead box p3; GATA‐3: GATA binding protein 3; ROR‐γT: RAR‐related orphan receptor γ; STAT: Signal Transducers and Activators of Transcription proteins; T‐bet: T box transcription factor; TLR: Toll like receptor.
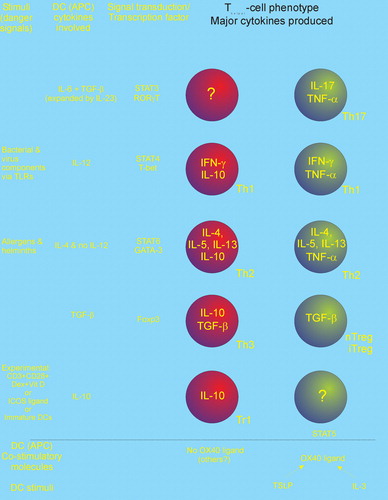
Dendritic cells of both the plasmacytoid and the myeloid lineages seem to have the capability to act as major histocompatiblility complex (MHC)‐bearing antigen‐presenting cells for naive CD4+ T cells. Besides this primary signal, obviously being recognized by the T cell receptor, a number of other—antigen unspecific—signals are exchanged between the dendritic cell and the T cell leading to the differentiation of the T cell into different phenotypes. These signals comprise soluble—cytokine‐cytokine‐receptor—interactions and cell‐to‐cell contacts, mediated by pairs of surface‐bound, costimulatory molecules (Table ). The latter comprise members of the immunoglobulin, the TNF, and the TNF‐receptor (TNFR) superfamilies Citation50, all of which include many soluble and cell‐bound ligand‐receptor pairs that are found throughout the immune system of both human and rodents. As for many other cytokines and chemokines and their receptors, there seems to be a certain promiscuity in the ligand‐receptor preferences in the TNF/TNFR superfamilies with receptor activator of NFKB ligand/TNP‐related apoptosis‐inducing ligand (RANKL/TRAIL)‐ and B‐cell activating factor of the TNF‐family/a proliferation inducing ligand (BAFF/APRIL)‐networks as notable examples Citation51. Signals delivered by CD80/CD86 to CD28 have been described as ‘the second signal’, but the relative importance of the different costimulatory signals is not clear. What is important, however, is that the combination of costimulatory signals from the dendritic cell is able to instruct the naive CD4 T cell to differentiate. It is likely that this differentiation is not just a choice between Th1 and Th2 but more modular, in the sense that presence/absence of certain stimuli will instruct the T cell to produce different classes of cytokines. Besides the Th1 and Th2 type cytokines—induced by IL‐12 and IL‐4‐without‐concomitant‐IL‐12—it seems likely that instructions for secretion of IL‐17A and F (Th17 cells generated by IL‐6+TGF‐β) can be induced, as can IL‐10 and/or TGF‐β (Tregs, Th3, or Tr1 cells by IL‐10). Interestingly, new data suggest that stimulation via the OX40 ligand in combination with the above‐mentioned stimulation schemes selects between TNF‐α and IL‐10 induction in Th2, Th1 Citation52, and Tr1 cells Citation53. This has given rise to subtypes of the previously described Th1, Th2, and Tr1 phenotypes (described as ‘inflammatory’ for TNF‐α+ and ‘regulatory’ for IL‐10+ Citation52), but it is not yet known whether the phenomenon is more general and also extendable to cells described as Th17 and Tregs, or whether the expression of TNF‐α in Th17 and IL‐10 in Tregs can be explained by the action of OX40 ligand (Figure ). Neither can it be excluded that other costimulatory pairs are involved even though the glucocorticoid‐induced TNF receptor (GITR)‐GITR ligand and 4‐1BB‐4‐1BB ligand pairs did not influence the expression of TNF‐α/IL‐10 in Tr1 cells Citation53.
Table I. Pairs of costimulatory molecules on antigen‐presenting cells and T cells.
As discussed below, IL‐4 or IL‐13 seems to be unique in inducing IgE isotype switch but the T cell secretion of other cytokines such as TNF‐α versus IL‐10 would have profound influence on the humoral immune response, since the combination of IL‐4 and IL‐10 would preferentially increase the IgG4 on behalf of the IgE response. It is tempting to speculate that this might happen during allergen immunotherapy and helminth infestation, where high IgG4 levels are seen. Thus, in spite of the appearance of newly defined Th cell subtypes, the Th2 cell still seems to be central in the sensitization phase of the allergic reaction. It should be emphasized, however, that during the efferent phase more (Th and other) cell types may become activated and move into the inflamed areas in the mucous membranes. Several of the Th cell subsets secrete cytokines which may act as positive or negative signals for the development of other Th cell subsets, and in this way an intricate regulatory network may emerge, which may in time change the inflammatory profile of the involved cells. This ‘downstream’ part of the allergic reaction is, however, beyond the scope of this paper.
Going one step back to the dendritic cell it is interesting that strong OX40 ligand expression can be induced by the cytokine thymic stromal lymphopoietin (TSLP). Besides inducing the costimulatory molecule, OX40 ligand, CD80/86, and MHC class II, TSLP has an interesting effect on myeloid dendritic cells in that cytokines such as IL‐1, IL‐6, IL‐12, and IFN‐α/β are not induced as opposed to activators such as CD40 ligand or Toll‐like receptor ligands (lipopolysaccharide, polyinosinic, policytidylic acid; a synthetic polymer that resembles RNA of infectious viruses and which is used experimentally to model viral infections (poly I:C), and R848) Citation54. This gives the TSLP‐stimulated dendritic cell exactly the right phenotype to instruct a naive CD4+ T cell to become a Th2 cell. More interestingly, the major source of TSLP seems to be epithelial cells, in particular in the lungs, skin, and gut, and upregulation of TSLP has been found in atopic eczema Citation54, that is often characterized by very high levels of serum IgE. Also lipid components in pollen Citation44 have been demonstrated to reduce the IL‐12‐producing capacity of dendritic cells, and terpenes from wood have the ability to induce the OX40 ligand and reduce the IL‐12 production from dendritic cells Citation55.
B lymphocyte development
The heated debate on the various aspects of the hygiene hypothesis and different T cell subsets (Figure ) has taken some focus away from the cell that actually produces IgE, i.e. the end stage of B lymphocyte differentiation: the plasma cell. From both a preventive and therapeutic point of view this cell is important, however. Increasing evidence is accumulating that long‐lived IgE‐producing plasma cells do exist, and these may explain the previously mentioned finding of IgE with specificities to allergens to which the patient has not been exposed for years. Such cells are not easily targeted by therapeutics since plasma cells are located in special niches such as the bone marrow and do not divide Citation56. The longevity of the IgE response may severely compromise therapeutic strategies such as various forms of immunotherapy that are directed against the T cell component of the immune system.
B cells' development is divided into three main stages: 1) generation of mature immunocompetent B cells within the bone marrow, 2) activation of mature B cells upon interaction with antigen, and 3) differentiation of activated B cells into memory and plasma B cells Citation57–60.
Generation of B cells
The development of B cells begins in the bone marrow with a lymphoid progenitor (pro‐B cell) (Figure ). These cells express CD45 (called B220 in mice) and the unique B cell marker CD19, which will be expressed throughout the rest of the life of the B cells, with the exception of plasma cells. Pro‐B cells require direct contact with stromal cells in early stages via the c‐kit receptor on the pro‐B cell interacting with the stem cell factor on the stromal cell Citation61. Immunoglobulin (Ig) DNA rearrangement is required for further B cell maturation and when the Ig heavy chain genes are rearranged, the pro‐B cells differentiate to pre‐B cells. Appropriate expression of the surrogate light chain proteins, λ5 and VpreB, is essential for B cell differentiation. Together with Igα and Igβ these molecules form the pre‐B cell receptor that appears on the surface of the cell during the pre‐B cell stage of development Citation62, Citation63. Once a light chain gene has been rearranged successfully, its product combines with the heavy chain to form intact IgM, which is expressed at the cell surface in a complex with Igα and Igβ. Immature B cells expressing self‐reactive IgM that binds to self‐Ag in the bone marrow are eliminated. Downregulation of adhesion molecules results in the separation of pre‐B cells from the stromal cells and naïve (mature) B cells will leave the bone marrow and travel to secondary lymphoid tissues. A change in RNA processing permits the production of two mRNAs resulting in the coexpression of membrane‐bound IgM and IgD, making the B cells fully (naïve) mature. If these B cells fail to encounter their specific antigen, most of them will die within a few days. The B cells will travel to the secondary lymphoid tissues for activation Citation57, Citation60. In adults the majority of surviving immature bone marrow B cells ultimately yield two subsets of mature peripheral B cells, termed follicular (FO) and marginal zone (MZ) B cells Citation64. FO B cells, which are located in the follicles, are relatively long‐lived and cooperate with T cells to generate the adaptive immune response. In contrast, MZ B cells are strategically positioned at the blood‐lymphoid interface and are programmed to initiate a fast and intense antibody response to blood‐borne viral and bacterial agents, independent of T cell help and are considered important in the early immune response.
B cell activation
Antigen from tissue is filtered by the lymph nodes, whereas the spleen filters blood‐borne antigen. Antigen is presented to B cells which, along with Th cells, causes the B cells to become activated and to differentiate (Figure ). Antigen binding to B cell receptor (BCR) stimulates upregulation of CD80/CD86 and major histocompatiblility complex (MHC) class II, which help to activate the T cells. The contact between the Th cell and B cells causes an upregulation of CD40 ligand (CD40L) on the Th cell membrane. Interaction of the B cell's CD40 with CD40L, along with cytokines and BCR cross‐linking, induce the B cell to proliferate. A few activated B cells differentiate into short‐living plasma cells that migrate directly to the medullary cords and begin secreting IgM and later IgG. Most activated B cells travel to the B cell areas of the lymph node, called primary follicles, which provide a favorable environment for interactions between B cells, Th cells, and dendritic cells. Within a few days of antigen stimulation, B cell blasts (dividing cells) form germinal centers. Germinal centers are sites of intensive B cell proliferation and are surrounded by the Th cells that activated the B cells and migrated with them. By a week after initial antigen contact, many germinal centers are present. Somatic hypermutation (SHM), class switch recombination (CSR), and formation of plasma and memory B cells occur in these germinal centers Citation58, Citation59, Citation65.
Generation of Ig diversity
Somatic hypermutation (SHM)
The antigen‐binding specificity can be optimized by SHM, which occurs in rapidly dividing B cells in the germinal centers. Mutation occurs in the hypervariable regions of the heavy and light chains at a rate high enough that about half of B cells undergo mutation of their Ig antigen‐binding regions Citation66. B cells which remain specific for the stimulating antigen are selected by binding antigen held on follicular dendritic cells (FDC), which do not express MHC II nor act as antigen‐presenting cells (APC) for T cells Citation67. Only B cells, which bind antigen with high enough affinity, continue to secrete antibody and differentiate into plasma cells; low‐affinity B cells undergo apoptosis.
Class switch recombination (CSR)
Antibodies can be classified according to their heavy (H) chain constant regions into nine classes of isotype: IgM, IgD, IgG1, IgG2, IgG3, IgG4, IgA1, IgA2 and IgE, each with different features (Figure ). After exposure to an antigen, the first antibodies to appear are IgM and IgD. Later, antibodies of other classes emerge as a result of CSR. This involves a change in the isotype of the antibody, so that the variable region, which determines the specificity of an antibody, becomes associated with the constant regions of different isotypes with different effector functions. To understand the molecular mechanisms in CSR it is important to know the structure of the immunoglobulin regions. Each class of Ig is composed of a short germline exon (I), a repetitive GC‐rich switch (S) region, and several exons encoding the H chain constant region (CH) (Figures and ).
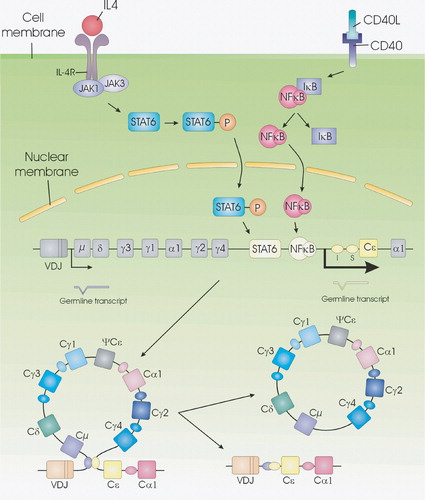
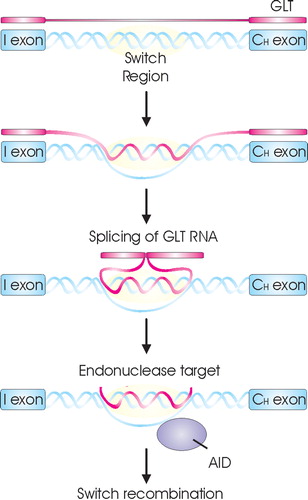
CSR is induced by two signals. The first signal is delivered by T cell antigen‐specific interaction with the B cell via CD40L/CD40 Citation68, Citation69. The second signal is delivered by cytokines secreted by activated T cells Citation70. Binding of cytokines to their receptors initiates a signaling cascade resulting in translocation to the nucleus of STAT (signal transducer and activator of transcription) molecules (Figure ) which become active dimers after phosphorylation Citation71–74. In the nucleus STAT binds to DNA sequences present in the promoter regions of cytokine responsive genes initiating sterile germline mRNA transcripts (GLT) (precursor mRNA for Ig), which are not translated into a functional protein Citation75. The first signal via CD40 leads to activation of NF‐κB (nuclear factor kappa B) (Figure ) that also binds to one of the germline gene promoter and synergizes with STAT for Ig transcription and maximal Ig production Citation76. The transcription of GLTs is initiated at the I exon, transcribed through the S and CH genes, and terminated at the normal termination sites for mature mRNAs Citation77, Citation78. They are spliced, polyadenylated, and exported to the cytoplasm, although they are not translated. Different cytokines have been found to initiate different GLTs Citation79, Citation80. The GLTs have been shown to be necessary for CSR to occur Citation81–83, and the requirement of these transcripts appears to explain how cytokines direct CSR to specific isotypes.
Indirect evidence suggests that GLTs might associate with the genomic S region to form RNA‐DNA hybrids (R‐loops) with the transcribed S region stand in vitro and in vivoCitation84, Citation85 (Figure ). The R‐loop structure is purposed to result in a single stranded DNA segment, which has been shown to be a target for activation‐induced cytidine deaminase (AID) Citation86–90. By deamination, AID converts dC to dU residues within S regions and antibody variable regions Citation86, Citation90–92. A consequence of this DNA deamination, an U:G mismatch is generated in the Ig locus that is likely to be processed through uracil removal uracil‐N‐DNA glycosylase (UNG) or by alternative pathways, including base‐excision repair and mismatch repair leading to CSR and SHM Citation91–96. Because CSR occurs by an intrachromosomal deletion, double‐strand DNA breaks must be introduced into both the Sμ and a downstream S region to obtain CSR. Once a break has been made, CSR occurs.
Figure 5 Rearrangement at the immunoglobulin heavy chain locus. The human immunoglobulin heavy chain locus contains clusters of heavy chain variable(V), diversity (D), and joining (J) cassettes that are rearranged during B cell development. This process results in the assembly of a complete rearranged VDJ region that encodes an antigen‐binding domain capable of producing intact μ and δ heavy chains. STAT‐6 and NF‐κB are activated upon stimulation with IL‐4 and aCD40, initiating the production of μ and ϵ GLTs resulting in CSR. CSR is a process that exchanges the constant region of the heavy chain (CH) with a set of downstream constant‐region genes (here CSR to IgE is shown). For IgE isotype switching, this process involves the excision of a large piece of genomic DNA spanning from Sμ sequences to the Sϵ sequence. Ligation of the VDJ sequences to the constant (C) ϵ locus gives rise to an intact ϵ heavy chain gene and the production of intact IgE antibodies.
The findings that AID is essential for both CSR and SHM provided one of the recent major breakthroughs for this field Citation95, Citation96.
Memory and plasma cells
Upon activation, some B cells become memory cells, which can be rapidly restimulated by antigen and are present in higher frequency than the naïve resting B cells. Memory B cells are functionally and physically distinguishable from naïve B cells and often have membrane‐bound IgG, IgA, or IgE. Low levels of antigen may remain on follicular dendritic cells (FDC) for years, so that B cells may be continually activated at low levels to replenish memory cell populations Citation60, Citation97, Citation98. Other B cells become plasma cells. Plasma cells are antibody‐producing cells that no longer divide or respond to antigen. They are larger than B cells and have more ribosomes, endoplasmic reticulum, and golgi, but no membrane Ig. Some survive only a few weeks, while others migrate to the bone marrow, continuing to produce antibody for longer periods (the so‐called long‐lived plasma cells), providing an explanation of the continued raised levels of specific IgE described above. Plasma cells that develop after a germinal center reaction provide high‐affinity antibody and often survive many months in the bone marrow Citation56, Citation57, Citation97. The transcription factors Bcl‐6 (B cell lymphoma 6) and Pax5 (Paired box protein 5), which are required for germinal center B cells, block plasmacytic differentiation and repress BLIMP‐1 (B lymphocyte induced maturation protein 1) and XBP‐1 (X‐box binding protein 1), respectively Citation56, Citation99–101. When Bcl‐6‐dependent repression of Blimp‐1 is relieved, Blimp‐1 ensures the irreversible plasmacytic development by repressing Bcl‐6 and Pax5 Citation102–105. In plasma cells, Blimp‐1 Citation106, Citation107, XBP‐1 Citation99, Citation100, interferon regulating factor‐4 (IRF4) Citation108, and other regulators cause cessation of cell cycle, decrease signaling from the B cell receptor, and communication with T cells. Thus, commitment to plasmacytic differentiation involves inhibition of activities associated with earlier B cell developmental stages as well as expression of the plasma cell phenotype Citation101, Citation103. This leads to decreased expression of MHC class II, CD19, CD20, CD22, CD44, and CD45 Citation101, while surface molecules such as CD138/Syndecan‐1 and CD38 Citation109 are upregulated. CD138 mediates cellular adhesion to collagen type 1 and might play a role in adhesion to bone marrow stromal cells Citation110, Citation111.
Regulators of Ig production
Extracellular signals play prominent roles in the process of CSR, and cytokines such as interleukin 4 (IL‐4), IL‐10, IL‐13, interferon γ (IFN‐γ), and transforming growth factor β (TGF‐β) appear to play particularly critical roles by directing the isotype specificity of CSR. IL‐4 and IL‐13 induce high rates of IgG4 and IgE Citation112–117, but the IL‐4‐induced IgE production is easily inhibited by a number of cytokines including IL‐12, TGF‐β, IFN‐γ and IFN‐α Citation70, Citation115, Citation118–122, which is likely to explain the low levels of IgE compared to the other immunoglobulin classes. IFN‐γ is known to induce IgG2 Citation123, Citation124, whereas IL‐10 induces high amounts of IgG1, IgG3, IgA, and IgM, but not IgE Citation125–128. TGF‐β appears to have several roles. Generally, TGF‐β downregulates Ig secretion; at low doses, however, it induces CSR to IgA Citation129–133.
IL‐10 and related cytokines
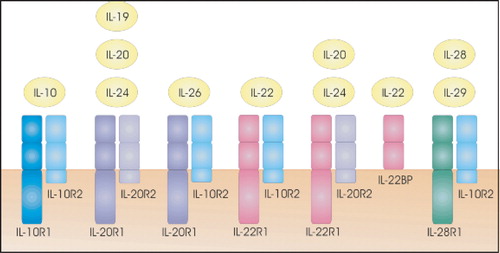
IL‐10 was initially described as an inhibitory factor for the production of Th1 cytokines. Subsequently, pleiotropic inhibitory and stimulatory effects on various types of blood cells were discovered for IL‐10, including its role as a survival and differentiation factor for B cells. IL‐10, which is produced by a diverse set of cells including activated monocytes and T cells, appears to be a crucial factor for at least some forms of peripheral tolerance and act as a major suppressor of the immune response and inflammation Citation134. The inhibitory function of IL‐10 is believed to be mediated by the induction of regulatory T cells Citation135. Recently, a family of IL‐10‐related cytokines has been discovered by bioinformatic approaches and by functional assays. These cytokines include IL‐19, IL‐20, IL‐22, IL‐24, IL‐26, IL‐28, and IL‐29 Citation136–139. The proteins are distantly related to one another (20%–30% sequence identity) Citation138 and have quite diverse functions, only some of which are currently known. They signal through different combinations of cytokine receptor family class II (CRF2) proteins, with some sharing of receptor subunits (Figure ) Citation140. All functional receptors are heterodimers composed of a type 1 (R1) and a type 2 (R2) chain. Traditional nomenclature has often defined the R1 subunit as being the ligand‐binding molecule and the R2 subunit as being the signal transduction subunit. In spite of their homology and common use of the IL‐10 receptor subunits, no influence was found on the IL‐4‐induced isotype switch to IgE production Citation141.
Figure 6 A model for the role of GLT in CSR. Upon B cell activation GLT is induced. This GLT interferes with the switch region forming a R‐loop to create a stable single‐strand DNA (ssDNA) substrate for AID. AID then converts dC to dU residues, creating targets for endonuclease resulting in DNA breakage necessary for CSR.
IL‐21
Another recently discovered interleukin is IL‐21, which has pleiotropic effects on the immune system. IL‐21 was identified as a four‐helix bundle that has most similarities with IL‐2, IL‐4, and IL‐15 Citation85, Citation142. The receptor for IL‐21, IL‐21R, is a novel class I cytokine receptor that exerts its activity through interactions with the common gamma chain (γc) Citation143, Citation144. IL‐21R has been found on a variety of cells including resting and activated B cells, T cells, natural killer (NK) cells and dendritic cells Citation145–148.
In the murine system, IL‐21 was found to inhibit the production of IgE in IL‐4 and lipopolysaccharide (LPS)‐stimulated B cells in vivoCitation149, Citation150. Additionally, in isolated murine B cells, IL‐21 directly antagonizes IL‐4 and LPS‐induced ϵ GLT Citation150. Moreover, IL‐21R‐deficient mice were found to have increased level of serum IgE compared to wild‐type mice Citation145, Citation149. Interestingly, IL‐21R‐deficient mice have low levels of serum IgG1, the analog to human IgG4. The finding of low serum IgG1 in association with high IgE indicates that IL‐21 may differentially influence IgE versus IgG1 CSR.
In the human system, IL‐21 has been found to be a switch factor for the production of IgG1 and IgG3 Citation151. Furthermore, IL‐21 has been found to enhance IgE production from purified human B cells stimulated with aCD40 monoclonal antibody and either IL‐4 or IL‐13 (Citation152 and own unpublished results). In contrast, an inhibitory effect of IL‐21 was observed when phytohemagglutin (PHA)‐activated T cells were used as the source of costimulatory signals for IgE production Citation152.
Taken together, these studies show that IL‐21 can either stimulate or inhibit IgE and IgG4 production in murine or human B cells depending on activation conditions, implying the importance of further studies in this field.
Conclusion
Considerable knowledge has accumulated on the molecular level of Th2 cell formation and B cell differentiation which leads to the IgE‐producing plasma cell and thus the sensitization of the individual to mount an allergic response when reexposed to allergens. The research has revealed a number of potential molecular targets for interfering with the process which would allow us to prevent and/or cure allergies. It is a sad fact, however, that we still only have a limited understanding of both the environmental determinants—whether related to hygiene or other modern lifestyle factors—and the molecular events leading to the high prevalence of IgE‐mediated allergy in a large and increasing part of the world.
References
- Holt P. G., Sly P. D., Martinez F. D., Weiss S. T., Bjorksten B., von Mutius E., et al. Drug development strategies for asthma: in search of a new paradigm. Nat Immunol 2004; 5: 695–8
- Krause T., Koch A., Friborg J., Poulsen L. K., Kristensen B., Melbye M. Frequency of atopy in the Arctic in 1987 and 1998. Lancet 2002; 360: 691–2
- Yazdanbakhsh M., van den Biggelaar A., Maizels R. M. Th2 responses without atopy: immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol 2001; 22: 372–7
- Bhalla R. B., Rappaport I., DeFilippi I., Schwartz M. K. Serum IgE levels in a Northeast United States caucasian population. Advanced interpretation of clinical laboratory data, C Heusghem, A Albert, E. S Benson. Marcel Dekker, Inc., New York 1982; 295–305
- Hansen D., Hornnes P., Poulsen L. K. IgE levels in cord blood in capillary blood at 4–6 days of age. Pediatr Allergy Immunol 1993; 4: 30–3
- Waldman T. A., Strober W., Blaese R. M. Variations in the metabolism of immunoglobulins measured by turnover rates. Immunoglobulins, E Merler. National Academy of Science, Washington 1970; 33–51
- Dreskin S. C., Goldsmith P. K., Strober W., Zech L. A., Gallin J. I. Metabolism of immunoglobulin E in patients with markedly elevated serum immunoglobulin E levels. J Clin Invest 1987; 79: 1764–72
- Johansson S. G., Nopp A., van Hage M., Olofsson N., Lundahl J., Wehlin L., et al. Passive IgE‐sensitization by blood transfusion. Allergy 2005; 60: 1192–9
- Ramirez M. A. Horse asthma following blood tranfusion. JAMA 1919; 73: 984
- Savliwala M. N., Reisman R. E. Studies of the natural history of stinging‐insect allergy: Long‐term follow‐up of patients without immunotherapy. J Allergy Clin Immunol 1987; 80: 741–5
- Hansen T. K., Bindslev‐Jensen C., Poulsen L. K. Long‐term changes in specific IgE to codfish. 2004, Proceedings from 9th International Symposium on Immunological, Chemical and Clinical Problems of Food Allergy. Budapest, Hungary. April 18–21, 2004
- Ober C., Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun 2006; 7: 95–100
- Bodtger U., Poulsen L. K., Malling H. J. Asymptomatic skin sensitization to birch predicts later development of birch pollen allergy in adults: A 3‐year follow‐up study. J Allergy Clin Immunol 2003; 111: 149–54
- Bodtger U. Prognostic value of asymptomatic skin sensitization to aeroallergens. Curr Opin Allergy Clin Immunol 2004; 4: 5–10
- Lynch N. R., Hagel I., Vargas M., Perez M., Lopez R. I., Garcia N. M., et al. Effect of age and helminthic infection on IgE levels in slum children. J Investig Allergol Clin Immunol 1993; 3: 96–9
- Kartasamita C. B., Rosmayudi O., Demedts M. Total serum IgE and eosinophil count in children with and without a history of asthma, wheezing, or atopy in an urban community in Indonesia. The Respiratory Disease Working Group. J Allergy Clin Immunol 1994; 94: 981–8
- Hagel I., Lynch N. R., Di P. M., Rojas E., Perez M., Alvarez N. Ascaris reinfection of slum children: relation with the IgE response. Clin Exp Immunol 1993; 94: 80–3
- Pedersen M. Type 1 allergiske in vitro reaktioner overfor mikororganismer hos patienter med AIDS [Thesis]. Københavns Universitet, Copenhagen 1990
- Szczeklik A., Jawien J. Immunoglobulin E in acute phase response to surgical stress. Clin Exp Allergy 1996; 26: 303–7
- Kampen G. T., Poulsen L. K., Nielsen H. J., Schulze F., Petersen L. J. IgE levels in surgery. Effect of ranitidine and prednisolone. Allergy 1999; 544: 171–6
- Hällgren R., Lundin L. Increased total serum IgE in alcoholics. Acta Med Scand 1983; 213: 99–103
- Strachan D. P. Hay fever, hygiene, and household size. BMJ 1989; 299: 1259–60
- Matricardi P. M., Rosmini F., Ferrigno L., Nisini R., Rapicetta M., Chionne P., et al. Cross sectional retrospective study of prevalence of atopy among Italian military students with antibodies against hepatitis A virus. BMJ 1997; 314: 999–1003
- Matricardi P. M., Rosmini F., Riondino S., Fortini M., Ferrigno L., Rapicetta M., et al. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological study. BMJ 2000; 320: 412–7
- Riedler J., Braun‐Fahrlander C., Eder W., Schreuer M., Waser M., Maisch S., et al. Exposure to farming in early life and development of asthma and allergy: a cross‐sectional survey. Lancet 2001; 358: 1129–33
- Alm J. S., Swartz J., Lilja G., Scheynius A., Pershagen G. Atopy in children of families with an anthroposophic lifestyle. Lancet 1999; 353: 1485–8
- Yazdanbakhsh M., Matricardi P. M. Parasites and the hygiene hypothesis: regulating the immune system?. Clin Rev Allergy Immunol 2004; 26: 15–24
- Olesen A. B., Juul S., Birkebaek N., Thestrup‐Pedersen K. Association between atopic dermatitis and insulin‐dependent diabetes mellitus: a case‐control study. Lancet 2001; 357: 1749–52
- Sheikh A., Smeeth L., Hubbard R. There is no evidence of an inverse relationship between TH2‐mediated atopy and TH1‐mediated autoimmune disorders: Lack of support for the hygiene hypothesis. J Allergy Clin Immunol 2003; 111: 131–5
- Tirosh A., Mandel D., Mimouni F. B., Zimlichman E., Shochat T., Kochba I. Autoimmune diseases in asthma. Ann Intern Med 2006; 144: 877–83
- Yazdanbakhsh M., Kremsner P. G., Van Ree R. Allergy, parasites, and the hygiene hypothesis. Science 2002; 296: 490–4
- Macpherson A. J., Harris N. L. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol 2004; 4: 478–85
- Poulsen L. K., Pedersen M. F., Malling H‐J., Søndergaard I., Weeke B. Maxisorp RAST. A sensitive method for detection of absolute quantities of antigen‐specific IgE. Allergy 1989; 44: 178–89
- Paull B. R., Jacob G. L., Yunginger J. W., Gleich G. J. Comparison between binding of IgE and IgG antibodies to honey bee venom phospholipase‐A. J Immunol 1978; 120: 1917–23
- Zeiss C. R., Pruzansky J. J., Patterson R., Roberts M. A solid phase radioimmunoassay for the quantitation of human reaginic antibody against ragweed antigen E. J Immunol 1973; 110: 414–21
- Schellenberg R. R., Adkinson N. F. Measurement of absolute amounts of antigen‐specific human IgE by a radioallergosorbent test (RAST) elution technique. J Immunol 1975; 115: 1577–83
- Eder W., Ege M. J., von Mutius E. The asthma epidemic. N Engl J Med 2006; 355: 2226–35
- Skov P. S., Pelck I., Ebbesen F., Poulsen L. K. Case report: Hypersensitivity to the diphteria component in the Di‐Te‐Pol vaccine. A type I allergic reaction demonstrated by basophil histamine release. Pediatr Allergy Immunol 1997; 8: 156–8
- Wahn U., Lau S., Bergmann R., Kulig M., Forster J., Bergmann K., et al. Indoor allergen exposure is a risk factor for sensitization during the first three years of life. J Allergy Clin Immunol 1997; 99: 763–9
- Johnsen C. R., Sorensen T. B., Ingemann L. A., Bertelsen S. A., Andreasen E., Kofoed G. S., et al. Allergy risk in an enzyme producing plant: a retrospective follow up study. Occup Environ Med 1997; 54: 671–5
- Paulsen E., Skov P. S., Bindslev‐Jensen C., Voitenko V., Poulsen L. K. Occupational type I allergy to Christmas cactus (Schlumbergera). Allergy 1997; 52: 656–60
- Diaz‐Sanchez D., Garcia M. P., Wang M., Jyrala M., Saxon A. Nasal challenge with diesel exhaust particles can induce sensitization to a neoallergen in the human mucosa. J Allergy Clin Immunol 1999; 104: 1183–8
- Riedl M. A., Landaw E. M., Saxon A., Diaz‐Sanchez D. Initial high‐dose nasal allergen exposure prevents allergic sensitization to a neoantigen. J Immunol 2005; 174: 7440–5
- Traidl‐Hoffmann C., Mariani V., Hochrein H., Karg K., Wagner H., Ring J., et al. Pollen‐associated phytoprostanes inhibit dendritic cell interleukin‐12 production and augment T helper type 2 cell polarization. J Exp Med 2005; 201: 627–36
- Van Der Kleij D., Latz E., Brouwers J. F., Kruize Y. C., Schmitz M., Kurt‐Jones E. A., et al. A novel host‐parasite lipid cross talk: schistosomal lyso‐phosphatidylserine activates Toll‐like receptor‐2 and affects immune polarization. J Biol Chem 2002; 277: 48122–9
- Seong S. Y., Matzinger P. Hydrophobicity: an ancient damage‐associated molecular pattern that initiates innate immune responses. Nat Rev Immunol 2004; 4: 469–78
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clones. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 1986; 136: 2348–57
- Asseman C., Mauze S., Leach M. W., Coffman R. L., Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 1999; 190: 995–1004
- Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell‐mediated tissue damage. Nat Med 2007; 13: 139–45
- Cosman D. A family of ligands for the TNF receptor superfamily. Stem Cells 1994; 12: 440–55
- Bossen C., Ingold K., Tardivel A., Bodmer J. L., Gaide O., Hertig S., et al. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem 2006; 281: 13964–71
- Ito T., Wang Y. H., Duramad O., Hori T., Delespesse G. J., Watanabe N., et al. TSLP‐activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med 2005; 202: 1213–23
- Ito T., Wang Y. H., Duramad O., Hanabuchi S., Perng O. A., Gilliet M., et al. OX40 ligand shuts down IL‐10‐producing regulatory T cells. Proc Natl Acad Sci U S A 2006; 103: 13138–43
- Liu Y. J., Soumelis V., Watanabe N., Ito T., Wang Y. H., Malefyt R. D., et al. TSLP: An Epithelial Cell Cytokine that Regulates T Cell Differentiation by Conditioning Dendritic Cell Maturation. Annu Rev Immunol 2007; 25: 193–219
- Takei M., Umeyama A., Arihara S. Diterpenes inhibit IL‐12 production by DC and enhance Th2 cells polarization. Biochem Biophys Res Commun 2007; 355: 603–10
- Shapiro‐Shelef M., Calame K. Regulation of plasma‐cell development. Nat Rev Immunol 2005; 5: 230–42
- Rolink A., Streb M., Nishikawa S., Melchers F. The c‐kit‐encoded tyrosine kinase regulates the proliferation of early pre‐B cells. Eur J Immunol 1991; 21: 2609–12
- Duchosal M. A. B‐cell development and differentiation. Semin Hematol 1997; 34: 2–12
- Janeway J., Travers P. The humoral immune response. Publishing Inc., New York 1997; 8:2–8:51
- Kelsoe G., Zheng B. Sites of B‐cell activation in vivo. Curr Opin Immunol 1993; 5: 418–22
- Henderson A., Calame K. Transcriptional regulation during B cell development. Annu Rev Immunol 1998; 16: 163–200
- Karasuyama H., Rolink A., Shinkai Y., Young F., Alt F. W., Melchers F. The expression of Vpre‐B/lambda 5 surrogate light chain in early bone marrow precursor B cells of normal and B cell‐deficient mutant mice. Cell 1994; 77: 133–43
- Melchers F., Haasner D., Grawunder U., Kalberer C., Karasuyama H., Winkler T., et al. Roles of IgH and L chains and of surrogate H and L chains in the development of cells of the B lymphocyte lineage. Annu Rev Immunol 1994; 12: 209–25
- Thomas M. D., Srivastava B., Allman D. Regulation of peripheral B cell maturation. Cell Immunol 2006; 239: 92–102
- Garraud O., Nutman T. B. The role of cytokines in human B‐cell differentiation into immunoglobulin‐secreting cells. Bull Inst Pasteur 1996; 94: 285–309
- Wabl M., Jack H. M., Meyer J., Beck‐Engeser G., von Borstel R. C., Steinberg C. M. Measurements of mutation rates in B lymphocytes. Immunol Rev 1987; 96: 91–107
- Wu J., Qin D., Burton G. F., Szakal A. K., Tew J. G. Follicular dendritic cell‐derived antigen and accessory activity in initiation of memory IgG responses in vitro. J Immunol 1996; 157: 3404–11
- Warren W. D., Berton M. T. Induction of germ‐line gamma 1 and epsilon Ig gene expression in murine B cells. IL‐4 and the CD40 ligand‐CD40 interaction provide distinct but synergistic signals. J Immunol 1995; 155: 5637–46
- Iciek L. A., Delphin S. A., Stavnezer J. CD40 cross‐linking induces Ig epsilon germline transcripts in B cells via activation of NF‐kappaB: synergy with IL‐4 induction. J Immunol 1997; 158: 4769–79
- Armitage R. J., Macduff B. M., Spriggs M. K., Fanslow W. C. Human B cell proliferation and Ig secretion induced by recombinant CD40 ligand are modulated by soluble cytokines. J Immunol 1993; 150: 3671–80
- Ivashkiv L. B. Cytokines and STATs: how can signals achieve specificity?. Immunity 1995; 3: 1–4
- Schindler C., Kashleva H., Pernis A., Pine R., Rothman P. STF‐IL‐4: a novel IL‐4‐induced signal transducing factor. EMBO J 1994; 13: 1350–6
- Quelle F. W., Shimoda K., Thierfelder W., Fischer C., Kim A., Ruben S. M., et al. Cloning of murine Stat6 and human Stat6, Stat proteins that are tyrosine phosphorylated in responses to IL‐4 and IL‐3 but are not required for mitogenesis. Mol Cell Biol 1995; 15: 3336–43
- Wick K. R., Berton M. T. IL‐4 induces serine phosphorylation of the STAT6 transactivation domain in B lymphocytes. Mol Immunol 2000; 37: 641–52
- Oettgen H. C. Regulation of the IgE isotype switch: new insights on cytokine signals and the functions of epsilon germline transcripts. Curr Opin Immunol 2000; 12: 618–23
- Bacharier L. B., Jabara H., Geha R. S. Molecular mechanisms of immunoglobulin E regulation. Int Arch Allergy Immunol 1998; 115: 257–69
- Gauchat J. F., Lebman D. A., Coffman R. L., Gascan H., de Vries J. E. Structure and expression of germline epsilon transcripts in human B cells induced by interleukin 4 to switch to IgE production. J Exp Med 1990; 172: 463–73
- Lutzker S., Alt F. W. Structure and expression of germ line immunoglobulin gamma 2b transcripts. Mol Cell Biol 1988; 8: 1849–52
- Stavnezer‐Nordgren J., Sirlin S. Specificity of immunoglobulin heavy chain switch correlates with activity of germline heavy chain genes prior to switching. EMBO J 1986; 5: 95–102
- Fear D. J., McCloskey N., O'Connor B., Felsenfeld G., Gould H. J. Transcription of Ig germline genes in single human B cells and the role of cytokines in isotype determination. J Immunol 2004; 173: 4529–38
- Stavnezer J. Immunoglobulin class switching. Curr Opin Immunol 1996; 8: 199–205
- Stavnezer J., Radcliffe G., Lin Y. C., Nietupski J., Berggren L., Sitia R., et al. Immunoglobulin heavy‐chain switching may be directed by prior induction of transcripts from constant‐region genes. Proc Natl Acad Sci U S A 1988; 85: 7704–8
- Jung S., Rajewsky K., Radbruch A. Shutdown of class switch recombination by deletion of a switch region control element. Science 1993; 259: 984–7
- Yu K., Chedin F., Hsieh C. L., Wilson T. E., Lieber M. R. R‐loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nat Immunol 2003; 4: 442–51
- Daniels G. A., Lieber M. R. RNA:DNA complex formation upon transcription of immunoglobulin switch regions: implications for the mechanism and regulation of class switch recombination. Nucleic Acids Res 1995; 23: 5006–11
- Dickerson S. K., Market E., Besmer E., Papavasiliou F. N. AID mediates hypermutation by deaminating single stranded DNA. J Exp Med 2003; 197: 1291–6
- Chaudhuri J., Tian M., Khuong C., Chua K., Pinaud E., Alt F. W. Transcription‐targeted DNA deamination by the AID antibody diversification enzyme. Nature 2003; 422: 726–30
- Pham P., Bransteitter R., Petruska J., Goodman M. F. Processive AID‐catalysed cytosine deamination on single‐stranded DNA simulates somatic hypermutation. Nature 2003; 424: 103–7
- Ramiro A. R., Stavropoulos P., Jankovic M., Nussenzweig M. C. Transcription enhances AID‐mediated cytidine deamination by exposing single‐stranded DNA on the nontemplate strand. Nat Immunol 2003; 4: 452–6
- Bransteitter R., Pham P., Scharff M. D., Goodman M. F. Activation‐induced cytidine deaminase deaminates deoxycytidine on single‐stranded DNA but requires the action of RNase. Proc Natl Acad Sci U S A 2003; 100: 4102–7
- Petersen‐Mahrt S. K., Harris R. S., Neuberger M. S. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature 2002; 418: 99–103
- Rada C., Williams G. T., Nilsen H., Barnes D. E., Lindahl T., Neuberger M. S. Immunoglobulin isotype switching is inhibited and somatic hypermutation perturbed in UNG‐deficient mice. Curr Biol 2002; 12: 1748–55
- Di Noia J., Neuberger M. S. Altering the pathway of immunoglobulin hypermutation by inhibiting uracil‐DNA glycosylase. Nature 2002; 419: 43–8
- Kenter A. L. Class‐switch recombination: after the dawn of AID. Curr Opin Immunol 2003; 15: 190–8
- Muramatsu M., Kinoshita K., Fagarasan S., Yamada S., Shinkai Y., Honjo T. Class switch recombination and hypermutation require activation‐induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000; 102: 553–63
- Revy P., Muto T., Levy Y., Geissmann F., Plebani A., Sanal O., et al. Activation‐induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper‐IgM syndrome (HIGM2). Cell 2000; 102: 565–75
- McHeyzer‐Williams M. G., Ahmed R. B cell memory and the long‐lived plasma cell. Curr Opin Immunol 1999; 11: 172–9
- Heyzer‐Williams L. J., Heyzer‐Williams M. G. Antigen‐specific memory B cell development. Annu Rev Immunol 2005; 23: 487–513
- Reimold A. M., Iwakoshi N. N., Manis J., Vallabhajosyula P., Szomolanyi‐Tsuda E., Gravallese E. M., et al. Plasma cell differentiation requires the transcription factor XBP‐1. Nature 2001; 412: 300–7
- Iwakoshi N. N., Lee A. H., Vallabhajosyula P., Otipoby K. L., Rajewsky K., Glimcher L. H. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP‐1. Nat Immunol 2003; 4: 321–9
- Calame K. L., Lin K. I., Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells. Annu Rev Immunol 2003; 21: 205–30
- Nutt S. L., Heavey B., Rolink A. G., Busslinger M. Commitment to the B‐lymphoid lineage depends on the transcription factor Pax5. Nature 1999; 401: 556–62
- Morrison A. M., Nutt S. L., Thevenin C., Rolink A., Busslinger M. Loss‐ and gain‐of‐function mutations reveal an important role of BSAP (Pax‐5) at the start and end of B cell differentiation. Semin Immunol 1998; 10: 133–42
- Nutt S. L., Eberhard D., Horcher M., Rolink A. G., Busslinger M. Pax5 determines the identity of B cells from the beginning to the end of B‐lymphopoiesis. Int Rev Immunol 2001; 20: 65–82
- Barberis A., Widenhorn K., Vitelli L., Busslinger M. A novel B‐cell lineage‐specific transcription factor present at early but not late stages of differentiation. Genes Dev 1990; 4: 849–59
- Turner C. A Jr., Mack D. H., Davis M. M. Blimp‐1, a novel zinc finger‐containing protein that can drive the maturation of B lymphocytes into immunoglobulin‐secreting cells. Cell 1994; 77: 297–306
- Angelin‐Duclos C., Cattoretti G., Lin K. I., Calame K. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp‐1 expression in vivo. J Immunol 2000; 165: 5462–71
- Eisenbeis C. F., Singh H., Storb U. Pip, a novel IRF family member, is a lymphoid‐specific, PU.1‐dependent transcriptional activator. Genes Dev 1995; 9: 1377–87
- Calame K. L. Plasma cells: finding new light at the end of B cell development. Nat Immunol 2001; 2: 1103–8
- Bernfield M., Sanderson R. D. Syndecan, a developmentally regulated cell surface proteoglycan that binds extracellular matrix and growth factors. Philos Trans R Soc Lond B Biol Sci 1990; 327: 171–86
- Ridley R. C., Xiao H., Hata H., Woodliff J., Epstein J., Sanderson R. D. Expression of syndecan regulates human myeloma plasma cell adhesion to type I collagen. Blood 1993; 81: 767–74
- Finkelman F. D., Katona I. M., Urban J. F Jr., Holmes J., Ohara J., Tung A. S., et al. IL‐4 is r113. equired to generate and sustain in vivo IgE responses. J Immunol 1988; 141: 2335–41
- Jabara H. H., Fu S. M., Geha R. S., Vercelli D. CD40 and IgE: synergism between anti‐CD40 monoclonal antibody and interleukin 4 in the induction of IgE synthesis by highly purified human B cells. J Exp Med 1990; 172: 1861–4
- Punnonen J., de Vries J. E. IL‐13 induces proliferation, Ig isotype switching, and Ig synthesis by immature human fetal B cells. J Immunol 1994; 152: 1094–102
- Pene J., Rousset F., Briere F., Chretien I., Bonnefoy J. Y., Spits H., et al. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proc Natl Acad Sci U S A 1988; 85: 6880–4
- Gascan H., Gauchat J. F., Aversa G., Van Vlasselaer P., de Vries J. E. Anti‐CD40 monoclonal antibodies or CD4+ T cell clones and IL‐4 induce IgG4 and IgE switching in purified human B cells via different signaling pathways. J Immunol 1991; 147: 8–13
- Lebman D. A., Coffman R. L. Interleukin 4 causes isotype switching to IgE in T cell‐stimulated clonal B cell cultures. J Exp Med 1988; 168: 853–62
- Gauchat J. F., Aversa G., Gascan H., de Vries J. E. Modulation of IL‐4 induced germline epsilon RNA synthesis in human B cells by tumor necrosis factor‐alpha, anti‐CD40 monoclonal antibodies or transforming growth factor‐beta correlates with levels of IgE production. Int Immunol 1992; 4: 397–406
- Punnonen J., Punnonen K., Jansen C. T., Kalimo K. Interferon (IFN)‐alpha, IFN‐gamma, interleukin (IL)‐2, and arachidonic acid metabolites modulate IL‐4‐induced IgE synthesis similarly in healthy persons and in atopic dermatitis patients. Allergy 1993; 48: 189–95
- Xu L., Rothman P. IFN‐gamma represses epsilon germline transcription and subsequently down‐regulates switch recombination to epsilon. Int Immunol 1994; 6: 515–21
- de Boer B. A., Kruize Y. C., Rotmans P. J., Yazdanbakhsh M. Interleukin‐12 suppresses immunoglobulin E production but enhances immunoglobulin G4 production by human peripheral blood mononuclear cells. Infect Immun 1997; 65: 1122–5
- Kiniwa M., Gately M., Gubler U., Chizzonite R., Fargeas C., Delespesse G. Recombinant interleukin‐12 suppresses the synthesis of immunoglobulin E by interleukin‐4 stimulated human lymphocytes. J Clin Invest 1992; 90: 262–6
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN‐gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol 1988; 140: 1022–7
- Bossie A., Vitetta E. S. IFN‐gamma enhances secretion of IgG2a from IgG2a‐committed LPS‐stimulated murine B cells: implications for the role of IFN‐gamma in class switching. Cell Immunol 1991; 135: 95–104
- Briere F., Servet‐Delprat C., Bridon J. M., Saint‐Remy J. M., Banchereau J. Human interleukin 10 induces naive surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3. J Exp Med 1994; 179: 757–62
- Malisan F., Briere F., Bridon J. M., Harindranath N., Mills F. C., Max E. E., et al. Interleukin‐10 induces immunoglobulin G isotype switch recombination in human CD40‐activated naive B lymphocytes. J Exp Med 1996; 183: 937–47
- Fujieda S., Saxon A., Zhang K. Direct evidence that gamma 1 and gamma 3 switching in human B cells is interleukin‐10 dependent. Mol Immunol 1996; 33: 1335–43
- Banchereau J. Converging and diverging properties of human interleukin‐4 and interleukin‐10. Behring Inst Mitt 1995; 58–77
- Defrance T., Vanbervliet B., Briere F., Durand I., Rousset F., Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti‐CD40‐activated naive human B cells to secrete immunoglobulin A. J Exp Med 1992; 175: 671–82
- Kitani A., Strober W. Differential regulation of C alpha 1 and C alpha 2 germ‐line and mature mRNA transcripts in human peripheral blood B cells. J Immunol 1994; 153: 1466–77
- McIntyre T. M., Kehry M. R., Snapper C. M. Novel in vitro model for high‐rate IgA class switching. J Immunol 1995; 154: 3156–61
- Stavnezer J. Regulation of antibody production and class switching by TGF‐beta. J Immunol 1995; 155: 1647–51
- Van Vlasselaer P., Punnonen J., de Vries J. E. Transforming growth factor‐beta directs IgA switching in human B cells. J Immunol 1992; 148: 2062–7
- Conti P., Kempuraj D., Kandere K., Di Gioacchino M., Barbacane R. C., Castellani M. L., et al. IL‐10, an inflammatory/inhibitory cytokine, but not always. Immunol Lett 2003; 86: 123–9
- Moore K. W., de Waal M. R., Coffman R. L., O'Garra A. Interleukin‐10 and the interleukin‐10 receptor. Annu Rev Immunol 2001; 19: 683–765
- Dumoutier L., Louahed J., Renauld J. C. Cloning and characterization of IL‐10‐related T cell‐derived inducible factor (IL‐TIF), a novel cytokine structurally related to IL‐10 and inducible by IL‐9. J Immunol 2000; 164: 1814–9
- Dumoutier L., Van Roost E., Colau D., Renauld J. C. Human interleukin‐10‐related T cell‐derived inducible factor: molecular cloning and functional characterization as an hepatocyte‐stimulating factor. Proc Natl Acad Sci U S A 2000; 97: 10144–9
- Fickenscher H., Hor S., Kupers H., Knappe A., Wittmann S., Sticht H. The interleukin‐10 family of cytokines. Trends Immunol 2002; 23: 89–96
- Kotenko S. V. The family of IL‐10‐related cytokines and their receptors: related, but to what extent?. Cytokine Growth Factor Rev 2002; 13: 223–40
- Wang M., Tan Z., Zhang R., Kotenko S. V., Liang P. Interleukin 24 (MDA‐7/MOB‐5) signals through two heterodimeric receptors, IL‐22R1/IL‐20R2 and IL‐20R1/IL‐20R2. J Biol Chem 2002; 277: 7341–7
- Hummelshoj L., Ryder L. P., Poulsen L. K. The role of the interleukin‐10 subfamily members in immunoglobulin production by human B cells. Scand J Immunol 2006; 64: 40–7
- Parrish‐Novak J., Dillon S. R., Nelson A., Hammond A., Sprecher C., Gross J. A., et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000; 408: 57–63
- Asao H., Okuyama C., Kumaki S., Ishii N., Tsuchiya S., Foster D., et al. Cutting edge: the common gamma‐chain is an indispensable subunit of the IL‐21 receptor complex. J Immunol 2001; 167: 1–5
- Kasaian M. T., Whitters M. J., Carter L. L., Lowe L. D., Jussif J. M., Deng B., et al. IL‐21 limits NK cell responses and promotes antigen‐specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity 2002; 16: 559–69
- Brandt K., Bulfone‐Paus S., Jenckel A., Foster D. C., Paus R., Ruckert R. Interleukin‐21 inhibits dendritic cell‐mediated T cell activation and induction of contact hypersensitivity in vivo. J Invest Dermatol 2003; 121: 1379–82
- Brandt K., Bulfone‐Paus S., Foster D. C., Ruckert R. Interleukin‐21 inhibits dendritic cell activation and maturation. Blood 2003; 102: 4090–8
- Ozaki K., Spolski R., Feng C. G., Qi C. F., Cheng J., Sher A., et al. A critical role for IL‐21 in regulating immunoglobulin production. Science 2002; 298: 1630–4
- Kovanen P. E., Leonard W. J. Cytokines and immunodeficiency diseases: critical roles of the gamma(c)‐dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol Rev 2004; 202: 67–83
- Suto A., Nakajima H., Hirose K., Suzuki K., Kagami S., Seto Y., et al. Interleukin 21 prevents antigen‐induced IgE production by inhibiting germ line C (epsilon) transcription of IL‐4‐stimulated B cells. Blood 2002; 100: 4565–73
- Pene J., Gauchat J. F., Lecart S., Drouet E., Guglielmi P., Boulay V., et al. Cutting edge: IL‐21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J Immunol 2004; 172: 5154–7
- Wood N., Bourque K., Donaldson D. D., Collins M., Vercelli D., Goldman S. J., et al. IL‐21 effects on human IgE production in response to IL‐4 or IL‐13. Cell Immunol 2004; 231: 133–45
- Romagnani S. Atopic allergy and other hypersensitivities interactions between genetic susceptibility, innocuous and/or microbial antigens and the immune system. Curr Opin Immunol 1997; 9: 773–5
- Behringwerke A. G. Enzygnost‐IgE. Enzymimmunoassay zur Bestimmung von Human‐IgE [manual for Behringwerke IgE assay]. Behringwerke AG, Marburg 1985, June 1985 ed
- Kjellman N‐I., Johansson S. G. O., Roth A. Serum IgE levels in healthy children quantified by a sandwich technique (PRIST). Clin Allergy 1976; 6: 51–9
- Kjellman N‐I. Atopic allergy and serum IgE concentrations in randomly selected children followed from 8 to 12 years of age. Allergy 1984; 39: 443–50
- Berciano F. A., Crespo M., Bao C. G., Alvarez F. V. Serum levels of total IgE in non‐allergic children. Influence of genetic and environmental factors. Allergy 1987; 42: 276–83
- Holford‐Strevens V., Warren P., Wong C., Manfreda J. Serum total immunoglobulin E levels in Canadian adults. J Allergy Clin Immunol 1984; 73: 516–22
- Lindberg R. E., Arroyave C. Levels of IgE in serum from normal children and allergic children as measured by an enzyme immunoassay. J Allergy Clin Immunol 1986; 78: 614–8
- Klink M., Cline M. G., Halonen M., Burrows B. Problems in defining normal limits for serum IgE. J Allergy Clin Immunol 1990; 85: 440–4