Abstract
Background. Abnormal indices of angiogenesis have been reported in chronic heart failure (CHF). We tested the hypothesis that circulating angiogenin (a potent inducer of neovascularization in vivo) is higher in CHF patients compared with controls and associated with indices of CHF severity: brain natriuretic peptide (BNP), Simpson's left ventricular ejection fraction (EF), and New York Heart Association (NYHA) class.
Methods. Using a cross-sectional approach, we measured serum angiogenin and BNP levels in 109 consecutive patients with CHF (85 males; mean age 60 (standard deviation (SD) 10 yrs) and 112 asymptomatic controls with normal cardiac function and related levels to echocardiographic parameters.
Results. Angiogenin was significantly higher in CHF patients compared to controls (P<0.001). On univariate analysis, angiogenin was positively associated with age, plasma glucose, insulin, and BNP (all P<0.001); and negatively correlated with diastolic blood pressure (P=0.04) and EF (P=0.002). Angiogenin levels increased in an ordinal fashion with NYHA class, exaggerated by the presence of diabetes mellitus (pseudo R2=0.15, P<0.001). In multivariate analysis, angiogenin levels were only associated with deteriorating NYHA classification (beta=0.14 (95% confidence interval (CI) 0.09–0.19), P<0.001). Angiogenin was also a modest discriminator for the presence of CHF (area under the curve 0.72; 95% CI 0.62–0.82), P<0.001).
Conclusion. Angiogenin is related to worsening heart failure severity (NYHA classification), with the highest levels in NYHA class III. Further research is warranted to determine the validity of angiogenin in a diagnostic and prognostic capacity in CHF.
Introduction
Chronic heart failure (CHF) is an increasing cause of cardiovascular disease (CVD) morbidity and mortality within the Western world, where its prognosis is recognized to be worse than that of prostate and breast cancer Citation1. While there are increased efforts to identify individuals at risk of CHF, clinical assessment is limited by symptoms, which are non-specific in a disease presentation that can be asymptomatic in its early stages Citation1. To address this challenge of CHF diagnosis, there has been interest in the evaluation of circulating biological markers ‘biomarkers’—measurable and quantifiable biological parameters—that can aid a clinician in disease management, risk stratification, and prevention Citation2.
The process of angiogenesis—the stimulation of the endothelium to form new vessels—appears to be an integral component in the pathophysiology of CVD Citation3, with increasing evidence to support the view that markers of angiogenesis are aberrant in CHF Citation4–7. However, atherosclerotic vascular disease is a common predisposing factor for CHF and presents a localized area that is rich with inflammatory infiltrates that can activate angiogenesis Citation3. It is unknown whether underlying CVD may confound the association between angiogenesis and CHF.
Key messages
Abnormal indices of angiogenesis have been reported in chronic heart failure (CHF).
In this study, levels of angiogenin (a potent inducer of neovascularization in vivo) are related to worsening heart failure severity, with the highest levels in New York Heart Association (NYHA) class III.
Further research is warranted to determine the validity of angiogenin in a diagnostic and prognostic capacity in CHF.
Angiogenin is a 14,124-Da soluble protein and a member of the ribonuclease (RNAse) superfamily, ‘RNAse 5’ Citation8. In normal subjects, angiogenin is ubiquitous in plasma, circulating free at concentrations between 250 µg/L and 360 µg/L Citation8. Angiogenin is recognized to be a potent inducer of neovascularization in vivo, and circulating levels reflect various angiogenic activities that include increased vessel permeability, endothelial proliferation, and vascular maturation Citation8. The role of angiogenin in CHF is unclear, but there is some evidence that circulating levels are raised in vascular disease, diabetes, and obesity Citation8–11.
Given the relationship between CHF and other indices associated with angiogenesis (vascular endothelial growth factor, angiopoietin Citation4–7), we hypothesized that circulating angiogenin would be elevated in CHF patients compared with controls with normal cardiac function, and this association between CHF and angiogenin would be related to indices of CHF severity (i.e. brain natriuretic peptide (BNP, a biomarker of left ventricular dysfunction), left ventricular ejection fraction, and New York Heart Association (NYHA) classification).
Methods
We recruited consecutive patients with mild to moderate CHF attending our specialist heart failure clinic. CHF was defined according to European Society of Cardiology criteria (symptoms and signs consistent with the diagnosis, and a record of previous (within 6 months) cardiac function investigations, such as echocardiography or cardiac catheterization, confirming left ventricular (LV) systolic impairment, defined as an ejection fraction <40%) Citation12, and stratified according to the NYHA criteria Citation13. Patients were excluded if they had one or more of the following: 1) recent admission (<6 months) due to exacerbation of heart failure; 2) severe or life-threatening inflammatory illnesses, such as rheumatoid arthritis or cancer; 3) acute coronary syndrome, myocardial infarction, or stroke within the last 6 months; or 4) atrial fibrillation. Age, gender, and ethnically matched controls with normal cardiac function were recruited from relatives of patients, hospital staff, and local community groups. Controls were asymptomatic and could have treated risk factors (e.g. hypertension, hyperlipidaemia) but were excluded if found to have any history of ischaemic heart disease, diabetes, or significant inflammatory, neoplastic, or endocrine disease.
Echocardiography and clinical investigation
The ethnicity of the patients and controls was determined by grandparental origin (i.e. at least three of four grandparents to be of a particular ethnicity). Participants were invited to a morning clinic session fasted (no food from 10 p.m. the previous evening), asked to refrain from smoking (more than 8 hours), and vasoactive medication was withheld for 24 hours prior to their clinic appointment. Measurements of height and weight were taken, and blood pressure was measured with a validated semi-automatic monitor (Omron Healthcare Europe, Mannheim, Germany). Diabetes, hypertension, and hyperlipidaemia status was determined by either self-reported histories or evidence within hospital case notes. Echocardiography was performed in the left lateral position, where systolic function was quantified by measurement of fractional shortening by the modified biplane Simpson's method Citation14. All subjects provided written informed consent. The protocol was approved by the West Birmingham Local Research Ethics Committee.
Laboratory data
Serum cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, and plasma glucose were all determined using routine clinical auto-analyser assays in the Biochemistry Department, Sandwell Hospital (West Midlands, UK). Separated fasting serum and ethylenediamine tetra-acetic acid (EDTA) plasma were stored at −70°C for batch analysis. Serum insulin levels were determined using a monoclonal immuno-auto-analyser technique (ADVIA Centaur, Bayer Healthcare, Newbury, UK). Plasma B-type natriuretic peptide (BNP) was assayed using an automated two-site sandwich immuno-assay using direct chemiluminescence (inter- and intra-assay coefficients of variation of the assay were <5%, and the sensitivity and assay range were 0.58–1445 pmol/L) (ADVIA Centaur, Bayer Healthcare, Newbury, UK). Angiogenin levels were measured by enzyme-linked immunosorbent assay (ELISA) in ethylenediamine tetra-acetic acid (EDTA) plasma, using commercially available antibodies (R&D Systems, Abingdon, UK). Coefficients for intra- and inter-assay variation were <3% and <5%, respectively. The lower limit of detection using this ELISA for angiogenin was 7.8 pg/mL.
Power calculation and statistical analysis
To test our hypothesis of a significant difference in levels of angiogenin between CHF patients and controls, a sample size of 87 was needed to detect 0.5 standard deviation in angiogenin geometric means, at 90% power, P< 0.05 using a two-sided test. The required number of patients to observe a statistically significant (P < 0.05) correlation coefficient of at least 0.35, using a two-sided test with a power of 80%, was 51 subjects.
Data were analysed in SPSS v.14 (SPSS Inc., Chicago, IL) using standard and non-parametric (Mann-Whitney) t tests and multiple regression analyses as appropriate. Angiogenin levels were of non-parametric distribution, as determined by normality plots (Kolmogorov-Smirnov). For non-parametric data, central tendencies were reported as medians, interquartile range (IQR). Similarly, for normally distributed variables, the mean and standard deviation (SD) are reported. Correlations were performed using Spearman's method. Linear regression models were calculated to test the strength of association—beta (95% CI)—from independent predictors of angiogenin. Ordinal regression analysis was used to determine changes in angiogenin levels in relation to CHF severity, where the pseudo R-square value was reported to explain the variation in angiogenin in response to CHF classification. Receiver operator characteristic (ROC) curves were used to evaluate the performance of angiogenin, depicted by the mean area under the curve (AUC) with 95% CI.
Results
We studied 109 CHF and 112 controls (). There were no differences in age, gender, ethnicity, systolic blood pressure (BP), heart rate, or total-to-HDL cholesterol ratio. CHF patients had a higher prevalence of known diabetes mellitus, hypertension, and previous myocardial infarction. The main underlying cause for CHF was coronary heart disease (80 patients), where hypertensive heart disease (9 patients) and dilated cardiomyopathy (22 patients) were other causes. Levels of serum cholesterol and HDL cholesterol were lower in the CHF group (P<0.001), whilst triglyceride levels were higher (P<0.001). Levels of angiogenin for CHF patients were non-parametrically distributed and significantly higher than levels in controls (P<0.001). As expected, levels of BNP were highest in CHF (P<0.001).
Table I. Angiogenin levels and cardiovascular and metabolic characteristics of patients with chronic heart failure and control subjects
Amongst CHF patients, there were no significant differences in angiogenin levels amongst those with and without reported myocardial infarction, diabetes, hyperlipidaemia, or a positive smoking history (). Whilst there were no differences in angiogenin levels by diabetes status amongst the CHF patients, levels of angiogenin amongst CHF patients with diabetes were still significantly higher than those in controls (P<0.001; full data not shown). Similarly, angiogenin levels were not different by the active use of cardiovascular medications. Amongst control subjects, levels of angiogenin were only higher amongst those with a positive smoking history (Mann-Whitney test, P < 0.05).
Table II. Angiogenin levels in patients with chronic heart failure.
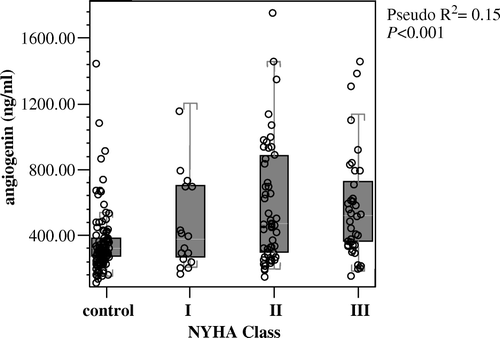
Levels of angiogenin increased with worsening heart failure severity (NYHA classification), with highest levels seen in NYHA class III (). On ordinal regression analysis levels of angiogenin increased with deteriorating NYHA classification (pseudo R2=0.15, P<0.001); thus, median angiogenin levels increased from 377 ng/mL (NYHA class I), 474 ng/mL (NYHA class II), to 519 ng/mL (NYHA class III). The presence of diabetes mellitus further elevated angiogenin levels, where median levels were 575 ng/mL (NYHA class I) and 647 ng/mL (NYHA class II). With quartiles of BNP, median levels of angiogenin increased in an ordinal fashion (P<0.001), from 306 ng/mL for those with a BNP concentration <4.7 pmol/L, to 463 ng/mL for those with BNP >43.3 pmol/L.
Figure 1. Ordinal regression analysis of New York Heart Association (NYHA) classification and serum angiogenin.
Univariate and multivariate analysis
On bivariate analysis of patients and controls, levels of angiogenin were positively associated with adverse metabolic factors (fasting plasma glucose, insulin), deteriorating cardiac function (BNP, ejection fraction), and age, and negatively with diastolic blood pressure (). In relation to cardiac function, with separate analysis of CHF patients and controls, angiogenin levels were associated with ejection fraction amongst CHF patients, r= − 0.25, P=0.01. In multivariate analysis using a linear regression model (that included insulin, age, gender, ethnicity, diastolic blood pressure, glucose, and history of myocardial infarction), variation in angiogenin levels were only associated with deteriorating NYHA classification (beta = 0.14 (95% CI 0.09–0.19), P<0.001).
Table III. Correlation between levels of angiogenin and cardiac risk markers in patients and controls.
Receiver-operating characteristics of angiogenin as a discriminator of CHF
With respect to the presence of CHF across patients and controls, angiogenin was a significant but modest discriminator (AUC 0.72, 95% CI 0.62–0.82, P<0.001), with a poorer performance compared to ejection fraction (AUC 0.99 (1.00–0.98), P < 0.001) or BNP levels (0.92 (0.87–0.98), P<0.001). Neither biomarker was a significant discriminator between NYHA classifications amongst patients with CHF (that is, class I versus II, or class II versus III).
Discussion
We found higher angiogenin levels amongst patients with mild to moderate CHF compared to controls with normal cardiac function, and this difference was associated with measures of cardiac function amongst CHF patients. This study therefore forms a basis for further research into the diagnostic and prognostic performance of angiogenin as a biomarker in the setting of heart failure.
Aberrant levels of angiogenesis markers such as vascular endothelial growth factor (VEGF) and the angiopoietin family (Ang) are evident in heart failure, particularly in those with acute, decompensated symptoms Citation4–7. Even though angiogenesis appears to improve symptoms and regress the progression of heart failure Citation15, markers of angiogenesis have not been convincingly associated with cardiac function and the severity of CHF per se. Further confounding comes from the observations that VEGF and Ang-2 are raised amongst those at an increased risk of myocardial infarction Citation16, as they are potential markers of subclinical atherosclerosis. Whilst VEGF and Ang-2 may also be related to the endothelium, angiogenin also executes many angiogenic capabilities that includes the increased permeability of endothelial and basement membrane layers, endothelial and smooth muscle cell proliferation and migration, and the differentiation and maturation of fragile capillaries into to multilayered vessels Citation8. In CHF, angiogenin could reflect the vascular processes affected by cardiac remodelling and tissue repair, which are not restricted to the endothelium.
The disease presentation of CHF is considered to progress with metabolic deterioration Citation17, and, in the present study, angiogenin levels were associated with features of both metabolism and cardiac function. The performance of angiogenin as a discriminator of CHF was less impressive compared to BNP, and both markers were poor at distinguishing NYHA class. However, the ubiquitous nature of this angiogenic factor within the blood makes it an attractive target for biomarker development. The cohort of patients presented here are representative of ‘typical’ CHF patients, where the majority had survived a myocardial infarction, and there is a high proportion of diabetes mellitus and cardiovascular medications. However, these underlying co-morbidities are likely to also impact on angiogenin levels Citation8, Citation11.
Angiogenin is a potent inducer of neovascularization Citation8, and, within the context of heart failure, raised levels are likely to reflect underlying compensatory remodelling of the coronary microvasculature. Hence, raised angiogenin in heart failure may underline a beneficial pathophysiological response to ischaemia. The implication is that raised angiogenin levels amongst CHF patients may provide the potential for novel therapeutic approaches. Similarly, this may also explain elevated angiogenin levels in acute coronary syndrome but relatively lower levels in patients with stable coronary artery disease Citation11. Further research could determine whether raised levels of angiogenin reflect the acute physiological inflammatory response to ischaemia across the spectrum of cardiovascular disease, and its prognostic value.
This study is limited in its cross-sectional approach, and it is difficult to fully evaluate angiogenin in CHF through this initial study. Further research is needed to determine the prognostic utility of angiogenin in CHF, specifically its diagnostic utility in the acute setting of heart failure. As much of the research on angiogenin in disease states thus far relates to malignancy Citation8, our data add to the use of this biomarker in CVD. The cross-sectional design of the study may overestimate the potential discriminating value of the proposed biomarker, and additional weaknesses could include the selection of patients with left ventricular dysfunction only (that is, systolic heart failure only), the presence of multiple significant differences between the two groups, including multiple variables that may be independently associated with neoangiogenesis, etc.
In conclusion, we propose that angiogenin is related to the deterioration of cardiac function, and further research is warranted to determine the validity of angiogenin in a diagnostic and prognostic capacity in CHF.
Acknowledgements
None of the authors have any conflicts of interest to declare.We are grateful for the laboratory support of Dr Nimai C Panja and Wendy Hoi-Yan Wan, as well as the Sandwell and West Birmingham Hospitals NHS Trust research and development programme.
References
- McMurray JJ, Stewart S. Epidemiology, aetiology and prognosis of heart failure. Heart. 2000; 83: 596–602
- Vasan RS. Biomarkers of Cardiovascular Disease: molecular basis and practical considerations. Circulation. 2006; 11: 2335–62
- Felmeden DC, Blann AD, Lip GYH. Angiogenesis: basic pathophysiology and implications for disease. Eur Heart J. 2003; 24: 586–603
- Chong AY, Caine GJ, Freestone B, Blann AD, Lip GY. Plasma angiopoietin-1, angiopoietin-2, and angiopoietin receptor tie-2 levels in congestive heart failure. J Am Coll Cardiol. 2004; 43: 423–8
- Chong AY, Caine GJ, Lip GY. Angiopoietin/tie-2 as mediators of angiogenesis: a role in congestive heart failure?. Eur J Clin Invest. 2004; 34: 9–13
- Chin BS, Blann AD, Gibbs CR, Chung NA, Conway DG, Lip GY. Prognostic value of interleukin-6, plasma viscosity, fibrinogen, von Willebrand factor, tissue factor and vascular endothelial growth factor levels in congestive heart failure. Eur J Clin Invest. 2003; 33: 941–8
- Chin BS, Chung NA, Gibbs CR, Blann AD, Lip GY. Vascular endothelial growth factor and soluble P-selectin in acute and chronic congestive heart failure. Am J Cardiol. 2002; 90: 1258–60
- Tello-Montoliu A, Patel JV, Lip GYH. Angiogenin: a review of the pathophysiology and potential clinical applications. J Thromb Haemost. 2006; 4: 1864–74
- Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond) 2005; 29: 1308–14
- Burgmann H, Hollenstein U, Maca T, Zedwitz-Liebenstein K, Thalhammer F, Koppensteiner R, et al. Increased serum laminin and angiogenin concentrations in patients with peripheralarterial occlusive disease. J Clin Pathol. 1996; 49: 508–10
- Tello-Montoliu A, Marin F, Patel J, Mainar L, Roldan V, Chung I, et al. Prognostic role of plasma angiogenin in non-ST-elevation acute coronary syndrome. Eur Heart J. 2007; 28: 3006–11
- Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, et al. Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005; 26: 1115–40
- The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagonsis of Diseases of the Heart and Great Vessels. 9th ed. Boston, Mass: Little Brown & Co Inc; 1994.
- Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantification of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr. 1989; 2: 358–67
- Pearlman JD, Hibberd MG, Chuang ML. Magnetic resonance mapping demonstrates benefits of VEGF-induced myocardial angiogenesis. Nat Med. 1995; 1: 1085–9
- Lim HS, Blann AD, Chong AY, Freestone B, Lip GYH. Plasma vascular endothelial growth factor, angiopoietin-1, and angiopoietin-2 in diabetes: implications for cardiovascular risk and effects of multifactorial intervention. Diabetes Care. 2004; 27: 2918–24
- Opie LH. The metabolic vicious cycle in heart failure. Lancet. 2004; 364: 1733–34
