Abstract
Background and aim. Hypertension-induced left ventricular structural remodelling associates with repolarization abnormalities. We investigated if antihypertensive drugs can modulate ventricular repolarization.
Methods. A total of 183 hypertensive men received for 4 weeks drugs (losartan 50 mg, bisoprolol 5 mg, amlodipine 5 mg, hydrochlorothiazide (HCTZ) 25 mg) in a randomized order, separated by 4-week placebo periods. Electrocardiograms (ECG) were recorded at the end of placebo and drug periods. Measurements of repolarization duration (QT intervals), repolarization heterogeneity (T-wave peak to T-wave end (TPE) intervals), and T-wave morphology (T-wave principal component analysis (PCA) ratio, T-wave morphology dispersion (TMD), and total cosine R-to-T (TCRT)) during each drug were compared to placebo measurements.
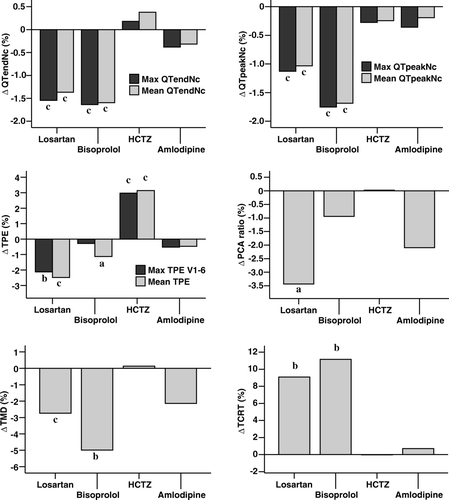
Results. Losartan and bisoprolol shortened maximum and mean rate-adjusted QT intervals as well as mean TPE interval, decreased TMD, and increased TCRT. Losartan also shortened precordial maximum TPE interval and decreased PCA ratio. Amlodipine had no repolarization effects, whereas HCTZ prolonged precordial maximum TPE interval and mean TPE interval.
Conclusion. Losartan and bisoprolol have beneficial short-term ECG repolarization effects. Amlodipine seems to have no repolarization effects. HCTZ seems to prolong the ECG TPE interval, potentially reflecting increased repolarization heterogeneity. These findings show that antihypertensive drugs may relatively rapidly and treatment-specifically modulate ECG markers of ventricular repolarization.
Introduction
Hypertension-induced left ventricular hypertrophy (LVH) increases the risk of sudden cardiac death Citation1, probably mediated by adverse changes in repolarization of the hypertrophied myocardium Citation2–5. Left ventricular (LV) mass increase is linearly associated with repolarization abnormalities Citation6, and repolarization remodelling may even precede clinically evident LVH Citation6–8. On the other hand, LVH regression with long-term antihypertensive drug treatment improves prognosis Citation9 and is associated with beneficial electrocardiographic (ECG) repolarization effects Citation10.
Previous studies have shown that angiotensin II and aldosterone have several proarrhythmic effects, including modulation of the cardiac potassium channel activity Citation11–13. Angiotensin II receptor blockers antagonize these effects, whereas thiazide diuretics activate the renin-angiotensin-aldosterone (RAA) system and may in higher doses increase the risk of sudden cardiac death in hypertensive patients Citation11, Citation12, Citation14–16. In addition, β-blockers have favourable effects on ECG ventricular repolarization in long QT syndrome (LQTS) type 1 Citation17. One may assume, therefore, that commonly used antihypertensive drugs may also have short-term effects on ventricular repolarization in hypertensive patients. Because antihypertensive drugs are widely used and already a mild LV mass increase reduces repolarization reserve exposing hypertensive patients to acquired LQTS Citation6, Citation18, even minor repolarization effects of antihypertensive drugs could be significant at population level. The purpose of this study was to investigate the short-term electrophysiological effects of four commonly used antihypertensive drugs, an angiotensin II receptor blocker (losartan), a β1-adrenergic receptor blocker (bisoprolol), a calcium-channel blocker (amlodipine), and a thiazide diuretic (hydrochlorothiazide (HCTZ)), in hypertensive men in a unique randomized cross-over design. We used digital ECG with simultaneously recorded 12 leads and hypothesized that an angiotensin II receptor blocker and a β-blocker may cause favourable effects whereas a thiazide diuretic might cause adverse electrophysiological repolarization effects, with none of the drugs affecting depolarization in the short term.
Key messages
There is an increased risk of ventricular repolarization abnormalities in patients with arterial hypertension, but little has been known about repolarization effects of antihypertensive drugs.
Our study shows that commonly used antihypertensive drugs may relatively rapidly and treatment-specifically modulate ECG markers of ventricular repolarization.
Patients and methods
Study population and protocol
The present study population originated from the GENRES study (a randomized, double-blind, cross-over, single-centre, placebo-controlled study on molecular genetics of drug responsiveness in essential hypertension). The main study design, power calculations, inclusion and exclusion criteria, and blood pressure (BP) measurement methods have been published in detail elsewhere Citation19. In brief, the study subjects were white men with either prior antihypertensive drug treatment or diastolic BP ≥95 mmHg on three separate measurements. All subjects were in sinus rhythm. Secondary hypertension, drug-treated diabetes mellitus, congestive heart failure, and coronary heart disease were among the exclusion criteria Citation19.
The GENRES study drugs were, with once-a-day doses, losartan 50 mg (an angiotensin II receptor blocker), bisoprolol 5 mg (a β1-adrenergic receptor blocker), amlodipine 5 mg (a calcium-channel blocker), and HCTZ 25 mg (a thiazide diuretic). The subjects received daily for 4 weeks one of the four study drugs in a randomized order. Placebo periods of 4 weeks were included before and between drug periods. A 12-lead digital ECG was recorded and BP measured at the same visit at the end of each period Citation19. Thus, for the current study, ECG data and BP data were gathered during eight different occasions (during four different antihypertensive drugs and during four placebo periods) from every study subject.
Of the GENRES study population, 188 subjects had an ECG recorded at the end of all four drug periods. We excluded subjects with complete bundle branch block (n = 1) and those without BP data from all four drug periods (n = 4). Thus, the present study included 183 subjects. Of the study subjects, 141 (77%) had been on antihypertensive medication before entering the 4-week run-in placebo period at the beginning of the GENRES study. None of the 183 study subjects had to discontinue the study because of insufficient BP control and, during the study, all of them used only the study drugs for their hypertension. As a BP measurement, we used the office systolic and office diastolic BP. Office BP measurements (Omron® M4; Omron Healthcare, Tokyo, Japan) were performed after a 30-minute rest in sitting position three times with 1-minute intervals. The mean of the last two measurements was used in the analyses. For all study subjects, we used the average values of BP and ECG measurements from all four placebo periods as a reference (later named as placebo) to which measurements during each drug period were compared. The GENRES study protocol included serum potassium measurements during the run-in placebo period and at the end of two drug periods for all patients. Hence, serial serum potassium measurements were available for nearly half of all drug periods in the study (n = 91 for bisoprolol and losartan, n = 92 for amlodipine and HCTZ). Clinical characteristics of the study population are shown in .
Table I. Clinical characteristics of the study population (n = 183). Values are mean±SD or proportions.
The study was approved by the Ethics Committee of Helsinki University Central Hospital and the National Agency for Medicines of Finland. All subjects gave their signed informed consent before entering the study.
Echocardiography
Echocardiography was performed during the run-in placebo period as previously described Citation6, Citation19. LV mass was calculated using an anatomically validated formula Citation20 and LV mass index (LVMI) by dividing LV mass by body surface area. LVMI >116 g/m2 was defined as LVH Citation21.
Electrocardiography
A digital standard 12-lead ECG was recorded with Marquette MAC 5000 (GE Marquette Medical Systems, Milwaukee, Wisconsin, USA). The 12 leads were recorded simultaneously, and a digital median QRS-T complex was used for analyses. We used QT Guard software (GE Marquette Medical Systems) to measure heart rate and T-wave principal component analysis (PCA) ratio, and a custom-made software for all other ECG measurements. Sum of QRS areas in all 12 ECG leads (QRS area sum) was calculated as previously described Citation22. In the study group, QRS area sum during placebo correlated with echocardiographically determined LVMI (r = 0.41, P < 0.001) and served as an estimate for LV mass changes.
Electrocardiographic repolarization measurements
Measurements of repolarization duration (QT intervals) and heterogeneity (TPE intervals)
We performed QT interval measurements based on a previously described algorithm Citation23. The software calculated QTpeak (from QRS onset to T-wave peak), QTend (to T-wave end), and T-wave peak to T-wave end (TPE) interval (as well as QRS duration) in each lead. A single observer (K.P.), blinded to clinical data and on-going treatment, reviewed measurements on-screen. We have recently reported a low coefficient of intraobserver variability of our ECG measurement techniques Citation6, and the same study subjects and measurement methods were used also in the present study. For final analyses, we used QTend interval (maximum (max) and mean) of all leads, QTpeak interval (max and mean) of all leads, max TPE interval of precordial leads Citation24, and mean TPE interval of all leads Citation25. QT intervals were nomogram-corrected (Nc) for heart rate with a nomogram derived from a study population similar to ours Citation26. TPE intervals were not rate-adjusted. To compare our results to earlier studies, also QTc dispersion (max QTend interval–minimum QTend interval, Bazett formula heart-rate adjustment) was calculated.
Measurements of T-wave morphology (PCA ratio, TMD, TCRT)
All T-wave morphology parameters were calculated fully automatically. PCA ratio was calculated as the ratio of the second to first eigenvalues of the spatial T-wave vector Citation27, with a higher PCA ratio indicating more complex T-wave morphology. Two additional T-wave morphology parameters were calculated by a previously described algorithm Citation28–30. In brief, the analysis is based on singular value decomposition of eight independent ECG leads (I, II, V1 to V6). T-wave morphology dispersion (TMD) measures the average angle between all T-wave reconstruction vector pairs excluding lead V1, with higher values indicating increasing differences in T-wave morphology between the leads Citation28. Total cosine R-to-T (TCRT) measures the deviation between potential directions during depolarization and repolarization phases, with lower values indicating increasing angle between R- and T-wave vector loops Citation28.
Statistical analysis
We analysed data with SPSS software 15.0 (SPSS Inc., Chicago, Illinois, USA) and present values as mean±SD. One-way analysis of variance (ANOVA) with the Scheffe post-hoc test was used to compare the antihypertensive responses between the study drugs. Drug treatment effect on serum potassium was analysed with the paired samples t test. The Kolmogorov-Smirnov test was used to test variable distributions. For normally distributed variables, we used the repeated measures ANOVA to compare drug treatment data to placebo data. In TMD and TCRT (both non-normally distributed), the non-parametric Friedman test showed that there were significant differences in at least one of the four drug periods. For these parameters, we used the Wilcoxon signed ranks test to further compare drug treatment data to placebo data. Changes in repolarization measurements during drug treatment (placebo value subtracted from drug value) were analysed (all drug periods combined) with correlation coefficients and regression analyses. We calculated univariate correlations, depending on the variable distribution, either with Pearson's or Spearman's test, and entered significant univariate correlates in stepwise multiple linear regression analyses to identify independent determinants of changes in repolarization measurements. A two-tailed P < 0.05 was considered statistically significant.
Results
Clinical characteristics
shows the effects of drug treatment on clinical and ECG parameters. Compared to placebo, both systolic and diastolic BPs were significantly lower during study drugs, but as seen in , HCTZ lowered BP the least. Overall, there was no systematic mean BP change over time during placebo periods (data not shown). Bisoprolol decreased heart rate, whereas other study drugs increased it. QRS duration did not change significantly during any of the study drugs. HCTZ increased and losartan decreased QRS area sum, whereas there were no significant changes during amlodipine or bisoprolol. Compared to serum potassium during the study run-in placebo period, serum potassium increased during bisoprolol and decreased during HCTZ, whereas it did not change during losartan and amlodipine.
Figure 1. Blood pressure changes after 4-week drug treatment. (BP = blood pressure; HCTZ = hydrochlorothiazide. P-values between all comparisons are not shown.)
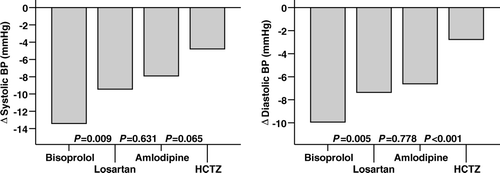
Table II. Effects of 4-week antihypertensive drug treatment (drug compared to placebo) on clinical and ECG parameters (n = 183). Values are mean±SD.
Effects of antihypertensive drugs on electrocardiographic repolarization measurements
shows that, compared to placebo, both losartan and bisoprolol shortened all studied QT intervals and mean TPE interval, decreased TMD, and increased TCRT. In addition, losartan shortened precordial max TPE interval and decreased PCA ratio. HCTZ prolonged both studied TPE intervals, but did not have an effect on the other ECG repolarization measurements. Amlodipine had no significant ECG repolarization effects. QTc dispersion was shortened by both losartan and bisoprolol but was not changed statistically significantly by amlodipine or HCTZ. The results remained the same also when max TPE interval of all leads was used instead of max TPE interval of precordial leads (data not shown). There was no systematic change over time in repolarization measurements during placebo periods (data not shown). shows the changes in ECG repolarization measurements during each drug compared to placebo in percentages.
Correlates of changes in electrocardiographic repolarization measurements
To determine factors associated with changes in ECG repolarization measurements, we correlated the changes in these measurements to the changes in systolic BP, QRS area sum, and serum potassium level after 4-week treatment. A decrease in systolic BP was associated with shortening of QT intervals (r from 0.09 to 0.16, P from 0.011 to <0.001) and TPE intervals (r from 0.10 to 0.13, P from 0.005 to <0.001) as well as with an increase in TCRT (r = −0.09, P = 0.015). A decrease in QRS area sum was associated with shortening of QTend intervals (r from 0.14 to 0.16, P < 0.001) and TPE intervals (r from 0.17 to 0.28, P < 0.001). A decrease in serum potassium level was associated with lengthening of QT intervals (r from -0.12 to -0.27, P from 0.020 to <0.001) and TPE intervals (r from -0.28 to -0.37, P < 0.001). In multivariate analyses, a decrease in systolic BP and QRS area sum as well as an increase in serum potassium level remained as weak, but still significant independent predictors of the shortening of QTend and TPE intervals, also after adjusting for the potential effects of baseline echocardiographic LVMI and placebo systolic BP on these variables (data not shown).
Discussion
Our results show that short-term treatments with different antihypertensive drugs have divergent electrophysiological effects in a mildly to moderately hypertensive middle-aged white male population. An angiotensin II blocker losartan and a β-blocker bisoprolol had beneficial effects on ECG estimates of ventricular repolarization duration and heterogeneity as well as on ECG T-wave morphology parameters. A thiazide diuretic HCTZ prolonged ECG TPE interval, potentially reflecting an increase in repolarization heterogeneity. A calcium-channel blocker amlodipine seemed to have no measurable ECG repolarization effects.
Effects of antihypertensive drugs on electrocardiographic depolarization parameters
We used 12-lead QRS area sum changes to evaluate potential LV mass changes, since QRS area sum seems to perform better than standard voltage criteria and voltage-duration products in ECG LVH detection Citation22, Citation31. Although BP decreased with all treatments, we observed that HCTZ treatment increased and losartan decreased QRS area sum, whereas bisoprolol and amlodipine had no significant effect, suggesting that direct BP-dependent effects cannot explain this finding. In addition, no change in QRS duration was observed, suggesting that none of the studied drugs influenced ventricular conduction.
QRS amplitudes are determined by LV mass, but also by the geometric relation between LV wall thickness and chamber dilatation, intracardiac blood volume, and proximity of the heart to the chest wall Citation32. The short-term increase of QRS area sum with HCTZ is surprising and may reflect volume changes Citation32. However, since losartan antagonizes and HCTZ activates the RAA system, and angiotensin II has cardiotrophic effects Citation33, it is possible that these observed changes in QRS area sum may also reflect true changes in electrically active LV mass, although significant LV mass changes were not expected on short-term Citation14, Citation31, Citation32. A decrease in QRS area sum was independently associated with shortening of QTend and TPE intervals, suggesting also the possibility that angiotensin II could directly mediate both these depolarization and repolarization ECG changes Citation11, Citation12.
Effects of antihypertensive drugs on electrocardiographic repolarization parameters
Previous studies have suggested that long-term treatment with antihypertensive drugs, particularly with angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers, is associated with beneficial changes in ventricular repolarization duration and heterogeneity, changes that are associated also with reverse remodelling of LV structure Citation11, Citation34–36. However, the present study indicates that the favourable repolarization changes with losartan treatment occur rapidly and before significant LV structural remodelling. Angiotensin II and aldosterone have direct proarrhythmic effects also by several BP-independent mechanisms, such as an increase in sympathetic activation, increases in extracellular Ca2 + entry and Ca2 + release from intracellular stores, and modulation of voltage-dependent potassium channels Citation11. Drugs that inhibit the RAA system have shown potentially rapid antiarrhythmic actions by blunting these mechanisms, and losartan with its metabolite also have direct complex effects on cardiac potassium channels Citation11, Citation37. The present results suggest that clinically, in hypertensive patients, losartan has beneficial direct short-term electrophysiological net effects on ventricular repolarization that are likely to be independent of structural remodelling and only partly related to BP reduction per se.
Beta-blockers are generally considered to be antiarrhythmic because they decrease the incidence of presumably arrhythmic sudden cardiac death, for example in patients with congestive heart failure and with the congenital LQTS Citation38. Beta-blockers seem to be particularly effective in preventing arrhythmic sudden deaths in type 1 LQTS patients Citation39, and in these patients β-blockers have been shown to have favourable ECG repolarization effects Citation17. In addition to preventing arrhythmia-promoting actions of β-adrenergic stimulation, β-blockers have been also reported to decrease RAA activity Citation40. The present findings indicate that bisoprolol has short-term protective electrophysiological repolarization effects not dependent on LV reverse remodelling also in hypertensive patients. In an earlier study with hypertensive subjects, QT dispersion remained unchanged during 48-week atenolol treatment Citation36. Our differing QT dispersion finding is probably explained by study population difference or by the different study drug or both.
HCTZ had potentially unfavourable effects on ECG repolarization heterogeneity. Experimentally, in hypertensive LVH, prolongation of action potential duration predisposes to early afterdepolarizations Citation3, Citation4. The changes in action potential durations are spatially non-uniform, thus also creating a substrate for re-entry Citation2, Citation4, Citation5. Although the clinical significance of prolonged TPE interval needs to be studied further, our results suggest that HCTZ monotherapy, by prolonging the TPE intervals and therefore by increasing repolarization heterogeneity, may modulate the arrhythmogenic substrate in hypertensive patients potentially unfavourably. This could be partly mediated by the potassium-wasting effect or by the RAA system activation or both Citation11–14.
Effects of blood pressure and serum potassium changes on electrocardiographic repolarization parameters
In our study population, after a 4-week treatment, with the drug doses used, bisoprolol showed the greatest reduction in systolic BP, and the similar effects of losartan and amlodipine were of greater magnitude than that of HCTZ. A decrease in systolic BP may directly affect ventricular repolarization favourably by stretch-mediated mechanisms Citation41, but in our study this effect evaluated from office BP measurements on QT and TPE intervals was weak, and showed an effect only on TCRT of the T-wave morphology parameters.
Bisoprolol treatment was associated with an increase in serum potassium level, whereas HCTZ had an opposite effect, as expected. A decrease in serum potassium was associated with prolongation of the TPE interval independently of BP changes. Hypokalemia is a well known risk factor in acquired LQTS-related arrhythmias Citation18. Additionally, in epidemiological studies, relatively short-term HCTZ treatment with much higher drug doses has been associated with an increased incidence of sudden cardiac death in hypertensive patients Citation15, Citation16. Our results suggest that, in addition to the effects of angiotensin II, the hypokalemic effect increasing ventricular repolarization heterogeneity may partly mediate the increased risk of sudden death with high-dose HCTZ treatment. Our study thus provides a potential mechanism explaining these epidemiological observations. However, increased incidence of sudden death during low-dose HCTZ therapy has not been reported.
Study limitations
Our results may not be generalized to other races and women. Our study does not provide information on the repolarization effects of antihypertensive drug combinations or repolarization effects of long-term treatment, and the results are directly applicable only to the drugs and doses used. Serial echocardiographic measurement of LV mass was not included in the study protocol. However, considering the randomized cross-over study design with intervening placebo wash-out periods and that mean QRS area sum did not change over time in the study, it is unlikely that changes in LV mass phenotype could have biased the repolarization results. The study design was also suitable to minimize the potential carry-over effects from previous antihypertensive treatment. The antihypertensive drug doses used did not lower BP equally but, on the other hand, BP change affected only weakly and only some of the studied ECG repolarization measurements. In addition, with a higher dose of HCTZ, the potentially favourable BP-lowering effect could have been offset by the potassium-lowering effect. The relationship between the observed T-wave morphology changes and the electrophysiological changes at the myocardial level needs to be clarified. Finally, our study used ECG surrogate markers of arrhythmia susceptibility and does not provide direct arrhythmia data.
In conclusion, our results show that commonly used antihypertensive drugs have measurable divergent short-term ECG repolarization effects and therefore may modulate ventricular repolarization also in the beginning of the therapy, before LVH regression with potentially beneficial repolarization remodelling of long-term treatment occurs.
Acknowledgements
This study was supported by the Finnish Academy, the Sigrid Juselius Foundation, the Helsinki University Central Hospital, the Aarne Koskelo Foundation, the Finnish Foundation for Cardiovascular Research, the Ida Montin Foundation, and the Paavo and Eila Salonen Foundation.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998; 32: 1454–9
- Kowey PR, Friechling TD, Sewter J, Wu Y, Sokil A, Paul J, et al. Electrophysiological effects of left ventricular hypertrophy. Effect of calcium and potassium channel blockade. Circulation. 1991; 83: 2067–75
- Ben-David J, Zipes DP, Ayers GM, Pride HP. Canine left ventricular hypertrophy predisposes to ventricular tachycardia induction by phase 2 early afterdepolarizations after administration of BAY K 8644. J Am Coll Cardiol. 1992; 20: 1576–84
- Yan GX, Rials SJ, Wu Y, Liu T, Xu X, Marinchak RA, et al. Ventricular hypertrophy amplifies transmural repolarization dispersion and induces early afterdepolarization. Am J Physiol Heart Circ Physiol. 2001; 281: H1968–75
- Kozhevnikov DO, Yamamoto K, Robotis D, Restivo M, El-Sherif N. Electrophysiological mechanism of enhanced susceptibility of hypertrophied heart to acquired torsade de pointes arrhythmias: tridimensional mapping of activation and recovery patterns. Circulation. 2002; 105: 1128–34
- Porthan K, Virolainen J, Hiltunen TP, Viitasalo M, Väänänen H, Dabek J, et al. Relationship of electrocardiographic repolarization measures to echocardiographic left ventricular mass in men with hypertension. J Hypertens. 2007; 25: 1951–7
- Schoenmakers M, Ramakers C, van Opstal JM, Leunissen JD, Londono C, Vos MA. Asynchronous development of electrical remodeling and cardiac hypertrophy in the complete AV block dog. Cardiovasc Res. 2003; 59: 351–9
- Perrier E, Kerfant BG, Lalevee N, Bideaux P, Rossier MF, Richard S, et al. Mineralocorticoid receptor antagonism prevents the electrical remodeling that precedes cellular hypertrophy after myocardial infarction. Circulation. 2004; 110: 776–83
- Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004; 292: 2343–9
- Oikarinen L, Nieminen MS, Toivonen L, Viitasalo M, Wachtell K, Papademetriou V, et al. Relation of QT interval and QT dispersion to regression of echocardiographic and electrocardiographic left ventricular hypertrophy in hypertensive patients: the Losartan Intervention For Endpoint Reduction (LIFE) study. Am Heart J. 2003; 145: 919–25
- Delpon E, Caballero R, Gomez R, Nunez L, Tamargo J. Angiotensin II, angiotensin II antagonists and spironolactone and their modulation of cardiac repolarization. Trends Pharmacol Sci. 2005; 26: 155–61
- Fischer R, Dechend R, Gapelyuk A, Shagdarsuren E, Gruner K, Gruner A, et al. Angiotensin II-induced sudden arrhythmic death and electrical remodeling. Am J Physiol Heart Circ Physiol. 2007; 293: H1242–53
- Domenighetti AA, Boixel C, Cefai D, Abriel H, Pedrazzini T. Chronic angiotensin II stimulation in the heart produces an acquired long QT syndrome associated with IK1 potassium current downregulation. J Mol Cell Cardiol. 2007; 42: 63–70
- Burnier M, Brunner HR. Neurohormonal consequences of diuretics in different cardiovascular syndromes. Eur Heart J. 1992;13 Suppl G:S28–33.
- Siscovick DS, Raghunathan TE, Psaty BM, Koepsell TD, Wicklund KG, Lin X, et al. Diuretic therapy for hypertension and the risk of primary cardiac arrest. N Engl J Med. 1994; 330: 1852–7
- Hoes AW, Grobbee DE, Lubsen J, Man in ‘t Veld AJ, van der Does E, Hofman A. Diuretics, beta-blockers, and the risk for sudden cardiac death in hypertensive patients. Ann Intern Med. 1995; 123: 481–7
- Viitasalo M, Oikarinen L, Swan H, Väänänen H, Järvenpää J, Hietanen H, et al. Effects of beta-blocker therapy on ventricular repolarization documented by 24-h electrocardiography in patients with type 1 long-QT syndrome. J Am Coll Cardiol. 2006; 48: 747–53
- Roden DM. Long QT syndrome: reduced repolarization reserve and the genetic link. J Intern Med. 2006; 259: 59–69
- Hiltunen TP, Suonsyrjä T, Hannila-Handelberg T, Paavonen KJ, Miettinen HE, Strandberg T, et al. Predictors of antihypertensive drug responses: initial data from a placebo-controlled, randomized, cross-over study with four antihypertensive drugs (the GENRES study). Am J Hypertens. 2007; 20: 311–8
- Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986; 57: 450–8
- Liu JE, Devereux RB. Clinical assessment of cardiac hypertrophy. In: Sheridan DJ Left ventricular hypertrophy. London: Churchill Livingstone; 1998. p. 11–16.
- Oikarinen L, Karvonen M, Viitasalo M, Takala P, Kaartinen M, Rossinen J, et al. Electrocardiographic assessment of left ventricular hypertrophy with time-voltage QRS and QRST-wave areas. J Hum Hypertens. 2004; 18: 33–40
- Oikarinen L, Paavola M, Montonen J, Viitasalo M, Mäkijärvi M, Toivonen L, et al. Magnetocardiographic QT interval dispersion in postmyocardial infarction patients with sustained ventricular tachycardia: validation of automated QT measurements. Pacing Clin Electrophysiol. 1998; 21: 1934–42
- Antzelevitch C, Fish J. Electrical heterogeneity within the ventricular wall. Basic Res Cardiol. 2001; 96: 517–27
- Zabel M, Portnoy S, Franz MR. Electrocardiographic indexes of dispersion of ventricular repolarization: an isolated heart validation study. J Am Coll Cardiol. 1995; 25: 746–52
- Karjalainen J, Viitasalo M, Mänttäri M, Manninen V. Relation between QT intervals and heart rates from 40 to 120 beats/min in rest electrocardiograms of men and a simple method to adjust QT interval values. J Am Coll Cardiol. 1994; 23: 1547–53
- Okin PM, Devereux RB, Fabsitz RR, Lee ET, Galloway JM, Howard BV. Principal component analysis of the T wave and prediction of cardiovascular mortality in American Indians: the Strong Heart Study. Circulation. 2002; 105: 714–9
- Acar B, Yi G, Hnatkova K, Malik M. Spatial, temporal and wavefront direction characteristics of 12-lead T-wave morphology. Med Biol Eng Comput. 1999; 37: 574–84
- Zabel M, Malik M, Hnatkova K, Papademetriou V, Pittaras A, Fletcher RD, et al. Analysis of T-wave morphology from the 12-lead electrocardiogram for prediction of long-term prognosis in male US veterans. Circulation. 2002; 105: 1066–70
- Zabel M, Acar B, Klingenheben T, Franz MR, Hohnloser SH, Malik M. Analysis of 12-lead T-wave morphology for risk stratification after myocardial infarction. Circulation. 2000; 102: 1252–7
- Okin PM, Roman MJ, Devereux RB, Pickering TG, Borer JS, Kligfield P. Time-voltage QRS area of the 12-lead electrocardiogram: detection of left ventricular hypertrophy. Hypertension. 1998; 31: 937–42
- Surawicz B. Electrocardiographic diagnosis of chamber enlargement. J Am Coll Cardiol. 1986; 8: 711–24
- Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007; 292: C82–97
- Gonzalez-Juanatey JR, Garcia-Acuna JM, Pose A, Varela A, Calvo C, Cabezas-Cerrato J, et al. Reduction of QT and QTc dispersion during long-term treatment of systemic hypertension with enalapril. Am J Cardiol. 1998; 81: 170–4
- Rials SJ, Xu X, Wu Y, Liu T, Marinchack RA, Kowey PR. Restoration of normal ventricular electrophysiology in renovascular hypertensive rabbits after treatment with losartan. J Cardiovasc Pharmacol. 2001; 37: 317–23
- Malmqvist K, Kahan T, Edner M, Bergfedt L. Comparison of actions of irbesartan versus atenolol on cardiac repolarization in hypertensive left ventricular hypertrophy: results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation Versus Atenolol (SILVHIA). Am J Cardiol. 2002; 90: 1107–12
- Caballero R, Delpon E, Valenzuela C, Longobardo M, Tamargo J. Losartan and its metabolite E3174 modify cardiac delayed rectifier K(+) currents. Circulation. 2000; 101: 1199–205
- Zicha S, Tsuji Y, Shiroshita-Takeshita A, Nattel S. Beta-blockers as antiarrhythmic agents. Handb Exp Pharmacol. 2006:235–66.
- Priori SG, Napolitano C, Schwartz PJ, Grillo M, Bloise R, Ronchetti E, et al. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. JAMA. 2004; 292: 1341–4
- Blumenfeld JD, Sealey JE, Mann SJ, Bragat A, Marion R, Pecker MS, et al. Beta-adrenergic receptor blockade as a therapeutic approach for suppressing the renin-angiotensin-aldosterone system in normotensive and hypertensive subjects. Am J Hypertens. 1999; 12: 451–9
- Eckardt L, Kirchhof P, Breithardt G, Haverkamp W. Load-induced changes in repolarization: evidence from experimental and clinical data. Basic Res Cardiol. 2001; 96: 369–80