Abstract
Non-alcoholic fatty liver disease or NAFLD is a chronic liver condition characterized by hepatic steatosis and associated with insulin resistance and type 2 diabetes mellitus (T2DM). In many patients fat accumulation leads to steatohepatitis (NASH) with chronic necrosis, inflammation, and fibrosis, and eventually to the development of cirrhosis. Obese and T2DM patients are at the greatest risk for NASH and progressive disease. New diagnostic techniques, such as magnetic resonance imaging and spectroscopy (MRS), have enhanced our way to non-invasively quantify liver fat and suggest that the epidemic of NAFLD is much larger than previously believed. However, the diagnosis of NAFLD for clinicians remains difficult due to a number of factors: limited awareness, non-specific symptoms, few laboratory findings, and the need for a liver biopsy to confirm the diagnosis. Traditional treatment approaches have centered on weight loss, but data are limited on its long-term efficacy, and the overall compliance is poor. Recently, pioglitazone has been shown to be safe and effective in patients with NASH and may radically change our approach to the disease. Still, many aspects remain poorly understood. Taken together, wider use of new diagnostic methods and treatment approaches appears to signal the dawn of a new era in the management of NAFLD.
Introduction
Ludwig et al. Citation[1] about 30 years ago described a liver disease that resembled alcoholic hepatitis in its histopathologic features but without a history of significant alcohol consumption. Since then, literature about non-alcoholic fatty liver disease (NAFLD) has expanded exponentially in attempts to understand its pathophysiology and treat the condition. NAFLD is a chronic liver condition that spans a spectrum of abnormalities ranging from simple hepatic steatosis to a predominantly lobular necroinflammation, with or without centrilobular fibrosis (termed non-alcoholic steatohepatitis or NASH), which can eventually lead to cirrhosis and its associated complications. Hepatocellular carcinoma is also a recognized complication of fatty liver disease Citation[2–4], and emerging evidence suggests that cardiovascular disease may also be more common, even when adjusted for traditional risk factors Citation[5], Citation[6]. However, until today, clinical awareness among primary care providers is poor, and many aspects remain incompletely understood. The worldwide epidemic of obesity and type 2 diabetes (T2DM) has driven health care providers to give increasing attention to the treatment of cardiovascular disease and other complications related to obesity and diabetes, but not until recently has NAFLD been recognized as another frequent complication that requires special attention. As discussed below, the disease can be easily overlooked, as there are inherent difficulties in making the diagnosis of NAFLD. There is also a perception among primary care providers that the disease belongs only to the realm of specialists in liver disease. This view is likely to change with the wider availability of magnetic resonance imaging and spectroscopy (MRS) to non-invasively quantify liver fat, combined with the emergence of thiazolidinediones as a viable therapeutic option for NASH.
Epidemiology
There are no exact numbers about the magnitude of the problem in the United States. The estimated prevalence varies widely depending on the methods used for screening, i.e. elevated liver enzymes and/or imaging techniques such as ultrasound (US), computed tomography (CT), or magnetic resonance spectroscopy (MRS). In general it is accepted that NAFLD affects between ~30% and 40% of the adult population Citation[7–9], with higher prevalence rates among patients with the metabolic syndrome or T2DM. It is estimated that about 40% of patients with NAFLD may actually have NASH, which may progress to cirrhosis in 10%–15% of cases Citation[10], Citation[11]. With the ensuing epidemic of obesity, dyslipidemia, and T2DM, the prevalence on NAFLD is expected only to rise worldwide and acquire epidemic proportions. NAFLD is already the most common cause of patient referral to specialists for unexplained chronic liver enzyme elevation in the United States Citation[12]. summarizes the clinical characteristics of subjects with a higher risk of having NASH in the general population.
Table I. Clinical characteristics that increase the risk of developing NASH.
A better estimate of the true prevalence of NAFLD is emerging by using MRS. Browning et al. Citation[9] have carried out the largest population-based study using MRS to present, reporting an overall 34% prevalence of NAFLD after screening 2,287 subjects by MRS. Ethnic differences were evident, as steatosis was much more common in Hispanics (45%) compared to Caucasians (33%) and African-Americans (24%). The chances of having NAFLD increased markedly (~2-fold) in subjects that were obese or had T2DM.
Key messages
Non-alcoholic fatty liver disease (NAFLD) is a chronic liver condition characterized by hepatic fat accumulation, insulin resistance, and frequently by type 2 diabetes mellitus (T2DM). T2DM patients are at the greatest risk for non-alcoholic steatohepatitis (NASH) and progressive disease.
New diagnostic techniques, such as magnetic resonance imaging and spectroscopy, have enhanced our way to non-invasively quantify liver fat in NAFLD.
Pioglitazone has been shown to be safe and effective in patients with NASH and may radically change our approach to the disease.
One important gap in our knowledge is the limited information available on the natural history of the disease. Only a handful of studies with paired biopsies are available Citation[3], Citation[4], Citation[13], Citation[14]. These studies only involve from 22 to 103 patients with an average follow-up ranging from 3.2 years Citation[4] to 13.8 years Citation[3]. In these studies, fibrosis progressed over time in 32%–41% of patients with NAFLD Citation[3], Citation[4], Citation[13–15]. However, disease remained stable in 34%–50% of patients and even improved in a minority. This calls for an urgent need to try to understand better the factors that lead to disease progression, of which obesity Citation[3], Citation[4], Citation[13–15] and T2DM Citation[3], Citation[4], Citation[13–15] have been the two most prominent factors of poor prognosis. In the study with the largest number of patients and longest follow-up, fibrosis progression happened more often in those gaining >5 kg over time, having more liver fat infiltration, and being more insulin-resistant. Of note, 78% had diabetes when re-examined after a decade of follow-up, and 89% were overweight or obese.
Hyperglycemia has been identified as a key factor in disease progression in large early studies by Marceau et al. (n=551) Citation[16] and Luyckx et al. (n=505) Citation[17]. Dixon et al. Citation[18] reported that 60% of patients with T2DM and NAFLD had biopsy-proven NASH, and that advanced fibrosis was present in 75% of those with diabetes and hypertension versus 7% without either condition. Of note, elevated liver enzymes (alanine aminotransferase (ALT) or aspartate aminotransferase (AST)/alanine aminotransferase ratio) have been less reliable than one may have expected to predict those with a worse prognosis. In general, the presence of elevated liver enzymes in NAFLD is accepted as evidence of more advanced disease, but liver transaminase levels may frequently be normal. Mofrad et al. Citation[19] and Sorrentino et al. Citation[20] compared NAFLD patients with persistently elevated to normal ALT levels and found that the prevalence of advanced fibrosis and cirrhosis was similar in both groups. Mofrad et al. Citation[19] also reported that in patients with NAFLD and normal liver function tests (LFTs) , 24% had bridging fibrosis and >10% cirrhosis, concluding that normal plasma ALT levels do not guarantee freedom from underlying advanced fibrosis. Sorrentino et al. Citation[20] reported that in 80 patients with NAFLD and normal liver transaminases, 65% already had NASH and 35% fibrosis. The presence of the metabolic syndrome and long-standing history of obesity were the strongest predictors of disease (but not plasma transaminase levels). Gholam et al. Citation[21] reported in 97 obese individuals a 36% prevalence of NASH and 25% of fibrosis, with insulin resistance and the metabolic syndrome being strongly associated with NASH and fibrosis. This is consistent with the role of obesity described in earlier findings by Frantzides et al. Citation[10], in which the biopsy-proven prevalence of steatosis in morbidly obese subjects [body mass index (BMI) ≥ 40 kg/m2] was 90%, with 42% of them also having steatohepatitis.
Others have also reported that in the setting of diabetes, liver enzymes are poor predictors of disease activity Citation[22], Citation[23], with necroinflammation and fibrosis being much more common in the presence of long-standing metabolic syndrome and/or T2DM. Many studies have confirmed the strong impact of both factors, but in particular diabetes, to the progression of disease (e.g. fibrosis) Citation[2–4], Citation[13–20], Citation[22–25]. More recently, Haukeland et al. Citation[26] reported that the presence of diabetes or impaired glucose tolerance increased by 3.8-fold the risk of fibrosis in patients with an unexplained elevation in LFT, diabetes being the only independent risk factor for NASH. Angulo et al. Citation[24] found that diabetes mellitus, obesity, advanced age and AST/ALT ratio of greater than 1 to be significant predictors of more severe liver fibrosis, so that when diabetes and obesity coexisted 66% of patients had advanced fibrosis.
NASH is now recognized as a plausible explanation for the majority of cases of cryptogenic cirrhosis, even when evidence for steatosis disappears with the progressive development of cirrhosis Citation[4], Citation[27]. The potential for mislabeling patients with NASH that evolve to end stage liver disease as ‘cryptogenic cirrhosis’ was initially highlighted in a long-term prospective study by Powell et al. Citation[2] in 42 patients with NAFLD, as steatosis cleared in many as patients progressed to cirrhosis. The link between cryptogenic cirrhosis and NASH also arises from the fact that both conditions share risk factors such as obesity, metabolic syndrome, and T2DM Citation[4], suggesting that many cases of cryptogenic cirrhosis may just represent an advanced stage of NASH. This was clearly illustrated in the study by Caldwell et al. Citation[28] in which obesity or T2DM was present in 73% of the patients that developed cryptogenic cirrhosis compared to those with hepatitis C-related cirrhosis (28%) or primary biliary cirrhosis patients (33%). In the same study, 70% of patients diagnosed with NASH had T2DM or obesity.
Increased cardiovascular disease (CVD) is also emerging as an important feature to keep in mind in the management of these patients. In many studies involving patients with NAFLD, those with diabetes had not only increased liver-related mortality but cardiovascular mortality was increased as well Citation[6], Citation[29–31]. A link between CVD and the severity of NAFLD is suggested from studies in which the severity of the histological features of NASH can be predicted based on carotid intima-medial thickness (CIMT), a marker of atherosclerosis burden Citation[32]. Long-term studies have indicated that CVD is the most common cause of death of patients with NAFLD, even after adjusting for classical cardiovascular (CV) risk factors Citation[3]. NAFLD may increase CVD in T2DM by inducing more systemic insulin resistance, chronic hyperinsulinemia, dyslipidemia [elevated triglycerides (TG), low high density lipoprotein (HDL)-cholesterol, and promoting the formation of small dense low density lipoprotein (LDL)-cholesterol], and subclinical inflammation, all risk factors that promote atherosclerosis. Taken together, the available evidence suggests that NAFLD should be aggressively pursued in patients that are either obese and/or have T2DM.
Pathogenesis of fatty liver disease
The mechanisms leading to NAFLD and NASH are only partially understood and exceed the goals of this review, so the reader is referred to several excellent in-depth reviews on the topic Citation[33–37]. However, some aspects are important in order to understand the mechanism of action of thiazolidinediones, currently the most promising class of pharmacological agents for the treatment of NASH.
Thiazolidinediones exert their metabolic effects through binding to peroxisome proliferator-activated receptors gamma (PPARγ). These nuclear receptors are particularly abundant in adipose tissue and much less in hepatocytes. They have multiple effects on adipocyte biology Citation[38], which translate at the clinical level into a reversal of the severe adipose tissue insulin resistance so characteristic of patients with fatty liver disease Citation[39]. In NAFLD and NASH, dysfunctional insulin-resistant adipocytes overload the liver with free fatty acids (FFA) and also release a number of cytokines that promote a state of insulin resistance and systemic chronic inflammation, both important determinants of hepatic fat accumulation. Adipose stores account for about 70% of the FFA used for hepatic fat synthesis in the setting of obesity and T2DM Citation[40]. Steatosis appears to be way the liver adapts to excessive FFA supply. There is an increasing consensus that this excess FFA load places mitochondria within hepatocytes under severe functional stress, as their ability to increase fatty acid oxidation is limited under normal living conditions in humans. An alternative adaptive mechanism by the liver in the setting of excessive FFA supply, chronic hyperinsulinemia, and hyperglycemia (all factors associated with increased hepatic triglyceride synthesis) is to increase the very low density lipoprotein (VLDL) secretion. This is likely an adaptive mechanism to prevent massive steatosis and helps explain the frequent association of high triglycerides and low HDL-C in NAFLD. When hepatic adaptation to chronic FFA supply is overwhelmed, reactive oxygen species (ROS) stimulate Kupffer cells (local macrophages) and activate multiple inflammatory pathways [i.e. c-Jun N-terminal kinases (JNK) and nuclear factor-kappa beta (NF-κβ)]. In this way, hepatic insulin resistance is exacerbated by the activation of intracellular proinflammatory signaling pathways Citation[34], Citation[36], Citation[37], Citation[40], Citation[41]. Steatohepatitis (NASH) with progressive liver damage frequently follows the collapse of mitochondrial function to excessive lipid accumulation (or more likely of toxic lipid metabolite accumulation) in patients with insulin resistance and T2DM.
In patients with NASH, insulin stimulates sterol regulatory element-binding protein-1c (SREBP-1c) which promotes hepatic fatty acid synthesis. Insulin-resistant ob/ob mice and transgenic mice that overexpress SREBP-1c have a clear increase of fatty acid synthesis and develop hepatic steatosis Citation[42]. As a proof-of-concept, ob/ob mice deficient in SREBP-1 retain their obesity and insulin resistance phenotype, but had less than one-half of the liver triglyceride content of the control ob/ob mice Citation[43]. Hepatic fatty acids synthesis is also driven by hyperglycemia as glucose stimulates the transcription factor carbohydrate response element-binding protein (ChREBP) and a sequence of steps leading to increased liver-type pyruvate kinase (L-PK). L-PK is involved in the synthesis of pyruvate from exogenous glucose, which is converted into citrate within the mitochondria to be transported to the cytosol to feed fatty acid synthesis. There is also altered insulin signaling and diminished activity of key energy regulators within the hepatocyte, such as adenosine monophosphate (AMP)-activated protein kinase (AMPK), eroxisome proliferator-activated receptor g coactivator-1 α (PGC-1α), PPARα, PPARγ, and other key transcription factors in NAFLD Citation[41].
Thiazolidinediones are a good fit to counteract the myriad of abnormalities described by ameliorating insulin resistance at the level of adipose tissue Citation[38], Citation[39], Citation[44], liver Citation[39], Citation[45–48], and muscle Citation[39], Citation[44], Citation[48]. This results in lower plasma FFA, glucose, and insulin concentrations. Thiazolidinediones reduce excessive hepatic fat deposition by inhibiting fatty acid synthesis (i.e. inhibiting SREBP-1c) and by stimulating fatty acid oxidation via activation ofAMPK Citation[49], Citation[50]. The increase in AMPK and reduction in hepatic fat accumulation may be also mediated by adiponectin Citation[51], which is markedly increased during treatment with thiazolidinediones Citation[39], Citation[45], Citation[52], Citation[53]. Adiponectin is a key regulator of many of these metabolic pathways, and plasma levels are abnormally low as the result of dysfunctional adipose tissue in obesity and T2DM. Consistent with this, patients with NASH have low plasma adiponectin levels Citation[54], Citation[55] as well as a reduced adiponectin receptor (adipo RII) expression in liver tissue Citation[56]. Adiponectin may also exert its beneficial effects in NASH by decreasing elevated plasma tumor necrosis factor alpha (TNF-α) concentrations and its activity within target tissues such as the liver Citation[34]. Against the background of systemic inflammation, pioglitazone reduces plasma levels of transforming growth factor (TGF)-β and TNF-α in patients with NASH Citation[39] and antagonizes the effects of several proinflammatory cytokines in rodent models of fatty liver Citation[57–59].
Despite the accepted role of excessive fat supply and inflammation to the pathogenesis of NAFLD, one must bear in mind that the disease remains overall poorly understood. The framework offered above arises largely from observations in animals, although there is no ideal animal model of NASH. Results are frequently conflicting, and there are limited data in humans. Note that the above framework does not satisfactorily explain why there are some lean subjects that have NASH, or that in many obese (or T2DM) individuals the disease does not progress over time or they are even spared completely from developing the disease. It is likely that steatosis is the result of the interplay between the genetic background and the metabolic milieu, so that a similar degree of hepatic fat accumulation may develop in two given individuals with a wide range of metabolic factors, insulin sensitivity, and overall adiposity. Thus, more work is needed to understand why hepatic fat accumulates if we are to develop effective ways to prevent its development.
Diagnosis
Clinical and laboratory findings ()
In making the diagnosis of NAFLD, a careful medical history is of paramount importance. One major characteristic and prerequisite for the diagnosis of NAFLD or NASH is the absence of significant alcohol intake. How much alcohol intake can induce liver steatosis has been a matter of extensive debate, but it is generally accepted that alcohol consumption should not exceed 15 g per day (>360 ml of beer, >150 ml of wine, or >1.5 of distilled spirits), while others discriminate cut-offs for males (two standard drinks per day or 140 g/week of ethanol) and for females (one standard drink per day or 70 g/week of ethanol) Citation[60]. One must also rule out other culprits of liver steatosis (e.g. medications like corticosteroids and estrogen, viral hepatitis, autoimmune hepatitis, alpha-1 antitrypsin deficiency, and other conditions (see list in ) before labeling a patient with NAFLD.
Table II. Diagnostic considerations in NASH.
Table III. Causes of fatty liver (other than NAFLD).
The symptoms of NASH are non-specific and frequently overlooked. They include general malaise and vague right upper abdominal pain. Findings on physical examination also are sparse with the most common abnormality being an enlarged liver. In the late stages of cirrhosis, findings of chronic liver disease dominate the picture. Obesity and signs of insulin resistance (e.g. acanthosis nigricans) may help give consideration to the diagnosis in some patients Citation[61]. As discussed earlier, a high level of suspicion should be kept when evaluating patients with T2DM as they are not only at higher risk of developing NAFLD and NASH, but they also carry a poorer long-term prognosis. Mild elevations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), usually in the range of ~1.5–2-fold above the upper limit of normal, commonly trigger the work-up in most patients with NAFLD. While a 2–3-fold elevation in liver transaminases in the absence of other diagnosis strongly suggests NASH, relying only on AST and ALT for the diagnosis of the disease may be misleading as they can fluctuate over the course of the disease, and, as mentioned earlier, may be even normal in end stage cirrhosis Citation[62]. Therefore, mild elevations in liver enzymes are unreliable for the diagnosis and monitoring of disease activity.
Imaging
A non-invasive test to diagnose liver steatosis is useful to aid in the evaluation of patients with NAFLD. Abdominal ultrasound commonly detects changes in liver parenchyma as increased echogenicity when compared to the spleen or the kidney and is the most frequently used tool in clinical practice. Advantages of ultrasound include no radiation exposure and being relatively inexpensive and widely available. Depending on the studies, its sensitivity ranges from 60% to 94% with a specificity between 88% and 95% Citation[25], Citation[63–66]. The major drawbacks of ultrasonography are being operator-dependent and affected considerably by enlarging body mass. The sensitivity of ultrasound improves considerably to ~80% when liver fat exceeds 30%, but drops to ~50% in morbid obesity or when liver fat content is less than 20% Citation[65–67]. Thus, ultrasonography leaves many patients undiagnosed, as steatosis of ≤20% is common in many patients with NAFLD Citation[9], Citation[39], Citation[52], Citation[55]. It may also lead to an incorrect diagnosis of NAFLD in 10%–30% of cases Citation[64].
Computed tomography provides a better picture when estimating liver fat. Estimation of liver fat content is based on the comparison between hepatic and splenic attenuations. The characteristic finding on a non-contrast CT scan is a decrease in liver attenuation which makes it appear darker than the spleen. It can predict moderate to severe degrees of steatosis Citation[68] with greater sensitivity when steatosis is above 33% of liver parenchyma Citation[69]. Both ultrasound and CT are considered ‘qualitative’ tests, best suited to detect liver steatosis, but not to quantify the amount of fat or to be used to follow disease progression or patients’ response to treatment.
Magnetic resonance imaging and spectroscopy (MRS) takes non-invasive testing of liver fat a step further, being a highly sensitive and reproducible technique to measure liver fat Citation[70]. The test can usually be performed within 20–30 minutes. This technique uses the same MRI technology available in magnets devoted to clinical care, but data acquisition and analysis are different requiring specially trained personnel. It uses the localized 1H spectra of water relative to that of fat obtained by magnetic resonance. MRS is currently limited to academic centers, although this is likely to change in the near future. The upper limit of hepatic triglycerides content is considered to be ~5% of the liver wet weight Citation[9]. This is in agreement with studies we have performed in our laboratory in recent years Citation[39], Citation[52], Citation[55], Citation[71]. MRS has a very good correlation with the amount of liver fat estimated by liver biopsy Citation[72] and has been extensively validated in the Dallas Heart Study, a large (n=2,287) multiethnic population-based study Citation[9], Citation[73]. This is in agreement with our own experience, in which the correlation with steatosis as compared within a given individual with liver biopsies is very good (r>0.80, P < 0.0001, unpublished). In fact, at lower fat content levels, it quantifies liver triglyceride content even before macrovesicular steatosis may be detected by microscopic exam Citation[72–74]. In our hands, we typically measure three areas of interest (upper right lobe, lower right lobe, and left lobe) each covering 3 cm×3 cm. The liver fat correlation among the three areas is extremely high (r>0.90, P < 0.0001, unpublished), a reason why just imaging one area may be considered an acceptable approach to reduce scanning time and overall cost. Because MRS samples a larger area of liver tissue than a liver biopsy does, it is likely that it provides even a more accurate estimate of fat content.
By MRS we have found in the predominantly Hispanic population of San Antonio, Texas, that NAFLD is present in >75% of unselected patients with T2DM, although it lacks good sensitivity and specificity for the presence of necroinflammation or fibrosis. This may improve in the near future with the combination of contrast agents with MRI to this end Citation[75], Citation[76]. Systematic screening for hepatic steatosis by MRS in subjects at the highest risk of progressive disease (i.e. obese patients with T2DM) is a novel approach for early intervention being under investigation in research settings to prevent progressive liver damage in high-risk subjects.
Role of liver biopsy
As can be appreciated, the diagnosis of NAFLD is not straightforward in many patients, requiring a rigorous medical history and laboratory evaluation. In the absence of solid clinical features or laboratory abnormalities in NASH patients, the diagnosis almost always mandates a liver biopsy. A liver biopsy should be considered to confirm the diagnosis and grade the liver abnormalities, including the presence and stage of fibrosis, and to assess response to treatment during follow-up. In 2005, Kleiner et al. Citation[77] proposed a scoring system of the pathologic features of fatty liver disease to grade its severity that is currently widely used to assess disease activity and progression or regression of this condition in prospective studies. The invasive nature of a liver biopsy is its major drawback which explains the reluctance by physicians and patients to undergo the procedure, in particular, given the limited therapeutic options beyond life-style modification. This may change in the future if thiazolidinediones establish themselves as safe and effective for long-term use. In the meantime, efforts have been made to combine clinical, metabolic, and plasma biomarkers of disease activity to allow a non-invasive diagnosis of NASH and discriminate between patients with simple steatosis that follow an indolent and rather benign course, from those evolving to severe steatohepatitis and fibrosis (reviewed by Wieckowska et al. Citation[8] and Adams et al. Citation[78]). Different scoring systems have met variable success in terms of accuracy and predictive value, being particularly helpful to separate the extremes of the disease spectrum, benign from fairly advanced disease. Unfortunately, they have not been tested to monitor response to treatment interventions and have been largely tested in non-diabetic Caucasian populations, awaiting validation in different ethnic groups and in patients with T2DM. So far no test has completely fulfilled the ideal criteria of a non-invasive test (i.e. simple, inexpensive, not affected by co-morbidities, able to closely mirror histological changes, sensitive, and reproducible), but this is a rapidly evolving area likely to change in the near future.
Interventions in NAFLD
The view of a patient with NAFLD has changed over the years from being considered an innocent condition of ‘just fat in the liver’, to a current view of a serious finding associated with a myriad of metabolic abnormalities that may carry considerable morbidity and a risk of increased mortality ranging from liver-related conditions (e.g. cirrhosis, liver cancer) to increased cardiovascular disease Citation[5]. This calls for a concentrated effort to find more effective strategies in NAFLD, combining life-style changes with safe and effective pharmacological interventions.
Life-style intervention
Diet and exercise have been considered the standard of care for fatty liver disease for many years; still no long-term large multicenter controlled trials have been conducted to date. Early studies showed that weight loss led to the resolution of liver steatosis Citation[79]. These early reports also suggested that it could exacerbate portal inflammation in patients with higher fatty infiltration and faster weight loss Citation[80]. However, this concern is much less now as massive weight loss following bariatric surgery in recent series report significant reduction in steatosis, although less of inflammation or fibrosis Citation[81–84]. Large, long-term studies have shown that bariatric surgery is associated with a significant decrease in overall and diabetes-related mortality Citation[85], Citation[86], although similar studies in NASH are unavailable.
The caveat about bariatric surgery studies is that patients are severely obese and probably do not reflect the majority of patients suffering from NAFLD. While weight reduction in NAFLD is felt to be beneficial, there has been a wide range of responses, and treatment success has been usually limited to an improvement in liver transaminases and in hepatic fatty infiltration Citation[87–89]. In a meta-analysis of 13 weight reduction studies, Wang et al. Citation[87] reported that most studies were small (only 3 had more than 50 patients, while 9 had 25 or fewer), uncontrolled (10 were case series), and frequently used a surrogate primary end-point (i.e. liver aminotransferase levels instead of liver histology in 8 of the 13 trials). In the few studies that performed a liver biopsy before and after weight loss, only steatosis improved, but not necroinflammation or fibrosis. Moreover, improvement in aminotransferase levels did not necessarily translate into improved liver histologic scores, something well documented in our recent trial in patients with NASH Citation[39]. Overall, intervention studies in patients with NAFLD face the same kind of difficulties in achieving and maintaining weight loss that clinicians face in clinical practice.
A few studies have included exercise as part of their treatment program Citation[87], Citation[90–94], but again, studies have typically been small, of short duration (less than 16 weeks), and only used surrogate end-points such as liver transaminases and/or liver ultrasound. Reduction in steatosis has also been reported in recent short-term (2–12 weeks), small studies (n=7–10), as assessed by MRS Citation[95–97]. Only Ueno et al. Citation[92] performed liver biopsies before and after diet and exercise for 3 months. He reported only a reduction in steatosis, but not in liver inflammation or fibrosis. Similarly, Huang et al. Citation[98] reported a trend toward a histologic improvement but not a significant benefit after a year-long, intense nutritional counseling program in patients with non-alcoholic steatohepatitis. Another study examined the effects of exercise in 14 patients with T2DM after 2 weeks of hypocaloric diet with or without moderate exercise (30-minute exercise program, 5–6 times per week) Citation[95]. Such an exercise program led to a modest reduction in liver fat but not beyond that of diet alone. However, the metabolic effects of such a short intervention were rather modest.
Finally, low-carbohydrate diets are of particular benefit to reduce steatosis and hepatic transaminases in subjects with NAFLD Citation[99], Citation[100]. Ryan et al. Citation[101] compared the effect of two hypocaloric diets containing either 60% carbohydrate/25% fat or 40% carbohydrate/45% fat (15% protein) for 16 weeks in 52 insulin-resistant obese subjects. While both diets resulted in significant decreases in weight, insulin resistance, and serum transaminases, the low-carbohydrate diet improved all three parameters much more than the high-carbohydrate diet.
Pharmacological treatments
Weight loss remains the standard of care in NAFLD. In part, this is because no pharmacological therapy (until recently) conclusively proved to be effective in NASH. Agents tested that have showed modest benefit included pentoxifylline, orlistat, vitamin E, cytoprotective agents, ursodeoxycholic acid, and lipid-lowering agents Citation[102]. The anti-TNF-α properties of pentoxifylline prompted its use in NASH Citation[103], Citation[104] in small 6–12 month open-label trials with some improvement in liver enzymes and TNF-α levels, but overall minimal clinical effects. Antioxidants such as vitamin E and C gained interest as a means to reduce oxidative stress causing liver cell injury and death. In a well designed study in 49 patients by Harrison et al. Citation[105] there was no significant effect of vitamin E and C on necroinflammation or fibrosis compared to the placebo group. Orlistat is an agent approved for weight loss that inhibits gastric and pancreatic lipases and impairs fat absorption. However, there was only a small reduction in hepatic steatosis in a pilot study in 10 patients with NASH Citation[106]. The efficacy of orlistat therapy plus a hypocaloric diet was minimal when compared to dietary management alone in a double-blind, randomized, placebo-controlled trial Citation[107]. Both control and active treatment groups lost weight in comparable magnitudes. In those patients who agreed to undergo a repeat liver biopsy (n=22), steatosis improved by ~50% without significant differences between the groups and no significant change in inflammation. Ursodeoxycholic acid Citation[108], Citation[109] is a popular medication in the liver field due to its possible cytoprotective effect on liver cells. However, in a 2-year large randomized trial in 166 patients with liver biopsy-proven NASH, ursodeoxycholic acid did not demonstrate any significant advantage over placebo on repeat liver biopsies Citation[110].
Another pilot trial evaluating betaine Citation[111] (a metabolite of choline) as a therapy for NASH reported some improvement in liver enzymes and histology, but the lack of a control group and the small number of patients did not bring this agent to focus in the NAFLD field. The renin-angiotensin system plays an important role in modulating insulin resistance as angiotensin receptor blockers (ARBs) may lower insulin resistance Citation[112]. ARBs may reduce the number of active hepatic stellate cells, the cells responsible for the synthesis of collagen and hepatic fibrosis when activated Citation[113], as reported in a pilot study of 7 patients with NASH in which losartan (50 mg/d) reduced hepatic inflammation (n=5) and fibrosis (n=4), although there was no change in steatosis Citation[114].
The strong association of NASH with insulin resistance and glucose intolerance led to the consideration of the insulin-sensitizer metformin as a treatment for NAFLD. Metformin is a biguanide that lowers hepatic, and to a lesser extent muscle, insulin resistance Citation[115], Citation[116]. In addition, as discussed earlier, it is a known activator of hepatic AMPK, a key metabolic target for the inhibition of hepatic lipogenesis Citation[117]. When first used in a small group of non-diabetic patients with NAFLD, there was a reduction in liver transaminases over a period of 4 months of treatment Citation[118], and a similar effect was noted in another open-label randomized trial Citation[119]. However, no significant improvement in hepatic inflammation was found on histology. In another small open-label study, a 1-year metformin trial, hepatic steatosis improved in 3 and inflammation in 2 out of the 15 patients Citation[120]. The largest study with metformin was an open-label trial of 110 patients randomized to receive either metformin 2 g/day (55 patients), vitamin E 800 IU/day (28 patients), or prescriptive weight-reducing diet (27 patients) for 12 months Citation[121]. Liver transaminases improved more with metformin than in the other arms. Steatosis, and to a lesser extent inflammation, also improved in a subset of 17 patients that underwent a liver biopsy before and after metformin treatment. Taken together, the effect of metformin has been rather modest and overall disappointing in improving liver histology in patients with NASH.
Use of thiazolidinediones in NASH
Thiazolidinediones (TZDs) have gained significant attention in recent years due to their beneficial metabolic effects in patients with T2DM. As mentioned earlier, TZDs exert positive changes on adipocytes (i.e. increasing plasma adiponectin levels, restoring insulin sensitivity, reducing excessive lipolysis and plasma FFA levels, among others) and improve hepatic and peripheral (muscle) insulin sensitivity. Troglitazone (later withdrawn due to its idiosyncratic hepatotoxic effect) was the first agent to be tested in humans with NASH. In a pilot study of 10 patients with NASH Citation[122], ALT was normalized in 7 subjects by the end of the study (6 months). However, necroinflammation was essentially unchanged and histological benefit was minimal. Neuschwander-Tetri et al. Citation[46] tested rosiglitazone in an uncontrolled open-label study in 22 subjects with biopsy-proven NASH. All of the patients were overweight, and 50% had impaired glucose tolerance (IGT). There was a significant reduction in liver enzymes and in steatosis, ballooning, and inflammation scores, but no effect on liver fibrosis. Pioglitazone was reported in two small uncontrolled studies to be of benefit as well. In 18 non-diabetic patients with biopsy-proven NASH, pioglitazone normalized liver enzymes and led to a significant histological response in approximately two-thirds of subjects Citation[47]. Sanyal et al. Citation[123] compared pioglitazone to daily vitamin E supplementation in another pilot study of 20 non-diabetic subjects. Both treatment groups had reduction in liver steatosis, but reduction in inflammation was only significant in the pioglitazone plus vitamin E group. No significant effect was seen on hepatic fibrosis.
It was not until a larger controlled trial in NASH was conducted that TZDs received wider attention as a potential treatment in NASH Citation[124], Citation[125]. In the first double-blind, placebo-controlled trial testing the full spectrum of metabolic and histological effects of pioglitazone, our group treated 55 subjects with NASH Citation[39]. In the wake of the liver toxicity associated with troglitazone and uncertainties relating to the response to treatment in patients with already chronic liver disease such as in NASH (the study was started in 2002), we felt at the time that exposure to a TZD would be only justified in patients with either IGT or T2DM, who would eventually also benefit from improved glycemic control. Therefore, all patients underwent a screening oral glucose tolerance test (OGTT). It was only after this systematic OGTT screening that we found that most patients with NASH had IGT or T2DM, most patients being previously unaware. At study entry, 8 out of 25 patients were first diagnosed with T2DM (5 in the pioglitazone and 3 in the placebo group), and of the 23 patients with IGT only 2 had a fasting glucose >110 mg/dL (in which one would more readily suspect IGT or diabetes). The proportion of patients with IGT and T2DM was evenly distributed with 13 and 14 in the pioglitazone arm and 12 and 9 in the placebo arm, respectively. Histology scores at base-line as well as the response to pioglitazone were similar whether patients had IGT or T2DM.
Both groups received identical nutritional counseling to mimic accepted standards of care for patients with NASH, and it was against this background that placebo versus pioglitazone were compared. The study design included before and after treatment a double-tracer OGTT (administration of oral glucose radio-labeled with 14C to assess its absorption and intravenous 3H glucose to measure hepatic glucose production), a total body fat measurement by dual X-ray absorptiometry (DXA), liver fat measured by MRS, and a repeat liver biopsy after 6 months of treatment. Pioglitazone significantly lowered liver transaminases (). The TZD markedly improved fasting and postprandial insulin levels and insulin resistance, as well as glucose tolerance. Liver fat content measured by MRS was not changed in the diet plus placebo group, but was reduced by 54% in the pioglitazone group despite a statistically significant weight gain (2.5±0.5 kg) in the pioglitazone group. However, visceral fat was significantly reduced. Pioglitazone reduced the plasma levels of inflammatory markers such as tumor necrosis factor (TNF)-α and transforming growth factor (TGF)-β and increased plasma adiponectin levels, while all were unchanged in the placebo group. Hepatic insulin sensitivity improved by 48% (P < 0.01 versus placebo). All histology scores improved significantly with pioglitazone (). Liver fibrosis improved in the pioglitazone group when compared to base-line, but did not reach statistical significance when compared to placebo. The combined overall necroinflammation score was reduced by 85% with the TZD (P < 0.0001). The results of this study have generated a substantial interest in the use of pioglitazone for the treatment for NASH Citation[124], Citation[125]. Larger clinical trials of longer duration are under way in both non-diabetics (e.g. PIVENS trial) and in patients with T2DM (at our center) to gain insights about the overall safety and efficacy of TZDs in NASH.
Figure 1. Effect of diet plus pioglitazone compared to diet alone (plus placebo) on liver transaminases. Note that during the run-in, dietary advice rapidly lowers AST and ALT. However, pioglitazone compared to modest weight reduction has a much more significant impact in improving AST and ALT levels, normalizing both enzymes with 6 months of treatment. Shaded area represents pioglitazone or placebo administration period.
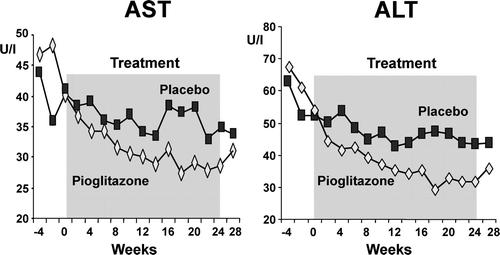
Figure 2. Mean scores for inflammation, ballooning necrosis, steatosis, and fibrosis in liver biopsy specimens before and after treatment with a hypocaloric diet (-500 kcal per day) plus pioglitazone, or a hypocaloric diet plus placebo, in 55 patients with impaired glucose tolerance or T2DM and liver biopsy-confirmed NASH. Between-group differences were compared by means of the Wilcoxon rank-sum test. Within-group differences (before versus after treatment) were compared by means of the Wilcoxon signed-rank test. Reproduced, with permission, from Belfort R et al. Citation[39].
![Figure 2. Mean scores for inflammation, ballooning necrosis, steatosis, and fibrosis in liver biopsy specimens before and after treatment with a hypocaloric diet (-500 kcal per day) plus pioglitazone, or a hypocaloric diet plus placebo, in 55 patients with impaired glucose tolerance or T2DM and liver biopsy-confirmed NASH. Between-group differences were compared by means of the Wilcoxon rank-sum test. Within-group differences (before versus after treatment) were compared by means of the Wilcoxon signed-rank test. Reproduced, with permission, from Belfort R et al. Citation[39].](/cms/asset/3099f41f-e7b1-457a-847e-7fd7070c3064/iann_a_355411_f0002_b.gif)
Conclusion and future directions
Non-alcoholic fatty liver disease is a disease of large magnitude and potentially very serious outcomes. The presence of obesity, diabetes, and Hispanic ancestry are factors associated with the highest risk of developing NASH and end stage liver disease. The availability of MRS has uncovered an epidemic demanding new diagnostic and treatment algorithms, although the best approach remains controversial given the gaps in our knowledge about its natural history, factors related to disease progression, and efficacy of long-term pharmacological treatment with a TZD. Awareness by health care providers is an essential starting-point for early diagnosis. The epidemic of T2DM call for fatty liver disease be included into our routine work-up of such patients, in the same way as we do for microvascular complications such as retinopathy, nephropathy, and neuropathy, or for cardiovascular disease. Although the data on the efficacy of diet and exercise are limited, it is essential for the successful management of fatty liver disease and its related metabolic conditions. Thiazolidinediones are emerging as a promising therapy in NASH, but a better understanding of their long-term safety and efficacy is mandatory before TZDs can be routinely incorporated into clinical practice.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980; 55: 434–8
- Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990; 11: 74–80
- Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006; 44: 865–73
- Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005; 129: 113–21
- Targher G, Lorenzo B, Roberto P, Stefano R, Roberto T, Luciano Z, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007; 30: 1212–8
- Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005; 54: 3541–6
- Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002; 122: 1649–57
- Wieckowska A, McCullough A, Feldstein A. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007; 46: 582–9
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004; 40: 1387–95
- Frantzides CT, Carlson MA, Moore RE, Zografakis JG, Madan AK, Puumala S, et al. Effect of body mass index on nonalcoholic fatty liver disease in patients undergoing minimally invasive bariatric surgery. J Gastrointest Surg. 2004; 8: 849–55
- Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology. 1999; 116: 1413–9
- Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003; 98: 960–7
- Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003; 98: 2042–7
- Fassio E, Alvarez E, Domínguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004; 40: 820–6
- Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005; 42: 132–8
- Marceau P, Biron S, Hould FS, Marceau S, Simard S, Thung SN, et al. Liver pathology and the metabolic syndrome X in severe obesity. J Clin Endocrinol Metab. 1999; 84: 1513–7
- Luyckx FH, Desaive C, Thiry A, Dewe W, Scheen AJ, Gielsen JE, et al. Liver abnormalities in severely obese subjects: effects of drastic weight loss after gastroplasty. Int J Obes Relat Metab Disord. 1998; 22: 222–6
- Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001; 121: 91–100
- Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003; 37: 1286–92
- Sorrentino P, Tarantino G, Conca P, Perrella A, Terracciano ML, Vecchione R, et al. Silent non-alcoholic fatty liver disease–-a clinical-histological study. J Hepatol. 2004; 41: 751–7
- Gholam PM, Flancbaum L, Machan JT, Charney DA, Kotler DP. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol. 2007; 102: 399–408
- Amarapurkar D, Patel N. Clinical spectrum and natural history of non-alcoholic steatohepatitis with normal alanine aminotransferase values. Trop Gastroenterol. 2004; 25: 130–4
- Kunde S, Larenzby A, Clements R, Abrams G. Spectrum of NAFLD and diagnostic implications of the proposed new normal range for serum ALT in obese women. Hepatology. 2005; 42: 650–6
- Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999; 30: 1356–62
- Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004; 2(3)262–5
- Haukeland JW, Konopski Z, Linnestad P, Azimy S, Marit Loberg E, Haaland T, et al. Abnormal glucose tolerance is a predictor of steatohepatitis and fibrosis in patients with non-alcoholic fatty liver disease. Scand J Gastroenterol. 2005; 40: 1469–77
- Maheshwari A, Paul JT. Cryptogenic cirrhosis and NAFLD: are they related?. Am J Gastroenterol. 2006; 101: 664–8
- Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999; 29: 664–9
- Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007; 13: 1579–84
- Targher G, Bertolini L, Padovani R, Poli F, Scala L, Tessari R, et al. Increased prevalence of cardiovascular disease in Type 2 diabetic patients with non-alcoholic fatty liver disease. Diabet Med. 2006; 23: 403–9
- Targher G. Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: the plot thickens. Diabet Med. 2007; 24: 1–6
- Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Zenari L, et al. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2006; 29: 1325–30
- Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004; 114: 147–52
- Diehl AM, Li ZP, Lin HZ, Yang SQ. Cytokines and the pathogenesis of non-alcoholic steatohepatitis. Gut. 2005; 54: 303–6
- Pessayre D, Fromenty B. NASH: a mitochondrial disease. J Hepatol. 2005; 42: 928–40
- Elsharkawy AM, Mann DA. Nuclear factor-kappaB and the hepatic inflammation-fibrosis-cancer axis. Hepatology. 2007; 46: 590–7
- Carter-Kent C, Zein NN, Feldstein AE. Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: implications for treatment. Am J Gastroenterol. 2008; 103: 1036–42
- Bogacka I, Xie H, Bray GA, Smith SR. The effect of pioglitazone on peroxisome proliferator-activated receptor-gamma target genes related to lipid storage in vivo. Diabetes Care. 2004; 27: 1660–7
- Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006; 355: 2297–307
- Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005; 115: 1343–51
- Fromenty B, Robin M, Igoudjil A, Mansouri A, Pessayre D. The ins and outs of mitochondrial dysfunction in NASH. Diabetes Metab. 2004; 30: 121–38
- Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999; 274: 30028–32
- Yahagi N, Shimano H, Hasty AH, Matsuzaka T, Ide T, Yoshikawa T, et al. Absence of sterol regulatory element-binding protein-1 (SREBP-1) ameliorates fatty livers but not obesity or insulin resistance in Lep(ob)/Lep(ob) mice. J Biol Chem. 2002; 277: 19353–7
- Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, et al. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002; 51: 2968–74
- Tiikkainen M, Hakkinen A-M, Korsheninnikova E, Nyman T, Makimattila S, Yki-Jarvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004; 53: 2169–76
- Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003; 38: 1008–17
- Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004; 39: 188–96
- Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, et al. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002; 87: 2784–91
- Fryer LG, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002; 277: 25226–32
- Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun. 2004; 314: 580–5
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002; 8: 1288–95
- Bajaj M, Suraamornkul S, Piper P, Hardies LJ, Glass L, Cersosimo E, et al. Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J Clin Endocrinol Metab. 2004; 89: 200–6
- Abbasi F, Chang SA, Chu JW, Ciaraldi TP, Lamendola C, McLaughlin T, et al. Improvements in insulin resistance with weight loss, in contrast to rosiglitazone, are not associated with changes in plasma adiponectin or adiponectin multimeric complexes. Am J Physiol Regul Integr Comp Physiol. 2006; 290: R139–44
- Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005; 48: 634–42
- Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007; 133: 496–506
- Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, et al. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005; 54: 117–21
- Cabrero A, Laguna JC, Vazquez M. Peroxisome proliferator-activated receptors and the control of inflammation. Curr Drug Targets Inflamm Allergy. 2002; 1: 243–8
- Murase K, Odaka H, Suzuki M, Tayuki N, Ikeda H. Pioglitazone time-dependently reduces tumour necrosis factor-( level in muscle and improves metabolic abnormalities in Wistar fatty rats. Diabetologia. 1998; 41: 257–64
- Enomoto N, Takei Y, Hirose M, Konno A, Shibuya T, Matsuyama S, et al. Prevention of ethanol-induced liver injury in rats by an agonist of peroxisome proliferator-activated receptor-gamma, pioglitazone. J Pharmacol Exp Ther. 2003; 306: 846–54
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology. 2003; 37: 1202–19
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002; 346: 1221–31
- Ipekci SH, Basaranoglu M, Sonsuz A. The fluctuation of serum levels of aminotransferase in patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2003; 36: 371
- Gupte P, Amarapurkar D, Agal S, Baijal R, Kulshrestha P, Pramanik S, et al. Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol. 2004; 19: 854–8
- Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005; 143: 722–8
- Palmentieri B, de Sio I, La Mura V, Masarone M, Vecchione R, Bruno S. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig Liver Dis. 2006; 38: 485–9
- Adams LA, Paul A. Role of liver biopsy and serum markers of liver fibrosis in non-alcoholic fatty liver disease. Clin Liver Dis. 2007; 11: 25–35
- Ryan CK, Johnson LA, Germin BI, Marcos A. One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl. 2002; 8: 1114–22
- Limanond P, Raman SS, Lassman C, Sayre J, Ghobrial RM, Busuttil RW, et al. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology. 2004; 230: 276–80
- Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002; 123: 745–50
- Longo R, Pollesello P, Ricci C, Masutti F, Kvam BJ, Bercich L, et al. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging. 1995; 5: 281–5
- Bajaj M, Suraamornkul S, Hardies J, Glass L, Musi N, Defronzo R. Effects of peroxisome proliferator-activated receptor (PPAR)-alpha and PPAR-gamma agonists on glucose and lipid metabolism in patients with type 2 diabetes mellitus. Diabetologia. 2007; 50: 1723–31
- Thomsen C, Becker U, Winkler K, Christoffersen P, Jensen M, Henriksen O. Quantification of liver fat using magnetic resonance spectroscopy. Magn Reson Imaging. 1994; 12: 487–95
- Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005; 288: E462–8
- Szczepaniak LS, Babcock EE, Schick F, Dobbins RL, Garg A, Burns DK, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999; 276: E977–89
- Noren B, Dahlqvist O, Lundberg P, Almer S, Kechagias S, Ekstedt M, et al. Separation of advanced from mild fibrosis in diffuse liver disease using 31P magnetic resonance spectroscopy. Eur J Radiol. 2008; 66: 313–20
- Ohno A, Ohta Y, Ohtomo K, Hirata K, Takatsuki K, Mochida S, et al. Magnetic resonance imaging in chronic liver disease evaluated in relation to hepatic fibrosis–-clinical and experimental results. Radiat Med. 1990; 8: 159–63
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005; 41: 1313–21
- Adams LA, Talwalkar JA. Diagnostic evaluation of nonalcoholic fatty liver disease. J Clin Gastroenterol. 2006; 40(Suppl 1)S34–8
- Keeffe EB, Adesman PW, Stenzel P, Palmer RM. Steatosis and cirrhosis in an obese diabetic. Resolution of fatty liver by fasting. Dig Dis Sci. 1987; 32: 441–5
- Andersen T, Gluud C, Franzmann MB, Christoffersen P. Hepatic effects of dietary weight loss in morbidly obese subjects. J Hepatol. 1991; 12: 224–9
- Shaffer E. Bariatric surgery: a promising solution for nonalcoholic steatohepatitis in the very obese. J Clin Gastroenterol. 2006; 40: S44–50
- Dixon JB, Bhathal PS, Hughes NR, O'Brien PE. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology. 2004; 39: 1647–54
- Klein S, Mittendorfer B, Eagon JC, Patterson B, Grant L, Feirt N, et al. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 2006; 130: 1564–72
- Mathurin P, Gonzalez F, Kerdraon O, Leteurtre E, Arnalsteen L, Hollebecque A, et al. The evolution of severe steatosis after bariatric surgery is related to insulin resistance. Gastroenterology. 2006; 130: 1617–24
- Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007; 357: 741–52
- Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007; 357: 753–61
- Wang R, Koretz R, Yee H. Is weight reduction an effective therapy for nonalcoholic fatty liver? A systematic review. Am J Med. 2003; 115: 554–9
- Zivkovic AM, German JB, Sanyal AJ. Comparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver disease. Am J Clin Nutr. 2007; 86: 285–300
- Harrison SA, Day CP. Benefits of lifestyle modification in NAFLD. Gut. 2007; 56: 1760–9
- Palmer M, Schaffner F. Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology. 1990; 99: 1408–13
- Park HS, Kim MW, Shin ES. Effect of weight control on hepatic abnormalities in obese patients with fatty liver. J Korean Med Sci. 1995; 10: 414–21
- Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, et al. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997; 27: 103–7
- Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003; 38: 413–9
- Sreenivasa Baba C, Alexander G, Kalyani B, Pandey R, Rastogi S, Pandey A, et al. Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic steatohepatitis. J Gastroenterol Hepatol. 2006; 21: 191–8
- Tamura Y, Tanaka Y, Sato F, Choi JB, Watada H, Niwa M, et al. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2005; 90: 3191–6
- Westerbacka J, Lammi K, Hakkinen AM, Rissanen A, Salminen I, Aro A, et al. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J Clin Endocrinol Metab. 2005; 90: 2804–9
- Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005; 54: 603–8
- Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, et al. One-year intense nutritional counseling results in histological improvement in patients with non–alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005; 100: 1072–81
- Browning JD, Davis J, Saboorian MH, Burgess SC. A low-carbohydrate diet rapidly and dramatically reduces intrahepatic triglyceride content. Hepatology. 2006; 44: 487–8
- Tendler D, Lin S, Jr, Yancy WS, Mavropoulos J, Sylvestre P, Rockey DC, et al. The effect of a low-carbohydrate, ketogenic diet on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. 2007; 52: 589–93
- Ryan MC, Abbasi F, Lamendola C, Carter S, McLaughlin TL. Serum alanine aminotransferase levels decrease further with carbohydrate than fat restriction in insulin-resistant adults. Diabetes Care. 2007; 30: 1075–80
- Bugianesi E, Marzocchi R, Villanova N, Marchesini G. Non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH): treatment. Best Pract Res Clin Gastroenterol. 2004; 18: 1105–16
- Crespo J, Cayon A, Fernandez-Gil P, Hernandez-Guerra M, Mayorga M, Dominguez-Diez A, et al. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001; 34: 1158–63
- Adams LA, Zein CO, Angulo P, Lindor KD. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol. 2004; 99: 2365–8
- Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003; 98: 2485–90
- Harrison SA, Ramrakhiani S, Brunt EM, Anbari MA, Cortese C, Bacon BR. Orlistat in the treatment of NASH: a case series. Am J Gastroenterol. 2003; 98: 926–30
- Zelber-Sagi S, Kessler A, Brazowsky E, Webb M, Lurie Y, Santo M, et al. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006; 4: 639–44
- Laurin J, Lindor KD, Crippin JS, Gossard A, Gores GJ, Ludwig J, et al. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996; 23: 1464–7
- Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, et al. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006; 4: 1537–43
- Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004; 39: 770–8
- Abdelmalek MF, Angulo P, Jorgensen RA, Sylvestre PB, Lindor KD. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: results of a pilot study. Am J Gastroenterol. 2001; 96: 2711–7
- Zandbergen AA, Lamberts SW, Janssen JA, Bootsma AH. Short-term administration of an angiotensin-receptor antagonist in patients with impaired fasting glucose improves insulin sensitivity and increases free IGF-I. Eur J Endocrinol. 2006; 155: 293–6
- Yokohama S, Tokusashi Y, Nakamura K, Tamaki Y, Okamoto S, Okada M, et al. Inhibitory effect of angiotensin II receptor antagonist on hepatic stellate cell activation in non-alcoholic steatohepatitis. World J Gastroenterol. 2006; 12: 322–6
- Yokohama S, Yoneda M, Haneda M, Okamoto S, Okada M, Aso K, et al. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology. 2004; 40: 1222–5
- Cusi K, DeFronzo R. Metformin: a review of its metabolic effects. Diabetes Rev. 1998; 6: 89–131
- Cusi K, Consoli A, DeFronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996; 81: 4059–67
- Towler M, Hardie D. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007; 100: 328–41
- Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001; 358: 893–4
- Uygun A, Kadayifci A, Isik AT, Ozgurtas T, Deveci S, Tuzun A, et al. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2004; 19: 537–44
- Nair S, Diehl AM, Wiseman M, Farr GH, Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004; 20: 23–8
- Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005; 100: 1082–90
- Caldwell SH, Hespenheide EE, Redick JA, Iezzoni JC, Battle EH, Sheppard BL. A pilot study of a thiazolidinedione, troglitazone, in nonalcoholic steatohepatitis. Am J Gastroenterol. 2001; 96: 519–25
- Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, et al. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004; 2: 1107–15
- Serfaty L. Pioglitazone: the beginning of a new era for NASH?. J Hepatol. 2007; 47: 160–2
- Lang L. Pioglitazone trial for NASH: results show promise. Gastroenterology. 2007; 132: 836–8
