Abstract
Because of the obvious negative relation between high-density lipoprotein (HDL) cholesterol and cardiovascular disease and the substantial residual risk of this disease even during treatment with high-dose statin there has been an urgent need to investigate the possible therapeutic benefit of increasing HDL. Even if treatment with nicotinic acid with its marked HDL-increasing effect has been encouraging, there is no evidence so far that specific increase of HDL cholesterol results in less cardiovascular disease. Treatment with the cholesterol ester transfer protein (CETP) inhibitor and HDL-increasing drug torcetrapib resulted in increased risk of cardiovascular disease. These negative results were followed by a lively discussion regarding the possible benefit of HDL-increasing treatment in general and CETP inhibition in particular. Suggested possible causes for the negative outcome by torcetrapib treatment are off-target non-CETP-related effect of this particular inhibitor, inability of very high blood HDL cholesterol levels to protect, induction of dysfunctional HDL, and direct atherogenic effect of CETP inhibition. It is concluded that still today little is known about the effect of specific therapeutic elevation of HDL cholesterol, particularly so through CETP inhibition on cardiovascular risk. New interventional studies on this therapeutic principle are welcomed and under way.
Introduction
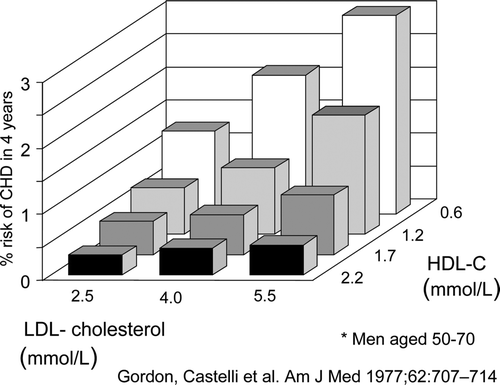
The observation that high-density lipoprotein (HDL) cholesterol serves a protective role in coronary heart disease (CHD) pathogenesis and development is almost as old as lipoprotein fractionation itself. The first observation was published in a paper by Barr et al. Citation1. This was soon followed by Nikkilä in his doctoral thesis Citation2. However, these seminal observations were largely forgotten for many years because the finding was perceived as counterintuitive: cholesterol accumulated in the coronary artery wall and was associated with CHD, and the kinetics and physiological roles of individual lipoprotein fractions in these processes were unknown. The concept was brought to life again by the brothers George and Norman Miller in 1975 in a widely cited Lancet article Citation3 in which they proposed that a reduction of plasma HDL concentration may accelerate the development of atherosclerosis, and hence CHD, by impairing the clearance of cholesterol from the arterial wall. The first prospective observations of the relation of HDL cholesterol to outcome came concomitantly in 1977 from the Tromsö Heart Study Citation4 and the Framingham study Citation5. Since then, HDL has been a main focus in lipoprotein research ().
Figure 1. Four-year coronary heart disease risk in men aged 50–70 years according to HDL and LDL cholesterol concentrations at base-line in the Framingham study Citation5.
Accumulated evidence from observational studies now indicates unequivocally that low plasma concentration of HDL cholesterol (HDL-C) marks increased risk of cardiovascular disease (CVD) in populations in industrialized countries and conversely that high HDL-C marks lower CVD risk.
The protective effect of HDL-C has been attributed to its role in reverse cholesterol transport (RCT). This is a multistep process resulting in the net movement of cholesterol from peripheral tissues back to the liver via the plasma compartment Citation6. Cell biological and molecular mechanisms behind RCT have been characterized. Cellular surface receptors for the main apolipoprotein in HDL, apolipoprotein A-I (apoA-I), have been identified, characterized, and cloned. These include scavenger receptor B1 (SRB1) Citation6, adenosine triphosphate (ATP)-binding cassette transporter A1 (ABCA1) Citation7, and ABCG1 Citation8. An excellent review of RCT was recently published by Tall et al. Citation9. In addition to their role in RCT, HDL particles contain many components that could contribute to cardioprotection through antioxidant, anti-inflammatory, antithrombotic, and endothelial stabilizing effects Citation10. HDL is also enriched in several proteins involved in the complement cascade, as well as in a variety of protease inhibitors, supporting the concept that HDL plays a role in innate immunity and in the regulation of proteolytic cascades involved in inflammatory and coagulation processes Citation11.
A large body of evidence amassed over the past two decades indicates that treatment to lower atherogenic low-density lipoprotein (LDL) cholesterol, particularly with statin drugs, reduces CHD risk. Despite the efficacy of statins, it is sobering to note the high residual risk that remains despite optimal statin treatment, usually about two-thirds of the risk observed in the placebo group in controlled clinical trials.
Key messages
In spite of the convincing epidemiological evidence of a protective effect of high-density lipoprotein (HDL) against atherosclerotic disease there are a number of findings indicating that a high HDL cholesterol may not always be of benefit.
Possible reasons for the negative outcome of the ILLUMINATE study are off-target effects of torcetrapib itself, proatherogenic effects of cholesterol ester transfer protein (CETP) inhibition, production of dysfunctional HDL particles, or inability of very high HDL cholesterol levels to provide protection.
More research is necessary, particularly on the effect of cholesterol ester transfer inhibition on the risk of cardiovascular disease, and is under way.
Abbreviations
CHD prevention by increasing HDL
The residual risk on statin treatment has remained a challenge to clinicians and to investigators. Data such as the Framingham three-dimensional risk chart () suggest that CHD risk might be further reduced by interventions to increase HDL-C. Early studies with nicotinic acid, which increases HDL cholesterol by about 25%, have been promising but inconclusive Citation12, Citation13. Numerous reviews have addressed potential approaches to reduce CHD risk by increasing HDL-C Citation14, Citation15.
Inhibition of cholesterol ester transfer protein (CETP)
Mutations of the CETP gene
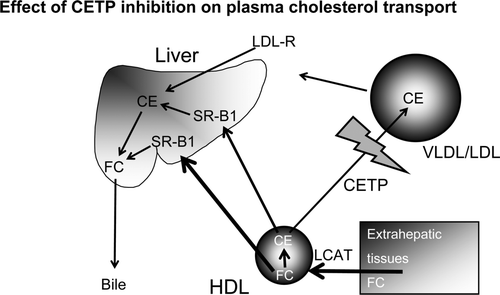
One way of increasing HDL would be modulate the transfer of cholesteryl ester from HDL to very-low-density lipoprotein (VLDL) and LDL in exchange for triglycerides via cholesteryl ester transfer protein (CETP) (). The CETP reaction increases circulating VLDL and LDL cholesterol levels and decreases HDL-C levels. As a result, CETP has often been considered a ‘proatherogenic’ factor. Genetic deficiency of CETP results in a marked increase in HDL-C and a decrease in LDL cholesterol Citation16. These observations led to the hypothesis that CETP inhibition would prevent the development of atherosclerotic disease Citation16. However, a later account of a Japanese population with heterozygous CETP deficiency demonstrated no protective effect of the mutation Citation17. Furthermore, in 3,469 men of Japanese ancestry in the Honolulu Heart Program, CETP mutation D442G was associated with decreased CETP activity (-35%) and increased HDL-C levels (+10%). The overall prevalence of definite CHD was 21% in men with mutations and 16% in men without mutations. However, among individuals with HDL-C above 1.5 mmol/L there was an apparent protective effect of the mutation. Subsequently, the Honolulu Heart Program published a 7-year follow-up of 2,340 men aged 71–93 Citation18. The age-adjusted CHD incidence rates were significantly lower in men with high versus low HDL-C levels. After adjustment for age, hypertension, smoking, and total cholesterol the relative risk of CHD for those with HDL-C levels above 1.5 mmol/L compared with those with HDL-C levels below 1.0 mmol/L was 0.6. Men with a CETP mutation had the lowest rates of CHD, although this was not statistically significant.
Figure 2. CETP inhibition. By blocking the transfer of cholesterol esters from HDL to VLDL/LDL the cholesterol content of HDL increases and that of VLDL/LDL decreases. (CE = cholesteryl ester; CETP = cholesteryl ester transfer protein; FC = free cholestrol; LCAT = lecithin cholesteryl acyl transferase, SR-B1 = scavenger receptor B1). By courtesy of Professor Philip Barter, Sydney.
An important inference from animal experiments and human studies is that the role of CETP is conditional upon the metabolic setting Citation19. For example, higher CETP has been associated with increased CHD risk in subjects with hypertriglyceridaemia Citation20. Conversely, in a nested case-control analysis of the prospective PREVEND study, higher plasma CETP was associated with reduced CHD risk among men with low triglycerides Citation21. Therefore, experiments of nature fail to provide a definitive answer to the question of whether raising HDL-C by CETP inhibition protects against CHD. Instead, there has been a sustained and lively debate regarding the pros and cons of CETP inhibition in man Citation22. One suggestion has been that CETP not only promotes cholesterol ester transfer from HDL to other lipoproteins but also promotes the dissociation of apoA-I from mature HDL to pre-beta HDL Citation23. The latter HDL subfraction has the ability to accelerate the transfer of cellular cholesterol to HDL. Thus, this action of CETP would potentially constitute a protection. It has been pointed out that genetic deficiencies in CETP need not exert the same effect on atherogenesis as pharmacological CETP inhibition Citation24. In fact, there has been continuing interest among scientists and the pharmaceutical industry to develop CETP-inhibiting drugs. To date, three CETP inhibitors have been or are being tested in man: torcetrapib, anacetrapib, and dalcetrapib.
Pharmacological CETP inhibition
Torcetrapib, ILLUMINATE, and its interpretation
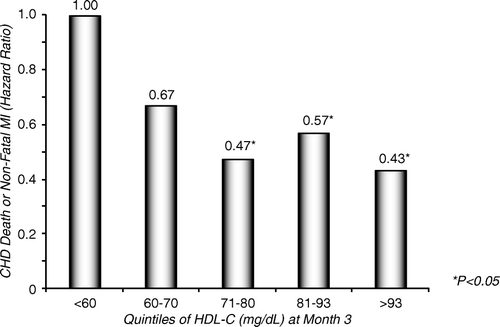
The development of torcetrapib was halted on 2 December 2006 because of adverse effects of the drug in the Investigation of Lipid Level Management to Understand its iMpact IN ATherosclerosis Events (ILLUMINATE) trial Citation25. Despite an increase of HDL-C by about 25%, there were 93 deaths in the atorvastatin/torcetrapib group versus 59 in the atorvastatin/placebo group. These negative results ignited much speculation and debate, particularly since the role of CETP inhibition was already quite contentious. The key question raised by ILLUMINATE is whether CETP inhibition, per se, is harmful, or whether torcetrapib exerted a particular adverse off-target effect. Post hoc analyses have shed some light on this question. Even prior to the commencement of ILLUMINATE it was clear that torcetrapib increased blood pressure. The average blood pressure increase of a few mmHg was considered of minor importance in view of the dramatic and presumed favourable effects of the drug on lipoproteins. In fact, post hoc analyses of ILLUMINATE showed that patients in the torcetrapib arm of ILLUMINATE who had the largest increases in HDL-C and apoA-I also had the lowest event rates (). These findings suggest that HDL-C formed through CETP inhibition is protective. However, ILLUMINATE revealed other unexpected effects of torcetrapib: serum potassium decreased and serum sodium and bicarbonate increased significantly among patients treated with torcetrapib. Importantly, serum aldosterone, as determined on frozen samples in a post hoc setting, also increased in the torcetrapib-treated group. Further post hoc analysis revealed that risk in the torcetrapib group of ILLUMINATE was related to the degree of decrease in potassium and increase in bicarbonate. Furthermore, it was speculated that if torcetrapib influenced other corticoids in a similar manner to aldosterone and thus induced an immunocompromised state, it might explain the higher observed rates of death from infections and cancer in the torcetrapib-treated group of ILLUMINATE. In sum, the data from ILLUMINATE suggest an off-target effect of torcetrapib—unrelated to the CETP inhibition—that could be responsible for the negative clinical outcomes with the drug.
Figure 3. Data from the ILLUMINATE trial. Post-hoc exploratory analyses in the torcetrapib/atorvastatin group hazard ratios for CHD death or non-fatal myocardial infarction by quintile of on-trial HDL cholesterol (referent group is HDL cholesterol <60 mg/dL stratum). Cox proportional hazard model adjusted for age, gender, and base-line HDL-C. In spite of the overall negative outcome of the ILLUMINATE trial the figure demonstrates a significant negative relation between on-trial HDL cholesterol and CHD death + non-fatal myocardial infarctions. By courtesy of Dr P Barter, AHA 2007. Unpublished.
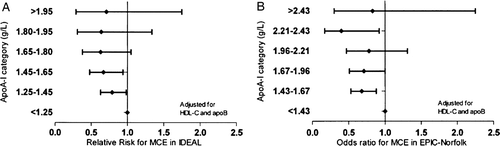
In view of the negative outcome of ILLUMINATE, we studied the relation of very high HDL-C to outcome in the Incremental Decrease in Endpoints through Aggressive Lipid lowering (IDEAL) trial and European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort Citation26, the former a prospective, randomized clinical trial comparing two statins and the latter an observational study not involving any drug under study. We investigated the relationships of HDL-C, HDL particle size, and apoA-I with risk of CHD. After adjustments for apoA-I and apoB, HDL-C (in IDEAL) and HDL particle size (in EPIC Norfolk) were significantly and positively related to the occurrence of CHD, particularly in the highest categories of the respective HDL distributions. In contrast, apoA-I exhibited no positive relationship with CHD in any model or at any concentration ( and ). It is important to note that this positive relation is not apparent with conventional stratification of HDL into quartiles or quintiles without adjustment for apolipoproteins; the apparent adverse effect of high HDL is only apparent after adjustment for apoA-I and apoB and then only at very high HDL-C concentration or particle size. The finding is at variance with the relation of HDL-C to outcome in the Honolulu Heart Study of patients with CETP mutants, which demonstrated increased risk at moderately high HDL-C levels but decreased risk at very high HDL-C levels. Also, the relation of HDL-C to outcome in the ILLUMINATE trial is at variance with these results.
Figure 4. Risk estimates for subgroups of HDL-C in IDEAL and EPIC-Norfolk studies. A: In IDEAL the relationships of HDL-C with major coronary event were calculated by a Cox proportional hazards model, yielding values for relative risk for a 1-SD increase of HDL-C with 95% confidence intervals. The basic regression model included covariates for age, gender, and smoking status (current, former, never) recorded at base-line. B: In EPIC-Norfolk, the relationships of HDL-C with major coronary events were determined by conditional logistic regression analysis that took into account the matching for age, gender, and enrolment period and included the covariates smoking status (current, former, never), body mass index, and alcohol consumption (number of units per week). The major coronary event risk estimates were expressed as odds ratios for a 1-SD increase of HDL-C with 95% confidence intervals. EPIC = European prospective investigation of cancer; IDEAL = Incremental decrease in endpoints through aggressive lipid lowering; MCE = major coronary event.
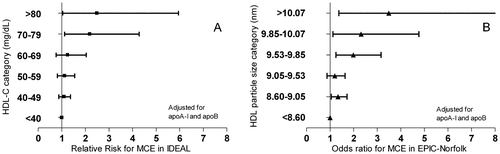
The likely reconciliation of these seemingly opposite findings is that apoA-I is the key protective component among HDL constituents. If apoA-I increases in concert with HDL-C, as it did in ILLUMINATE, then HDL-C is more likely to be protective. In fact, the ratio of apoB/apoA-I has been shown to be an excellent predictor of cardiovascular risk Citation27, Citation28. ApoA-I-Milano treatment has demonstrated favourable effects on coronary atheroma Citation29. Therefore it is reasonable that more focus is paid to the apoA-I moiety of HDL than HDL-C concentration in future research.
Another interpretation of the ILLUMINATE results was recently published by Sirtori and Mombelli Citation30. In this paper, the authors take a clear stance against CETP inhibition as a fruitful avenue to cardiovascular prevention. An interesting aspect of the authors’ reasoning is related to the effect of probucol on HDL-C and atherosclerosis. Probucol decreases HDL-C due to activation of CETP Citation31, an effect which the authors judge is of greater importance than the well known antioxidant effect of the drug. The authors then note that probucol exerted an inhibitory effect on carotid atherosclerosis progression Citation32. Sirtori and Mombelli further argue that in the FAST 2-year study of Japanese hypercholesterolaemic men, probucol treatment decreased both LDL and HDL cholesterol while carotid intima-media thickness decreased significantly and CHD was significantly lower than in an untreated control group Citation33. Sirtori and Mombelli conclude that CETP deficiency is associated with higher HDL cholesterol levels, but whether these elevated HDL-C levels may in themselves be beneficial is totally unproven. However, the probucol argument is less convincing when one examines data from the Probucol Quantitative Regression Swedish Trial (PQRST). In patients with femoral atherosclerosis and hypercholesterolaemia we found that probucol had an unfavourable effect on atherosclerosis Citation34 and that there was a clear relation between the probucol-induced decrease in HDL cholesterol and HDL2b particle size and increased measures of femoral atherosclerosis Citation35.
Other doubts about the benefits of HDL have been put forward by research groups that have pursued the concept of dysfunctional, proinflammatory HDL Citation36. In a recent review, Fogelman and colleagues point out that HDL from patients with CHD demonstrates proinflammatory properties in specific in vitro assays and that such proinflammatory properties are independent of in vivo HDL-C levels Citation36. The urgent need for meaningful assays of the HDL functionality is also stressed by other researchers Citation37. These investigators postulate that specific pathologic chemical and structural changes in HDL particles impede their physiologic role in promoting reverse cholesterol transport, reducing oxidation of LDL, and suppressing vascular inflammation. According to these authors HDL can be viewed as a shuttle that can be either anti-inflammatory or proinflammatory, depending on its cargo of proteins, enzymes, and lipids. The authors raise questions as to whether torcetrapib may have generated proinflammatory HDL particles that actually contributed to progression of atherosclerosis. At present, however, the concept of proinflammatory HDL remains largely an in vitro laboratory phenomenon. For example, nothing has been published on the qualitative changes of HDL following torcetrapib treatment in ILLUMINATE, and little is known about the chemical composition or function of HDL particles in EPIC-Norfolk or the IDEAL trial.
Another potential explanation for the negative results of ILLUMINATE is based on a recent observation that certain gene variants of CETP (B2 allele) and of hepatic lipase (LIPC-T allele) cause both increased HDL-C and increased risk of CHD Citation38. Combined homozygosity of these two gene variants occurred in 1.3% and 0.2% in CAD patients and controls, respectively, P = 0.033. The authors concluded that high HDL-C does not protect against CAD when associated with combined CETP and hepatic lipase-lowering gene variants. The authors also suggested that therapeutic interventions to reduce CAD aimed at raising HDL-C by CETP inhibition may be unfavourable for patients who are homozygous for these gene variants. Because of the relatively low prevalence of these gene variants and the maintained relation in ILLUMINATE with lower risk of CAD with higher HDL-C it is unlikely that this gene variation could have had a major impact on the negative outcome.
The unexpected and disappointing results of the ILLUMINATE trial may never be explained fully, but to this author the data to date most strongly support an off-target effect of torcetrapib involving the aldosterone system.
Dalcetrapib
Dalcetrapib (formerly known as JTT-705) has been studied extensively in the preclinical setting, and clinical studies are now under way. Treatment with 900 mg dalcetrapib for 4 weeks led to a 37% decrease in CETP activity (P<0.0001), a 34% increase in HDL-C (P<0.0001), and a 7% decrease in LDL cholesterol (P=0.017) Citation39, i.e. the HDL-C-increasing effect is less pronounced than exerted by torcetrapib and anacetrapib (see below). Dalcetrapib lacks the blood pressure-increasing effect seen on torcetrapib treatment and does not influence the renin-angiotensin system. Dalcetrapib is now being tested in a large international study comprising 15,600 participants with acute coronary syndrome.
Anacetrapib
Anacetrapib (MK-0859) is the latest CETP inhibitor currently under development Citation40. Anacetrapib produced dose-dependent lipid-altering effects with peak lipid-altering effects of 129% increase in HDL-C and a 38% decrease in LDL cholesterol in patients with dyslipidaemia. Despite greater lipid-altering effects relative to other members of this class, anacetrapib seems not to increase blood pressure, suggesting that potent CETP inhibition by itself might not lead to increased blood pressure.
Conclusion
The answer to the question initially posed, ‘Is high HDL cholesterol always good?’ remains unknown.
The high residual CHD risk despite high-dose statin therapy highlights the urgent need to find new avenues to reduce this risk. Based on epidemiologic data, increasing HDL is an appealing option. However there is still no conclusive evidence for the efficacy of drugs that increase HDL-C. Niacin may be a fruitful approach but is limited by tolerability, particularly due to flushing. Treatments aimed at increasing apoA-I through concentration or functionality are promising. Hardly any question in cardiovascular medicine is as contentious as whether CETP inhibition will yield benefit. The recently launched large morbidity and mortality trial of dalcetrapib in patients with acute coronary syndrome will be a pivotal proof-of-concept test of CETP inhibition, provided that that drug does not demonstrate the off-target effects observed with torcetrapib.
Acknowledgements
The author is indebted to Dr Gregory Schwartz for critical review of the manuscript.
Declaration of interest: Dr Olsson has received consultation fees or support for clinical trials from Artery Therapeutics, AstraZeneca, Genzyme, Karobio, MSD, Pfizer, Roche, Sanofi, and Takeda. The author alone is responsible for the content and writing of the paper.
References
- Barr DP, Russ EM, Eder HA. Protein-lipid relationships in human plasma: II. In atherosclerosis and related conditions. Am J Med. 1951; 11: 480–93
- Nikkilä E. Studies on the lipid-protein relationship in normal and pathological sera and the effect of heparin on serum lipoproteins. Scand J Clin Lab Invest. 1953; 5(Suppl 8)9–100
- Miller GJ, Miller NE. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1975; 305: 16–19
- Miller NE, Thelle DS, Forde OH, Mjos OD. The Tromsø heart-study. High-density lipoprotein and coronary heart-disease: a prospective case-control study. Lancet. 1977; 1: 965–8
- Gordon T, Castelli W, Hjortland M, Kannel W, Dawber T. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977; 62: 707–14
- Tall A. An overview of reverse cholesterol transport. Eur Heart J 1998; 19(Suppl A)A31–5
- Schmitz G, Kaminski W, Porsch-Ozcürümez M, Klucken J, Orsó E, Bodzioch M, et al. ATP-binding cassette transporter A1 (ABCA1) in macrophages: a dual function in inflammation and lipid metabolism?. Pathobiology. 1999; 67: 236–40
- Klucken J, Buchler C, Orso E, Kaminski WE, Porsch-Ozcurumez M, Liebisch G, et al. ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. Proc Natl Acad Sci U S A. 2000; 97: 817–22
- Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008; 7: 365–75
- Barter PJ, Nicholls S, Rye K-A, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004; 95: 764–72
- Vaisar T, Pennathur S, Green P, Gharib SA, Hoofnagle AN, Cheung MC, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007; 117: 746–56
- Canner P, Berge K, Wenger N, Stamler J, Friedman L, Prineas RJ, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986; 8: 1245–55
- Carlson L, Rosenhamer G. Reduction of mortality in the Stockholm Ischaemic Heart Disease Secondary Prevention Study by combined treatment with clofibrate and nicotinic acid. Acta Medica Scandinavica. 1988; 223: 405–18
- Hausenloy DJ, Yellon DM. Targeting residual cardiovascular risk: raising high-density lipoprotein cholesterol levels. Heart. 2008; 94: 706–14
- Wild S, Byrne CD. Time to rethink high-density lipoprotein?. Heart. 2008; 94: 692–4
- Inazu A, Brown M, Hesler C, Agellon LB, Koizumi J, Takata K, et al. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N Engl J Med. 1990; 323: 1234–8
- Hirano K-I, Yamashita S, Nakajima N, Arai T, Maruyama T, Yoshida Y, et al. Genetic cholesteryl ester transfer protein deficiency is extremely frequent in the Omagari area of Japan: marked hyperalphalipoproteinemia caused by CETP gene mutation is not associated with longevity. Arterioscler Thromb Vasc Biol. 1997; 17: 1053–9
- Curb JD, Abbott RD, Rodriguez BL, Masaki K, Chen R, Sharp DS, et al. A prospective study of HDL-C and cholesteryl ester transfer protein gene mutations and the risk of coronary heart disease in the elderly. J Lipid Res. 2004; 45: 948–53
- Watts G. The Yin and Yang of cholesteryl ester transfer protein and atherosclerosis. Clin Sci (Lond) 2002; 103: 595–7
- Boekholdt SM, Kuivenhoven J-A, Wareham NJ, Peters RJG, Jukema JW, Luben R, et al. Plasma levels of cholesteryl ester transfer protein and the risk of future coronary artery disease in apparently healthy men and women: the prospective EPIC (European Prospective Investigation into Cancer and nutrition)-Norfolk Population Study. Circulation. 2004; 110: 1418–23
- Borggreve SE, Hillege HL, Dallinga-Thie GM, de Jong PE, Wolffenbuttel BHR, Grobbee DE, et al. High plasma cholesteryl ester transfer protein levels may favour reduced incidence of cardiovascular events in men with low triglycerides. Eur Heart J. 2007; 28: 1012–8
- Fielding CJ, Havel RJ. Cholesteryl ester transfer protein: friend or foe?. J Clin Invest. 1996; 97: 2687–88
- Hennessy L, Kunitake S, Kane J. Apolipoprotein A-I-containing lipoproteins, with or without apolipoprotein A-II, as progenitors of pre-beta high-density lipoprotein particles. Biochemistry. 1993; 32: 5759–65
- Hirano K, Yamashita S, Matsuzawa Y. Pros and cons of inhibiting CETP. Curr Opin Lipidol. 2000; 11: 589–96
- Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJP, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007; 357: 2109–22
- van der Steeg WA, Holme I, Boekholdt SM, Larsen ML, Lindahl C, Stroes ESG, et al. High-density lipoprotein cholesterol, high-density lipoprotein particle size, and apolipoprotein A-I: significance for cardiovascular risk: The IDEAL and EPIC-Norfolk Studies. J Am Coll Cardiol. 2008; 51: 634–42
- Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004; 364: 937–52
- McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008; 372: 224–33
- Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003; 290: 2292–300
- Sirtori C, Mombelli G. Viability of developing CETP inhibitors. Cardiovasc Ther. 2008; 26: 135–46
- Franceschini G, Sirtori M, Vaccarino V, Gianfranceschi G, Rezzonico L, Chiesa G, et al. Mechanisms of HDL reduction after probucol. Changes in HDL subfractions and increased reverse cholesteryl ester transfer. Arteriosclerosis. 1989; 9: 462–9
- Baldassarre D, Franceschini G, Peruzzotti G, Brusoni B, Sirtori CR. Clinical evaluation of probucol in hypercholesteremia: individual lipoprotein responses and inhibitory effect on carotid atherosclerosis progression. J Cardiovasc Pharmacol. 1997; 30: 784–9
- Sawayama Y, Shimizu C, Maeda N, Tatsukawa M, Kinukawa N, Koyanagi S, et al. Effects of probucol and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia: Fukuoka atherosclerosis trial (FAST). J Am Coll Cardiol. 2002; 39: 610–6
- Walldius G, Erikson U, Olsson AG, Bergstrand L, Hådell K, Johansson J, et al. The effect of probucol on femoral atherosclerosis: the Probucol Quantitative Regression Swedish Trial (PQRST). Am J Cardiol. 1994; 74: 875–83
- Johansson J, Olsson AG, Bergstrand L, Schafer Elinder L, Nilsson S, Erikson U, et al. Lowering of HDL2b by probucol partly explains the failure of the drug to affect femoral atherosclerosis in subjects with hypercholesterolemia: a Probucol Quantitative Regression Swedish Trial (PQRST) Report. Arterioscler Thromb Vasc Biol. 1995; 15: 1049–56
- Ansell B, Fonarow G, Fogelman A. The paradox of dysfunctional high-density lipoprotein. Curr Opin Lipidol. 2007; 18: 427–34
- deGoma EM, deGoma RL, Rader DJ. Beyond high-density lipoprotein cholesterol levels: evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J Am Coll Cardiol. 2008; 51: 2199–211
- van Acker BAC, Botma G-J, Zwinderman AH, Kuivenhoven JA, Dallinga-Thie GM, Sijbrands EJG, et al. High HDL cholesterol does not protect against coronary artery disease when associated with combined cholesteryl ester transfer protein and hepatic lipase gene variants. Atherosclerosis. 2008; 200: 161–7
- de Grooth GJ, Kuivenhoven JA, Stalenhoef AFH, de Graaf J, Zwinderman AH, Posma JL, et al. Efficacy and safety of a novel cholesteryl ester transfer protein inhibitor, JTT-705, in humans: a randomized phase ii dose-response study. Circulation. 2002; 105: 2159–65
- Krishna R, Anderson MS, Bergman AJ, Jin B, Fallon M, Cote J, et al. Effect of the cholesteryl ester transfer protein inhibitor, anacetrapib, on lipoproteins in patients with dyslipidaemia and on 24-h ambulatory blood pressure in healthy individuals: two double-blind, randomised placebo-controlled phase I studies. Lancet. 2007; 370: 1907–14