Abstract
Background. Abnormal adipocyte function is implicated in the coalition of multiple cardiovascular risk factors, where aberrant circulating levels of the adipose-derived hormones adiponectin, leptin, and tumour necrosis factor (TNF) α may provide the putative link between hypertension and increased cardiovascular risk. The pragmatic utility of these ‘adipocytokines’ in the clinical setting of hypertension is unclear, and we hypothesized a relationship of circulating adipocytokines to hypertension, and associated cardiovascular morbidity.
Method. Using a cross-sectional approach, we measured plasma adipocytokines in 278 ‘high-risk’ treated hypertensive participants of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) study (mean (SD) age 62.9 (7.7) years), who were compared to 54 newly diagnosed untreated hypertensives (61.3 (10.9) years) and 55 healthy controls (48.3 (12.3) years).
Results. Levels of all three adipocytokines were lower amongst treated hypertensives compared to newly diagnosed hypertensives and healthy controls (P<0.001 for leptin and adiponectin), and varied with gender, co-morbidities (e.g. diabetes, cardiovascular disease (CVD), left ventricular hypertrophy) and by treatments (e.g. statins and beta-blockade). Levels of adiponectin (P<0.001) and leptin (P=0.02) rose in an ordinal fashion with increasing hypertension severity (grade). Levels of leptin were associated with diastolic blood pressure in a positive fashion (P<0.001).
Conclusions. While hypertension affects adipocytokine levels, the clinical interpretation of circulating levels in hypertension is confounded by a range of factors. The positive relation between leptin and adiponectin with hypertension severity may reflect an underlying adaptive response that is attenuated during pharmacological hypertension management.
Introduction
Despite advances in the understanding of hypertension and its treatment, the pathophysiological mechanisms by which hypertension confers an increased risk of cardiovascular disease (CVD) morbidity and mortality remain unclear. Indeed, effective blood pressure control by antihypertensive treatment in a hypertensive patient still does not reduce CVD risk back to the levels found in the normotensive population Citation[1–3], and current clinical approaches in blood pressure measurement underestimate the magnitude of atherosclerotic damage Citation[4]. Reasons for this disparity are linked to co-morbidities such as obesity, insulin resistance and abnormal glucose and lipid metabolism, which commonly co-manifest with hypertension Citation[5–9]. The early identification of hypertensive patients, and their susceptibility to multiple CVD risk factors—or the so-called ‘metabolic syndrome’—is therefore an arduous challenge. However, there is evidence that changes in the secretory activity of the adipose tissue are co-ordinated with the development of a coalition of CVD risk factors in these hypertensive patients Citation[10].
Adiponectin, leptin, and tumour necrosis factor (TNF) α are hormones produced by adipocytes—termed ‘adipocytokines’—that elicit a diverse range of metabolic changes that often engage multiple CVD risk factors Citation[11]. Circulating concentrations of adiponectin are ubiquitous in the circulation and are reduced in obesity Citation[12] and hypertension Citation[13], Citation[14], but it is not clear whether there is an independent role in blood pressure regulation per se Citation[15], Citation[16]. Leptin is a centrally acting mediator of satiety, energy expenditure, and adipocyte form Citation[17], where it is proposed to impact on blood pressure through sympathetic outflow Citation[18]. Similarly, levels of the pro-inflammatory cytokine TNF-α are implicated in the modulation of biomarkers of energy balance that includes the adipocytokines Citation[19].
Key messages
Abnormal adipocyte function is implicated in the coalition of multiple cardiovascular risk factors, where aberrant circulating levels of the adipose-derived hormones adiponectin, leptin, and tumour necrosis factor (TNF) α may provide the putative link between hypertension and increased cardiovascular risk.
In this study, we find that hypertension affects adipocytokine levels, but the clinical interpretation of circulating levels in hypertension is confounded by a range of factors.
The positive relation between leptin and adiponectin with hypertension severity may reflect an underlying adaptive response that is attenuated during pharmacological hypertension management.
We hypothesized a relationship of circulating adipocytokines to hypertension and associated cardiovascular morbidity, and that plasma concentrations would discriminate between high blood pressure and normality. We tested this using cross-sectional and case control approaches, by measuring plasma adipocytokines in 278 ‘high-risk’ treated hypertensive participants of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) study, who were compared to newly diagnosed untreated hypertensives and healthy controls.
Methods
All 278 hypertension patients were participating in the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT), and attending the Birmingham study centre. Patients were aged 40–80 years, with hypertension, either treated (systolic blood pressure (BP) > 140 mmHg and/or diastolic BP > 90 mmHg) or newly diagnosed (systolic BP > 160 mmHg and/or diastolic BP > 100 mmHg) subjects. Details of ASCOT are published elsewhere Citation[20]. Briefly, the inclusion criteria for ASCOT required hypertensive patients with three or more risk factors to be included, that is: 1) left ventricular hypertrophy; 2) other electrocardiogram (ECG) abnormalities (left ventricle strain pattern, abnormal Q waves, left bundle branch block, ST-T changes compatible with ischemic heart disease); 3) history of diabetes mellitus (DM) according to World Health Organization criteria; 4) past medical history of a cerebrovascular event, including transient ischaemic attack; 5) male gender; 6) age > 55 years; 7) microalbuminuria/proteinuria; 8) positive smoking history; 9) raised plasma total cholesterol/high-density lipoprotein (HDL) cholesterol ratio; 10) family history of cardiovascular disease; and 11) peripheral vascular disease. Patients were excluded if they had a history of malignant or secondary hypertension, congestive cardiac failure, fasting serum triglycerides > 4.5 mmol/L, major concomitant, non-cardiovascular disease or were taking warfarin. All ASCOT patients were receiving antihypertensive therapy at the time of recruitment.
These patients were compared to 54 newly diagnosed, untreated hypertensives (defined as average BP > 140/90 mmHg on two or more separate occasions) without any overt CVD risk features/medications as highlighted above, recruited from a local community health-screening project. To allow a comparison to ‘normal’ values of the adipocytokines, 55 apparently healthy controls were also recruited from relatives of patients and hospital staff, and were ‘healthy’ by virtue of detailed clinical history and examination, as well as basic blood tests and ECG. Controls were excluded if found to have any history of CVD, a history of hypertension or active antihypertensive medication, diabetes or significant inflammatory, neoplastic, or endocrine disease.
Both patients and controls attended a morning clinic session fasted (no food from 10 p.m. the previous evening). Blood pressure for patients and controls was measured after the subject was seated in a quiet room for 10 minutes, using the OMRON 705-CP (Omron Healthcare Europe, Mannheim, Germany), as per the ASCOT protocol; a minimum of three readings were performed, and the average of the last two readings was used. Mean arterial blood pressure was calculated as the mean pulse pressure added to one-third of the diastolic blood pressure. Body mass was measured on Seca scales (Seca Ltd, Birmingham UK). A Leicester standard rule (Seca Ltd, Birmingham UK) was used to determine the height of the subject's ‘vertex’ (during which time the subject was asked to stand upright with their head in the Frankfort plane). Body mass index (BMI) was calculated as the weight (kg) divided by height (m) squared, and obesity was defined as those subjects with a BMI ≥ 27 kg/m2. Details of the following cardiovascular events were used to define overt CVD: angina, myocardial infarction, non-haemorrhagic stroke, coronary intervention, and peripheral vascular disease. Impaired fasting glucose was used to classify non-diabetic subjects with a fasting plasma glucose ≥6.1 mmol/L and <7 mmol/L. All subjects provided written informed consent. The protocol was approved by the West Birmingham Research Ethics Committee.
Laboratory
Blood was drawn with minimal trauma from the antecubital vein. Blood was centrifuged at 3000 rpm for 20 minutes within 30 minutes of collection, and the serum and plasma separated and stored at −80°C prior to analysis. Serum cholesterol (CHOD-PAP method), triglycerides (GPO-PAP method), HDL (direct method) and plasma glucose (glucose oxidase method) were determined using routine autoanalyser assays in the Biochemistry Department (Sandwell and West Birmingham Hospitals NHS Trust, UK). TNF-α, leptin, and adiponectin levels were measured by enzyme-linked immunosorbent assay (ELISA) in ethylenediamine tetra-acetic acid (EDTA) plasma, using commercially available antibodies (R&D Systems, Abingdon, UK). Coefficients for intra- and interassay variation was <3% and <5%, respectively. The lower limits of detection by ELISA were 62.5 pg/mL for adiponectin, and 15.6 pg/mL for both leptin and TNF-α.
Power calculations
The study was of a cross-sectional design, with no provision for demographic or disease stratification (e.g. gender, obesity). We hypothesized significant differences in levels of adipocytokines between hypertension patients and controls. A sample size of 60 was needed to detect half a standard deviation in adipocytokine means, at 80% power, P<0.05 using a two-sided test.
Statistical analysis
Data were analysed in SPSS v14 (SPSS Inc., Chicago, IL) using standard and non-parametric tests as appropriate. Adipocytokines were of non-parametric distribution, as determined by normality plots (Kolmogorov-Smirnov test). Differences in adipocytokine concentrations between groups were determined using Mann-Whitney (two independent samples) or by the Kruskall-Wallis test (for more than two groups). For non-parametric data, central tendencies were reported as medians, and variation by interquartile range (IQR). Similarly, for normally distributed variables, the mean and standard deviation (SD) are reported. Spearman rank correlation method was used to determine statistical correlations between adipocytokines with CVD risk. Multivariate logistic regression was used to determine the contribution of various risk factors to the presence of hypertension and CVD. Similarly, linear regression was used to determine the predictors for variations in circulating adipocytokines, where beta (95% confidence intervals (CI)) is reported to reflect the strength of association from independent predictors. Receiver operator characteristic (ROC) curves were use to evaluate the performance of adipocytokines with conventional CVD risk factors in this population, depicted by the mean area under the curve (AUC) with 95% CI. Polytomous Universal Model (PLUM) ordinal regression analysis was used to determine the association between adiponectin levels and ordinal variables such as hypertension grade, where a pseudo R2 was reported to reflect the strength of the association. A P-value < 0.05 was considered as statistically significant.
Results
A total of 278 ASCOT patients (mean (SD) age 62.9 (7.7) years), 54 newly diagnosed untreated hypertensives (61.3 (10.9) years), and 55 healthy controls (48.3 (12.3) years) were studied. Amongst ASCOT patients, 17.3% had diabetes mellitus, 15.6% had overt CVD, and 9.5% had left ventricular hypertrophy (LVH). Numbers of hypertensive patients vary between Tables due to the selection of gender and disease-specific subgroups.
Factors influencing variations in adipocytokines amongst treated patients
Across combined cohorts of patients and controls, levels of adipocytokines were markedly different by gender (P≤0.03) and BMI (P<0.05), and previous cardiovascular event (P≤0.02 for adiponectin and leptin only). Amongst patients treated for hypertension (in ASCOT), adiponectin levels were lower amongst those with diabetes, impaired fasting glucose, and those on statin therapy, and non-significantly so amongst those with previous CVD (see ). For leptin, levels were higher in female patients, and lower amongst those with multiple metabolic CVD risk factors. For TNF-α, levels were higher in those patients with LVH.
Table I. Circulating concentrations of adiponectin, leptin, and tumour necrosis factor α amongst 278 patients treated for hypertension (ASCOT trial participants).
Given the confounding impact of diabetes and CVD on adipocytokine levels, a subgroup of 198 non-diabetic hypertension patients (ASCOT), without CVD were compared to 54 untreated, newly diagnosed hypertensives and 55 healthy controls (see Table II). Compared to healthy controls, treated hypertensive patients had markedly lower levels of adiponectin, leptin, and TNF-α. Similarly, between the two hypertensive cohorts, levels of adipocytokines were significantly lower amongst the treated compared to the untreated ‘newly diagnosed’ hypertensives—despite comparable measures of BMI and serum lipids (). Levels of adipocytokines were not significantly different between healthy controls and newly diagnosed hypertensives. The above differences remained significant even after using smaller cohorts of age-matched males ().
Table IIA. Adipocytokines and characteristics of healthy controls compared with treated hypertension patients (ASCOT trial participants) compared with untreated, newly diagnosed hypertensives.
Table IIB. Adipocytokines of male patients with hypertension without diabetes and without a previous cardiovascular event compared with matched controls.
Adipocytokines in obese and non-obese males treated for hypertension
Amongst male patients treated for hypertension (ASCOT) only, circulating adipocytokines were compared between 120 obese and 64 non-obese patients, who were matched for age and comparable for smoking habit, CVD, and CVD therapy (, ). Adipocytokine levels were comparable between these groups, despite higher systolic and arterial blood pressure amongst obese patients. Amongst controls, levels of adiponectin were comparable between obese and non-obese subjects, but were altogether higher than in patients with hypertension ().
Figure 1. Median circulating adiponectin levels by obesity status in male and female hypertension patients and controls.
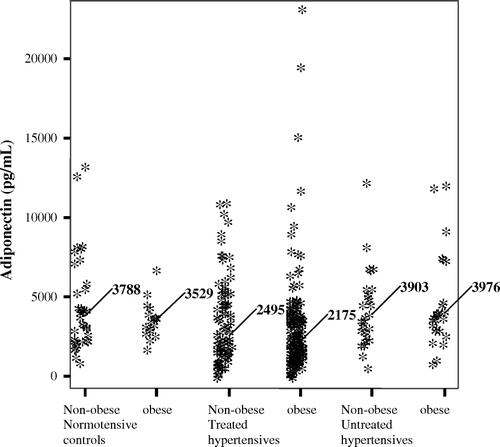
Table III. Cardiovascular risk factors, cardiovascular medication and adipocytokines in male hypertension patients (ASCOT trial participants) by obesity status.
Adipocytokines as discriminators of cardiovascular risk
On ROC analysis of a combined cohort of treated hypertension patients (ASCOT) and healthy controls, levels of adipocytokines and fasting plasma glucose were strong discriminators of the presence of hypertension (). Using a similar approach, adipocytokines did not discriminate between healthy controls and newly diagnosed hypertensives. On ROC analysis of treated hypertensive patients only, levels of adipocytokines did not significantly discriminate the presence of raised cholesterol, raised triglycerides, raised plasma glucose, low HDL cholesterol, or combinations of these metabolic risk factors.
Table IV. Area under the curve for discriminators of hypertension amongst males without diabetes and without a previous cardiovascular event.
Regression analysis
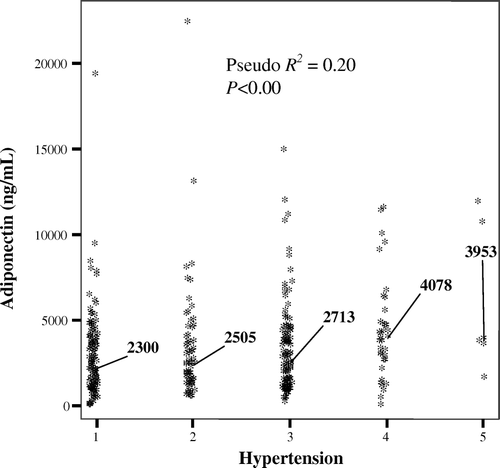
On logistic regression analysis of treated hypertension patients (ASCOT) and healthy controls, the presence of hypertension was independently predicted by leptin (beta, 95% CI: 0.1, 0.1–0.1; P<0.001), BMI (1.5, 1.1–2.0; P=0.006), and age (1.1, 1.0–1.2; P=0.008), in a model that included adipocytokines, gender, lipids, diabetes, and smoking habit. On similar analysis of treated hypertension patients (ASCOT), adipocytokines did not feature in a logistic regression model that investigated predictors of overt CVD. On bivariate analysis across patients and controls, adipocytokines were largely unrelated to direct measures of systolic, diastolic, arterial, and pulse pressure. Amongst treated hypertensive patients alone, leptin levels were associated with diastolic blood pressure (P<0.001, r=0.21), and this association remained after controlling for gender, age, and cardiovascular treatment effects. On ordinal regression analysis, levels of adiponectin (pseudo R2=0.20, P < 0.001) and leptin (pseudo R2=0.02, P=0.02) increased in an ordinal fashion with deteriorating hypertension grade ().
Figure 2. Median circulating adiponectin levels by hypertension grade status in hypertension patients (ASCOT). (1 = normal: systolic BP ≤ 120 mmHg, diastolic BP ≤ 80 mmHg; 2 = high-normal: systolic BP between 135 and 139 mmHg, diastolic BP between 85 and 89 mmHg; 3 = mild hypertension: systolic BP between 140 and 159 mmHg, diastolic BP between 90 and 99 mmHg; 4 = moderate hypertension: systolic BP between 160 and 179 mmHg, diastolic BP between 100 and 109 mmHg; 5 = severe hypertension: systolic BP > 180 mmHg, diastolic BP > 110 mmHg.). ASCOT = Anglo-Scandinavian Cardiac Outcomes Trial; BP = Blood Pressure
Discussion
This study supports the view that hypertension has an impact on circulating levels of adipocytokines. However, this relationship appears to be explained by (unmeasured) cardiovascular management consequence(s) rather than blood pressure per se. For example, active antihypertensive therapy may reduce levels of adipocytokines in treated hypertension, but, paradoxically, levels of adiponectin and leptin increase with untreated or unmanaged blood pressure. Pragmatically, the measurement of adipocytokines in hypertension would not appear to improve the value of a clinician's existing approach to holistic CVD risk assessment, nor does it contribute to the inferred pathophysiological relationship between obesity and hypertension. While the data support our hypothesis that circulating adipocytokines are related to hypertension, the measurement of plasma concentrations is unlikely to discriminate between high blood pressure and normality in a clinical setting. The implication is that it remains unclear how these hormones provide common ground between the developmental relationship between hypertension and CVD risk.
While adipocytokines have been linked to hypertension Citation[10], a pragmatic understanding of their clinical utility is not apparent. Variability in the levels of adipocytokines measured here was evident by a diverse range of variables (gender, BMI, co-morbidities, and CVD treatment), which would serve to complicate the interpretation of circulating measures in a risk stratification setting. Moreover, there appears to be a paradoxical relationship, where levels of adipocytokines are reduced in patients with hypertension (treated), but, in the same patients, are positively moderated by the increasing severity of blood pressure or blood pressure itself. Levels of leptin and adiponectin ordinally increased with hypertension grade in the present study, supported by previous reports of an independent positive association with blood pressure Citation[21]. A possible explanation for the differences in adipocytokine levels may be the positive influence of the sympathetic nervous system (SNS) on blood pressure Citation[22], whereas leptin levels may reflect an adaptive response via hypothalamic pathways. Leptin is a catabolic hormone and mobilizes triglyceride stores, and increases in this hormone may also be co-ordinated by similar changes in adiponectin concentrations (due to its lipolytic influences on triglyceride-rich lipoproteins). Hence, it would appear here that the pharmacological management of hypertension attenuates a physiological response to increasing blood pressure which is in itself cardioprotective Citation[12], Citation[22]. Whether this then ‘drives’ an environment for weight gain in the patient is debatable, but the interplay between the adipocytokines, physiological response, and pharmacological treatment underlines the difficulty in the use of these hormones in a biochemical setting.
While adipocytokines did not have a significant role the discrimination of CVD risk factors, levels of adiponectin and leptin were adversely different in treated hypertensive patients by CVD status. Adipocytokines are associated with abnormal haemostasis and fibrinolysis. For example, plasma levels of plasminogen activator inhibitor (PAI-1) are related to visceral adiposity Citation[23], and this may also underline the relationship between aberrant levels of adipocytokines and hypertension reported here. Adiponectin has been described as a marker of endothelial dysfunction, and this may also provide a direct role for this adipocytokine in the CVD complications associated with hypertension Citation[22].
Consistent with data elsewhere, adiponectin levels were reduced amongst subjects with obesity Citation[12], diabetes Citation[24], CVD Citation[25], and hypertension Citation[13]. Adiponectin level differences between treated hypertension patients (ASCOT) and healthy controls were consistent with the 40% reduction reported in studies of essential hypertension Citation[13], Citation[15]. However, absolute levels in this study are lower than those previously reported, which may underline differences between commercially available antibody kits. Comprehensively, there was no direct regulatory role for adiponectin on blood pressure amongst normotensives, newly detected hypertensives, or treated hypertensives. While low levels of adiponectin have been reported in essential hypertension, it has been debated as to whether this is due to bias from renal function Citation[15]. In this study, amongst patients with hypertension, we found no relationship between glomerular filtration rate and adipocytokine concentrations. While previous studies have investigated the relationship between adipocytokines and hypertension Citation[10], in this study we were interested to look at the impact of demographic and pathological confounding. For example, amongst hospital patients, adiponectin has been implicated in the aging process, where increased levels in the elderly may reflect a physiological protective response to combat low-level inflammation and oxidative stress. In this study, cohorts were devised to be comparable or matched for gender, age, and cardiovascular morbidities.
Centripetal fat accumulation has been proposed as the etiological driver for this metabolic risk factor clustering, as studies consistently link waist circumference (a surrogate for centripetal visceral fat) with increased cardiovascular risk Citation[5]. The absence of a central adiposity measure in the present study is a limitation as a ‘metabolically obese but normal weight’ phenotype is well described Citation[26]. Comparisons across cohorts of normotensives, newly diagnosed, and treated hypertensives proceeded by post hoc selection biases relating to gender and co-morbidities which are not readily representative of the general patient population that a clinician would experience. Moreover, the period of time for the presence of hypertension amongst patients (treated and untreated) was unclear. While cross-sectional studies can reveal an association between two variables, they cannot purport cause and effect. Therefore, adipocytokine levels may affect blood pressure but hypertension per se does not affect adipocytokine levels.
Limitations
This paper is limited by its cross-sectional design, and as this substudy is part of the main ASCOT clinical trial the investigations and treatment regimes allowed are very much protocol-driven, and the substudy was secondary to the main ASCOT trial. The patients in the present analysis were consecutive patients over the initial period of the trial recruitment at the Birmingham study site, and we accept that males were over-represented (as with many clinical trials). We also recognize that there are a whole range of substances secreted by adipocytes. Clearly, we cannot measure an unlimited number of biomarkers, and the present paper addresses a specific hypothesis on the relationship of circulating adipocytokines (i.e. adiponectin, leptin, and TNF-α) to hypertension, and associated cardiovascular morbidity, and that plasma concentrations would discriminate between high blood pressure and normality.
In conclusion, circulating adipocytokine concentrations are aberrant in hypertension, but are unrelated to blood pressure per se. While these hormones may reflect the increased CVD risk in this disease group, the utility of this approach in a clinical setting is not supported by data presented here.
Acknowledgements
We acknowledge the support of the Sandwell and West Birmingham Hospitals NHS Trust Research and Development programme for the Haemostasis Thrombosis and Vascular Biology Unit. We thank Professor D. G. Beevers, Dr C. Spencer, Dr S. Nadar, and Dr D. Felmeden for help with data collection. Other ASCOT Investigators are listed in reference 20. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, et al. Blood pressure, stroke, and coronary heart disease. Part 1. Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990; 335: 765–74
- Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, et al. Blood pressure, stroke, and coronary heart disease. Part 2. Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990; 335: 827–38
- Patel JV, Lim HS, Gunarathne A, Tracey I, Durrington PN, Hughes EA, et al. Ethnic differences in myocardial infarction in patients with hypertension: effects of diabetes mellitus. QJM. 2008; 101: 231–6
- Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, et al. CAFE Investigators; Anglo-Scandinavian Cardiac Outcomes Trial Investigators; CAFE Steering Committee and Writing Committee. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006; 113: 1213–25
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. National Heart, Lung, and Blood Institute; American Heart Association. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004; 109: 433–8
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002; 287: 356–9
- Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002; 288: 2709–16
- Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001; 24: 683–9
- Alberti KGMM, Zimmet P, Shaw J. for the IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—a new worldwide definition. Lancet. 2005; 366: 1059–62
- Patel JV, Lim HS, Hughes EA, Lip GY. Adiponectin and hypertension: a putative link between adipocyte function and atherosclerotic risk?. J Hum Hypertens. 2007; 21: 1–4
- Wassink AM, Olijhoek JK, Visseren FL. The metabolic syndrome: metabolic changes with vascular consequences. Eur J Clin Invest. 2007; 37: 8–17
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999; 257: 79–83
- Adamczak M, Wiecek A, Funahashi T, Chudek J, Kokot F, Matsuzawa Y. Decreased plasma adiponectin concentration in patients with essential hypertension. Am J Hypertens. 2003; 6: 72–5
- Mahmud A, Feely J. Adiponectin and arterial stiffness. Am J Hypertens. 2005; 18: 1543–8
- Mallamaci F, Zoccali C, Cuzzola F, Tripepi G, Cutrupi S, Parlongo S, et al. Adiponectin in essential hypertension. J Nephrol. 2002; 15: 507–11
- Furuhashi M, Ura N, Higashiura K, Murakami H, Tanaka M, Moniwa N, et al. Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003; 42: 76–81
- Banks WA. The many lives of leptin. Peptides. 2004; 25: 331–8
- Quilliot D, Böhme P, Zannad F, Ziegler O. Sympathetic-leptin relationship in obesity: effect of weight loss. Metabolism. 2008; 57: 555–62
- Takahashi N, Waelput W, Guisez Y. Leptin is an endogenous protective protein against the toxicity exerted by tumour necrosis factor. J Exp Med. 1999; 189: 207–12
- Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. ASCOT Steering Committee, Anglo-Scandinavian Cardiac Outcomes Trial. Anglo-Scandinavian Cardiac Outcomes Trial: a brief history, rationale and outline protocol. J Hum Hypertens 2001; 15(Suppl 1)S11–2
- Agata J, Masuda A, Takada M, Higashiura K, Murakami H, Miyazaki Y, et al. High plasma immunoreactive leptin level in essential hypertension. Am J Hypertens. 1997; 10: 1171–4
- Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003; 42: 231–4
- Cigolini M, Targher G, Bergamo Andreis IA, Tonoli M, Agostino G, et al. Visceral fat accumulation and its relation to plasma hemostatic factors in healthy men. Arterioscler Thromb Vasc Biol. 1996; 16: 368–74
- Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000; 20: 1595–9
- Lim HS, Tayebjee MH, Tan KT, Patel JV, Macfadyen RJ, Lip GYH. Severity differences and relation to coronary artery disease serum adiponectin in coronary heart disease: Heart. 2005; 91: 1605–6
- Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003; 163: 427–36
