Abstract
The Study on Cognition and Prognosis in the Elderly (SCOPE) assessed the effect of candesartan on cardiovascular and cognitive outcomes in elderly patients (aged 70–89 years) with mild to moderate hypertension. Patients were randomized to treatment with candesartan 8–16 mg daily (n = 2477) or placebo (n = 2460) and followed for 3.7 years on average. In agreement with the study protocol, other antihypertensive drugs were added if blood pressure remained ⩾160 mHg systolic and/or ⩾90 mmHg diastolic. Due to extensive add‐on therapy, particularly in patients randomized to placebo, the between‐treatment difference in blood pressure was only 3.2/1.6 mmHg. Nevertheless, the main analysis showed that non‐fatal stroke was reduced by 28% (p = 0.04) in the candesartan group compared with the control group, and there was a non‐significant 11% reduction in the primary endpoint, major cardiovascular events (p = 0.19). This review article presents different predefined and post hoc analyses made so far. Of particular interest are significant risk reductions with candesartan in major cardiovascular events (32%, p = 0.013), cardiovascular mortality (29%, p = 0.049) and total mortality (27%, p = 0.018) in patients who did not receive add‐on therapy after randomization, and in whom the difference in blood pressure was 4.7/2.6 mmHg. Other analyses suggest positive effects of candesartan‐based treatment on cognitive function, quality of life and new‐onset diabetes. In conclusion, SCOPE strongly suggests that candesartan treatment reduces cardiovascular morbidity and mortality in old and very old patients with mild to moderate hypertension. Candesartan‐based antihypertensive treatment may also have positive effects on cognitive function and quality of life.
Introduction
Hypertension increases the risk of cardiovascular morbidity and mortality Citation[1]. It is also a risk factor for cognitive decline and dementia Citation[2,3]. Randomized controlled trials show that blood pressure lowering treatment reduces cardiovascular morbidity and mortality in patients with hypertension Citation[4]. However, there has been little data from randomized controlled trials regarding the effect in certain subgroups of patients, e.g. in old and very old patients with mild hypertension. It is unknown whether antihypertensive treatment can retard cognitive decline or prevent dementia.
The main objectives of the Study on COgnition and Prognosis in the Elderly (SCOPE) were to assess the effect of treatment with the angiotensin II type 1 (AT1)‐receptor antagonist candesartan in old patients (aged 70–89 years) with mild to moderate hypertension on:
Cardiovascular morbidity and mortality;
Cognitive decline and dementia.
The purpose of this review article is to summarize information from a number of previous publications based on SCOPE, including study design, patient characteristics, methods, main results and findings from different explorative analyses. The various results and their implications will be discussed.
Study design, patients and methods
SCOPE was initially designed as a comparison of candesartan and placebo. However, due to changing treatment guidelines and for ethical reasons, a large proportion of patients in both treatment groups were given open‐label active antihypertensive therapy when needed to control blood pressure, as recommended in a protocol amendment issued already during the recruitment phase. Therefore, the study was in fact a comparison between candesartan‐based therapy and other active antihypertensive therapy (mostly diuretic based).
Details of design, patient inclusion and exclusion criteria, procedures, endpoints and clinical measures in SCOPE have been reported previously Citation[5]. Briefly, the study was a multinational, prospective, randomized, double‐blind, controlled trial with a parallel‐group design. A total of 527 centres in 15 countries, mainly in Europe, participated in the study. Patients were recruited between March 1997 and January 1999, and the follow‐up phase ended in March 2002. SCOPE was approved by all ethics committees concerned and conducted in accordance with the principles stated in the Declaration of Helsinki and Good Clinical Practice.
Both untreated and previously treated men and women with mild to moderate primary hypertension, aged 70–89 years, could be enrolled providing no exclusion criteria applied. The latter included stroke or myocardial infarction within 6 months, decompensated heart failure, impaired renal or hepatic function, dementia, conditions precluding MMSE, certain brain and mental disorders, psycho‐pharmacological treatment started within 6 months, and serious concomitant diseases affecting survival.
The study consisted of an open run‐in period (1–3 months) followed by double‐blind treatment for 3–5 years. During the run‐in period, patients with previous antihypertensive medication (approximately 50% of all patients) had their therapy standardized to hydrochlorothiazide (HCT), 12.5 mg once daily. This baseline therapy was maintained throughout the study. Patients with systolic blood pressure (SBP) 160–179 mmHg, or diastolic blood pressure (DBP) 90–99 mmHg, or both, and good cognitive function [Mini Mental State Examination (MMSE) score ⩾24] at the end of the run‐in period were then randomized to receive either candesartan or placebo. The initial dose was 8 mg once daily, which was increased to 16 mg once daily in case of inadequate blood pressure reduction. Consistent with the revised study protocol, many patients went on to receive additional open‐label antihypertensive therapy because their blood pressure remained too high (SBP⩾160 mmHg and/or DBP⩾90 mmHg) despite candesartan 16 mg once daily or corresponding placebo.
Patients returned for check‐up visits at 1 and 3 months, and then on a 6‐monthly basis. Blood pressure and adverse events were assessed at these visits, and all suspected clinical events were reported. Blood pressure was measured in triplicate after 5–10 min rest using a cuff of appropriate size, and the mean of the last two readings was used in all decisions and analyses. The DBP was taken as the pressure at which the Korotkoff sounds disappeared (phase V). Cardiovascular events, deaths and cases of dementia were adjudicated by an independent clinical event committee, which was blinded with respect to the patients treatment group allocation. The primary outcome measure was the (time to) occurrence of a first major cardiovascular event (cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke). Secondary outcome measures included the occurrence of a first non‐fatal or fatal stroke (together and separately), change in MMSE score, development of significant cognitive decline (reduction in MMSE score ⩾4 at two consecutive visits compared with baseline), dementia and new‐onset diabetes.
Statistics
The analyses were conducted according to the intention‐to‐treat and last value carried forward principles. Differences between the treatment groups in “time to event” were analysed with a log‐rank test. Differences between proportions of patients with an event were analysed with the chi‐square test. Changes in blood pressure and MMSE score from baseline were symmetrically distributed and tested in an analysis of covariance (ANCOVA) model, with prespecified factors adjusting for country and baseline value. Analysis of covariance was also used to analyse changes from baseline in health‐related quality of life scores. Cox regression analysis was used to calculate p‐values for the interaction between treatment and subgroups of patients. Two‐sided p‐values and 95% confidence intervals (CI) were used.
Results
Patient characteristics
The intention‐to‐treat population consisted of 4937 patients, of whom 2477 were randomized to the candesartan arm and 2460 to the control arm. The two treatment groups were well balanced regarding a number of characteristics at baseline Citation[6] (). Almost two‐thirds of the patients were women. The mean age was 76 years in both treatment groups, and 21% of the patients were aged 80 or above. About 30% of the patients in both groups had isolated systolic hypertension (SBP⩾160 mmHg and DBP<90 mmHg).
Table I. Demographics and clinical characteristics at baseline. Means (±SD) or per cent are given.
Eight patients only were lost to follow‐up, i.e. their vital status at the end of the study was unknown. The mean duration of the trial was 3.7 years, resulting in 18 445 patient‐years of observation.
Antihypertensive treatment, blood pressure reduction
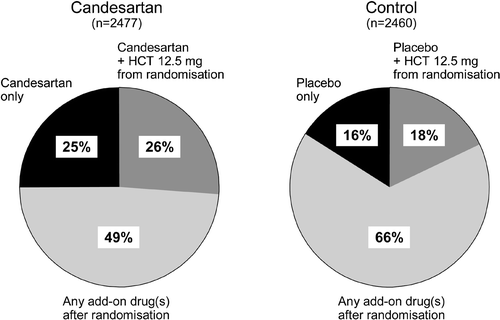
The mean (±SD) dose of candesartan was 11.6±4.0 mg once daily. As a consequence of the treatment schedule specified in the study protocol, only 16% of the patients in the control group received placebo alone and the vast majority of control patients (84%) received active antihypertensive treatment (). Eighteen per cent remained on the low dose HCT (12.5 mg o.d.) already given at baseline, and 66% received open‐label add‐on antihypertensive treatment after randomization. The corresponding figures for the candesartan group were 26% and 49%, respectively.
The mean blood pressure was reduced from 166.0/90.3 mmHg to 145.2/79.9 mmHg in the candesartan group and from 166.5/90.4 mmHg to 148.5/81.6 mmHg in the control group. The mean difference between the treatment groups in adjusted blood pressure reduction was 3.2/1.6 mmHg in favour of the candesartan group (p<0.001 for both) Citation[6].
Cardiovascular events and total mortality
Main analysis
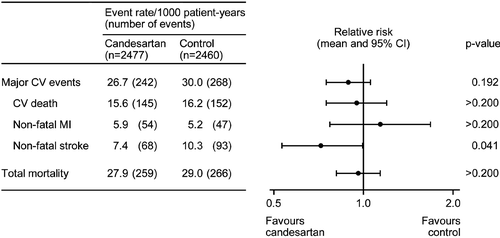
The main results of SCOPE were published in 2003 Citation[6]. shows data on cardiovascular events and total mortality. Despite the relatively small difference in blood pressure reductions between the treatment groups, the mean relative risk (RR) of non‐fatal stroke in the candesartan group compared to the control group was 0.72, i.e. there was a 28% RR reduction (p = 0.04). However, there were no significant RR reductions in the primary endpoint (major cardiovascular events; a composite of cardiovascular death, non‐fatal myocardial infarction and non‐fatal stroke), other cardiovascular endpoints, or total mortality.
Pre‐specified subgroups
The SCOPE study protocol stated that the results in clinical events should be analysed for consistency between a number of subgroups of patients as defined by baseline characteristics. Pre‐specified pairs of subgroups included; age 80–89/70–79 years, gender male/female, diabetes yes/no, previous stroke yes/no, isolated systolic hypertension (ISH) yes/no, and current smoker yes/no. Results for cardiovascular events and total mortality in these subgroups of patients have been reported previously Citation[7,8].
A significant interaction between treatment and subgroups was found for one pair of subgroups only; the reduction in major cardiovascular events with candesartan was greater in patients with a previous stroke (64% RR reduction, p = 0.004) than in those without (5% RR reduction, p>0.20). Although RR reductions in major cardiovascular events and stroke with candesartan‐based therapy were indicated in all subgroups, they were mostly non‐significant. This was expected considering the limited number of patients and events in most subgroups. Particularly large RR reductions in stroke with candesartan‐based treatment were observed in patients with ISH (42%, p = 0.050), atrial fibrillation (56%, p = 0.074) or a previous stroke (62%, p = 0.047) at baseline.
Patients without add‐on therapy
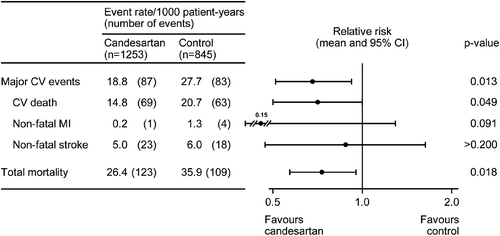
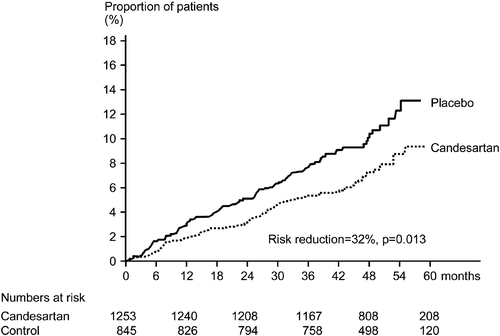
To avoid the confounding factor represented by the more frequent use of add‐on therapy after randomization in the control group (66% of patients) than in the candesartan group (49% of patients), patients who did not receive add‐on therapy were subject for a separate analysis, which reflects the original intention of a placebo‐controlled trial Citation[9]. This analysis included 1253 patients treated with candesartan and 845 patients treated with placebo. The two treatment groups were generally similar with respect to baseline characteristics, although blood pressure must have been particularly easy to control in patients who only received placebo during follow‐up. The difference in blood pressure reductions between the treatment groups (4.7/2.6 mmHg) was greater in this subset of patients than in the total SCOPE study population. There were significant RR reductions with candesartan compared to placebo treatment in major cardiovascular events (32%, p = 0.013), cardiovascular mortality (29%, p = 0.049) and total mortality (27%, p = 0.018), . The time courses for a first major cardiovascular event in the two treatment groups are shown in .
Cognitive function and dementia
Main analysis
Cognitive function was high at baseline and well maintained during follow‐up in both treatment groups Citation[6]. The mean MMSE score fell from 28.5 to 28.0 in the candesartan group and from 28.5 to 27.9 in the control group, with no significant difference between the groups in adjusted change (mean 0.15; 95% CI –0.08 to 0.38). Neither were there any significant differences between the treatment groups in the proportions of patients who had a significant cognitive decline (candesartan 4.7%, control 5.2%) or developed dementia (candesartan 2.5%, control 2.3%).
Effects of baseline cognitive function on cognitive outcomes
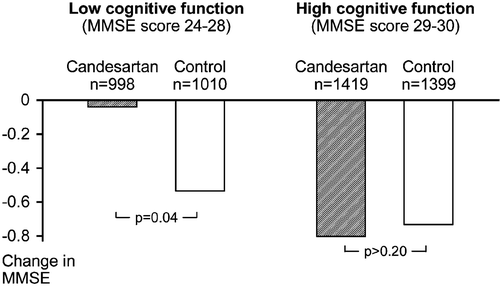
Limited sensitivity of MMSE to measure change in cognitive function in subjects with maximal score, or close to maximal score, was the main reason to investigate the effect of candesartan treatment, compared with control treatment, on MMSE score in two subgroups of patients; those with MMSE score 24–28 (“low cognitive function”) at baseline and those with MMSE score 29–30 (“high cognitive function”) at baseline Citation[10]. The incidence of significant cognitive decline and dementia in relation to baseline MMSE score was also examined since low cognitive function has been claimed to increase the risk of these outcomes.
In patients with low cognitive function at baseline (n = 2070), the adjusted change in MMSE score was significantly smaller in the candesartan group (−0.04) than in the control group (−0.53); the mean difference in change was 0.49 (95% CI 0.02 to 0.97, p = 0.04; ). However, in patients with high cognitive function at baseline (n = 2867) there was no significant difference in change in MMSE score between the candesartan group (−0.80) and the control group (−0.73). The proportions of patients with significant cognitive decline did not differ significantly between the candesartan and control groups, either in patients with low cognitive function (candesartan group 6.1%, control group 7.0%), or in patients with high cognitive function (candesartan group 3.5%; control group 3.7%). Likewise, the proportions of patients who developed dementia did not differ significantly between the candesartan and control groups, either in patients with low cognitive function (candesartan group 4.3%, control group 4.5%), or in patients with high cognitive function (candesartan group 1.2%; control group 0.7%).
Significant cognitive decline during the study was almost twice as high in patients with low cognitive function as in those with high cognitive function at baseline (6.6% vs 3.6%, p<0.001), and dementia was more than four times more common in patients with low cognitive function (4.4% vs 1.0%, p<0.001). These differences remained significant also after adjustment for covariates at baseline.
Other tests of cognitive function than MMSE
One large centre (Newcastle, UK) that participated in SCOPE used a comprehensive battery of tests for the assessment of cognitive function in 257 patients. Using tests of five cognitive domains, which were sensitive to change and free from ceiling effects, the candesartan group showed significantly less decline during the study in Attention and Episodic Memory compared to the placebo group, with a similar trend for Speed of Cognition Citation[11]. However, there were no significant differences between the treatment groups in the decline in Working Memory or Executive Function.
Brain atrophy
The rate of whole brain atrophy was assessed using 1.5T MRI scans at baseline and after 2 years at the SCOPE centre in Newcastle, UK Citation[12]. A total of 32 patients from the SCOPE candesartan group, 36 patients from the SCOPE control group and 27 normotensive subjects were examined. The mean blood pressures were 131/73 mmHg in the normotensive group, 146/77 mmHg in the SCOPE candesartan group and 154/81 mmHg in the SCOPE control group. The mean brain atrophy rate was 0.37%, 0.46% and 0.62% per year, respectively (p = 0.038 for trend).
New‐onset diabetes
In SCOPE, new‐onset diabetes mellitus was reported in 106 of the 2477 patients (4.3%) in the candesartan group and in 131 of the 2460 patients (5.3%) in the control group Citation[6]. Thus, candesartan‐based antihypertensive treatment, compared to control treatment, was associated with a relative reduction in new‐onset diabetes of 19% (95% CI −2 to 42%, p = 0.09) during a mean follow‐up period of 3.7 years.
Health‐related quality of life
Health‐related quality of life (HRQL) was assessed in a substudy of SCOPE using three well‐recognized and validated scales; the Psychological General Well‐Being (PGWB) Index, the Subjective Symptom Assessment Profile (SSA‐P) and the EuroQol Health Utility Index (EuroQol). This substudy included 1428 patients in the candesartan group and 1422 in the control group, i.e. more than 50% of the total SCOPE study population. The HRQL was generally good at baseline and well preserved during the mean follow‐up period of 3.7 years in the presence of substantial blood pressure reductions in both treatment groups Citation[13]. In fact, several of the observed changes in score during the observation period favoured candesartan‐based treatment compared to control treatment, particularly the changes in PGWB Anxiety (−0.5 vs −1.0, p = 0.01), PGWB Positive Well‐being (−0.8 vs −1.1, p = 0.04), SSA‐P Cardiac Symptoms (0.03 vs 0.10, p = 0.03) and EuroQoL Current Health (−3.1 vs −5.3, p = 0.008).
Cost‐effectiveness
A cost‐effectiveness analysis of candesartan‐based antihypertensive treatment for the prevention of non‐fatal stroke based on data from SCOPE has been reported Citation[14]. About half of the higher cost of antihypertensive treatment in the candesartan group was offset by lower costs for treating strokes, resulting in a net cost of 370 EUR per patient. As candesartan‐based antihypertensive treatment was associated with 0.029 additional quality adjusted life‐years (QALYs) per patient, the incremental cost per QUALY gained was approximately 13.000 EUR, which lies within the range of society’s willingness to pay for health gains.
Discussion
Although initially intended as a placebo‐controlled outcome study in elderly patients with mild to moderate hypertension, SCOPE was in fact a comparison between two antihypertensive treatment regimens, one based on candesartan and the other based on other drugs, mostly HCT. This was consistent with the study protocol and the ethical requirement not to leave high blood pressure untreated long‐term. The more frequent use of add‐on therapy after randomization in order to control blood pressure in the control group (66% of patients) than in the candesartan group (49% of patients) explains why the difference in blood pressure reduction between the treatment groups was small 3.2/1.6 mmHg, and less than originally anticipated, and why the relative risk reduction in the primary endpoint in the candesartan group compared with the control group was modest and statistically non‐significant.
Since the main hypothesis that candesartan‐based treatment reduces major cardiovascular events in elderly patients with mild to moderate hypertension was not confirmed in the primary analysis of SCOPE, all secondary analyses must be interpreted with caution. Nevertheless, the pre‐specified and post hoc secondary analyses done so far indicate favourable effects of candesartan‐based antihypertensive treatment in the elderly and must not be neglected. Some points that deserve special attention are discussed below.
In the analysis of all patients, non‐fatal stroke, a pre‐specified secondary outcome variable, was reduced by 28% (p = 0.04) in the candesartan group compared with the control group. The reduction in stroke in the candesartan group is most likely a true finding. It is an expected effect that can be explained, at least partly, by the slightly greater blood pressure reduction in the candesartan group than in the control group. In addition, AT1‐receptor antagonists may have a cerebro‐protective effect “beyond blood pressure reduction” as also suggested by animal experimental data Citation[15,16] and in the Losartan Intervention For Endpoint (LIFE) reduction in hypertension clinical trial Citation[17].
Major differences in the effects of candesartan‐based treatment on major cardiovascular events and stroke in the pre‐specified subgroups of patients (defined by baseline characteristics such as age, gender and medical history) seem less likely, with one exception. The reduction in major cardiovascular events in patients who entered the study with a previous stroke was significantly greater (64% reduction) than that in patients without a previous stroke (5% reduction). However, modest differences in effect of candesartan also in other subgroups of patients cannot be excluded since several subgroups were relatively small with low number of events and interaction tests are generally weak.
The analysis of the subgroup of patients without add‐on therapy after randomization is of special interest since it reflects the original intention of a placebo‐controlled trial. The results, significant reductions in major cardiovascular events (32% reduction), cardiovascular mortality (29% reduction) and total mortality (27% reduction), are consistent with what can be expected in a comparison between active antihypertensive treatment and placebo treatment in elderly patients, based on previous observations Citation[18,19]. This post‐hoc analysis therefore strongly supports the conclusion that antihypertensive treatment with candesartan reduces cardiovascular morbidity and mortality. Importantly, the reduction in cardiovascular mortality seems not offset by any increase in non‐vascular mortality since total mortality was also reduced by candesartan treatment.
The post hoc analysis of the influence of baseline cognitive function on cognitive outcomes indicates that elderly patients with mild to moderate hypertension and slightly impaired cognitive function (MMSE score 24–28) are at increased risk of dementia, and that antihypertensive therapy may reduce cognitive decline in these patients. Thus, patients with somewhat low cognitive function appear an important target group for antihypertensive treatment and must not be excluded from treatment because of concern for deteriorating cognition secondary to blood pressure lowering. This interpretation is supported by the favourable effects of candesartan treatment on cognitive function found in the Newcastle subgroup using a comprehensive battery of neuropsychological tests in addition to the MMSE. The other observation in the Newcastle subgroup, i.e. that hypertension in older people is associated with increased rate of whole brain atrophy and that candesartan‐based blood pressure lowering therapy may reduce the rate of atrophy, gives further support for the hypothesis that antihypertensive treatment in the elderly may reduce the risk of cognitive decline. However, the most convincing evidence for such an effect comes from the Systolic Hypertension in Europe (Syst‐Eur) study Citation[20].
The indication of less new‐onset diabetes with candesartan‐based antihypertensive treatment compared with other treatment (mainly HCT) in SCOPE is consistent with observations in other studies of blocking the renin–angiotensin–aldosterone system Citation[21]. For example, lower rates of new‐onset diabetes were found in hypertensive patients with losartan‐based treatment compared to atenolol‐based treatment in the LIFE study Citation[17], with candesartan‐based treatment compared to HCT‐based treatment in the Antihypertensive treatment and Lipid Profile in a North of Sweden Efficacy evaluation (ALPINE) study Citation[22], and with valsartan‐based treatment compared to amlodipine‐based treatment in the Valsartan Antihypertensive Long‐term Use Evaluation (VALUE) study Citation[23]. Also in patients with heart failure, candesartan treatment compared to placebo treatment reduced new‐onset diabetes in the Candesartan in Heart failure – Assessment of Reduction in Morbidity and mortality (CHARM) study programme Citation[24].
As indicated by the favourable results on HRQL in SCOPE, there should be no concern for a negative effect on HRQL by modern antihypertensive therapy, such as candesartan treatment. This is an important message to both doctors and patients who have been unsure about the balance between cardiovascular benefit and risk of impaired HRQL of antihypertensive treatment, especially in elderly patients.
The health economy analysis in SCOPE shows that antihypertensive treatment based on candesartan is cost‐effective because of the greater reduction in stroke in comparison with treatment mainly based on a diuretic. Similar results were found in a health economy analysis in LIFE; losartan‐based antihypertensive treatment was cost‐effective in comparison with beta‐blocker‐based treatment Citation[25].
In conclusion, SCOPE strongly suggests that candesartan compared to placebo treatment reduces cardiovascular morbidity and mortality in old and very old patients with mild and moderate hypertension. In addition, candesartan‐based antihypertensive treatment may have positive effects on cognitive function and quality of life.
References
- MacMahon S., Peto R., Cutler J., Collins R., Sorlie P., Neaton J., et al. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: Prospective observational studies corrected for the regression dilution bias. Lancet 1990; 335: 765–774
- Kilander L., Nyman H., Boberg M., Hansson L., Lithell H. Hypertension is related to cognitive impairment: A 20‐year follow‐up of 999 men. Hypertension 1998; 31: 780–786
- Skoog I., Lernfelt B., Landahl S., Palmertz B., Andreasson L‐A., Nilsson L., et al. 15‐year longitudinal study of blood pressure and dementia. Lancet 1996; 347: 1141–1145
- Blood Pressure Lowering Treatment Trialists Collaboration. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: Results of prospectively‐designed overviews of randomised trials. Lancet 2003; 362: 1527–1535
- Hansson L., Lithell H., Skoog I., Baro F., Bánki C. M., Breteler M., et al. Study on Cognition and Prognosis in the Elderly (SCOPE). Blood Press 1999; 8: 177–183
- Lithell H., Hansson L., Skoog I., Elmfeldt D., Hofman A., Olofsson B., , for the SCOPE study group, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): Principal results of a randomized double‐blind intervention trial. J Hypertens 2003; 21: 875–886
- Trenkwalder P., Elmfeldt D., Hofman A., Lithell H., Olofsson B., Papademetriou V., et al. The Study on Cognition and Prognosis in the Elderly (SCOPE) – Major CV events and stroke in subgroups of patients. Blood Press 2005; 14: 31–37
- Papademetriou V., Farsang C., Elmfeldt D., Hofman A., Lithell H., Olofsson B., , for the SCOPE study group, et al. Stroke prevention with angiotensin II Type1‐receptor blocker candesartan in elderly patients with isolated systolic hypertension – the Study on Cognition and Prognosis in the Elderly (SCOPE). J Am Coll Cardiol 2004; 44: 1175–1180
- Lithell H., Hansson L., Skoog I., Elmfeldt D., Hofman A., Olofsson B., , for the SCOPE study group, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): Outcomes in patients not receiving add‐on therapy after randomization. J Hypertens 2004; 22: 1605–1612
- Skoog I., Lithell H., Hansson L., Elmfeldt D., Hofman A., Olofsson B., , for the SCOPE study group, et al. Effect of baseline cognitive function and antihypertensive treatment on cognition and cardiovascular outcomes: Study on Cognition and Prognosis in the Elderly (SCOPE). AJH 2005; 18: 1052–1059
- Saxby B. K., Harrington F., McKeith I. G., Wesnes K., Ford G. A. The effects of candesartan cilexetil on cognitive function in older adults with mild hypertension. J Hypertens 2003; 21(Suppl 4)S18–S19, abstract 3B.5
- Wiseman R. M., Burton E. J., Saxby B. K., O’Brien J. T., Ford G. A. Increased brain atrophy rate in untreated older hypertensive subjects. J Hypertens 2003; 21(Suppl 4)S17–S18, abstract 3B.1
- Degl’Innocenti A., Elmfeldt D., Hofman A., Lithell H., Olofsson B., Skoog I., et al. Health‐related quality of life during treatment of elderly patients with hypertension: Results from the Study on Cognition and Prognosis in the Elderly (SCOPE). J Hum Hypertens 2004; 18: 239–245
- Lundkvist J., Ekman M., Kartman B., Carlsson J., Jönsson L., Lithell H. The cost‐effectiveness of candesartan‐based antihypertensive treatment for the prevention of nonfatal stroke: Results from the Study on Cognition and Prognosis in the Elderly (SCOPE). J Hum Hypertens 2005; 19: 569–576
- Inada Y., Wada T., Ojima M., Sanada T., Shibouta Y., Kanagawa R., et al. Protective effects of candesartan cilexetil (TCV‐116) against stroke, kidney dysfunction and cardiac hypertrophy in stroke‐prone spontaneously hypertensive rats. Clin Exp Hypertens 1997; 19: 1079–1099
- Culman J., Blume A., Gohlke P., Unger T. The renin–angiotensin system in the brain: Possible therapeutic implications for AT1‐receptor blockers. J Hum Hypertens 2002; 16: S64–S70
- Dahlöf B., Devereux R. B., Kjeldsen S. E., Julius S., Beevers G., de Faire U., , for the LIFE study group, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet 2002; 359: 995–1003
- Dahlöf B., Lindholm L. H., Hansson L., Scherstén B., Ekbom T., Wester P‐O. Morbidity and mortality in the Swedish trial in Old Patients with Hypertension (STOP‐Hypertension). Lancet 1991; 338: 1281–1285
- Staessen J. A., Fagard R., Thijs L., Celis H., Arabidze G. G., Birkenhäger W. H., , for the Systolic Hypertension in Europe (Syst‐Eur) trial investigators, et al. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet 1997; 350: 757–764
- Forette F., Seux M‐L., Staessen J. A., Thijs L., Birkenhäger W. A., Babarskiene M‐R., , on behalf of the Syst‐Eur investigators, et al. Prevention of dementia in randomised double‐blind placebo‐controlled Systolic Hypertension in Europe (Syst‐Eur) trial. Lancet 1998; 352: 1347–1351
- Mancia G., Grassi G., Zanchetti A. New onset diabetes and antihypertensive drugs. J Hypertens 2006; 24: 3–10
- Lindholm L. H., Persson M., Alaupovic P., Carlberg B., Svensson A., Samuelsson O. Metabolic outcome during 1 year in newly detected hypertensives: Results of the Antihypertensive Treatment and Lipid Profile in a North of Sweden Efficacy Evaluation (ALPINE study). J Hypertens 2003; 21: 1563–1574
- Julius S., Kjeldsen S. E., Weber M., Brunner H. R., Ekman S., Hansson L., , for the VALUE trial group, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: The VALUE randomised trial. Lancet 2004; 363: 2022–2031
- Yusuf S., Östergren J. B., Gerstein H. C., Pfeffer M. A., Swedberg K., Granger C. B., , on behalf of the Candesartan in Heart Failure – Assessment of Reduction in Mortality and Morbidity Program (CHARM) investigators, et al. Effects of candesartan on the development of a new diagnosis of diabetes mellitus in patients with heart failure. Circulation 2005; 112: 48–53
- Jönsson B., Carides G. W., Burke T. A., Dasbach E. J., Lindholm L. H., Dahlöf B. Cost effectiveness of losartan in patients with hypertension and LVH: An economic evaluation for Sweden of the LIFE trial. J Hypertens 2005; 23: 1425–1431