Abstract
Introduction. Insomnia may increase risk of cardiovascular events. There is little data available reporting the prevalence and clinical relevance of insomnia in patients with essential hypertension. Therefore, the aim of the study was to investigate the relationship between insomnia and different clinical and biochemical parameters in essential hypertension patients. Methods. Four hundred and thirty‐two patients (mean age: 47±13 years; 253 male, 179 female) with essential hypertension were screened for insomnia using the Athens Insomnia Scale (AIS). Several variables including age, sex, known duration of hypertension, body mass index, creatinine, left ventricular mass index, coexisting disorders, smoking status and alcohol use were analysed. Twenty‐four‐hour ambulatory blood pressure measurements (ABPM) were performed. Results. Among patients included in the study, 207 subjects (mean age: 49±13 years; 47.9%) had an AIS score of 15 or higher and were identified as insomniacs. Insomnia was more frequent in women than in men (60.9% vs 38.7%, p<0.001) and was reported more frequently in patients with coronary artery disease. Subjects with insomnia were older and had longer duration of hypertension. There were no differences between insomniacs and non‐insomniacs in ABPM parameters. A relationship was found between the number of antihypertensive drugs and insomnia frequency. There were correlations between AIS score and age (r = 0.21; p<0.001) and duration of hypertension (r = 0.22; p<0.001). In the sub‐group of untreated essential hypertension patients, there were negative correlations between AIS score and night fall in systolic and diastolic blood pressure. Conclusion. Our results showed that insomnia is common in patients with essential hypertension and indicate an association between insomnia and gender, known duration of hypertension and number of antihypertensive drugs taken. Untreated essential hypertension insomniacs were characterized by less pronounced nocturnal fall in both systolic and diastolic blood pressure compared with non‐insomniacs.
Introduction
Insomnia is regarded to be the most common sleep disorder, especially in Europe and in the United States. Based on epidemiological studies performed so far, the reported prevalence for this disorder ranges from 10% to 34% Citation[1], Citation[2]. Several studies showed that subjective complaint of insomnia appears to increase the risk of future coronary events. Recent prospective surveys indicate that there is an association between insomnia and coronary artery disease (CAD) mortality Citation[3], Citation[4]. Cross‐sectional epidemiological studies have demonstrated that various psychosocial, medical and psychiatric conditions are correlated with insomnia. It has also been demonstrated that hypertension was one of the single factors associated with insomnia Citation[1], Citation[2], Citation[5], Citation[6].
Several prospective studies have demonstrated a higher cardiovascular complication rate in patients with a non‐dipping compared with a dipping blood pressure (BP) profile. It has been postulated that differences in sleep patterns should be considered a potential determinant of the magnitude of the “dipping” phenomenon Citation[7–11]. However, it should be stressed that there are little data available reporting the prevalence and clinical relevance of insomnia in patients with essential hypertension. Therefore, the aim of the study was to investigate insomnia in hypertensive patients in relation to different clinical and biochemical parameters.
Methods
Four hundred and thirty‐two consecutive patients (mean age: 47±13 years, range: 17–83 years; 253 males, 179 females) with essential hypertension (according to the recent ESH/ESC classification) were included in the study. All patients were diagnosed in seven clinical hypertension centres in Poland (tertiary referral centres) and secondary forms of arterial hypertension were excluded. The study was approved by the local ethics committee. All patients gave written informed consent before enrolment into the study.
All patients were free of any known clinical disorder that would affect sleep or mobility including congestive heart failure, narcolepsy or night shift work. Patients were clinically screened to exclude subjects with sleep apnoea; 115 patients (27% – mean age: 39±11 years; range 19–68 years; 92 males, 23 females) were untreated. Several variables including age, sex, known duration of hypertension, body mass index (BMI), creatinine, left ventricular mass index (LVMI), coexisting disorders, smoking status and alcohol use (⩾1 drink a day on average) were analysed. Creatinine clearance (CrCl) was calculated according to Cockcroft and Gault formula.
In all patients, 24‐h ambulatory BP measurements (ABPM) were recorded using SpaceLabs 90207 or 90217 monitors (Redmond, Washington, USA). Readings were obtained every 15 min during the day and every 20 min during the night. In order to study the nocturnal decrease in BP in a quantitative way, we calculated the relative decrease in nocturnal BP for both systolic (SBP) and diastolic BP (DBP): [(daytime pressure−night‐time pressure)/daytime pressure]×100 and expressed it in percentage. Subjects were classified as dippers if the proportional change from awake to asleep BP fell by ⩾10%.
All participants of the study were screened for insomnia using the Athens Insomnia Scale (AIS). This self‐assessment questionnaire has been previously shown high consistency, reliability and external validity for simultaneous measurement of severity of insomnia and establishing the diagnosis of insomnia Citation[12]. The AIS can be used in epidemiological studies, clinical practice and research for the evaluation of the intensity of sleep difficulty during the previous month. It has been reported that AIS in overall case identification corresponded to high indices of both sensitivity (93%) and specificity (85%). The scale consists of several items assessing: difficulty with sleep induction, awakenings during the night, early morning awakening, total sleep time, overall quality of sleep, problems with sense of well‐being, overall functioning and sleepiness during the day. In our study, a total AIS score higher than 15 indicates that a person is insomniac Citation[12], Citation[13].
Since the present study lacks a control group, the incidence of insomnia was compared with age‐, sex‐ and BMI‐matched normotensive subjects from a large cross‐sectional study in which insomnia was assessed by means of the same questionnaire. In brief, the POL‐MONICA BIS study was carried out in a randomly selected sample of a Warsaw district (eastern side) population aged 20–74 years, and was carried out as a cross‐sectional study in year 2001 (1206 subjects were screened) Citation[5]. Hypertension was diagnosed according to WHO criteria (BP>140/90 mmHg or current antihypertensive treatment). Insomnia as in present study was screened by means of the AIS. Insomnia was defined as an AIS score of 16 or more points (as in the hypertensive cohort). Normotensive subjects from POL‐MONICA BIS study were selected to the control group according to the same distribution of age, sex and BMI as in the present study; 577 normotensive subjects (325 male, 252 female, mean age 46±13 years) were matched for age, sex and BMI (p = NS for differences in age, sex and BMI between those two groups).
For statistical comparison between the groups means and medians, the t‐test for independent samples and Mann–Whitney test were employed. Comparison of the prevalence rates among groups was performed using the chi‐square test with Yates's correction for continuity. Correlations were obtained using Spearman's equation. Multivariate analysis using forward stepwise logistic regression was employed to determine the effect of one variable or the simultaneous effect of several variables on the prevalence of insomnia. Ninety‐five per cent confidence intervals were calculated for the odds ratios derived from multiple logistic regression model. The results throughout are presented as mean±standard deviation. p<0.05 was considered statistically significant.
Results
The characteristics of the whole group, the insomniacs and the non‐insomniacs are presented in the Tables and . Our results indicate that among the 432 hypertensive patients included in the study, 207 subjects (mean age: 49±13 years; 109 female, 98 male) (47.9%) had AIS score of 15 and higher and were identified as insomniacs; 225 patients (mean age: 45±13 years; 155 male, 70 female) were defined as non‐insomniacs (Table ).
Table I. Characteristics of studied patients.
Table II. Characteristics of untreated and treated patients.
In the total sample, insomnia was more frequent in women than in men (60.9% vs 38.7% p<0.001), was reported more frequently by hypertensive patients with coexisting CAD (62.5% vs 37.5%; p<0.01) and tended to be more prevalent in those with coexisting diabetes mellitus (59.1% vs 40.9%; p = 0.1) compared with subjects without these two clinical conditions. The prevalence of insomnia was higher in patients older than 50 years (57.7% vs 42.3%; p<0.01).
Insomnia was also more frequent in the studied group compared with age‐, sex‐and BMI‐matched normotensive subjects from the POL‐MONICA BIS study (47.9% vs 24.4%; p<0.0001).
Subjects with insomnia were older (49±13 vs 45±13; p<0.01) and had significantly longer known duration of hypertension (8.8±7.8 vs 6.3±6.1; p<0.01) compared with those without insomnia. There were no differences in smoking status as well as in alcohol use between insomniacs and non‐insomniacs (Table ).
There were no statistically significant differences between insomniacs and non‐insomniacs in 24‐h, daytime as well as night‐time SBP and DBP. There were no statistical significant differences between groups in nocturnal fall in BP (Table ). However, it should be noted that insomniacs were characterized by higher median number of antihypertensive drugs taken compared with non‐insomniacs (2 vs 1; p<0.001). There was relationship between the increasing number of antihypertensive drugs and percentage of patients with insomnia (Figure ).
Figure 1 Percentage of patients suffering from insomnia depending on the quantity of used antihypertensive drugs.
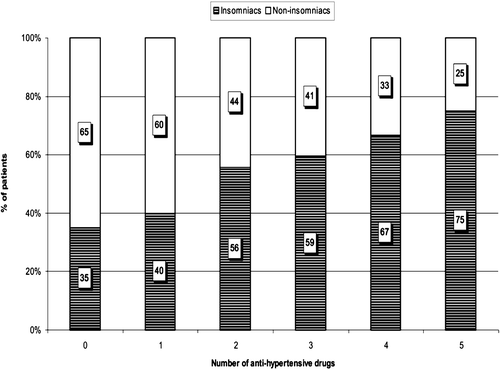
There were no statistical differences in serum electrolytes, glucose, creatinine concentrations and other biochemical parameters between insomniacs and non‐insomniacs. No statistically significant difference was found in BMI and LVMI between insomniacs and non‐insomniacs. The insomniacs were characterized by lower CrCl when they were compared with the non‐insomniacs (Table ). The difference was significant also when adjusted for age.
In the whole group, there were significant correlations between AIS score and age (r = 0.21; p<0.001), known duration of hypertension (r = 0.22; p<0.001), CrCl (r = −0.20; p<0.001) and LVMI (r = 0.12; p<0.01), respectively.
In the stepwise binary logistic regression model that included age, sex, BMI, known duration of hypertension and number of antihypertensive drugs taken, the only significant independent factors related to the prevalence of insomnia were female sex (OR 1.82; 95% CI 1.16–2.87; p<0.01) and number of antihypertensive drugs taken (OR 1.34; 95% CI 1.14–1.56; p<0.001) (Table ).
Table III. Stepwise regression results showing variables predicting occurrence of insomnia among all patients.
In the studied group, a total of 115 subjects were untreated (newly diagnosed essential hypertension). Of these 40 patients (34.8%) had an AIS score over 15 and were identified as insomniacs. There was a significant difference in the prevalence of insomnia between this group and the treated patients with essential hypertension (Table ).
In this subset, there were no statistically significant differences between insomniacs and non‐insomniacs in terms of age, known duration of hypertension and LVMI.
Insomniacs with untreated essential hypertension had significantly less pronounced nocturnal blood fall in SBP and DBP than non‐insomniacs. In the untreated group, there were negative correlations between the AIS score and the night fall in SBP (r = −0.0.22; p<0.05) and night fall in DBP (r = −0.24, p<0.01).
In the 115 untreated patients with essential hypertension, 18 subjects (15.7%) were characterized as non‐dippers and patients with insomnia were characterized by a higher percentage of non‐dippers (72.2%) compared with non‐insomniacs (27.8%; p<0.001).
In the stepwise binary logistic regression model that included age, sex, BMI, known duration of hypertension and dipping status, the only significant independent factors related to the prevalence of insomnia were female sex (OR 3.0, 95% CI 1.1–8.3), p<0.05) and lack of nocturnal BP fall (OR 6.4; 95% CI 2.0–20.5; p<0.01).
Discussion
In the present study, the prevalence of insomnia in patients with essential hypertension was 47.9%. In the general population, the reported prevalence of insomnia depends on the methods and definitions used. Rates of insomnia with associated daytime dysfunction range from 10% to as high as 34% depending on descriptions used, severity, chronicity, age group and sampling bias Citation[1], Citation[2]. There are only few other studies available to compare the rate of insomnia in patients with essential hypertension with our data Citation[5], Citation[6]. In the POL‐MONICA BIS study, performed in a randomly selected Warsaw metropolitan area (1206 subjects aged 20–74 years), insomnia was identified in 30.7% subjects. In this study, patients with hypertension reported insomnia more frequently than normotensives did (39.2% vs 24.2%, p<0.01) Citation[5]. Comparison of the incidence of insomnia between our hypertensive patients and age‐, sex‐ and BMI‐matched normotensive subjects from POL‐MONICA BIS study disclosed a higher incidence of insomnia in our studied hypertensive patients.
Clinicians may use various diagnostic methods to assess insomnia, including polysomnography, actigraphy or sleep questionnaires Citation[1], Citation[2], Citation[12]. In our study, the Athens Insomnia Scale (AIS) was used to screen patients with essential hypertension for insomnia. The AIS was designed simultaneously to measure the severity of insomnia and to establish its diagnosis based on commonly accepted criteria. The standard length of the self‐assessment period for rating sleep difficulties in AIS is 1 month and is identical to the time criterion for the diagnosis of insomnia in both ICD‐10 and DSM‐IV. The percentage of subjects correctly identified by the AIS is comparable with the respective percentage (89%) obtained with the Pittsburgh Sleep Quality Index (PSQI) Citation[12].
Our results indicate that in the total sample, insomnia was more frequent in hypertensive women than in men. Also, the same relationship was observed in the group of untreated hypertensives characterized by lower female proportion compared with the whole group. Our results confirm other reports indicating a gender difference and higher rates of insomnia in women. In a study performed in a large random sample of Central Pennsylvania, a multivariate logistic regression analysis indicated that female gender was one of the strongest factors associated with insomnia Citation[6]. However, it should be noted that in contrast to other studies describing the general population, we found a higher rate of insomnia in women in the group of patients with essential hypertension. It has also been reported that insomnia is occurring about 1.5 times more often in women than in men, especially in menopausal and postmenopausal women compared with middle‐aged men Citation[1], Citation[2]. In a study carried out in a community‐based sample of 301 women between 35 and 55 years, one of the most common perimenopausal symptoms was insomnia Citation[14]. It should be noted that the mean age of our group of hypertensive women with insomnia was similar to that reported in the study mentioned above.
Our study also showed that patients with essential hypertension and insomnia were older and had a significantly longer known duration of hypertension than those without insomnia. This observation is in accordance with other reports, indicating a high prevalence of insomnia in older people Citation[1], Citation[2].
We also found a significant difference in CrCl between hypertensive patients with and without insomnia. There was also a negative correlation between the global AIS score and renal function assessed by CrCl but not by the creatinine plasma level. Lower CrCl in the group of hypertensive insomniacs may be partially explained by more advanced age of hypertensive insomniacs compared with non‐insomniacs. However, the difference remained significant after adjusting for age. It may be therefore speculated that lower CrCl may result from longer duration of hypertension. Iliescu et al. reported no association between renal function and the global PSQI score Citation[15]. However, it should be noted that it is difficult to compare our group of patients with essential hypertension and preserved renal function with subjects with chronic renal disease as described by Iliescu et al. Citation[15].
In the investigated group, there were no significant differences between insomniacs and non‐insomniacs in both SBP and DBP levels as assessed by ABPM during whole day, daytime and night‐time. There were also no significant differences between insomniacs and non‐insomniacs in their dipper status and mean fall of BP between daytime and night time. Bixler et al observed no differences in diastolic BP between untreated insomniacs and non‐insomniacs but a significant difference in SBP in insomniacs compared with non‐insomniacs Citation[6]. However, it should be noted that in our study, BP was evaluated by ABPM method.
It is of interest that insomniacs with essential hypertension were characterized by higher median number of antihypertensive drugs taken. We also found that when divided according to the number of antihypertensive drugs taken, there was relationship between percentage of patients with insomnia and increasing number of medications taken. Since in most patients, antihypertensive therapy was based on combination of two and more drugs, it was not possible to analyse any association between insomnia and effect of particular class of antihypertensive drugs used. In many epidemiological studies, insomnia has been correlated with frequent use of medical resources and increased use of drugs Citation[1], Citation[2], Citation[16].
Our study provides novel information that untreated hypertensive insomniacs had a significantly lower fall in both SBP and DBP compared with non‐insomniacs. We have observed negative correlations between the AIS score and night fall in both SBP and DBP. We also found that in the untreated group of patients with essential hypertension, the prevalence of insomnia was higher in non‐dippers than in dippers. However, it should be noted that in our study there was a small number of untreated hypertensive patients with non‐dipping profile.
The study of Pedulla et al., based on polysomnographic recording, demonstrated that non‐dipper essential hypertensive patients suffer from sleep disturbances Citation[11]. Non‐dippers patients had a worse sleep pattern than dippers and their sleep was characterized by a decrease of stage 4 with an increased duration of stage 2 compared with dippers.
Kario et al. found that sleep activity was related to nocturnal BP dipping status as evaluated by actigraphic measures Citation[9]. The non‐dippers exhibited greater sleep activity than extreme dippers and an increase sleep/awake activity ratio compared with extreme dippers and dippers. The authors hypothesize that the association of sleep activity to sleep BP and dipping reflects differences in sleep quality. Also the results of Mansoor raised the possibility that sleep itself is disturbed in patients classified as non‐dippers based on actigraphic measures Citation[10]. Taken together, these results indicate an association between sleep quality, assessed by objective (polysomnography and actigraphy) and subjective (questionnaires) measures, and nocturnal BP fall.
Furthermore, our data indicate that insomnia was reported more frequently by patients with essential hypertension and coexisting CAD. Insomnia also tended to be reported more frequently by hypertensive patients with coexisting diabetes or compared with subjects without these two clinical conditions. It should be underlined that in several epidemiological studies insomnia has been correlated with diabetes and CAD. A subjective complaint of insomnia appears to increase one's risk of a future coronary events and this increase in risk cannot be explained by classic coronary risk factors. Reported risk ratios for coronary events in patients with various sleep complaints ranged from 1.2 to 3.9, and adjustment for conventional risk factors did not reduce the magnitude of this relationship. Problems falling asleep increased the risk of myocardial infarction and cardiac death, and a higher risk of developing myocardial infarction was found in males with severe sleep disturbances Citation[3], Citation[4], Citation[17], Citation[18].
The limitations of the present study may be the limited sample size of untreated hypertensive patients that does not permit the meaningful evaluation of variables that can influence dipping status. It should also be mentioned that no methods other than careful clinical screening were used to exclude patients suspected for sleep apnoea. The design of the study allows only describing associations between hypertension and insomnia but cannot provide any data on causality. Since our study is not based on an epidemiological design, it is difficult to generalize our results to larger populations. Also, the control group of normotensives matched for age, sex and BMI does not permit comparison with other parameters or BP profile in relation to insomnia.
In conclusion, the results of this study suggest that insomnia is a common complaint in patients with essential hypertension, indicating an association between insomnia and gender, known duration of hypertension and number of antihypertensive drugs taken. However, the pathophysiological mechanisms of this association remain unknown. We also found that untreated hypertensive insomniacs were characterized by less pronounced fall in both SBP and DBP compared with non‐insomniacs. These data may suggest a relationship between insomnia and an abnormal BP profile. Large prospective longitudinal studies of insomnia in patients with essential hypertension are needed to confirm the high prevalence of insomnia in hypertensives and to examine any association between insomnia and the 24‐h BP profile.
Acknowledgement
This study was supported by scientific grant of Polish Science Committee No. 4 P05B 143 18.
References
- Sateia M. J., Nowell P. D. Insomnia. Lancet 2004; 364: 1959–1973
- Partinen M., Hublin C. Epidemiology of sleep disorders. Principle and practice of sleep medicine, M Kryger, T Roth, W. C Dement, 2000; 558–580, Philadelphia, W.B. Saunders Company
- Nilsson P. M., Nilsson J. A., Hedblad B., Berglund G. Sleep disturbances in association with elevated pulse rate for prediction of mortality – Consequences of mental strain?. J Int Med 2001; 250: 521–529
- Mallon L., Broman J. E., Hetta J. Sleep complaints predict coronary artery disease mortality in males: A 12‐year follow‐up study of a middle‐aged Swedish population. J Int Med 2002; 251: 207–216
- Piwoński J., Piotrowski W., Prejbisz A., et al. Prevalence of insomnia in the Warsaw metropolitan area (Pol‐Monica Bis) – Relationship to hypertension and other cardiovascular risk factors. J Hypertens 2003; 21(Suppl 4)S171 (abstract)
- Bixler E. O., Vgontzas A. N., Lin H. M., Vela‐Bueno A., Kales A. Insomnia in central Pennsylvania. J Psychosom Res 2002; 53: 589–592
- Ohkubo T., Hozawa A., Yamaguchi J., Kikuya M., Ohmori K., Michimata M., et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24‐h blood pressure: The Ohasama study. J Hypertens 2002; 20: 2183–2189
- Weir M. R., Blantz R. C. Blood pressure and cardiovascular risk: Implications of the presence or absence of a nocturnal dip in blood pressure. Curr Opin Nephrol Hypertens 2003; 12: 57–60
- Kario K., Schwartz J. E., Pickering T. G. Ambulatory physical activity as a determinant of diurnal blood pressure variation. Hypertension 1999; 34: 685–691
- Mansoor G. A. Sleep actigraphy in hypertensive patients with the ‘non‐dipper’ blood pressure profile. J Hum Hypertens 2002; 16: 237–242
- Pedulla M., Silvestri R., Lasco A., Mento G., Lanuzza B., Sofia L., et al. Sleep structure in essential hypertensive patients: Differences between dippers and non‐dippers. Blood Press 1995; 4: 232–237
- Soldatos C. R., Dikeos D. G., Paprrigopoulos T. J. The diagnostic validity of the Athens Insomnia Scale. J Psychosom Res 2003; 55: 263–267
- Szelenberger W., Niemcewicz S. Severity of insomnia correlates with cognitive impairment. Acta Neurobiol Exp 2000; 60: 373
- Mitchell E. S., Woods N. F. Symptom experiences of midlife women: Observations from the Seattle Midlife Women's Health Study. Maturitas 1996; 25: 1–10
- Iliescu E. A., Yeates K. E., Holland D. C. Quality of sleep in patients with chronic kidney disease. Nephrol Dial Transplant 2004; 19: 95–99
- Kapur V. K., Redline S., Nieto F. J., Young T. B., Newman A. B., Henderson J. A. The relationship between chronically disrupted sleep and healthcare use. Sleep 2002; 25: 289–296
- Schwartz S. W., Cornoni‐Huntley J., Cole S. R., Hays J. C., Blazer D., Schocken D. D. Are sleep complaints an independent risk factor for myocardial infarction?. Ann Epidemiol 1998; 384–392, 8
- Schwartz S., Anderson W. M., Cole S. R., Cornoni‐Huntley J., Hays J. C., Blazer D. Insomnia and heart disease: A review of epidemiologic studies. J Psychosom Res 1999; 47: 313–333
