Authors/Task Force Members: Giuseppe Mancia, Co‐Chairperson (Italy), Guy De Backer, Co‐Chairperson (Belgium), Anna Dominiczak (UK), Renata Cifkova (Czech Republic) Robert Fagard (Belgium), Giuseppe Germano (Italy), Guido Grassi (Italy), Anthony M. Heagerty (UK), Sverre E. Kjeldsen (Norway), Stephane Laurent (France), Krzysztof Narkiewicz (Poland), Luis Ruilope (Spain), Andrzej Rynkiewicz (Poland), Roland E. Schmieder (Germany), Harry A.J. Struijker Boudier (Netherlands), Alberto Zanchetti (Italy)
ESC Committee for Practice Guidelines (CPG): Alec Vahanian, Chairperson (France), John Camm (United Kingdom), Raffaele De Caterina (Italy), Veronica Dean (France), Kenneth Dickstein (Norway), Gerasimos Filippatos (Greece), Christian Funck‐Brentano (France), Irene Hellemans (Netherlands), Steen Dalby Kristensen (Denmark), Keith McGregor (France), Udo Sechtem (Germany), Sigmund Silber (Germany), Michal Tendera (Poland), Petr Widimsky (Czech Republic), Jose Luis Zamorano (Spain)
ESH Scientific Council: Sverre E. Kjeldsen, President (Norway), Serap Erdine, Vice‐President (Turkey), Krzysztof Narkiewicz, Secretary (Poland), Wolfgang Kiowski, Treasurer (Switzerland), Enrico Agabiti‐Rosei (Italy), Ettore Ambrosioni (Italy), Renata Cifkova (Czech Republic), Anna Dominiczak (United Kingdom), Robert Fagard (Belgium), Anthony M. Heagerty, Stephane Laurent (France), Lars H. Lindholm (Sweden), Giuseppe Mancia (Italy), Athanasios Manolis (Greece), Peter M. Nilsson (Sweden), Josep Redon (Spain), Roland E. Schmieder (Germany), Harry A.J. Struijker‐Boudier (The Netherlands), Margus Viigimaa (Estonia)
Document Reviewers: Gerasimos Filippatos (CPG Review Coordinator) (Greece), Stamatis Adamopoulos (Greece), Enrico Agabiti‐Rosei (Italy), Ettore Ambrosioni (Italy), Vicente Bertomeu (Spain), Denis Clement (Belgium), Serap Erdine (Turkey), Csaba Farsang (Hungary), Dan Gaita (Romania), Wolfgang Kiowski (Switzerland), Gregory Lip (UK), Jean‐Michel Mallion (France), Athanasios J. Manolis (Greece), Peter M. Nilsson (Sweden), Eoin O'Brien (Ireland), Piotr Ponikowski (Poland), Josep Redon (Spain), José Rodicio (Spain), Frank Ruschitzka (Switzerland), Juan Tamargo (Spain), Pieter van Zwieten (Netherlands), Margus Viigimaa (Estonia), Bernard Waeber (Switzerland), Bryan Williams (UK), Jose Luis Zamorano (Spain)
1. Introduction and purposes
For several years the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC) decided not to produce their own guidelines on the diagnosis and treatment of hypertension but to endorse the guidelines on hypertension issued by the World Health Organization (WHO) and International Society of Hypertension (ISH) Citation[1,2] with some adaptation to reflect the situation in Europe. However, in 2003 the decision was taken to publish ESH/ESC specific guidelines Citation[3] based on the fact that, because the WHO/ISH Guidelines address countries widely varying in the extent of their health care and availability of economic resource, they contain diagnostic and therapeutic recommendations that may be not totally appropriate for European countries. In Europe care provisions may often allow a more in‐depth diagnostic assessment of cardiovascular risk and organ damage of hypertensive individuals as well as a wider choice of antihypertensive treatment.
The 2003 ESH/ESC Guidelines Citation[3] were well received by the clinical world and have been the most widely quoted paper in the medical literature in the last two years. Citation[4] However, since 2003 considerable additional evidence on important issues related to diagnostic and treatment approaches to hypertension has become available and therefore updating of the previous guidelines has been found advisable.
In preparing the new guidelines the Committee established by the ESH and ESC has agreed to adhere to the principles informing the 2003 Guidelines, namely 1) to try to offer the best available and most balanced recommendation to all health care providers involved in the management of hypertension, 2) to address this aim again by an extensive and critical review of the data accompanied by a series of boxes where specific recommendations are given, as well as by a concise set of practice recommendations to be published soon thereafter as already done in 2003; Citation[5] 3) to primarily consider data from large randomized trials but also to make use, where necessary, of observational studies and other sources of data, provided they were obtained in studies meeting a high scientific standard; 4) to emphasize that guidelines deal with medical conditions in general and therefore their role must be educational and not prescriptive or coercive for the management of individual patients who may differ widely in their personal, medical and cultural characteristics, thus requiring decisions different from the average ones recommended by guidelines; 5) to avoid a rigid classification of recommendations by the level or strength of scientific evidence. Citation[6] The Committee felt that this is often difficult to apply, that it can only apply to therapeutic aspects and that the strength of a recommendation can be judged from the way it is formulated and from reference to relevant studies. Nevertheless, the contribution of randomized trials, observational studies, meta‐analyses and critical reviews or expert opinions has been identified in the text and in the reference list.
The members of the Guidelines Committee established by the ESH and ESC have participated independently in the preparation of this document, drawing on their academic and clinical experience and applying an objective and critical examination of all available literature. Most have undertaken and are undertaking work in collaboration with industry and governmental or private health providers (research studies, teaching conferences, consultation), but all believe such activities have not influenced their judgement. The best guarantee of their independence is in the quality of their past and current scientific work. However, to ensure openness, their relations with industry, government and private health providers are reported in the ESH and ESC websites (www.eshonline.org and www.escardio. org) Expenses for the Writing Committee and preparation of these guidelines were provided entirely by ESH and ESC.
2. Definition and classification of hypertension
Historically more emphasis was placed on diastolic than on systolic blood pressure as a predictor of cardiovascular morbid and fatal events. Citation[7] This was reflected in the early guidelines of the Joint National Committee which did not consider systolic blood pressure and isolated systolic hypertension in the classification of hypertension. Citation[8,9] It was reflected further in the design of early randomized clinical trials which almost invariably based patient recruitment criteria on diastolic blood pressure values. Citation[10] However, a large number of observational studies has demonstrated that cardiovascular morbidity and mortality bear a continuous relationship with both systolic and diastolic blood pressures. Citation[7],Citation[11] The relationship has been reported to be less steep for coronary events than for stroke which has thus been labelled as the most important ‘hypertension related’ complication. Citation[7] However, in several regions of Europe, though not in all of them, the attributable risk, that is the excess of death due to an elevated blood pressure, is greater for coronary events than for stroke because heart disease remains the most common cardiovascular disorder in these regions. Citation[12] Furthermore, both systolic and diastolic blood pressures show a graded independent relationship with heart failure, peripheral artery disease and end stage renal disease. Citation[13–16] Therefore, hypertension should be considered a major risk factor for an array of cardiovascular and related diseases as well as for diseases leading to a marked increase in cardiovascular risk. This, and the wide prevalence of high blood pressure in the population, Citation[17–19] explain why in a WHO report high blood pressure has been listed as the first cause of death worldwide. Citation[20]
2.1 Systolic versus diastolic and pulse pressure
In recent years the simple direct relationship of cardiovascular risk with systolic and diastolic blood pressure has been made more complicated by the findings of observational studies that in elderly individuals the risk is directly proportional to systolic blood pressure and, for any given systolic level, outcome is inversely proportional to diastolic blood pressure, Citation[21–23] with a strong predictive value of pulse pressure (systolic minus diastolic). Citation[24–27] The predictive value of pulse pressure may vary with the clinical characteristics of the subjects. In the largest meta‐analysis of observational data available today (61 studies in almost 1 million subjects without overt cardiovascular disease, of which 70% are from Europe) Citation[11] both systolic and diastolic blood pressures were independently and similarly predictive of stroke and coronary mortality, and the contribution of pulse pressure was small, particularly in individuals aged less than 55 years. By contrast, in middle aged Citation[24,25] and elderly Citation[26,27] hypertensive patients with cardiovascular risk factors or associated clinical conditions, pulse pressure showed a strong predictive value for cardiovascular events. Citation[24–27]
It should be recognized that pulse pressure is a derived measure which combines the imperfection of the original measures. Furthermore, although figures such as 50 or 55 mmHg have been suggested, Citation[28] no practical cutoff values separating pulse pressure normality from abnormality at different ages have been produced. As discussed in section 3.1.7 central pulse pressure, which takes into account the ‘amplification phenomena’ between the peripheral arteries and the aorta, is a more precise assessment and may improve on these limitations.
In practice, classification of hypertension and risk assessment (see sections 2.2 and 2.3) should continue to be based on systolic and diastolic blood pressures. This should be definitely the case for decisions concerning the blood pressure threshold and goal for treatment, as these have been the criteria employed in randomized controlled trials on isolated systolic and systolic‐diastolic hypertension. However, pulse pressure may be used to identify elderly patients with systolic hypertension who are at a particularly high risk. In these patients a high pulse pressure is a marker of a pronounced increase of large artery stiffness and therefore advanced organ damage Citation[28] (see section 3.6).
2.2 Classification of hypertension
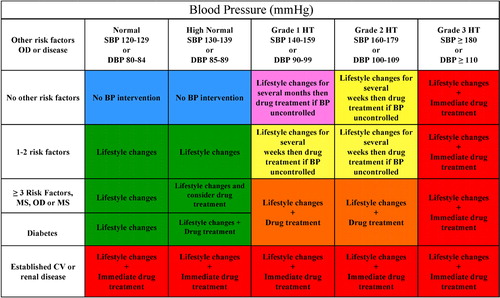
Blood pressure has a unimodal distribution in the population Citation[29] as well as a continuous relationship with cardiovascular risk down to systolic and diastolic levels of 115–110 mmHg and 75–70 mmHg, respectively. Citation[7],Citation[11] This fact makes the word hypertension scientifically questionable and its classification based on cutoff values arbitrary. However, changes of a widely known and accepted terminology may generate confusion while use of cutoff values simplifies diagnostic and treatment approaches in daily practice. Therefore the classification of hypertension used in the 2003 ESH/ESC Guidelines has been retained (Table) with the following provisos:
when a patient's systolic and diastolic blood pressures fall into different categories the higher category should apply for the quantification of total cardiovascular risk, decision about drug treatment and estimation of treatment efficacy;
isolated systolic hypertension should be graded (grades 1, 2 and 3) according to the same systolic blood pressure values indicated for systolic‐diastolic hypertension. However, as mentioned above, the association with a low diastolic blood pressure (e.g. 60–70 mmHg) should be regarded as an additional risk;
the threshold for hypertension (and the need for drug treatment) should be considered as flexible based on the level and profile of total cardiovascular risk. For example, a blood pressure value may be considered as unacceptably high and in need of treatment in high risk states, but still acceptable in low risk patients. Supporting evidence for this statement will be presented in the section on therapeutic approach (Section 5).
Table 1. Definitions and classification of blood pressure (BP) levels (mmHg)
The USA Joint National Committee Guidelines (JNC 7) on hypertension published in 2003 Citation[30] unified the normal and high normal blood pressure categories into a single entity termed ‘prehypertension’. This was based on the evidence from the Framingham study Citation[31,32] that in such individuals the chance of developing hypertension is higher than in those with a blood pressure <120/80 mmHg (termed ‘normal’ blood pressure) at all ages. The ESH/ESC Committee has decided not to use this terminology for the following reasons: 1) even in the Framingham study the risk of developing hypertension was definitely higher in subjects with high normal (130–139/85–89 mmHg) than in those with normal blood pressure (120–129/80–84 mmHg) Citation[32,33] and therefore there is little reason to join the two groups together; 2) given the ominous significance of the word hypertension for the layman, the term ‘prehypertension’ may create anxiety and request for unnecessary medical visits and examinations in many subjects; Citation[34] 3) most importantly, although lifestyle changes recommended by the 2003 JNC 7 Guidelines for all prehypertensive individuals may be a valuable population strategy, Citation[30] in practice this category is a highly differentiated one, with the extremes consisting of subjects in no need of any intervention (e.g. an elderly individual with a blood pressure of 120/80 mmHg) as well as of those with a very high or high risk profile (e.g. after stroke or with diabetes) in whom drug treatment is required.
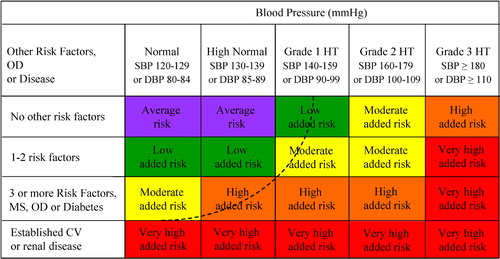
In conclusion, it might be appropriate to use a classification of blood pressure without the term ‘hypertension’. However, this has been retained in Table for practical reasons and with the reservation that the real threshold for hypertension must be considered as flexible, being higher or lower based on the total cardiovascular risk of each individual. This is further illustrated in section 2.3 and in Figure.
Figure 1. Stratification of CV Risk in four categories. SBP: systolic blood pressure; DBP: diastolic blood pressure; CV: cardiovascular; HT: hypertension. Low, moderate, high and very high risk refer to 10 year risk of a CV fatal or non‐fatal event. The term ‘added’ indicates that in all categories risk is greater than average. OD: subclinical organ damage; MS: metabolic syndrome. The dashed line indicates how definition of hypertension may be variable, depending on the level of total CV risk.
2.3 Total cardiovascular risk (Box 1)
2.3.1 Concept
For a long time, hypertension guidelines focused on blood pressure values as the only or main variables determining the need and the type of treatment. Although this approach was maintained in the 2003 JNC 7 Guidelines, Citation[30] the 2003 ESH‐ESC Guidelines Citation[3] emphasized that diagnosis and management of hypertension should be related to quantification of total (or global) cardiovascular risk. This concept is based on the fact that only a small fraction of the hypertensive population has an elevation of blood pressure alone, with the great majority exhibiting additional cardiovascular risk factors, Citation[35–39] with a relationship between the severity of the blood pressure elevation and that of alterations in glucose and lipid metabolism. Citation[40] Furthermore, when concomitantly present, blood pressure and metabolic risk factors potentiate each other, leading to a total cardiovascular risk which is greater than the sum of its individual components. Citation[35],Citation[41,42] Finally, evidence is available that in high risk individuals thresholds and goals for antihypertensive treatment, as well as other treatment strategies, should be different from those to be implemented in lower risk individuals. Citation[3] In order to maximize cost‐efficacy of the management of hypertension the intensity of the therapeutic approach should be graded as a function of total cardiovascular risk. Citation[43,44]
Box 1 Position statement: Total cardiovascular risk
Dysmetabolic risk factors and subclinical organ damage are common in hypertensive patients.
All patients should be classified not only in relation to the grades of hypertension but also in terms of the total cardiovascular risk resulting from the coexistence of different risk factors, organ damage and disease.
Decisions on treatment strategies (initiation of drug treatment, BP threshold and target for treatment, use of combination treatment, need of a statin and other non‐antihypertensive drugs) all importantly depend on the initial level of risk.
There are several methods by which total cardiovascular risk can be assessed, all with advantages and limitations. Categorization of total risk as low, moderate, high, and very high added risk has the merit of simplicity and can therefore be recommended. The term ‘added risk’ refers to the risk additional to the average one.
Total risk is usually expressed as the absolute risk of having a cardiovascular event within 10 years. Because of its heavy dependence on age, in young patients absolute total cardiovascular risk can be low even in the presence of high BP with additional risk factors. If insufficiently treated, however, this condition may lead to a partly irreversible high risk condition years later. In younger subjects treatment decisions should better be guided by quantification of relative risk, i.e. the increase in risk in relation to average risk in the population.
2.3.2 Assessment
Estimation of total cardiovascular risk is simple in particular subgroups of patients such as those with 1) a previous diagnosis of cardiovascular disease, 2) type 2 diabetes, 3) type 1 diabetes, and 4) individuals with severely elevated single risk factors. In all these conditions the total cardiovascular risk is high, calling for the intense cardiovascular risk reducing measures that will be outlined in the following sections. However, a large number of hypertensive patients does not belong to one of the above categories and identification of those at high risk requires the use of models to estimate total cardiovascular risk so as to be able to adjust the intensity of the therapeutic approach accordingly.
Several computerized methods have been developed for estimating total cardiovascular risk, i.e. the absolute chance of having a cardiovascular event usually over 10 years. However, some of them are based on Framingham data Citation[45] which are only applicable to some European populations due to important differences in the incidence of coronary and stroke events. Citation[12] More recently, a European model has become available based on the large data‐base provided by the SCORE project. Citation[46] SCORE charts are available for high and low risk countries in Europe. They estimate the risk of dying from cardiovascular (not just coronary) disease over 10 years and allow calibration of the charts for individual countries provided that national mortality statistics and estimates of the prevalence of major cardiovascular risk factors are known. The SCORE model has also been used in the HeartScore, the official ESC management tool for implementation of cardiovascular disease prevention in clinical practice. This is available on the ESC Web Site (www.escardio.org).
The 2003 ESH/ESC Guidelines Citation[3] classified the total cardiovascular risk based on the scheme proposed by the 1999 WHO/ISH Guidelines on hypertension Citation[2] with the extension to subjects with ‘normal’ or ‘high normal’ blood pressure. This classification is retained in the present Guidelines (Figure). The terms ‘low’, ‘moderate’, ‘high’ and ‘very high’ risk are used to indicate an approximate risk of cardiovascular morbidity and mortality in the coming 10 years, which is somewhat analogous to the increasing level of total cardiovascular risk estimated by the Framingham Citation[45] or the SCORE Citation[46] models. The term ‘added’ is used to emphasize that in all categories relative risk is greater than average risk. Although use of a categorical classification provides data that are in principle less precise than those obtained from equations based on continuous variables, this approach has the merit of simplicity. The 2003 WHO/ISH Guidelines Citation[47] have further simplified the approach by merging the high and very high risk categories which were regarded as similar when it came to making treatment decisions. The distinction between high and very high risk categories has been maintained in the present guidelines, thereby preserving a separate place for secondary prevention, i.e. prevention in patients with established cardiovascular disease. In these patients, compared with the high risk category, not only can total risk be much higher, but multidrug treatment may be necessary throughout the blood pressure range from normal to high. The dashed line drawn in Figure illustrates how total cardiovascular risk evaluation influences the definition of hypertension when this is correctly considered as the blood pressure value above which treatment does more good than harm. Citation[48]
Table indicates the most common clinical variables that should be used to stratify the risk. They are based on risk factors (demographics, anthropometrics, family history of premature cardiovascular disease, blood pressure, smoking habits, glucose and lipid variables), measures of target organ damage, and diagnosis of diabetes and associated clinical conditions as outlined in the 2003 Guidelines. Citation[3] The following new points should be highlighted:
Table 2. Factors influencing prognosis
The metabolic syndrome Citation[49] has been mentioned because it represents a cluster of risk factors often associated with high blood pressure which markedly increases cardiovascular risk. No implication is made that it represents a pathogenetic entity.
Further emphasis has been given to identification of target organ damage, since hypertension‐related subclinical alterations in several organs indicate progression in the cardiovascular disease continuum Citation[50] which markedly increases the risk beyond that caused by the simple presence of risk factors. A separate Section (3.6) is devoted to searching for subclinical organ damage where evidence for the additional risk of each subclinical alteration is discussed and the proposed cutoff values are justified.
The list of renal markers of organ damage has been expanded, to include estimates of creatinine clearance by the Cockroft‐Gault formula Citation[51] or of glomerular filtration rate by the MDRD formula, Citation[52] because of the evidence that these estimated values are a more precise index of the cardiovascular risk accompanying renal dysfunction.
Microalbuminuria has now been considered as an essential component in the assessment of organ damage because its detection is easy and relatively inexpensive.
Concentric left ventricular hypertrophy has been identified as the cardiac structural parameter that more markedly increases cardiovascular risk.
Whenever possible the recommendation is made to measure organ damage in different tissues (e.g. heart, blood vessels, kidney and brain) because multiorgan damage is associated with a worse prognosis. Citation[53]
Increased pulse wave velocity is added to the list of factors influencing prognosis as an early index of large artery stiffening, Citation[54,55] although with the caveat that it has a limited availability in the clinical practice.
A low ankle to brachial blood pressure ratio (<0.9) is listed as a relatively easy to obtain marker of atherosclerotic disease and increased total cardiovascular risk. Citation[56]
Not only is assessment of organ damage recommended pre‐treatment (in order to stratify risk) but also during therapy because of the evidence that regression of left ventricular hypertrophy and reduction of proteinuria indicate treatment‐induced cardiovascular protection. Citation[57–61]
There may be reasons to include an elevated heart rate as a risk factor because of a growing body of evidence that elevated heart rate values relate to the risk of cardiovascular morbidity and mortality as well as to all cause mortality. Citation[62–65] Also, there is evidence that an elevated heart rate increases the risk of new onset hypertension Citation[66,67] and is frequently associated with metabolic disturbances and the metabolic syndrome. Citation[67–69] However, because of the wide range of accepted resting heart rate normality values (60 to 90 beats/min), no cutoff heart rate can be offered presently to increase the accuracy of total cardiovascular risk stratification.
The major diagnostic elements for classifying subjects in the high or very high risk categories are summarized in Table. It is worth noticing that multiple risk factors, diabetes or organ damage invariably place a subject with hypertension, and even with high normal blood pressure, in the high risk category.
Table 3. High/Very high risk subjects
2.3.3 Limitations
All currently available models for cardiovascular risk assessment have limitations which must be appreciated. Total cardiovascular risk models do not consider the duration of exposure to a risk factor or disease and their quantification is usually based on some risk factors only, while paying limited attention to other variables linked to cardiovascular outcome (e.g. physical activity and stress). Citation[70] Furthermore, the significance of target organ damage in determining calculation of overall risk is dependent on how carefully the damage is assessed, based on available facilities. Also, there are several additional markers of target organ damage that have not been listed in Table because of a difficulty in measurement, less well established prognostic importance or practical problems (low availability, high dependence on operator's skill, lack of standardization, time requirement, invasiveness, cost, etc.). However, because these measurements are currently the object of extensive research, which may make them more useful in the near future they have been discussed in section 3.6 and listed in Table together with an assessment of their clinical value and limitations. The issue is further discussed in Section 3.6.
Table 4. Availability, prognostic value and cost of some markers of organ damage (scored from 0 to 4 pluses)
Conceptual limitations should also be mentioned. One should never forget that the rationale of estimating total cardiovascular risk is to govern the best use of limited resources to prevent cardiovascular disease, that is to grade preventive measures in relation to the increased risk. Yet, stratification of absolute risk is often used by private or public healthcare providers to establish a barrier below which treatment is discouraged. The threshold of 20% risk of cardiovascular disease in 10 years is arbitrary and simplistic, and use of a cutoff value leading to intense interventions above this threshold and no action at all below cannot be supported. One should be aware of the strong effect of age on total cardiovascular risk models. It is so strong that younger adults (particularly women) are unlikely to reach high risk levels even when they have more than one major risk factor and a clear increase in relative risk (i.e. the existing risk compared to their peers). By contrast, most elderly men (e.g. >70 years) will often reach a high total risk level whilst being at very little increased risk relative to their peers. The consequences are that most resources are concentrated on older subjects, whose potential lifespan is relatively short despite intervention, and little attention is given to young subjects at high relative risk despite the fact that, in the absence of intervention, their long term exposure to an increased risk may lead to a high and partly irreversible risk situation in middle age, with potential shortening of their otherwise longer life expectancy. As already suggested in the 2003 ESH‐ESC Guidelines, Citation[3] these shortcomings may be avoided by using the relative risk as a guide to the need and the intensity of therapeutic interventions in young subjects. This is possible with the HeartScore management tool (www.escardio. org), with the update provided by the guidelines on cardiovascular disease prevention in clinical practice issued by the Fourth Joint European Task Force. Citation[71] It is important to remember that in young individuals who are at low absolute risk just because of their age but who carry important risk factors, non‐pharmacological and, if necessary, pharmacological interventions should be implemented to improve their risk profile and prevent the development of a high risk condition later in life. In the absence of treatment, this can occur even earlier than indicated in risk charts because risk factors tend to become more pronounced with ageing and a life time blood pressure elevation is frequently accompanied by development of organ damage.
3. Diagnostic evaluation
Diagnostic procedures aim at: 1) establishing blood pressure levels; 2) identifying secondary causes of hypertension; 3) evaluating the overall cardiovascular risk by searching for other risk factors, target organ damage and concomitant diseases or accompanying clinical conditions.
The diagnostic procedures comprise:
repeated blood pressure measurements
medical history
physical examination
laboratory and instrumental investigations. Some of these should be considered part of the routine approach in all subjects with high blood pressure; some are recommended and may be used extensively in the developed health systems of Europe; some are indicated only when suggested by the basic examination or the clinical course of the patient.
3.1 Blood pressure measurement
Blood pressure is characterized by large spontaneous variations both during the day and between days, months and seasons. Citation[72–74] Therefore the diagnosis of hypertension should be based on multiple blood pressure measurements, taken on separate occasions over a period of time. If blood pressure is only slightly elevated, repeated measurements should be obtained over a period of several months to define the patients ‘usual’ blood pressure as accurately as possible. On the other hand, if the patient has a more marked blood pressure elevation, evidence of hypertension‐related organ damage or a high or very high cardiovascular risk profile, repeated measurements should be obtained over shorter periods of time (weeks or days). In general, the diagnosis of hypertension should be based on at least 2 blood pressure measurements per visit and at least 2 to 3 visits, although in particularly severe cases the diagnosis can be based on measurements taken at a single visit. Blood pressures can be measured by the doctor or the nurse in the office or in the clinic (office or clinic blood pressure), by the patient or a relative at home, or automatically over 24 h. Based on specific recommendations of the European Society of Hypertension, Citation[75] these procedures can be summarized as follows:
3.1.1 Office or clinic blood pressure
Blood pressure can be measured by a mercury sphygmomanometer the various parts of which (rubber tubes, valves, quantity of mercury, etc.) should be kept in proper working order. Other non‐invasive devices (auscultatory or oscillometric semiautomatic devices) can also be used and will indeed become increasingly important because of the progressive banning of the medical use of mercury. However, these devices should be validated according to standardized protocols (Citation[76] and website: www.dableducational.org), and their accuracy should be checked periodically by comparison with mercury sphygmomanometric values. Instructions for correct office blood pressure measurements are summarized in Box 2.
Box 2 Blood pressure (BP) measurement
When measuring BP, care should be taken to:
Allow the patients to sit for several minutes in a quiet room before beginning BP measurements
Take at least two measurements spaced by 1–2 minutes, and additional measurements if the first two are quite different
Use a standard bladder (12–13 cm long and 35 cm wide) but have a larger and a smaller bladder available for fat and thin arms, respectively. Use the smaller bladder in children
Have the cuff at the heart level, whatever the position of the patient
Use phase I and V (disappearance) Korotkoff sounds to identify systolic and diastolic BP, respectively
Measure BP in both arms at first visit to detect possible differences due to peripheral vascular disease. In this instance, take the higher value as the reference one
Measure BP 1 and 5 min after assumption of the standing position in elderly subjects, diabetic patients, and in other conditions in which postural hypotension may be frequent or suspected
Measure heart rate by pulse palpation (at least 30 sec) after the second measurement in the sitting position
3.1.2 Ambulatory blood pressure (Box 3)
Several devices (mostly oscillometric) are available for automatic blood pressure measurements in patients allowed to conduct a near normal life. They provide information on 24‐hour average blood pressure as well as on mean values over more restricted periods such as the day, night or morning. This information should not be regarded as a substitute for information derived from conventional blood pressure measurements. However, it may be considered of important additional clinical value because cross‐sectional and longitudinal studies have shown that office blood pressure has a limited relationship with 24‐h blood pressure and thus with that occurring in daily life. Citation[77–79] These studies have also shown that ambulatory blood pressure 1) correlates with hypertension‐related organ damage and its changes by treatment more closely than does office blood pressure, Citation[80–85] 2) has a relationship with cardiovascular events that is steeper than that observed for clinic blood pressure, with a prediction of cardiovascular risk greater than, and additional to the prediction provided by office blood pressure values in populations as well as in untreated and treated hypertensives, Citation[86–96] and 3) measures more accurately than clinic blood pressure the extent of blood pressure reduction induced by treatment, because of a higher reproducibility over time Citation[97,98] and an absent or negligible ‘white coat’ Citation[99] and placebo effect. Citation[100,101] Although some of the above advantages can be obtained by increasing the number of office blood pressure measurements, Citation[82],Citation[98] 24‐hour ambulatory blood pressure monitoring may be useful at the time of diagnosis and at varying intervals during treatment. Effort should be made to extend ambulatory blood pressure monitoring to 24 hours in order to obtain information on both daytime and nighttime blood pressure profiles, day‐night blood pressure difference, morning blood pressure rise and blood pressure variability. Daytime and nighttime blood pressure values and changes by treatment are related to each other, Citation[78,79] but the prognostic value of nighttime blood pressure has been found to be superior to that of daytime blood pressure. Citation[87],Citation[89–92],Citation[94] In addition, subjects in whom nocturnal decrease in blood pressure is blunted (non‐dippers) Citation[102] have been reported to have a greater prevalence of organ damage and a less favourable outcome, although in some studies the prognostic value of this phenomenon was lost when multi‐variate analysis included 24‐h average blood pressure. Citation[87,88],Citation[90],Citation[92,93],Citation[103–106] Evidence is also available that cardiac and cerebrovascular events have a peak prevalence in the morning, Citation[107–110] possibly in relation to the sharp blood pressure rise occurring at awaking from sleep, Citation[72],Citation[111–113] as well as to an increased platelet aggregability, a reduced fibrinolytic activity and a sympathetic activation. Citation[114–118] Worsening of organ damage and the incidence of events have also been related to blood pressure variability as quantified by the standard deviation around mean values. Citation[119–121] Although in these studies the role of confounding factors was not always excluded, an independent role of blood pressure variability has recently been confirmed by a long‐term observational study. Citation[122]
Box 3 Position statement: Ambulatory and home BP measurements
Ambulatory BP
Although office BP should be used as reference, ambulatory BP may improve prediction of cardiovascular risk in untreated and treated patients
Normal values are different for office and ambulatory BP (Table)
24‐h ambulatory BP monitoring should be considered, in particular, when
considerable variability of office BP is found over the same or different visits
high office BP is measured in subjects otherwise at low total cardiovascular risk
there is a marked discrepancy between BP values measured in the office and at home
resistance to drug treatment is suspected
hypotensive episodes are suspected, particu larly in elderly and diabetic patients
office BP is elevated in pregnant women and preeclampsia is suspected
Home BP
Self‐measurement of BP at home is of clinical value and its prognostic significance is now demonstrated. These measurements should be encouraged in order to:
provide more information on the BP lowering effect of treatment at trough, and thus on therapeutic coverage throughout the dose‐to‐dose time interval
improve patient's adherence to treatment regimens
there are doubts on technical reliability/environmental conditions of ambulatory BP data
Self‐measurement of BP at home should be discouraged whenever:
it causes anxiety to the patient
it induces self‐modification of the treatment regimen
Normal values are different for office and home BP (Table)
When measuring 24‐hour blood pressure Citation[75] care should be taken to:
Use only devices validated by international standardized protocols.
Use cuffs of appropriate size and compare the initial values with those from a sphygmomanometer to check that the differences are not greater than ±5 mmHg.
Set the automatic readings at no more than 30 min intervals to obtain an adequate number of values and have most hours represented if some readings are rejected because of artefact.
Automatic deflation of the equipment should be at a rate of no more than 2 mmHg/s.
Instruct the patients to engage in normal activities but to refrain from strenuous exercise, and to keep the arm extended and still at the time of cuff inflations.
Ask the patient to provide information in a diary on unusual events and on duration and quality of night sleep.
Obtain another ambulatory blood pressure if the first examination has less than 70% of the expected number of valid values because of frequent artefacts. Ensure that the proportion of valid values is similar for the day and night periods.
Remember that ambulatory blood pressure is usually several mmHg lower than office blood pressure. Citation[123–125] As shown in Table, different population studies indicate that office values of 140/90 mmHg correspond to average 24‐h values of either 125–130 mmHg systolic and 80 mmHg diastolic, the corresponding average daytime and nighttime values being 130–135/85 and 120/70 mmHg. These values may be regarded as approximate threshold values for diagnosing hypertension by ambulatory blood pressure.
Clinical judgement should be mainly based on average 24‐hour, day and/or night values. Other information derived from ambulatory blood pressure (e.g. morning blood pressure surge and blood pressure standard deviations) is clinically promising, but the field should still be regarded as in the research phase.
Table 5. Blood pressure thresholds (mmHg) for definition of hypertension with different types of measurement
3.1.3 Home blood pressure (Box 3)
Self‐measurement of blood pressure at home cannot provide the extensive information on daily life blood pressure values provided by ambulatory blood pressure monitoring. However, it can provide values on different days in a setting close to daily life. When averaged over a period of a few days these values share some of the advantages of ambulatory blood pressure, that is they are free of a significant white coat effect, are more reproducible and predict the presence and progression of organ damage as well as the risk of cardiovascular events better than office values. Citation[81],Citation[89,90],Citation[92],Citation[126,127] Therefore, home blood pressure measurements for suitable periods can be recommended before and during treatment also because this relatively cheap procedure may improve patient adherence to treatment. Citation[128]
When advising self‐measurement of blood pressure at home: Citation[75]
Suggest the use of validated devices. Few of the presently available wrist devices for measurement of blood pressure have been validated satisfactorily; Citation[76] should any of these wrist devices be used, the subject should be recommended to keep the arm at heart level during the measurement.
Prefer semiautomatic devices rather than a mercury sphygmomanometer to avoid the difficulty posed by having to educate the patient on its use and the error derived from hearing problems in elderly individuals.
Instruct the patient to make measurements in the sitting position after several minutes rest, preferably in the morning and in the evening. Inform him or her that values may differ between measurements because of spontaneous blood pressure variability.
Avoid requesting that an excessive number of values are measured and ensure that those measurements include the period prior to drug intake so as to have information on the duration of treatment effects.
Remember that, as for ambulatory blood pressure, normal values are lower for home than for office blood pressure. Take 130–135/85 mmHg as the values that approximately correspond to 140/90 mmHg measured in the office or clinic (Table).
Give the patient clear instructions on the need to provide the doctor with proper documentation of the measured values and to avoid self‐alterations of the treatment regimens.
3.1.4 Isolated office or white coat hypertension
In some patients office blood pressure is persistently elevated while daytime or 24‐hour blood pressure, or home blood pressure, are within their normal range. This condition is widely known as ‘white coat hypertension’, Citation[129] although the more descriptive and less mechanistic term ‘isolated office (or clinic) hypertension’ is preferable because the office ambulatory blood pressure difference does not correlate with the office blood pressure elevation induced by the alerting response to a doctor or a nurse, Citation[130] that is the true ‘white coat effect’. Citation[131,132] Regardless of the terminology, evidence is now available that isolated office hypertension may be present in about 15% of the general population and that it may account for a noticeable fraction (one third or more) of individuals in whom hypertension is diagnosed. Citation[106],Citation[133,134] There is evidence that in individuals with isolated office hypertension cardiovascular risk is less than in individuals with both raised office and ambulatory blood pressure. Citation[90],Citation[92],Citation[106],Citation[133–138] However, several, although not all studies, have reported this condition to be associated with a prevalence of organ damage and metabolic abnormalities greater than that of normotensive subjects, which suggests that it may not be a clinically innocent phenomenon. Citation[133] Evidence of its adverse prognostic relevance is less consistent in outcome studies when data are properly adjusted for age and gender Citation[92],Citation[106],Citation[133],Citation[138] but there is one report of its association with a rate of cardiovascular events that is intermediate between that of subjects in whom normal blood pressure and hypertension are found both in and out of office. Citation[133]
It is difficult to predict which patients found to be hypertensive in the office will have isolated office hypertension, but this condition is more common when there is a grade 1 (mild) hypertension in females, at older ages, in non‐smokers, in hypertension of recent onset and when there is a limited number of office blood pressure measurements. Citation[75] Isolated office hypertension should be diagnosed whenever office blood pressure is ⩾140/90 mmHg on at least 3 occasions, while 24‐hour mean and daytime blood pressures are within their normal range. Its diagnosis can also be based on home blood pressure values (when the average of several home readings is <135/85 mmHg and office values ⩾140/90 mmHg), bearing in mind that subjects with isolated office hypertension diagnosed by ambulatory blood pressure monitoring may not be entirely the same group identified by home blood pressure measurements. Citation[133],Citation[139] Some individuals may have a high home and a normal ambulatory blood pressure and vice versa. Identification of isolated office hypertension should be followed by a search for metabolic risk factors and organ damage. Drug treatment should be instituted when there is evidence of organ damage or a high cardiovascular risk profile. However, lifestyle changes and a close follow‐up are recommended in all patients with isolated office hypertension even when it is decided not to start pharmacological treatment.
3.1.5 Isolated ambulatory or masked hypertension
The reverse phenomenon of ‘white coat hypertension’ has also been described: individuals with normal office blood pressure (<140/90 mmHg) may have elevated ambulatory or home blood pressure values, a condition termed ‘isolated ambulatory hypertension’ or ‘masked hypertension’. Citation[92],Citation[95],Citation[106],Citation[132–134],Citation[137],Citation[139–41] The prevalence in the population is about the same as that of isolated office hypertension Citation[106],Citation[133,134],Citation[141] and it has been calculated that about 1 in 7 or 8 subjects with a normal office blood pressure may fall into this category. Citation[133] Although limited information exists on the persistence of this condition over time, Citation[142] such individuals have been shown to have greater than normal prevalence of organ damage, Citation[139] with an increased prevalence of metabolic risk factors Citation[133] compared with subjects with a truly normal blood pressure. Outcome studies have suggested that masked hypertension increases cardiovascular risk, which appears to be close to that of in‐ and out‐of‐office hypertension. Citation[92], Citation[106],Citation[133,134],Citation[137],Citation[141]
In conclusion, studies made in the last few years have provided a growing body of evidence on the clinical importance of out‐of‐office blood pressure measurements as these characterize more precisely the severity of hypertension and identify a higher risk profile in some apparently normotensive individuals. In a recent long‐term observational study the 12‐year risk of death progressively increased from the condition of being normotensive on office, home, and 24‐hour definitions to the condition of being found hypertensive by one, two and all three blood pressure measurement modalities. Citation[133] Ambulatory and home blood pressures may provide useful information even when there is no apparent elevation in clinic blood pressure, particularly in subjects in whom multiple risk factors and organ damage are present.
3.1.6 Blood pressure during exercise and laboratory stress
Both physical and mental stressors have been applied in the laboratory to assess the blood pressure response to challenging stimuli and its potential clinical utility. Physical stress involves active physical activity (dynamic or static exercise) or passive physical stress, such as the cold pressor test. Mental stress is evoked via a problem of mathematical, technical or decisional nature. Citation[143]
All stressors increase blood pressure and the variable individual blood pressure response has been evaluated with regard to the prediction of new onset hypertension, target organ damage and incident cardiovascular disease or death.
Data on the prediction of future hypertension are conflicting. Citation[144] Some studies reported a significant and independent risk for incident hypertension in subjects who showed disproportionate exercise blood pressure responses, Citation[145] and in male civil servants blood pressure reactions to mental stress predicted future blood pressure values and hypertension at a 10 year follow‐up. Citation[146] However, only a small fraction of the variance of future blood pressure values was explained by the different response to mental stress, and other studies Citation[147] have led to negative results.
As to organ damage, most studies on normotensive and hypertensive subjects did not observe a significant relationship between the pressor effect of dynamic exercise and left ventricular hypertrophy after proper adjustment for resting blood pressure, Citation[148–154] but in a recent report the change of systolic blood pressure from rest to submaximal exercise was found to be a strong predictor of left ventricular hypertrophy in prehypertensive individuals. Citation[155] The significance of blood pressure reactivity to static exercise has been rarely addressed but no significant association between the blood pressure response to handgrip and left ventricular mass has been reported in one study, Citation[156] while the blood pressure increase induced by the cold pressor test predicted left ventricular mass Citation[153] in one but not another report. Citation[157] The blood pressure effect of an arithmetic task was significantly related to left ventricular concentric remodelling but not to left ventricular mass in one study, Citation[158] while other studies failed to find positive associations between left ventricular structure and this type of blood pressure reactivity. Citation[153],Citation[157]
There is conflicting evidence as to whether an exaggerated blood pressure response to bicycle exercise can predict cardiovascular morbidity and mortality independent of resting values, Citation[149],Citation[159] although the results of a 21‐year follow up have recently shown that both supine and 6‐min exercise systolic blood pressures provide predictive information on cardiovascular death, particularly in subjects with mild blood pressure elevation. Citation[160] However, the matter may be different in more severe hypertension. Whether an excessive blood pressure rise during exercise adds prognostic information to blood pressure at rest may depend on the effect of exercise on cardiac output. If the exercise‐induced rise in cardiac output is impaired, as it can be seen in severe hypertension, exercise blood pressure can no longer carry an independent prognostic significance. There is some evidence that an impaired reduction of systemic vascular resistance during exercise carries a worse prognosis. Citation[159],Citation[161]
In conclusion, the results on the independent relationships of the blood pressure response to physical and mental stressors, future hypertension and target organ damage are not consistent and, if significant, the additional explained variance is small. As to the prediction of cardiovascular events, the 21‐year follow‐up study mentioned above Citation[160] suggests that an exercise test may provide some additional prognostic information at least in subjects with mild blood pressure elevation, because in the absence of other risk factors or organ damage a decision on the need for therapeutic intervention may be difficult. Finally, it should not be forgotten that non‐invasive blood pressure measurements during exercise are limited to systolic values, and that their accuracy is much less than that of resting values.
3.1.7 Central blood pressure
Due to the variable superimposition of incoming and reflected pressure waves along the arterial tree, aortic systolic and pulse pressure (i.e. the pressure exerted at the level of the heart, brain and kidney) may be different from the conventionally measured brachial pressure. Citation[162] Furthermore, the claim has long been made that peripheral and central systolic and pulse pressures may be differently affected by antihypertensive drugs. Citation[163] The need for invasive measurement of central blood pressure has confined this issue to research. However, recently a method has been described to non‐invasively estimate aortic blood pressure by calculating the ‘augmentation index’ from the pulse wave pressure contour recorded from a peripheral artery. Citation[164,165] Use of this method has confirmed that the effects of antihypertensive drugs on central systolic and pulse pressure do not invariably reflect those seen at the brachial artery level. Citation[166,167] Furthermore, the results obtained in a large substudy performed within a randomized trial have shown that central pulse pressure as assessed from the ‘augmentation index’ is significantly related to cardiovascular events. Citation[166] However, the prognostic role of central as opposed to peripheral blood pressure needs to be further confirmed in more large‐scale observational and interventional studies.
3.2 Family and clinical history (Box 4)
A comprehensive family history should be obtained with particular attention to hypertension, diabetes, dyslipidaemia, premature coronary heart disease, stroke, peripheral artery or renal disease.
The clinical history should include: a) duration and previous levels of high blood pressure; b) symptoms suggestive of secondary causes of hypertension and intake of drugs or substances that can raise blood pressure, such as liquorice, nasal drops, cocaine, amphetamines, oral contraceptives, steroids, non‐steroidal anti‐inflammatory drugs, erythropoietin, and cyclosporin; c) lifestyle factors, such as dietary intake of fat (animal fat in particular), salt and alcohol, quantification of smoking and physical activity, weight gain since early adult life; d) past history or current symptoms of coronary disease, heart failure, cerebrovascular or peripheral vascular disease, renal disease, diabetes mellitus, gout, dyslipidaemia, asthma or any other significant illnesses, and drugs used to treat those conditions; e) previous antihypertensive therapy, its results and adverse effects; and f) personal, family and environmental factors that may influence blood pressure, cardiovascular risk, as well as the course and outcome of therapy. Also, physicians should enquire of the patient and/or partner about snoring which may be a sign of sleep apnoea syndrome and increased cardiovascular risk.
Box 4 Guidelines for family and clinical history
Duration and previous level of high BP
Indications of secondary hypertension:
family history of renal disease (polycystic kidney)
renal disease, urinary tract infection, haematuria, analgesic abuse (parenchymal renal disease)
drug/substance intake: oral contraceptives, liquorice, carbenoxolone, nasal drops, cocaine, amphetamines, steroids, non‐steroidal anti‐inflammatory drugs, erythropoietin, cyclosporin
episodes of sweating, headache, anxiety, palpitation (phaeochromocytoma)
episodes of muscle weakness and tetany (aldosteronism)
Risk factors:
family and personal history of hypertension and cardiovascular disease
family and personal history of dyslipidaemia
family and personal history of diabetes mellitus
smoking habits
dietary habits
obesity; amount of physical exercise
snoring; sleep apnoea (information also from partner)
personality
Symptoms of organ damage
brain and eyes: headache, vertigo, impaired vision, transient ischaemic attacks, sensory or motor deficit
heart: palpitation, chest pain, shortness of breath, swollen ankles
kidney: thirst, polyuria, nocturia, haematuria
peripheral arteries: cold extremities, intermittent claudication
Previous antihypertensive therapy:
Drug(s) used, efficacy and adverse effects
Personal, family and environmental factors
3.3 Physical examination (Box 5)
In addition to blood pressure heart rate should be carefully measured (pulse counting over at least 30 s or longer if arrhythmias are reported) because the repeated finding of values above normal may be an indication of greater risk, increased sympathetic or decreased parasympathetic activity, Citation[62–65] or of heart failure. Physical examination should search for evidence of additional risk factors, for signs suggesting secondary hypertension, and for evidence of organ damage. Waist circumference should be measured with the patient standing and body weight and height should be obtained to calculate body mass index by a standard formula.
Box 5 Physical examination for secondary hypertension, organ damage and visceral obesity
Signs suggesting secondary hypertension and organ damage
Features of Cushing syndrome
Skin stigmata of neurofibromatosis (phaeochromocytoma)
Palpation of enlarged kidneys (polycystic kidney)
Auscultation of abdominal murmurs (renovascular hypertension)
Auscultation of precordial or chest murmurs (aortic coarctation or aortic disease)
Diminished and delayed femoral pulses and reduced femoral BP (aortic coarctation, aortic disease)
Signs of organ damage
Brain: murmurs over neck arteries, motor or sensory defects
Retina: fundoscopic abnormalities
Heart: location and characteristics of apical impulse, abnormal cardiac rhythms, ventricular gallop, pulmonary rales, peripheral oedema
Peripheral arteries: absence, reduction, or asymmetry of pulses, cold extremities, ischaemic skin lesions
Carotid arteries: systolic murmurs
Evidence of visceral obesity
Body weight
Increased waist circumference (standing position) M: >102 cm; F: >88 cm
Increased body mass index [body weight (kg)/height (m)2]
Overweight ⩾25 kg/m2; Obesity ⩾30 kg/m2
3.4 Laboratory investigations (Box 6)
Laboratory investigations are directed at providing evidence for additional risk factors, searching for secondary hypertension and looking for the absence or presence of organ damage. Investigations should progress from the most simple to the more complicated. The younger the patient, the higher the blood pressure and the faster the development of hypertension, the more detailed the diagnostic work‐up should be. However, the minimum laboratory investigations needed remain a matter of debate.
Box 6 Laboratory investigations
Routine tests
Fasting plasma glucose
Serum total cholesterol
Serum LDL‐cholesterol
Serum HDL‐cholesterol
Fasting serum triglycerides
Serum potassium
Serum uric acid
Serum creatinine
Estimated creatinine clearance (Cockroft‐Gault formula) or glomerular filtration rate (MDRD formula)
Haemoglobin and haematocrit
Urinalysis (complemented by microalbuminuria via dipstick test and microscopic examination)
Electrocardiogram
Recommended tests
Echocardiogram
Carotid ultrasound
Quantitative proteinuria (if dipstick test positive)
Ankle‐brachial BP Index
Fundoscopy
Glucose tolerance test (if fasting plasma glucose >5.6 mmol/L (100 mg/dL)
Home and 24 h ambulatory BP monitoring
Pulse wave velocity measurement (where available)
Extended evaluation (domain of the specialist)
Further search for cerebral, cardiac, renal and vascular damage. Mandatory in complicated hypertension
Search for secondary hypertension when suggested by history, physical examination or routine tests: measurement of renin, aldosterone, corticosteroids, catecholamines in plasma and/or urine; arteriographies; renal and adrenal ultrasound; computer‐assisted tomography; magnetic resonance imaging
In the rather uniform European context, where cardiovascular diseases are the primary cause of morbidity and mortality, routine laboratory investigations should include: blood chemistry for fasting glucose, total cholesterol, LDL‐cholesterol, HDL‐cholesterol, triglycerides (fasting), urate, creatinine, potassium, haemoglobin and haematocrit; urinalysis by a dipstick test that permits the detection of microalbuminuria; urine microscopic examination and an electrocardiogram. Serum creatinine is an imprecise measure of renal function. Nevertheless, even a small elevation may indicate substantial renal damage and an increased risk of cardiovascular disease. Serum creatinine values should also be used to estimate creatinine clearance via the Cockroft Gault formula or glomerular filtration rate by the abbreviated MDRD formula, Citation[51,52] easy procedures allowing identification of patients with reduced glomerular filtration and increased cardiovascular risk but in whom serum creatinine values are still in the normal range (see also section 3.6.3). When fasting plasma glucose is ⩾5.6 mmol/L (100 mg/dL), a post‐load plasma glucose (glucose tolerance test) is recommended. Citation[168] The repeated finding of a fasting plasma glucose ⩾7.0 mmol/L (126 mg/dL), and an abnormal glucose tolerance test are considered indicative of diabetes mellitus. Citation[168] Although high sensitivity C reactive protein (hsCRP) has been reported to predict the incidence of cardiovascular events in several clinical settings, Citation[169] its added value in determining total cardiovascular risk is uncertain, Citation[170] except in patients with metabolic syndrome in whom hsCRP values have been reported to be associated with a further marked increase in risk. Citation[171,172] The value of other inflammatory markers (fibrinogen, cytokines, homeocysteine and brain natriuretic peptide levels etc.) Citation[173] for cardiovascular risk stratification is the object of active research, but at present their measurement for clinical guidance in hypertension cannot be recommended.
3.5 Genetic analysis
There is often a family history of high blood pressure in hypertensive patients, suggesting that inheritance contributes to the pathogenesis of this disorder. Essential hypertension is a highly heterogeneous disorder, which points to a multi‐factorial aetiology and polygenic abnormalities. Citation[174,175] Variants in some genes might render an individual sensitive to a given factor in the environment. A number of mutations in genes encoding for major blood pressure controlling systems has been recognized in humans, but their exact role in the pathogenesis of essential hypertension is still unclear. However, the patient's genetic predisposition might influence drug‐metabolizing enzymes and this in turn might affect both efficacy and adverse effects of antihypertensive agents. There are emerging examples of pharmacogenetic and pharmacogenomic studies that tackle these issues as summarized recently. Citation[176] Moreover, several rare monogenic forms of hypertension have been described such as glucocorticoid‐remediable aldosteronism, Liddle's syndrome and others where single gene mutation fully explains the pathogenesis of hypertension and dictates the best treatment modality. Citation[177]
3.6 Searching for subclinical organ damage (Box 7)
Due to the importance of subclinical organ damage as an intermediate stage in the continuum of vascular disease and as a determinant of overall cardiovascular risk, signs of organ involvement should be sought carefully. It should be pointed out that a large body of evidence is now available on the crucial role of subclinical organ damage in determining the cardiovascular risk of individuals with and without high blood pressure.
Box 7 Position statement: Searching for subclinical organ damage
Due to the importance of subclinical organ damage as an intermediate stage in the continuum of vascular disease and as a determinant of total cardiovascular risk, signs of organ involvement should be sought carefully by appropriate techniques:
Heart ‐ Electrocardiography should be part of all routine assessment of subjects with high BP in order to detect left ventricular hypertrophy, patterns of ‘strain’, ischaemia and arrhythmias. Echocardiography is recommended when a more sensitive detection of left ventricular hypertrophy is considered useful. Geometric patterns can be defined echocardiographically, of which concentric hypertrophy carries the worse prognosis. Diastolic dysfunction can be evaluated by transmitral Doppler.
Blood vessels ‐ Ultrasound scanning of carotid arteries is recommended when detection of vascular hypertrophy or asymptomatic atherosclerosis is deemed useful. Large artery stiffening (leading to isolated systolic hypertension in the elderly) can be measured by pulse wave velocity. It might be more widely recommended if its availability were greater. A low ankle‐brachial BP index signals advanced peripheral artery disease.
Kidney ‐ Diagnosis of hypertension‐related renal damage is based on a reduced renal function or an elevated urinary excretion of albumin. Estimation from serum creatinine of glomerular filtration rate (MDRD formula, requiring age, gender, race) or creatinine clearance (Cockroft‐Gault formula, requiring also body weight) should be routine procedure. Urinary protein should be sought in all hypertensives by dipstick. In dipstick negative patients low grade albuminuria (microalbuminuria) should be determined in spot urine and related to urinary creatinine excretion.
Fundoscopy ‐ Examination of eye grounds is recommended in severe hypertensives only. Mild retinal changes are largely non‐specific except in young patients. Haemorrhages, exudates and papilloedema, only present in severe hypertension, are associated with increased CV risk.
Brain ‐ Silent brain infarcts, lacunar infarctions, microbleeds and white matter lesions are not infrequent in hypertensives, and can be detected by MRI or CT. Availability and costs do not allow indiscriminate use of these techniques. In elderly hypertensives, cognitive tests may help to detect initial brain deterioration.
Table summarizes availability, prognostic value and cost of procedures to detect subclinical organ damage.
Microalbuminuria has been shown repeatedly to be associated with an increased incidence of cardiovascular disease not only in diabetes but also in non‐diabetic subjects. Citation[178–184] In addition, an increased risk has been documented for urinary protein levels lower than those defined as microalbuminuria. Citation[181,182],Citation[185,186]
There has been further confirmation of the adverse prognostic role of left ventricular hypertrophy, Citation[187–189] as well as of carotid intima‐media thickness Citation[190–193] together with evidence that their prevalence in ordinary hypertensive individuals is much more common than observed when only routine investigations are performed. Citation[194] Without ultrasound investigations for left ventricular hypertrophy and vascular thickening or plaques, up to 50% of hypertensive subjects may be mistakenly classified as at low or moderate added risk, whereas the presence of cardiac or vascular damage classifies them within a higher risk group. Citation[194]
Retrospective analyses of prospective trials Citation[57–61],Citation[195] have shown that treatment‐induced reductions in proteinuria and left ventricular hypertrophy are accompanied by a reduced incidence of cardiovascular events, suggesting that measuring organ damage is advisable not only to quantify total cardiovascular risk initially but also to monitor treatment‐induced protection.
For these reasons the present guidelines, as was the case in 2003, Citation[3] devote a special section to a discussion on the evidence of the risk represented by various organ abnormalities and the methods for their detection. In general, screening for microalbuminuria should now be considered a routine procedure to be done in all hypertensive patients as well as in subjects with metabolic syndrome even in presence of high normal blood pressure. Echocardiography and vascular ultrasonography can be considered as recommended tests, particularly in patients in whom organ damage is not detected by routine investigations such as the electrocardiogram, and in the elderly in whom cardiac hypertrophy and arterial disease are frequent. Also, useful information on vascular damage may be obtained by measuring arterial stiffness by pulse wave velocity. However, this technique is currently not sufficiently widespread, and thus the information it provides remains desirable but difficult to obtain.
Methods for evaluating organ damage are mentioned in detail below.
3.6.1 Heart
Electrocardiography should be part of all routine assessment of subjects with high blood pressure. Its sensitivity in detecting left ventricular hypertrophy is low, but nonetheless hypertrophy detected by the Sokolow‐Lyons index (SV1+RV5_6>38 mm) or by the Cornell voltage QRS duration product (>2440 mm*ms) is an independent predictor of cardiovascular events, Citation[187] and its use as a marker of cardiac damage as well as of regression of this damage and patients' protection by treatment appears to be valuable, at least in patients over 55 years of age. Citation[195,196] Electrocardiography can also be used to detect patterns of ventricular overload or ‘strain’ (known to indicate more severe risk), Citation[187] ischaemia, conduction defects and arrhythmias, including atrial fibrillation which are not rare in elderly hypertensives. Holter electrocardiography is indicated in hypertension when arrhythmias or ischaemic episodes are to be detected. It may also provide evidence of reduced heart rate variability, which can occur in severe hypertension. Citation[72] However, the negative prognostic significance of this finding, although demonstrated in heart failure and after a myocardial infarction Citation[197–199] is unproved.
Although not immune from technical limitations (inter‐observer variability, low quality imaging in obese subjects and in subjects with obstructive lung disease, etc.) echocardiography is more sensitive than electrocardiography in diagnosing left ventricular hypertrophy Citation[200] and predicting cardiovascular risk, Citation[188] and may help in the more precise stratification of overall risk and in determining therapy. Citation[194] Proper evaluation includes measurements of the interventricular septum, left ventricular posterior wall thickness and end diastolic diameter, with calculation of left ventricular mass according to current formulae. Citation[201] Although the relation between left ventricular mass index and cardiovascular risk is continuous, thresholds of 125 g/m2 for men, and 110 g/m2 for women are widely used for conservative estimates of left ventricular hypertrophy. Concentric hypertrophy (wall to radius ratio ⩾0.42 with an increased left ventricular mass), Citation[202] eccentric hypertrophy (increased left ventricular mass with a wall‐to‐radius ratio <0.42) and concentric remodelling (a wall‐to‐radius ratio ⩾0.42 with a normal left ventricular mass) all predict an increased incidence of cardiovascular disease, but concentric hypertrophy has consistently been shown to be the condition which most markedly increases the risk. Citation[203,204]
In addition, echocardiography provides a means of assessing left ventricular systolic function; ejection fraction as well as endocardial and midwall fractional shortening have been proposed as possible additional predictors of cardiovascular events. Citation[205,206] Left ventricular diastolic filling (a measure of the so‐called ‘diastolic function’) can also be assessed by Doppler measurement of the ratio between the E and A waves of transmitral blood flow velocity, of early diastolic relaxation time and of pulmonary vein outflow into the left atrium. Citation[207] Useful information can also be derived from tissue Doppler imaging at the lateral mitral annulus. Citation[208] All these measurements are of great current interest because it is now recognized that a considerable proportion (about 50%) of heart failure may be explained by ‘diastolic dysfunction’, with no or little impairment of systolic function, and that so called ‘diastolic heart failure’ is an ominous condition. Citation[209] Alterations of diastolic function are frequent among hypertensives, and in elderly individuals with elevated blood pressure at least one in four patients may be affected. Citation[210] These alterations may occur in the absence of systolic function alterations and even without left ventricular hypertrophy. There is evidence that diastolic dysfunction increases the risk of atrial fibrillation. Citation[211] Furthermore, two studies have reported that diastolic dysfunction predicts subsequent heart failure, Citation[206] and is associated with an increased incidence of all cause mortality, Citation[212] although in another study this association was found not to be independent of covariates. Citation[213] Finally, echocardiography provides some information on the presence and degree of left atrial enlargement, which is related to the risk of atrial fibrillation, cardiovascular disease and death. Citation[214–216] Also, data can be obtained on segmental defects of left ventricular wall contraction due to ischaemia or previous infarction.
Other diagnostic cardiac procedures, such as nuclear magnetic resonance, cardiac scintigraphy, exercise testing and coronary angiography are reserved for specific indications. An X‐ray of the thorax may be a useful additional diagnostic procedure, when dyspnoea is the presenting complaint or information on large intrathoracic arteries or the pulmonary circulation is sought, but in general chest X‐ray is an obsolete standard procedure for the identification of hypertensive heart disease.
In recent years interest has grown in the possibility of assessing the degree of cardiac fibrosis in order to improve the ability of increased left ventricular mass to predict outcome. Techniques based on reflectivity of cardiac ultrasound imaging have been used: Citation[217,218] cyclic variations of the backscattering signal may reflect to some extent the contractile properties of the myocardium more than collagen content, whereas echoreflectivity more directly correlates with histologically measured fibrosis. Echoreflectivity has shown that the tissue constitution of left ventricular hypertrophy may vary and that drugs favouring its regression may differ in reducing fibrosis. Citation[219] To date the most precise measurement of cardiac tissue constitution is provided by nuclear magnetic resonance, the cost of which, however, prevents large scale use. Also, under investigation are circulating markers of collagen tissue composition, Citation[219] but they are only partly derived from the heart.
3.6.2 Blood vessels
Several non‐invasive screening tests are available for identifying the abnormal structure and function of large arteries in hypertension. Ultrasound examination of the carotid arteries with measurement of intima‐media thickness (IMT) or the presence of plaques has been shown to predict the occurrence of both stroke and myocardial infarction. Citation[190–193] The relationship between carotid IMT and cardiovascular events is a continuous one but for the common carotid arteries an IMT>0.9 mm can be taken as a conservative estimate of existing abnormalities. Ultrasound scannings limited to the common carotid arteries (an infrequent site of atherosclerosis) are likely to measure vascular hypertrophy only, whereas assessment of atherosclerosis also requires scanning of the bifurcations and/or internal carotids where plaques are more frequent. Citation[220–222] Presence of a plaque can be identified by an IMT>1.3 or 1.5 mm or by a focal increase in thickness of 0.5 mm or 50% of the surrounding IMT value. Citation[220–222] There is evidence that, in untreated hypertensive individuals without target organ damage at routine examinations, these alterations are common, and thus carotid ultrasound examination may often detect vascular damage and make risk stratification more precise. Citation[194] Also, evidence of arterial damage may be suggested by an ankle‐brachial blood pressure index <0.9, using a continuous wave Doppler unit and a blood pressure manometer. A low ankle‐brachial blood pressure index signals peripheral artery disease and, in general, advanced atherosclerosis, Citation[56] whereas carotid IMT measurements are able to detect earlier changes. Citation[220] Nevertheless, a reduced ankle‐brachial index relates to further development of angina, myocardial infarction, congestive heart failure, need for coronary bypass surgery, stroke, carotid and peripheral vascular surgery, Citation[15],Citation[223–226] and in patients with multi‐vessel coronary disease it confers additional risk. Citation[227]
Over the past 10 years, a large body of evidence has been collected on large artery stiffening and the wave reflection phenomenon, which have been identified as being the most important pathophysiological determinants of isolated systolic hypertension and pulse pressure increases. Citation[228] Measuring arterial stiffness via changes in vessel diameter in relation to blood pressure changes is complex and not suitable for standard clinical use. On the other hand, measurement of carotid‐femoral pulse wave velocity provides a comprehensive non‐invasive assessment of arterial stiffness, which is simple and accurate enough to be considered as a diagnostic procedure. Citation[28] This is because this measure has been shown to have an independent predictive value for all cause mortality and cardiovascular morbidity, coronary events and strokes in patients with uncomplicated essential hypertension. Citation[54,55],Citation[229,230] Although the relationship between aortic stiffness and events is continuous, a threshold >12 m/s has been suggested as a conservative estimate of significant alterations of aortic function in middle age hypertensives. Though a wider clinical use of pulse wave velocity and augmentation index measurements may add further precision to the assessment of arterial damage, the availability of these technique is largely limited to research centres.
As shown in Table several other methods for detecting vascular organ damage cannot be supported for clinical use for a variety of reasons. An increase in the wall to lumen ratio of small arteries can be measured in subcutaneous tissues obtained through gluteal biopsies. These measurements can demonstrate early alterations in diabetes and hypertension Citation[231–234] and have a predictive value for cardiovascular morbidity and mortality, Citation[235] but the invasiveness of the method makes this approach unsuitable for general use. Increase in coronary calcium as quantified by high resolution cardiac computer tomography has also been prospectively validated as a predictor of cardiovascular disease Citation[236] but the limited availability and high cost of the necessary instrumentations are serious problems. Endothelial dysfunction predicts outcome in several cardiovascular diseases, Citation[237,238] although data on hypertension are still rather scant. Citation[239] Furthermore, the techniques available for investigating endothelial responsiveness to various stimuli are invasive, laborious and time consuming. Finally, methods are not yet standardized and no certainty exists as to whether endothelial function assessed in one organ is representative of other vascular beds. Thus, assessment of endothelial function cannot be advocated as currently useful in the clinical evaluation of the hypertensive patient. However, current studies on circulating markers of endothelial activity as well as on progenitors of endothelial cells are promising Citation[240] and simpler tests or markers of endothelial dysfunction or damage may become available in the future. This might favour prospective assessment of their prognostic role on a larger scale, and a more widespread clinical use.
3.6.3 Kidney
The diagnosis of hypertension‐induced renal damage is based on the finding of a reduced renal function and/or the detection of elevated urinary excretion of albumin. Citation[241] Renal insufficiency is now classified according to the estimated glomerular filtration rate calculated by the abbreviated MDRD formula that requires age, gender, race and serum creatinine. Citation[52] Values of estimated glomerular filtration rate below 60 ml/min/1.73 m2 indicate chronic renal disease stage 3, whilst values below 30 and 15 ml/min/1.73 m2 indicate chronic renal disease stages 4 and 5, respectively. Citation[242] The other formula (the so called Cockcroft‐Gault formula) estimates creatinine clearance and is based on age, gender, body weight and serum creatinine. Citation[51] This formula is valid in the range >60 ml/min, but it overestimates creatinine clearance in chronic kidney disease stage 3 to 5. Citation[242] Both formulae help to detect mild impaired renal function in the face of serum creatinine values that are still in the normal range. Citation[242] A reduction in glomerular filtration rate and an increase in cardiovascular risk may also be inferred from the increased serum levels of cystatin C. Citation[243]
A slight increase in serum creatinine (up to 20%) may sometimes occur when antihypertensive therapy is instituted or potentiated, but this should not be taken as a sign of progressive renal deterioration. Hyperuricaemia is frequently seen in untreated hypertensives (particularly in pre‐eclampsia), and has also been shown to correlate with reduced renal blood flow and the presence of nephrosclerosis. Citation[244]
While an elevated serum creatinine concentration or a low estimated glomerular filtration rate (or creatinine clearance) points to a reduced rate of glomerular filtration, an increased rate of urinary albumin or protein excretion points to a derangement in the glomerular filtration barrier. Microalbuminuria (see Table) has been shown to predict the development of overt diabetic nephropathy in both type 1 and type 2 diabetics, Citation[245] while the presence of overt proteinuria generally indicates the existence of established renal parenchymatous damage. Citation[246] In both diabetic and non‐diabetic hypertensive patients, microalbuminuria, even below the threshold values currently considered, Citation[247] has been shown to predict cardiovascular events, Citation[178–186],Citation[248] and a continuous relationship between cardiovascular, as well as non‐cardiovascular, mortality and urinary protein/creatinine ratios ⩾3.9 mg/g in men and 7.5 mg/g in women has been reported in several studies. Citation[185,186] Thus the term microalbuminuria may be misleading (also because it falsely suggests a minor damage) and should in theory be replaced by ‘low grade albuminuria’. Citation[248] Microalbuminuria can be measured from spot urine samples (24‐hour or night urine samples are discouraged due to the inaccuracy of urinary sampling) by indexing the urinary albumin concentration to the urinary creatinine concentration. Citation[242] Classic dipstick tests detect albuminuria above 300 mg/g creatinine and the ‘micro‐albuminuric’ dipstick test above 30 mg/g creatinine. Sensitive dipsticks for the lower range of low grade albuminuria are under investigation.
In conclusion, the finding of impaired renal function in a hypertensive patient, expressed as any of the abnormalities mentioned above, is frequent and constitutes a very potent predictor of future cardiovascular events and death even in treated patients. Citation[179],Citation[249–253] Therefore, it is recommended that glomerular filtration rate is estimated, and the presence of urinary protein (by dipstick) sought in all hypertensive patients. In dipstick negative patients low grade albuminuria should also be determined in spot urine by using one of the validated commercial methods at least twice on separate occasions. Albuminuria should be related to urinary creatinine excretion, with application of sex specific criteria.
3.6.4 Fundoscopy
In contrast to the 1930s, when Keith, Wagener and Barker classified hypertensive retinal changes into four grades Citation[254] most hypertensive patients today present early in the process of their illness, and haemorrhages and exudates (grade 3), not to mention papilloedema (grade 4), are observed very rarely. Grade 1 (arteriolar narrowing either focal or general in nature) and 2 (arterio‐venous nipping) retinal changes, on the contrary, are much more frequently reported than markers of organ damage with documented clinical significance (left ventricular hypertrophy, carotid plaques and microalbuminuria), Citation[255] but the ability of these milder degrees of retinopathy detected by fundal analysis to be used for prognosis has been questioned. Citation[255–257] This is because these changes appear to be largely non‐specific arteriolar alterations, except perhaps in young patients in whom deviation from an entirely normal retina should raise concern. In contrast, grades 3 and 4 retinal changes are associated with an increased risk of cardiovascular events. Citation[258,259] More selective methods for objectively investigating ocular damage in hypertension have been developed and studied. Citation[260] For instance, digitized retinal photographs can be analysed by a semiautomated program to quantify geometric and topological properties of arteriolar and venular tree. This method has identified hypertension‐related topological alterations of retinal vasculature Citation[261] and showed that retinal arteriolar and venular narrowing may precede the development of hypertension. Citation[262,263] However, its use is still mainly confined to research.
3.6.5 Brain
In patients who have suffered a stroke, imaging techniques allow improved diagnosis of the existence, nature and location of a lesion. Citation[264,265] Cranial computed tomography (CT) is the standard procedure for diagnosis of a stroke but, with the exception of prompt recognition of an intracranial haemorrhage, CT is progressively being replaced by magnetic resonance imaging (MRI) techniques. Diffusion‐weighted MRI can identify ischaemic injury within minutes after arterial occlusion. Furthermore MRI, particularly in fluid attenuated inversion recovery (FLAIR) sequences, is much superior to CT in discovering silent brain infarcts, the large majority of which are small and deep (lacunar infarctions). Several studies have shown that MRI‐detected small silent brain infarcts, microbleeds and white matter lesions are not rare in the general population, Citation[266,267] and that their prevalence increases with age and hypertension, and is associated with an increased risk of stroke, cognitive decline and dementia. Citation[267–269] Availability and cost considerations do not allow a widespread use of MRI in the evaluation of elderly hypertensives, but silent brain infarcts should be sought in all hypertensives with neural disturbance and, particularly, memory loss. As cognitive disturbances in the elderly are, at least in part, hypertension related, Citation[270–272] suitable cognitive evaluation tests should be used in the clinical assessment of the elderly hypertensive.
4. Evidence for therapeutic management of hypertension
4.1 Introduction
Recommendations about therapy for hypertension are here preceded by some considerations on the strength of available evidence on the benefits associated with antihypertensive treatment as well as on the comparative benefits of the various classes of drugs. There is a consensus that large randomized trials measuring fatal and non‐fatal events represent the strongest type of evidence available. However, it is commonly recognized that event based randomized therapeutic trials also have limitations. Citation[3],Citation[273,274]
These include the need to select elderly or otherwise high risk patients in order to maximize the number of events collected and thus the power of trials, which means that uncomplicated, younger and lower risk patients are rarely represented, with the unfortunate consequence that little direct information is available on treatment benefits in a large sector of the hypertensive population. Furthermore, the therapeutic programmes of trials often diverge from usual therapeutic practice because drugs randomly allocated at the beginning of a trial are continued even in absence of blood pressure lowering effects, while in practice physicians normally do not continue prescribing drugs that are not effective; therefore in trials, but not in practice, benefits occurring in subjects responsive to the allocated treatment are diluted by the lack of benefit in non‐responsive subjects.
Perhaps the most important limitation is the necessarily short duration of a trial (in most cases 4 to 5 years) whereas additional life expectancy, and hence expectancy of treatment duration, for middle age hypertensives is 20 to 30 years. Long term therapeutic benefits, as well as differences in benefit between various drug classes, have recently been investigated by prolonging the observation of patients after the end of trials, Citation[275,276] but this can only be done in an uncontrolled fashion, which limits the value of the results.
An additional approach to the assessment of treatment benefit is use of intermediate endpoints such as subclinical organ damage. The evidence from studies using such end‐points does not have the same weight as that based on ‘hard’ endpoints (fatal or non‐fatal myocardial infarction or stroke and cardiovascular or all cause mortality). However, a large body of evidence demonstrates that several measures of subclinical organ damage have a strong predictive value for subsequent fatal and non‐fatal events, and that changes in proteinuria and echocardiographic or electrocardiographic left ventricular hypertrophy induced by treatment are predictive of a reduction in ‘hard’ endpoints (see Sections 3.6 and 4.5). This, and the simple consideration that events cannot occur in a healthy cardiovascular system, but must always be preceded by alterations in organ structure or function, makes this approach a valuable one, and thus information from trials using organ damage as end points has been considered. Similarly, a valuable approach to extend evidence of the benefit of treatment over a longer time scale, is to use as endpoint the incidence or worsening of diseases with an adverse prognostic impact such as diabetes, metabolic disorders and end stage renal disease. End stage renal disease is associated with a striking increase in cardiovascular risk Citation[186],Citation[277] and has indeed been used as endpoint in several therapeutic trials. New onset diabetes is also being used as intermediate end‐point, and its predictive value is discussed in depth in Section 4.5.5.
Finally, whenever useful, information provided by meta‐analyses has been given due attention, but meta‐analyses have not been considered to necessarily represent the top level of evidence. Indeed, although meta‐analyses have a greater statistical power than individual trials, and may provide useful average measurements of treatment effects, they also have limitations. By definition, they are post‐hoc analyses, the choice of the trials to be included is often arbitrary, the trials included are not homogeneous, with differences not always susceptible to being assessed by statistical tests. Therefore, meta‐analysis data have been reviewed critically, as have all other sources of information.
4.2 Event based trials comparing active treatment to placebo
Randomized placebo controlled trials investigating the benefits of blood pressure lowering have been numerous and have given unequivocal results. Citation[278–291] They have been included in several meta‐analyses which are based on an impressively large number of patients. Citation[10],Citation[292–299] The findings can be summarized as follows: 1) antihypertensive treatment translates into significant reductions of cardiovascular morbidity and mortality while having a less significant effect on all cause mortality; 2) the benefit can also be seen at older ages, including patients with isolated systolic hypertension; 3) the proportional reduction of cardiovascular risk is similar in men and women and treatment has a beneficial effect in Caucasian, Asian and black populations, which suggests that it is present across various ethnic groups; and 4) with regard to cause‐specific events antihypertensive treatment is associated with a major reduction in the risk of fatal or non‐fatal stroke (about 30–40%), but, coronary events are reduced as well, though to a lesser degree (20%). Finally, treatment appears to cause a large reduction in the incidence of heart failure.
Meta‐analyses of placebo controlled trials have also separately addressed the effect of treatment initiated with different drugs, though comparisons are difficult because of variable blood pressure differences between active and placebo treatments in the various trials. However, the overall results show a beneficial effect on cardiovascular morbidity and mortality, as well as on cause‐specific events, when a thiazide diuretic or a β‐blocker was given as first drug. Beneficial effects, however, have also been found when treatment was initiated with a calcium channel blocker or an ACE inhibitor. Citation[292,293]
The demonstration of the beneficial effects of blood pressure lowering has made it ethically unacceptable to perform placebo controlled trials according to the previous design, i.e. with an untreated placebo group. For this reason in more recent trials the drug under investigation was compared with placebo in groups of patients already treated with other antihypertensive agents. This has provided additional evidence on the beneficial effect of various antihypertensive drugs also documenting that the benefit may be substantial even when blood pressure reductions are small and the initial blood pressure is below the traditional cutoff defining hypertension. In the HOPE trial in patients with high cardiovascular risk (mostly because of a history of myocardial infarction) and thus multiple drug treatment, administration of ramipril caused a modest blood pressure reduction (about 3 mmHg systolic blood pressure) and a clearcut reduction (−22%) in the incidence of cardiovascular events compared to the placebo group. Citation[300] In the FEVER trial the calcium antagonist felodipine was compared to placebo in moderate risk hypertensive patients whose blood pressure had been brought below 160/90 mmHg by background therapy. Citation[301] In the felodipine group in which blood pressure achieved slightly lower values than in the placebo group (−3.5/−1.5 mmHg) the incidence of all cardiovascular endpoints was significantly reduced by about 28%. In the EUROPA trial, Citation[302] in patients with coronary disease (and thus multiple background treatment), blood pressure lowering (−5/−2 mmHg) by an ACE inhibitor (perindopril with the possible addition of indapamide) was accompanied by beneficial cardiovascular effects compared with placebo, independent of the baseline blood pressure value. In the ACTION trial in patients with angina pectoris, a modest blood pressure lowering obtained by slow‐release nifedipine on the top of other agents also reduced the incidence of cardiovascular events compared to placebo, although only in the subgroup with baseline hypertension. Citation[303,304] A reduction of cardiovascular events was also observed in the CAMELOT trial in treated coronary patients in whom the addition of amlodipine reduced blood pressure by few mmHg compared to placebo. Citation[305] Surprisingly, another trial in coronary patients and with similar blood pressure differences in which an ACE inhibitor was compared to placebo was unable to show any benefit. Citation[306]
A similar approach has been used to study newer drugs such as angiotensin receptor antagonists. In the SCOPE study Citation[307] in elderly hypertensive patients (age >70 years) the angiotensin receptor antagonist candesartan, often administered on top of a diuretic, reduced blood pressure modestly more than placebo also frequently administered on top of diuretic‐based conventional therapy (difference 3.2/1.6 mmHg), with a significant concomitant reduction in non‐fatal stroke. In the RENAAL and IDNT studies on hypertensive patients with type 2 diabetes and nephropathy, addition of the angiotensin receptor antagonists losartan Citation[308] and irbesartan Citation[309] on top of multiple antihypertensive therapies slowed down the progression of renal disease (the primary end‐point), while showing no significant beneficial effect on most secondary cardiovascular endpoints, for the evaluation of which, however, the studies were not sufficiently powered. Yet, when these two studies were combined in a meta‐analysis a significant reduction of cardiovascular morbidity was found in the angiotensin receptor antagonist treatment group. Citation[310] Thus, it can be concluded that blood pressure lowering by angiotensin receptor antagonists is also beneficial.
4.3 Event based trials comparing more and less intense blood pressure lowering
Most of the available information still relies on the largest trial of this type, the HOT study, Citation[311] but additional data from smaller trials, mostly in diabetic patients, are also available. Data from five trials on about 22,000 patients have been included in the Blood Pressure Lowering Treatment Trialists' (BPLTT) collaboration meta‐analyses, Citation[292],Citation[296] the results showing significant benefits from a more intense blood pressure reduction as far as stroke and major cardiovascular events are concerned, particularly in diabetics. Further information can also be derived from recent placebo‐controlled trials (see above), in which the placebo group often received a somewhat less intensive antihypertensive therapy. Finally, some indirect evidence may be provided by trials such as the HDFP Citation[312] which compared active treatment regimens of different intensity and did not achieve equal blood pressures in the treatment arms. Almost invariably, a lower blood pressure was accompanied by at least a trend towards less strokes (see Section 4.4).
4.4 Event based trials comparing different active treatments
After publication of the 2003 ESH/ESC Guidelines a large meta‐analysis of trials comparing active regimens Citation[220],Citation[222],Citation[313–327] was published by the BPLTT’ collaboration. Citation[292] We have taken this meta‐analysis as the basis for the following discussion. However, we have also discussed results of more recent trials not included in the BPLTT meta‐analysis and critically addressed some of the problems inherent in many of these trials as well as in the various types of analyses. Citation[328,329]
Indeed, these studies provide important information on the relative efficacy of the various classes of antihypertensive agents, but their straightforward interpretation is often made difficult by the failure to achieve comparable blood pressure values with the different treatments. Admittedly, differences are commonly small, but even small blood pressure differences may be accompanied by large differences in outcome, Citation[273,274] and statistical adjustment is an imperfect way to cope with failure of achieving a protocol requirement. Meta‐regression analyses can provide information that takes into account differences in blood pressure effects, if it is understood that homogeneity of the trials included in a meta‐regression is even lower than homogeneity in classical meta‐analyses. Finally, trials comparing different agents actually compare regimens only initiated on different agents, since the majority of randomized subjects ends up with combination therapy including agents similarly distributed in the comparison groups.
4.4.1 Calcium antagonists versus thiazide diuretics and β‐blockers
A recent meta‐analysis of 9 trials comparing calcium antagonists with conventional drugs made use of data on more than 68000 patients. Citation[292] For reductions in blood pressure that were similar or only slightly different between groups the odds ratios expressing the possible benefit of calcium antagonists over conventional drugs were close to unity and non‐significant for total mortality, cardiovascular mortality, all cardiovascular events and myocardial infarction. Calcium antagonists provided a slightly better protection against stroke, but they showed a reduced ability, as compared with conventional therapy, to protect against the incidence of heart failure. Results were similar when diabetic and non‐diabetic patients were separately analysed. Citation[296] The ASCOT trial has more recently added further information on the comparative efficacy of treatment initiated by either a calcium antagonist (amlodipine) or a conventional drug. Citation[330] INVEST, not included in the meta‐analysis, also showed equal incidence of cardiovascular events in patients with coronary heart disease in whom treatment was started with a calcium antagonist (verapamil, often combined with an ACE inhibitor) or with a β‐blocker (atenolol, often combined with a diuretic). Citation[331] The amlodipine based treatment resulted in a slightly greater blood pressure reduction accompanied by a significant reduction in stroke, cardiovascular and all cause mortality. As in most trials, the majority of ASCOT patients received combination therapy (calcium antagonist with ACE inhibition versus β‐blocker with thiazide diuretic).
4.4.2 ACE inhibitors versus thiazide diuretics and β‐blockers
The BPLTT collaboration analysis includes 6 trials with a total of about 47000 randomized patients comparing ACE inhibitors with diuretics and beta‐blockers. Citation[292] The pooled odds ratios expressing the possible benefits of ACE inhibitors versus conventional treatment were very close to unity and non significant for total mortality, all cardiovascular events, cardiovascular mortality and coronary heart disease. However, there were non‐significant trends towards less effective protection of ACE inhibitors as far as stroke and congestive heart failure were concerned. Nonsignificant differences in odds ratio for total and cause specific cardiovascular events have also been reported by the meta‐analysis that has separately examined diabetic and non‐diabetic patients. Citation[296]
It should be mentioned that trials comparing ACE inhibitors with diuretics have not always given entirely consistent results. In the second Australian blood pressure study Citation[327] hypertensive patients randomized to an ACE inhibitor had a reduced number of cardiovascular events compared with those randomized to thiazide diuretics, although the difference was small, only evident in men, and significant only if recurrent events were included. In the ALLHAT trial, Citation[322] on the contrary, hypertensive patients on the diuretic chlorthalidone showed a similar incidence of coronary heart disease (the primary end point) as compared to those randomized to the ACE inhibitor lisinopril, but heart failure and stroke were significantly lower in the diuretic treated group (which also showed a greater blood pressure reduction).
4.4.3 ACE inhibitors versus calcium antagonists
Comparisons of these two drug classes as performed in the BPLTT meta‐analysis are based on a total of almost 26000 patients from 6 studies. Citation[292] The results show the odds ratio expressing relative benefits of the two regimens to be close to unity and non‐significant for total coronary events, cardiovascular mortality, total mortality as well as coronary heart disease. Protection against stroke was, on the other hand, significantly more effective for calcium antagonists, whilst protection against heart failure was better for ACE inhibitors.
4.4.4 Angiotensin receptor antagonists versus other drugs
Five trials have compared angiotensin receptor antagonists with other antihypertensive agents. The different comparators used make meta‐analysis of these studies difficult. In the LIFE study Citation[332] in more than 9000 hypertensive patients with electrocardiographic left ventricular hypertrophy mean blood pressure was reduced to the same degree in the groups in which treatment was initiated with either losartan or the β‐blocker atenolol. Over the about 5 years of follow‐up losartan‐treated patients showed a significant 13% reduction in major cardiovascular events (the primary end point) with no difference in the incidence of myocardial infarction, but a 25% difference in the incidence of stroke. A significant reduction in non‐fatal stroke (although not in the primary end‐point) was also reported in the elderly patients of the SCOPE trial, in whom candesartan lowered blood pressure slightly more than placebo and usual treatment. Citation[307] In the MOSES trial Citation[333] on about 1500 hypertensive patients with a previous cerebrovascular event comparison was made of treatment initiated by either eprosartan or the calcium antagonist nitrendipine. During a mean follow‐up of 2.5 years, and for a similar blood pressure decrease, cardiovascular events were significantly less in eprosartan‐treated patients, whereas incidence of stroke was found to be decreased only if strokes recurrently seen in the same patient were considered. In the JIKEI HEART trial Citation[334] on more than 3000 Japanese treated hypertensive patients at high risk because of the concomitance of coronary heart disease, heart failure, diabetes or multiple risk factors, addition of valsartan reduced blood pressure from 139/81 mmHg to 132/78 mmHg. Over a 3 year treatment period this was accompanied by a marked reduction in the incidence of stroke (40%) compared to the group in which only slightly greater blood pressure values (132/78 mmHg) were achieved by the addition of drugs other than angiotensin receptor antagonists. Finally, in the VALUE trial Citation[335] more than 15000 hypertensive patients at high risk were randomized to treatment with either valsartan or the calcium antagonist amlodipine. Over the 5 year follow‐up amlodipine‐treated patients showed a slightly lower blood pressure value than valsartan‐treated patients. The incidence of cardiac events and death (the primary outcome) was not significantly different between the two groups, but there was a significant reduction in myocardial infarction and a non‐significant trend towards a lower incidence of stroke in the amlodipine group; on the other hand, the risk of heart failure showed a trend in favour of valsartan. Pooled data have shown that the benefits of angiotensin receptor antagonists for heart failure prevention are particularly large in diabetic patients, but the number of observations is small. Citation[296]
The claim has recently been made that angiotensin receptor antagonists would provide less protection against myocardial infarction than other antihypertensive agents. Citation[336] However, this has not been confirmed by comprehensive meta‐analyses published recently, which show the incidence of myocardial infarction to be similar to that occurring with other drugs. Citation[337,338] Direct comparisons between the overall and cause‐specific beneficial effects of angiotensin receptor antagonists and ACE inhibitors (i.e. the classes specifically opposing the cardiovascular influences of the renin‐angiotensin‐system) in hypertension are not available, however; this makes the results of an ongoing large trial on high risk hypertensive and normotensive patients randomized to ramipril or telmisartan (ONTARGET) of major importance. Citation[339] Comparative randomized trials in heart failure or post‐myocardial infarction patients with left ventricular dysfunction show no significant between‐treatment differences in the incidence of stroke, major coronary events and heart failure in patients treated either with ACE inhibitors or angiotensin receptor antagonists. Citation[340–342] A recent meta‐regression analysis by the BPLTT indicates that angiotensin receptor antagonists have the same blood pressure dependent beneficial effect on coronary events as ACE inhibitors, although the latter may exert a small blood pressure independent effect. Citation[329]
4.4.5 Trials with β‐blockers
The benefit of β‐blockers compared with that of other antihypertensive agents has recently been questioned on the basis of the results of two large randomized trials, the LIFE study Citation[332] and the ASCOT study, Citation[330] both of which showed superiority of an angiotensin receptor antagonist and, respectively, a calcium antagonist over therapy initiated by a β‐blocker as far as stroke (LIFE) or stroke and mortality (ASCOT) were concerned. These two large trials have strongly influenced a recent meta‐analysis Citation[343] which concluded that β‐blocker initiated therapy is inferior to others in stroke prevention, but not in prevention of myocardial infarction and reduction in mortality. On the basis of a similar meta‐analysis, the National Institute for Health and Clinical Excellence (NICE) in the United Kingdom has advised the use of β‐blockers only as fourth line antihypertensive agents. Citation[344] These conclusions must be considered with care but also with a critical mind. Both the LIFE and the ASCOT studies were characterized by a design implicating early use of combination therapy, so that the vast majority of patients randomized to a β‐blocker actually received a β‐blocker‐thiazide combination. A similar combination was often used in the chlorthalidone treatment group of the ALLHAT trial, Citation[322] which failed to find inferiority of this combination even concerning stroke prevention. Also, in the INVEST trial, Citation[331] a treatment strategy based on the initial administration of a β‐blocker followed by the addition, in most patients, of a thiazide diuretic was accompanied by an incidence of all cardiovascular and cause‐specific events similar to that of a treatment initiated with the calcium antagonist verapamil followed by the addition of the ACE inhibitor trandolapril. Finally, a recent meta‐analysis shows that, when compared with placebo, β‐blocker based therapy did indeed reduce stroke significantly. Citation[297] This suggests that at least part of the inferiority of the β‐blocker‐thiazide combination reported in ASCOT may be due to a lesser blood pressure reduction, Citation[330] particularly of central blood pressure, Citation[166] that occurred in this trial with this therapeutic regimen.
β‐blocker‐thiazide combinations have nevertheless been consistently associated with metabolic disturbances and new onset diabetes (see Section 4.5.5) and may have specific contraindications in patients prone to diabetes. In any case, the above quoted meta‐analyses of β‐blocker initiated trials Citation[297],Citation[343] well illustrate the difficulties inherent in many recent trials in which combination therapy hinders the attribution of either benefits or harms to individual compounds.
4.4.6 Conclusions
Comparative randomized trials show that for similar blood pressure reductions, differences in the incidence of cardiovascular morbidity and mortality between different drug classes are small, thus strengthening the conclusion that their benefit largely depends on blood pressure lowering per se. Because of the unfortunate failure of several comparative trials to lower blood pressure to the same extent in the two active treatment arms, recourse has been made to meta‐regression analysis in which differences in achieved blood pressures are taken into account.
Despite some limitations in this approach, as previously outlined, all recent meta‐regression analyses Citation[292],Citation[328,329] underline the important role of blood pressure lowering for all cause‐specific events, with the exception of heart failure: whenever systolic blood pressure is reduced by 10 mmHg, independent of the agent used, both stroke and coronary events are markedly reduced. Citation[328,329] These meta‐regression analyses also suggest that some antihypertensive agents may exert some cause‐specific beneficial effects that are blood pressure independent (i.e. an outcome reduction at no blood pressure difference), calcium antagonists on stroke and ACE inhibitors on coronary events. This effect, however, is definitively smaller (5–10%) than the dominant protective effect exerted by lowering blood pressure. On the other hand, individual trials and their meta‐analyses Citation[292],Citation[296] are generally concordant in reporting less protection of calcium antagonists compared with diuretics/β‐blockers, ACE inhibitors and angiotensin receptor antagonists with respect to prevention of new onset heart failure, independent of possible differences in blood pressure between treatments. It has been remarked that new onset heart failure is often a difficult diagnosis and, when calcium antagonists are administered, diagnosis may be confounded by ankle oedema dependent on vasodilatation. Furthermore, drugs such as diuretics may not prevent new onset heart failure but just mask its symptoms. Citation[3],Citation[345,346] This has led recent trials, such as VALUE, Citation[335] to consider only hospitalization for heart failure as a suitable endpoint, thus providing more convincing evidence of a limited protective effect of calcium antagonists as compared with angiotensin receptor antagonists on appearance of this clinical condition. It is reasonable to suppose that in prevention of heart failure humoral effects, differently influenced by different antihypertensive agents, may play a relevant direct role. Even under this circumstance, however, blood pressure lowering probably remains of paramount importance because in the hypertensive coronary patients of the ACTION trial a blood pressure reduction of 14.6/7.6 mmHg in the group randomized to slow‐release nifedipine administration was associated with a 38% reduction in the incidence of hospitalized heart failure compared with placebo. Citation[304]
4.5 Randomized trials based on intermediate endpoints
The possibility of clinically relevant differences in the beneficial effects of various classes of antihypertensive agents should not be explored by event based trials only. Subclinical organ damage occurs much earlier than events in the continuum of cardiovascular disease and may be more susceptible to specific, differential actions of the various antihypertensive compounds. Citation[274] For this reason, randomized trials using subclinical organ damage as endpoint are discussed.
4.5.1 Heart
Many studies have continued to test the effects of various antihypertensive agents on hypertension associated left ventricular hypertrophy, mostly evaluated by measuring left ventricular mass on the echocardiogram, but only a few of them have followed strict enough criteria to provide reliable information. As studies in hypertensive patients with left ventricular hypertrophy cannot be placebo controlled but must compare active treatments, 1) a large number of patients must be included in order to have sufficient power to detect presumably small between‐treatment differences, 2) treatment duration must be of at least 9–12 months, 3) blood pressure must be equally reduced by the compared treatments, and 4) special precautions must be taken in order to avoid regression to the mean and reading bias if the sequence of scans is not blinded. Citation[347,348] Because of the limitations of many studies, meta‐analyses cannot offer indisputable evidence of advantages of specific drug classes. Citation[349]
More reliable information is provided by a number of large and adequately designed studies. Three of these studies Citation[350–352] have shown equal regression with ACE inhibitors (lisinopril, enalapril and fosinopril, respectively) and with calcium antagonists (amlodipine, nifedipine and amlodipine, respectively), one study Citation[347] equal regression with an angiotensin receptor antagonist (candesartan) and an ACE inhibitor (enalapril), and another study Citation[353] equal regression of left ventricular mass with a calcium antagonist (lacidipine) and a β‐blocker (atenolol). Several studies Citation[354–356] have reproducibly shown a greater regression with several angiotensin receptor antagonists (valsartan, irbestartan, losartan, respectively) than with a β‐blocker (atenolol in all studies), and this conclusion has been greatly strengthened by the large echocardiographic LIFE substudy (involving 960 patients) confirming a significantly greater reduction of left ventricular hypertrophy with losartan than atenolol. Citation[357] Two other large studies have compared an ACE inhibitor‐diuretic fixed combination (perindopril‐indapamide) with the β‐blocker atenolol or, respectively, the ACE inhibitor enalapril, but the greater reduction of left ventricular mass with the combination was associated with a greater blood pressure reduction, Citation[358,359] and significantly correlated with a greater reduction in central blood pressure. Citation[360] Further information is provided by two studies using magnetic resonance imaging to evaluate left ventricular mass. In a relatively large‐size study Citation[361] the aldosterone blocker, eplerenone, and the ACE inhibitor, enalapril, were found equally effective, and their combination more effective than either agent (but with a greater blood pressure reduction). A smaller study compared the angiotensin receptor antagonist, telmisartan, with the β‐blocker (with α‐blocking properties) carvedilol, reporting a significantly greater effect of telmisartan, for a similar 24 h blood pressure reduction. Citation[362]
In conclusion, information from adequate trials shows that blood pressure lowering by whatever agent or agent combination can be accompanied by reduction of increased left ventricular mass, that equivalent efficacy appears to be provided by ACE inhibitors, angiotensin receptor antagonists and calcium antagonists, and probably by aldosterone antagonists, while at least angiotensin receptor antagonists are superior to β‐blockers. As to diuretics, the only adequately powered study Citation[363] shows a significant efficacy of indapamide; the same study also showed a superiority of indapamide over the ACE inhibitor, enalapril. As this is the only study in which an ACE inhibitor was found not to induce left ventricular mass reduction, no conclusion can be drawn on the comparative efficacy of diuretics versus ACE inhibitors in regressing left ventricular hypertrophy.
Recent studies have provided further clinically useful information: two long‐term trials Citation[353],Citation[357] have shown that regression of left ventricular hypertrophy is maintained over time (but achieves a maximum by 2–3 years). A large‐sized study such as LIFE has been able to show that a treatment‐induced reduction in left ventricular mass is significantly and independently associated with a reduction of major cardiovascular events, stroke and cardiovascular and all‐cause mortality Citation[57] thus substantiating findings from other long‐term observational studies. Citation[61],Citation[364,365]
Interest in the fibrotic component of left ventricular hypertrophy has been raised by the availability of non‐invasive methodologies: two recent randomized controlled trials of left ventricular hypertrophy regression Citation[347],Citation[356] have been re‐analysed by the echoreflectivity technique, and have found the angiotensin receptor antagonist, losartan, to be significantly more effective than the β‐blocker, atenolol, Citation[219] in decreasing an echoreflectivity index of myocardial fibrosis, Citation[217],Citation[366] and another angiotensin receptor antagonist, candesartan, to be effective on the same index to an equal extent as an ACE inhibitor, enalapril. Citation[367] Biochemical indices of fibrosis e.g. propeptide of procollagen types I and III, were found to change in the direction of decreased collagen content in patients receiving losartan but not in those receiving atenolol in one study, Citation[219] but not in another one. Citation[368] In two comparative studies, natriuretic peptides decreased with losartan and increased with atenolol, Citation[356],Citation[369] suggesting opposite effects on left ventricular compliance.
Some evidence for different effects of various antihypertensive agents on left ventricular hypertrophy is also available from electrocardiographic studies. The LIFE trial showed that losartan was significantly more effective than atenolol in inducing regression of electrocardiographic indices of left ventricular hypertrophy, Citation[370] in parallel with what was shown in the echocardiographic substudy. Citation[357] Lower values of in‐treatment electrocardiographic hypertrophy were significantly associated with lower rates of cardiovascular morbidity and mortality. Citation[195] In two smaller studies, another angiotensin receptor antagonist, irbesartan, has also been found to be more effective than atenolol, Citation[371] and the ACE inhibitor enalapril more than the calcium antagonist nisoldipine on electrocardiographic indices of left ventricular hypertrophy. Citation[372]
Much less information is available on the comparative effects of different antihypertensive treatments on the diastolic abnormalities frequently occurring in hypertensive patients, often but not always concomitant with ventricular hypertrophy. Citation[210] Two studies that showed a greater reduction of left ventricular mass with angiotensin receptor blockers (losartan, irbesartan) than with atenolol were both unable to show different effects of the compared regimens on echocardiographic indices of diastolic function, Citation[356],Citation[373] but neither required recruited patients to have signs of diastolic abnormalities. Large trials having left ventricular diastolic dysfunction as primary endpoint are currently ongoing.
Attention has recently been concentrated on echocardiographic measurement of left atrial size, as a frequent correlate of left ventricular hypertrophy Citation[374] and a predictor of cardiovascular events, Citation[375] in parallel to growing evidence that antihypertensive agents may exert different effects on development of atrial fibrillation. Citation[376] Two large hypertension trials Citation[377,378] have shown that the angiotensin receptor blockers, losartan and valsartan, are associated with a lower incidence of new atrial fibrillation than the β‐blocker, atenolol, and the calcium antagonist, amlodipine, respectively. A lower incidence of new atrial fibrillation was also observed in three heart failure trials, when the ACE inhibitor, enalapril Citation[379] or the angiotensin receptor antagonists, candesartan Citation[380] and valsartan Citation[381] were compared with placebo as add‐on therapy. In the LIFE trial decreased incidence of atrial fibrillation correlated with regression of left ventricular hypertrophy. Citation[382] Smaller studies have addressed the effects of angiotensin receptor antagonists on recurrent atrial fibrillation in patients with previous episodes of arrhythmia. They have reported favourable effects of either irbesartan versus placebo Citation[383] and losartan versus amlodipine, Citation[384] the drugs being in both cases added to amiodarone. Thus there is strong evidence concerning new atrial fibrillation and less strong evidence concerning recurrent atrial fibrillation in favour of beneficial effects of angiotensin receptor blockers as compared with β‐blockers, calcium antagonists or placebo. No comparative data are available between angiotensin receptor blockers and ACE inhibitors. In this area, more information may come from ongoing specific trials. Citation[385]
4.5.2 Arterial wall and atherosclerosis
Meta‐analyses of randomized studies using carotid artery intima‐media thickness as an endpoint Citation[386] are made difficult by the remarkable differences between studies: a number of them are of insufficient statistical power for assessing small differences between difficult measurements, others have not used internal controls to avoid reading bias and regression to the mean, and finally those having used the common carotid only as an endpoint (index of vascular hypertrophy) can hardly be analysed together with those that have used a composite endpoint including the bifurcation and/or the internal carotid (more reliable index of atherosclerosis).
As far as the common carotid is concerned, three studies of active therapy versus placebo were unable to find any greater efficacy of ACE inhibitors Citation[387,388] or a β‐blocker. Citation[389] Comparison of different antihypertensive regimens has shown no different effect of an ACE inhibitor versus a thiazide diuretic Citation[390] and a consistently greater effect of various calcium antagonists over, respectively, a thiazide, Citation[391] a β‐blocker Citation[220,221] and an ACE inhibitor. Citation[392] Therefore, current evidence suggests that calcium antagonists may have a greater effect on hypertension related thickening (presumably hypertrophy) of the carotid artery than other antihypertensive agents.
As to the composite endpoint of carotid intima‐media thickening including bifurcation and/or internal carotid (therefore a likely index of atherosclerosis), placebo controlled studies have shown a greater effect of active treatment with a calcium antagonist, Citation[393] an ACE inhibitor, Citation[394] and a β‐blocker, Citation[389] possibly indicating the antiatherosclerotic effect of blood pressure lowering. Comparison of different antihypertensive regimens achieving the same blood pressure levels has also shown consistently greater effects of calcium antagonists than, respectively, hydrochlorothiazide, Citation[395] chlorthalidone Citation[222] and atenolol, Citation[220,221] but a recent study has also shown a greater effect of an ACE inhibitor than of a thiazide diuretic. Citation[390] The ELSA study Citation[220,221] has also found that lower progression of the composite carotid intima‐media thickness is paralleled by lower progression and greater regression of plaque number with lacidipine than with atenolol. Composition of the carotid wall, investigated by an echoreflectivity approach histologically tested, Citation[396] did not show significantly different changes with both lacidipine and atenolol, however. Citation[397] In conclusion, sufficient evidence appears to be available to conclude that progression of carotid atherosclerosis can be delayed by lowering blood pressure, but that calcium antagonists have a greater efficacy than diuretics and β‐blockers, and ACE inhibitors more than diuretics.
Although pulse wave velocity is acknowledged as a valid clinical method for assessing large artery distensibility, there is a paucity of adequate studies investigating the effects of antihypertensive therapy per se and of different antihypertensive regimens on this vascular parameter. Many of the studies have been small, non‐comparative or non‐randomized, and it is difficult to conclude whether the described decrease in pulse wave velocity (hence in stiffness) was due to the blood pressure decrease, to specific properties of the agents employed or to regression to the mean.
A number of small, placebo‐controlled, relatively short‐term (only a few weeks) studies suggests that several antihypertensive agents can indeed favourably affect pulse wave velocity, Citation[398] but the observed decrease could well be due to blood pressure reduction. This conclusion is strengthened by a recent study of more or less intense blood pressure lowering, in which a significant reduction in pulse wave velocity was only found in the more intensely treated group. Citation[399] Whether different agents exert different effects is still largely unclear; four recent comparative studies have given conflicting results, Citation[400–403] probably due to insufficient statistical power of each study.
4.5.3 Brain and cognitive function
A limited number of randomized trials of antihypertensive therapy have used brain lesions and cognitive dysfunction as endpoints. Citation[404] One small substudy of the PROGRESS trial has explored the effect of blood pressure lowering on progression of white matter disease (magnetic resonance imaging) and shown a significant reduction in mean total volume of new lesions in the group in which perindopril plus indapamide treatment reduced blood pressure by 11/4 mmHg more than placebo. Citation[405]
Trials using cognitive measurements as endpoints have been the object of a recent meta‐analysis. Citation[406] The three studies on 13143 subjects that have used the Mini Mental State Evaluation Test for cognitive performance Citation[283],Citation[407,408] found a small but significant improvement for a blood pressure difference (versus placebo) of −4.8/−2.6 mmHg. The five studies on 717 subjects that have investigated the effect of blood pressure reduction on logical memory test Citation[409–413] found that a reduction in blood pressure of 3.2/1.5 mmHg (versus placebo) was associated with a significantly better performance both on the immediate and the delayed task results. On the other hand, the four randomized studies on 2396 individuals Citation[409–412],Citation[414] that have analysed perceptual processing and sequential abilities found that a mean blood pressure reduction of 17.1/7.0 mmHg was associated with a small but significant decline in the test. Therefore, it appears that lowering blood pressure may improve performance on screening tests for dementia and memory, further supporting the benefits of antihypertensive therapy on cerebrovascular morbidity. However, performance or perceptual processing and learning capacity may not benefit from blood pressure lowering, suggesting that different cognitive functions may be differently influenced. It should be emphasized that trials showing no benefit in perceptual and learning tests were associated with a much greater blood pressure reduction, and thus a J‐shaped effect cannot be excluded. Citation[406]
Finally, many of the trials testing cognitive function compared active antihypertensive agents with placebo, and those comparing different antihypertensive regimens are few. Therefore there is no firm evidence on whether some antihypertensive agents are more beneficial than others in preserving or improving cognition. However, it should be mentioned that the only placebo‐controlled study that reported a significant reduction in incident dementia used the calcium antagonist nitrendipine as an active agent. Citation[275],Citation[407]
4.5.4 Renal function and disease
A very large number of randomized studies has investigated the effects of antihypertensive therapy on a diversity of renal endpoints such as microalbuminuria or proteinuria, glomerular filtration rate and end stage renal disease in a variety of conditions, such as diabetes, diabetic nephropathy, non‐diabetic renal disease, or simply hypertension. Because of the diversity of the clinical conditions, of the endpoint used, as well as of the size and statistical power of the studies, the issue is not an ideal one for meta‐analyses, as shown by the hot debate raised by a recent meta‐analysis. Citation[415–417] Probably the best approach is that of critical and selective reviews of available data. Citation[418,419]
A major issue is whether in the presence of renal disease renal function is better preserved by a blood pressure goal lower than in uncomplicated hypertension, i.e. below 130/80 mmHg rather than 140/90 mmHg. Although this is recommended by all current guidelines, Citation[3],Citation[30],Citation[420] it must be recognized that evidence from trials having randomized renal patients to more versus less intensive blood pressure lowering is scanty. Evidence is mostly based on the long‐term follow‐up of the MDRD trial, Citation[421] showing a significant reduction of end stage renal disease in patients with predominantly non‐diabetic kidney disease when randomized to mean blood pressure reduction <92 mmHg mHg (i.e. below 120/80 mmHg) rather than <107 mmHg (i.e. below 140/90). However, in other trials randomization to these goals in patients with non‐diabetic renal disease Citation[318] or with diabetes Citation[422] was not accompanied by greater preservation of renal function than randomization to a somewhat higher blood pressure. In a further trial in diabetic normotensive patients, bringing blood pressure to <120/80 mmHg by valsartan did not influence creatinine clearance to a greater extent than less intense treatment achieving blood pressures slightly above 120/80 mmHg, but urinary protein excretion was favourably influenced by more aggressive therapy. Citation[423] In another trial on non‐diabetic nephropathy, further blood pressure lowering by adding a calcium antagonist to an ACE inhibitor Citation[424] did not further reduce the incidence of end stage renal disease and proteinuria. However, the positive data of the MDRD are strengthened by analyses, admittedly retrospective and observational, of the IDNT trial Citation[425] and of 11 trials in non‐diabetic renal patients, showing that systolic blood pressure reduction down to a least 120 mmHg may be beneficial. Citation[426] Finally the dispute about the blood pressure goal to preserve renal function in diabetic patients may be futile in view of the evidence available about the benefits of intense blood pressure reduction in these patients, even below 130 mmHg systolic and 80 mmHg diastolic as far as cardiovascular events are concerned. Citation[311],Citation[422],Citation[427–429]
Nephroprotective properties of antihypertensive agents, mostly ACE inhibitors or angiotensin receptor antagonists, have been investigated in a large number of randomized trials. Several placebo controlled studies have shown angiotensin receptor antagonists, ACE inhibitors or a low dose ACE inhibitor‐diuretic combination to delay end stage renal disease or a significant increase in serum creatinine, and to reduce or prevent microalbuminuria or proteinuria, in patients with both diabetic and non‐diabetic nephropathy. Citation[308,309],Citation[428], Citation[430–435] An antiproteinuric effect versus placebo has been shown also with the use of spironolactone. Citation[436] Except in one study, Citation[430] in all other placebo controlled studies the renal effects of the active drug were accompanied by a slightly greater blood pressure reduction, which may have been at least partly responsible for the renal effects. In fact, also a calcium antagonist (nitrendipine) has been shown in the SYST‐EUR trial to better preserve renal function than placebo. Citation[437]
Comparison of different active regimens has provided less clear results. Two trials, one in patients with proteinuric diabetic nephropathy Citation[309] the other in non‐diabetic nephropathy Citation[317] have shown superiority of an angiotensin receptor antagonist or an ACE inhibitor over a calcium antagonist in delaying end stage renal disease and significant increases in serum creatinine, but a post‐hoc subanalysis of the ALLHAT trial on those hypertensive patients who had reduced renal function at baseline (but proteinuria was unknown) showed equal incidence of these endpoints in patients treated with a diuretic, a calcium antagonist or an ACE inhibitor. Citation[438] Studies measuring changes in glomerular filtration rate have also produced inconsistent results: only one study has shown significantly less decline with an ACE inhibitor than a β‐blocker or a calcium antagonist, Citation[317,318] while other studies were unable to demonstrate different effects of ACE inhibitors compared with a calcium antagonist, Citation[319],Citation[422] or a β‐blocker Citation[316] or an angiotensin receptor antagonist Citation[439] or both a calcium antagonist and a diuretic; Citation[438] equal effect of a calcium antagonist and a diuretic was also shown by another study. Citation[322]
More clear results were obtained when the effects of different antihypertensive regimens on microalbuminuria or proteinuria were compared. Angiotensin receptor blockers were found to be more effective in reducing urinary protein excretion than a β‐blocker, Citation[440] a calcium antagonist Citation[441] or a thiazide, Citation[442] an aldosterone antagonist more than a calcium antagonist, Citation[443] and an ACE inhibitor more than a calcium antagonist. Citation[432] Divergent results should be mentioned, however, as ACE inhibitors were reported to be equally effective as calcium antagonists in three trials, Citation[319],Citation[422],Citation[444] or as a diuretic in another one. Citation[445]
Of interest are several recent studies that have investigated the combination of an angiotensin receptor antagonist with an ACE inhibitor (compared with monotherapies). The COOPERATE study has reported a reduced progression of non‐diabetic nephropathy by the combination versus the combination components in monotherapy without a blood pressure difference between treatment groups. Citation[446] Other studies have shown a greater antiproteinuric action of the combination, associated, however, with a greater blood pressure reduction; Citation[447,448] indeed, when the ACE inhibitor dose was titrated to obtain the same blood pressure decrease as the combination, no difference in the antiproteinuric effect was observed. Citation[449] Available studies have been included in a recent meta‐analysis Citation[450] which has confirmed the greater antiproteinuric action of the combination, associated with a greater blood pressure lowering. On the other hand, two small studies suggest that very high doses of angiotensin receptor antagonists may exert a significantly greater antiproteinuric action than a standard dose without any increment of the antihypertensive effect. Citation[451,452] These studies deserve to be confirmed by larger trials.
4.5.5 New onset diabetes
Diabetes and hypertension are often associated, Citation[453] and their combination is known to have ominous consequences. Citation[454] Awareness that several antihypertensive agents may exert undesirable metabolic effects has prompted investigation (often post‐hoc) of the incidence of new diabetes in antihypertensive treatment trials. Citation[455] Almost all trials of antihypertensive therapy using new onset diabetes as an endpoint have shown a significantly greater incidence in patients treated with diuretics and/or β‐blockers compared with ACE inhibitors, Citation[313],Citation[322],Citation[327],Citation[456] angiotensin receptor antagonists Citation[307],Citation[332],Citation[457] or calcium antagonists. Citation[315],Citation[321,322],Citation[331] Recently, angiotensin receptor antagonists Citation[335] and ACE inhibitors Citation[322] have been shown to be associated with significantly less new diabetes than calcium antagonists. It is difficult to conclude whether agents interfering with the renin‐angiotensin system exert a real antidiabetogenic action, or whether they simply lack a diabetogenic action possessed by β‐blockers and diuretics and, to a lesser degree, by calcium antagonists. Citation[455],Citation[458] The only placebo‐controlled antihypertensive therapy trial that has reported new diabetes, the SHEP trial, has recently described a greater incidence of diabetes in the actively treated arm (with a diuretic and often a β‐blocker). Citation[459] Similar observations appear to have been made in the MRC trial in the elderly Citation[288] according to a recent meta‐analysis, Citation[460] which reports less new diabetes in the placebo than either in the diuretic or the β‐blocker group. Other placebo controlled trials in conditions different from hypertension (high cardiovascular risk, chronic heart failure) have also shown a lower new diabetes incidence in patients treated with ACE inhibitors Citation[306],Citation[461,462] or angiotensin receptor antagonists Citation[463] than in placebo patients, but in all these trials placebo (as well as the active agent) was added on top of multiple drug therapies, among which diuretics and β‐blockers predominated at baseline and could be varied to an unknown extent during the trial. The same confounding factor makes interpretation of the recent negative finding of the DREAM trial Citation[464] difficult: in this trial administration of ramipril to subjects with impaired glucose tolerance was not associated with a lower subsequent incidence of diabetes than administration of placebo. However, almost half of DREAM subjects had hypertension and one third dyslipidaemia, and a large number of them received various types of antihypertensive agents and lipid lowering drugs. A very recent network meta‐analysis of 22 trials with more that 160,000 participants Citation[460] has calculated that the association of antihypertensive agents with new diabetes is lowest for angiotensin receptor antagonists and ACE inhibitors followed by calcium antagonists and placebo, β‐blockers and diuretics in rank order.
It has been suggested that treatment‐related new diabetes may not have the same adverse prognostic effect as ‘spontaneously’ occurring diabetes. This claim is based on the observation that during controlled trials patients developing diabetes have not had a greater morbidity than patients without new onset diabetes. Citation[322] However, it is known that cardiovascular complications follow the onset of diabetes after a time delay (more than 10 years) longer than that possible in controlled randomized trials. Citation[465] Longer term (16–30 years) observational studies have shown a significantly higher incidence of cardiovascular complication in patients having developed diabetes during antihypertensive treatment predominantly with diuretics and/or β‐blockers. Citation[466–470] A notable exception is a 14‐year follow up of the SHEP study, Citation[459] during which newly occurring diabetes among actively treated patients (chlorthalidone plus, eventually, atenolol) was reported not to be associated with increased mortality. A limitation of the above long‐term follow‐up studies is that microvascular endpoints, i.e. complications highly related to hyperglycaemia, were not assessed. Furthermore, in long‐term studies follow‐up cannot be done under controlled conditions and confounding factors may be frequent and unknown. Therefore the claim that treatment‐induced and ‘spontaneous’ onset diabetes may be prognostically different appears impossible to confirm or confute. In the absence of more compelling evidence of an innocuous nature, the increased diabetes incidence with some antihypertensive agents currently raises concerns that would be imprudent to disregard.
5. Therapeutic approach
5.1 When to initiate antihypertensive treatment
The decision to start antihypertensive treatment should be based on two criteria, i.e. 1) the level of systolic and diastolic blood pressure as classified in Table, and ) the level of total cardiovascular risk. This is illustrated in Figure.
All patients in whom repeated blood pressure measurements show grade 2 or 3 hypertension are definite candidates for antihypertensive treatment because, as detailed in the 2003 ESH/ESC Guidelines, Citation[3] a large number of placebo controlled trials have conclusively demonstrated that in patients with these blood pressure values blood pressure reduction lowers the incidence of cardiovascular morbid and fatal events, independently of their level of total risk (i.e. moderate, high or very high). Citation[10],Citation[23],Citation[292],Citation[471] Evidence for the benefit of treating grade 1 hypertensives is admittedly more scant, as specific trials have not addressed the issue. However, the recent finding of the FEVER study on the protective effect of lowering systolic blood pressure to <140 rather than slightly >140 mmHg even in hypertensive patients at moderate risk Citation[301] lends support to the recommendation to consider antihypertensive interventions when systolic blood pressure is ⩾140 mmHg.
In all grade 1 to 3 hypertensives, lifestyle instructions should be given as soon as hypertension is diagnosed or suspected, while promptness in the initiation of pharmacological therapy depends on the level of total cardiovascular risk. In the high risk hypertensives of the VALUE study the treatment arm in which blood pressure control was somewhat delayed was associated with a trend towards more cardiovascular events. Citation[335] Furthermore, in the hypertensive patients of the ASCOT study (who had additional risk factors although total cardiovascular risk was less than that of the VALUE patients) the beneficial effect of the treatment associated with a better blood pressure control was evident within a few months. Citation[472] Therefore in Figure the acceptable time delay to assess the results of life style changes is prudently shorter than indicated in previous guidelines. Citation[3] Drug treatment should be initiated promptly in grade 3 hypertension, as well as in grade 1 and 2 when total cardiovascular risk is high or very high. In grade 1 or 2 hypertensives with moderate total cardiovascular risk drug treatment may be delayed for several weeks and in grade 1 hypertensives without any other risk factor (low added risk) for several months. However, even in these patients lack of blood pressure control after a suitable period of non‐pharmacological interventions should lead to the institution of drug treatment in addition to lifestyle changes.
When initial blood pressure is in the high normal range (130–139/85–89 mmHg), the decision on drug intervention heavily depends on the level of risk. In case of diabetes, history of cerebrovascular, coronary or peripheral artery disease, randomized trials Citation[283],Citation[300],Citation[302],Citation[305],Citation[319] have shown that antihypertensive treatment is associated with a reduction in cardiovascular fatal and non‐fatal events, although in two other trials on coronary patients no benefit of blood pressure lowering was reported Citation[306] or a reduction of cardiovascular events was only seen when initial blood pressure was in the hypertensive range. Citation[304] Evidence is also available that in diabetic patients with an increased urinary protein excretion reductions in blood pressure to very low values (<125/75 mmHg) are associated with reductions in microalbuminuria or proteinuria (i.e. predictors of renal deterioration and cardiovascular risk) Citation[473] as well as with a reduced rate of progression to more severe proteinuric states. This is the case also when initial blood pressure values are below 140/90 mmHg and drugs with a direct antiproteinuric effect such as blockers of the renin‐angiotensin system are used. Citation[319],Citation[474,475] This justifies the recommendation to start administration of blood pressure lowering drugs (together with intense lifestyle changes) even in patients in whom blood pressure is not elevated but in the high normal (and sometimes normal) range, provided that there is associated cardiovascular disease or diabetes.
Whether a similar therapeutic approach (i.e. intense lifestyle changes combined with antihypertensive drug treatment) may also benefit individuals with high normal blood pressure who are at high risk because of the presence of three or more additional risk factors, the metabolic syndrome or organ damage is uncertain. It should be emphasized that prospective observational studies have demonstrated that subjects with high normal blood pressure have a greater incidence of cardiovascular disease compared to people with normal or optimal blood pressure. Citation[7],Citation[11],Citation[33] Furthermore, the risk of developing hypertension is greater in subjects with high normal than in those with normal or optimal blood pressure with an additional increase in risk when, as often occurs, concurrent multiple risk factors and the metabolic syndrome are present. Citation[31,32],Citation[69] Finally, new onset hypertension can be delayed by some time by administration of an antihypertensive agent. Citation[476] In contrast with these potentially favourable arguments stand the negative results of the DREAM trial, Citation[464] which showed that administration of ramipril to subjects with metabolic disturbances (mostly with high normal blood pressure or grade 1 and 2 hypertension) did not significantly delay onset of diabetes or reduced cardiovascular events despite blood pressure lowering. Unfortunately, the DREAM study was not powered for assessing effects on cardiovascular events, and sufficiently powered trials are necessary to clarify this important issue. For the time being, subjects with a high cardiovascular risk due to factors other than diabetes but a blood pressure still in the high normal range should be advised to implement intense lifestyle measures (including smoking cessation) and blood pressure should be closely monitored because of the relatively high chance these individuals have to progress to hypertension, Citation[31,32] which will then require drug treatment. However, physicians and patients may sometimes consider antihypertensive drugs, particularly those more effective in protecting against organ damage, new onset hypertension and new onset diabetes. Lifestyle measures and close blood pressure monitoring should be the intervention procedures in subjects with a normal blood pressure who are at low or moderate added risk.
5.2 Goals of treatment (Box 8)
The primary goal of treatment of the hypertensive patient is to achieve the maximum reduction in the long‐term total risk of cardiovascular morbidity and mortality. This requires treatment of all the reversible risk factors identified, including smoking, dyslipidaemia, abdominal obesity or diabetes, and the appropriate management of associated clinical conditions, as well as treatment of the raised blood pressure per se.
Box 8 Position statement: Goals of treatment
In hypertensive patients, the primary goal of treatment is to achieve maximum reduction in the long‐term total risk of cardiovascular disease.
This requires treatment of the raised BP per se as well as of all associated reversible risk factors.
BP should be reduced to at least below 140/90 mmHg (systolic/diastolic), and to lower values, if tolerated, in all hypertensive patients.
Target BP should be at least <130/80 mmHg in diabetics and in high or very high risk patients, such as those with associated clinical conditions (stroke, myocardial infarction, renal dysfunction, proteinuria).
Despite use of combination treatment, reducing systolic BP to <140 mmHg may be difficult and more so if the target is a reduction to <130 mmHg. Additional difficulties should be expected in elderly and diabetic patients, and, in general, in patients with cardiovascular damage.
In order to more easily achieve goal BP, antihypertensive treatment should be initiated before significant cardiovascular damage develops.
5.2.1 Blood pressure target in the general hypertensive population
The 2003 ESH‐ESC Guidelines, Citation[3] while recommending to lower blood pressure below 140/90 in all hypertensive subjects, admitted that this was only a prudent recommendation, since trial evidence of the benefit of achieving this goal was limited to patients with diabetes or previous cardiovascular disease, and to a post‐hoc analysis of the HOT trial, Citation[311] indicating the lowest event incidence to be at blood pressures around 138/83 mmHg. In addition to the evidence reviewed in the 2003 guidelines, Citation[3] further indirect evidence supporting a blood pressure goal <140 mmHg has been provided by post‐hoc analyses of the VALUE and INVEST trials. In the VALUE study Citation[477] hypertensive patients in whom blood pressure was ‘controlled’ by treatment (<140/90 mmHg) had a significantly lower incidence of stroke, myocardial infarction, heart failure as well as cardiovascular morbidity and mortality than those remaining ‘uncontrolled’, independent of the antihypertensive regimens to which they were allocated. Lower rates of non‐fatal and fatal cardiovascular events have also been reported in ‘controlled’ versus ‘uncontrolled’ hypertensive patients of the INVEST study. Citation[478] All this is consistent with what has been reported in studies on hypertensive patients followed in the setting of clinical practice, those achieving blood pressure values <140/90 mmHg showing a cardiovascular morbidity and mortality rate much less than those treated but uncontrolled. Citation[479] Admittedly, data obtained outside intention‐to‐treat analyses of randomized trials should be interpreted with caution. However, it should be pointed out that the recommendation of achieving a target blood pressure below 140/90 mmHg is founded now on direct data, since the recent FEVER study Citation[301] showed that hypertensive patients randomized to active treatment, who achieved blood pressure values of 138.1/82.3 mmHg, had a 28% reduction in stroke, coronary events, and cardiovascular mortality as compared with those randomized to placebo, who remained at values of 141.6/83.9 mmHg.
There are also arguments in favour of trying to achieve values below 90 mmHg diastolic and 140 mmHg systolic, i.e. as close as possible to optimal blood pressure, if well tolerated by the patient. 1) The results of the HOT study Citation[311] have shown that there was no increase in cardiovascular risk in patients randomized to the lowest target blood pressure group, a finding that is relevant to clinical practice because setting lower blood pressure goals would allow a greater number of subjects to at least meet the traditional goals. 2) Observational studies show a direct linear relationship with cardiovascular events of systolic and diastolic blood pressure values as low as 115–110 and 75–70 mmHg, respectively, without evidence within this range of a J curve phenomenon. Citation[7],Citation[11] 3) Evidence that achieving lower blood pressure targets by treatment may enhance protection in hypertensive patients at higher risk, is detailed below.
5.2.2 Blood pressure targets in diabetic and very high or high risk patients
In order to maximize cardiovascular protection, in diabetic patients it has been recommended that antihypertensive treatment should be more intense, and a goal blood pressure of <130/80 mmHg has been proposed. There is very solid evidence of a beneficial effect (reduction in macro and microvascular complications) of a greater versus a smaller blood pressure reduction in type 2 diabetic patients as demonstrated by the HOT and UKPDS trials, Citation[311],Citation[427] and confirmed by the ABCD studies. Citation[319],Citation[422] A recent meta‐analysis of available trials in diabetic patients has calculated a reduced incidence of cardiovascular events (particularly stroke) with more versus less intense treatment, for a between‐group difference in systolic and diastolic blood pressure averaging 6.0 and 4.6 mmHg, respectively. Citation[296] Nevertheless, evidence on the benefit of the strict goal of <130/80 mmHg is more limited. Several randomized trials have shown the benefit of reducing diastolic blood pressure to values very close to or even below 80 mmHg, Citation[311],Citation[319],Citation[422],Citation[427] but very few data are available on the beneficial effect of systolic blood pressure targets <130 mmHg. However, 1) in the ABCD studies Citation[319],Citation[422] on diabetic hypertensives or nor‐motensives achieved systolic blood pressure values of 132 and 128 mmHg, respectively, were associated with lower incidence of outcomes (total mortality and stroke, respectively) than in the groups with slightly less rigorous blood pressure control (systolic blood pressure of 138 and 137 mmHg, respectively), and 2) a prospective observational study within the UKPDS programme has found a significant relationship between follow‐up systolic blood pressure and incidence of macro and microvascular complications in diabetic patients, with a continuous increment in complications for values >120 mmHg. Citation[429]
Data favouring lower blood pressure targets in patients in whom a high risk condition is due to factors other than diabetes are of variable strength. The most clear evidence concerns patients with previous stroke or transient ischaemic attack, since in the PROGRESS study Citation[283] subjects with a history of cerebrovascular disease in whom treatment reduced blood pressure from 147/86 mmHg to 138/82 mmHg showed a 28% reduction in stroke recurrence and 26% reduction in the incidence of major cardiovascular events compared with placebo in which the blood pressure reduction was negligible. There were substantial cardiovascular benefits also in normotensive patients in whom on‐treatment values were reduced to 127/75 mmHg. Furthermore, in a recent post‐hoc analysis of the PROGRESS data a progressive reduction in the incidence of stroke recurrence (particularly haemorrhagic stroke) has been reported until achieved systolic blood pressure values of about 120 mmHg. Citation[480] Lower levels of evidence are available for other high risk groups. In a post‐hoc subgroup analysis of the HOT study Citation[481] greater reductions in diastolic and systolic blood pressure (82 versus 85 mmHg and 142–145 versus 145–148 mmHg) were associated with a greater benefit in patients with a high or very high total cardiovascular risk (50% of the HOT population) but not in patients at a lower level of risk. In placebo controlled trials in survivors from a myocardial infarction administration of β‐blockers or ACE inhibitors Citation[482,483] reduced the incidence of recurrent myocardial infarction and death even when blood pressure was normal. However, because of the assumption of a protective effect of these drugs per se, blood pressure was seldom considered as a possible mechanism and often unreported, although when mentioned it was lower in the actively treated than in the placebo groups. Yet, it has been noted in section 5.1 that most placebo controlled trials in patients with angina pectoris or coronary heart disease Citation[302],Citation[304,305] have provided evidence of reduced incidence of cardiovascular events by bringing blood pressure to rather low levels (EUROPA: 128/78 rather than 133/80 mmHg; ACTION‐hypertensives: 137/77 rather than 144/81 mmHg; CAMELOT: 124/76 rather than 130/77 mmHg) although in another trial on anginal patients similar blood pressure targets (129/74 mmHg rather than 132/76 mmHg) provided no further benefit. Citation[306]
There are no sufficient cardiovascular outcome data upon which to recommend a lower target blood pressure in patients with non‐diabetic renal disease, but sufficient though not conclusive evidence suggests that values lower than 130/80 mmHg may help preserve renal function, especially in the presence of proteinuria (see Section 4.5.4).
5.2.3 Home and ambulatory blood pressure targets
The growing evidence of the prognostic importance of home and ambulatory blood pressure makes these measurements more and more commonly employed to evaluate efficacy of treatment. For ambulatory blood pressure this approach is supported by the evidence that for similar achieved office blood pressure values, lower achieved ambulatory blood pressures are associated with a lower rate of cardiovascular outcomes. Citation[88] However, no evidence is so far available indicating which values of home and ambulatory blood pressure should be considered as optimal targets. Home and ambulatory blood pressures are several mmHg lower than office blood pressures (Table), but these differences are proportional to the level of office blood pressure values, Citation[484] i.e. they are usually larger when office blood pressure is high and smaller at the lower office blood pressure values representing treatment goals. Citation[77] This, and the greater blood pressure lowering effect of treatment on clinic as compared with 24‐h blood pressure values, Citation[485] makes it likely that optimal target blood pressures are not too different when measured in‐ and out‐of‐office.
5.2.4 Conclusions
On the basis of current evidence it can be recommended that blood pressure be lowered at least to below 140/90 mmHg in all hypertensive patients and that lower values be pursued if tolerated. Antihypertensive treatment should be more aggressive in diabetics, in whom a target blood pressure of <130/80 mmHg appears a reasonable one. Similar targets should be adopted in individuals with a history of cerebrovascular disease and can at least be considered in patients with coronary disease. Although differences between individual patients may exist, the risk of underperfusion of vital organs is very low, except in episodes of postural hypotension that should be avoided, particularly in the elderly and diabetic. The existence of a J‐shaped curve relating outcomes to achieved blood pressure has so far been suspected as a result of post‐hoc analyses Citation[486–490] which have reported, however, the rate of events to increase at quite low diastolic pressures. Further evidence that an inflection of the curve may only occur at blood pressure levels much lower than those aimed at with intense antihypertensive therapy is provided by randomized studies in post‐myocardial infarction or chronic heart failure patients, in whom β‐blockers or ACE inhibitors reduced the incidence of cardiovascular events despite lowering blood pressure from already quite low initial systolic and diastolic values. Citation[482],Citation[491]
It should be mentioned that, despite wide use of multidrug treatment, in most trials the achieved average systolic blood pressure remained above 140 mmHg, Citation[492] and even in trials achieving average blood pressure values <140 mmHg, the control rate included at most 60–70% of recruited patients. In diabetic subjects average on‐treatment values <130 mmHg were never obtained, Citation[492] except in the ABCD normotensive trial that recruited subjects with initially normal or high normal blood pressures. Citation[319] Reaching the target blood pressures recommended above may thus be difficult and the difficulty may be greater when initial blood pressures are higher and in the elderly since age makes the elevation in systolic blood pressure strictly dependent on increased aortic fibrosis and stiffness. Trial evidence also shows that for the same or even a greater use of combination treatment achieved systolic blood pressure remains usually somewhat higher in diabetics than in non‐diabetics. Citation[249],Citation[428],Citation[493]
5.3 Cost‐effectiveness of antihypertensive treatment
Several studies have shown that in high or very high risk patients, treatment of hypertension is largely cost effective, that is that the reduction in the incidence of cardiovascular disease and death largely offsets the cost of treatment despite its lifetime duration. Citation[494] Indeed, it is likely that the benefit is even greater than that calculated by the number of events saved per year of treatment and expressed by the so called number needed to treat or ‘NNT’. Citation[495] 1) In several placebo‐controlled trials a substantial number of patients randomized to placebo received treatment and a number of patients allocated to active treatment actually withdrew from it while continuing to be considered in the original groups according to the intention‐to‐treat principle; Citation[273] 2) Some trials show that the difference in event incidence between treated and placebo groups increases progressively over the few years of the trial duration, raising the possibility of a greater long‐term protective effect of blood pressure reductions; 3) In younger low risk hypertensives what appears to be as a relatively small benefit when calculated over a treatment period of 5 years may translate into a more substantial number of added life years compared with elderly high risk hypertensives. Citation[274] This implies that in younger subjects actuarial information may provide a better assessment of the benefit than data obtained in trials. Citation[496] In young patients the purpose of treatment is not to prevent an unlikely morbid or fatal event in the subsequent few years, but rather to prevent onset and/or progression of organ damage that will, in the long term, convert the low risk patient into a higher risk one. Several trials of antihypertensive therapy, foremost the HDFP Citation[312] and HOT Citation[497] studies, have shown that despite intense blood pressure lowering the incidence of cardiovascular events remains much higher in high risk hypertensives or hypertensives with complications than in hypertensives with initial low or moderate risk. This suggests that some of the major cardiovascular risk changes may be difficult to reverse, and that restricting antihypertensive therapy to patients at high or very high risk may be far from an optimal strategy. Finally, the cost of drug treatment of hypertension is often contrasted to lifestyle measures, which are considered cost‐free. However, real implementation, and therefore effectiveness, of lifestyle changes requires behavioural support, counselling and reinforcement, the cost of which may not be negligible. Citation[498,499]
6. Treatment strategies
6.1 Lifestyle changes (Box 9)
Lifestyle measures should be instituted, whenever appropriate, in all patients, including subjects with high normal blood pressure and patients who require drug treatment. The purpose is to lower blood pressure, to control other risk factors and clinical conditions, and to reduce the number and doses of antihypertensive agents which might have to be subsequently used. The lifestyle measures that are widely agreed to lower blood pressure or cardiovascular risk, and that should be considered in all patients are: 1) smoking cessation, 2) weight reduction in the overweight, 3) moderation of alcohol consumption, 4) physical activity, 5) reduction of salt intake and 6) increase in fruit and vegetable intake and decrease in saturated and total fat intake. Citation[500] Healthy eating habits should always be promoted. However, lifestyle measures are unproved in preventing cardiovascular complications in hypertensive patients, and long‐term compliance with their implementation is notoriously low. Citation[501] They should never delay unnecessarily the initiation of drug treatment, especially in patients at higher levels of risk.
Box 9 Position statement: Lifestyle changes
Lifestyle measures should be instituted, whenever appropriate, in all patients, including those who require drug treatment. The purpose is to lower BP, to control other risk factors and to reduce the number of doses of antihypertensive drugs to be subsequently administered.
Lifestyle measures are also advisable in subjects with high normal BP and additional risk factors to reduce the risk of developing hypertension.
The lifestyle measures that are widely recognized to lower BP or cardiovascular risk, and that should be considered are:
smoking cessation
weight reduction (and weight stabilization)
reduction of excessive alcohol intake
physical exercise
reduction of salt intake
increase in fruit and vegetable intake and decrease in saturated and total fat intake
Lifestyle recommendations should not be given as lip service but instituted with adequate behavioural and expert support, and reinforced periodically.
Because long‐term compliance with lifestyle measures is low and the BP response highly variable, patients under non‐pharmacological treatment should be followed‐up closely to start drug treatment when needed and in a timely fashion.
6.1.1 Smoking cessation
Smoking causes an acute increase in blood pressure and heart rate, persisting for more than 15 minutes after smoking one cigarette. Citation[502] The mechanism is likely to be a stimulation of the sympathetic nervous system at central level and at nerve endings, which is responsible for an increase in plasma catecholamines parallel to the blood pressure increase. Citation[503,504] Paradoxically, several epidemiological studies have found that blood pressure levels among cigarette smokers were the same as, or lower than, those in non‐smokers. Citation[505] However, studies using ambulatory blood pressure monitoring have shown that both untreated hypertensive and normotensive smokers present higher daily blood pressure values than non‐smokers, Citation[506–508] the increase being particularly pronounced in heavy smokers. Citation[502] Smoking has also been reported to predict a future rise in systolic blood pressure, Citation[509] but no independent chronic effect of smoking on blood pressure has been found in all studies Citation[510] and smoking cessation does not lower blood pressure. Citation[511]
Smoking is a powerful cardiovascular risk factor Citation[512] and smoking cessation is probably the single most effective lifestyle measure for the prevention of a large number of cardiovascular diseases including stroke and myocardial infarction. Citation[512–514] This is supported by the observation that those who quit smoking before middle age typically have a life expectancy that is not different from that of lifelong non‐smokers. Citation[515,516] Therefore, hypertensive smokers should be counselled regarding smoking cessation.
Where necessary, nicotine replacement Citation[517] or bupropion therapy should be considered since they appear to facilitate smoking cessation. Citation[518] Varenicline is a novel selective nicotine acetylcholine receptor partial agonist developed specifically for smoking cessation, with documented short‐ and long‐term efficacy versus placebo. Citation[519] Passive smoking has now been shown to produce an increase in the risk of coronary and other smoking‐related diseases. Citation[520,521] Exposure to passive smoking may have declined in those countries where regulations have been introduced to protect the non‐ and ex‐smokers from environmental tobacco smoke. It is desirable that this become commonplace all over Europe.
6.1.2 Moderation of alcohol consumption
Many studies have shown a U or J shaped association of mortality with alcohol consumption, in which light and moderate drinking results in a reduced mortality compared with non‐drinkers, while heavy drinkers have a rising death rate, Citation[522] but this relationship has recently been challenged by a meta‐analysis of available data. Citation[523] The relationship between alcohol consumption, blood pressure levels and the prevalence of hypertension is linear in populations. Citation[524] Beyond that, high levels of alcohol consumption are associated with high risk of stroke; Citation[525] this is particularly so for binge drinking. Alcohol attenuates the effects of antihypertensive drug therapy, but this effect is at least partially reversible within 1–2 weeks by moderation of drinking by around 80%. Citation[526] Heavier drinkers (five or more standard drinks per day) may experience a rise in blood pressure after acute alcohol withdrawal and be more likely to be diagnosed as hypertensive at the beginning of the week if they have a weekend drinking pattern. Trials of alcohol reduction have shown a significant reduction in systolic and diastolic blood pressures. Citation[500] Hypertensive men who drink alcohol should be advised to limit their consumption to no more than 20–30 g ethanol per day, and hypertensive women to no more than 10–20 g ethanol per day. They should be warned against the increased risk of stroke associated with binge drinking.
6.1.3 Sodium restriction
Epidemiological studies suggest that dietary salt intake is a contributor to blood pressure elevation and to the prevalence of hypertension. Citation[527,528] Randomized controlled trials in hypertensive patients Citation[500] indicate that reducing sodium intake by 80–100 mmol (4.7–5.8 g of sodium chloride) per day from an initial intake of around 180 mmol (10.5 g of sodium chloride) per day reduces blood pressure by an average of 4–6 mmHg, Citation[529–533] although with a large between patient variability. Sodium restriction may have a greater antihypertensive effect if combined with other dietary counselling Citation[500] and may allow reduction of doses and number of antihypertensive drugs employed to control blood pressure. The effect of sodium restriction on blood pressure is greater in blacks, middle‐aged and older people as well as in individuals with hypertension, diabetes, or chronic kidney disease, i.e. groups that have a less responsive renin‐angiotensin‐aldosterone system, Citation[534] whose activation, together with an activation of the sympathetic nervous system, Citation[535,536] may counteract the blood pressure lowering effect of sodium restriction. In a restricted salt diet, patients should be advised to avoid added salt, and obviously oversalted food (particularly processed food) and to eat more meals cooked directly from natural ingredients containing more potassium. Citation[537] An excessive intake of salt may be a cause of resistant hypertension. The recommended adequate daily sodium intake has been recently reduced from 100 to 65 mmol/day corresponding to 3.8 g/day of sodium chloride, which may be currently difficult to achieve. An achievable recommendation is less than 5 g/day sodium chloride (85 mmol/day). Citation[538]
6.1.4 Other dietary changes
Over the past decade, increased potassium intake and dietary patterns based on the DASH diet (a diet rich in fruits, vegetables, and low‐fat dairy products, with a reduced content of dietary cholesterol as well as saturated and total fat) Citation[539] have emerged as also having blood pressure lowering effects. Several small clinical trials and their meta‐analyses have documented that high‐dose omega‐3 polyunsaturated fatty acid supplements (commonly called fish oil) can lower blood pressure in hypertensive individuals although the effect can usually be seen only at relatively high doses (⩾3 g/day). Citation[500],Citation[540,541] In hypertensive individuals, average systolic and diastolic blood pressure reductions were 4.0 and 2.5 mmHg, respectively. Citation[542] As to an increased intake of fibre alone, Citation[543,544] the data are insufficient to recommend it for blood pressure lowering. Supplemental calcium or magnesium Citation[500],Citation[545,546] has been proposed as a means to lower blood pressure, but data are not entirely consistent and additional research is warranted before recommendations on other specific diets can be made, including diets with a modified content in carbohydrates. Citation[500],Citation[547,548] As a general measure, hypertensive patients should be advised to eat more fruits and vegetables (4–5 servings or 300 grams of vegetables per day), Citation[549] to eat more fish Citation[550] and to reduce intake of saturated fat and cholesterol. Counselling by trained dieticians may be useful.
6.1.5 Weight reduction
A substantial body of evidence from observational studies documents that body weight is directly associated with blood pressure Citation[551] and that excess body fat predisposes to increased blood pressure and hypertension. Citation[552] There is also conclusive evidence that weight reduction lowers blood pressure in obese patients and has beneficial effects on associated risk factors such as insulin resistance, diabetes, hyperlipidaemia, left ventricular hypertrophy, and obstructive sleep apnoea. In a meta‐analysis of available studies, the mean systolic and diastolic blood pressure reductions associated with an average weight loss of 5.1 kg were 4.4 and 3.6 mmHg, respectively. Citation[553] In a further subgroup analysis, blood pressure reductions were similar for non‐hypertensive and hypertensive individuals, but were greater in those who lost more weight. Within trial dose‐response analyses Citation[554,555] and prospective observational studies Citation[556] also document that greater weight loss leads to a greater blood pressure reduction. Modest weight loss, with or without sodium reduction, can prevent hypertension in overweight individuals with high normal blood pressure, Citation[557] and can facilitate medication step‐down and drug withdrawal. Citation[558,559] Because in middle aged individuals body weight frequently shows a progressive increase (0.5–1.5 kg per year), weight stabilization may also be considered a useful goal to pursue.
6.1.6 Physical exercise
Lack of physical fitness is a strong predictor of cardiovascular mortality independent of blood pressure and other risk factors. Citation[560] A recent meta‐analysis of randomized controlled trials Citation[561] concluded that dynamic aerobic endurance training reduces resting systolic and diastolic blood pressures by 3.0/2.4 mmHg, and daytime ambulatory blood pressure by 3.3/3.5 mmHg. The reduction in resting blood pressure was more pronounced in the hypertensive group (−6.9/−4.9 mmHg) than in the normotensive one (−1.9/−1.6 mmHg). Even moderate levels of exercise lowered blood pressure, Citation[562] and this type of training also reduced body weight, body fat and waist circumference, and increased insulin sensitivity and HDL‐cholesterol levels. Dynamic resistance training decreased resting blood pressure by 3.5/3.2 mmHg. Citation[563] Thus, sedentary patients should be advised to take up exercise of moderate intensity on a regular basis, e.g. 30–45 min daily. Citation[564] The type of exercise should be primarily endurance physical activity (walking, jogging, swimming) supplemented by resistance exercise. Citation[144],Citation[564,565] The extent of pre‐training evaluation of the cardiovascular status will depend on the extent of the envisaged exercise and on the patient's symptoms and signs, total cardiovascular risk and associated clinical conditions. However, intensive isometric exercise such as heavy weight lifting can have a marked pressor effect and should be avoided. If hypertension is poorly controlled, heavy physical exercise as well as maximal exercise testing should be discouraged or postponed until appropriate drug treatment has been instituted and blood pressure lowered. Citation[566]
6.2 Pharmacological therapy (Boxes 10 and 11)
6.2.1 Choice of antihypertensive drugs
The large number of randomized trials of antihypertensive therapy, both those comparing active treatment versus placebo and those comparing treatment regimens based on different compounds, confirm the conclusion of the 2003 ESH/ESC Guidelines Citation[3] that 1) the main benefits of antihypertensive treatment are due to lowering of blood pressure per se, and are largely independent of the drugs employed, and 2) thiazide diuretics (as well as chlorthalidone and indapamide), β‐blockers, calcium antagonists, ACE inhibitors and angiotensin receptor antagonists can adequately lower blood pressure and significantly and importantly reduce cardiovascular outcomes. Therefore all these drugs are suitable for the initiation and maintenance of antihypertensive treatment either as monotherapy or in some combinations with each other. Each of the recommended classes may have specific properties, advantages and limitations, which are discussed in the following paragraphs so that doctors may make the most appropriate choice in individual patients.
Box 10 Position statement: Choice of antihypertensive drugs
The main benefits of antihypertensive therapy are due to lowering of BP per se.
Five major classes of antihypertensive agents – thiazide diuretics, calcium antagonists, ACE inhibitors, angiotensin receptor antagonists and β‐blockers – are suitable for the initiation and maintenance of antihypertensive treatment, alone or in combination. β‐blockers, especially in combination with a thiazide diuretic, should not be used in patients with the metabolic syndrome or at high risk of incident diabetes.
Because in many patients more than one drug is needed, emphasis on identification of the first class of drugs to be used is often futile. Nevertheless, there are many conditions for which there is evidence in favour of some drugs versus others either as initial treatment or as part of a combination.
The choice of a specific drug or a drug combination, and the avoidance of others, should take into account the following:
The previous favourable or unfavourable experience of the individual patient with a given class of compounds.
The effect of drugs on cardiovascular risk factors in relation to the cardiovascular risk profile of the individual patient.
The presence of subclinical organ damage, clinical cardiovascular disease, renal disease or diabetes which may be more favourably treated by some drugs than others (Box 11 and Table).
The presence of other disorders that may limit the use of particular classes of antihypertensive drugs (Table).
The possibilities of interactions with drugs used for other conditions.
The cost of drugs, either to the individual patient or to the health provider, but cost considerations should never predominate over efficacy, tolerability, and protection of the individual patient.
Continuing attention should be given to side effects of drugs, because they are the most important cause of non‐compliance. Drugs are not equal in terms of adverse effects, particularly in individual patients.
The BP lowering effect should last 24 hours. This can be checked by office or home BP measurements at trough or by ambulatory BP monitoring.
Drugs which exert their antihypertensive effect over 24 hours with a once‐a‐day administration should be preferred because a simple treatment schedule favours compliance.
Box 11 Position statement: Antihypertensive treatment: Preferred drugs
We have mentioned in Section 4.4.5 that in two recent large scale trials Citation[330],Citation[332] and in a recent meta‐analysis Citation[343] β‐blockers had a reduced ability to protect against stroke, though being equally effective for protection from coronary events and mortality. Administration of beta‐blockers has proved to be beneficial in patients with angina pectoris, heart failure and a recent myocardial infarction, important hypertension‐related complications. Citation[482,483],Citation[567] Thus β‐blockers may still be considered an option for initial and subsequent antihypertensive treatment strategies. Because they favour an increase in weight, Citation[568] have adverse effects on lipid metabolism and increase (compared with other drugs) the incidence of new onset diabetes, Citation[455],Citation[458] they should not be preferred, however, in hypertensives with multiple metabolic risk factors including the metabolic syndrome and its major components, i.e. abdominal obesity, high normal or impaired fasting glucose, and impaired glucose tolerance, conditions that make the risk of incident diabetes higher. Citation[569,570] This applies also to thiazide diuretics, which have dyslipidaemic and diabetogenic effects when used at high doses. Citation[455] Thiazides have often been administered together with β‐blockers in trials showing a relative excess of new diabetes, thus making a distinction between the contribution of the two agents difficult. It may not apply, however, to vasodilator β‐blockers, such as carvedilol and nebivolol, which have less or no dysmetabolic action, as well as a reduced incidence of new onset diabetes compared with classical β‐blockers. Citation[571,572] β‐blockers, ACE inhibitors and angiotensin receptor antagonists are less effective in blacks in whom diuretics and calcium antagonists should be preferred. Citation[299],Citation[573]
Trials assessing intermediate endpoints (subclinical organ damage) suggest other differences between various antihypertensive agents or compounds: ACE inhibitors and angiotensin receptor antagonists have been reported to be particularly effective in reducing left ventricular hypertrophy, Citation[349] including the fibrotic component; Citation[219],Citation[367] they are also quite effective in reducing microalbuminuria and proteinuria Citation[308,309],Citation[430–432],Citation[437] and in preserving renal function and delaying renal disease; Citation[308,309],Citation[430,431],Citation[434] calcium antagonists, beside being effective on left ventricular hypertrophy, appear beneficial in slowing down the progression of carotid hypertrophy and atherosclerosis. Citation[220–222],Citation[391,392],Citation[395]
Evidence concerning the benefits of other classes of antihypertensive agents is much more limited. α1‐blockers, central agents (α2‐adrenoreceptor agonists and modulators of imidazoline receptors) have been shown to adequately lower blood pressure and to also have favourable metabolic effects. Citation[574] A blood pressure lowering effect has also been demonstrated with aldosterone antagonists. Citation[575] As the only trial testing an α1‐blocker (the doxazosin arm of the ALLHAT trial) was interrupted before crucial evidence could be obtained, Citation[576] the overall benefits or harm of α1‐blockers for antihypertensive therapy remain unproved. This is the case also for centrally acting drugs and aldosterone antagonists. However, all these agents have been frequently used as added drugs in trials documenting cardiovascular protection and can thus be employed for combination treatment. α1‐blockers have a specific indication in the presence of benign prostatic hypertrophy. Aliskiren, a new drug that is targeting the renin system at its point of activation Citation[577] is already available in the USA and may soon be made available in Europe. This drug has been shown to effectively lower blood pressure in hypertension, both alone and in combination with a thiazide diuretic, Citation[578–580] and also to have an antiproteinuric effect in pre‐clinical studies. Citation[581] It has been suggested that renin may have effects not connected to the classical renin‐angiotensin cascade Citation[577] and be a prognostic factor independent of angiotensin II production. Citation[582] Conclusive evidence that this is the case as well as data on the cardiovascular protective effects of renin inhibition are not yet available.
Identification of the first class of drugs to be used in the management of hypertension has always been a debated issue. However, there is now conclusive evidence from trials that combination treatment is needed to control blood pressure in the majority of patients. Citation[583] Thus, if two or more drugs are taken for the lifetime of the patients it is of marginal relevance which is the one used alone for the first few weeks of therapy. However, drug classes (and even compounds within a given class) differ in type and frequency of adverse effects they may induce, and different individuals may be differently prone to develop a given adverse effect. Furthermore, drugs may have different effects on risk factors, organ damage and cause‐specific events and show specific protective influences in special groups of patients. This makes selection of a given agent alone or in association with other drugs mandatory or advisable according to the circumstances. As a general scenario the choice or the avoidance of drugs should take into account the following: 1) the previous favourable or unfavourable experience of the individual patient with a given class of compounds both in relation to blood pressure lowering and side effects; 2) the effect of drugs on cardiovascular risk factors in relation to the cardiovascular risk profile of the individual patient; 3) the presence of subclinical organ damage, clinical cardiovascular disease, renal disease or diabetes which may be more favourably treated by some drugs than others; 4) the presence of other disorders that may limit the use of particular classes of antihypertensive drugs; 5) the possibility of interactions with drugs used for other conditions present in the patient; 6) the cost of drugs, either to the individual patient or to the health provider. Cost considerations, however, should never predominate over efficacy, tolerability, and protection of the individual patient. Physicians should give preference to drugs that have a long lasting effect and a documented ability to effectively lower blood pressure over the 24 hours with once a day administration. Simplification of treatment improves adherence to therapy, Citation[584] while effective 24‐hour blood pressure control is prognostically important in addition to office blood pressure control. Citation[88] Long‐acting drugs also make the antihypertensive effect more homogeneous over the 24 hours, thus minimizing blood pressure variability. Citation[585]
The criteria listed in this section allow the selection of specific drugs or drug combinations in many patients. Conditions favouring or not favouring, and sometimes contraindicating, various agents are known and listed in detail in Tables and , and in Box 11 while specific therapeutic approaches in special conditions and groups of patients are discussed in more detail in Section 7.
Table 6. Conditions favouring use of some antihypertensive drugs versus others
Table 7. Compelling and possible contraindications to use of antihypertensive drugs
In the initial choice of drugs as well as in the subsequent treatment modifications, particular attention should be given to adverse events, even when of a purely subjective nature, because adverse events are the most important cause of non‐compliance. Citation[584],Citation[586] Adverse events during antihypertensive treatment are not entirely avoidable because they may have, in part, a psychological nature and indeed are also reported during administration of placebo. Citation[291] Great effort should be devoted, however, to limitation of drug‐related side effects and preservation of the quality of life either by switching treatment from the responsible drug to another agent or by avoiding unnecessary increases of the dose of the drug employed. Side effects of thiazide diuretics, β‐blockers and calcium antagonists are dose related whereas there is little or no dose‐dependent increase in side effects with angiotensin receptor antagonists and ACE inhibitors. Citation[587]
6.2.2 Monotherapy (Box 12)
Treatment can start with a single drug, which should initially be administered at low dose. If blood pressure is not controlled, either a full dose of the initial agent can be given or patients can be switched to an agent of a different class (which should also be administered, first at low and then at full dose). Switching to an agent from a different class is mandatory in case the first agent had no blood pressure lowering or induced important side effects. This ‘sequential monotherapy’ approach may allow to find the drug to which any individual patient best responds both in terms of efficacy and tolerability. However, although the so called ‘responder rate’ (systolic and diastolic blood pressure reduction ⩾20 and 10 mmHg, respectively) to any agent in monotherapy is approximately 50%, Citation[588] the ability of any agent used alone to achieve target blood pressure values (<140/90 mmHg) does not exceed 20–30% of the overall hypertensive population except in subjects with grade 1 hypertension. Citation[589,590] Furthermore the procedure is laborious and frustrating for both doctors and patients, leading to low compliance and unduly delaying urgent control of blood pressure in high risk hypertensives. Hopes are placed on pharmacogenomics, which in the future may succeed in identifying the drugs having the best chance of being effective and beneficial in individual patients. Research in this area should be encouraged.
Box 12 Position statement: Monotherapy versus combination therapy
Regardless of the drug employed, monotherapy allows to achieve BP target in only a limited number of hypertensive patients.
Use of more than one agent is necessary to achieve target BP in the majority of patients. A vast array of effective and well tolerated combinations is available.
Initial treatment can make use of monotherapy or combination of two drugs at low doses with a subsequent increase in drug doses or number, if needed (Figures and ).
Monotherapy could be the initial treatment for a mild BP elevation with a low or moderate total cardiovascular risk. A combination of two drugs at low doses should be preferred as first step treatment when initial BP is in the grade 2 or 3 range or total cardiovascular risk is high or very high (Figure).
Fixed combinations of two drugs can simplify treatment schedule and favour compliance.
In several patients BP control is not achieved by two drugs, and a combination of three of more drugs is required.
In uncomplicated hypertensives and in the elderly, antihypertensive therapy should normally be initiated gradually. In higher risk hypertensives, goal blood pressure should be achieved more promptly, which favours initial combination therapy and quicker adjustment of doses.
6.2.3 Combination treatment (Box 12)
In most trials combination of two or more drugs has been the most widely used treatment regimen to reduce blood pressure effectively and reach the predetermined goal. Use of combination therapy has been found to be even more frequently needed in diabetic, renal and high risk patients and in general whenever lower blood pressure targets are pursued. Citation[311] For example, in a recent large scale trial on high risk hypertensives about 9 out of 10 patients were given two or more antihypertensive drugs in order to reduce blood pressure to <140/90 mmHg. Citation[330]
In the 2003 ESH/ESC Guidelines Citation[3] the recommendation was given not to limit two‐drug treatment to a frequently necessary step after attempting monotherapy, but also to consider two‐drug treatment as an alternative to monotherapy as a first choice therapeutic approach (Figure). An obvious disadvantage of initiating treatment with two drugs is that of potentially exposing some patients to an unnecessary agent. The advantages, however, are that 1) by using a combination both the first and the second drug can be given in the low dose range which is more likely to be free of side effects compared to full dose monotherapy; 2) the frustration of repetitively and vainly searching for effective monotherapies in patients with very high blood pressure values or organ damage may be avoided; 3) fixed low dose combinations are available, allowing the two agents to be administered in a single tablet, the treatment simplification optimizing compliance; and 4) starting treatment with a two‐drug combination may allow blood pressure targets to be reached earlier than with monotherapy. This may be of critical importance in high risk patients, because in the VALUE trial greater blood pressure reduction (−3.8/−2.2 mmHg) seen in amlodipine versus valsartan‐treated patients in the first 6 months was accompanied by a difference in cardiovascular event rate in favour of the more effectively treated group. Citation[335] Accordingly, combination treatment should be considered as first choice particularly when there is a high cardiovascular risk, i.e. in individuals in whom blood pressure is markedly above the hypertension threshold (e.g. more than 20 mmHg systolic or 10 mmHg diastolic), or milder degrees of blood pressure elevation are associated with multiple risk factors, subclinical organ damage, diabetes, renal or associated cardiovascular disease. In all these conditions, there is the need to obtain a large blood pressure reduction (due to the high initial values or the low targets), which is difficult to achieve with monotherapy.
Antihypertensive drugs of different classes can be combined if 1) they have different and complementary mechanisms of action, 2) there is evidence that the antihypertensive effect of the combination is greater than that of either combination component, 3) the combination may have a favourable tolerance profile, the complementary mechanisms of action of the components minimizing their individual side effects. The following two‐drug combinations have been found to be effective and well tolerated, and have been favourably used in randomized efficacy trials. They are indicated with a continuous thick line in the diagram of Figure
Figure 4. Possible combinations between some classes of antihypertensive drugs. The preferred combinations in the general hypertensive population are represented as thick lines. The frames indicate classes of agents proven to be beneficial in controlled intervention trials.
Thiazide diuretic and ACE inhibitor
Thiazide diuretic and angiotensin receptor antagonist
Calcium antagonist and ACE inhibitor
Calcium antagonist and angiotensin receptor antagonist
Calcium antagonist and thiazide diuretic
β‐blocker and calcium antagonist (dihydropiridine)
The combination of a thiazide diuretic and a β‐blocker is also a time honoured combination which has been used successfully in many placebo and actively controlled trials, but evidence is now available that these drugs have dysmetabolic effects which may be even more pronounced when they are administered together (Sections 4.4.5 and 4.5.5). Thus, this combination, although still valid as a therapeutic alternative, should be avoided in patients with the metabolic syndrome and when there is a high risk of incident diabetes. The combination of a thiazide and a potassium sparing diuretic (amiloride, triamterene or spironolactone) has been widely used for years in order to prevent the loss of potassium associated with thiazide administration, possibly reducing the incidence of sudden death, Citation[591] preventing glucose intolerance and decreasing the incidence of diabetes associated with thiazide‐induced hypokalaemia. Citation[592,593] The combination of an ACE inhibitor and an angiotensin receptor antagonist has become a focus of recent studies. Although the drugs included in this combination may interfere, albeit at different levels, with the same physiological mechanism, nevertheless their combination has been reported to exert a somewhat greater blood pressure reduction and a more pronounced antiproteinuric effect than either component alone both in diabetic and non‐diabetic nephropathy. Citation[446],Citation[594] This combination has also been shown to improve survival in heart failure. Citation[595] Although it remains unclear whether the advantage of this combination can be replicated by simply increasing the dose of either component in monotherapy, Citation[449],Citation[596] more evidence on the benefits of combining an angiotensin receptor antagonist and an ACE inhibitor will be provided by the ONTARGET trial. Citation[339] Other combinations are possible, but these are less frequently used and evidence on their therapeutic efficacy is more limited. Some of these combinations are indicated by the dotted line in the diagram of Figure.
Finally, combinations between two drugs in a single tablet, usually at low doses, (but sometimes both at lower and at higher doses), are now widely available, particularly those of an angiotensin receptor antagonist with a thiazide diuretic, or of an ACE inhibitor with a thiazide diuretic or with a calcium antagonist, of a β‐blocker with a diuretic, and of a thiazide with a potassium sparing diuretic. Although the fixed dose of the combination components limits the flexibility of upward and downward treatment strategies, fixed combinations reduce the number of tablets to be taken by the patient, and this has some advantage for compliance with treatment. Citation[584],Citation[597] Fixed dose combinations can substitute extemporaneous combinations that have successfully controlled blood pressure, but, when at low doses, they can also be considered for first step treatment, provided that initial use of two drugs rather than monotherapy is indicated. It should be emphasized that two‐drug combinations are not invariably capable of controlling blood pressure and use of three or four drugs may be necessary in several patients, particularly in those with renal disease and other complicated types of hypertension. Further information on the advantages of this therapeutic approach will be available after completion of the ACCOMPLISH trial, Citation[598] which compares the effect on cardiovascular morbidity and mortality of treatment initiated with a fixed dose combination of an ACE inhibitor with a calcium antagonist or a diuretic.
7. Therapeutic approach in special conditions
7.1 Elderly (Box 13)
Older patients benefit from antihypertensive drug treatment in terms of reduced cardiovascular morbidity and mortality, irrespective of whether they have systolic‐diastolic hypertension or isolated systolic hypertension. Citation[294],Citation[471] This has been shown in a large number of randomized trials that have included patients aged 60 or 70 years or more. A meta‐analysis of these trials has shown that a reduction of fatal and non‐fatal cardiovascular events, as well as of stroke, also occurred in treated patients aged 80 years or more although all cause mortality was not reduced. Citation[599] Beneficial effects on morbidity but not on mortality in the very elderly have recently been confirmed in the HYVET Citation[600] pilot trial.
Box 13 Antihypertensive treatment in the elderly
Randomized trials in patients with systolic‐diastolic or isolated systolic hypertension aged ⩾60 years have shown that a marked reduction in cardiovascular morbidity and mortality can be achieved with antihypertensive treatment.
Drug treatment can be initiated with thiazide diuretics, calcium antagonists, angiotensin receptor antagonists, ACE inhibitors, and β‐blockers, in line with general guidelines. Trials specifically addressing treatment of isolated systolic hypertension have shown the benefit of thiazides and calcium antagonists but sub‐analysis of other trials also shows efficacy of angiotensin receptor antagonists.
Initial doses and subsequent dose titration should be more gradual because of a greater chance of undesirable effects, especially in very old and frail subjects.
BP goal is the same as in younger patients, i.e. <140/90 mmHg or below, if tolerated. Many elderly patients need two or more drugs to control blood pressure and reductions to <140 mmHg systolic may be particularly difficult to obtain.
Drug treatment should be tailored to the risk factors, target organ damage and associated cardiovascular and non‐cardiovascular conditions that are frequent in the elderly. Because of the increased risk of postural hypotension, BP should always be measured also in the erect posture.
In subjects aged 80 years and over, evidence for benefits of antihypertensive treatment is as yet inconclusive. However, there is no reason for interrupting a successful and well tolerated therapy when a patient reaches 80 years of age.
The randomized controlled trials that have shown the benefit of antihypertensive treatment versus placebo or no treatment in elderly patients with systolic‐diastolic hypertension used either a diuretic or a β‐blocker as first line therapy. Citation[281,282,],Citation[287,288] A recent meta‐analysis has suggested that in the elderly β‐blockers may have a less pronounced preventive effect on cardiovascular events than diuretics, but in many of these patients diuretics and β‐blockers were used together. Citation[601] In trials of isolated systolic hypertension, first‐line drugs comprised a diuretic Citation[280] or a dihydropyridine calcium channel blocker. Citation[284] Treatment was initiated with the latter drug class also in two Chinese trials, one in systolic‐diastolic hypertension Citation[285] and the other in isolated systolic hypertension, Citation[286] in which alternate rather than random allocation was used. In all these trials active therapy was superior to placebo or no treatment. Other drug classes have only been used in trials in which ‘newer’ drugs were compared with ‘older’ drugs. The STOP‐2 trial Citation[314] found that the incidence of cardiovascular events was similar in elderly hypertensives randomized to a calcium antagonist, an ACE inhibitor, or conventional treatment with a diuretic or a β‐blocker, and ALLHAT Citation[322] showed a diuretic, a calcium channel antagonist and an ACE inhibitor influenced cardiovascular events to the same extent also in the subgroup of patients older than 65 years. The LIFE trial Citation[332] showed that, in 55‐to‐80‐year old hypertensive patients with evidence of left ventricular hypertrophy, the angiotensin receptor antagonist losartan was more effective in reducing cardiovascular events, particularly stroke, than the β‐blocker atenolol, this being also true for patients with isolated systolic hypertension. Citation[602] SCOPE Citation[307] showed a reduction in non‐fatal strokes in hypertensive patients aged 70 years or older treated with an antihypertensive regimen containing the angiotensin receptor antagonist candesartan, in comparison with patients receiving an antihypertensive treatment without candesartan. A subgroup analysis of SCOPE patients with isolated systolic hypertension showed a significant 42% reduction of stroke in candesartan‐treated patients. Citation[603] Therefore, it appears that benefits have been demonstrated in older hypertensive patients for at least one representative agent of several drug classes, i.e. diuretics, β‐blockers, calcium antagonists, ACE inhibitors and angiotensin receptor antagonists. Thus there are insufficient grounds for an age‐depending strategy in the choice of antihypertensive agents. Citation[344]
Initiation of antihypertensive treatment in elderly patients should follow the general guidelines. Before and during treatment blood pressure should always be measured both in the sitting and in the standing position, because their higher risk of postural hypotension may be enhanced by antihypertensive drugs. Citation[604] Older patients more frequently have other risk factors, target organ damage and associated cardiovascular or non cardiovascular clinical conditions than younger ones. This means that the choice of the first drug often needs to be precisely tailored to individual characteristics. Furthermore, many patients will need two or more drugs to control blood pressure, since in the elderly it is often particularly difficult to lower systolic pressure to below 140 mmHg. Citation[492],Citation[605]
The optimum diastolic blood pressure to be achieved by treatment is not clear. In a post‐hoc analysis the SHEP investigators assessed the role of the on‐treatment diastolic blood pressure in patients with isolated systolic hypertension. Citation[606] They concluded that an achieved diastolic pressure of less than 70 mmHg, and especially below 60 mmHg, identifies a high‐risk group that has a poorer outcome. They suggested that this was possibly due to overtreatment. However, in the Syst‐Eur trial there was no evidence of harm down to a diastolic blood pressure of 55 mmHg (below which there were insufficient data), except in the presence of a history of coronary heart disease at baseline. Citation[607] In addition, in the same trial a low diastolic blood pressure was associated with higher non‐cardiovascular mortality also in the placebo group, suggesting that the excess risk of these patients is not due to overtreatment. A higher cardiovascular and non‐cardiovascular mortality for diastolic and systolic blood pressure values below 60 and 120 mmHg, respectively, has been reported in a meta‐analysis of several thousand patients. Citation[487] This suggests reverse casualty, i.e. that an initially high risk may be responsible for an excessive blood pressure reduction during treatment and not vice versa. Further studies are needed to determine how far blood pressure can be safely lowered in elderly patients and, in particular, which levels of diastolic blood pressure can be accepted in order to pursue optimal control of isolated systolic hypertension by treatment.
7.2 Diabetes mellitus (Boxes 14 and 15)
Diabetes consists of two distinct forms, ‘type 1’, which occurs usually in younger subjects and is characterized by (β cell destruction and absolute insulin deficiency, and ‘type 2’, which is more typical of the middle to older age range and is characterized by a reduction in the ability of insulin to favour transportation of glucose across the membrane of skeletal muscle cells, although insulin secretory defects may also be present. Citation[168] By far the commoner form of the disease is type 2 diabetes, which occurs about 10–20 times more frequently than insulin dependent type 1 diabetes, and has a prevalence of hypertension up to 70–80%. Citation[453]
Box 14 Antihypertensive treatment in diabetics
Where applicable, intense non‐pharmacological measures should be encouraged in all diabetic patients, with particular attention to weight loss and reduction of salt intake in type 2 diabetes.
Goal BP should be <130/80 mmHg and antihypertensive drug treatment may be started already when BP is in the high normal range.
To lower BP, all effective and well tolerated drugs can be used. A combination of two or more drugs is frequently needed.
Available evidence indicates that lowering BP also exerts a protective effect on appearance and progression of renal damage. Some additional protection can be obtained by the use of a blocker of the renin‐angiotensin system (either an angiotensin receptor antagonist or an ACE inhibitor).
A blocker of the renin‐angiotensin system should be a regular component of combination treatment and the one preferred when monotherapy is sufficient.
Microalbuminuria should prompt the use of antihypertensive drug treatment also when initial BP is in the high normal range. Blockers of the renin‐angiotensin system have a pronounced antiproteinuric effect and their use should be preferred.
Treatment strategies should consider an intervention against all cardiovascular risk factors, including a statin.
Because of the greater chance of postural hypotension, BP should also be measured in the erect posture.
Box 15 Antihypertensive therapy in patients with renal dysfunction
Renal dysfunction and failure are associated with a very high risk of cardiovascular events.
Protection against progression of renal dysfunction has two main requirements: a) strict blood pressure control (<130/80 mmHg and even lower if proteinuria is >1 g/day); b) lowering proteinuria to values as near to normal as possible.
To achieve the blood pressure goal, combination therapy of several antihypertensive agents (including loop diuretics) is usually required.
To reduce proteinuria, an angiotensin receptor blocker, an ACE inhibitor or a combination of both are required.
There is controversial evidence as to whether blockade of the renin–angiotensin system has a specific beneficial role in preventing or retarding nephrosclerosis in non‐diabetic non‐proteinuric hypertensives, except perhaps in Afro‐American individuals. However, inclusion of one of these agents in the combination therapy required by these patients appears well founded.
An integrated therapeutic intervention (antihypertensive, statin and antiplatelet therapy) has to be frequently considered in patients with renal damage because, under these circumstances, cardiovascular risk is extremely high.
It has been clearly established that the co‐existence of hypertension and diabetes mellitus of either type substantially increases the risk of developing renal and other organ damage, leading to a much greater incidence of stroke, coronary heart disease, congestive heart failure, peripheral artery disease and cardiovascular mortality. Citation[454] As described in section 3.6.3, the presence of microalbuminuria is an early marker of renal disease Citation[245] and an indicator of increased cardiovascular risk. Citation[178],Citation[186],Citation[248] Data on cardiovascular protection by antihypertensive treatment are limited in type 1 diabetes, in which, however, there is evidence that conventional and ACE inhibitor treatment delay progression of nephropathy. Citation[434],Citation[608]
Available evidence discussed in section 4.4 leaves no doubt that in type 2 diabetes blood pressure lowering has a remarkable cardiovascular protective effect regardless of the drug(s) employed. Citation[296],Citation[609] Placebo controlled studies with positive results have used diuretics (often combined with β‐blockers), calcium antagonists, and ACE inhibitors. This allows the conclusion that even in diabetes cardiovascular benefit largely originates from blood pressure lowering per se. A recent meta‐analysis suggests that lower blood pressure goals may induce even greater cardiovascular benefits in type 2 diabetics than in non‐diabetics. Citation[296] The recommendation of initiating treatment when blood pressure is still in the high normal range and of bringing blood pressure to below 130/80 mmHg is supported by the data discussed in Sections 5.1 and 5.2. Whether these lower blood pressure levels also help retard diabetic nephropathy is less clearly established (see Section 4.5.4).
Several controlled randomized trials have investigated whether in type 2 diabetes some antihypertensive drugs may have specific renal protective properties that could enhance the protection associated with blood pressure lowering per se. As discussed in Section 4.5.4 there is evidence for superiority of angiotensin receptor antagonists and ACE inhibitors, which is particularly strong for prevention and reduction of microalbuminuria and proteinuria.
In conclusion, in type 2 diabetic patients it can be recommended to lower blood pressure, whenever possible, to <130/80 mmHg. Intensive lifestyle measures should be implemented with particular emphasis on interventions (caloric restriction and increased physical activity) favouring weight reduction, because overweight and obesity are common in type 2 diabetes, and weight reduction is associated with some decrease in blood pressure and an improvement in glucose tolerance. Citation[168] Antihypertensive drugs should also be considered when blood pressure is in the high normal range and in the presence of microalbuminuria. Citation[319],Citation[473–475] All antihypertensive agents can in principle be considered, keeping in mind that effective blood pressure control can be particularly difficult to achieve in diabetes and that combination of two or more agents may frequently be needed. β‐blockers and thiazide diuretics should not be preferred as the first step drugs because they may worsen insulin resistance and lead to increased doses or numbers of anti‐diabetic agents. Citation[316],Citation[331] Available evidence suggests that in the presence of microalbuminuria or diabetic nephropathy, treatment must start with or include a drug acting against the renin‐angiotensin system. Because of the recent evidence that in type 2 diabetics ACE inhibition prevents appearance of microalbuminuria Citation[432] ACE inhibitors may be recommended also as primary preventive intervention against nephropathy. Lipid lowering agents should also be considered because of the result of the CARDS trial, which indicated that diabetic patients benefit from having their lipids tightly controlled. Citation[610]
7.3 Cerebrovascular disease (Box 16)
7.3.1 Stroke and transient ischaemic attacks
The 2003 ESH‐ESC Guidelines already presented evidence that antihypertensive therapy provides a benefit in patients with a history of stroke or transient ischaemic attacks. This was based on the results of two double‐blind placebo‐controlled randomized trials (PATS using the diuretic indapamide Citation[289] and PROGRESS using the ACE inhibitor perindopril in frequent association with indapamide Citation[283]), both showing an about 30% reduction in recurrent stroke in actively treated patients. These two trials reported benefits both in patients who were hypertensive and in those who were normotensive at baseline. A trend towards a beneficial effect of ACE inhibitors versus placebo was also observed in a subgroup of patients recruited in the HOPE trial who had a history of stroke. Citation[611] Thus blood pressure reduction represents an effective secondary preventive strategy in patients with cerebrovascular disease even when initial blood pressure is below 140/90 mmHg, as discussed in Section 5.1.
Box 16 Antihypertensive treatment in patients with cerebrovascular disease
In patients with a history of stroke or transient ischaemic attacks, antihypertensive treatment markedly reduces the incidence of stroke recurrence and also lowers the associated high risk of cardiac events.
Antihypertensive treatment is beneficial in hypertensive patients as well as in subjects with BP in the high normal range. BP goal should be <130/80 mmHg.
Because evidence from trials suggests that the benefit largely depends on BP lowering per se, all available drugs and rational combinations can be used. Trial data have been mostly obtained with ACE inhibitors and angiotensin receptor antagonists, in association with or on the top of diuretic and conventional treatment, but more evidence is needed before their specific cerebrovascular protective properties are established.
There is at present no evidence that BP lowering has a beneficial effect in acute stroke but more research is under way. Until more evidence is obtained antihypertensive treatment should start when post‐stroke clinical conditions are stable, usually several days after the event. Additional research in this area is necessary because cognitive dysfunction is present in about 15% and dementia in 5% of subjects aged ⩾65 years.
In observational studies, cognitive decline and incidence of dementia have a positive relationship with BP values. There is some evidence that both can be somewhat delayed by antihypertensive treatment.
Since the publication of the 2003 Guidelines, further evidence has accumulated to clarify the role of antihypertensive therapy in patients with cerebrovascular disease. Additional analysis of PROGRESS shows that the benefit involves both ischaemic and haemorrhagic stroke, Citation[283] and that its size is proportional to the magnitude of the blood pressure reduction. Citation[480] In this trial combination treatment with perindopril and indapamide lowered systolic blood pressure by 12.3 mmHg and stroke incidence by 43% (36% ischaemic stroke and 76% haemorrhagic stroke), whereas perindopril alone caused only a small systolic blood pressure reduction and a non significant (5%) stroke protective effect. The level to which blood pressure should be lowered to achieve maximal benefits among survivors from stroke and transient ischaemic attacks is not precisely known, though this post‐hoc analysis of the PROGRESS Citation[480] suggests a goal of below 130 mmHg systolic.
Data concerning the use of angiotensin receptor antagonists have also accumulated. A subgroup analysis of the SCOPE trial has shown a significant reduction of strokes and major cardiovascular events in patients with a history of stroke, who were randomized to candesartan rather than to control therapy plus placebo. Citation[612] As summarized in section 4.4.4, in the MOSES trial Citation[333] in hypertensive patients with previous cerebrovascular events incidence of cardiovascular events was 31% less with the angiotensin receptor antagonist eprosartan than with the calcium antagonist nitrendipine, but reduction of stroke recurrence (12%) did not reach the level of statistical significance. Overall, if the role of blood pressure reduction appears to be very well established, the comparative efficacy of different antihypertensive agents in preventing recurrence of stroke requires to be investigated further.
Limited information is available on the desired extent and best methods of blood pressure lowering in acute stroke. Anecdotal evidence and pathophysiological data suggest that, because in acute stroke cerebral autoregulation is impaired (particularly in and around the infarcted or haemorrhagic area), rapid blood pressure reductions may lead to underperfusion of the penumbra area and extension of the damage. Citation[613] However, in a recent trial on 339 hypertensive patients, administration of candesartan from the first day after stroke significantly and markedly reduced cumulative 12 months mortality and number of cardiovascular events. Citation[614] As candesartan was administered to both treatment groups, except during the first few days during which one group only received the angiotensin receptor antagonist, this might have exerted either a blood pressure independent protective effect or a protective effect due to prompter blood pressure control. Other randomized studies on blood pressure management during acute stroke are necessary to clarify the matter, and a few are under way. Citation[615,616] For the time being, caution should be used in lowering blood pressure in the first hours after a stroke, also in view of the finding that the elevated blood pressure values often seen in these circumstances tend to spontaneously decrease over the following days. Citation[614] On the other hand, marked blood pressure elevations may be life threatening in these severely compromised patients, and a prompt blood pressure reduction is necessary in the presence of pulmonary oedema, aortic dissection and a recent myocardial infarction. Under all circumstances blood pressure should be reduced slowly under carefully controlled conditions.
7.3.2 Cognitive dysfunction and dementia
Several observational studies show that high blood pressure is associated with cognitive dysfunction and that in hypertensive subjects or in subjects with a history of hypertension several forms of dementia are more frequent than in people with normal blood pressure. Citation[270–272] High blood pressure is known to lead to small vessel disease which is responsible for lacunar infarcts and white matter lesions, both of which are more frequent in hypertensive individuals and associated with cognitive deterioration. Citation[270],Citation[617–620]
Whilst there is unequivocal evidence that reduction in blood pressure is associated with a decrease in the risk of stroke, more subtle forms of cerebrovascular disease such as white matter lesions, cognitive impairment and dementia progression are influenced less clearly. In Section 4.5.3 results of trials that have explored the effects of antihypertensive therapy, mostly versus placebo, on various cognitive functions have been discussed, with the help of a recent meta‐analysis. Citation[406] Overall, lowering blood pressure was found to slightly improve cognitive performance and memory, but not to benefit learning capacity. For the present time cognitive impairment in hypertensives may be considered as an indication for blood pressure reduction, but additional research in this area is necessary because evidence is preliminary and cognitive dysfunction is present in about 15% of individuals aged ⩾65 years with a 5% prevalence of dementia rising to 25% at age ⩾85 years. Citation[621]
7.4 Coronary heart disease and heart failure (Box 17)
Patients with coronary heart disease often have elevated blood pressure values or a history of hypertension, Citation[622] and after a myocardial infarction the risk of a subsequent fatal or non‐fatal coronary event is greater if blood pressure is raised. Citation[623,624] Immediately or some time after a myocardial infarction, several β‐blockers and ACE‐inhibitors and angiotensin receptor antagonists have been tested in placebo or active controlled randomized trials, commonly with significant reductions in cardiovascular morbidity or mortality. Citation[340,341],Citation[482,483],Citation[625] In many instances the trial design focused on investigating direct organ protective properties of the agents rather than the effect of blood pressure reduction, to the point that in some of these studies blood pressure changes were unreported. When blood pressure changes were reported, almost invariably blood pressure was found to be lower in actively treated patients, so that the relative weight of direct and blood pressure mediated benefits cannot be easily unravelled. Independently of the mechanisms, there is clear evidence favouring administration of antihypertensive agents such as β‐blockers, ACE inhibitors and angiotensin receptor antagonists in patients with a recent myocardial infarction, particularly if complicated by systolic dysfunction. Citation[482,483],Citation[625]
Box 17 Antihypertensive treatment in patients with coronary heart disease and heart failure
In patients surviving a myocardial infarction, early administration of β‐blockers, ACE inhibitors or angiotensin receptor antagonists reduces the incidence of recurrent myocardial infarction and death. These beneficial effects can be ascribed to the specific protective properties of these drugs but possibly also to the associated small BP reduction.
Antihypertensive treatment is also beneficial in hypertensive patients with chronic coronary heart disease. The benefit can be obtained with different drugs and drug combinations (including calcium antagonists) and appears to be related to the degree of BP reduction. A beneficial effect has been demonstrated also when initial BP is <140/90 mmHg and for achieved BP around 130/80 mmHg or less.
A history of hypertension is common while a raised BP is relatively rare in patients with congestive heart failure. In these patients, treatment can make use of thiazide and loop diuretics, as well as of β‐blockers, ACE inhibitors, angiotensin receptor antagonists and antialdosterone drugs on top of diuretics. Calcium antagonists should be avoided unless needed to control BP or anginal symptoms.
Diastolic heart failure is common in patients with a history of hypertension and has an adverse prognosis. There is at present no evidence on the superiority of specific antihypertensive drugs.
As to patients with chronic coronary heart disease, the results of four recent placebo‐controlled trials have been summarized in Section 4.2, with three trials, Citation[302–305] but not the fourth one, Citation[306] showing improved cardiovascular outcome associated with blood pressure lowering. The important role of blood pressure lowering in patients with coronary heart disease is supported by a post‐hoc analysis of the INVEST study showing that, irrespective of the type of treatment, in hypertensive patients with known coronary heart disease the incidence of cardiovascular events decreased steeply in relation to the achieved blood pressure value and was markedly less in patients with blood pressure control versus those without control. Citation[478]
Among trials comparing different antihypertensive regimens the INVEST study reported the incidence of coronary and cardiovascular events to be similar in hypertensive coronary patients treated with verapamil (plus eventually trandolapril) or atenolol (plus eventually hydrochlorothiazide). Citation[330] This finding has been complemented by the data from a large subgroup of hypertensive coronary patients in the ALLHAT trial which showed similar incidences of coronary and cardiovascular events by treatment with chlorthalidone, lisinopril or amlodipine. Citation[322]
Thus it appears that patients with coronary heart disease benefit from blood pressure lowering interventions and that it does not matter much by which drug blood pressure is reduced; in particular claims that calcium antagonists may be dangerous in coronary patients have been disproved. Obviously, in coronary patients it may be prudent to lower blood pressure gradually and to avoid tachycardia.
Raised blood pressure is infrequently seen in patients with overt heart failure because of pump failure and reduction in cardiac output. A number of randomized trials has shown improved survival or less hospitalization by the administration of antihypertensive drugs. Treatment can make use of thiazide and loop diuretics, as well as β‐blockers, antialdosterone drugs, ACE inhibitors and angiotensin receptor antagonists administered on top of diuretic therapy (see Section 4). In patients with heart failure, if hypertension persists after the use of these agents, dihydropiridine calcium antagonists can be added, particularly if there is concomitant angina. Evidence is growing, however, that a significant proportion of chronic heart failure patients, particularly hypertensive and elderly subjects, do not present with systolic dysfunction, but rather with ‘diastolic’ dysfunction of the left ventricle (see Section 3.6.1). A recent trial has reported angiotensin receptor antagonist administration to be associated with a modest degree of benefit in patients with heart failure and preserved systolic function, Citation[626] but the evidence is still limited and the advantage of antihypertensive drug administration in this common form of heart failure needs confirmation from ongoing trials.
7.5 Atrial fibrillation
Hypertension is the most important risk factor for atrial fibrillation on a population basis. Citation[627] Atrial fibrillation increases the risk of cardiovascular morbidity and mortality by approximately 2 to 5 fold with a marked increase in the risk of embolic stroke. Citation[628] Increased left ventricular mass and enlargement of the left atrium have been identified as independent determinants of new onset atrial fibrillation. Citation[215] Hypertensive patients with these alterations appear to require intensive antihypertensive therapy. Blood pressure control appears to be strictly required when anticoagulant treatment is given because stroke and bleeding episodes are more frequent when systolic blood pressure is ⩾140 mmHg. Citation[629] In view of the results of post‐hoc analyses of two recent trials, Citation[376–378] showing less incidence of new atrial fibrillation with angiotensin receptor antagonists (see Section 4.5.1), these agents may be preferable, although confirmation from ongoing trials is desirable.
In patients with previous atrial fibrillation, two studies have reported less recurrence by adding angiotensin receptor antagonists to amiodarone Citation[383,384] (see Section 4.5.1). The studies mentioned above were both relatively small, and confirmation by large ongoing trials is desirable before administration of these agents is firmly recommended for secondary prevention of atrial fibrillation. For the time being, however, angiotensin receptor antagonists may be preferred also in patients with previous episodes of atrial fibrillation who require antihypertensive therapy. In a meta‐analysis involving published data on primary and secondary prevention of atrial fibrillation, ACE inhibitors and angiotensin receptor antagonists reduced the incidence of these episodes to a similar extent in patients with paroxis‐mal atrial fibrillation and congestive heart failure. Citation[630] This suggests that blockade of the renin‐angiotensin system by either class of agents is beneficial. In permanent atrial fibrillation, β‐blockers and non‐dihydropiridine calcium antagonists (verapamil and diltiazem) remain important classes of drugs in order to control ventricular rate.
7.6 Non‐diabetic renal disease (Box 15)
Before antihypertensive treatment became available, renal involvement was frequent in patients with primary hypertension. In 1955 Perera Citation[631] described that proteinuria was present in 42%, and chronic renal failure in 18%, in a series of 500 patients he had followed until death. In this series, life expectancy after the onset of renal involvement was reported to be no more than 5–7 years. After the advent of antihypertensive agents, renal complications of hypertension were considered to be relatively infrequent, but with the introduction of formulae estimating either glomerular filtration rate or creatinine clearance it has been realized that a not insignificant proportion of hypertensive patients has deranged renal function, which in turn is an important risk factor for cardiovascular disease. Citation[252]
As summarized in Section 4.5.4, there is sufficient evidence to recommend that blood pressure be lowered to at least 120/80 mmHg in these patients, particularly when proteinuria is present. In several studies blockade of the renin‐angiotensin system has been shown to be superior in delaying end stage renal disease and increase of serum creatinine, and in reducing proteinuria and microalbuminuria. Citation[318],Citation[430],Citation[442] Admittedly, this has not been found in other studies, e.g. in ALLHAT, Citation[438] but reaching a very low blood pressure goal usually requires combination therapy, and therefore it appears reasonable to suggest that any combination should include either an ACE inhibitor or an angiotensin receptor antagonist and that in the few cases in which a single agent can be used, this should be a blocker of the renin‐angiotensin system. If the blood pressure goal is achieved, but proteinuria remains >1.0 g/day (or >1 g/g creatinine) therapy should be further intensified. Citation[632] In this regard, there are promising data by the use of ACE inhibitors and angiotensin receptor antagonists in combination Citation[446],Citation[450] or of high doses of angiotensin receptor antagonists, Citation[451,452] provided careful attention is paid to possible rises in serum creatinine and potassium. However, this is an area where additional research is required before firm recommendations can be made.
7.7 Hypertension in women (Box 18)
Women typically have lower systolic blood pressure levels than men in the 30‐ to 44 year age groups. Citation[633] However, systolic blood pressure rises more steeply with age in females than in males Citation[634] which means that at or beyond 60 years of age, women have a higher blood pressure and greater prevalence of hypertension. The continuous relationship between blood pressure and cardiovascular disease is similar in females and males, except for the lower absolute incidence of coronary disease in females before old age. Citation[635] In a meta‐analysis of individual patients, the beneficial effect of antihypertensive treatment versus placebo was found to be similar in the two genders. Citation[295] No gender‐based meta‐analysis has yet been made of trials comparing different active treatments but most studies have shown similar risk reductions by the various regimens in either gender group, with the exception of the ANBP 2 trial, which reported the benefit of enalapril‐ over the hydrochlorothiazide‐based treatment to be limited to males, Citation[327] and the VALUE trial, which reported amlodipine to be more effective than valsartan in lowering blood pressure and reducing cardiac events in women but not in men. Citation[636]
Box 18 Hypertension in women
Treatment of hypertension in women
Response to antihypertensive agents and beneficial effects of BP lowering appear to be similar in women and in men. However, ACE inhibitors and angiotensin receptor antagonists should be avoided in pregnant and pregnancy planning women because of potential teratogenic effects during pregnancy.
Oral contraceptives
Even low oestrogen oral contraceptives are associated with increased risk of hypertension, stroke and myocardial infarction. The progestogen‐only pill is a contraceptive option for women with high BP, but influence on cardiovascular outcomes has been insufficiently investigated.
Hormone replacement therapy
There is evidence that the only benefit of this therapy is a decreased incidence of bone fractures and colon cancer, accompanied, however, by increased risk of coronary events, stroke, thromboembolism, breast cancer, gallbladder disease, and dementia. This therapy is not recommended for cardioprotection in postmenopausal women.
Hypertension in pregnancy
Hypertensive disorders in pregnancy, particularly pre‐eclampsia, may adversely affect neonatal and maternal outcomes.
Non‐pharmacological management (including close supervision and restriction of activities) should be considered for pregnant women with SBP 140–149 mmHg or DBP 90–95 mmHg. In the presence of gestational hypertension (with or without proteinuria) drug treatment is indicated at BP levels ⩾140/90 mmHg. SBP levels ⩾170 or DBP ⩾110 mmHg should be considered an emergency requiring hospitalization.
In non‐severe hypertension, oral methyldopa, labetalol, calcium antagonists and (less frequently) β‐blockers are drugs of choice.
In pre‐eclampsia with pulmonary oedema, nitroglycerine is the drug of choice. Diuretic therapy is inappropriate because plasma volume is reduced.
As emergency, intravenous labetalol, oral methyldopa and oral nifedipine are indicated. Intravenous hydralazine is no longer the drug of choice because of an excess of perinatal adverse effects. Intravenous infusion of sodium nitroprusside is useful in hypertensive crises, but prolonged administration should be avoided.
Calcium supplementation, fish oil and low dose aspirin are not recommended. However, low dose aspirin may be used prophylactically in women with a history of early onset pre‐eclampsia.
A most important recommendation about antihypertensive treatment in women is avoidance of potentially teratogenic drugs in the child bearing age. Among current antihypertensive agents, ACE inhibitors and angiotensin receptor antagonists should be avoided in fertile women, or immediately withdrawn in case of pregnancy.
7.7.1 Oral contraceptives
Oral contraceptives result in a mild elevation of blood pressure in most women and in established hypertension in about 5%. Citation[637,638] The risk of cardiovascular complications is found primarily in women over 35 years of age and in those who smoke. Citation[638] Hypertension induced by oral contraception is usually mild and blood pressure returns to normal within 6 months of withdrawal. There are conflicting reports on the role of oral contraceptives in the induction of accelerated hypertension Citation[639] whereas some studies have related oral contraceptives to biopsy‐proven renal damage in the absence of primary renal disease. Citation[640] Oestrogens are commonly believed to be the main factor responsible for the blood pressure raising effect, but the mechanisms are as yet unknown. Citation[640] Although oestrogens have been reported to improve endothelial function, Citation[641] their administration may also stimulate the hepatic synthesis of angiotensinogen. Citation[642] Furthermore, arterial distensibility fluctuates during the menstrual cycle in relation to changes in oestrogen concentration, Citation[643] and the use of oral contraceptives has been reported to be associated with an increased albuminuria. Citation[644]
Preparations with an oestrogen content of 30 µg and progestogen of 1 mg or less are regarded to be relatively safe. However, a cross‐sectional survey of a stratified random sample of English women showed that, despite the fact that most combined oral contraceptives used in England in 1994 contained low‐dose oestrogen, there were slightly but significantly higher blood pressure values (2.3/1.6 mmHg) among oral contraceptive users. Citation[637] In a large prospective cohort study in American nurses, a doubling in the adjusted relative risk for hypertension was documented in current users of low‐dose oral contraceptives. Citation[638]
Several case‐control studies performed in the late 1960s supported an association between use of oral contraceptives and stroke. Citation[645–647] Despite recent data Citation[648] questioning whether this association is clinically important when low‐dose oral contraceptives are used, a recent systematic review of combined oral contraceptive use in women with hypertension does show a higher risk for stroke and acute myocardial infarction in contraceptive users than in non‐users. Citation[649] Thrombotic stroke has also been reported to be more frequent with use of oral contraceptives which is associated with a 2‐ to 6‐fold increase in the relative risk of venous thromboembolic disease. Citation[650]
The progestogen‐only pill is a contraceptive option for women shown to have high blood pressure, either induced by use of combined oral contraceptives or due to other causes. So far no significant association between hypertension and use of progestogen‐only pills has been found over 2–4 years of follow‐up, Citation[651] but this matter has not been addressed by randomized studies because family planning is largely a matter of personal choice, which makes random allocation to interventional and control arms difficult and ethically questionable.
7.7.2 Hormone replacement therapy
In Western societies, women show a steeper increase in systolic blood pressure after the menopause, but whether this is due to the effect of age or the menopause is debated because studies that have explored this issue have obtained diverging results, i.e. an association of the menopause with higher blood pressure values, Citation[652–655] but also no significant blood pressure differences. Citation[656–658] The most recent cross‐sectional study in 18,326 women Citation[652] indicates that the menopause has some blood pressure increasing effects, but this is small (about 3/3 mmHg) and largely masked by the pressor effect of ageing.
There is no question, however, that after the menopause women are at an increased risk of cardiovascular disease and that the menopause has an adverse impact on many cardiovascular risk factors. This has brought about the interest in investigating the cardiovascular impact of hormone replacement therapy. A number of observational studies showed that women taking hormone replacement therapy had better cardiovascular risk profiles Citation[659] and a reduced prevalence of coronary disease Citation[660] and stroke Citation[661,662] compared to those not taking hormone replacement therapy. Furthermore, a smaller increase in systolic blood pressure over time was reported in postmenopausal women taking hormone replacement therapy compared to controls. Citation[663] However, rather than confirming cardiovascular benefit, recent large intervention trials have shown an increased risk of cancer and cardiovascular disease with hormone replacement therapy. Citation[664,665] A recent Cochrane systematic review indicates that the only significant benefit of this therapy was a decreased incidence of bone fractures and colon cancer, accompanied, however, by a significantly increased risk of coronary events, stroke, thromboembolism, breast cancer, gallbladder disease and, in women over 65 years of age, dementia. Citation[666] Therefore, at the present time, hormone replacement therapy is not recommended forcardioprotection in postmenopausal women. Citation[667]
7.7.3 Hypertension in pregnancy
Hypertensive disorders in pregnancy remain a major cause of maternal, fetal and neonatal morbidity and mortality worldwide. Blood pressure normally falls in the second trimester, reaching values that are approximately 15 mmHg lower than before pregnancy. In the third trimester values return to, or may exceed, the pre‐pregnancy levels. The above fluctuations occur in normotensive women as well as in those who were previously hypertensive or develop pregnancy‐specific hypertension.
The definition of hypertension in pregnancy is not uniform. Citation[2],Citation[668] However, while in the past the definition was based on an elevation in blood pressure during the second trimester from a baseline reading in the first trimester or before pregnancy, a definition based on absolute blood pressure values (systolic blood pressure ⩾140 mmHg or diastolic blood pressure ⩾90 mmHg) is now preferred. Citation[669] The diagnosis of hypertension in pregnancy should be based on at least two high blood pressure readings on two separate occasions. However, 24 hour blood pressure values have been shown to be superior to conventional measurements in predicting proteinuria, risk of pre‐term delivery, infant weight at birth and in general outcome of pregnancy. Citation[670–672] For both diagnostic and treatment purposes it may thus be useful to perform ambulatory blood pressure monitoring, particularly in high‐risk pregnant women with hypertension, or those with diabetic or renal damage. Until recently, the recommendation was to identify diastolic blood pressure by the Korotkoff phase IV (muffling of the sound), which was reported to more closely correspond to intra‐arterial diastolic blood pressure, in contrast to phase V (disappearance of the sound) which was believed to often indicate too low values. Citation[673] However, phase IV is more difficult to detect and has a limited reproducibility. Citation[674] Korotkoff phase V is now recommended for the measurement of diastolic blood pressure in pregnancy, Citation[675,676] with phase IV being indicated only if Korotkoff sounds persist at cuff pressures approaching 0 mmHg.
Hypertension in pregnancy comprises:
Pre‐existing hypertension, which complicates 1–5% of pregnancies and is defined as blood pressure ⩾140/90 mmHg that either predates pregnancy or develops before 20 weeks of gestation, usually persisting more than 42 days post partum. It may be associated with proteinuria.
Gestational hypertension, which is pregnancy‐induced hypertension without proteinuria. Gestational hypertension associated with significant proteinuria (>300 mg/l or >500 mg/24‐h or dipstick 2+ or more) is known as pre‐eclampsia. Hypertension develops after 20 weeks of gestation and, in most cases, it resolves within 42 days post partum. Gestational hypertension is characterized by poor organ perfusion.
Pre‐existing hypertension plus superimposed gestational hypertension with proteinuria. Pre‐existing hypertension is associated with further worsening of blood pressure and a protein excretion rate ⩾3 g/day in 24‐hour urine collection after 20 weeks of gestation. It corresponds to the previous definition of ‘chronic hypertension with superimposed pre‐eclampsia’.
Antenatally unclassifiable hypertension. Hypertension with or without systemic manifestations based on blood pressure measurements after 20 weeks of gestation with no confirmation of previous values. Under these circumstances re‐assessment is necessary at or after 42 days post partum. If hypertension is resolved, the condition should be re‐classified as gestational hypertension with or without proteinuria. If hypertension is not resolved, the condition should be reclassified as pre‐existing hypertension.
Oedema occurs in up to 60% of normal pregnancies, and is no longer used in the diagnosis of pre‐eclampsia.
Hypertensive disorders in pregnancy, particularly gestational hypertension with or without proteinuria, may produce haematologic, renal and hepatic alterations that may adversely affect both neonatal and maternal outcomes.
Non‐pharmacologic management Citation[677] should be considered for pregnant women with systolic blood pressure of 140–149 mmHg and/or diastolic blood pressure of 90–95 mmHg as measured in the clinical setting. Depending on the blood pressure level, gestational age and presence of maternal and fetal risk factors, management may include close supervision and limitation of activities. A normal diet without salt restriction is advised. Interventions aimed at reducing the incidence of gestational hypertension, especially pre‐eclampsia, such as calcium supplementation (2 g/d), Citation[678] fish oil supplementation Citation[679] and low‐dose acetylsalicylic acid therapy Citation[680] have failed to consistently produce the benefits expected, especially on the fetus, and are thus not recommended. However, low‐dose aspirin is used prophylactically in women who have a history of early onset (<28 weeks) pre‐eclampsia. Although helpful in reducing blood pressure, weight reduction is not recommended during pregnancy in obese women because of its possible association with reduced neonatal weight and lower subsequent infant growth. Citation[681]
The value of continued administration of antihypertensive drugs to pregnant women with pre‐existing mild to moderate blood pressure elevations continues to be an area of debate. First, these women are at low risk for cardiovascular complications within the short time frame of pregnancy with good maternal and neonatal outcomes. Citation[682], Citation[683] Second, although it might be beneficial for the hypertensive mother, a reduction in blood pressure may impair uteropla‐cental perfusion and thereby jeopardize fetal development. Citation[684,685] Finally, data on pharmacological treatment of mild to moderate hypertensive pregnant women largely originate from trials that were too small to be able to detect a predictably modest reduction in obstetrical complications. Nevertheless, it appears reasonable to recommend drug treatment when systolic blood pressure is ⩾150 mmHg or diastolic blood pressure is ⩾95 mmHg. However, a lower threshold (140/90 mmHg) is indicated in women with gestational hypertension (with or without proteinuria), pre‐existing hypertension with the superimposition of gestational hypertension, or hypertension with subclinical organ damage or symptoms at any time during pregnancy. A systolic blood pressure ⩾170 or a diastolic blood pressure ⩾110 mmHg should be considered an emergency requiring hospitalization. Under emergency circumstances, a reduction in blood pressure may be obtained by intravenous labetalol, oral methyldopa, or oral nifedipine. Intravenous hydralazine should no longer be considered because its use is associated with more perinatal adverse effects than use of other drugs. Citation[686] Intravenous infusion of sodium nitroprusside remains the treatment of choice in hypertensive crises, although its prolonged administration carries an increased risk of fetal cyanide poisoning since nitroprusside is metabolized into thiocyanate. Citation[687] In pre‐eclampsia associated with pulmonary oedema, nitroglycerin is the drug of choice. In non‐severe hypertension and out‐of‐emergency situations, methyldopa, labetalol, and calcium antagonists are the preferred drugs. Atenolol should be given with caution during pregnancy because of reports of an association with fetal growth retardation which is related to the duration of treatment. Citation[688] ACE inhibitors and angiotensin receptor antagonists should never be used in pregnancy. Unless there is oliguria, diuretic therapy is inappropriate in pre‐eclampsia, in which plasma volume is reduced. Magnesium sulfate i.v. has been proved effective in the prevention of eclampsia and the treatment of seizures. Citation[689] Induction of delivery is appropriate in gestational hypertension with proteinuria and adverse conditions such as visual disturbances, coagulation abnormalities or fetal distress.
All administered antihypertensive agents are excreted into breast milk. However, for most antihypertensive drugs, concentration in breast milk is very low, except for propranolol and nifedipine whose concentrations are similar to those in maternal plasma.
Women with previous gestational hypertension seem to be at increased risk for cardiovascular disease in later life. Citation[690,691] This may depend on a relative hyperandrogenic state. It may further depend on alterations in endothelial function, carbohydrate and lipid metabolism, which have been shown in otherwise healthy women with previous gestational hypertension.
7.8 Metabolic syndrome (Box 19)
The metabolic syndrome embraces conditions characterized by various combinations of abnormalities in glucose metabolism, lipid metabolism, and blood pressure, a simple and widely (though not universally) adopted definition being that proposed by the National Cholesterol Education Program Adult Treatment. Citation[49] The most common features of the metabolic syndrome are: 1) a high age‐related prevalence (up to 30–40%) in middle aged and elderly population; 2) cardiovascular morbidity and mortality markedly higher than those of individuals without the syndrome; Citation[69],Citation[692–694] 3) a 3‐ to 6‐fold increase in the risk of developing diabetes Citation[695,696] as well as a greater risk of new onset hypertension; Citation[31–33],Citation[476] and 4) a frequent association with subclinical organ damage such as microalbuminuria and reduced glomerular filtration rate, Citation[697–699] arterial stiffening, Citation[700] left ventricular hypertrophy, diastolic dysfunction, atrial enlargement Citation[69],Citation[697,698],Citation[701–703] and, in some studies, carotid artery wall thickening, Citation[704] with some types of damage being detectable irrespective of the presence or absence of hypertension as a metabolic syndrome component. Citation[69],Citation[705] The presence of left ventricular hypertrophy confers a higher risk Citation[69] as does an elevation in home and ambulatory blood pressure levels in addition to the office values. Citation[69] The metabolic syndrome is also frequently accompanied by an increased level of inflammatory markers such as hsCRP which may contribute to its atherogenic effect Citation[706] and cause a further increase in cardiovascular risk. Citation[172],Citation[707]
Box 19 The metabolic syndrome
The metabolic syndrome is characterized by the variable combination of visceral obesity and alterations in glucose metabolism, lipid metabolism and BP. It has a high prevalence in the middle age and elderly population.
Subjects with the metabolic syndrome also have a higher prevalence of microalbuminuria, left ventricular hypertrophy and arterial stiffness than those without the metabolic syndrome. Their cardiovascular risk is high and the chance of developing diabetes markedly increased.
In patients with a metabolic syndrome diagnostic procedures should include a more in‐depth assessment of subclinical organ damage. Measuring ambulatory and home BP is also desirable.
In all individuals with metabolic syndrome, intense lifestyle measures should be adopted. When there is hypertension drug treatment should start with a drug unlikely to facilitate onset to diabetes. Therefore a blocker of the renin‐angiotensin system should be used followed, if needed, by the addition of a calcium antagonist or a low‐dose thiazide diuretic. It appears desirable to bring BP to the normal range.
Lack of evidence from specific clinical trials prevents firm recommendations on use of antihypertensive drugs in all metabolic syndrome subjects with a high normal BP. There is some evidence that blocking the renin‐angiotensin system may also delay incident hypertension.
Statins and antidiabetic drugs should be given in the presence of dyslipidaemia and diabetes, respectively. Insulin sensitizers have been shown to markedly reduce new onset diabetes, but their advantages and disadvantages in the presence of impaired fasting glucose or glucose intolerance as a metabolic syndrome component remain to be demonstrated.
Current guidelines consider a reduction in body weight by low caloric diet and physical exercise as the first and main treatment strategy in subjects with the metabolic syndrome. Citation[708] A realistic goal is to reduce body weight by 7–10% over 6 to 12 months via a relatively modest reduction of caloric intake (by 500–1000 calories/day), which is usually more effective than an extreme dietary approach. Citation[709] Nutritional therapy also calls for low intake of saturated fats, trans‐fatty acids, cholesterol, and simple carbohydrates with an increased consumption of fruits, vegetables, and whole grains. Citation[710] Long‐term maintenance of weight loss can be best achieved if regular exercise (e.g. a minimum of 30 min of daily moderate physical activity) is also implemented. Citation[711] In the Diabetic Prevention Program and in the Finnish Diabetes Prevention Study, Citation[712,713] behavioural modifications reduced progression to type 2 diabetes by almost 60%, the effect being greater than that obtained by metformin. In a secondary analysis of the Diabetes Prevention Program, the prevalence of the metabolic syndrome decreased over 3.2 years from 51–43% in the lifestyle intervention group whereas, in the conventional care group, an increase from 55–61% was observed. Citation[714] Thus lifestyle modifications have a protective effect.
In patients with the metabolic syndrome, additional administration of antihypertensive, antidiabetic or lipid lowering drugs is required when there is hypertension, diabetes or frank dyslipidaemia, respectively. Because cardiovascular risk is high in hypertensive patients with the metabolic syndrome it would appear advisable to pursue a rigorous blood pressure control, i.e. to lower blood pressure to values less than the high normal ones that are a common component of the syndrome. Citation[69] However, the optimal blood pressure values to achieve in these patients have never been investigated. As mentioned in sections 4.4.5, 5.5 and 6.2.1, unless required by specific indications, β‐blockers should be avoided in subjects with the metabolic syndrome because of their adverse effect on the incidence of new onset diabetes as well as on body weight, Citation[715] insulin sensitivity and the lipid profile. Citation[716] However, these effects appear to be less pronounced or absent with the new vasodilating β‐blockers such as carvedilol and nebivolol. Citation[572],Citation[717] Diabetogenic and other dysmetabolic actions also characterize thiazide diuretics, especially at high doses, Citation[455] and therefore their use as the first‐line treatment is not recommended in subjects with a metabolic syndrome. Classes to be considered are angiotensin receptor antagonists or ACE inhibitors, which are associated with a lower incidence of diabetes compared to other antihypertensive drugs Citation[455],Citation[458],Citation[460],Citation[718] and can also have a favourable effect on organ damage (see Section 4.5). If blood pressure is not controlled by monotherapy with one of these agents, a dihydropyridine or a non‐dihydropyridine calcium antagonist can be added, because calcium antagonists are metabolically neutral and also have favourable effects on organ damage (see Section 4.5). In addition, the combination of a blocker of the renin‐angiotensin system and a calcium antagonist has been shown to be associated with a lower incidence of diabetes than conventional treatment with a diuretic and a β‐blocker. Citation[330,331] Because subjects with the metabolic syndrome are frequently obese and have a salt‐sensitive blood pressure, Citation[719] a low‐dose thiazide diuretic might also represent a reasonable second or third step therapy. Thiazide diuretics at low dose, although they may still have some dysmetabolic effect, Citation[331],Citation[455],Citation[720] reduce serum potassium concentration to a lower degree, which attenuates the adverse effect of hypokalaemia on insulin resistance, carbohydrate tolerance and new onset diabetes. Citation[721] Maintenance of body potassium has been shown to prevent the glucose intolerance induced by thiazides, Citation[592,593] which suggests that the combination of thiazide and potassium‐sparing diuretics may have a metabolic advantage compared to thiazide diuretics alone.
Lack of specific intervention trials in the metabolic syndrome prevents any firm recommendation to be given on whether lifestyle modifications should be associated with antihypertensive drug treatment in non‐hypertensive and non‐diabetic patients with the metabolic syndrome, although the clustering of various risk factors and the frequent presence of organ damage make the cardiovascular risk of these patients rather high. The pros and cons of administration of a blocker of the renin‐angiotensin system when these subjects have blood pressure in the high normal range have been summarized in Section 5. It has been concluded that, for the time being, intense lifestyle measures should remain the main treatment approach, but that, in some cases, consideration might be given to drugs such as blockers of the renin‐angiotensin system for their potential ability of preventing new onset hypertension and new onset diabetes, and some of the organ damage that is particularly common in this high risk condition. Evidence is also inconclusive as to whether, in the absence of diabetes, metabolic syndrome subjects might benefit from the use of antidiabetic drugs. In a review of five prospective trials using alpha‐glucosidase inhibitors in individuals with impaired fasting glucose, a decreased incidence of type 2 diabetes has been reported. No significant difference was found, however, on mortality, other types of morbidity, glycated haemoglobin and blood pressure. Citation[722] The insulin sensitizers thiazolidinediones have received approval to be used for the treatment of type 2 diabetes, because of their ability to stimulate the peroxisome proliferator‐activated receptor‐gamma (PPRγ), which is, to a lesser extent, also a property of some angiotensin receptor antagonists. Citation[723,724] One of these compounds (rosiglitazone) has been tested in patients with impaired glucose tolerance and has been shown to be significantly effective in preventing new onset diabetes. Citation[725] However, these agents increase weight and induce fluid retention, which makes the balance of their benefits and disadvantages in the absence of overt diabetes unclear. In diabetic patients, however, pioglitazone has been shown to induce a significant reduction in the incidence of major cardiovascular events Citation[726] and this class of drugs has been reported to exert a small but significant blood pressure lowering effect. Citation[727] Long‐term reductions in body weight and waist circumference, as well as favourable changes in other metabolic risk factors for cardiovascular disease, such as plasma glucose, HDL‐cholesterol, serum triglycerides and insulin resistance, have recently been reported with the use of the endocannabinoid C1‐receptor blocker rimonabant in placebo controlled studies. Citation[728–731] There is also some evidence that administration of the drug does not increase and may even cause some blood pressure reduction. The impact of rimonabant on cardiovascular risk is currently being investigated in a prospective study. Citation[732]
In conclusion, in hypertensive subjects with the metabolic syndrome, diagnostic procedures should be more extensive than usual because of the higher prevalence of multiple organ damage and increased levels of inflammatory markers. Intense lifestyle measures should be adopted and antihypertensive drug treatment instituted whenever blood pressure is ⩾140/90 mmHg, by preferably blocking the renin‐angiotensin system with the addition, when needed, of a calcium antagonist or a low dose thiazide diuretic. Administration of a renin‐angiotensin system blocker when blood pressure is still in the high normal range, in order to protect against organ damage and prevent new onset diabetes or hypertension, cannot be generally recommended at present. Similarly, antidiabetic drug treatment should be instituted in metabolic syndrome patients with type‐2 diabetes, but no firm recommendation can as yet be given on use of antidiabetic drugs or insulin sensitizers in subjects who only have an impaired glucose tolerance. A lower incidence of events has been reported in subjects who were given a statin, which suggests that lipid lowering treatment may also be considered. Citation[733] Pharmacological approaches to subjects with the metabolic syndrome who are not hypertensive or diabetic are worth being investigated in consideration of the fact that, at variance with results of clinical trials, in real life adherence to lifestyle modifications is low and persistent reduction in body weight rare. Citation[734]
7.9 Resistant hypertension
Hypertension is usually defined as resistant or refractory to treatment when a therapeutic plan that has included attention to lifestyle measures and the prescription of at least three drugs (including a diuretic) in adequate doses has failed to lower systolic and diastolic blood pressure to goal. According to this definition prevalence of resistant hypertension is high: for instance in the ALLHAT cohort 8% of the patients were prescribed 4 or more drugs, and it has been calculated that a minimum of 15% would have been classified as having resistant hypertension. Citation[322] In such situations, referral to a specialist or a hypertension centre should be considered, because resistant hypertension is recognized to be often associated with subclinical organ damage and a high added cardiovascular risk. Citation[735]
Box 20 Causes of resistant hypertension
Poor adherence to therapeutic plan
Failure to modify lifestyle including:
weight gain
heavy alcohol intake (NB: binge drinking)
Continued intake of drugs that raise blood pressure (liquorice, cocaine, glucocorticoids, non‐steroid anti‐inflammatory drugs, etc.)
Obstructive sleep apnoea
Unsuspected secondary cause
Irreversible or scarcely reversible organ damage
Volume overload due to:
inadequate diuretic therapy
progressive renal insufficiency
high sodium intake
hyperaldosteronism
Causes of spurious resistant hypertension:
Isolated office (white‐coat) hypertension
Failure to use large cuff on large arm
Pseudohypertension
Causes of resistant hypertension are listed in 20. One of the most common causes of resistant hypertension is poor compliance or adherence to drug treatment or recommended lifestyle changes (particularly elimination of alcohol abuse). In this situation two options are possible. It can be helpful to suspend all drug therapy under close medical supervision, and begin again with a new simpler regimen; or arrange a brief admission to hospital to administer therapy under supervised conditions whilst monitoring blood pressure. Another not infrequent cause of resistant hypertension is obstructive sleep apnoea, Citation[736–739] possibly because of the long term effects of night time hypoxia and chemoreceptor stimulation as well as of sleep deprivation. In addition, it is imperative that secondary causes of hypertension are excluded (see section 9). For example, an occult renal artery stenosis can lead to blood pressure being refractory to therapy and, although the chances of ameliorating blood pressure are greater in younger subjects, it is still possible to reduce treatment load as a result of interventions such as a revascularization procedures, which can often be done by balloon angioplasty and stenting. Difficulties in lowering blood pressure to goal may also be due to extensive cardiovascular damage scarcely or very slowly reversible. Volume overload may be due to progressing renal insufficiency, excessive salt intake, hyperaldosteronism, and, most often, insufficient diuretic therapy. Finally, one must also consider the possibility of a spurious hypertension, such as isolated office (white coat) hypertension, and failure to use large cuffs on large arms (which leads to an overestimation of blood pressure values). In elderly patients one must exclude also the possibility of pseudohypertension, a condition in which an extreme degree of stiffness makes compression of the vascular wall by an external cuff difficult, with blood pressure readings falsely higher than the real intra‐arterial ones.
In consequence, the first step in managing resistant hypertension lies in a careful elicitation of the history, a meticulous examination of the patient and good investigational back‐up, primarily to exclude secondary causes of hypertension. Investigation should include ambulatory blood pressure monitoring, which may further characterize the degree of blood pressure elevation and increase in cardiovascular risk. Citation[96] It will be necessary to test whether compliance is good or not, and careful history taking may provide the key to the cause: binge drinking of alcohol, for example, may explain why blood pressure of an individual is difficult to control.
Ultimately, many patients will need administration of more than three drugs. At present, the optimal choice of the 3rd, 4th and 5th line antihypertensive agents has not been addressed by proper randomized trials. However, recent observational studies suggest that the aldosterone antagonist spironolactone provides significant additional blood pressure reduction when added to multidrug treatment regimens of patients with resistant hypertension. Citation[575],Citation[740] In the only placebo‐controlled randomized trial, Saha et al.Citation[741] found a greater additional antihypertensive effect of amiloride than spironolactone. Spironolactone, however, was found to cause a good additional antihypertensive response when given at a relatively small dose (25–50 mg/day). Citation[742] A good response to amiloride has also been reported. Citation[743] Whether the good response to antialdosterone agents of some resistant hypertensives is due to undiscovered primary aldosteronism or to secondary aldosteronism induced by multiple therapy is at present unknown. The reported effectiveness of small doses of these agents makes the adverse effects of spironolactone less likely to occur, but attention to serum potassium and creatinine concentrations is necessary because many of these patients may have poor renal function and are likely to concomitantly take renin‐angiotensin system blockers. The advantage of administering endothelin antagonists in patients defined as having resistant hypertension is under investigation. In these patients a blood pressure reduction has recently been reported by chronic field stimulation of carotid sinus nerves via implanted electrical devices. Citation[744]
7.10 Hypertensive emergencies
Hypertensive emergencies are observed when severe forms of high blood pressure are associated with acute damage to target organs. Marked rises in blood pressure associated with acute worsening of organ damage, such as those sometimes occurring in the elderly with isolated systolic hypertension, are improperly defined emergencies, and should be treated promptly but in the same way as chronic blood pressure elevations are. The most important emergencies are listed in Table. Such emergencies are rare but can be life threatening. In these conditions, the management of hypertension must be rapid. Care should be taken, however, that extremely rapid falls in blood pressure may not be associated with complications such as underperfusion of the brain and cerebral infarction or damage to the myocardium and kidneys.
Table 8. Hypertensive Emergencies
Excessive or rapid reductions in blood pressure should be avoided in acute stroke (see section 7.3.1).
7.11 Malignant hypertension
Whilst there is a clear overlap between resistant and malignant hypertension, in most developed societies malignant phase hypertension is observed infrequently and mostly in economically deprived strata. Malignant hypertension embraces a syndrome of severe elevation of arterial blood pressure (diastolic blood pressure usually but not always >140 mmHg) with vascular damage that can be particularly manifest as retinal haemorrhages, exudates and/or papilloedema. Citation[745] Some physicians use the term accelerated hypertension when such a syndrome appears but papilloedema on retinal examination is absent. Malignant hypertension may be seen in a variety of conditions. Severe or poorly treated essential hypertension is usually the commonest harbinger of malignant phase hypertension, although in various studies the presence of a secondary cause of hypertension has probably been underestimated. Citation[746] Anecdotally, it has been reported that a large number of patients with malignant hypertension are current smokers and blacks are known to be more frequently affected than Caucasians. Citation[747] The prevalence of this condition amongst hypertensive patients has obviously diminished as a result of earlier treatment of hypertension and more efficient therapeutic programmes, as well as a decrease of most predisposing causes. What causes malignant hypertension to be a condition with such a sinister prognosis is the breakdown of autoregulation as a result of the arterial wall being continuously exposed to extremely high levels of blood pressure. Pathological studies of the vascular wall demonstrate that there is myointimal proliferation and fibrinoid necrosis. The severity of the proliferative response parallels the severity and length of exposure to the high blood pressure. Citation[748] The fibrinoid necrosis represents spasm and forced dilatation of small arterioles. The leaking of fluid into the extracellular space is associated with small haemorrhages and of course target organ damage. Citation[748]
The most dangerous condition that is associated with malignant phase hypertension is hypertensive encephalopathy. Citation[745],Citation[747] It is associated with reversible alterations in neurological function and can include headache, disturbed mental status and visual impairment. Also associated with this condition is a deterioration in renal function, which has been described as being prognostically important, with more severe forms of renal failure being associated with reduced life expectancy despite prompt and effective management of the hypertension. In some patients there is irreversible renal damage necessitating renal replacement therapy including dialysis on a permanent basis. Malignant phase hypertension is also associated with haemolysis, red blood cell fragmentation and evidence of disseminated intravascular coagulation.
When malignant hypertension is untreated, its prognosis is extremely poor, with 50% of individuals dying within 12 months. Citation[254],Citation[749] However, following the institution of effective management programmes the incidence of such initial problems has declined. Citation[750,751] Survival is better and reflects not only improved blood pressure control, but also good identification of secondary causes and more widely available services such as renal dialysis and transplantation.
Malignant phase hypertension must be regarded as a hypertension emergency. Oral medication can be used if blood pressure is responsive, with the goal to bring diastolic blood pressure down to 100–110 mmHg over 24 hours.
8. Treatment of associated risk factors (Box 21)
8.1 Lipid lowering agents
Several randomized secondary and primary prevention trials have allowed to be analysed the effect of lipid lowering interventions with statins. Citation[752–754] Although epidemiological data show serum cholesterol concentration to be closely associated with coronary events but not with stroke, Citation[755] statins have been shown to be effective in preventing both coronary and cerebrovascular events, prevention of both outcomes being similar in hypertensives and normotensives. Citation[752–754] In the largest randomized trial so far performed with a statin, the Heart Protection Study, Citation[756] administration of simvastatin to patients with established cardiovascular disease markedly reduced cardiac and cerebrovascular events compared to placebo. The effects were manifest in the hypertensive subpopulation (41% of the total cohort) regardless of the type of antihypertensive treatment employed. Similar results were obtained with pravastatin in the elderly patients of the PROSPER study, Citation[757] 62% of whom were hypertensive. Effective prevention was also found with another statin, atorvastatin, in patients with a previous stroke. Citation[758] Therefore, patients up to the age of at least 80 years who have an established cardiovascular disease such as coronary heart disease, peripheral artery disease, previous stroke or long‐term (at least 10 years) diabetes should receive a statin. In all these patients the goal for total and LDL serum cholesterol should be set at respectively <4.5 mmol/l (175 mg/dl) and <2.5 mmol/l (100 mg/dl), and lower goals may also be considered, i.e. <4.0 and <2 mmol/l (155 and 80 mg/dl).
Box 21 Position statement: Treatment of associated risk factors
Lipid lowering agents
All hypertensive patients with established cardiovascular disease or with type 2 diabetes should be considered for statin therapy aiming at serum total and LDL cholesterol levels of, respectively, <4.5 mmol/l (175 mg/dl) and <2.5 mmol/l (100 mg/dl), and lower, if possible.
Hypertensive patients without overt cardiovascular disease but with high cardiovascular risk (⩾20% risk of events in 10 years) should also be considered for statin treatment even if their baseline total and LDL serum cholesterol levels are not elevated.
Antiplatelet therapy
Antiplatelet therapy, in particular low‐dose aspirin, should be prescribed to hypertensive patients with previous cardiovascular events, provided that there is no excessive risk of bleeding.
Low‐dose aspirin should also be considered in hypertensive patients without a history of cardiovascular disease if older than 50 years, with a moderate increase in serum creatinine or with a high cardiovascular risk. In all these conditions, the benefit‐to‐risk ratio of this intervention (reduction in myocardial infarction greater than the risk of bleeding) has been proven favourable.
To minimize the risk of haemorrhagic stroke, antiplatelet treatment should be started after achievement of BP control.
Glycaemic control
Effective glycaemic control is of great importance in patients with hypertension and diabetes.
In these patients dietary and drug treatment of diabetes should aim at lowering plasma fasting glucose to values ⩽6 mmol/l (108 mg/dl) and a glycated haemoglobin of <6.5%.
Two trials, ALLHATand ASCOT, have evaluated the benefits associated with the use of statins specifically among patients with hypertension. In ALLHAT the administration of 40 mg/day of pravastatin to 10,000 hypertensive patients (about two thirds of whom had established vascular disease) reduced serum total and LDL cholesterol (by 11% and 17%, respectively) compared to usual care, but had no significant effect on coronary heart disease, stroke and all cause mortality. Citation[759] In contrast, in ASCOT Citation[760] administration of 10 mg/day of atorvastatin in over 10,000 hypertensive patients with additional cardiovascular risk factors and a serum total cholesterol <6.5 mmol/l reduced serum total cholesterol by 19.9% compared to placebo. This was accompanied by substantial benefits both with regard to total cardiovascular events (36% reduction) and stroke (27% reduction). The beneficial effect seen in the ASCOT trial as compared to the lack of benefit reported in ALLHAT may depend on the greater relative difference in total and LDL cholesterol achieved among the actively treated versus the control group.
In view of the results of the ASCOT trial Citation[760] it seems reasonable to consider statin therapy in hypertensive patients aged less than 80 years who have an estimated 10 years risk of cardiovascular disease ⩾20% or of cardiovascular death (based on the SCORE model) of 5% or more. There are reports that the benefit of statin administration in hypertensive patients may include some blood pressure reduction, Citation[761] although in the ASCOT Citation[760] and the PHYLLIS Citation[390] trials addition of statin to antihypertensive treatment was not accompanied by a further clear blood pressure lowering effect. Target levels should be a serum total and LDL cholesterol of respectively <5 mmol/l (190 mg/dl) and <3 mmol/l (115 mg/dl). The majority of patients will reach these targets using a statin at appropriate doses in combination with non‐pharmacological measures. For patients who do not reach targets or whose HDL‐cholesterol or triglyceride levels remain abnormal (e.g. <1.0 mmol/l, >2.3 mmol/l, respectively) addition of ezetimibe Citation[762] or other therapies as well as referral to a specialist service may be indicated.
8.2 Antiplatelet therapy
Antiplatelet therapy, in particular low dose aspirin (i.e. 75–100 mg/day), has been shown to reduce the risk of stroke and/or myocardial infarction in several populations ranging from asymptomatic middle‐aged subjects at low cardiovascular risk to patients with established cardiovascular disease. Citation[763] The risk of a serious vascular event is reduced by approximately 25%. However, long‐term therapy with low‐dose aspirin approximately doubles the risk of major extracranial bleedings. For patients with established cardiovascular disease taking low dose aspirin, the number in whom a serious vascular event would be avoided clearly outweighs the number with major bleeding problems. Citation[764,765] Whether the benefits of aspirin exceed the risks of bleeding in lower risk subjects is uncertain. Therefore the decision to add aspirin in hypertensive patients should be taken in accordance with the total cardiovascular risk and/or with the presence of organ damage. Evidence about benefits and possible harms of administering low dose aspirin to hypertensive patients was obtained from the HOT study. Citation[311] Overall, the study showed a 15% reduction in major cardiovascular events, and a 36% reduction in acute myocardial infarction, with no effect on stroke and no increased risk of intracerebral haemorrhage but an associated 65% increased risk of major haemorrhagic events. However, subgroup analyses of the HOT data Citation[764] identified subgroups of hypertensive patients who are likely to have greater absolute benefits than harm. Patients with serum creatinine >115 µmol/l (>1.3 mg/dl) had a significantly greater reduction of cardiovascular events and myocardial infarction (−13 and −7 events/1000 patient‐years) while the risk of bleeding was not significantly increased. A favourable balance between benefits and harm of aspirin was also found in patients at higher global baseline risk and higher baseline systolic or diastolic blood pressure (benefit −3.1 to −3.3 cardiovascular events/1000 patient‐years versus harm: 1.0 to 1.4 bleeds/1000 patient‐years), while in hypertensives at lower baseline risk the harm of aspirin counterbalanced the benefits. These observations are in line with those of several meta‐analyses of primary prevention studies, also including non‐hypertensive patients, Citation[766–769] and with the recent results of the Women Prevention Study in a large cohort of very low risk subjects, showing little net benefit of aspirin. Citation[766] Therefore, treatments with a low‐dose aspirin have favourable benefit/risk ratios only if given to patients above a certain threshold of total cardiovascular risk (15–20% in 10 years). This is the case for hypertensive patients with a moderate increase in serum creatinine, hypertensive patients aged 50 years or more at high or very high total cardiovascular risk or with higher initial blood pressure values. It should be stressed that in the HOT study low dose aspirin did not interfere with a blood pressure lowering effect of concomitant antihypertensive therapy. Citation[770] The benefits were seen in patients with effective blood pressure control (virtually all patients had a diastolic blood pressure ⩽90 mmHg) and it is possible that this control was instrumental in avoiding an increment in intracerebral haemorrhage which was reported in some studies. Citation[311],Citation[765],Citation[766–769] It thus appears reasonable to suggest that in high or very high risk hypertensive individuals aspirin be introduced only when effective blood pressure control has been achieved.
8.3 Glycaemic control
Diabetes but also impaired glucose tolerance are major risk factors for cardiovascular disease. Citation[771–773] As mentioned in Section 7.2, hypertension is common with type 2 diabetes and diabetic hypertensive patients have a marked increase in total cardiovascular risk. Moreover, hypertension per se is associated with a doubling of risk of developing type 2 diabetes. Citation[774] Effective glycaemic control is of great importance in patients with hypertension and diabetes. In the UKPDS study hypertensive patients with type 2 diabetes benefited from intensive blood glucose control mainly in terms of microvascular complications. Citation[775] However, other studies have shown that more intense lifestyle or drug interventions to normalize the deranged glucose metabolism protect against macrovascular complications as well Citation[776–778] and the EDIC follow‐up has recently shown this to be true at least in type 1 diabetics. Citation[779] A direct association exists between macro and microvascular complications and the mean HbA1c, with no indication of a threshold of HbA1c values below which the risk no longer decreases. Citation[778],Citation[780] According to Guidelines for the management of diabetes the treatment goals are set to ⩽6.0 mmol/l (108 mg/dl) for plasma preprandial glucose concentrations (average of several measurements), and at less than 6.5% for glycated haemoglobin. Citation[168],Citation[781] Because of the known effect of thiazide diuretics and β‐blockers on glucose metabolism, use of these antihypertensive agents in subjects with impaired glucose tolerance may require earlier and more intense antidiabetic medication. Citation[316],Citation[331] Further information on the cardiovascular beneficial effects of tight blood glucose control will be available after the completion of two large scale randomized trials on type 2 diabetic patients, ACCORD (www.accord‐trial.org) and ADVANCE, Citation[782] which also examine the additional protective effects of tight blood pressure control.
9. Screening and treatment of secondary forms of hypertension
A specific cause of blood pressure elevation can be identified in a small proportion of adult patients with hypertension. Simple screening for secondary forms of hypertension can be obtained from clinical history, physical examination and routine laboratory investigations. Furthermore, a secondary form of hypertension is suggested by a severe blood pressure elevation, sudden onset or worsening of hypertension and blood pressure responding poorly to drug therapy. In these cases, specific diagnostic procedures may become necessary, as outlined below.
9.1 Renal parenchymal disease
Renal parenchymal disease is the most common cause of secondary hypertension. The finding of bilateral upper abdominal masses at physical examination is consistent with polycystic kidney disease and should lead to an abdominal ultrasound examination. Renal ultrasound has now almost completely replaced intravenous urography in the anatomical exploration of the kidney. While the latter requires the injection of potentially nephrotoxic contrast medium, ultrasound is non‐invasive and provides all the necessary anatomic data about kidney size and shape, cortical thickness, urinary tract obstruction and renal masses. Citation[783] Assessing the presence of protein, erythrocytes and leucocytes in the urine, as well as measuring serum creatinine concentration, are the appropriate functional screening tests for renal parenchymal disease. Citation[784,785] These tests should be performed in all patients with hypertension (see Section 3.4). Renal parenchymal disease may be excluded if urine analysis and serum creatinine concentration are normal on repeated determinations. The presence of erythrocytes and leucocytes should be confirmed by microscopic examination of the urine. If the screening tests for renal parenchymal hypertension are positive, a detailed work‐up for kidney disease should ensue.
9.2 Renovascular hypertension
Renovascular hypertension is the second most common cause of secondary hypertension, its prevalence being approximately 2% of adult patients with blood pressure elevation when assessed in specialized centres. Citation[786] This is caused by one or more stenoses of the extra‐renal arteries which in the elderly population have frequently an atherosclerotic nature. Fibromuscular dysplasia accounts for up to 25% of total cases and is the most common variety in young adults. Hypertension of abrupt onset or worsening as well as high blood pressures increasingly difficult to treat suggest the presence of this condition. Signs of renal artery stenosis include abdominal bruit with lateralization, hypokalaemia and progressive decline in renal function. However, these signs are not present in many patients with renovascular hypertension. Determination of the longitudinal diameter of the kidney using ultrasound can be used as a screening procedure. However, a difference of more than 1.5 cm in length between the two kidneys, which is usually considered as being diagnostic for renal artery stenosis is only found in 60–70% of the patients with renovascular hypertension. Citation[787] Colour Doppler ultrasonography is often able to detect stenosis of the renal artery, particularly when localized close to the origin of the vessel. Citation[788] In addition, it allows determination of the resistance index that can be predictive of outcome from angioplasty and stenting. There is evidence that investigations of the renal vasculature by breath‐hold three‐dimensional, gadolinium‐enhanced magnetic resonance angiography is the diagnostic procedure of choice for renovascular hypertension. Citation[789] Another imaging procedure with similar sensitivity is spiral computed tomography, which, however, requires the application of contrast media and the use of relatively high X‐ray doses. Once there is a strong suspicion of renal artery stenosis, intra‐arterial digital subtraction angiography should be performed for confirmation. This invasive procedure is still the gold standard for the detection of renal artery stenosis. The determination of the renal vein renin ratio requires multiple catheterization and its invasiveness and complexity is not compensated by an acceptable level of sensitivity or specificity. It cannot thus be recommended as a screening procedure.
Treatment of patients with renovascular hypertension is a controversial issue due to the limited number of large scale long‐term outcome trials comparing different therapeutic approaches, and to the difficulty of predicting the blood pressure response to renal revascularization procedures in individual patients. Citation[786] However, available data justify the following recommendations: 1) refractory hypertension (i.e. elevated blood pressure values despite administration of at least three drugs, including a diuretic at adequate doses) as well as a progressive decline in renal function represent an indication for revascularization; 2) although different opinions exist, surgical revascularization is now performed less frequently and is being progressively replaced by angioplasty; Citation[790] 3) angioplasty alone is the treatment of choice in fibromuscular dysplasia in which it is followed by a high rate of therapeutic success, i.e. persistent blood pressure normalization or reduction with values more responsive to drug treatment. Citation[787],Citation[791] Success rate is less common in atherosclerotic disease, which has a greater incidence of restenosis, Citation[791] but restenosis can be reduced by stenting which is thus now almost regularly added to angioplasty in renovascular stenoses of atherosclerotic nature. 4) Medical treatment has been compared with angioplasty in a number of trials, Citation[792–794] the meta‐analysis of which has shown a modest but significant advantage of angioplasty. Citation[795] The result of this procedure, however, heavily depends on the physician's skill and experience, and medical treatment remains of paramount importance for patients with atherosclerotic renovascular disease. It should be regarded as the preferable option when renal function is preserved, blood pressure control can be achieved, renal artery stenosis is not tight or there is a long (e.g. >10 years) history of hypertension. Because of the high risk of progression of atherosclerotic lesions, their treatment consists of intense lifestyle modifications, low dose aspirin, statin and multiple antihypertensive drug administration. Use should be made of a thiazide diuretic at appropriate doses and a calcium antagonist with the possible addition of a renin‐angiotensin blocker, except in the presence of bilateral renal artery stenosis. This treatment can lower blood pressure in the majority of patients with renovascular disease. The main risk is acute deterioration of renal function and increase in serum creatinine due to a marked reduction in perfusion pressure beyond the stenotic site. This is more common when a blocker of the renin‐angiotensin system is used, but the serum creatinine change normally reverts when treatment is withdrawn.
9.3 Phaeochromocytoma
Phaeochromocytoma is a very rare secondary hypertensive state (0.2–0.4% of all cases of elevated blood pressure) with an estimated annual incidence of 2–8 per million population. Citation[796] It can be inherited or acquired. Hypertension occurs in about 70% of all cases of phaeochromocytoma, being stable or paroxysmal (presenting with symptoms such as headache, sweating, palpitations and pallor) in approximately equal proportions. The diagnosis is based on establishing an increase in plasma or urinary catecholamines or their metabolites. It can be supported by pharmacological tests which should precede the carrying out of functional imaging procedures designed to localize the tumour. The test that achieves the highest sensitivity (97–98%) is the measurement of plasma free metanephrines, together with urinary fractionated metanephrines. However, because measurement of plasma free metanephrines is not available for routine diagnosis, measurement of urinary fractionated metanephrines and urinary cathecolamines remains the diagnostic test of choice. Citation[797] Very high values require no further testing. Citation[798] On the other hand, when plasma or urine values are only modestly elevated, despite there being a strong clinical suspicion of phaeochromocytoma, then stimulation or suppression tests with glucagon or clonidine, respectively, can be carried out, although in case of borderline results of biochemical tests (and given the limited specificity of the responses to pharmacological tests) many clinicians prefer to proceed directly to imaging methods. Citation[799] The glucagon test must be performed after the patient has been effectively treated with an a‐adrenoreceptor antagonist to prevent marked blood pressure increases after injection of the hormone. The clonidine suppression test is regarded as negative when there is a marked reduction of plasma catecholamines. Citation[800]
After the diagnosis of phaeochromocytoma has been made, localization of the tumour is mandatory. Citation[801] Ninety‐five per cent are located in or close to the adrenal glands and, since they are often large tumours, they can sometimes be detected by ultrasound. However, the most sensitive procedures (98–100%) are CT and, particularly, magnetic resonance imaging (MRI), which, however, has a low specificity (50%). Complementary to a CT scan or MRI, isotopic scanning using meta‐iodobenzylguanidine may be useful in localizing extra‐adrenal phaeochromocytomas and metastases from the 10% of phaeochromocytomas that are malignant, or to functionally confirm phaeochromocytomas localized by CTor MRI. There are several familial disorders that are associated with an increased incidence of phaeochromocytoma, and these include multiple endocrine neoplasia type 2 (MEN2), von Hippel‐Lindau disease (VHL), and neurofibromatosis type 1. Familial paragangliomas also cluster with pheochromocytoma. It is therefore recommended to offer genetic tests to patients and their family members, especially if phaeochromocytoma is associated with hereditary syndromes. To date, germline mutations in five genes have been described leading to familial disorders associated with phaechromocytomas. Citation[802] Definite treatment requires excision of the tumour. In advance of this the patient must be adequately prepared. This requires the introduction of an α adrenoreceptor blocker and, after adequate treatment with this blocker, β‐blockers can be introduced. Surgical excision, these days often carried out laparoscopically, can then follow, but after adequate fluid replacement had been effected. This is necessary because protracted exposure to phaeochromocytoma causes pressure natriuresis and venoconstriction with a marked volume depletion.
9.4 Primary aldosteronism
Primary aldosteronism has become a prominent area of controversy in hypertension management in recent years. This is because the prevalence varies in different studies of unselected primary hypertensives from 1% to 11%. Citation[803,804] As a screening test the determination of serum potassium levels is regarded as important but only a small number of patients will have hypokalaemia at an early stage in their disease. Citation[805,806] Thirty per cent of cases of primary aldosteronism are caused by adrenal adenomas which are commoner in women and rarer in children. Seventy per cent of cases are caused by adrenal hyperplasia and there are rare cases of adrenal carcinoma and the autosomal dominant condition of glucocorticoid remediable aldosteronism. Citation[806] The blood pressure profile is one of a moderate or marked elevation resistant to treatment. Glucocorticoid remediable hypertension appears early in life and usually in childhood. There are associations of primary aldosteronism with phaeochromocytoma, hyperparathyroidism and acromegaly. It has been suggested Citation[807] that only patients with unprovoked hypokalaemia or truly resistant hypertension should be evaluated for primary aldosteronism. The condition should be suspected in resistant hypertension and in unprovoked hypokalaemia. It can be confirmed by the fludrocortisone suppression test (failure of 4 day administration of the hormone to reduce plasma aldosterone below its threshold value), and measurement of aldosterone and renin under standardized conditions. Citation[808] In recent years there has been a move to measure the aldosterone‐to‐renin ratio. Citation[809] However, aldosterone can be high or the renin low in elderly people or black patients. Also, a high aldosterone‐to‐renin ratio is seen in chronic renal disease, where a high potassium stimulates aldosterone release, and in the case of rare genetic mutations leading to increased aldosterone levels. In a meta‐analysis carried out on 19 studies including 10,396 patients, there was a high variation in the aldosterone‐to‐renin ratio. High ratios were observed in 5.5 to 39% of patients, but adenomas were only established in between 0 to 6.5% of individuals. Citation[810] The usefulness of these measurements is therefore controversial. Imaging of the adrenal glands is now usually carried out using CT, magnetic resonance imaging or isotopic techniques using radio labelled cholesterol. However, adenomas on CT or magnetic resonance imaging can turn out to be due to hyperplasia. False positive results are likely to be relatively frequent, because nodular hyperplasia of the zona glomerulosa is reported even in the presence of functioning adenomas, and observed adenomas may actually be non‐functioning. Citation[811] This means that, if imaging is used, it may have to be supplemented with adrenal venous sampling. There are reports suggesting that unless this is carried out, on the basis of CT alone, 25% of patients would have had unnecessary adrenalectomy. Citation[812] The surgical technique for removal of a suspected adenoma is laparoscopic adrenalectomy. Series report no deaths and minimal morbidity with a mean post‐operative stay of 2.6 days. Prior to surgery or in the case of adrenal hyperplasia, treatment with an aldosterone antagonist such as spironolactone is advised. However, this is associated with side effects such as gynaecomastia which may reduce its usefulness. In this case eplerenone may be considered, although at recommended doses its effect is less than that of spironolactone. Citation[813]
9.5 Cushing's syndrome
Cushing's syndrome affects <0.1% of the total population. Citation[814] Hypertension is a very common finding and is reported in about 80% of such patients, with a 50% prevalence when the disease occurs in children and adolescents. Usually, the syndrome is suggested by the typical body habitus of the patient. The determination of 24‐hour urinary cortisol excretion is the most practical and reliable diagnostic test and a value exceeding 110 mmol (40 µg) is highly suggestive of Cushing's syndrome. The diagnosis is confirmed by the 2‐day, low‐dose dexamethasone suppression test (0.5 mg every 6 h for eight doses) or the overnight dexamethasone suppression test (1 mg at 23.00 h). In the 2‐day test, a urinary cortisol excretion higher than 27 mmol (10 µg) per day on day 2 indicates Cushing's syndrome. The same is true if plasma cortisol concentration is greater than 140 mmol/l (5 µg/dl) at 8.00 h in the overnight test. A normal result excludes the possibility of Cushing's syndrome. Recently the determination of mid/late‐night serum or salivary cortisol has been suggested as a simpler approach to the diagnosis. Citation[815] Further tests and imaging procedures have to be used to differentiate the various forms of the syndrome.
9.6 Obstructive sleep apnoea
Obstructive sleep apnoea (OSA) is characterized by recurrent episodes of cessation of respiratory airflow caused by upper airway inspiratory collapse during sleep, with a consequent decrease in oxygen saturation. Citation[816] It is important to consider sleep apnoea in the characterization of obese patients, especially those with hypertension resistant to conventional drug therapy. Citation[736–739] Furthermore, hypertensive patients, who are classified as ‘non‐dippers’ on ambulatory pressure measurements, should be investigated for obstructive sleep apnoea. Signs and symptoms include daytime somnolence, impaired concentration, unrefreshing and restless sleep, choking episodes during sleep, witnessed apnoeas, nocturia, irritability and personality changes, decreased libido and increased motor vehicle accidents. Where suspected, one should use one of the validated questionnaires: the Epworth Sleepiness Scale or the Berlin Questionnaire. Polysomnography remains the ‘gold standard’ diagnostic tool for assessing sleep‐disordered breathing. The apnoea‐hypopnoea index (i.e. the number of apnoeic and hypopnoeic events per hour) is used as an index of the presence and severity of the syndrome. An apnoea‐hypopnoea index of 5 to 15 indicates mild apnoea; of 15 to 30, moderate apnoea; and of greater than 30, severe apnoea. Untreated obstructive sleep apnoea may have direct and deleterious effects on cardiovascular function and structure through several mechanisms, including sympathetic activation, oxidative stress, inflammation and endothelial dysfunction. Citation[738] The syndrome may contribute to the elevated pressure in a large proportion of hypertensive patients, Citation[817,818] the pressor effect being possibly generated by an impairment of reflex cardiovascular regulation and endothelial dysfunction. Citation[819] Weight loss in obese subjects ameliorates the syndrome, which is also improved by using positive pressure breathing equipment.
9.7 Coarctation of the aorta
Coarctation of the aorta is a rare form of hypertension in children and young adults. The diagnosis is usually evident from physical examination. A midsystolic murmur, which may become continuous with time, is heard over the anterior part of the chest and also over the back. The femoral pulse is absent or delayed relative to the radial pulse. Hypertension is found in the upper extremities concomitantly with low or unmeasurable blood pressure in the legs. After repair or stenting, especially in adults, hypertension may persist due to haemodynamic and vascular effects, and many patients need to continue antihypertensive therapy.
9.8 Drug‐induced hypertension
Substances or drugs that can raise blood pressure include: liquorice, oral contraceptives, steroids, non steroidal anti‐inflammatory drugs, cocaine and amphetamines, erythro‐poietin, cyclosporins, tacrolimus. The patient should be asked about their medication at the time their clinical history is taken, and the use of drugs that can raise blood pressure should be monitored carefully.
10. Follow‐up (Box 22)
During the drug titration phase patients should be seen often (e.g. every 2 to 4 weeks) in order to adjust the chosen treatment regimen (increase in drug dose, addition of other drugs, dose reduction or drug withdrawal) in accordance with the achieved blood pressure or the appearance of side effects. In this phase dose titration and goal achievement may be helped by instructing the patient to self‐measure blood pressure at home. Once the goals of therapy have been reached, including the achievement of target blood pressure and control of all correctable risk factors, the frequency of visits can be reduced considerably. Patients with low cardiovascular risk and mild degrees of blood pressure elevation may be seen every 6 months whereas patients with a higher initial blood pressure or a high or very high cardiovascular risk should be seen more often. Frequent follow‐up visits are also needed in patients on non‐pharmacological treatment because 1) compliance with this intervention is low, Citation[500],Citation[584] 2) the blood pressure response is variable, Citation[820] and 3) this treatment requires reinforcement, and in case of failure, timely shift to drug administration.
Box 22 Patients' follow‐up
Titration to BP control requires frequent visits in order to modify the treatment regimen in relation to BP changes and appearance of side effects.
Once target BP has been obtained, the frequency of visits can be considerably reduced. However, excessively wide intervals between visits are not advisable because they interfere with a good doctor‐patient relationship, which is crucial for patient's compliance.
Patients at low risk or with grade 1 hypertension may be seen every 6 months and regular home BP measurements may further extend this interval. Visits should be more frequent in high or very high risk patients. This is the case also in patients under non‐pharmacological treatment alone due to the variable antihypertensive response and the low compliance with this intervention.
Follow‐up visits should aim at maintaining control of all reversible risk factors as well as at checking the status of organ damage. Because treatment‐induced changes in left ventricular mass and carotid artery wall thickness are slow, there is no reason to perform these examinations at less than 1 year intervals.
Treatment of hypertension should be continued for life because in correctly diagnosed patients cessation of treatment is usually followed by return to the hypertensive state. Cautious downward titration of the existing treatment may be attempted in low risk patients after long‐term BP control, particularly if non‐pharmacological treatment can be successfully implemented.
Home measurement of blood pressure may allow the periods between visits to be extended and further simplification of the follow‐up visit schedule may be offered by new technologies, such as teletransmission of home blood pressure values to the physician's office, which has been shown to further improve patient's adherence to treatment. Citation[821] In general, however, it is not advisable that follow‐up visits be at excessively wide intervals, because treatment crucially depends on a good doctor‐patient relationship, which frequent visits help to maintain. If blood pressure goals are not achieved within 6 months, or a previously good control is lost, referral to a hypertension specialist or clinic should be considered. Although it is recognized that this poses considerable difficulties, follow‐up may take advantage of periodical assessment of organ damage because its regression or lack of progression has favourable prognostic implications (see Section 4.5). No definite time schedule can be given, but it is useful to remember that treatment‐induced changes in urinary protein excretion can be expected to occur in weeks Citation[473] whereas changes in left ventricular hypertrophy are usually not evident before one year with some further modification thereafter. Citation[357] Cessation of treatment by patients who have been correctly diagnosed as hypertensive is usually followed, sooner or later, by the return of blood pressure to the elevated pretreatment levels. Nevertheless, after prolonged blood pressure control it may be possible to attempt a careful progressive reduction in the dose or number of drugs used, particularly among patients strictly observing lifestyle measures. This can be done because blood pressure control may reverse, at least in part, the anatomical vascular changes (i.e. arteriolar remodelling) that help maintaining blood pressure at elevated values on a structural basis. Citation[476] However, attempts to ‘step down’ treatment should be done prudently and accompanied by continued supervision of blood pressure values, preferably associated with home monitoring.
11. Implementation of guidelines Closing the gap between experts'recommendations and poor blood pressure control in medical practice
Despite overwhelming evidence that hypertension is a major cardiovascular risk factor and that blood pressure lowering strategies substantially reduce the risk, studies performed on various continents, as well as in several European countries, Citation[822] consistently show that 1) a noticeable proportion of hypertensive individuals are unaware of their condition or, if aware, do not undergo treatment, Citation[605],Citation[823] and 2) goal blood pressure levels are seldom achieved, regardless of whether treatment is prescribed and patients are followed by specialists or practitioners. Citation[824,825] Systolic blood pressure control is particularly rare, and the lower values (<130 mmHg) recommended in diabetics and very high risk patients almost exceptionally reached. Citation[825] This explains why high blood pressure remains a leading cause of death and cardiovascular morbidity both worldwide and in industrialized countries. It also emphasizes the strong need to extend to a larger fraction of the population the procedures that allow hypertension to be detected, as well as to ‘capture’ for effective treatment a substantially greater number of patients (Box 23).
Box 23 How to improve compliance with treatment
Inform the patient of the risk of hypertension and the benefit of effective treatment.
Provide clear written and oral instructions about treatment.
Tailor the treatment regimen to patient's lifestyle and needs.
Simplify treatment by reducing, if possible, the number of daily medicaments.
Involve patient's partner or family in information on disease and treatment plans.
Make use of self measurement of BP at home and of behavioural strategies such as reminder systems.
Pay great attention to side effects (even if subtle) and be prepared to change drug doses or types if needed.
Dialogue with patient regarding adherence and be informed of his/her problems.
Provide reliable support system and affordable prices.
The purpose of the present guidelines is to help achieve this goal. However, producing guidelines alone is insufficient to address the above problem. There must be a continuous process of implementation involving education and audit. The successful implementation of guidelines requires a concerted effort of medical professionals to realize its full potential. With regard to hypertension the approach may differ between European countries. In some countries prevention of cardiovascular disease, including detection and control of hypertension, is carried out in the primary care setting under the responsibility of general practitioners as well as dedicated nurses and other health professionals. In other countries specialists and hospital physicians may be more extensively involved. Therefore guidelines issued by an international expert committee should be adapted at the national level, depending on local cultural background, socioeconomic situations, and health care organization.
A broad acceptance of the present guidelines by national hypertension societies and leagues is a prerequisite to promote management implementation in practice and improve patient outcomes. In this context, the present guidelines have been prepared in close cooperation with the Fourth Joint Task Force of European and other Societies of Cardiovascular Disease Prevention. Citation[71] Their recommendations are thus consistent with the recommendations that will appear in the Fourth Joint Task Force Guidelines which will also be published in 2007. Also important is that the ESH and the ESC are both part of a platform for Societies interested in the implementation of prevention of cardiovascular disease in clinical practice in the Joint Prevention Committee. The other partners in that platform are: the European Atherosclerosis Society, the European Association for the Study of Diabetes, the International Diabetes Federation‐Europe, WONCA‐Europe (European Society of General Practice/Family Medicine), the European Heart Network and the International Society of Behavioural Medicine. This partnership is crucial because general practitioners are more likely to accept and to use guidelines when these are developed with the involvement of those known to them.
Successful implementation of guidelines requires awareness of the barriers interposed between recommendations and practice. The first barrier is knowledge and acceptance by physicians. Knowledge is hampered by the high number of guidelines doctors receive, by their duplication by too many scientific societies, local organizations, health providing agencies. Confusion is raised by even small differences in the recommendations, and the suspicion is cultivated that some guidelines may be excessively influenced by the scientific biases of the experts, or by extrinsic influences such as those of the pharmaceutical industry or of private or public health providers. Furthermore, doctors are correctly aware that their task is to manage individuals, so often different from each other, while guidelines, by necessity, are dealing with a medical condition in general. This aspect was carefully considered when the 2003 ESH‐ESC Guidelines Citation[3] were prepared, and the choice of making them widely informative and minimally prescriptive has likely been an important reason for their acceptance. This choice has been reiterated when preparing the current guidelines.
Barriers to implementation relate not only to the clinician but also to the patient. Adherence to lifestyle changes and longterm compliance with multiple drugs are major problems. Lifestyle changes have too often been conceived as an object of preaching rather than an approach to be implemented, and as a cheap alternative to the costs of drug therapy, while a costly professional approach guided by experts in behavioural medicine is often needed.
Besides the doctor and the patient, the health care system by itself may be a barrier. Indeed, health providers sometimes wrongly consider the management of hypertension as the matter of few minute visits, and reimburse doctors accordingly. They often see guidelines as an instrument to reduce cost and limit reimbursement to high risk conditions defined by arbitrary cutoffs. Therefore policy makers and all those responsible for the organization of the system should be involved in the development of a comprehensive preventive programme.
The Committee is well aware of the fact that issuing these guidelines on its own may not make the difference, but it can be helpful as part of a more comprehensive strategy of evidence based preventive medicine where it may serve as:
a consensus among all partners involved in detection and control of arterial hypertension,
a basis for education and training,
a template for national joint task forces to adopt and/or adapt these guidelines in accord with national health policies and available resources,
a reference point based on scientific evidence to identify the most appropriate management tools for hypertension control,
a good basis for health economic purposes.
Key to References
CT: controlled trial; GL: guidelines/experts' opinion; MA: meta‐analysis; OS: observational study; RT: randomized trial; RV: review.
Notes
The affiliations of Task Force members are listed in the Appendix. Their Disclosure forms are available on the respective society Web Sites. These guidelines also appear in the Journal of Hypertension, doi:10.1097/HJH.0b013e3281fc975a and in the European Heart Journal (2007) 28, 1462‐1536 doi:10.1093/eurheartj/ehm236
* Correspondence to Giuseppe Mancia, Clinica Medica, Ospedale San Gerardo, Universita Milano‐Bicocca, Via Pergolesi, 33 ‐ 20052 MONZA (Milano), Italy. Tel: ±39 039 233 3357; fax: ±39 039 32 22 74, e‐mail: [email protected]
* Correspondence to Guy de Backer, Dept. of Public Health, University Hospital, De Pintelaan 185, 9000 Ghent, Belgium. Tel: ±32 9 240 3627; fax: ±32 9 240 4994; e‐mail: [email protected]
The content of these European Guidelines has been published for personal and educational use only. No commercial use is authorized. Whole or parts of the Guidelines may be translated or reproduced with permission from the ESH or the ESC.
Disclaimer. The Guidelines represent the views of the ESH and the ESC and were arrived at after careful consideration of the available evidence at the time they were written. Health professionals are encouraged to take them fully into account when exercising their clinical judgement. The guidelines do not, however, override the individual responsibility of health professionals to make appropriate decisions in the circumstances of the individual patients, in consultation with that patient, and where appropriate and necessary the patient's guardian or carer. It is also the health professional's responsibility to verify the rules and regulations applicable to drugs and devices at the time of prescription.
© 2007 The European Society of Hypertension (ESH) and European Society of Cardiology (ESC). All rights reserved.
References
- Guidelines Sub‐Committee. 1993 Guidelines for the management of mild hypertension: memorandum from a World Health Organization/International Society of Hypertension meeting. J Hypertens 1993; 11: 905–918, GL
- Guidelines Sub‐Committee. 1999 World Health Organization/International Society of Hypertension Guidelines for the management of hypertension. J Hypertens 1999; 17: 151–183, GL
- Guidelines Committee 2003. European Society of Hypertension‐European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 2003; 21: 1011–1053, GL
- Top 10 papers published. The Scientist 2005; 19: 26, OS
- ESH/ESC Hypertension Practice Guidelines Committee. Practice guidelines for primary care physicians: 2003 ESH/ESC hypertension guidelines. J Hypertens 2003; 21: 1779–1786, GL
- Simoons M. L., van der Putten N., Wood D., Boersma E., Bassand J. P. The Cardiology Information System: the need for data standards for integration of systems for patient care, registries and guidelines for clinical practice. Eur Heart J 2002; 23: 1148–1152, GL
- MacMahon S., Peto R., Cutler J., Collins R., Sorlie P., Neaton J., Abbott R., Godwin J., Dyer A., Stamler J. Blood pressure stroke coronary heart disease. Part 1 prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990; 335: 765–774, MA
- Report of the Joint National Committee on Detection Evaluation. Treatment of High Blood Pressure: a cooperative study. JAMA 1977; 237: 255–261, GL
- The 1980 report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med 1980; 140: 1280–1285, GL
- Collins R., Peto R., MacMahon S., Herbert P., Fieback N. H., Eberlein K. A., Godwin J., Qizilbash N., Taylor J. O., Hennekens C. H. Blood pressure stroke coronary heart disease. Part 2, short‐term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990; 335: 827–839, MA
- Prospective Studies Collaboration. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360: 1903–1913, MA
- European cardiovascular disease statistics, British Heart Foundation 2000 www.dphpc.ox.ac/UKbhfhprg. RV,
- Kannel W. B. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA 1996; 275: 1571–1576, OS
- Levy D., Larson M. G., Vasan R. S., Kannel W. B., Ho K. K. The progression from hypertension to congestive heart failure. JAMA 1996; 275: 1557–1562, OS
- Criqui M. H., Langer R. D., Fronek A., Feigelson H. S., Klauber M. R., McCann T. J., Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992; 326: 381–386, OS
- Klag M. J., Whelton P. K., Randall B. L., Neaton J. D., Brancati F. L., Ford C. E., Shulman N. B., Stamler J. Blood pressure and end‐stage renal disease in men. N Engl J Med 1996; 334: 13–18, OS
- Kearney P. M., Whelton M., Reynolds K., Muntner P., Whelton P. K., He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365: 217–223, OS
- Martiniuk A. L., Lee C. M., Lawes C. M., Ueshima H., Suh I., Lam T. H., Gu D., Feigin V., Jamrozik K., Ohkubo T., Woodward M., for the Asia‐Pacific Cohort Studies Collaboration. Hypertension: its prevalence and population‐attributable fraction for mortality from cardiovascular disease in the Asia‐Pacific region. J Hypertens 2007; 25: 73–79, OS
- Wolf‐Maier K., Cooper R. S., Banegas J. R., Giampaoli S., Hense H. W., Joffres M., Kastarinen M., Poulter N., Primatesta P., Rodriguez‐Artalejo F., Stegmayr B., Thamm M., Tuomilehto J., Vanuzzo D., Vescio F. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA 2003; 289: 2363–2369, OS
- Ezzati M., Lopez A. D., Rodgers A., Vander Hoorn S., Murray C. J., Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet 2002; 360: 1347–1360, RV
- Franklin S. S. Ageing and hypertension: the assessment of blood pressure indices in predicting coronary heart disease. J Hypertens 1999; 17((Suppl 5))S29–S36, RV
- Benetos A., Zureik M., Morcet J., Thomas F., Bean K., Safar M., Ducimetiere P., Guize L. A decrease in diastolic blood pressure combined with an increase in systolic blood pressure is associated with a higher cardiovascular mortality in men. J Am Coll Cardiol 2000; 35: 673–680, OS
- Staessen J. A., Gasowski J., Wang J. G., Thijs L., Den Hond E., Boissel J. P., Coope J., Ekbom T., Gueyffier F., Liu L., Kerlikowske K., Pocock S., Fagard R. H. Risks of untreated and treated isolated systolic hypertension in the elderly: meta‐analysis of outcome trials. Lancet 2000; 355: 865–872, MA
- Darne B., Girerd X., Safar M., Cambien F., Guize L. Pulsatile versus steady component of blood pressure: a cross‐sectional analysis and a prospective analysis on cardiovascular mortality. Hypertension 1989; 13: 392–400, OS
- Benetos A., Safar M., Rudnichi A., Smulyan H., Richard J. L., Ducimetieere P., Guize L. Pulse pressure: a predictor of long‐term cardiovascular mortality in a French male population. Hypertension 1997; 30: 1410–1415, OS
- Gasowski J., Fagard R. H., Staessen J. A., Grodzicki T., Pocock S., Boutitie F., Gueyffier F., Boissel J. P., INDANA Project Collaborators. Pulsatile blood pressure component as predictor of mortality in hypertension: a meta‐analysis of clinical trial control groups. J Hypertens 2002; 20: 145–151, MA
- Blacher J., Staessen J. A., Girerd X., Gasowski J., Thijs L., Liu L., Wang J. G., Fagard R. H., Safar M. E. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med 2000; 160: 1085–1089, MA
- Laurent S., Cockcroft J., Van Bortel L., Boutouyrie P., Giannattasio C., Hayoz D., Pannier B., Vlachopoulos C., Wilkinson I., Struijker‐Boudier H., on behalf of the European Network for non invasive investigation of large arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27: 2588–2605, GL
- Pickering G. The nature of essential hypertension. J & A. Churchill Ltd, London 1961; 1–151, RV
- Chobanian A. V., Bakris G. L., Black H. R., Cushman W. C., Green L. A., Izzo L., Jr., Jones D. W., Materson B. J., Oparil S., Wright J. T., Jr., Roccella E. J., National Heart, Lung, Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42: 1206–1252, GL
- Vasan R. S., Beiser A., Seshadri S., Larson M. G., Kannel W. B., D'Agostino R. B., Levy D. Residual lifetime risk for developing hypertension in middle‐aged women and men: The Framingham Heart Study. JAMA 2002; 287: 1003–1010, OS
- Vasan R. S., Larson M. G., Leip E. P., Kannel W. B., Levy D. Assessment of frequency of progression to hypertension in non‐hypertensive participants in the Framingham Heart Study: a cohort study. Lancet 2001; 358: 1682–1686, OS
- Vasan R. S., Larson M. G., Leip E. P., Evans J. C., O'Donnell C. J., Kannel W. B., Levy D. Impact of high‐normal blood pressure on the risk of cardiovascular disease. N Engl J Med 2001; 345: 1291–1297, OS
- Mancia G., Grassi G. European, American and British Guidelines: similarities and differences. Hypertension. A companion to Braunwald's Heart diseases, H. R Black, W. J Elliott. Saunders‐Elsevier, Amsterdam 2007; 571–575
- Kannel W. B. Risk stratification in hypertension: new insights from the Framingham Study. Am J Hypertens 2000; 13((Suppl 1))S3–S10, OS
- Thomas F., Rudnichi A., Bacri A. M., Bean K., Guize L., Benetos A. Cardiovascular mortality in hypertensive men according to presence of associated risk factors. Hypertension 2001; 37: 1256–1261, OS
- Wei M., Mitchell B. D., Haffner S. M., Stern M. P. Effects of cigarette smoking, diabetes, high cholesterol, and hypertension on all‐cause mortality and cardiovascular disease mortality in Mexican Americans. The San Antonio Heart Study. Am J Epidemiol 1996; 144: 1058–1065, OS
- Assmann G., Schulte H. The Prospective Cardiovascular Munster (PROCAM) study: prevalence of hyperlipidemia in persons with hypertension and/or diabetes mellitus and the relationship to coronary heart disease. Am Heart J 1988; 116: 1713–1724, OS
- Mancia G., Parati G., Borghi C., Ghironzi G., Andriani E., Marinelli L., Valentini M., Tessari F., Ambrosioni E. Hypertension prevalence, awareness, control and association with metabolic abnormalities in the San Marino population: the SMOOTH study. J Hypertens 2006; 24: 837–843, OS
- Mancia G., Facchetti R., Bombelli M., Friz H. P., Grassi G., Giannattasio C., Sega R. Relationship of office, home, and ambulatory blood pressure to blood glucose and lipid variables in the PAMELA population. Hypertension 2005; 45: 1072–1077, OS
- Asia Pacific Cohort Studies Collaboration. Joint effects of systolic blood pressure and serum cholesterol on cardiovascular disease in the Asia Pacific region. Circulation 2005; 112: 3384–3390, OS
- Multiple Risk Factor Intervention Trial Research Group Relationship between baseline risk factors coronary heart disease total mortality in the Multiple Risk Factor Intervention Trial. Multiple Risk Factor Intervention Trial Research Group. Prev Med 1986; 15: 254–273, OS
- Wood D., De Backer G., Faergeman O., Graham I., Mancia G., Pyorala K. Prevention of coronary heart disease in clinical practice. Summary of recommendations of the Second Joint Task Force of European and other Societies on Coronary Prevention. J Hypertens 1998; 16: 1407–1414, GL
- De Backer G., Ambrosioni E., Borch‐Johnsen K., Brotons C., Cifkova R., Dallongeville J., Ebrahim S., Faergeman O., Graham I., Mancia G., Manger Cats V., Orth‐Gomer K., Perk J., Pyorala K., Rodicio J. L., Sans S., Sansoy V., Sechtem U., Silber S., Thomsen T., Wood D. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J 2003; 24: 1601–1610, GL
- D'Agostino R. B. S., Grundy S., Sullivan L. M., Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA 2001; 286: 180–187, OS
- Conroy R. M., Pyorala K., Fitzgerald A. P., Sans S., Menotti A., De Backer G., De Bacquer D., Ducimetiere P., Jousilahti P., Keil U., Njolstad I., Oganov R. G., Thomsen T., Tunstall‐Pedoe H., Tverdal A., Wedel H., Whincup P., Wilhelmsen L., Graham I. M. Estimation of ten‐year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003; 24: 987–1003, OS
- World Health Organization/International Society of Hypertension. 2003. World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003; 21: 1983–1992, GL
- Evans J. G., Rose G. Hypertension. Br Med Bull 1971; 27: 37–42, RV
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation. Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Expert Panel on Detection Evaluation Treatment of High Blood Cholesterol in Adults. JAMA 2001; 285: 2486–2497, GL
- Dzau V. J., Antman E. M., Black H. R., Hayes D. L., Manson J. E., Plutzky J., Popma J. J., Stevenson W. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation 2006; 114: 2850–2870, RV
- Cockroft D. W., Gault M. H. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41
- Hallan S., Asberg A., Lindberg M., Johnsen H. Validation of the Modification of Diet in Renal Disease formula for estimating GFR with special emphasis on calibration of the serum creatinine assay. Am J Kidney Dis 2004; 44: 84–93
- Olsen M. H., Wachtell K., Bella J. N., Palmieri V., Gerdts E., Smith G., Nieminen M. S., Dahlof B., Ibsen H., Devereux R. B. Albuminuria predicts cardiovascular events independently of left ventricular mass in hypertension: a LIFE substudy. J Hum Hypertens 2004; 18: 453–459, OS
- Willum‐Hansen T., Staessen J. A., Torp‐Pedersen C., Rasmussen S., Thijs L., Ibsen H., Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 2006; 113: 664–670, OS
- Laurent S., Boutouyrie P., Asmar R., Gautier I., Laloux B., Guize L., Ducimetiere P., Benetos A. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension 2001; 37: 1236–1241, OS
- Feringa H. H., Bax J. J., van Waning V. H., Boersma E., Elhendy A., Schouten O., Tangelder M. J., van Sambeek M. H., van den Meiracker A. H., Poldermans D. The long‐term prognostic value of the resting and postexercise ankle‐brachial index. Arch Intern Med 2006; 166: 529–535, OS
- Devereux R. B., Wachtell K., Gerdts E., Boman K., Nieminen M. S., Papademetriou V., Rokkedal J., Harris K., Aurup P., Dahlof B. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA 2004; 292: 2350–2356, OS
- Ibsen H., Olsen M. H., Wachtell K., Borch‐Johnsen K., Lindholm L. H., Mogensen C. E., Dahlof B., Devereux R. B., de Faire U., Fyhrquist F., Julius S., Kjeldsen S. E., Lederballe‐Pedersen O., Nieminen M. S., Omvik P., Oparil S., Wan Y. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: Losartan Intervention For Endpoint reduction in hypertension study. Hypertension 2005; 45: 198–202, OS
- de Zeeuw D., Remuzzi G., Parving H. H., Keane W. F., Zhang Z., Shahinfar S., Snapinn S., Cooper M. E., Mitch W. E., Brenner B. M. Albuminuria, a Therapeutic Target for Cardiovascular Protection in Type 2 Diabetic Patients With Nephropathy. Circulation 2004; 110: 921–927, OS
- Olsen M. H., Wachtell K., Ibsen H., Lindholm L. H., Dahlof B., Devereux R. B., Kjeldsen S. E., Oikarinen L., Okin P. M. LIFE Study Investigators. Reductions in albuminuria and in electrocardiographic left ventricular hypertrophy independently improve prognosis in hypertension: the LIFE study. J Hypertens 2006; 24: 775–781, OS
- Verdecchia P., Angeli F., Borgioni C., Gattobigio R., de Simone G., Devereux R. B., Porcellati C. Changes in cardiovascular risk by reduction of left ventricular mass in hypertension: a meta‐analysis. Am J Hypertens 2003; 16: 895–899, MA
- Benetos A., Rudnichi A., Thomas F., Safar M., Guize L. Influence of heart rate on mortality in a French population: role of age, gender, and blood pressure. Hypertension 1999; 33: 44–52, OS
- Palatini P., Thijs L., Staessen J. A., Fagard R. H., Bulpitt C. J., Clement D. L., de Leeuw P. W., Jaaskivi M., Leonetti G., Nachev C., O'Brien E. T., Parati G., Rodicio J. L., Roman E., Sarti C., Tuomilehto J., Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. Predictive value of clinic, ambulatory heart rate for mortality in elderly subjects with systolic hypertension. Arch Intern Med 2002; 162: 2313–2321, OS
- Kannel W. B., Kannel C., Paffenbarger R. S., Jr., Cupples L. A. Heart rate and cardiovascular mortality: the Framingham study. Am Heart J 1987; 113: 1489–1494, OS
- Palatini P., Benetos A., Grassi G., Julius S., Kjeldsen S. E., Mancia G., Narkiewicz K., Parati G., Pessina A. C., Ruilope L. M., Zanchetti A. European Society of Hypertension Identification and management of the hypertensive patient with elevated heart rate: statement of a European Society of Hypertension Consensus Meeting. J Hypertens 2006; 24: 603–610, GL
- Levy R. L., White P. D., Stroud W. D., Hillman C. C. Transient tachycardia: prognostic significance alone and in association with transient hypertension. JAMA 1945; 129: 585–588, OS
- King D. E., Everett C. J., Mainous A. G., 3rd., Liszka H. A. Long‐term prognostic value of resting heart rate in subjects with prehypertension. Am J Hypertens 2006; 19: 796–800, OS
- Palatini P., Casiglia E., Pauletto P., Staessen J., Kaciroti N., Julius S. Relationship of tachycardia with high blood pressure and metabolic abnormalities: a study with mixture analysis in three populations. Hypertension 1997; 30: 1267–1273, OS
- Mancia G., Bombelli M., Corrao G., Facchetti R., Madotto F., Giannattasio C., Trevano F. Q., Grassi G., Zanchetti A., Sega R. Metabolic syndrome in the Pressioni Arteriose Monitorate E Loro Associazioni (PAMELA) study: daily life blood pressure, cardiac damage, and prognosis. Hypertension 2007; 49: 40–47, OS
- Yusuf S., Hawken S., Ounpuu S., Dans T., Avezum A., Lanas F., McQueen M., Budaj A., Pais P., Varigos J., Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEARTstudy): case‐control study. Lancet 2004; 364: 937–952, OS
- Fourth Joint Task Force of European other Societies on Cardiovascular Disease Prevention in Clinical Practice. European Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2007, in preparation. GL
- Mancia G., Ferrari A., Gregorini L., Parati G., Pomidossi G., Bertinieri G., Grassi G., di Rienzo M., Pedotti A., Zanchetti A. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ Res 1983; 53: 96–104. OS
- Sega R., Cesana G., Bombelli M., Grassi G., Stella M. L., Zanchetti A., Mancia G. Seasonal variations in home and ambulatory blood pressure in the PAMELA population. Pressione Arteriose Monitorate E Loro Associazioni. J Hypertens 1998; 16: 1585–1592. OS
- Modesti P. A., Morabito M., Bertolozzi I., Massetti L., Panci G., Lumachi C., Giglio A., Bilo G., Caldara G., Lonati L., Orlandini S., Maracchi G., Mancia G., Gensini G. F., Parati G. Weather‐related changes in 24‐hour blood pressure profile: effects of age and implications for hypertension management. Hypertension 2006; 47: 155–161. OS
- O'Brien E., Asmar R., Beilin L., Imai Y., Mallion J. M., Mancia G., Mengden T., Myers M., Padfield P., Palatini P., Parati G., Pickering T., Redon J., Staessen J., Stergiou G., Verdecchia P. European Society of Hypertension Recommendations for Conventional, Ambulatory and Home Blood Pressure Measurement. J Hypertens 2003; 21: 821–848. GL
- O'Brien E., Waeber B., Parati G., Staessen J., Myers M. G. Blood pressure measuring devices: recommendations of the European Society of Hypertension. Br Med J 2001; 322: 531–536. GL
- Mancia G., Omboni S., Parati G., Clement D. L., Haley W. E., Rahman S. N., Hoogma R. P. Twenty‐four hour ambulatory blood pressure in the Hypertension Optimal Treatment (HOT) study. J Hypertens 2001; 19: 1755–1763. OS
- Mancia G., Omboni S., Ravogli A., Parati G., Zanchetti A. Ambulatory blood pressure monitoring in the evaluation of antihypertensive treatment: additional information from a large data base. Blood Press 1995; 4: 148–156. OS
- Mancia G., Parati G., Bilo G., Maronati A., Omboni S., Hennig M., Zanchetti A. Assessment of long‐term antihypertensive treatment by clinic an ambulatory blood pressure. Data from the ELSA Study. J Hypertens 2007; 25: 1087–1094. OS
- Fagard R. H., Staessen J. A., Thijs L. Relationships between changes in left ventricular mass and in clinic and ambulatory blood pressure in response to antihypertensive therapy. J Hypertens 1997; 15: 1493–1502. OS
- Mancia G., Zanchetti A., Agabiti‐Rosei E., Benemio G., De Cesaris R., Fogari R., Pessina A., Porcellati C., Rappelli A., Salvetti A., Trimarco B. Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment induced regression of left ventricular hypertrophy. Circulation 1997; 95: 1464–1470. OS
- Fagard R. H., Staessen J. A., Thijs L. Prediction of cardiac structure and function by repeated clinic and ambulatory blood pressure. Hypertension 1997; 29: 22–29. OS
- Verdecchia P., Schillaci G., Guerrieri M., Gatteschi C., Benemio G., Boldrini F., Porcellati C. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation 1990; 81: 528–536. OS
- Mancia G., Parati G., Hennig M., Flatau B., Omboni S., Glavina F., Costa B., Scherz R., Bond G., Zanchetti A. Relation between blood pressure variability and carotid artery damage in hypertension: baseline data from European Lacidipine Study on Atherosclerosis (ELSA). J Hypertens 2001; 19: 1981–1989. OS
- Redon J., Baldo E., Lurbe E., Bertolin V., Lozano J. V., Miralles A., Pascual J. M. Microalbuminuria, left ventricular mass and ambulatory blood pressure in essential hypertension. Kidney Int Suppl 1996; 55: S81–S84. OS
- Imai Y., Ohkubo T., Sakuma M., Tsuji I. I., Satoh H., Nagai K., Hisamichi S., Abe K. Predictive power of screening blood pressure, ambulatory blood pressure and blood pressure measured at home for overall and cardiovascular mortality: a prospective observation in a cohort from Ohasama, Northern Japan. Blood Press Monit 1996; 1: 251–254. OS
- Staessen J. A., Thijs L., Fagard R., O'Brien E. T., Clement D., de Leeuw P. W., Mancia G., Nachev C., Palatini P., Parati G., Tuomilehto J., Webster J. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA 1999; 282: 539–546. OS
- Clement D. L., De Buyzere M. L., De Bacquer D. A., de Leeuw P. W., Duprez D. A., Fagard R. H., Gheeraert P. J., Missault L. H., Braun J. J., Six R. O., Van Der Niepen P., O'Brien E. Prognostic value of ambulatory blood pressure recordings in patients with treated hypertension. New Engl J Med 2003; 348: 2407–2415. OS
- Sega R., Facchetti R., Bombelli M., Cesana G., Corrao G., Grassi G., Mancia G. Prognostic value of ambulatory and home blood pressure compared with office blood pressure in the general population: follow‐up results from the PAMELA study. Circulation 2005; 111: 1777–1783. OS
- Fagard R. H., Celis H. Prognostic significance of various characteristics of out‐of‐the‐office blood pressure. J Hypertens 2004; 22: 1663–1666. OS
- Dolan E., Stanton A., Thijs L., Hinedi K., Atkins N., McClory S., Den Hond E., McCormack P., Staessen J. A., O'Brien E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality. Hypertension 2005; 46: 156–161. OS
- Fagard R. H., Van Den Broeke C., De Cort P. Prognostic significance of blood pressure measured in the office, at home and during ambulatory monitoring in older patients in general practice. J Hum Hypertens 2005; 19: 801–807. OS
- Hansen T. W., Jeppesen J., Rasmussen S., Ibsen H., Torp‐Pedersen C. Ambulatory blood pressure and mortality. A population‐based study. Hypertension 2005; 45: 499–504. OS
- Kikuya M., Ohkubo T., Asayama K., Metoki H., Obara T., Saito S., Hashimoto J., Totsune K., Hoshi H., Satoh H., Imai Y. Ambulatory blood pressure and 10‐year risk of cardiovascular and noncardiovascular mortality. The Ohasama Study. Hypertension 2005; 45: 240–245. OS
- Pickering T. G., Shimbo D., Haas D. Ambulatory blood pressure monitoring. New Engl J Med 2006; 354: 2368–2374. RV
- Redon J., Campos C., Narciso M. L., Rodicio J. L., Pascual J. M., Ruilope L. M. Prognostic value of ambulatory blood pressure monitoring in refractory hypertension: a prospective study. Hypertension 1998; 31: 712–718. OS
- Coats A. J. S., Radaelli A., Clark S. J., Conway J., Sleight P. The influence of ambulatory blood pressure monitoring on the design and interpretation of trials in hypertension. J Hypertension 1992; 10: 385–391. OS
- Mancia G., Ulian L., Parati G., Trazzi S. Increase in blood pressure reproducibility by repeated semi‐automatic blood pressure measurements in the clinic environment. J Hypertens 1994; 12: 469–473. OS
- Parati G., Pomidossi G., Casadei V., Mancia G. Lack of alerting reactions and pressor responses to intermittent cuff inflations during non‐invasive blood pressure monitoring. Hypertension 1985; 7: 597–601. OS
- Mancia G., Omboni S., Parati G., Ravogli A., Villani A., Zanchetti A. Lack of placebo effect on ambulatory blood pressure. Am J Hypertens 1995; 8: 311–315. OS
- Staessen J. A., Thijs L., Clement D., Davidson C., Fagard R., Lehtonen A., Mancia G., Palatini P., O'Brien E. T., Parati G., Webster J., Amery A. Ambulatory blood pressure decreases on long‐term placebo treatment in older patients with isolated systolic hypertension. J Hypertens 1994; 12: 1035–1039. OS
- O'Brien E., Sheridan J., O'Malley K. Dippers and non‐dippers. Lancet 1988; 2: 397. RV
- Ohkubo T., Hozawa A., Yamaguchi J., Kikuya M., Ohmori K., Michimata M., Matsubara M., Hashimoto J., Hoshi H., Araki T., Tsuji I., Satoh H., Hisamichi S., Imai Y. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24‐h blood pressure: the Ohasama study. J Hypertens 2002; 20: 2183–2189. OS
- Verdecchia P., Porcellati C., Schillaci G., Borgioni C., Ciucci A., Battistelli M., Guerrieri M., Gatteschi C., Zampi I., Santucci A. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension 1994; 24: 793–801. OS
- Metoki H., Ohkubo T., Kikuya M., Asayama K., Obara T., Hashimoto J., Totsune K., Hoshi H., Satoh H., Imai Y. Prognostic significance for stroke of a morning pressor surge and a nocturnal blood pressure decline, The Ohasama Study. Hypertension 2006; 47: 149–154. OS
- Hansen T. W., Jeppesen J., Rasmussen S., Ibsen H., Torp‐Pedersen C. Ambulatory blood pressure monitoring and risk of cardiovascular disease: a population based study. Am J Hypertens 2006; 19: 243–250. OS
- Willich S. N., Goldberg R. J., Maclure M., Perriello L., Muller J. E. Increased onset of sudden cardiac death in the first three hours after awakening. Am J Cardiol 1992; 70: 65–68. OS
- Rocco M. B., Barry J., Campbell S., Nabel E., Cook E. F., Goldman L., Selwyn A. P. Circadian variation of transient myocardial ischemia in patients with coronary artery disease. Circulation 1987; 75: 395–400. OS
- Muller J. E., Stone P. H., Turi Z. G., Rutherford J. D., Czeisler C. A., Parker C., Poole W. K., Passamani E., Roberts R., Robertson T. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985; 313: 1315–1322. OS
- Elliott W. J. Circadian variation in the timing of stroke onset: a meta‐analysis. Stroke 1998; 29: 992–996. MA
- Millar‐Craig M. W., Bishop C. N., Raftery E. B. Circadian variation of blood‐pressure. Lancet 1978; 1: 795–797. OS
- Kario K., Pickering T. G., Umeda Y., Hoshide S., Hoshide Y., Morinari M., Murata M., Kuroda T., Schwartz J. E., Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation 2003; 107: 1401–1406. OS
- Mancia G., Zanchetti A. Cardiovascular regulation during sleep. Handbook of Physiology during Sleep, J Orem. Academic Press, New York 1980; 1–55. RV
- El‐Tamimi H., Mansour M., Pepine C. J., Wargovich T. J., Chen H. Circadian variation in coronary tone in patients with stable angina. Protective role of the endothelium. Circulation 1995; 92: 3201–3205. OS
- Otto M. E., Svatikova A., Barretto R. B., Santos S., Hoffmann M., Khandheria B., Somers V. Early morning attenuation of endothelial function in healthy humans. Circulation 2004; 109: 2507–2510. OS
- Brown N. J., Agirbasli M. A., Williams G. H., Litchfield W. R., Vaughan D. E. Effect of activation and inhibition of the renin‐angiotensin system on plasma PAI‐1. Hypertension 1998; 32: 965–971. OS
- Weber M. A. The 24‐hour blood pressure pattern: does it have implications for morbidity and mortality?. Am J Cardiol 2002; 89: 27A–33A. RV
- Undar L., Turkay C., Korkmaz L. Circadian variation in circulating platelet aggregates. Ann Med 1989; 21: 429–433. OS
- Frattola A., Parati G., Cuspidi C., Albini F., Mancia G. Prognostic value of 24‐hour blood pressure variability. J Hypertens 1993; 11: 1133–1137. OS
- Sander D., Kukla C., Klingelhofer J., Winbeck K., Conrad B. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: A 3‐year follow‐up study. Circulation 2000; 102: 1536–1541. OS
- Verdecchia P., Borgioni C., Ciucci A., Gattobigio R., Schillaci G., Sacchi N., Santucci A., Santucci C., Reboldi G., Porcellati C. Prognostic significance of blood pressure variability in essential hypertension. Blood Press Monit 1996; 1: 3–11. OS
- Mancia G., Bombelli M., Facchetti R., Madotto F., Corrao G., Quarti‐Trevano F., Grassi G., Sega R. Long term prognostic value of blood pressure variability in the general population: result of the PAMELA study. Hypertension 2007, in press. OS
- Staessen J., Fagard R. H., Lijnen P. J., Van Hoof R., Amery A. K. Mean and range of the ambulatory pressure in normotensive subjects from a meta‐analysis of 23 studies. Am J Cardiol 1991; 67: 723–727. MA
- Mancia G., Sega R., Bravi C., De Vito G., Valagussa F., Cesana G., Zanchetti A. Ambulatory blood pressure normality: results from the PAMELA Study. J Hypertens 1995; 13: 1377–1390. OS
- Ohkubo T., Imai Y., Tsuji I., Nagai K., Ito S., Satoh H., Hisamichi S. Reference values for 24‐hour ambulatory blood pressure monitoring based on a prognostic criterion: the Ohasama Study. Hypertension 1998; 32: 255–259. OS
- Sakuma M., Imai Y., Nagai K., Watanabe N., Sakuma H., Minami N., Satoh H., Abe K. Reproducibility of home blood pressure measurements over a 1‐year period. Am J Hypertens 1997; 10: 798–803. OS
- Ohkubo T., Imai Y., Tsuji I., Nagai K., Kato J., Kikuchi N., Nishiyama A., Aihara A., Sekino M., Kikuya M., Ito S., Satoh H., Hisamichi S. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population‐based observation in Ohasama, Japan. J Hypertens 1998; 16: 971–975. OS
- Zarnke K. B., Feagan B. G., Mahon J. L., Feldman R. D. A randomized study comparing a patient‐directed hypertension management strategy with usual office‐based care. Am J Hypertens 1997; 10: 58–67. OS
- Pickering T., James G. D., Boddie C., Hrashfield G. A., Blank S., Laragh J. H. How common is white coat hypertension?. JAMA 1988; 259: 225–228. OS
- Parati G., Ulian L., Santucci C., Omboni S., Mancia G. Difference between clinic and daytime blood pressure is not a measure of the white coat effect. Hypertension 1998; 31: 1185–1189. OS
- Mancia G., Bertinieri G., Grassi G., Parati G., Pomidossi G., Ferrari A., Gregorini L., Zanchetti A. Effects of blood‐pressure measurement by the doctor on patient's blood pressure and heart rate. Lancet 1983; 2: 695–698. OS
- Mancia G., Parati G., Pomidossi G., Grassi G., Casadei R., Zanchetti A. Alerting reaction and rise in blood pressure during measurement by physician and nurse. Hypertension 1987; 9: 209–215. OS
- Mancia G., Facchetti R., Bombelli M., Grassi G., Sega R. Long‐term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension 2006; 47: 846–853. OS
- Ohkubo T., Kikuya M., Metoki H., Asayama K., Obara T., Hashimoto J., Totsune K., Hoshi H., Satoh H., Imai Y. Prognosis of masked hypertension and white‐coat hypertension detected by 24‐h ambulatory blood pressure monitoring. J Am Coll Cardiol 2005; 46: 508–515. OS
- Khattar R. S., Senior R., Lahiri A. Cardiovascular outcome in white‐coat versus sustained mild hypertension. A 10‐year follow‐up study. Circulation 1998; 98: 1892–1897. OS
- Fagard R. H., Staessen J. A., Thijs L., Gasowski J., Bulpitt C. J., Clement D., de Leeuw P. W., Dobovisek J., Jaaskivi M., Leonetti G., O'Brien E., Palatini P., Parati G., Rodicio J. L., Vanhanen H., Webster J. Response to antihypertensive treatment in older patients with sustained or nonsustained systolic hypertension. Circulation 2000; 102: 1139–1144. OS
- Bobrie G., Chatellier G., Genes N., Clerson P., Vaur L., Vaisse B., Menard J., Mallion J. M. Cardiovascular prognosis of ‘masked hypertension’ detected by blood pressure self‐measurement in elderly treated hypertensive patients. JAMA 2004; 291: 1342–1349. OS
- Verdecchia P., Reboldi G. P., Angeli F., Schillaci G., Schwartz J. E., Pickering T. G., Imai Y., Ohkubo T., Kario K. Short‐ and long‐term incidence of stroke in white‐coat hypertension. Hypertension 2005; 45: 203–208. OS
- Sega R., Trocino G., Lanzarotti A., Carugo S., Cesana G., Schiavina R., Valagussa F., Bombelli M., Giannattasio C., Zanchetti A., Mancia G. Alterations of cardiac structure in patients with isolated office, ambulatory or home hypertension. Data from the general PAMELA population. Circulation 2001; 104: 1385–1392. OS
- Wing L. M. H., Brown M. A., Beilin L. J., Ryan P., Reid C. Reverse white‐coat hypertension in older hypertensives. J Hypertens 2002; 20: 639–644. OS
- Bjorklund K., Lind L., Zethelius B., Andren B., Lithell H. Isolated ambulatory hypertension predicts cardiovascular morbidity in elderly men. Circulation 2003; 107: 1297–1302. OS
- Lurbe E., Torro I., Alvarez V., Nawrot T., Paya R., Redon J., Staessen J. A. Prevalence, persistence, and clinical significance of masked hypertension in youth. Hypertension 2005; 45: 493–498. OS
- Mancia G., Parati G. Reactivity to physical and behavioral stress and blood pressure variability in hypertension. Handbook of Hypertension. Vol 9. Behavioral Factors in Hypertension, S Julius, D. R Bassett, 1987; 104–122. RV
- Pescatello L. S., Franklin B. A., Fagard R., Farquhar W. B., Kelley G. A., Ray C. A. American College of Sports Medicine Position Stand: Exercise and Hypertension. Med Sci Sports Exerc 2004; 36: 533–553. GL
- Singh J. P., Larson M. G., Manolio T. A., O'Donnell C. J., Lauer M., Evans J. C., Levy D. Blood pressure response during treadmill testing as a risk factor for new‐onset hypertension: the Framingham Heart Study. Circulation 1999; 99: 1831–1836. OS
- Carroll D., Smith G. D., Shipley M. J., Steptoe A., Brunner E. J., Marmot M. G. Blood pressure reactions to acute psychological stress and future blood pressure status: a 10‐year follow‐up of men in the Whitehall II study. Psychosom Med 2001; 63: 737–743. OS
- Manolio T. A., Burke G. L., Savage P. J., Sidney S., Gardin J. M., Oberman A. Exercise blood pressure response and 5‐year risk of elevated blood pressure in a cohort of young adults: the CARDIA study. Am J Hypertens 1994; 7: 234–241. CT
- Fagard R., Staessen J., Amery A. Exercise blood pressure and target organ damage in essential hypertension. J Hum Hypertension 1991; 5: 69–75. OS
- Filipovsky J., Ducimetiere P., Safar M. Prognostic significance of exercise blood pressure and heart rate in middle‐aged men. Hypertension 1992; 20: 337–339. OS
- Lauer M. S., Levy D., Anderson K. M., Plehn J. F. Is there a relationship between exercise systolic blood pressure response and left ventricular mass?. Ann Intern Med 1992; 116: 203–210. OS
- Smith D. H. G., Neutel J. M., Graettinger W. F., Myers J., Froelicher V. F., Weber M. A. Impact of left ventricular hypertrophy on blood pressure responses to exercise. Am J Cardiol 1992; 69: 225–228. OS
- Fagard R., Staessen J., Thijs L., Amery A. Relation of left ventricular mass and filling to exercise blood pressure and rest blood pressure. Am J Cardiol 1995; 75: 53–57. OS
- Markovitz J. H., Raczynski J. M., Lewis C. E., Flack J., Chesney M., Chettur V., Hardin J. M., Johnson E. Lack of independent relationships between left ventricular mass and cardiovascular reactivity to physical and psychological stress in the CARDIA study. Am J Hypertens 1996; 9: 915–923. OS
- Fagard R. H., Pardaens K., Staessen J. A., Thijs L. Should exercise blood pressure be measured in clinical practice?. J Hypertens 1998; 16: 1215–1217. RV
- Kokkinos P., Pittaras A., Narayan P., Faselis C., Singh S., Manolis A. Exercise capacity and blood pressure associations with left ventricular mass in prehypertensive individuals. Hypertension 2007; 49: 55–61. OS
- Al'Absi M., Devereux R. B., Lewis C. E., Kitzman D. W., Rao D. C., Hopkins P., Markovitz J., Arnett D. K. Blood pressure responses to acute stress and left ventricular mass. Am J Cardiol 2002; 89: 536–540. OS
- Rostrup M., Smith G., Bjornstad H., Westheim A., Stokland O., Eide I. Left ventricular mass and cardiovascular reactivity in young men. Hypertension 1994; 23((Suppl I))I168–I171. OS
- Al'Absi M., Devereux R. B., Rao D. C., Kitzman D., Oberman A., Hopkins P., Arnett D. K. Blood pressure stress reactivity and left ventricular mass in a random community sample of African‐American and Caucasian men and women. Am J Cardiol 2006; 97: 240–244. OS
- Fagard R. H., Pardaens K., Staessen J. A., Thijs L. Prognostic value of invasive hemodynamic measurements at rest and during exercise in hypertensive men. Hypertension 1996; 28: 31–36. OS
- Kjeldsen S. E., Mundal R., Sandvik L., Erikssen G., Thaulow E., Erikssen J. Supine and exercise systolic blood pressure predict cardiovascular death in middle‐aged men. J Hypertens 2001; 19: 1343–1348. OS
- Palatini P. Exaggerated blood pressure response to exercise: pathophysiologic mechanisms and clinical relevance. J Sports Med Phys Fitness 1998; 38: 1–9. OS
- O' Rourke M. F. Principles and definitions of arterial stiffness, wave reflections and pulse pressure amplification. Arterial stiffness in hypertension. Handbook of Hypertension, M. E Safar, M. F O'Rourke, 2006; Vol 23: 3–19, Elsevier
- Morgan T., Lauri J., Bertram D., Anderson A. Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens 2004; 17: 118–123
- Chen C. H., Nevo E., Fetics B., Pak P. H., Yin F. C., Maughan W. L., Kass D. A. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation 1997; 95: 1827–1836
- Hope S. A., Tay D. B., Meredith I. T., Cameron J. D. Use of arterial transfer functions for the derivation of aortic waveform characteristics. J Hypertens 2003; 21: 1299–1305
- Williams B., Lacy P. S., Thom S. M., Cruickshank K., Stanton A., Collier D., Hughes A. D., Thurston H., O'Rourke M., CAFE Investigators Anglo‐Scandinavian Cardiac Outcomes Trial Investigators CAFE Steering Committee, Writing Committee. Differential impact of blood pressure‐lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006; 113: 1213–1225. RT
- Dhakam Z., McEniery C. M., Yasmin, Cockcroft J. R., Brown M. J., Wilkinson I. B. Atenolol and eprosartan: differential effects on central blood pressure and aortic pulse wave velocity. Am J Hypertens 2006; 19: 214–219. RT
- Ryden L., Standl E., Bartnik M., Van den Berghe G., Betteridge J., de Boer M. J., Cosentino F., Jonsson B., Laakso M., Malmberg K., Priori S., Ostergren J., Tuomilehto J., Thrainsdottir I., Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) European Association for the Study of Diabetes (EASD). Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases: executive summary: The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J 2007; 28: 88–136. GL
- Ridker P. M. High‐sensitivity C‐reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 2001; 103: 1813–1818. OS
- Wang T. J., Gona P., Larson M. G., Tofler G. H., Levy D., Newton‐Cheh C., Jacques P. F., Rifai N., Selhub J., Robins S. J., Benjamin E. J., D'Agostino R. B., Vasan R. S. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med 2006; 355: 2631–2639. OS
- Ridker P. M., Buring J. E., Cook N. R., Rifai N. C‐reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8‐year follow‐up of 14719 initially healthy American women. Circulation 2003; 107: 391–397. OS
- Sattar N., Gaw A., Scherbakova O., Ford I., O'Reilly D. S., Haffner S. M., Isles C., Macfarlane P. W., Packard C. J., Cobbe S. M., Shepherd J. Metabolic syndrome with and without C‐reactive protein as a predictor of coronary heart disease and diabetes in the West Of Scotland Coronary Prevention Study. Circulation 2003; 108: 414–419. OS
- Olsen M. H., Wachtell K., Tuxen C., Fossum E., Bang L. E., Hall C., Ibsen H., Rokkedal J., Devereux R. B., Hildebrandt P. N‐terminal pro‐brain natriuretic peptide predicts cardiovascular events in patients with hypertension and left ventricular hypertrophy: a LIFE study. J Hypertens 2004; 22: 1597–1604. OS
- Luft F. C. Molecular genetics of human hypertension. J Hypertens 1998; 16: 1871–1878. RV
- Melander O. Genetic factors in hypertension‐what is known and what does it mean?. Blood Press 2001; 10: 254–270. RV
- Cadman P. E., O'Connor D. T. Pharmacogenomics of hypertension. Curr Opin Nephrol Hypertens 2003; 12: 61–70. RV
- Lifton R. P., Gharavi A. G., Geller D. S. Molecular mechanisms of human hypertension. Cell 2001; 104: 545–556. RV
- Jensen J. S., Feldt‐Rasmussen B., Strandgaard S., Schroll M., Borch‐Johnsen K. Arterial hypertension microalbuminuria risk of ischemic heart disease. Hypertension 2000; 35: 898–903. OS
- De Leeuw P. W., Ruilope L. M., Palmer C. R., Brown M. J., Castaigne A., Mancia G., Rosenthal T., Wagener G. Clinical significance of renal function in hypertensive patients at high risk: results from the INSIGHT trial. Arch Intern Med 2004; 164: 2459–2464. RT
- Sarnak M. J., Levey A. S., Schoolwerth A. C., Coresh J., Culleton B., Hamm L. L., McCullough P. A., Kasiske B. L., Kelepouris E., Klag M. J., Parfrey P., Pfeffer M., Raij L., Spinosa D. J., Wilson P. W., American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003; 108: 2154–2169. GL
- Gerstein H. C., Mann J. F., Yi Q., Zinman B., Dinneen S. F., Hoogwerf B., Halle J. P., Young J., Rashkow A., Joyce C., Nawaz S., Yusuf S., HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286: 421–426. OS
- Wachtell K., Ibsen H., Olsen M. H., Borch‐Johnsen K., Lindholm L. H., Mogensen C. E., Dahlof B., Devereux R. B., Beevers G., de Faire U., Fyhrquist F., Julius S., Kjeldsen S. E., Kristianson K., Lederballe‐Pedersen O., Nieminen M. S., Okin P. M., Omvik P., Oparil S., Wedel H., Snapinn S. M., Aurup P. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann Intern Med 2003; 139: 901–906. OS
- Jager A., Kostense P. J., Ruhe H. G., Heine R. J., Nijpels G., Dekker J. M., Bouter L. M., Stehouwer C. D. Microalbuminuria and peripheral arterial disease are independent predictors of cardiovascular and all‐cause mortality, especially among hypertensive subjects: five‐year follow‐up of the Hoorn Study. Arterioscler Thromb Vasc Biol 1999; 19: 617–624. OS
- Bigazzi R., Bianchi S., Baldari D., Campese V. M. Microalbuminuria predicts cardiovascular events and renal insufficiency in patients with essential hypertension. J Hypertens 1998; 16: 1325–1333. OS
- Hillege H. L., Fidler V., Diercks G. F., van Gilst W. H., de Zeeuw D., van Veldhuisen D. J., Gans R. O., Janssen W. M., Grobbee D. E., de Jong P. E., Prevention of Renal, Vascular End Stage Disease (PREVEND) Study Group. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002; 106: 1777–1782. OS
- National Kidney Foundation. Executive summary. Am J Kid Dis 2004; 43((Suppl. 1))S16–S33. RV
- Levy D., Salomon M., D'Agostino R. B., Belanger A. J., Kannel W. B. Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation 1994; 90: 1786–1793. OS
- Levy D., Garrison R. J., Savage D. D., Kannel W. B., Castelli W. P. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322: 1561–1566. OS
- Koren M. J., Devereux R. B., Casale P. N., Savage D. D., Laragh J. H. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 1991; 114: 345–352. OS
- Salonen J. T., Salonen R. Ultrasound B‐mode imaging in observational studies of atherosclerotic progression. Circulation 1993; 87((Suppl II))II56–II65. OS
- Bots M. L., Hoes A. W., Koudstaal P. J., Hofman A., Grobbee D. E. Common carotid intima‐media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation 1997; 96: 1432–1437. OS
- Hodis H. N., Mack W. J., LaBree L., Selzer R. H., Liu C. R., Liu C. H., Azen S. P. The role of carotid arterial intima‐media thickness in predicting clinical coronary events. Ann Intern Med 1998; 128: 262–269. OS
- O'Leary D. H., Polak J. F., Kronmal R. A., Manolio T. A., Burke G. L., Wolfson S. K., Jr. Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 1999; 340: 14–22. OS
- Cuspidi C., Ambrosioni E., Mancia G., Pessina A. C., Trimarco B., Zanchetti A. Role of echocardiography and carotid ultrasonography in stratifying risk in patients with essential hypertension: the Assessment of Prognostic Risk Observational Survey. J Hypertens 2002; 20: 1307–1314. OS
- Okin P. M., Devereux R. B., Jern S., Kjeldsen S. E., Julius S., Nieminen M. S., Snapinn S., Harris K. E., Aurup P., Edelman J. M., Wedel H., Lindholm L. H., Dahlof B., LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA 2004; 292: 2343–2349. OS
- Fagard R. H., Staessen J. A., Thijs L., Celis H., Birkenhager W. H., Bulpitt C. J., de Leeuw P. W., Leonetti G., Sarti C., Tuomilehto J., Webster J., Yodfat Y., Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. Prognostic significance of electrocardiographic voltages and their serial changes in elderly with systolic hypertension. Hypertension 2004; 44: 459–464. OS
- La Rovere M. T., Pinna G. D., Maestri R., Mortara A., Capomolla S., Febo O., Ferrari R., Franchini M., Gnemmi M., Opasich C., Riccardi P. G., Traversi E., Cobelli F. Short‐term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 2003; 107: 565–570. OS
- Bigger J. T., Jr., Fleiss J. L., Steinman R. C., Rolnitzky L. M., Kleiger R. E., Rottman J. N. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 1992; 85: 164–171. OS
- Kleiger R. E., Miller J. P., Bigger J. T., Jr., Moss A. J., for the Multicentre Post‐Infarction Research Group. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 1987; 59: 256–262. OS
- Reichek N., Devereux R. B. Left ventricular hypertrophy: relationship of anatomic, echocardiographic and electrocardiographic findings. Circulation 1981; 63: 1391–1398. OS
- Devereux R. B., Alonso D. R., Lutas E. M., Gottlieb G. J., Campo E., Sachs I., Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57: 450–458. OS
- Lang R. M., Bierig M., Devereux R. B., Flachskampf F. A., Foster E., Pellikka P. A., Picard M. H., Roman M. J., Seward J., Shanewise J., Solomon S., Spencer K. T., St John Sutton M., Stewart W. American Society of Echocardiography's Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography. European Society of Cardiology. Recommendations for chamber quantification. Eur J Echocardiogr 2006; 7: 79–108. GL
- Jennings G., Wong J. Reversibility of left ventricular hypertrophy and malfunction by antihypertensy treatment. Handbook of Hypertension, L Hansson, W. H Birkenhager. Elsevier Science, Amsterdam 1997; 184–223. RV, Vol 18
- Muiesan M. L., Salvetti M., Monteduro C., Bonzi B., Paini A., Viola S., Poisa P., Rizzoni D., Castellano M., Agabiti‐Rosei E. Left ventricular concentric geometry during treatment adversely affects cardiovascular prognosis in hypertensive patients. Hypertension 2004; 43: 731–738. OS
- De Simone G., Devereux R. B., Koren M. J., Mensah G. A., Casale P. N., Laragh J. H. Midwall left ventricular mechanics. An independent predictor of cardiovascular risk in arterial hypertension. Circulation 1996; 93: 259–265. OS
- Aurigemma G. P., Gottdiener J. S., Shemanski L., Gardin J., Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: The Cardiovascular Health Study. J Am Coll Cardiol 2001; 37: 1042–1048. OS
- Swedberg K., Cleland J., Dargie H., Drexler H., Follath F., Komajda M., Tavazzi L., Smiseth O. A., Gavazzi A., Haverich A., Hoes A., Jaarsma T., Korewicki J., Levy S., Linde C., Lopez‐Sendon J. L., Nieminen M. S., Pierard L., Remme W. J., Task Force for the Diagnosis, Treatment of Chronic Heart Failure of the European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005). The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J 2005; 26: 1115–1140. GL
- Ogunyankin K. O., Burggraf G. W., Abiose A. K., Malik P. G. Validity of revised Doppler echocardiographic algorithms and composite clinical and angiographic data in diagnosis of diastolic dysfunction. Echocardiography 2006; 23: 817–828. OS
- Bursi F., Weston S. A., Redfield M. M., Jacobsen S. J., Pakhomov S., Nkomo V. T., Meverden R. A., Roger V. L. Systolic and diastolic heart failure in the community. JAMA 2006; 296: 2209–2216. OS
- Zanchetti A., Agabiti‐Rosei E., Ambrosioni E., Chiariello N., Leonetti G., Mancia G., Pessina A. C., Rizzon P., Salvetti A., Trimarco B., Volpe M. Left ventricular diastolic dysfunction in a cohort of hypertensive patients attending hospital outpatient clinics in Italy, the APROS‐DIADYS project. J Hypertension 2006; 24((suppl 6))41. (abstract). OS
- Tsang T. S., Barnes M. E., Gersh B. J., Bailey K. R., Seward J. B. Risks for atrial fibrillation and congestive heart failure in patients ⩾65 years of age with abnormal left ventricular diastolic relaxation. Am J Cardiol 2004; 93: 54–58. OS
- Redfield M. M., Jacobsen S. J., Burnett J. C., Jr., Mahoney D. W., Bailey K. R., Rodeheffer R. J. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003; 289: 194–202. OS
- Bella J. N., Palmieri V., Roman M. J., Liu J. E., Welty T. K., Lee E. T., Fabsitz R. R., Howard B. V., Devereux R. B. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle‐aged and elderly adults. The Strong Heart Study. Circulation 2002; 105: 1928–1933. OS
- Laukkanen J. A., Kurl S., Eranen J., Huttunen M., Salonen J. T. Left atrium size and the risk of cardiovascular death in middle‐aged men. Arch Intern Med 2005; 165: 1788–1793. OS
- Verdecchia P., Reboldi G., Gattobigio R., Bentivoglio M., Borgioni C., Angeli F., Carluccio E., Sardone M. G., Porcellati C. Atrial fibrillation in hypertension: predictors and outcome. Hypertension 2003; 41: 218–223. OS
- Kizer J. R., Bella J. N., Palmieri V., Liu J. E., Best L. G., Lee E. T., Roman M. J., Devereux R. B. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle‐aged and elderly adults: the Strong Heart Study (SHS). Am Heart J 2006; 151: 412–418. OS
- Ciulla M., Paliotti R., Hess D. B., Tjahja E., Campbell S. E., Magrini F., Weber K. T. Echocardiographic patterns of myocardial fibrosis in hypertensive patients: endomyocardial biopsy versus ultrasonic tissue characterization. J Am Soc Echocardiogr 1997; 10: 657–664. OS
- Hoyt R. M., Skorton D. J., Collins S. M., Melton H. E. Ultrasonic backscatter and collagen in normal ventricular myocardium. Circulation 1984; 69: 775–782. OS
- Ciulla M. M., Paliotti R., Esposito A., Diez J., Lopez B., Dahlof B., Nicholls M. G., Smith R. D., Gilles L., Magrini F., Zanchetti A. Different effects of antihypertensive therapies based on losartan or atenolol on ultrasound and biochemical markers of myocardial fibrosis: results of a randomized trial. Circulation 2004; 110: 552–557. RT
- Zanchetti A., Bond M. G., Hennig M., Neiss A., Mancia G., Dal Palu C., Hansson L., Magnani B., Rahn K. H., Reid J. L., Rodicio J., Safar M., Eckes L., Rizzini P., European Lacidipine Study on Atherosclerosis investigators. Calcium antagonist lacidipine slows down progression of asymptomatic carotid atherosclerosis: principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double‐blind, long‐term trial. Circulation 2002; 106: 2422–2427. RT
- Zanchetti A., Bond M. G., Hennig M., Tang R., Hollweck R., Mancia G., Eckes L., Micheli D., ELSA Investigators. Absolute and relative changes in carotid intima‐media thickness and atherosclerotic plaques during long‐term antihypertensive treatment: further results of the European Lacidipine Study on Atherosclerosis (ELSA). J Hypertens 2004; 22: 1201–1212. RT
- Zanchetti A., Agabiti Rosei E., Dal Palu C., Leonetti G., Magnani B., Pessina A. The Verapamil in Hypertension and Atherosclerosis Study (VHAS): results of long‐term randomized treatment with either verapamil or chlorthalidone on carotid intima‐media thickness. J Hypertens 1998; 16: 1667–1676. RT
- Hiatt W. R. Medical treatment of peripheral arterial disease and claudication. N Engl J Med 2001; 344: 1608–1621. RV
- Vogt M. T., Cauley J. A., Newman A. B., Kuller L. H., Hulley S. B. Decreased ankle/arm blood pressure index and mortality in elderly women. JAMA 1993; 270: 465–469. OS
- McKenna M., Wolfson S., Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis 1991; 87: 119–128. OS
- Vogt M. T., McKenna M., Anderson S. J., Wolfson S. K., Kuller L. H. The relationship between ankle‐arm index and mortality in older men and women. J Am Geriatr Soc 1993; 41: 523–530. OS
- Burek K. A., Sutton‐Tyrrell K., Brooks M. M., Naydeck B., Keller N., Sellers M. A., Roubin G., Jandova R., Rihal C. S. Prognostic importance of lower extremity arterial disease in patients undergoing coronary revascularization in the Bypass Angioplasty Revascularization Investigation (BARI). J Am Coll Cardiol 1999; 34: 716–721. OS
- Safar M. E., Levy B. I., Struijker‐Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 2003; 107: 2864–2869. RV
- Laurent S., Katsahian S., Fassot C., Tropeano A. I., Laloux B., Boutouyrie P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke 2003; 34: 1203–1206. OS
- Boutouyrie P., Tropeano A. I., Asmar R., Gautier I., Benetos A., Lacolley P., Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 2002; 39: 10–15. OS
- Park J. B., Schiffrin E. L. Small artery remodeling is the most prevalent (earliest?) form of target organ damage in mild essential hypertension. J Hypertension 2001; 19: 921–930. OS
- Korsgaard N., Aalkjaer C., Heagerty A. M., Izzard A. S., Mulvany M. J. Histology of subcutaneous small arteries from patients with essential hypertension. Hypertension 1993; 22: 523–526
- Rizzoni D., Porteri E., Guelfi D., Muiesan M. L., Valentini U., Cimino A., Girelli A., Rodella L., Bianchi R., Sleiman I., Agabiti‐Rosei E. Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non‐insulin‐dependent diabetes mellitus. Circulation 2001; 103: 1238–1244
- Schofield I., Malik R., Izzard A., Austin C., Heagerty A. Vascular structural and functional changes in type 2 diabetes mellitus: evidence for the roles of abnormal myogenic responsiveness and dyslipidemia. Circulation 2002; 106: 3037–3043. OS
- Rizzoni D., Porteri E., Boari G. E., De Ciuceis C., Sleiman I., Muiesan M. L., Castellano M., Miclini M., Agabiti‐Rosei E. Prognostic significance of small‐artery structure in hypertension. Circulation 2003; 108: 2230–2235
- Greenland P., Gaziano J. M. Clinical practice. Selecting asymptomatic patients for coronary computed tomography or electrocardiographic exercise testing. N Engl J Med 2003; 349: 465–473. RV
- Heitzer T., Schlinzig T., Krohn K., Meinertz T., Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 2001; 104: 2673–2678. OS
- Halcox J. P., Schenke W. H., Zalos G., Mincemoyer R., Prasad A., Waclawiw M. A., Nour K. R., Quyyumi A. A. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002; 106: 653–665. OS
- Taddei S., Salvetti A. Endothelial dysfunction in essential hypertension: clinical implications. J Hypertens 2002; 20: 1671–1674. RV
- Werner N., Kosiol S., Schiegl T., Ahlers P., Walenta K., Link A., Bohm M., Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 2005; 353: 999–1007. OS
- Stevens L. A., Coresh J., Greene T., Levey A. S. Assessing kidney function‐measured and estimated glomerular filtration rate. N Engl J Med 2006; 354: 2473–2483
- Moe S., Drueke T., Cunningham J., Goodman W., Martin K., Olgaard K., Ott S., Sprague S., Lameire N., Eknoyan G., Kidney Disease: Improving Global Outcomes (KDIGO). Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67: 2089–2100. GL
- Shlipak M. G., Katz R., Sarnak M. J., Fried L. F., Newman A. B., Stehman‐Breen C., Seliger S. L., Kestenbaum B., Psaty B., Tracy R. P., Siscovick D. S. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med 2006; 145: 237–246. OS
- Culleton B. F., Larson M. G., Wilson P. W., Evans J. C., Parfrey P. S., Levy D. Cardiovascular disease and mortality in a community‐based cohort with mild renal insufficiency. Kidney Int 1999; 56: 2214–2219. OS
- Parving H. H. Initiation and progression of diabetic nephropathy. N Engl J Med 1996; 335: 1682–1683. RV
- Ruilope L. M., Rodicio J. L. Clinical relevance of proteinuria and microalbuminuria. Curr Opin Nephrol Hypertens 1993; 2: 962–967. RV
- Redon J., Williams B. Microalbuminuria in essential hypertension: redefining the threshold. J Hypertens 2002; 20: 353–355. RV
- Arnlov J., Evans J. C., Meigs J. B., Wang T. J., Fox C. S., Levy D., Benjamin E. J., D'Agostino R. B., Vasan R. S. Low‐grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 2005; 112: 969–975. OS
- Zanchetti A., Hansson L., Dahlof B., Elmfeldt D., Kjeldsen S., Kolloch R., Larochelle P., McInnes G. T., Mallion J. M., Ruilope L., Wedel H. Effects of individual risk factors on the incidence of cardiovascular events in the treated hypertensive patients of the Hypertension Optimal Treatment Study. HOT Study Group. J Hypertens 2001; 19: 1149–1159. OS
- Ruilope L. M., Salvetti A., Jamerson K., Hansson L., Warnold I., Wedel H., Zanchetti A. Renal function and intensive lowering of blood pressure in hypertensive participants of the Hypertension Optimal Treatment (HOT) study. J Am Soc Nephrol 2001; 12: 218–225. RT
- De Leeuw P. W., Thijs L., Birkenhager W. H., Voyaki S. M., Efstratopoulos A. D., Fagard R. H., Leonetti G., Nachev C., Petrie J. C., Rodicio J. L., Rosenfeld J. J., Sarti C., Staessen J. A., Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. Prognostic significance of renal function in elderly patients with isolated systolic hypertension: results from the Syst‐Eur trial. J Am Soc Nephrol 2002; 13: 2213–2222. OS
- Segura J., Ruilope L. M., Zanchetti A. On the importance of estimating renal function for cardiovascular risk assessment. J Hypertens 2004; 22: 1635–1639. RV
- Rahman M., Pressel S., Davis B. R., Nwachuku C., Wright J. T., Jr., Whelton P. K., Barzilay J., Batuman V., Eckfeldt J. H., Farber M. A., Franklin S., Henriquez M., Kopyt N., Louis G. T., Saklayen M., Stanford C., Walworth C., Ward H., Wiegmann T., ALLHAT Collaborative Research Group. Cardiovascular outcomes in high‐risk hypertensive patients stratified by baseline glomerular filtration rate. Ann Intern Med 2006; 144: 172–180. OS
- Keith N. H., Wagener H. P., Barker M. W. Some different types of essential hypertension: their course and prognosis. Am J Med Sci 1939; 197: 332–343. OS
- Cuspidi C., Macca G., Salerno M., Michev L., Fusi V., Severgnini B., Corti C., Meani S., Magrini F., Zanchetti A. Evaluation of target organ damage in arterial hypertension: which role for qualitative funduscopic examination?. Ital Heart J 2001; 2: 702–706. OS
- Dimmitt S. B., West J. N., Eames S. M., Gibson J. M., Gosling P., Littler W. A. Usefulness of ophthalmoscopy in mild to moderate hypertension. Lancet 1989; 1: 1103–1106. OS
- Fuchs F. D., Maestri M. K., Bredemeier M., Cardozo S. E., Moreira F. C., Wainstein M. V., Moreira W. D., Moreira L. B. Study of the usefulness of optic fundi examination of patients with hypertension in a clinical setting. J Hum Hypertens 1995; 9: 547–551. OS
- Wong T. Y., Klein R., Sharrett A. R., Duncan B. B., Couper D. J., Tielsch J. M., Klein B. E., Hubbard L. D. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA 2002; 287: 1153–1159. OS
- Wong T. Y., Klein R., Sharrett A. R., Couper D. J., Klein B. E., Liao D. P., Hubbard L. D., Mosley T. H., ARIC Investigators. Atheroslerosis Risk in Communities Study. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet 2001; 358: 1134–1140. OS
- Martinez‐Perez M. E., Hughes A. D., Stanton A. V., Thom S. A., Chapman N., Bharath A. A., Parker K. H. Retinal vascular tree morphology: a semi‐automatic quantification. IEEE Trans Biomed Eng 2002; 49: 912–917
- Hughes A. D., Martinez‐Perez E., Jabbar A. S., Hassan A., Witt N. W., Mistry P. D., Chapman N., Stanton A. V., Beevers G., Pedrinelli R., Parker K. H., Thom S. A. Quantification of topological changes in retinal vascular architecture in essential and malignant hypertension. J Hypertens 2006; 24: 889–894
- Antonios T. F., Singer D. R., Markandu N. D., Mortimer P. S., MacGregor G. A. Rarefaction of skin capillaries in borderline essential hypertension suggests an early structural abnormality. Hypertension 1999; 34: 655–658. OS
- Noon J. P., Walker B. R., Webb D. J., Shore A. C., Holton D. W., Edwards H. V., Watt G. C. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J Clin Invest 1997; 99: 1873–1879. OS
- Price T. R., Manolio T. A., Kronmal R. A., Kittner S. J., Yue N. C., Robbins J., Anton‐Culver H., O'Leary D. H. Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community‐dwelling older adults: the Cardiovascular Health Study. Stroke 1997; 28: 1158–1164. OS
- Liao D., Cooper L., Cai J., Toole J. F., Bryan N. R., Hutchinson R. G., Tyroler H. A. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control: The ARIC Study. Stroke 1996; 27: 2262–2270. OS
- Vermeer S. E., Koudstaal P. J., Oudkerk M., Hofman A., Breteler M. M. Prevalence and risk factors of silent brain infarcts in the population‐based Rotterdam Scan Study. Stroke 2002; 33: 21–25. OS
- Longstreth W. T., Jr., Manolio T. A., Arnold A., Burke G. L., Bryan N., Jungreis C. A., Enright P. L., O'Leary D., Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke 1996; 27: 1274–1282. OS
- Prins N. D., van Dijk E. J., den Heijer T., Vermeer S. E., Koudstaal P. J., Oudkerk M., Hofman A., Breteler M. M. Cerebral white matter lesions and the risk of dementia. Arch Neurol 2004; 61: 1531–1534. OS
- Vermeer S. E., Hollander M., van Dijk E. J., Hofman A., Koudstaal P. J., Breteler M. M., Rotterdam Scan Study. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke 2000; 34: 1126–1129. OS
- Skoog I., Lernfelt B., Landahl S., Palmertz B., Andreasson L. A., Nilsson L., Persson G., Oden A., Svanborg A. 15‐year longitudinal study of blood pressure dementia. Lancet 1996; 347: 1141–1145. OS
- Kilander L., Nyman H., Boberg M., Hansson L., Lithell H. Hypertension is related to cognitive impairment: A 20‐year follow‐up of 999 men. Hypertension 1998; 31: 780–786. OS
- Launer L. J., Masaki K., Petrovitch H., Foley D., Havlik R. J. The association between midlife blood pressure levels and late‐life cognitive function. The Honolulu‐Asia Aging Study. JAMA 1995; 274: 1846–1851. OS
- Mancia G. Role of outcome trials in providing information on antihypertensive treatment: importance and limitations. Am J Hypertens 2006; 19: 1–7. RV
- Zanchetti A. Evidence‐based medicine in hypertension: what type of evidence?. J Hypertens 2005; 23: 1113–1120. RV
- Forette F., Seux M. L., Staessen J. A., Thijs L., Babarskiene M. R., Babeanu S., Bossini A., Fagard R., Gil‐Extremera B., Laks T., Kobalava Z., Sarti C., Tuomilehto J., Vanhanen H., Webster J., Yodfat Y., Birkenhager W. H., Systolic Hypertension in Europe Investigators. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst‐Eur) study. Arch Intern Med 2002; 162: 2046–2052. CT
- Forette F., Seux M. L., Staessen J. A., Thijs L., Babarskiene M. R., Babeanu S., Bossini A., Fagard R., Gil‐Extremera B., Laks T., Kobalava Z., Sarti C., Tuomilehto J., Vanhanen H., Webster J., Yodfat Y., Birkenhager W. H., Systolic Hypertension in Europe Investigators. Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. Effects of immediate versus delayed antihypertensive therapy on outcome in the Systolic Hypertension in Europe Trial. J Hypertens 2004; 22: 847–857. CT
- Mann J. F., Gerstein H. C., Pogue J., Bosch J., Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med 2001; 134: 629–636. CT
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 115 through 129 mmHg. JAMA 1967; 202: 1026–1034. RT
- Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results. Medical Research Council. BMJ 1985; 291: 97–104. RT
- SHEP Collaborative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265: 3255–3264. RT
- Dahlof B., Lindholm L. H., Hansson L., Schersten B., Ekbom T., Wester P. O. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP‐Hypertension). Lancet 1991; 338: 1281–1285. RT
- Amery A., Birkenhager W., Brixko P., Bulpitt C., Clement D., Deruyttere M., De Schaepdryver A., Dollery C., Fagard R., Forette F. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985; 1: 1349–1354. RT
- PROGRESS Collaborative Study Group. Randomised trial of perindopril based blood pressure‐lowering regimen among 6108 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358: 1033–1041. RT
- Staessen J. A., Fagard R., Thijs L., Celis H., Arabidze G. G., Birkenhager W. H., Bulpitt C. J., de Leeuw P. W., Dollery C. T., Fletcher A. E., Forette F., Leonetti G., Nachev C., O'Brien E. T., Rosenfeld J., Rodicio J. L., Tuomilehto J., Zanchetti A., for the Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet 1997; 350: 757–764. RT
- Gong L., Zhang W., Zhu Y., Zhu J., Kong D., Page V., Ghadirian P., LeLorier J., Hamet P. Shanghai trial of nifedipine in the elderly (STONE). J Hypertens 1996; 16: 1237–1245. CT
- Liu L., Wang J. L., Gong L., Liu G., Staessen J. A., For the Syst‐China Collaborative Group. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. J Hypertens 1998; 16: 1823–1829. CT
- Coope J., Warrender T. S. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J 1986; 293: 1145–1151. RT
- MRC Working Party. Medical Research Council trial of treatmeant of hypertension in older adults: principal results. Br Med J 1992; 304: 405–412. RT
- PATS Collaborative Group. Post‐stroke antihypertensive treatment study. Chin Med J 1995; 108: 710–717. RT
- Helgeland A. Treatment of mild hypertension: a five year controlled drug trial. The Oslo study. Am J Med 1980; 69: 725–732. RT
- Management Committee. The Australian therapeutic trial in mild hypertension. Lancet 1980; 1: 1261–1267. RT
- Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of prospectively‐designed overviews of randomised trials. Lancet 2003; 362: 1527–1535. MA
- Staessen J. A., Wang J. G., Thijs L. Cardiovascular prevention and blood pressure reduction: a quantitative overview updated until 1 March 2003. J Hypertens 2003; 21: 1055–1076. MA
- Staessen J. A., Gasowski J., Wang J. G., Thijs L., Den Hond E., Boissel J. P., Coope J., Ekbom T., Gueyffier F., Liu L., Kerlikowske K., Pocock S., Fagard R. H. Risks of untreated and treated isolated systolic hypertension in the elderly: meta‐analysis of outcome trials. Lancet 2000; 355: 865–872. MA
- Gueyffier F., Boutitie F., Boissel J. P., Pocock S., Coope J., Cutler J., Ekbom T., Fagard R., Friedman L., Perry M., Prineas R., Schron E. The effect of antihypertensive drug treatment on cardiovascular outcomes in women and men. Results from a meta‐analysis of individual patient data randomised controlled trials. Ann Intern Med 1997; 126: 761–767. MA
- Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood pressure‐lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus. Results of prospectively designed overviews of randomized trials. Arch Intern Med 2005; 165: 1410–1419. MA
- Bradley H. A., Wiysonge C. S., Volmink J. A., Mayosi B. M., Opie L. H. How strong is the evidence for use of beta‐blockers as first‐line therapy for hypertension? Systematic review and meta‐analysis. J Hypertens 2006; 24: 2131–2141. MA
- Neal B., MacMahon S., Chapman N., Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood‐pressure‐lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists' Collaboration. Lancet 2000; 356: 1955–1964
- Brewster L. M., van Montfrans G. A., Kleijnen J. Systematic review: antihypertensive drug therapy in black patients. Ann Intern Med 2004; 141: 614–627. MA
- The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. N Engl J Med 2000; 342: 145–153. RT
- Liu L., Zhang Y., Liu G., Li W., Zhang X., Zanchetti A., FEVER Study Group. The Felodipine Event Reduction (FEVER) Study: a randomized long‐term placebo‐controlled trial in Chinese hypertensive patients. J Hypertens 2005; 23: 2157–2172. RT
- The EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double‐blind, placebo‐controlled, multicentre trial (the EUROPA study). Lancet 2003; 362: 782–788. RT
- Poole‐Wilson P. A., Lubsen J., Kirwan B. A., van Dalen F. J., Wagener G., Danchin N., Just H., Fox K. A., Pocock S. J., Clayton T. C., Motro M., Parker J. D., Bourassa M. G., Dart A. M., Hildebrandt P., Hjalmarson A., Kragten J. A., Molhoek G. P., Otterstad J. E., Seabra‐Gomes R., Soler‐Soler J., Weber S. A Coronary disease Trial Investigating Outcome with Nifedipine gastrointestinal therapeutic system investigators. Effect of long‐acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet 2004; 364: 849–857. RT
- Lubsen J., Wagener G., Kirwan B. A., de Brouwer S., Poole‐Wilson P. A. ACTION (A Coronary disease Trial Investigating Outcome with Nifedipine GITS) investigators. Effect of long‐acting nifedipine on mortality and cardiovascular morbidity in patients with symptomatic stable angina and hypertension: the ACTION trial. J Hypertens 2005; 23: 641–648. CT
- Nissen S. E., Tuzcu E. M., Libby P., Thompson P. D., Ghali M., Garza D., Berman L., Shi H., Buebendorf E., Topol E. J., CAMELOT Investigators. Effect of antihypertensive agents on cardiorprovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA 2004; 292: 2217–2225. RT
- The PEACE trial investigators. Angiotensin‐converting‐enzyme inhibition in stable coronary artery disease. New Engl J Med 2004; 351: 2058–2068. RT
- Lithell H., Hansson L., Skoog I., Elmfeldt D., Hofman A., Olofsson B., Trenkwalder P., Zanchetti A., SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE). Principal results of a randomised double‐blind intervention trial. J Hypertens 2003; 21: 875–886. RT
- Brenner B. M., Cooper M. E., de Zeeuw D., Keane W. F., Mitch W. E., Parving H. H., Remuzzi G., Snapinn S. M., Zhang Z., Shahinfar S., RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869. RT
- Lewis E. J., Hunsicker L. G., Clarke W. R., Berl T., Pohl M. A., Lewis J. B., Ritz E., Atkins R. C., Rohde R., Raz I., Collaborative Study Group. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860. RT
- Pourdjabbar A. M., Lapointe N., Rouleau J‐L. Angiotensin receptor blockers: Powerful evidence with cardiovascular outcomes?. Can J Cardiol 2002; 18((Suppl A))7A–14A. MA
- Hansson L., Zanchetti A., Carruthers S. G., Dahlof B., Elmfeldt D., Julius S., Menard J., Rahn K. H., Wedel H., Westerling S. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet 1998; 351: 1755–1762. RT
- Hypertension Detection, Follow‐up Program. The effect of treatment on mortality in ‘mild’ hypertension: results of the Hypertension Detection, Follow‐up Program. N Engl J Med 1982; 307: 976–980. RT
- Hansson L., Lindholm L. H., Niskanen L., Lanke J., Hedner T., Niklason A., Luomanmaki K., Dahlof B., de Faire U., Morlin C., Karlberg B. E., Wester P. O., Bjorck J. E. Effect of angiotensin‐converting‐enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet 1999; 353: 611–616. RT
- Hansson L., Lindholm L. H., Ekbom T., Dahlof B., Lanke J., Schersten B., Wester P. O., Hedner T., de Faire U. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity in the Swedish Trial in Old Patients with Hypertension‐2 study. Lancet 1999; 354: 175–1756. RT
- Hansson L., Hedner T., Lund‐Johansen P., Kjeldsen S. E., Lindholm L. H., Syvertsen J. O., Lanke J., de Faire U., Dahlof B., Karlberg B. E. Randomised trial of effects of calcium antagonists compared with diuretics and beta‐blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet 2000; 356: 359–365. RT
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascularand microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998; 317: 713–720. RT
- Agodoa L. Y., Appel L., Bakris G. L., Beck G., Bourgoignie J., Briggs J. P., Charleston J., Cheek D., Cleveland W., Douglas J. G., Douglas M., Dowie D., Faulkner M., Gabriel A., Gassman J., Greene T., Hall Y., Hebert L., Hiremath L., Jamerson K., Johnson C. J., Kopple J., Kusek J., Lash J., Lea J., Lewis J. B., Lipkowitz M., Massry S., Middleton J., Miller E. R., 3rd., Norris K., O'Connor D., Ojo A., Phillips R. A., Pogue V., Rahman M., Randall O. S., Rostand S., Schulman G., Smith W., Thornley‐Brown D., Tisher C. C., Toto R. D., Wright J. T., Jr., Xu S., African American Study of Kidney Disease, Hypertension (AASK) Study Group. Effect of Ramipril vs Amlodipine on Renal Outcomes in Hypertensive Nephrosclerosis. A Randomized Controlled Trial. JAMA 2001; 285: 2719–2728. RT
- Wright J. T., Jr., Bakris G., Greene T., Agodoa L. Y., Appel L. J., Charleston J., Cheek D., Douglas‐Baltimore J. G., Gassman J., Glassock R., Hebert L., Jamerson K., Lewis J., Phillips R. A., Toto R. D., Middleton J. P., Rostand S. G., African American Study of Kidney Disease, Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK Trial. JAMA 2002; 288: 2421–2431. RT
- Schrier R. W., Estacio R. O., Esler A., Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and stroke. Kidney Int 2002; 61: 1086–1097. RT
- Estacio R. O., Jeffers B. W., Hiatt W. R., Biggerstaff S. L., Gifford N., Schrier R. W. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non‐insulin independent diabetes and hypertension. N Engl J Med 1998; 338: 645–652. RT
- Brown M. J., Palmer C. R., Castaigne A., de Leeuw P. W., Mancia G., Rosenthal T., Ruilope L. M. Morbidity and mortality in patients randomised to double‐blind treatment with a long‐acting calcium‐channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet 2000; 356: 366–372. RT
- The ALLHAT Officers, Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: The Anti hypertensive and Lipid‐Lowering treatment to prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288: 2981–2997. RT
- Black H. R., Elliott W. J., Grandits G., Grambsch P., Lucente T., White W. B., Neaton J. D., Grimm R. H., Jr., Hansson L., Lacourciere Y., Muller J., Sleight P., Weber M. A., Williams G., Wittes J., Zanchetti A., Anders R. J., CONVINCE Research Group. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular Endpoints (CONVINCE) trial. JAMA 2003; 289: 2073–2082. RT
- Malacco E., Mancia G., Rappelli A., Menotti A., Zuccaro M. S., Coppini A., SHELL Investigators. Treatment of isolated systolic hypertension: the SHELL study results. Blood Press 2003; 12: 160–167. RT
- NICS Study Group. Randomized double‐blind comparison of a calcium antagonist and a diuretic in elderly hypertensives. National Intervention Cooperative Study in Elderly Hypertensives Study Group. Hypertension 1999; 34: 1129–1133. RT
- Yui Y., Sumiyoshi T., Kodama K., Hirayama A., Nonogi H., Kanmatsuse K., Origasa H., Iimura O., Ishii M., Saruta T., Arakawa K., Hosoda S., Kawai C., Japan Multicenter Investigation for Cardiovascular Diseases‐B Study Group. Comparison of nifedipine retard with angiotensin converting enzyme inhibitors in Japanese hypertensive patients with coronary artery disease: the Japan Multicenter Investigation for Cardiovascular Diseases‐B (JMIC‐B) randomized trial. Hypertens Res 2004; 27: 181–191. RT
- Wing L. M., Reid C. M., Ryan P., Beilin L. J., Brown M. A., Jennings G. L., Johnston C. I., McNeil J. J., Macdonald G. J., Marley J. E., Morgan T. O., West M. J., Second Australian National Blood Pressure Study Group. A comparison of outcomes with angiotensin‐converting‐enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med 2003; 348: 583–592. RT
- Verdecchia P., Reboldi G., Angeli F., Gattobigio R., Bentivoglio M., Thijs L., Staessen J. A., Porcellati C. Angiotensin‐converting enzyme inhibitors and calcium channel blockers for coronary heart disease and stroke prevention. Hypertension 2005; 46: 386–392
- Blood Pressure Lowering Treatment Trialists' Collaboration. Blood pressure dependent and independent effects of agents that inhibit the renin‐angiotensin system. J Hypertens 2007; 25: 951–958. MA
- Dahlof B., Sever P. S., Poulter N. R., Wedel H., Beevers D. G., Caulfield M., Collins R., Kjeldsen S. E., Kristinsson A., McInnes G. T., Mehlsen J., Nieminen M., O'Brien E., Ostergren J., ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendo‐flumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA): a multicentre randomized controlled trial. Lancet 2005; 366: 895–906. RT
- Pepine C. J., Handberg E. M., Cooper‐DeHoff R. M., Marks R. G., Kowey P., Messerli F. H., Mancia G., Cangiano J. L., Garcia‐Barreto D., Keltai M., Erdine S., Bristol H. A., Kolb H. R., Bakris G. L., Cohen J. D., Parmley W. W., INVEST Investigators. A calcium antagonist vs a non‐calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil‐Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003; 290: 2805–2816. RT
- Dahlof B., Devereux R. B., Kjeldsen S. E., Julius S., Beevers G., de Faire U., Fyhrquist F., Ibsen H., Kristiansson K., Lederballe‐Pedersen O., Lindholm L. H., Nieminen M. S., Omvik P., Oparil S., Wedel H., LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359: 995–1003. RT
- Schrader J., Luders S., Kulschewski A., Hammersen F., Plate K., Berger J., Zidek W., Dominiak P., Diener HCMOSES Study Group. Morbidity and Mortality After Stroke. Eprosartan Compared with Nitrendipine for Secondary Prevention: principal results of a prospective randomized controlled study (MOSES). Stroke 2005; 36: 1218–1226. RT
- Mochizuki S., Dahlof B., Shimizu M., Ikewaki K., Yoshikawa M., Taniguchi I., Ohta M., Yamada T., Ogawa K., Kanae K., Kawai M., Seki S., Okazaki F., Taniguchi M., Yoshida S., Tajima N for the Jikei Heart Study group. Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open‐label, blinded endpoint morbidity‐mortality study. Lancet 2007; 369: 1431–1439. RT
- Julius S., Kjeldsen S. E., Weber M., Brunner H. R., Ekman S., Hansson L., Hua T., Laragh J., McInnes G. T., Mitchell L., Plat F., Schork A., Smith B., Zanchetti A. VALUE trial group. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004; 363: 2022–2031. RT
- Verma S., Strauss M. Angiotensin receptor blockers and myocardial infarction. Br Med J 2004; 329: 1248–1249. RV
- Volpe M., Mancia G., Trimarco B. Angiotensin receptor blockers and myocardial infarction: the importance of dosage. J Hypertens 2006; 24: 1681–1682. RV
- Verdecchia P., Angeli F., Gattobigio R., Reboldi G. P. Do angiotensin II receptor blockers increase the risk of myocardial infarction?. Eur Heart J 2005; 26: 2381–2386. MA
- Teo K., Yusuf S., Sleight P., Anderson C., Mookadam F., Ramos B., Hilbrich L., Pogue J., Schumacher H., ONTARGET/TRANSCEND Investigators. Rationale, design, baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, their combination in high‐risk patients: the Ongoing Telmisartan Alone, in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trials. Am Heart J 2004; 148: 52–61. RT
- Dickstein K., Kjekshus J., OPTIMAAL Steering Committee of the OPTIMAAL Study Group. Effects of losartan and captopril on mortality and morbidity in high‐risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet 2002; 360: 752–760. RT
- Pfeffer M. A., McMurray J. J., Velazquez E. J., Rouleau J. L., Kober L., Maggioni A. P., Solomon S. D., Swedberg K., Van de Werf F., White H., Leimberger J. D., Henis M., Edwards S., Zelenkofske S., Sellers M. A., Califf R. M. Valsartan in Acute Myocardial Infarction Trial Investigators. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349: 1893–1896. RT
- Pitt B., Poole‐Wilson P. A., Segal R., Martinez F. A., Dickstein K., Camm A. J., Konstam M. A., Riegger G., Klinger G. H., Neaton J., Sharma D., Thiyagarajan B. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial‐the Losartan Heart Failure Survival Study ELITE II. Lancet 2000; 355: 1582–1587. RT
- Lindholm L. H., Carlberg B., Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta‐analysis. Lancet 2005; 366: 1545–1553. MA
- Hypertension: management of hypertension in adults in primary care NICE/BHS. GL. June 2006. wwww.nice.org.uk/CG034nice.org.uk/CG034.
- McInnes G. T., Kjeldsen S. E. Never mind the quality, feel the width–ALLHAT revisited. Blood Press 2004; 13: 330–334. RV
- Wright J. T., Jr., Dunn J. K., Cutler J. A., Davis B. R., Cushman W. C., Ford C. E., Haywood L. J., Leenen F. H., Margolis K. L., Papademetriou V., Probstfield J. L., Whelton P. K., Habib G. B., ALLHAT Collaborative Research Group. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA 2005; 293: 1595–1608. CT
- Cuspidi C., Muiesan M. L., Valagussa L., Salvetti M., Di Biagio C., Agabiti‐Rosei E., Magnani B., Zanchetti A. CATCH investigators. Comparative effects of candesartan and enalapril on left ventricular hypertrophy in patients with essential hypertension: the Candesartan Assessment in the Treatment of Cardiac Hypertrophy (CATCH) study. J Hypertens 2002; 20: 2293–2300. RT
- Jennings G. L., McMullen J. R. Left ventricular hypertrophy. Beyond the image and defining human cardiac phenotype in hypertension. J Hypertens 2007, in press. RV
- Klingbeil A. U., Schneider M., Martus P., Messerli F. H., Schmieder R. E. A meta‐analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med 2003; 115: 41–46. MA
- Terpstra W. F., May J. F., Smit A. J., de Graeff P. A., Havinga T. K., van den Veur E., Schuurman F. H., Meyboom‐de Jong B., Crijns H. J. Long‐term effects of amlodipine and lisinopril on left ventricular mass and diastolic function in elderly, previously untreated hypertensive patients: the ELVERA trial. J Hypertens 2001; 19: 303–309. RT
- Devereux R. B., Palmieri V., Sharpe N., De Quattro V., Bella J. N., de Simone G., Walker J. F., Hahn R. T., Dahlof B. Effects of once‐daily angiotensin‐converting enzyme inhibition and calcium channel blockade‐based antihypertensive treatment regimens on left ventricular hypertrophy and diastolic filling in hypertension. The Prospective Randomized Enalapril Study Evaluating Regression of Ventricular Enlargement (PRESERVE) trial. Circulation 2001; 104: 1248–1254. RT
- Zanchetti A., Ruilope L. M., Cuspidi C., Macca G., Verschuren J., Kerselaers W. Comparative effects of the ACE inhibitor fosinopril and the calcium antagonist amlodipine on left ventricular hypertrophy and urinary albumin excretion in hypertensive patients. Results of FOAM, a multicenter European study. J Hypertens 2001; 19((Suppl 2))S92. (abstract). RT
- Agabiti‐Rosei E., Trimarco B., Muiesan M. L., Reid J., Salvetti A., Tang R., Hennig M., Baurecht H., Parati G., Mancia G., Zanchetti A., ELSA Echocardiographic Substudy Group. Cardiac structural and functional changes during long‐term antihypertensive treatment with lacidipine and atenolol in the European Lacidipine Study on Atherosclerosis (ELSA). J Hypertens 2005; 23: 1091–1098. CT
- Thurmann P. A., Kenedi P., Schmidt A., Harder S., Rietbrock N. Influence of the angiotensin II antagonist valsartan on left ventricular hypertrophy in patients with essential hypertension. Circulation 1998; 98: 2037–2042. RT
- Malmqvist K., Kahan T., Edner M., Held C., Hagg A., Lind L., Muller‐Brunotte R., Nystrom F., Ohman K. P., Osbakken M. D., Ostergern J. Regression of left ventricular hypertrophy in human hypertension with irbesartan. J Hypertens 2001; 19: 1167–1176. RT
- Dahlof B., Zanchetti A., Diez J., Nicholls M. G., Yu C. M., Barrios V., Aurup P., Smith R. D., Johansson M., For the REGAAL Study Investigators. Effects of losartan and atenolol on left ventricular mass and neurohormonal profile in patients with essential hypertension and left ventricular hypertrophy. J Hypertens 2002; 20: 1855–1864. RT
- Devereux R. B., Dahlof B., Gerdts E., Boman K., Nieminen M. S., Papademetriou V., Rokkedal J., Harris K. E., Edelman J. M., Wachtell K. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation 2004; 110: 1456–1462. RT
- De Luca N., Mallion J. M., O'Rourke M. F., O'Brien E., Rahn K. H., Trimarco B., Romero R., De Leeuw P. W., Hitzenberger G., Battegay E., Duprez D., Sever P., Safar M. E. Regression of left ventricular mass in hypertensive patients treated with perindopril/indapamide as a first‐line combination: the REASON echocardiography study. Am J Hypertens 2004; 17: 660–667. RT
- Dahlof B., Gosse P., Gueret P., Dubourg O., de Simone G., Schmieder R., Karpov Y., Garcia‐Puig J., Matos L., De Leeuw P. W., Degaute J. P., Magometschnigg D., The PICXEL Investigators. Perindopril/indapamide combination more effective than enalapril in reducing blood pressure and left ventricular mass: the PICXEL study. J Hypertens 2005; 23: 2063–2070. RT
- De Luca N., Asmar R. G., London G. M., O'Rourke M. F., Safar M. E., REASON Project Investigators. Selective reduction of cardiac mass and central blood pressure on low‐dose combination perindopril/indapamide in hypertensive subjects. J Hypertens 2004; 22: 1623–1630. RT
- Pitt B., Reichek N., Willenbrock R., Zannad F., Phillips R. A., Roniker B., Kleiman J., Krause S., Burns D., Williams G. H. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E‐left ventricular hypertrophy study. Circulation 2003; 108: 1831–1838. RT
- Galzerano D., Tammaro P., del Viscovo L., Lama D., Galzerano A., Breglio R., Tuccillo B., Paolisso G., Capogrosso P. Three‐dimensional echocardiographic and magnetic resonance assessment of the effect of telmisartan compared with carvedilol on left ventricular mass a multicenter, randomized, longitudinal study. Am J Hypertens 2005; 18: 1563–1569. RT
- Gosse P., Sheridan D. J., Zannad F., Dubourg O., Gueret P., Karpov Y., de Leeuw P. W., Palma‐Gamiz J. L., Pessina A., Motz W., Degaute J. P., Chastang C. Regression of left ventricular hypertrophy in hypertensive patients treated with indapamide SR 1.5 mg versus enalapril 20 mg; the LIVE study. J Hypertens 2000; 18: 1465–1475. RT
- Muiesan M. L., Salvetti M., Rizzoni D., Castellano M., Donato F. Agabiti Rosei E. Association of change in left ventricular mass with prognosis during long‐term antihypertensive treatment. J Hypertens 1995; 13: 1091–1095. OS
- Koren M. J., Ulin R. J., Koren A. T., Laragh J. H., Devereux R. B. Left ventricular mass change during treatment and outcome in patients with essential hypertension. Am J Hypertens 2002; 15: 1021–1028. OS
- Cuspidi C., Ciulla M., Zanchetti A. Hypertensive myocardial fibrosis. Nephrol Dial Transplant 2006; 21: 20–23. RV
- Ciulla M. M., Paliotti R., Esposito A., Cuspidi C., Muiesan M. L., Salvetti M., Agabiti‐Rosei E., Magrini F., Zanchetti A. Effects of the angiotension receptor antagonist candesartan and the ACE inhibitor Enalapril on ultrasound markers of myocardial fibrosis in hypertensive patients with left ventricular hypertrophy. J Hypertens 2005; 23((Suppl 2))S381. (abstract). RT
- Christensen M. K., Olsen M. H., Wachtell K., Tuxen C., Fossum E., Bang L. E., Wiinberg N., Devereux R. B., Kjeldsen S. E., Hildebrandt P., Rokkedal J., Ibsen H. Does long‐term losartan‐ vs atenolol‐based antihypertensive treatment influence collagen markers differently in hypertensive patients? A LIFE substudy. Blood Press 2006; 15: 198–206. CT
- Olsen M. H., Wachtell K., Tuxen C., Fossum E., Bang L. E., Hall C., Ibsen H., Rokkedal J., Devereux R. B., Hildebrandt P. N‐terminal pro‐brain natriuretic peptide predicts cardiovascular events in patients with hypertension and left ventricular hypertrophy: a LIFE study. J Hypertens 2004; 22: 1597–1604. OS
- Okin P. M., Devereux R. B., Jern S., Kjeldsen S. E., Julius S., Nieminen M. S., Snapinn S., Harris K. E., Aurup P., Edelman J. M., Dahlof B. Losartan Intervention for Endpoint reduction in hypertension Study Investigations. Regression of electrocardiographic left ventricular hypertrophy by losartan versus atenolol: The Losartan Intervention For Endpoint reduction in Hypertension (LIFE) Study. Circulation 2003; 108: 684–690. RT
- Schneider M. P., Klingbeil A. U., Delles C., Ludwig M., Kolloch R. E., Krekler M., Stumpe K. O., Schmieder R. E. Effect of irbesartan versus atenolol on left ventricular mass and voltage: results of the CardioVascular Irbesartan Project. Hypertension 2004; 44: 61–66. RT
- Havranek E. P., Esler A., Estacio R. O., Mehler P. S., Schrier R. W., Appropriate Blood Pressure Control in Diabetes Trial. Differential effects of antihypertensive agents on electrocardiographic voltage: results from the Appropriate Blood Pressure Control in Diabetes (ABCD) trial. Am Heart J 2003; 145: 993–998. RT
- Muller‐Brunotte R., Edner M., Malmqvist K., Kahan T. Irbesartan and atenolol improve diastolic function in patients with hypertensive left ventricular hypertrophy. J Hypertens 2005; 23: 633–640. RT
- Cuspidi C., Meani S., Valerio C., Fusi V., Catini E., Sala C., Zanchetti A. Ambulatory blood pressure, target organ damage and left atrial size in never‐treated essential hypertensive individuals. J Hypertens 2005; 23: 1589–1595. OS
- Gerdts E., Wachtell K., Omvik P., Otterstad J. E., Oikarinen L., Boman K., Dahlof B., Devereux R. B. Left atrial size and risk of major cardiovascular events during antihypertensive treatment: losartan intervention for endpoint reduction in hypertension trial. Hypertension 2007; 49: 311–316. OS
- Aksnes T. A., Flaa A., Strand A., Kjeldsen S. E. Prevention of new‐onset atrial fibrillation and its predictors with angiotensin II‐receptor blockers in the treatment of hypertension and heart failure. J Hypertens 2007; 25: 15–23. RV
- Wachtell K., Lehto M., Gerdts E., Olsen M. H., Hornestam B., Dahlof B., Ibsen H., Julius S., Kjeldsen S. E., Lindholm L. H., Nieminen M. S., Devereux R. B. Angiotensin II receptor blockade reduces new‐onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol 2005; 45: 712–719. RT
- Schmieder R., Kjeldsen S. E., Julius S., McInnes G. T., Zanchetti A., Hua T. Reduced incidence of new onset atrial fibrillation with angiotensin II receptor blockade: the VALUE‐trial. J Hypertens 2006; 24: S3 (abstract). RT
- Vermes E., Tardif J. C., Bourassa M. G., Racine N., Levesque S., White M., Guerra P. G., Ducharme A. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the Studies Of Left Ventricular Dysfunction (SOLVD) trials. Circulation 2003; 107: 2926–2931. RT
- Ducharme A., Swedberg K., Pfeffer M. A., Cohen‐Solal A., Granger C. B., Maggioni A. P., Michelson E. L., McMurray J. J., Olsson L., Rouleau J. L., Young J. B., Olofsson B., Puu M., Yusuf S., CHARM Investigators. Prevention of atrial fibrillation in patients with symptomatic chronic heart failure by candesartan in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Am Heart J 2006; 152: 86–92. RT
- Maggioni A. P., Latini R., Carson P. E., Singh S. N., Barlera S., Glazer R., Masson S., Cere E., Tognoni G., Cohn J. N., Val‐HeFT Investigators. Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val‐HeFT). Am Heart J 2005; 149: 548–557. RT
- Okin P. M., Wachtell K., Devereux R. B., Harris K. E., Jern S., Kjeldsen S. E., Julius S., Lindholm L. H., Nieminen M. S., Edelman J. M., Hille D. A., Dahlof B. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new‐onset atrial fibrillation in patients with hypertension. JAMA 2006; 296: 1242–1248. OS
- Madrid A. H., Bueno M. G., Rebollo J. M., Marin I., Pena G., Bernal E., Rodriguez A., Cano L., Cano J. M., Cabeza P., Moro C. Use of irbesartan to maintain sinus rhythm in patients with long‐lasting persistent atrial fibrillation: a prospective, randomized study. Circulation 2002; 106: 331–336. RT
- Fogari R., Mugellini A., Destro M., Corradi L., Zoppi A., Fogari E., Rinaldi A. Losartan and prevention of atrial fibrillation recurrence in hypertensive patients. J Cardiovasc Pharmacol 2006; 47: 46–50. RT
- Disertori M., Latini R., Maggioni A. P., Delise P., Di Pasquale G., Franzosi M. G., Staszewsky L., Tognoni G., on behalf of the GISSI‐AF Investigators. Rationale, design of the GISSI‐Atrial Fibrillation Trial: a randomized, prospective, multicentre study on the use of valsartan, an angiotensin II AT 1‐receptor blocker, in the prevention of atrial fibrillation recurrence. J Cardiovasc Med 2006; 7: 29–38. RT
- Wang J. G., Staessen J. A., Li Y., Van Bortel L. M., Nawrot T., Fagard R., Messerli F. H., Safar M. Carotid intima‐media thickness and antihypertensive reatment: a meta‐analysis of randomized controlled trials. Stroke 2006; 37: 1933–1940. MA
- MacMahon S., Sharpe N., Gamble G., Clague A., Mhurchu C. N., Clark T., Hart H., Scott J., White H. Randomized, placebo‐controlled trial of the angiotensin‐converting enzyme inhibitor, ramipril, in patients with coronary or other occlusive arterial disease. PART‐2 Collaborative Research Group. Prevention of Atherosclerosis with Ramipril. J Am Coll Cardiol 2000; 36: 438–443. RT
- Asselbergs F. W., van Roon A. R., Hillege H. L., de Jong R. E., Gans R. O. B., Smit A. J., van Gilst W. H., on behalf of the PREVEND IT Investigators PREVEND IT Investigators. Effects of fosinopril and pravastatin on carotid intima‐media thickness in subjects with increased albuminuria. Stroke 2005; 36: 649–653. RT
- Hedblad B., Wikstrand J., Janzon L., Wedel H., Berglund G. Low‐dose metoprolol CR/XL and fluvastatin slow progression of carotid intima‐media thickness: Main results from the Beta‐Blocker Cholesterol‐Lowering Asymptomatic Plaque Study (BCAPS). Circulation 2001; 103: 1721–1726. RT
- Zanchetti A., Crepaldi G., Bond M. G., Gallus G., Veglia F., Mancia G., Ventura A., Baggio G., Sampietri L., Rubba P., Sperti G., Magni A., on behalf of PHYLLIS Investigators. Different effects of antihypertensive regimens based on fosinopril or hydrochlorothiazide with or without lipid lowering by pravastatin on progression of asymptomatic carotid atherosclerosis: principal results of PHYLLIS‐a randomized double‐blind trial. Stroke 2004; 35: 2807–2812. RT
- Simon A., Gariepy J., Moyse D., Levenson J. Differential effects of nifedipine and co‐amilozide on the progression of early carotid wall changes. Circulation 2001; 103: 2949–2954. CT
- Terpstra W. F., May J. F., Smit A. J., Graeff P. A., Meyboom‐de Jong B., Crijns H. J. Effects of amlodipine and lisinopril on intima‐media thickness in previously untreated, elderly hypertensive patients (the ELVERA trial). J Hypertens 2004; 22: 1309–1316. RT
- Pitt B., Byington R. P., Furberg C. D., Hunninghake D. B., Mancini G. B. J., Miller M. E., Riley W. Effect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. Circulation 2000; 102: 1503–1510. RT
- Lonn E. M., Yusuf S., Dzavik V., Doris C. I., Yi Q., Smith S., Moore‐Cox A., Bosch J., Riley W. A., Teo K. K. Effects of ramipril and vitamin E on atherosclerosis: The Study to Evaluate Carotid Ultrasound changes in patients treated with Ramipril and vitamin E SECURE). Circulation 2001; 103: 919–925. CT
- Borhani N. O., Mercuri M., Borhani P. A., Buckalew V. M., Canossa‐Terris M., Carr A. A., Kappagoda T., Rocco M. V., Schnaper H. W., Sowers J. R., Bond M. G. Final outcome results of the Multicenter Isradipine Diuretic Atherosclerosis Study (MIDAS). A randomized controlled trial. JAMA 1996; 276: 785–791. RT
- Ciulla M. M., Paliotti R., Ferrero S., Vandoni P., Magrini F., Zanchetti A. Assessment of carotid plaque composition in hypertensive patients by ultrasonic tissue characterization: a validation study. J Hypertens 2002; 20: 1589–1596
- Paliotti R., Ciulla M. M., Hennig M., Tang R., Bond M. G., Mancia G., Magrini F., Zanchetti A. Carotid wall composition in hypertensive patients after 4‐year treatment with lacidipine or atenolol: an echoreflectivity study. J Hypertens 2005; 23: 1203–1209. CT
- Asmar R. Effect of antihypertensive agents on arterial stiffness as evaluated by pulse wave velocity: clinical implications. Am J Cardiovasc Drugs 2001; 1: 387–397. RV
- Ichihara A., Hayashi M., Koura Y., Tada Y., Hirota N., Saruta T. Long‐term effects of intensive blood‐pressure lowering on arterial wall stiffness in hypertensive patients. Am J Hypertens 2003; 16: 959–965. OS
- Asmar R. G., London G. M., O'Rourke M. E., Safar M. E., REASON Project Coordinators and Investigators. Improvement in blood pressure, arterial stiffness and wave reflections with a very‐low‐dose perindopril/indapamide combination in hypertensive patient: a comparison with atenolol. Hypertension 2001; 38: 922–926. RT
- Rajzer M., Klocek M., Kawecka‐Jaszcz K. Effect of amlodipine, quinapril, and losartan on pulse wave velocity and plasma collagen markers in patients with mild‐to‐moderate arterial hypertension. Am J Hypertens 2003; 16: 439–444. RT
- Munakata M., Nagasaki A., Nunokawa T., Sakuma T., Kato H., Yoshinaga K., Toyota T. Effects of valsartan and nifedipine coat‐core on systemic arterial stiffness in hypertensive patients. Am J Hypertens 2004; 17: 1050–1055. RT
- Dhakam Z., McEniery C. M., Yasmin, Cockcroft J. R., Brown M. J., Wilkinson I. B. Atenolol and eprosartan: differential effects on central blood pressure and aortic pulse wave velocity. Am J Hypertens 2006; 19: 214–219. RT
- Staessen J. A., Richart T., Birkenhager W. H. Less atherosclerosis and lower blood pressure for a meaningful life perspective with more brain. Hypertension 2007; 49: 389–400. RV
- Dufouil C., Chalmers J., Coskun O., Besancon V., Bousser M. G., Guillon P., MacMahon S., Mazoyer B., Neal B., Woodward M., Tzourio‐Mazoyer N., Tzourio C., PROGRESS MRI Substudy Investigators. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation 2005; 112: 1644–1650. CT
- Birns J., Morris R., Donaldson N., Kalra L. The effects of blood pressure reduction on cognitive function: a review of effects based on pooled data from clinical trials. J Hypertens 2006; 24: 1907–1914. MA
- Forette F., Seux M. L., Staessen J. A., Thijs L., Birkenhager W. H., Babarskiene M. R., Babeanu S., Bossini A., Gil‐Extremera B., Girerd X., Laks T., Lilov E., Moisseyev V., Tuomilehto J., Vanhanen H., Webster J., Yodfat Y., Fagard R. Prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst‐Eur) study. Lancet 1998; 352: 1347–1351. RT
- Skoog I., Lithell H., Hansson L., Elmfeldt D., Hofman A., Olofsson B., Trenkwalder P., Zanchetti A., SCOPE Study Group. Effect of baseline cognitive function and antihypertensive treatment on cognitive and cardiovascular outcomes: Study on COgnition and Prognosis in the Elderly (SCOPE). Am J Hypertens 2005; 18: 1052–1059. RT
- Goldstein G., Materson B. J., Cushman W. C., Reda D. J., Freis E. D., Ramirez E. A., Talmers F. N., White T. J., Nunn S., Chapman R. H. Treatment of hypertension in the elderly: II. Cognitive and behavioral function. Results of a Department of Veterans Affairs Cooperative Study. Hypertension 1990; 15: 361–369. RT
- McCorvey E., Jr., Wright J. T., Jr., Culbert J. P., McKenney J. M., Proctor J. D., Annett M. P. Effect of hydrochlorothiazide, enalapril, and propranolol on quality of life and cognitive and motor function in hypertensive patients. Clin Pharm 1993; 12: 300–305. RT
- Leonetti G., Salvetti A. Effects of cilazapril and nitrendipine on blood pressure, mood, sleep, and cognitive function in elderly hypertensive patients: an Italian multicenter study. J Cardiovasc Pharmacol 1994; 24((Suppl 3))S73–S77RT
- Starr J. M., Whalley L. J., Deary I. J. The effects of antihypertensive treatment on cognitive function: results from the HOPE study. J Am GeriatrSoc 1996; 44: 411–415. CT
- Fogari R., Mugellini A., Zoppi A., Marasi G., Pasotti C., Poletti L., Rinaldi A., Preti P. Effects of valsartan compared with enalapril on blood pressure and cognitive function in elderly patients with essential hypertension. Eur J Clin Pharmacol 2004; 59: 863–868. RT
- Prince M. J., Bird A. S., Blizard R. A., Mann A. H. Is the cognitive function of older patients affected by antihypertensive treatment? Results from 54 months of the Medical Research Council's trial of hypertension in older adults. BMJ 1996; 312: 801–805. CT
- Casas J. P., Chua W., Loukogeorgakis S., Vallance P., Smeeth L., Hingorani A. D., MacAllister R. J. Effect of inhibitors of the renin‐angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta‐analysis. Lancet 2005; 366: 2026–2033. MA
- De Zeeuw D., Lewis E. J., Remuzzi G., Brenner B. M., Cooper M. E. Renoprotective effects of renin‐angiotensin‐system inhibitors. Lancet 2006; 367: 899–900
- Mann J. F., Ritz E., Kunz R. Renoprotective effects of renin‐angiotensin‐system inhibitors. Lancet 2006; 367: 900
- Zanchetti A., Ruilope L. M. Antihypertensive treatment in patients with type‐2 diabetes mellitus: what guidance from recent controlled randomized trials?. J Hypertens 2002; 20: 2099–2110. RV
- Karalliedde J., Viberti G. Evidence for renoprotection by blockade of the renin‐angiotensin‐aldosterone system in hypertension and diabetes. J Hum Hypertens 2006; 20: 239–253. RT
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension, antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43((5 Suppl 1))S1–290. GL
- Levey A. S., Greene T., Beck G. J., Caggiula A. W., Kusek J. W., Hunsicker L. G., Klahr S. Dietary protein restriction and the progression of chronic renal disease: what have all of the results of the MDRD study shown? Modification of Diet in Renal Disease Study group. J Am Soc Nephrol 1999; 10: 2426–2439. CT
- Estacio R. O., Jeffers B. W., Gifford N., Schrier R. W. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000; 23((Suppl 2))B54–B64. RT
- Estacio R. O., Coll J. R., Tran Z. V., Schrier R. W. Effect of intensive blood pressure control with valsartan on urinary albumin excretion in normotensive patients with type 2 diabetes. Am J Hypertens 2006; 19: 1241–1248. RT
- Ruggenenti P., Perna A., Loriga G., Ganeva M., Ene‐Iordache B., Turturro M., Lesti M., Perticucci E., Chakarski I. N., Leonardis D., Garini G., Sessa A., Basile C., Alpa M., Scanziani R., Sorba G., Zoccali C., Remuzzi G., REIN‐2 Study Group REIN‐2 Study Group. Blood‐pressure control for renoprotection in patients with non‐diabetic chronic renal disease (REIN‐2): multicentre, randomised controlled trial. Lancet 2005; 365: 939–946. RT
- Pohl M. A., Blumenthal S., Cordonnier D. J., De Alvaro F., Deferrari G., Eisner G., Esmatjes E., Gilbert R. E., Hunsicker L. G., de Faria J. B., Mangili R., Moore J., Jr., Reisin E., Ritz E., Schernthaner G., Spitalewitz S., Tindall H., Rodby R. A., Lewis E. J. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol 2005; 16: 3027–3037. CT
- Jafar T. H., Stark P. C., Schmid C. H., Landa M., Maschio G., de Jong P. E., de Zeeuw D., Shahinfar S., Toto R., Levey A. S., AIPRD Study Group. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin‐converting enzyme inhibition: a patient‐level meta‐analysis. Ann Intern Med 2003; 139: 244–252. MA
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascularand microvascular complications in Type 2 diabetes. UKPDS38. BMJ 1998; 317: 703–713. RT
- Heart Outcomes Prevention Evaluation (HOPE) Study investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO‐HOPE substudy. Lancet 2000; 355: 253–259. RT
- Adler A. I., Stratton I. M., Neil H. A., Yudkin J. S., Matthews D. R., Cull C. A., Wright A. D., Turner R. C., Holman R. R. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. Br Med J 2000; 321: 412–429. OS
- Randomised placebo‐controlled trial of effect of ramipril on decline in glomerular filtration rate risk of terminal renal failure in proteinuric non‐diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997; 349: 1857–1863. RT
- Mann J. F., Gerstein H. C., Yi Q. L., Franke J., Lonn E. M., Hoogwerf B. J., Rashkow A., Yusuf S., HOPE Investigators. Progression of renal insufficiency in type 2 diabetes with and without microalbuminuria: results of the Heart Outcomes and Prevention Evaluation (HOPE) randomized study. Am J Kidney Dis 2003; 42: 936–942. RT
- Ruggenenti P., Fassi A., Ilieva A. P., Bruno S., Iliev I. P., Brusegan V., Rubis N., Gherardi G., Arnoldi F., Ganeva M., Ene‐Iordache B., Gaspari F., Perna A., Bossi A., Trevisan R., Dodesini A. R., Remuzzi G., Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) Investigators. Preventing microalbuminuria in type 2 diabetes. N Engl J Med 2004; 351: 1941–1951. RT
- Mogensen C. E., Viberti G., Halimi S., Ritz E., Ruilope L., Jermendy G., Widimsky J., Sareli P., Taton J., Rull J., Erdogan G., De Leeuw P. W., Ribeiro A., Sanchez R., Mechmeche R., Nolan J., Sirotiakova J., Hamani A., Scheen A., Hess B., Luger A., Thomas S. M., Preterax in Albuminuria Regression (PREMIER) Study Group. Effect of low‐dose perindopril/indapamide on albuminuria in diabetes: Preterax in albuminuria regression: PREMIER. Hypertension 2003; 41: 1063–1071. RT
- Lewis E. J., Hunsicker L. G., Bain R. P., Rohde R. D. The effect of angiotensin‐converting‐enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329: 1456–1462. RT
- Parving H‐H., Lehnert H., Brochner‐Mortensen J., Gomis R., Andersen S., Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001; 345: 870–878. RT
- Schjoedt K. J., Rossing K., Juhl T. R., Boomsma F., Tarnow L., Rossing P., Parving H. H. Beneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathy. Kidney Int 2006; 70: 536–542. RT
- Voyaki S. M., Staessen J. A., Thijs L., Wang J. G., Efstratopoulos A. D., Birkenhager W. H., de Leeuw P. W., Leonetti G., Nachev C., Rodicio J. L., Tuomilehto J., Fagard R., Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. Follow‐up of renal function in treated and untreated older patients with isolated systolic hypertension. Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. J Hypertens 2001; 19: 511–519. RT
- Rahman M., Pressel S., Davis B. R., Nwachuku C., Wright J. T., Jr., Whelton P. K., Barzilay J., Batuman V., Eckfeldt J. H., Farber M., Henriquez M., Kopyt N., Louis G. T., Saklayen M., Stanford C., Walworth C., Ward H., Wiegmann T. Renal outcomes in high‐risk hypertensive patients treated with an angiotensin‐converting enzyme inhibitor or a calcium channel blocker vs a diuretic: a report from the Antihypertensive and Lipid‐ Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Intern Med 2005; 165: 936–946. CT
- Barnett A. H. Preventing renal complications in diabetic patients: the Diabetics Exposed to Telmisartan And enalaprIL (DETAIL) study 1. Acta Diabetol 2005; 42((Suppl 1))S42–S49. RT
- Ibsen H., Olsen M. H., Wachtell K., Borch‐Johnsen K., Lindholm L. H., Mogensen C. E., Dahlof B., Snapinn S. M., Wan Y., Lyle P. A. Does albuminuria predict cardiovascular outcomes on treatment with losartan versus atenolol in patients with diabetes, hypertension, and left ventricular hypertrophy? The LIFE study. Diabetes Care 2006; 29: 595–600. CT
- Viberti G., Wheeldon N. M., MicroAlbuminuria Reduction With VALsartan (MARVAL) Study Investigators. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure‐independent effect. Circulation 2002; 106: 672–678. RT
- Vogt L., Navis G., Koster J., Manolis A. J., Reid J. L., de Zeeuw D., on behalf of the Angiotensin II Receptor Antagonist Telmisartan Micardis in Isolated Systolic Hypertension (ARAMIS) Study Group. The angiotensin II receptor antagonist telmisartan reduces urinary albumin excretion in patients with isolated systolic hypertension: results of a randomized, double‐blind, placebo‐controlled trial. J Hypertens 2005; 23: 2055–2061. RT
- White W. B., Duprez D., St Hillaire R., Krause S., Roniker B., Kuse‐Hamilton J., Weber M. A. Effects of the selective aldosterone blocker eplerenone versus the calcium antagonist amlodipine in systolic hypertension. Hypertension 2003; 41: 1021–1026. RT
- Dalla Vestra M., Pozza G., Mosca A., Grazioli V., Lapolla A., Fioretto P., Crepaldi G. Effect of lercanidipine compared with ramipril on albumin excretion rate in hypertensive Type 2 diabetic patients with microalbuminuria: DIAL study. Diabetes Nutr Metab 2004; 17: 259–266. RT
- Marre M., Puig J. G., Kokot F., Fernandez M., Jermendy G., Opie L., Moyseev V., Scheen A., Ionescu‐Tirgoviste C., Saldanha M. H., Halabe A., Williams B., Mion Junior D., Ruiz M., Hermansen K., Tuomilehto J., Finizola B., Gallois Y., Amouyel P., Ollivier J. P., Asmar R. Equivalence of indapamide SR and enalapril on microalbuminuria reduction in hypertensive patients with type 2 diabetes: the NESTOR Study. J Hypertens 2004; 22: 1613–1622. RT
- Nakao N., Yoshimura A., Morita H., Takada M., Kayano T., Ideura T. Combination treatment of angiotensin‐I I receptor blocker and angiotensin‐converting‐enzyme inhibitor in non‐diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet 2003; 361: 117–124. RT
- Kincaid‐Smith P., Fairley K., Packham D. Randomized controlled crossover study of the effect on proteinuria and blood pressure of adding an angiotensin II receptor antagonist to an angiotensin converting enzyme inhibitor in normotensive patients with chronic renal disease and proteinuria. Nephrol Dial Transplant 2002; 17: 597–601. RT
- Mogensen C. E., Neldam S., Tikkanen I., Oren S., Viskoper R., Watts R. W., Cooper M. E. Randomised controlled trial of dual blockade of renin‐angiotensin system in patients with hypertension, microalbuminuria, and non‐insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. Br Med J 2000; 321: 1440–1444. RT
- Andersen N. H., Poulsen P. L., Knudsen S. T., Poulsen S. H., Eiskjaer H., Hansen K. W., Helleberg K., Mogensen C. E. Long‐term dual blockade with candesartan and lisinopril in hypertensive patients with diabetes: the CALM II study. Diabetes Care 2005; 28: 273–277. RT
- MacKinnon M., Shurraw S., Akbari A., Knoll G. A., Jaffey J., Clark H. D. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: a systematic review of the efficacy and safety data. Am J Kidney Dis 2006; 48: 8–20. MA
- Rossing K., Schjoedt K. J., Jensen B. R., Boomsma F., Parving H. H. Enhanced renoprotective effects of ultrahigh doses of irbesartan in patients with type 2 diabetes and microalbuminuria. Kidney Int 2005; 68: 1190–1198. RT
- Schmieder R. E., Klingbeil A. U., Fleischmann E. H., Veelken R., Delles C. Additional antiproteinuric effect of ultrahigh dose candesartan: a double‐blind, randomized, prospective study. J Am Soc Nephrol 2005; 16: 3038–3045. RT
- Kannel W. B., Wilson P. W., Zhang T. J. The epidemiology of impaired glucose tolerance and hypertension. Am Heart J 1991; 121: 1268–1273. OS
- Stamler J., Vaccaro O., Neaton J. D., Wentworth D. Diabetes, other riskfactors, and 12‐yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993; 16: 434–444. CT
- Mancia G., Grassi G., Zanchetti A. New‐onset diabetes and antihypertensive drugs. J Hypertens 2006; 24: 3–10. RV
- Norris K., Bourgoigne J., Gassman J., Hebert L., Middleton J., Phillips R. A., Randall O., Rostand S., Sherer S., Toto R. D., Wright J. T., Jr., Wang X., Greene T., Appel L. J., Lewis J., AASK Study Group. Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) Trial. Am J Kidney Dis 2006; 48: 739–751. RT
- Lindholm L. H., Persson M., Alaupovic P., Carlberg B., Svensson A., Samuelsson O. Metabolic outcome during 1 year in newly detected hypertensives: results of the Antihypertensive Treatment and Lipid Profile in a North of Sweden Efficacy Evaluation (ALPINE study). J Hypertens 2003; 21: 1563–1574. RT
- Opie L. H., Schall R. Old antihypertensives and new diabetes. J Hypertens 2004; 22: 1453–1458. MA
- Kostis J. B., Wilson A. C., Freudenberger R. S., Cosgrove N. M., Pressel S. L., Davis B. R., SHEP Collaborative Research Group. Long‐term effect of diuretic‐based therapy on fatal outcomes in subjects with isolated systolic hypertension with and without diabetes. Am J Cardiol 2005; 95: 29–35. CT
- Elliott W. J., Meyer P. M. Incident diabetes in clinical trials of antihypertensive drugs: a network meta‐analysis. Lancet 2007; 369: 201–207. MA
- Domanski M., Norman J., Pitt B., Haigney M., Hanlon S., Peyster E., Studies of Left Ventricular Dysfunction. Diuretic use, progressive heart failure, and death in patients in the Studies Of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol 2003; 42: 705–708. RT
- Yusuf S., Gerstein H., Hoogwerf B., Pogue J., Bosch J., Wolffenbuttel B. H., Zinman B., HOPE Study Investigators. Ramipril and the development of diabetes. JAMA 2001; 286: 1882–1885. RT
- Pfeffer M. A., Swedberg K., Granger C. B., Held P., McMurray J. J., Michelson E. L., Olofsson B., Ostergren J., Yusuf S., Pocock S., CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM‐Overall programme. Lancet 2003; 362: 759–766. RT
- DREAM Trial Investigators, Bosch J., Yusuf S., Gerstein H. C., Pogue J., Sheridan P., Dagenais G., Diaz R., Avezum A., Lanas F., Probstfield J., Fodor G., Holman R. R. Effect of ramipril on the incidence of diabetes. NEnglJMed 2006; 355: 1551–1562. RT
- Howard B. V., Rodriguez B. L., Bennett P. H., Harris M. I., Hamman R., Kuller L. H., Pearson T. A., Wylie‐Rosett J. Prevention Conference VI: Diabetes and Cardiovascular disease: Writing Group I: epidemiology. Circulation 2002; 105: 132–137. RV
- Alderman M. H., Cohen H., Madhavan S. Diabetes and cardiovascular events in hypertensive patients. Hypertension 1999; 33: 1130–1134. OS
- Dunder K., Lind L., Zethelius B., Berglund L., Lithell H. Increase in blood glucose concentration during antihypertensive treatment as a predictor of myocardial infarction: population based cohort study. Br Med J 2003; 326: 681. OS
- Eberly L. E., Cohen J. D., Prineas R., Yang L., Intervention Trial Research group. Impact of incident diabetes and incident nonfatal cardiovascular disease on 18‐year mortality: the multiple risk factor intervention trial experience. Diabetes Care 2003; 26: 848–854. CT
- Verdecchia P., Reboldi G., Angeli F., Borgioni C., Gattobigio R., Filippucci L., Norgiolini S., Bracco C., Porcellati C. Adverse prognostic significance of new diabetes in treated hypertensive subjects. Hypertension 2004; 43: 963–969. OS
- Almgren T., Willemsen O., Samuelsson O., Himmelmann A., Rosengren A., Anderson O. K. Diabetes in treated hypertension is common and carries a high cardiovascular risk: results from 20 years follow up. J Hypertens 2007, in press. OS
- Collins R., MacMahon S. Blood pressure, antihypertensive drug treatment and the risk of stroke and of coronary heart disease. Br Med Bull 1994; 50: 272–298. MA
- Sever P. S., Poulter N. R., Dahlof B., Wedel H., Anglo‐Scandinavian Cardiac Outcomes Trial Investigators. Different time course for prevention of coronary and stroke events by atorvastatin in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Lipid‐Lowering Arm (ASCOT‐LLA). Am J Cardiol 2005; 96: 39F–44F. RT
- Atkins R. C., Briganti E. M., Lewis J. B., Hunsicker L. G., Braden G., Champion de Crespigny P. J., DeFerrari G., Drury P., Locatelli F., Wiegmann T. B., Lewis E. J. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45: 281–287. OS
- The ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin‐converting enzyme inhibitors? A meta‐analysis of individual patient data. Ann Int Med 2001; 134: 370–379. MA
- Parving H. H., Hommel E., Jensen B. R., Hansen H. P. Long‐term beneficial effect of ACE inhibition on diabetic nephropathy in normotensive type 1 diabetic patients. Kidney Int 2001; 60: 228–234. OS
- Julius S., Nesbitt S. D., Egan B. M., Weber M. A., Michelson E. L., Kaciroti N., Black H. R., Grimm R. H., Jr., Messerli F. H., Oparil S., Schork M. A., Trial of Preventing Hypertension (TROPHY) Study Investigators. Feasibility of treating prehypertension with an angiotensin‐receptor blocker. N Engl J Med 2006; 354: 1685–1697. RT
- Weber M. A., Julius S., Kjeldsen S. E., Brunner H. R., Ekman S., Hansson L., Hua T., Laragh J. H., McInnes G. T., Mitchell L., Plat F., Schork M. A., Smith B., Zanchetti A. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet 2004; 363: 2049–2051. CT
- Pepine C. J., Kowey P. R., Kupfer S., Kolloch R. E., Benetos A., Mancia G., Coca A., Cooper‐DeHoff R. M., Handberg E., Gaxiola E., Sleight P., Conti C. R., Hewkin A. C., Tavazzi L., INVEST Investigators. Predictors of adverse outcome among patients with hypertension and coronary artery disease. J Am Coll Cardiol 2006; 47: 547–551. OS
- Benetos A., Thomas F., Bean K. E., Guize L. Why cardiovascular mortality is higher in treated hypertensives versus subjects of the same age, in the general population. J Hypertens 2003; 21: 1635–1640. OS
- Arima H., Chalmers J., Woodward M., Anderson C., Rodgers A., Davis S., Macmahon S., Neal B., PROGRESS Collaborative Group. Lower target blood pressures are safe and effective for the prevention of recurrent stroke: the PROGRESS trial. J Hypertens 2006; 24: 1201–1208. OS
- Zanchetti A., Hansson L., Clement D., Elmfeldt D., Julius S., Rosenthal T., Waeber B., Wedel H., HOT Study Group. Benefits and risks of more intensive blood pressure lowering in hypertensive patients of the HOT study with different risk profiles: does a J‐shaped curve exist in smokers?. J Hypertens 2003; 21: 797–804. CT
- Freemantle N., Cleland J., Young P., Mason J., Harrison J. Beta blockade after myocardial infarction: systematic review and meta regression analysis. Br Med Journal 1999; 318: 1730–1737. MA
- Shekelle P. G., Rich M. W., Morton S. C., Atkinson C. S., Tu W., Maglione M., Rhodes S., Barrett M., Fonarow G. C., Greenberg B., Heidenreich P. A., Knabel T., Konstam M. A., Steimle A., Warner Stevenson L. Efficacy of angiotensin‐converting enzyme inhibitors and beta‐blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta‐analysis of major clinical trials. JAm Coll Cardiol 2003; 41: 1529–1538. MA
- Sega R., Cesana G., Milesi C., Grassi G., Zanchetti A., Mancia G. Ambulatory and home blood pressure normality in the elderly: data from the PAMELA population. Hypertension 1997; 30: 1–6. OS
- Mancia G., Parati G. Office compared with ambulatory blood pressure in assessing response to antihypertensive treatment: a meta‐analysis. J Hypertens 2004; 22: 435–445. MA
- Messerli F. H., Mancia G., Conti C. R., Hewkin A. C., Kupfer S., Champion A., Kolloch R., Benetos A., Pepine C. J. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous?. Ann Intern Med 2006; 144: 884–893. OS
- Boutitie F., Gueyffier F., Pocock S., Fagard R., Boissel J. P., INDANA Project Steering Committee. INdividual Data ANalysis of Antihypertensive intervention. J‐shaped relationship between blood pressure and mortality in hypertensive patients: new insights from a meta‐analysis of individual‐patient data. Ann Intern Med 2002; 136: 438–448. MA
- Samuelsson O. G., Wilhelmsen L. W., Pennert K. M., Wedel H., Berglund G. L. The J‐shaped relationship between coronary heart disease and achieved blood pressure level in treated hypertension: further analyses of 12 years of follow‐up of treated hypertensives in the Primary Prevention Trial in Gothenburg, Sweden. J Hypertens 1990; 8: 547–555. OS
- Cruickshank J. M., Pennert K., Sorman A. E., Thorp J. M., Zacharias F. M., Zacharias F. J. Low mortality from all causes, including myocardial infarction, in well‐controlled hypertensives treated with a beta‐blocker plus other antihypertensives. J Hypertens 1987; 5: 489–498. OS
- Staessen J., Bulpitt C., Clement D., De Leeuw P., Fagard R., Fletcher A., Forette F., Leonetti G., Nissinen A., O'Malley K. Relation between mortality and treated blood pressure in elderly patients with hypertension: report of the European Working Party on High Blood Pressure in the Elderly. Br Med J 1989; 298: 1552–1556. CT
- Bonet S., Agusti A., Arnau J. M., Vidal X., Diogene E., Galve E., Laporte J. R. Beta‐adrenergic blocking agents in heart failure: benefits of vasodilating and non‐vasodilating agents according to patients' characteristics: a metaanalysis of clinical trials. Arch Intern Med 2000; 160: 621–627. MA
- Mancia G., Grassi G. Systolic and diastolic blood pressure control in antihypertensive drug trials. J Hypertens 2002; 20: 1461–1464. RV
- Mancia G., Brown M., Castaigne A., de Leeuw P., Palmer C. R., Rosenthal T., Wagener G., Ruilope L. M., INSIGHT. Outcomes with nifedipine GITS or Co‐amilozide in hypertensive diabetics and nondiabetics in Intervention as a Goal in Hypertension (INSIGHT). Hypertension 2003; 41: 431–436. RT
- Ambrosioni E. Pharmacoeconomic challenges in disease management of hypertension. J Hypertens 2001; 19((Suppl 3))S33–S40. RV
- Schulzer M., Mancini G. B. ‘Unqualified success’ and ‘unmitigated failure’: number‐needed‐to‐treat‐related concepts for assessing treatment efficacy in the presence of treatment‐induced adverse events. Int J Epidemiol 1996; 25: 704–712. RV
- Zanchetti A., Mancia G. Benefits and cost‐effectiveness of antihypertensive therapy. The actuarial versus the intervention trial approach. J Hypertens 1996; 14: 809–811. RV
- Zanchetti A., Hansson L., Menard J., Leonetti G., Rahn K. H., Warnold I., Wedel H. Risk assessment and treatment benefit in intensively treated hypertensive patients of the Hypertension Optimal Treatment (HOT) study. J Hypertens 2001; 19: 819–825. OS
- Zanchetti A. Costs of implementing recommendations on hypertension management given in recent guidelines. J Hypertens 2003; 21: 2207–2209. RV
- Moser M. Are lifestyle interventions in the management of hypertension effective? How long should you wait before starting specific medical therapy? An ongoing debate. J Clin Hypertens 2005; 7: 324–326. RV
- Dickinson H. O., Mason J. M., Nicolson D. J., Campbell F., Beyer F. R., Cook S. W., Williams B., Ford G. A. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomised controlled trials. J Hypertens 2006; 24: 215–233. MA
- Haynes R. B., McDonald H. P., Garg A. X. Helping patients follow prescribed treatment: clinical applications. JAMA 2002; 288: 2880–2883
- Groppelli A., Giorgi D. M., Omboni S., Parati G., Mancia G. Persistent blood pressure increase induced by heavy smoking. J Hypertens 1992; 10: 495–499
- Grassi G., Seravalle G., Calhoun D. A., Bolla G. B., Giannattasio C., Marabini M., Del Bo A., Mancia G. Mechanisms responsible for sympathetic activation by cigarette smoking in humans. Circulation 1994; 90: 248–253
- Narkiewicz K., van de Borne P. J., Hausberg M., Cooley R. L., Winniford M. D., Davison D. E., Somers V. K. Cigarette smoking increases sympathetic outflow in humans. Circulation 1998; 98: 528–534
- Seltzer C. C. Effect of smoking on blood pressure. Am Heart J 1974; 87: 558–564
- Verdecchia P., Schillaci G., Borgioni C., Ciucci A., Zampi I., Battistelli M., Gattobigio R., Sacchi N., Porcellati C. Cigarette smoking, ambulatory blood pressure and cardiac hypertrophy in essential hypertension. J Hypertens 1995; 13: 1209–1215. OS
- Mann S. J., James G. D., Wang R. S., Pickering T. G. Elevation of ambulatory systolic blood pressure in hypertensive smokers. A case‐control study. JAMA 1991; 265: 2226–2228. OS
- Bang L. E., Buttenschon L., Kristensen K. S., Svendsen T. L. Do we undertreat hypertensive smokers? A comparison between smoking and non‐smoking hypertensives. Blood Press Monit 2000; 5: 271–274
- Mundal R., Kjeldsen S. E., Sandvik L., Erikssen G., Thaulow E., Erikssen J. Predictors of 7‐year changes in exercise blood pressure: effects of smoking physical fitness pulmonary function. J Hypertens 1997; 15: 245–249. OS
- Primatesta P., Falaschetti E., Gupta S., Marmot M. G., Poulter N. R. Association between smoking and blood pressure: evidence from the health survey for England. Hypertension 2001; 37: 187–193. OS
- Omvik P. How smoking affects blood pressure. Blood Press 1996; 5: 71–77. RV
- Doll R., Peto R., Wheatley K., Gray R., Sutherland I. Mortality in relation to smoking: 40 years' observations on male British doctors. Br Med J 1994; 309: 901–911. OS
- Rosenberg L., Kaufman D. W., Helmrich S. P., Shapiro S. The risk of myocardial infarction after quitting smoking in men under 55 years of age. N Engl J Med 1985; 313: 1511–1514. OS
- Manson J. E., Tosteson H., Ridker P. M., Satterfield S., Hebert P., O'Connor G. T., Buring J. E., Hennekens C. H. The primary prevention of myocardial infarction. N Engl J Med 1992; 326: 1406–1416
- Wilson K., Gibson N., Willan A., Cook D. Effect of smoking cessation on mortality after myocardial infarction: meta‐analysis of cohort studies. Arch Intern Med 2000; 160: 939–944. MA
- Tsevat J., Weinstein M. C., Williams L. W., Tosteson A. N., Goldman L. Expected gains in life expectancy from various coronary heart disease risk factor modifications. Circulation 1991; 83: 1194–1201. OS
- Silagy C., Mant D., Fowler G., Lodge M. Meta‐analysis on efficacy of nicotine replacement therapies in smoking cessation. Lancet 1994; 343: 139–142. MA
- Tonstad S., Farsang C., Klaene G., Lewis K., Manolis A., Perruchoud A. P., Silagy C., van Spiegel P. I., Astbury C., Hider A., Sweet R. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicentre, randomised study. Eur Heart J 2003; 24: 946–955. RT
- Nides M., Oncken C., Gonzales D., Rennard S., Watsky E. J., Anziano R., Reeves K. R. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7‐week, randomized, placebo‐ and bupropion‐controlled trial with 1‐year follow‐up. Arch Intern Med 2006; 166: 1561–1568. RT
- Law M. R., Morris J. K., Wald N. J. Environmental tobacco smoke exposure and ischaemic heart disease: an evaluation of the evidence. Br Med J 1997; 315: 973–980. RV
- Stranges S., Bonner M. R., Fucci F., Cummings K. M., Freudenheim J. L., Dorn J. M., Muti P., Giovino G. A., Hyland A., Trevisan M. Lifetime cumulative exposure to secondhand smoke and risk of myocardial infarction in never smokers: results from the Western New York health study, 1995–2001. Arch Intern Med 2006; 166: 1961–1967. OS
- Rimm E. B., Williams P., Fosher K., Criqui M., Stampfer M. J. Moderate alcohol intake and lower risk of coronary heart disease: meta‐analysis of effects on lipids and haemostatic factors. Br Med J 1999; 319: 1523–1528. MA
- Fillmore K. M., Kerr W. C., Stockwell T., Chikritzhs T., Bostrom A. Moderate alcohol use and reduced mortality risk: Systematic error in prospective studies. Addiction Research & Theory 2006; 14: 101–132. RV
- Puddey I. B., Beilin L. J., Rakie V. Alcohol, hypertension and the cardiovascular system: a critical appraisal. Addiction Biol 1997; 2: 159–170. RV
- Wannamethee S. G., Shaper A. G. Patterns of alcohol intake and risk of stroke in middle‐aged British men. Stroke 1996; 27: 1033–1039. OS
- Puddey I. B., Beilin L. J., Vandongen R. Regular alcohol use raises blood pressure in treated hypertensive subjects. A randomised controlled trial. Lancet 1987; 1: 647–651. RT
- Law M. R. Epidemiologic evidence on salt and blood pressure. Am J Hypertens 1997; 10((Suppl 5))S42–S45. RV
- Joint WHO/FAO Expert report on diet, nutrition, the prevention of chronic disease. Executive Summary. www.who.int. RV
- Cutler J. A., Follman D., Alexander P. S. Randomized controlled trials of sodium reduction: an overview. Am J Clin Nutr 1997; 65((Suppl 2))S643–S651. MA
- Graudal N. A., Galloe A. M., Garred P. Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride: a meta‐analysis. JAMA 1998; 279: 1383–1391. MA
- He F. J., MacGregor G. A. How far should salt intake be reduced?. Hypertension 2003; 42: 1093–1099. RV
- Robertson J. I. J. Dietary salt and hypertension: a scientific issue or a matter of faith?. J Eval Clin Pract 2003; 9: 1–22. RV
- Australian National Health Medical Research Council Dietary Salt Study Management Committee. Effects of replacing sodium intake in subjects on a low sodium diet a crossover study. Clin Exp Hypertens 1989; A11: 1011–1024
- He F. J., Markandu N. D., MacGregor G. A. Importance of the renin system for determining blood pressure fall with acute salt restriction in hypertensive and normotensive whites. Hypertension 2001; 38: 321–325. OS
- Grassi G., Dell'Oro R., Seravalle G., Foglia G., Quarti Trevano F., Mancia G. Short‐ and long‐term neuroadrenergic effects of moderate dietary sodium restriction in essential hypertension. Circulation 2002; 106: 1957–1961
- Grassi G., Cattaneo B. M., Seravalle G., Lanfranchi A., Bolla G., Mancia G. Baroreflex impairment by low sodium diet in mild or moderate essential hypertension. Hypertension 1997; 29: 802–807
- Appel L. J., Brands M. W., Daniels S. R., Karanja N., Elmer P. J., Sacks F. M., American Heart Association. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 2006; 47: 296–308. GL
- Otten J., Pitzi Helliwig J., Meyers L. D. The dietary reference intakes: the essential guide to nutrient requirements. National Academies Press, Washington, DC 2006, RV
- Sacks F. M., Svetkey L. P., Vollmer W. M., Appel L. J., Bray G. A., Harsha D., Obarzanek E., Conlin P. R., Miller E. R., 3rd., Simons‐Morton D. G., Karanja N., Lin P. H., DASH‐Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH‐Sodium Collaborative Research Group. N Engl J Med 2001; 344: 3–10. RT
- Morris M. C., Sacks F., Rosner B. Does fish oil lower blood pressure? A meta‐analysis of controlled trials. Circulation 1993; 88: 523–533. MA
- Geleijnse J. M., Giltay E. J., Grobbee D. E., Donders A. R., Kok F. J. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens 2002; 20: 1493–1499. MA
- Appel L. J., Miller E. R., 3rd., Seidler A. J., Whelton P. K. Does supplementation of diet with ‘fish oil’ reduce blood pressure? A meta‐analysis of controlled clinical trials. Arch Intern Med 1993; 153: 1429–1438. MA
- He J., Whelton P. K. Effect of dietary fiber and protein intake on blood pressure: a review of epidemiologic evidence. Clin Exp Hypertens 1999; 21: 785–796. RV
- He J., Streiffer R. H., Muntner P., Krousel‐Wood M. A., Whelton P. K. Effect of dietary fiber intake on blood pressure: a randomized, double‐blind, placebo‐controlled trial. J Hypertens 2004; 22: 73–80. RT
- Griffith L. E., Guyatt G. H., Cook R. J., Bucher H. C., Cook D. J. The influence of dietary and nondietary calcium supplementation on blood pressure: an updated metaanalysis of randomized controlled trials. Am J Hypertens 1999; 12: 84–92. MA
- Jee S. H., Miller E. R., 3rd., Guallar E., Singh V. K., Appel L. J., Klag M. J. The effect of magnesium supplementation on blood pressure: a meta‐analysis of randomized clinical trials. Am J Hypertens 2002; 15: 691–696. MA
- Visvanathan R., Chen R., Horowitz M., Chapman I. Blood pressure responses in healthy older people to 50 g carbohydrate drinks with differing glycaemic effects. Br J Nutr 2004; 92: 335–340. OS
- Pereira M. A., Swain J., Goldfine A. B., Rifai N., Ludwig D. S. Effects of a low‐glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA 2004; 292: 2482–2490. OS
- Margetts B. M., Beilin L. J., Vandongen R., Armstrong B. K. Vegetarian diet in mild hypertension: a randomised controlled trial. Br Med J 1986; 293: 1468–1471. RT
- Bao D. Q., Mori T. A., Burke V., Puddey I. B., Beilin L. J. Effects of dietary fish and weight reduction on ambulatory blood pressure in overweight hypertensives. Hypertension 1998; 32: 710–717. OS
- Daniels S. R., Kimball T. R., Khoury P., Witt S., Morrison J. A. Correlates of the hemodynamic determinants of blood pressure. Hypertension 1996; 28: 37–41. OS
- Stamler J. Epidemiologic findings on body mass and blood pressure in adults. Ann Epidemiol 1991; 1: 347–362. OS
- Neter J. E., Stam B. E., Kok F. J., Grobbee D. E., Geleijnse J. M. Influence of weight reduction on blood pressure: a meta‐analysis of randomized controlled trials. Hypertension 2003; 42: 878–884. MA
- Stevens V. J., Corrigan S. A., Obarzanek E., Bernauer E., Cook N. R., Hebert P., Mattfeldt‐Beman M., Oberman A., Sugars C., Dalcin A. T. Weight loss intervention in phase 1 of the Trials of Hypertension Prevention. The TOHP Collaborative Research Group. Arch Intern Med 1993; 153: 849–858. CT
- Stevens V. J., Obarzanek E., Cook N. R., Lee I. M., Appel L. J., Smith West D., Milas N. C., Mattfeldt‐Beman M., Belden L., Bragg C., Millstone M., Raczynski J., Brewer A., Singh B., Cohen J., Trials for the Hypertension Prevention Research Group. Long‐term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 2001; 134: 1–11. RT
- Huang Z., Willett W. C., Manson J. E., Rosner B., Stampfer M. J., Speizer F. E., Colditz G. A. Body weight, weight change, and risk for hypertension in women. Ann Intern Med 1998; 128: 81–88. OS
- The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high‐normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med 1997; 157: 657–667. RT
- Langford H. G., Blaufox M. D., Oberman A., Hawkins C. M., Curb J. D., Cutter G. R., Wassertheil‐Smoller S., Pressel S., Babcock C., Abernethy J. D. Dietary therapy slows the return of hypertension after stopping prolonged medication. JAMA 1985; 253: 657–664. RT
- Whelton P. K., Appel L. J., Espeland M. A., Applegate W. B., Ettinger W. H., Jr., Kostis J. B., Kumanyika S., Lacy C. R., Johnson K. C., Folmar S., Cutler J. A. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA 1998; 279: 839–846. RT
- Sandvik L., Erikssen J., Thaulow E., Erikssen G., Mundal R., Rodahl K. Physical fitness as a predictor of mortality among healthy, middle‐aged Norwegian men. N Engl J Med 1993; 328: 533–537. OS
- Cornelissen V. A., Fagard R. H. Effects of endurance training on blood pressure, blood pressure‐regulating mechanisms, and cardiovascular risk factors. Hypertension 2005; 46: 667–675. OS
- Fagard R. H. Exercise characteristics and the blood pressure response to dynamic physical training. Med Sci Sports Exerc 2001; 33: S484–S492. OS
- Cornelissen V. A., Fagard R. H. Effect of resistance training on resting blood pressure: a meta‐analysis of randomized controlled trials. J Hypertens 2005; 23: 251–259. MA
- Jennings G. L. Exercise, blood pressure: Walk, run or swim?. J Hypertens 1997; 15: 567–569. RV
- Stringer W. W., Wasserman K. Statement on exercise: American College of Chest Physicians/American Thoracic Society‐exercise for fun or profit?. Chest 2005; 127: 1072–1073. GL
- Fagard R. H., Bjornstad H. H., Borjesson M., Carre F., Deligiannis A., Vanhees L., European Society of Cardiology. ESC Study Group of Sports Cardiology recommendations for participation in leisure‐time physical activities and competitive sports for patients with hypertension. Eur J Cardiovasc PrevRehabil 2005; 12: 326–331. GL
- Heidenreich P. A., McDonald K. M., Hastie T., Fadel B., Hagan V., Lee B. K., Hlatky M. A. Meta‐analysis of trials comparing beta‐blockers, calcium antagonists, and nitrates for stable angina. JAMA 1999; 281: 1927–1936. MA
- Sharma A. M., Pischon T., Hardt S., Kunz I., Luft F. C. Hypothesis: Beta‐adrenergic receptor blockers and weight gain: a systematic analysis. Hypertension 2001; 37: 250–254. RV
- Lindholm L. H., Ibsen H., Borch‐Johnsen K., Olsen M. H., Wachtell K., Dahlof B., Devereux R. B., Beevers G., de Faire U., Fyhrquist F., Julius S., Kjeldsen S. E., Kristianson K., Lederballe‐Pedersen O., Nieminen M. S., Omvik P., Oparil S., Wedel H., Aurup P., Edelman J. M., Snapinn S., For the LIFE study group. Risk of new‐onset diabetes in the Losartan Intervention For Endpoint reduction in hypertension study. J Hypertens 2002; 20: 1879–1886. RT
- Kjeldsen S. E., Julius S., Mancia G., McInnes G. T., Hua T., Weber M. A., Coca A., Ekman S., Girerd X., Jamerson K., Larochelle P., MacDonald T. M., Schmieder R. E., Schork M. A., Stolt P., Viskoper R., Widimsky J., Zanchetti A., VALUE Trial Investigators. Effects of valsartan compared to amlodipine on preventing type 2 diabetes in high‐risk hypertensive patients: the VALUE trial. J Hypertens 2006; 24: 1405–1412. RT
- Torp‐Pedersen C., Metra M., Charlesworth A., Spark P., Lukas M. A., Poole‐Wilson P. A., Swedberg K., Cleland J. G., Di Lenarda A., Remme W., Scherhaug A. Effects of metoprolol and carvedilol on preexisting and new onset diabetes in patients with chronic heartfailure Data from the Carvedilol or metoprolol European Trial (COMET). Heart 2007, in press. RT
- Kaiser T., Heise T., Nosek L., Eckers U., Sawicki P. T. Influence of nebivolol and enalapril on metabolic parameters and arterial stiffness in hypertensive type 2 diabetic patients. J Hypertens 2006; 24: 1397–1403. RT
- Cushman W. C., Reda D. J., Perry H. M., Williams D., Abdellatif M., Materson B. J. Regional and racial differences in response to antihypertensive medication use in a randomized controlled trial of men with hypertension in the United States. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. Arch Intern Med 2000; 160: 825–831. CT
- Van Zwieten P. A. Centrally acting antihypertensive drugs. Manual of Hypertension, G Mancia, J Chalmers, S Julius, T Saruta, M Weber. Churchill Livingston, London 2002; 401–410. RV
- Zannad F. Aldosterone antagonist therapy in resistant hypertension. J Hypertens 2007; 25: 747–750. RV
- The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the Antihypertensive and Lipid‐Lowering treatment to prevent Heart Attack Trial (ALLHAT). JAMA 2000; 283: 1967–1975. RT
- Nguyen G. Renin/prorenin receptors. Kidney Int 2006; 69: 1503–1506. RV
- Villamil A., Chrysant S. G., Calhoun D., Schober B., Hsu H., Matrisciano‐Dimichino L., Zhang J. Renin inhibition with aliskiren provides additive antihypertensive efficacy when used in combination with hydrochlorothiazide. J Hypertens 2007; 25: 217–226. RT
- O'Brien E., Barton J., Nussberger J., Mulcahy D., Jensen C., Dicker P., Stanton A. Aliskiren reduces blood pressure and suppresses plasma renin activity in combination with a thiazide diuretic, an angiotensin‐converting enzyme inhibitor, or an angiotensin receptor blocker. Hypertension 2007; 49: 276–284. RT
- Gradman A. H., Schmieder R. E., Lins R. L., Nussberger J., Chiang Y., Bedigian M. P. Aliskiren, a novel orally effective renin inhibitor, provides dose‐dependent antihypertensive efficacy and placebo‐like tolerability in hypertensive patients. Circulation 2005; 111: 1012–1018. RT
- Pilz B., Shagdarsuren E., Wellner M., Fiebeler A., Dechend R., Gratze P., Meiners S., Feldman D. L., Webb R. L., Garrelds I. M., Jan Danser A. H., Luft F. C., Muller D. N. Aliskiren, a human renin inhibitor, ameliorates cardiac and renal damage in double‐transgenic rats. Hypertension 2005; 46: 569–576
- Alderman M. H., Madhavan S., Ooi W. L., Cohen H., Sealey J. E., Laragh J. H. Association of the renin‐sodium profile with the risk of myocardial infarction in patients with hypertension. N Engl J Med 1991; 324: 1098–1104. OS
- Ruilope L. M., Agabiti‐Rosei E., Bakris G. L., Mancia G., Poulter N. R., Taddei S., Unger T., Volpe M., Waeber B., Zannad F. Angiotensin receptor blockers: therapeutic targets and cardiovascular protection. Blood Press 2005; 14: 196–209. RV
- Waeber B., Burnier M., Brunner H. R. Compliance with antihypertensive therapy. Clin Exp Hypertens 1999; 21: 973–985. RV
- Parati G., Omboni S., Rizzoni D., Agabiti‐Rosei E., Mancia G. The smoothness index: a new, reproducible and clinically relevant measure of the homogeneity of the blood pressure reduction with treatment for hypertension. J Hypertens 1998; 16: 1685–1691
- Ambrosioni E., Leonetti G., Pessina A. C., Rappelli A., Trimarco B., Zanchetti A. Patterns of hypertension management in Italy: results of a pharmacoepidemiological survey on antihypertensive therapy. Scientific Committee of the Italian Pharmacoepidemiological Survey on Antihypertensive Therapy. J Hypertens 2000; 18: 1691–1699. OS
- Law M. R., Wald N. J., Morris J. K., Jordan R. E. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. Br Med J 2003; 326: 1427. MA
- Materson B. J., Reda D. J., Cushman W. C. Department of Veterans Affairs single‐drug therapy of hypertension study. Revised figures and new data. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. Am J Hypertens 1995; 8: 189–192. RT
- Morgan T. O., Anderson A. I., MacInnis R. J. ACE inhibitors, beta‐blockers, calcium blockers, and diuretics for the control of systolic hypertension. Am J Hypertens 2001; 14: 241–247. RV
- Dickerson J. E., Hingorani A. D., Ashby M. J., Palmer C. R., Brown M. J. Optimisation of antihypertensive treatment by crossover rotation of four major classes. Lancet 1999; 353: 2008–2013. OS
- Hoes A. W., Grobbee D. E., Lubsen J., Man in 't Veld A. J., van der Does E., Hofman A. Diuretics, beta‐blockers, and the risk for sudden cardiac death in hypertensive patients. Ann Intern Med 1995; 123: 481–487. OS
- Helderman J. H., Elahi D., Andersen D. K., Raizes G. S., Tobin J. D., Shocken D., Andres R. Prevention of the glucose intolerance of thiazide diuretics by maintenance of body potassium. Diabetes 1983; 32: 106–111. OS
- Conn J. W. Hypertension, the potassium ion and impaired carbohydrate tolerance. N Engl J Med 1965; 273: 1135–1143. RV
- Ferrari P., Marti H. P., Pfister M., Frey F. J. Additive antiproteinuric effect of combined ACE, inhibition and angiotensin II receptor blockade. J Hypertens 2002; 20: 125–130. RT
- McMurray J. J., Ostergren J., Swedberg K., Granger C. B., Held P., Michelson E. L., Olofsson B., Yusuf S., Pfeffer M. A., CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left‐ventricular systolic function taking angiotensin‐converting‐enzyme inhibitors: the CHARM‐Added trial. Lancet 2003; 362: 767–771. RT
- Giannattasio C., Achilli F., Failla M., Capra A., Vincenzi A., Valagussa F., Mancia G. Radial, carotid, aortic distensibility in congestive heart failure: effects of high‐dose angiotensin‐converting enzyme inhibitor or low‐dose association with angiotensin type 1 receptor blockade. JAm Coll Cardiol 2002; 39: 1275–1282. OS
- Bangalore S., Kamalakkannan G., Panjrath G., Messerli F. H. Fixed‐dose combination improves medication compliance: a meta‐analysis. J Clin Hypertens 2006; 8((Suppl A))A72 (abstract). MA
- Jamerson K. A., Bakris G. L., Wun C. C., Dahlof B., Lefkowitz M., Manfreda S., Pitt B., Velazquez E. J., Weber M. A. Rationale and design of the avoiding cardiovascular events through combination therapy in patients living with systolic hypertension (ACCOMPLISH) trial: the first randomized controlled trial to compare the clinical outcome effects of first‐line combination therapies in hypertension. Am J Hypertens 2004; 17: 793–801. RT
- Gueyffier F., Bulpitt C., Boissel J. P., Schron E., Ekbom T., Fagard R., Casiglia E., Kerlikowske K., Coope J. Antihypertensive drugs in very old people: a subgroup analysis of randomised controlled trials. Lancet 1999; 353: 793–796. MA
- Bulpitt C. J., Beckett N. S., Cooke J., Dumitrascu D. L., Gil‐Extremera B., Nachev C., Nunes M., Peters R., Staessen J. A., Thijs L., Hypertension in the Very Elderly Trial Working Group. Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens 2003; 21: 2409–2417. RT
- Messerli F. H., Grossman E., Goldbourt U. Are beta‐blockers efficacious as first‐line therapy for hypertension in the elderly?. JAMA 1998; 279: 1903–1907. MA
- Kjeldsen S. E., Dahlof B., Devereux R. B., Julius S., Aurup P., Edelman J., Beevers G., de Faire U., Fyhrquist F., Ibsen H., Kristianson K., Lederballe‐Pedersen O., Lindholm L. H., Nieminen M. S., Omvik P., Oparil S., Snapinn S., Wedel H., LIFE (Losartan Intervention for Endpoint Reduction) Study Group. Effects of losartan on cardiovascular morbidity and mortality in patients with isolated systolic hypertension and left ventricular hypertrophy: a Losartan Intervention for Endpoint Reduction (LIFE) substudy. JAMA 2002; 288: 1491–1498. CT
- Papademetriou V., Farsang C., Elmfeldt D., Hofman A., Lithell H., Olofsson B., Skoog I., Trenkwalder P., Zanchetti A., Study on Cognition, Prognosis in the Elderly study group. Stroke prevention with the angiotensin II type 1‐receptor blocker candesartan in elderly patients with isolated systolic hypertension: the Study on Cognition and Prognosis in the Elderly (SCOPE). J Am Coll Cardiol 2004; 44: 1175–1180. CT
- Lakatta E. G. Deficient neuroendocrine regulation of the cardiovascular system with advancing age in healthy humans. Circulation 1993; 87: 631–636. RV
- Fagard R. H., Van den Enden M., Leeman M., Warling X. Survey on treatment of hypertension and implementation of WHO‐ISH risk stratification in primary care in Belgium. J Hypertens 2002; 20: 1297–1302. OS
- Somes G. W., Pahor M., Shorr R. I., Cushman W. C., Applegate W. B. The role of diastolic blood pressure when treating isolated systolic hypertension. Arch Intern Med 1999; 159: 2004–2009. OS
- Fagard R. H., Staessen J. A., Thijs L., Celis H., Bulpitt C. J., de Leeuw P. W., et al. On‐treatment diastolic blood pressure and prognosis in systolic Hypertension. Arch Intern Med 2007, in press. OS
- Mogensen C. E. Long‐term antihypertensive treatment inhibiting progression of diabetic nephropathy. Br Med J 1982; 285: 685–688. OS
- Mancia G. The association of hypertension and diabetes: prevalence, cardiovascular risk and protection by blood pressure reduction. Acta Diabetol 2005; 42((Suppl 1))S17–S25. RV
- Colhoun H. M., Betteridge D. J., Durrington P. N., Hitman G. A., Neil H. A., Livingstone S. J., Thomason M. J., Mackness M. I., Charlton‐Menys V., Fuller J. H., CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo‐controlled trial. Lancet 2004; 364: 685–696. RT
- Bosch J., Yusuf S., Pogue J., Sleight P., Lonn E., Rangoonwala B., Davies R., Ostergren J., Probstfield J., HOPE Investigators. Heart outcomes prevention evaluation. Use of ramipril in preventing stroke: double blind randomised trial. Br Med J 2002; 324: 699–701. RT
- Trenkwalder P., Elmfeldt D., Hofman A., Lithell H., Olofsson B., Papademetriou V., Skoog I., Zanchetti A., The Study on Cognition. Prognosis in the Elderly (SCOPE). The Study on Cognition and Prognosis in the Elderly (SCOPE) ‐ major cardiovascular events and stroke in subgroups of patients. Blood Press 2005; 14: 31–37. CT
- Bathl P., Chalmersl J., Powersl W., Beilinl L., Davisl S., Lenfantl C., Mancial G., Neall B., Whitworthl J., Zanchettil A., International Society of Hypertension Writing Group. International Society of Hypertension (ISH): statement on the management of blood pressure in acute stroke. J Hypertens 2003; 21: 665–672. GL
- Schrader J., Luders S., Kulschewski A., Berger J., Zidek W., Treib J., Einhaupl K., Diener H. C., Dominiak P., Acute Candesartan Cilexetil Therapy in Stroke Survivors Study Group. The ACCESS Study: evaluation of Acute Candesartan Cilexetil Therapy in Stroke Survivors. Stroke 2003; 34: 1699–1703. RT
- COSSACS Trial Group. COSSACS (Continue or Stop post‐Stroke Antihypertensives Collaborative Study): rationale and design. J Hypertens 2005; 23: 455–458. RT
- Potter J., Robinson T., Ford G., James M., Jenkins D., Mistri A., Bulpitt C., Drummond A., Jagger C., Knight J., Markus H., Beevers G., Dewey M., Lees K., Moore A., Paul S., The CHHIPS Trial Group. CHHIPS (Controlling Hypertension and Hypotension Immediately Post‐Stroke) Pilot Trial: rationale and design. J Hypertens 2005; 23: 649–655. RT
- van Dijk E. J., Breteler M. M., Schmidt R., Berger K., Nilsson L. G., Oudkerk M., Pajak A., Sans S., de Ridder M., Dufouil C., Fuhrer R., Giampaoli S., Launer L. J., Hofman A., CASCADE Consortium. The association between blood pressure, hypertension, and cerebral white matter lesions: cardiovascular determinants of dementia study. Hypertension 2004; 44: 625–630. OS
- Vermeer S. E., Hollander M., van Dijk E. J., Hofman A., Koudstaal P. J., Breteler M. M., Rotterdam Scan Study. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke 2003; 34: 1126–1129. OS
- Vermeer S. E., Prins N. D., den Heijer T., Hofman A., Koudstaal P. J., Breteler M. M. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003; 348: 1215–1222. OS
- Sierra C., de La Sierra A., Mercader J., Gomez‐Angelats E., Urbano‐Marquez A., Coca A. Silent cerebral white matter lesions in middle‐aged essential hypertensive patients. J Hypertens 2002; 20: 519–524. OS
- Qiu C., Winblad B., Fratiglioni L. The age‐dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 2005; 4: 487–499. RV
- Kannel W. B. Risk stratification in hypertension: new insights from the Framingham Study. Am J Hypertens 2000; 13((Suppl 1))S3–S10. RV
- Yap Y. G., Duong T., Bland J. M., Malik M., Torp‐Pederson C., Kober L., Connolly S. J., Gallagher M. M., Camm A. J. Prognostic value of blood pressure measured during hospitalization after acute myocardial infarction: an insight from survival trials. J Hypertens 2007; 25: 307–313. OS
- Domanski M. J., Mitchell G. F., Norman J. E., Exner D. V., Pitt B., Pfeffer M. A. Independent prognostic information provided by sphygmomanometrically determined pulse pressure and mean arterial pressure in patients with left ventricular dysfunction. J Am Coll Cardiol 1999; 33: 951–958. OS
- Lee V. C., Rhew D. C., Dylan M., Badamgarav E., Braunstein G. D., Weingarten S. R. Meta‐analysis: angiotensin‐receptor blockers in chronic heart failure and high‐risk acute myocardial infarction. Ann Intern Med 2004; 141: 693–704. MA
- Yusuf S., Pfeffer M. A., Swedberg K., Granger C. B., Held P., McMurray J. J., Michelson E. L., Olofsson B., Ostergren J., CHARM Investigators Committees. Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐Preserved Trial. Lancet 2003; 362: 777–781
- Kannel W. B., Wolf P. A., Benjamin E. J., Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population‐based estimates. Am J Cardiol 1998; 82: 2N–9N. OS
- Hankey G. J. Preventable stroke and stroke prevention. J Thromb Haemost 2005; 3: 1638–1645. RV
- Lip G. Y., Frison L., Grind M. Effect of hypertension on anticoagulated patients with atrial fibrillation. Eur Heart J 2007; 28: 752–759. OS
- Healey J. S., Baranchuk A., Crystal E., Morillo C. A., Garfinkle M., Yusuf S., Connolly S. J. Prevention of atrial fibrillation with angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers: a meta‐analysis. J Am Coll Cardiol 2005; 45: 1832–1839. MA
- Perera G. A. Hypertensive vascular disease: description and natural history. J Chronic Dis 1955; 1: 33–42
- Redon J., Rovira E., Miralles A., Julve R., Pascual J. M. Factors related to the occurrence of microalbuminuria during antihypertensive treatment in essential hypertension. Hypertension 2002; 39: 794–798. OS
- Lawes C. M. M., Vander Hoorn S., Law M. R., Elliott P., MacMahon S., Rodgers A. Blood pressure and the global burden of disease 2000. Part I: Estimates of blood pressure levels. J Hypertens 2006; 24: 413–422. OS
- Franklin S. S., Gustin W 4th., Wong N. D., Larson M. G., Weber M. A., Kannel W. B., Levy D. Hemodynamic patterns of age‐related changes in blood pressure. The Framingham Heart Study. Circulation 1997; 96: 308–315. OS
- Wilson P. W., Castelli W. P., Kannel W. B. Coronary risk prediction in adults (the Framingham Heart Study). Am J Cardiol 1987; 59: 91G–94G. RV
- Zanchetti A., Julius S., Kjeldsen S., McInnes G. T., Hua T., Weber M., Laragh J. H., Plat F., Battegay E., Calvo‐Vargas C., Cieslinski A., Degaute J. P., Holwerda N. J., Kobalava J., Pedersen O. L., Rudyatmoko F. P., Siamopoulos K. C., Storset O. Outcomes in subgroups of hypertensive patients treated with regimens based on valsartan and amlodipine: An analysis of findings from the VALUE trial. J Hypertens 2006; 24: 2163–2168. CT
- Dong W., Colhoun H. M., Poulter N. R. Blood pressure in women using oral contraceptives results from the Health Survey for England 1994. J Hypertens 1997; 15: 1063–1068. OS
- Chasan‐Taber L., Willett W. C., Manson J. A. E., Spiegelman D., Hunter D. J., Curhan G., Colditz G. A., Stampfer M. J. Prospective study of oral contraceptives and hypertension among women in the United States. Circulation 1996; 94: 483–489. OS
- Lip G. Y., Beevers M., Beevers D. G. Malignant hypertension in young women is related to previous hypertension in pregnancy, not oral contraception. Quart J Med 1997; 90: 571–575. OS
- Woods J. W. Oral contraceptives and hypertension. Hypertension 1998; 11: II11–II15. RV
- Kawano H., Motoyama T., Kugiyama K., Hirashima O., Ohgushi M., Fujii H., Ogawa H., Yasue H. Gender difference in improvement of endothelium‐dependent vasodilation after estrogen supplementation. J Am Coll Cardiol 1997; 30: 914–919. OS
- Skinner S. L., Lumbers E. R., Symonds E. M. Alteration by oral contraceptives of normal menstrual changes in plasma renin activity, concentration and substrate. Clin Sci 1969; 36: 67–76. OS
- Giannattasio C., Failla M., Grappiolo A., Stella M. L., Del Bo A., Colombo M., Mancia G. Fluctuations of radial artery distensibility throughout the menstrual cycle. Arterioscler Thromb Vasc Biol 1999; 19: 1925–1929
- Ribstein J., Halimi J‐M., Guilhem du Cailar, Mimran A. Renal characteristics and effect of angiotensin suppression in oral contraceptive users. Hypertension 1999; 33: 90–95
- Inman W. H. W., Vessey M. P. Investigation of deaths from pulmonary, coronary, and cerebral thrombosis and embolism in women of childbearing age. BMJ 1968; 2: 193–199. OS
- Vessey M. P., Doll R. Investigation of the relation between use of oral contraceptives and thromboembolic disease. BMJ 1968; 2: 199–205. OS
- Masi A. T., Dudgate M. Cerebrovascular disease associated with the use of oral contraceptives: a review of the English‐language literature. Ann Intern Med 1970; 72: 111–121. RV
- Han W‐S., Ray J., Wai E. K., Ginsburg S., Hannah M. E., Corey P. N., Ginsberg J. S. Risk of stroke in women exposed to low‐dose oral contraceptives. A critical evaluation of the evidence. Arch Intern Med 2004; 164: 741–747. MA
- Curtis K. M., Mohllajee A. P., Martins S. L., Peterson H. B. Combined oral contraceptive use among women with hypertension: a systematic review. Contraception 2006; 73: 179–188. MA
- Gomes M. P., Deitcher S. R. Risk of venous thromboembolic disease associated with hormonal contraceptives and hormone replacement therapy. Arch Intern Med 2004; 164: 1965–1976. OS
- Hussain S. F. Progestogen‐only pills and high blood pressure: is there an association? A literature review. Contraception 2004; 69: 89–97. RV
- Zanchetti A., Facchetti R., Cesana G. C., Modena G. M., Pirrelli A., Sega R. Menopause‐related blood pressure increase and its relationship to age and body mass index: the SIMONA epidemiological study. J Hypertens 2005; 23: 2269–2276. OS
- Shelley J. M., Green A., Smith A. M., Dudley E., Dennerstein L., Hopper J., Burger H. Relationship of sex hormones to lipids and blood pressure in mid‐aged women. Ann Epidemiol 1998; 8: 39–45. OS
- Grobbee D. E., Van Hemert A. M., Vanderbroucke J. P., Hofman A., Valkenburg H. A. Importance of body weight in determining risk and level of blood pressure in postmenopausal women. J Hypertens 1988; 6((Suppl))S614–S616. OS
- Staessen J. A., Ginocchio G., Thijs L., Fagard R. Conventional and ambulatory blood pressure and menopause in a prospective population study. J Hum Hypertens 1997; 11: 507–514. OS
- Casiglia E., d'Este D., Ginocchio G., Colangeli G., Onesto C., Tramontin P., Ambrosio G. B., Pessina A. C. Lack of influence of menopause on blood pressure and cardiovascular risk profile: a 16 year longitudinal study concerning a cohort of 568 women. J Hypertens 1996; 14: 729–736. OS
- Lindqvist O., Bengtsson C. Serum lipids arterial blood pressure body weight in relation to the menopause: results from a population study of women in Goteborg Sweden. Scand J Clin Invest 1980; 40: 629–636. OS
- Torng P. L., Su T. C., Sung F. G., Chien K. L., Huang S. C., Chon S. N., Lee Y. T. Effects of menopause on intraindividual changes in serum lipids, blood pressure and body weight: the Chin‐Shan community cardiovascular cohort study. Atherosclerosis 2002; 161: 409–415. OS
- The Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA 1995; 273: 199–208. RT
- Grodstein F., Manson J. E., Sampfer M. J. Postmenopausal hormone use and secondary prevention of coronary events in the nurses health study, a prospective, observational study. Ann Intern Med 2001; 135: 1–8. OS
- Falkenborn M., Persson I., Terent A., Adami H. O., Lithell H., Bergstrom R. Hormone replacement therapy and the risk of stroke, follow‐up of a population‐based cohort in Sweden. Arch Intern Med 1993; 153: 1201–1209. OS
- Finucane F. F., Madans J. H., Bush T. L., Wolf P. H., Kleinman J. C. Decreased risk of stroke among postmenopausal hormone users, results from a national cohort. Arch Intern Med 1993; 153: 73–79. OS
- Scuteri A., Bos A. J. G., Brant L. J., Talbot L., Lakatta E. G., Fleg J. L. Hormone replacement therapy and longitudinal changes in blood pressure in postmenopausal women. Ann Intern Med 2001; 135: 229–238. OS
- Hulley S., Grady D., Bush T., Furberg C., Herrington D., Riggs B., Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) research group. JAMA 1998; 280: 605–613. RT
- Rossouw J. E., Anderson G. L., Prentice R. L., LaCroix A. Z., Kopperberg C., Stefanick M. L., Jackson R. D., Beresford S. A., Howard B. V., Johnson K. C., Kotchen J. M., Ockene J., Writing Group of Womefts Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Womeris Health Initiative randomized controlled trial. JAMA 2002; 288: 321–333. RT
- Farquhar C. M., Marjoribanks J., Lethaby A., Lamberts Q., Suckling J. A., the Cochrane HT Study Group. Long term hormone therapy for perimeno‐pausal and postmenopausal women. Cochrane database of Systematic Reviews 2005, Issue 3. Art No CD004143. DOI 10.1002/1465868. CD004143.pub2. MA
- Stramba‐Badiale M., Fox K. M., Priori S. G., Collins P., Daly C., Graham I., Jonsson B., Schenck‐Gustafsson K., Tendera M. Cardiovascular disease in women: a statement from the policy conference of the European Society of Cardiology. Eur Heart J 2006; 27: 994–1005. GL
- Consensus Report: National High Blood Pressure Education Program Working Group Report on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 1990; 163: 1689–1712. GL
- Levine R. J., Ewell M. G., Hauth J. C., Curet L. B., Catalano P. M., Morris C. D., Choudhary G., Sibai B. M. Should the definition of preeclampsia include a rise in diastolic blood pressure of ⩾15 mm Hg to a level >90 mm Hg in association with proteinuria?. Am J Obstet Gynecol 2000; 183: 787–792. GL
- Staessen J. A., Asmar R., De Buyzere M., Imai Y., Parati G., Shimada K., Stergiou G., Redon J., Verdecchia P., Participants of the 2001 Consensus Conference on Ambulatory Blood Pressure Monitoring. Task Force II: blood pressure measurement and cardiovascular outcome. Blood Press Monit 2001; 6: 355–370. GL
- Churchill D., Perry I. J., Beevers D. G. Ambulatory blood pressure in pregnancy and fetal growth. Lancet 1997; 349: 7–10. OS
- Penny J. A., Halligan A. W., Shennan A. H., Lambert P. C., Jones D. R., de Swiet M., Taylor D. J. Automated, ambulatory, or conventional blood pressure measurement in pregnancy: which is the better predictor of severe hypertension?. Am J Obstet Gynecol 1998; 178: 521–526. OS
- Perry I. J., Stewart B. A., Brockwell J., Khan M., Davies P., Beevers D. G., Luesley D. M. Recording diastolic blood pressure in pregnancy. Br Med J 1990; 301: 1198
- Shennan A., Gupta M., Halligan A., Taylor D. J., de Swiet M. Lack of reproducibility in pregnancy of Korotkoff phase IV as measured by mercury sphygmomanometry. Lancet 1996; 347: 139–142. OS
- Higgins J. R., de Swiet M. Blood pressure measurement and classification in pregnancy. Lancet 2001; 357: 131–135
- Task Force Members, Oakley C., Child A., Lung B., Persbitero P., Tornos, Klein W., Garcia M. A. A., Blomstrom‐Lundqvist C., de Backer G., Dargie H., Deckers J., Flather M., Hradec J., Mazzotta G., Oto A., Parkhomenko A., Silber S., Torbicki A., Trappe H‐J., Dean V., Pourmeyrol‐Jumeau D. Expert consensus document on management of cardiovascular diseases during pregnancy. Eur Heart J 2003; 24: 761–781. GL
- Moutquin J‐M., Garner P. R., Burrows R. F., Rey E., Helewa M. E., Lange I. R., Rabkin S. W. Report of the Canadian Hypertension Society Consensus Conference: 2. Nonpharmacologic management and prevention of hypertensive disorders in pregnancy. Can Med Assoc J 1997; 157: 907–919. GL
- Atallah A. N., Hofmeyr G. J., Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems (Cochrane Review). In: The Cochrane Library, Issue 1. Update Software, Oxford 2000, MA
- Olsen S., Secher N. J., Tabor A., Weber T., Walker J. J., Gluud C. Randomised clinical trials offish oil supplementation in high risk pregnancies. Br J Obstet Gynaecol 2000; 107: 382–395. RT
- Knight M., Duley L., Henderson‐Smart D. J., King J. F. Antiplatelet agents and pre‐eclampsia (Cochrane Review). The Cochrane Library. 2000, Issue 1. Oxford, Update Software, MA
- Gilbert J. S., Cox L. A., Mitchell G., Nijland M. J. Nutrient‐restricted fetus and the cardio‐renal connection in hypertensive offspring. Expert Rev Cardiovasc Ther 2006; 4: 227–237. RV
- Sibai B. M., Mabie W. C., Shamsa F., Vilnar M. A., Anderson G. D. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol 1990; 162: 960–967. RT
- Gruppo di Studio Ipertensione in Gravidanza. Nifedipine versus expectant management in mild to moderate hypertension in pregnancy. Br J Obstet Gynaecol 1998; 105: 718–722. RT
- De Swiet M. Maternal blood pressure and birthweight. Lancet 2000; 355: 81–82. RV
- von Dadelszen P., Ornstein M. P., Bull S. B., Logan A. G., Koren G., Magee L. A. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta‐analysis. Lancet 2000; 355: 87–92. MA
- Magee L. A., Ornstein M. P., von Dadelszen P. Management of hypertension in pregnancy. Br Med J 1999; 318: 1332–1336. GL
- Coppage K. H., Sibai B. M. Treatment of hypertensive complications in pregnancy. Current Pharm Design 2005; 11: 749–757. RV
- Lydakis C., Lip G. Y., Beevers M., Beevers D. G. Atenolol and fetal growth in pregnancies complicated by hypertension. Am J Hypertension 1999; 12: 541–547. OS
- The Magpie Trial Collaborative Group. Do women with pre‐eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo‐controlled trial. Lancet 2002; 359: 1877–1890. RT
- Paradisi G., Biaggi A., Savone R., Ianniello F., Tomei C., Caforio L., Caruso A. Cardiovascular risk factors in healthy women with previous gestational hypertension. J Clin Endocrinol Metab 2006; 91: 1233–1238. OS
- Wilson B. J., Watson M. S., Prescott G. J., Sunderland S., Campbell D. M., Hannaford P., Smith W. C. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. Br MedJ 2003; 326: 845–851. OS
- Lakka H. M., Laaksonen D. E., Lakka T. A., Niskanen L. K., Kumpusalo E., Tuomilehto J., Salonen J. T. The metabolic syndrome and total and cardiovascular disease mortality in middle‐aged men. JAMA 2002; 288: 2709–2716. OS
- Girman C. J., Rhodes T., Mercuri M., Pyorala K., Kjekshus J., Pedersen T. R., Beere P. A., Gotto A. M., Clearfield M., 4S Group the AFCAPS/TexCAPS Research Group. The metabolic syndrome and risk of major coronary events in the Scandinavian Simvastatin Survival Study (4S) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). Am J Cardiol 2004; 93: 136–141. OS
- Dekker J. M., Girman C., Rhodes T., Nijpels G., Stehouwer C. D., Bouter L. M., Heine R. J. Metabolic syndrome and 10‐year cardiovascular disease risk in the Hoorn Study. Circulation 2005; 112: 666–673. OS
- Resnick H. E., Jones K., Ruotolo G., Jain A. K., Henderson J., Lu W., Howard B. V. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease in nondiabetic American Indians: the Strong Heart Study. Diabetes Care 2003; 26: 861–867. OS
- Schmidt M. I., Duncan B. B., Bang H., Pankow J. S., Ballantyne C. M., Golden S. H., Folsom A. R., Chambless L. E. Identifying individuals at high risk for diabetes: The Atherosclerosis Risk in Communities study. Diabetes Care 2005; 28: 2013–2018. OS
- Mule G., Nardi E., Cottone S., Cusimano P., Volpe V., Piazza G., Mongiovi R., Mezzatesta G., Andronico G., Cerasola G. Influence of metabolic syndrome on hypertension‐related target organ damage. J Intern Med 2005; 257: 503–513. OS
- Leoncini G., Ratto E., Viazzi F., Vaccaro V., Parodi D., Parodi A., Falqui V., Tomolillo C., Deferrari G., Pontremoli R. Metabolic syndrome is associated with early signs of organ damage in nondiabetic, hypertensive patients. J Intern Med 2005; 257: 454–460. OS
- Cuspidi C., Meani S., Fusi V., Severgnini B., Valerio C., Catini E., Leonetti G., Magrini F., Zanchetti A. Metabolic syndrome and target organ damage in untreated essential hypertensives. JHypertens 2004; 22: 1991–1998. OS
- Schillaci G., Pirro M., Vaudo G., Mannarino M. R., Savarese G., Pucci G., Franklin S. S., Mannarino E. Metabolic syndrome is associated with aortic stiffness in untreated essential hypertension. Hypertension 2005; 45: 1978–1982. OS
- de Simone G., Palmieri V., Bella J. N., Celentano A., Hong Y., Oberman A., Kitzman D. W., Hopkins P. N., Arnett D. K., Devereux R. B. Association of left ventricular hypertrophy with metabolic risk factors: the HyperGEN study. J Hypertens 2002; 20: 323–331. OS
- Schillaci G., Pirro M., Pucci G., Mannarino M. R., Gemelli F., Siepi D., Vaudo G., Mannarino E. Different impact of the metabolic syndrome on left ventricular structure and function in hypertensive men and women. Hypertension 2006; 47: 881–886. OS
- Cuspidi C., Meani S., Fusi V., Valerio C., Catini E., Sala C., Sampieri L., Magrini F., Zanchetti A. Prevalence and correlates of left atrial enlargement in essential hypertension: role of ventricular geometry and the metabolic syndrome: the Evaluation of Target Organ Damage in Hypertension study. J Hypertens 2005; 23: 875–882. OS
- Kawamoto R., Tomita H., Oka Y., Kodama A., Kamitani A. Metabolic syndrome amplifies the LDL‐cholesterol associated increases in carotid atherosclerosis. Intern Med 2005; 44: 1232–1238. OS
- Cuspidi C., Meani S., Valerio C., Fusi V., Catini E., Sala C., Zanchetti A. Ambulatory blood pressure, target organ damage and left atrial size in never‐treated essential hypertensive individuals. J Hypertens 2005; 23: 1589–1595. OS
- Pepys M. B., Hirschfield G. M. C‐reactive protein: a critical update. J Clin Invest 2003; 111: 1805–1812. RV
- Nesto R. C‐reactive protein its role in inflammation Type 2 diabetes cardiovascular disease the effects of insulin‐sensitizing treatment with thiazolidinediones. Diabet Med 2004; 21: 810–817. RV
- Mancia G., Bousquet P., Elghozi J. L., Esler M., Grassi G., Julius S., Reid J., Van Zwieten P. A. The sympathetic nervous system and the metabolic syndrome. J Hypertens 2007, in press. RV
- Clinical guidelines on the identification evaluation treatment of overweight obesity in adults‐the evidence report. National Institutes of Health. ObesRes 1998; 2((Suppl 6))51S–209S. GL
- Krauss R. M., Eckel R. H., Howard B., Appel L. J., Daniels S. R., Deckelbaum R. J., Erdman J. W., Jr., Kris‐Etherton P., Goldberg I. J., Kotchen T. A., Lichtenstein A. H., Mitch W. E., Mullis R., Robinson K., Wylie‐Rosett J., St Jeor S., Suttie J., Tribble D. L., Bazzarre. TL AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 2000; 102: 2284–2299. GL
- Thompson P. D., Buchner D., Pina I. L., Balady G. J., Williams M. A., Marcus B. H., Berra K., Blair S. N., Costa F., Franklin B., Fletcher G. F., Gordon N. F., Pate R. R., Rodriguez B. L., Yancey A. K., Wenger N. K., American Heart Association Council on Clinical Cardiology Subcommittee on Exercise Rehabilitation Prevention American Heart Association Council on Nutrition Physical Activity Metabolism Subcommittee on Physical Activity. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the American Heart Association Council on Clinical Cardiology (Subcommittee on Nutrition, Physical Activity and Metabolism (Subcommittee on Physical Activity). Circulation 2003; 107: 3109–3116. GL
- Knowler W. C., Barrett‐Connor E., Fowler S. E., Hamman R. F., Lachin J. M., Walker E. A., Nathan D. M., Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403. RT
- Tuomilehto J., Lindstrom J., Eriksson J. G., Valle T. T., Hamalainen H., Ilanne‐Parikka P., Keinanen‐Kiukaanniemi S., Laakso M., Louheranta A., Rastas M., Salminen V., Uusitupa M., Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343–1350. RT
- Orchard T. J., Temprosa M., Goldberg R., Haffner S., Ratner R., Marcovina S., Fowler S., Diabetes Prevention Program Research Group. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 2005; 142: 611–619. RT
- Pischon T., Sharma A. M. Use of beta‐blockers in obesity hypertension: potential role of weight gain. Obes Rev 2001; 2: 275–280. RV
- Jacob S., Rett K., Henriksen E. J. Antihypertensive therapy and insulin sensitivity: do we have to redefine the role of beta‐blocking agents?. Am J Hypertens 1998; 11: 1258–1265. RV
- Poole‐Wilson P. A., Swedberg K., Cleland J. G., Di Lenarda A., Hanrath P., Komajda M., Lubsen J., Lutiger B., Metra M., Remme W. J., Torp‐Pedersen C., Scherhag A., Skene A., Carvedilol Or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 2003; 362: 7–13. RT
- Abuissa H., Jones P. G., Marso S. P., O'Keefe J. H., Jr. Angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta‐analysis of randomized clinical trials. J Am Coll Cardiol 2005; 46: 821–826. MA
- Rocchini A. P. Obesity hypertension salt sensitivity insulin resistance. Nutr Metab Cardiovasc Dis 2000; 10: 287–294. RV
- Bakris G., Molitch M., Hewkin A., Kipnes M., Sarafidis P., Fakouhi K., Bacher P., Sowers J., STAR Investigators. Differences in glucose tolerance between fixed‐dose antihypertensive drug combinations in people with metabolic syndrome. Diabetes Care 2006; 29: 2592–2597
- Zillich A. J., Garg J., Basu S., Bakris G. L., Carter B. L. Thiazide diuretics potassium the development of diabetes: a quantitative review. Hypertension 2006; 48: 219–224. MA
- Van de Laar F. A., Lucassen P. L., Akkermans R. P., Van de Lisdonk E. H., De Grauw W. J. Alpha‐glucosidase inhibitors for people with impaired glucose tolerance or impaired fasting blood glucose. Cochrane Database SystRev 2006; 4: CD005061. RV
- Kurtz T. W., Pravenec M. Antidiabetic mechanisms of angiotensin‐converting enzyme inhibitors and angiotensin II receptor antagonists: beyond the renin‐angiotensin system. J Hypertens 2004; 22: 2253–2261. RV
- Schupp M., Janke J., Clasen R., Unger T., Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator‐activated receptor‐gamma activity. Circulation 2004; 109: 2054–2057
- DREAM (Diabetes REduction Assessment with ramipril rosiglitazone Medication) Trial Investigators; Gerstein HC, Yusuf S., Bosch J., Pogue J., Sheridan P., Dinccag N., Hanefeld M., Hoogwerf B., Laakso M., Mohan V., Shaw J., Zinman B., Holman R. R. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006; 368: 1096–1105. RT
- Dormandy J. A., Charbonnel B., Eckland D. J., Erdmann E., Massi‐Benedetti M., Moules I. K., Skene A. M., Tan M. H., Lefebvre P. J., Murray G. D., Standl E., Wilcox R. G., Wilhelmsen L., Betteridge J., Birkeland K., Golay A., Heine R. J., Koranyi L., Laakso M., Mokan M., Norkus A., Pirags V., Podar T., Scheen A., Scherbaum W., Schernthaner G., Schmitz O., Skrha J., Smith U., Taton J., PROactive investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglit Azone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005; 366: 1279–1289. RT
- Sarafidis P. A., Nilsson P. M. The effects of thiazolidinediones on blood pressure levels ‐ a systematic review. Blood Press 2006; 15: 135–150. RV
- Van Gaal L. F., Rissanen A. M., Scheen A. J., Ziegler O., Rossner S., RIO‐Europe Study Group. Effects of the cannabinoid‐1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1‐year experience from the RIO‐Europe study. Lancet 2005; 365: 1389–1397. RT
- Despres J. P., Golay A., Sjostrom L., Rimonabant in Obesity‐Lipids Study Group. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 2005; 353: 2121–2134. RT
- Pi‐Sunyer F. X., Aronne L. J., Heshmati H. M., Devin J., Rosenstock J., RIO‐North America Study Group. Effect of rimonabant, a cannabinoid‐1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO‐North America: a randomized controlled trial. JAMA 2006; 295: 761–775. RT
- Scheen A. J., Finer N., Hollander P., Jensen M. D., Van Gaal L. F., RIO‐Diabetes Study Group. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes. Lancet 2006; 368: 1160–1172. RT
- ClinicalTrials.gov.CRESCENDO: comprehensive rimonabant evaluation study of cardiovascular endopoints outcomes. December 2005: http//clinicaltrials.gov/. RT
- Deedwania P., Barter P., Carmena R., Fruchart J. C., Grundy S. M., Haffner S., Kastelein J. J., LaRosa J. C., Schachner H., Shepherd J., Waters D. D. Reduction of low‐density lipoprotein cholesterol in patients with coronary heart disease and metabolic syndrome: analysis of the Treating to New Targets study. Lancet 2006; 368: 919–928. RT
- Wing R. R., Phelan S. Long‐term weight loss maintenance. Am J Clin Nutr 2005; 82((1 Suppl))222S–225S. RV
- Cuspidi C., Macca G., Sampieri L., Michev I., Salerno M., Fusi V., Severgnini B., Meani S., Magrini F., Zanchetti A. High prevalence of cardiac and extracardiac target organ damage in refractory hypertension. J Hypertens 2001; 19: 2063–2070. OS
- Logan A. G., Perlikowski S. M., Mente A., Tisler A., Tkacova R., Niroumand M., Leung R. S., Bradley T. D. High prevalence of unrecognized sleep apnoea in drug‐resistant hypertension. J Hypertens 2001; 19: 2271–2277. OS
- Parati G., Ongaro G., Bonsignore M. R., Glavina F., Di Rienzo M., Mancia G. Sleep apnoea and hypertension. Curr Opin Nephrol Hypertens 2002; 11: 201–214. RV
- Narkiewicz K., Wolf J., Lopez‐Jimenez F., Somers V. K. Obstructive sleep apnea and hypertension. Curr Cardiol Rep 2005; 7: 435–440. RV
- Baguet J. P., Narkiewicz K., Mallion J. M. Update on hypertension management: obstructive sleep apnea and hypertension. J Hypertens 2006; 24: 205–208. RV
- Calhoun D. A. Low‐dose aldosterone blockade as a new treatment paradigm for controlling resistant hypertension. J Clin Hypertens 2007; 9((Suppl 1))19–24. RV
- Saha C., Eckert G. J., Ambrosius W. T., Chun T. Y., Wagner M. A., Zhao Q., Pratt J. H. Improvement in blood pressure with inhibition of the epithelial sodium channel in blacks with hypertension. Hypertension 2005; 46: 481–487. RT
- Lane D. A., Shah S., Beevers D. G. Low‐dose spironolactone in management of resistant hypertension: a surveillance study. J Hypertens 2007; 25: 891–894. OS
- Eide I. K., Torjesen P. A., Drolsum A., Babovic A., Lilledahl N. P. Low‐renin status in therapy‐resistant hypertension: a clue to efficient treatment. J Hypertens 2004; 22: 2217–2226. OS
- de Leeuw P. W., Kroon A. A., Scheffers I., Tordoir J., Schmidli, Mohaupt M., Allemann Y., Jordan J., Engeli S., Liebeskind U., Luft F. C., Eckert S., Hansky B., Kieval R., Cody R., Rossing M., Irwin E., Peters T. Baroreflex hypertension therapy with a cronically implanted system: preliminary efficacy and safety results from the rheos debut‐ht study in patients with resistant hypertension. J Hypertens 2006; 24((Suppl 4))S300. (abstract)
- Isles C. G. Malignant hypertension and hypertensive encephalophaty. Textbook of Hypertension, J. D Swales. Blackwell Scientific Publications, London 1994; 1233–1248. RV
- Davis B. A., Crook J. E., Vestal R. E., Oates J. A. Prevalence of renovascular hypertension in patients with grade III or IV hypertensive retinopathy. NEngl J Med 1979; 301: 1273–1276. OS
- Lip G. Y., Beevers M., Beevers G. The failure of malignant hypertension to decline: a survey of 24 years' experience in a multiracial population in England. J Hypertens 1994; 12: 1297–1305. OS
- Giese J. Acute hypertensive vascular disease, 2: Studies on vascular reaction patterns and permeability changes by means of vital microscopy and colloidal tracer technique. Acta Pathol Microbiol Scand 1964; 62: 497–515. OS
- Kincaid‐Smith P., McMichael J., Murphy E. A. The clinical cause and pathology of hypertension with papilloedema. Quart J Med 1958; 27: 117–154. OS
- Isles C. G., Liu K. G., Boulton‐Jones M., Cameron H., Lever A. F., Murray G., Robertson J. W. K. Factors influencing mortality in malignant hypertension. J Hypertens 1985; 3((Suppl 3))405–407. OS
- Lip G. Y., Beevers M., Beevers D. G. Complications and survival of 315 patients with malignant‐phase hypertension. J Hypertens 1995; 13: 915–924. OS
- Gotto A. M., Jr. Review of primary and secondary prevention trials with lovastatin, pravastatin, and simvastatin. Am J Cardiol 2005; 96: 34F–38F. RV
- Clearfield M. Statins and the primary prevention of cardiovascular events. Curr Atheroscler Rep 2006; 8: 390–396. RV
- Thavendiranathan P., Bagai A., Brookhart M. A., Choudhry N. K. Primary prevention of cardiovascular diseases with statin therapy: a meta‐analysis of randomized controlled trials. Arch Intern Med 2006; 166: 2307–2313. MA
- Gorelick P. B., Schneck M., Berglund L. F., Feinberg W., Goldstone J. Status of lipids as a risk factor for stroke. Neuroepidemiology 1997; 16: 107–115. RV
- Heart Protection Study Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet 2002; 360: 7–22. RT
- Shepherd J., Blauw G. J., Murphy M. B., Bollen E. L., Buckley B. M., Cobbe S. M., Ford I., Gaw A., Hyland M., Jukema J. W., Kamper A. M., Macfarlane P. W., Meinders A. E., Norrie J., Packard C. J., Perry I. J., Stott D. J., Sweeney B. J., Twomey C., Westendorp R. G., PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360: 1623–1630. RT
- Amarenco P., Bogousslavsky J., Callahan A., 3rd., Goldstein L. B., Hennerici M., Rudolph A. E., Sillesen H., Simunovic L., Szarek M., Welch K. M., Zivin J. A., Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High‐dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006; 355: 549–559. RT
- The ALLHAT Officers, Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care. The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT‐LLT). JAMA 2002; 288: 2998–3007. RT
- Sever P. S., Dahlof B., Poulter N. R., Wedel H., Beevers G., Caulfield M., Collins R., Kjeldsen S. E., Kristinsson A., McInnes G. T., Mehlsen J., Nieminen M., O'Brien E., Ostergren J., ASCOT investigators. The prevention of coronary events and stroke with atorvastatin in hypertensive subjects with average or below average cholesterol levels. The Anglo‐Scandinavian Cardiac Outcomes Trial: Lipid Lowering Arm (ASCOT:LLA). Lancet 2003; 361: 1149–1158. RT
- Borghi C., Dormi A., Veronesi M., Immordino V., Ambrosioni E. Use of lipid‐lowering drugs and blood pressure control in patients with arterial hypertension. J Clin Hypertens 2002; 4: 277–285. RV
- Kosoglou T., Statkevich P., Johnson‐Levonas A. O., Paolini J. F., Bergman A. J., Alton K. B. Ezetimibe: a review of its metabolism pharmacokinetics drug interactions. Clin Pharmacokinet 2005; 44: 467–494. RV
- Antithrombotic Trialists' Collaboration. Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324: 71–86. MA
- Zanchetti A., Hansson L., Dahlof B., Julius S., Menard J., Warnold I., Wedel H. Benefit and harm of low‐dose aspirin in well‐treated hypertensives at different baseline cardiovascular risk. J Hypertens 2002; 20: 2301–2307. CT
- Diener H. C., Bogousslavsky J., Brass L. M., Cimminiello C., Csiba L., Kaste M., Leys D., Matias‐Guiu J., Rupprecht H. J. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial. Lancet 2004; 364: 331–337. RT
- Ridker P. M., Cook N. R., Lee I. M., Gordon D., Gaziano J. M., Manson J. E., Hennekens C. H., Buring J. E. A randomized trial of low‐dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352: 1293–1304. RT
- Hayden M., Pignone M., Phillips C., Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 136: 161–172. MA
- Wald N. J., Law M. R. A strategy to reduce cardiovascular disease by more than 80%. BMJ 2003; 326: 1419. RV
- Sanmuganathan P. S., Ghahramani P., Jackson P. R., Wallis E. J., Ramsay L. E. Aspirin for primary prevention of coronary heart disease: safety and absolute benefit related to coronary risk derived from meta‐analysis of randomised trials. Heart 2001; 85: 265–271. MA
- Zanchetti A., Hansson L., Leonetti G., Rahn K. H., Ruilope L., Warnold I., Wedel H. Low‐dose aspirin does not interfere with the blood pressure‐lowering effects of antihypertensive therapy. J Hypertens 2002; 20: 1015–1022. RT
- Haffner S. M., Lehto S., Ronnemaa T., Pyorala K., Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in non‐diabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339: 229–234. OS
- Stamler J., Vaccaro O., Neaton J. D., Wentworth D. Diabetes other riskfactors 12‐yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993; 16: 434–444. OS
- Knowler W. C., Sartor G., Melander A., Schersten B. Glucose tolerance and mortality, including a substudy of tolbutamide treatment. Diabetologia 1997; 40: 680–686. OS
- Gress T. W., Nieto F. J., Shahar E., Wofford M. R., Brancati F. L. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. Atherosclerosis Risk in Communities Study. N Engl J Med 2000; 342: 905–912. OS
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. RT
- Chiasson J. L., Josse R. G., Gomis R., Hanefeld M., Karasik A., Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP‐ NIDDM trial. JAMA 2003; 290: 486–494. RT
- Gaede P., Vedel P., Larsen N., Jensen G. V., Parving H. H., Pedersen O. Multi‐factorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383–393. RT
- The Diabetes Control, Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. RT
- Nathan D. M., Cleary P. A., Backlund J. Y., Genuth S. M., Lachin J. M., Orchard T. J., Raskin P., Zinman B., Diabetes Control Complications Trial/Epidemiology of Diabetes Interventions Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643–2653. CT
- Balkau B., Shipley M., Jarrett R. J., Pyorala K., Pyorala M., Forhan A., Eschwege E. High blood glucose concentration is a risk factor for mortality in middle‐aged nondiabetic men. 20‐year follow‐up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care 1998; 21: 360–367. OS
- European Diabetes Policy Group 1999. A desktop guide to type 2 diabetes mellitus. Diabetes Med 1999; 16: 716–730. GL
- ADVANCE trial study group Rationale, design of the ADVANCE study: a randomised trial of blood pressure lowering, intensive glucose control in high‐risk individuals with type 2 diabetes mellitus. Action in Diabetes and Vascular Disease: PreterAx and DiamicroN Modified‐Release Controlled Evaluation. J Hypertens 2001; 19((Suppl))S21–S28. RT
- Campos C., Segura J., Rodicio J. L. Investigations in secondary hypertension: renal disease. Hypertension, A Zanchetti, L Hansson, J. L Rodicio. McGraw Hill International, London 2001; 119–126. RV
- Keane W. F., Eknoyan G. Proteinuria albuminuria risk assessment detection elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis 1999; 33: 1004–1010. GL
- Koler H., Wandel E., Brunck B. Acanthocyturia ‐ a characteristic marker for glomerular bleeding. Kidney Int 1991; 40: 115–120. OS
- Elliott W. J. Secondary hypertension: renovascular hypertension. Hypertension: a Companion to Braunwald's Heart Disease, W. G Blackh & Elliott, 2007; 93–105. RV, Saunders Elsevier
- Safian R. D., Textor S. C. Renal‐artery stenosis. N Engl J Med 2001; 344: 431–442. RV
- Krumme W., Blum U., Schwertfeger E., Fliigel P., Hollstin F., Schollmeyer P., Rump L. C. Diagnosis of renovascular disease by intra‐extrarenal Doppler scanning. Kidney Int 1996; 50: 1288–1292. OS
- Vasbinder B. G. C., Nelemans P. J., Kessels A. G. H., Kroon A. A., De Leeuw P. W., van Engelshoven J. M. A. Diagnostic tests for renal artery stenosis in patients suspected of having renovascular hypertension: a meta‐analysis. Ann Intern Med 2001; 135: 401–411. MA
- Bruce G. H. Intervention for renal artery stenosis: endovascular and surgical roles. J Hypertens 2005; 23((Suppl 3))S23–S29. RV
- Aurell M., Jensen G. Treatment of renovascular hypertension. Nephron 1997; 75: 373–383. RV
- Plouin P. F., Chatellier G., Darne B., Raynaud A. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Essai Multicentrique Medicaments vs Angioplastie (EMMA) Study Group. Hypertension 1998; 31: 823–829. RT
- Webster J., Marshall F., Abdalla M., Dominiczak A., Edwards R., Isles C. G., Loose H., Main J., Padfield P., Russell I. T., Walker B., Watson M., Wilkinson R. Randomised comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. Scottish and Newcastle Renal Artery Stenosis Collaborative Group. J Hum Hypertens 1998; 12: 329–335. OS
- van Jaarsveld B. C., Krijnen P., Pieterman H., Derkx F. H., Deinum J., Postma C. T., Dees A., Woittiez A. J., Bartelink A. K., Man in ’tVeld A. J., Schalekamp M. A. The effect of balloon angioplasty on hypertension in atherosclerotic renal‐artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000; 342: 1007–1014. OS
- Nordmann A. J., Woo K., Parkes R., Logan A. G. Balloon angioplasty or medical therapy for hypertensive patients with atherosclerotic renal artery stenosis? A meta‐analysis of randomized controlled trials. Am J Med 2003; 114: 44–50. MA
- Reisch N., Peczkowska M., Januszewicz A., Neumann H. P. Pheochromocytoma: presentation diagnosis treatment. J Hypertens 2006; 24: 2331–2339. RV
- Sawka A. M., Jaeschke R., Singh R. J., Young W. F., Jr. A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24‐hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 2003; 88: 553–558. OS
- Bravo E. L. Evolving concepts in the pathophysiology diagnosis treatment of pheochromocytoma. Endocrine Rev 1994; 15: 356–368. RV
- Goldstein D. S., Eisenhofer G., Flynn J. A., Wand G., Pacak K. Diagnosis and localization of pheochromocytoma. Hypertension 2004; 43: 907–910. RV
- Sjoberg R. J., Simcic K. J., Kidd G. S. The clonidine suppression test for pheochromocytoma. A review of its utility and pitfalls. Arch Intern Med 1992; 152: 1193–1197. RV
- Ilias I., Pacak K. Current approaches and recommended algorithm for the diagnostic localization of pheochromocytoma. J Clin Endocrinol Metab 2004; 89: 479–491. RV
- Gimm O., Koch C. A., Januszewicz A., Opocher G., Neumann H. P. The genetic basis of pheochromocytoma. Front Horm Res 2004; 31: 45–60. RV
- Rossi G. P., Bernini G., Caliumi C., Desideri G., Fabris B., Ferri C., Ganzaroli C., Giacchetti G., Letizia C., Maccario M., Mallamaci F., Mannelli M., Mattarello M. J., Moretti A., Palumbo G., Parenti G., Porteri E., Semplicini A., Rizzoni D., Rossi E., Boscaro M., Pessina A. C., Mantero F., PAPY Study Investigators. A prospective study of the prevalence of primary aldosteronism in 1125 hypertensive patients. J Am Coll Cardiol 2006; 48: 2293–2300. OS
- Stowasser M., Gordon R. D., Gunasekera T. G., Cowley D. C., Ward G., Archibald C., Smithers B. M. High rate of detection of primary aldosteronism, including surgically treatable forms, after ‘non‐selective’ screening of hypertensive patients. J Hypertens 2003; 21: 2149–2157. OS
- Bravo E. L., Tarazi R. C., Dustan H. P., Fouad F. M., Textor S. C., Gifford R. W., Vidt D. G. The changing clinical spectrum of primary aldosteronism. Am J Med 1983; 74: 641–651. RV
- Ganguly A. Primary aldosteronism. NEngl J Med 1998; 339: 1828–1834. RV
- Kaplan N. M. The current epidemic of primary aldosteronism: causes and consequences. J Hypertens 2004; 22: 863–869. RV
- Gordon R. D., Stowasser M., Tunny T. J., Klemm S. A., Rutherford J. C. High incidence of primary aldosteronism in 199 patients referred with hypertension. Clin Exp Pharmacol Physiol 1994; 21: 315–318. OS
- Lins P. E., Adamson U. Plasma aldosterone‐plasma renin activity ratio. A simple test to identify patients with primary aldosteronism. Acta Endocrinol 1986; 113: 564–569. OS
- Pimenta E., Calhoun D. A. Primary aldosteronism: diagnosis treatment. J Clin Hypertens 2006; 8: 887–893. RV
- Phillips J. L., Walther M. M., Pezzullo J. C., Rayford W., Choyke P. L., Berman A. A., Linehan W. M., Doppman J. L., Gill J. R., Jr. Predictive value of preoperative tests in discriminating bilateral adrenal hyperplasia from an aldosterone‐producing adrenal adenoma. J Clin Endocrinol Metab 2000; 85: 4526–4533. OS
- Bravo E. L. Secondary Hypertension: Mineralocorticoid excess states. Hypertension: A Companion to Braunwald's Heart diseases, H. R Black, W. J Elliott. Saunders‐Elsevier, Amsterdam 2007; 106–118. RV
- Krum H., Gilbert R. E. Novel therapies blocking the renin‐angiotensin‐aldosterone system in the management of hypertension and related disorders. J Hypertens 2007; 25: 25–35. RV
- Newell‐Price J., Bertagna X., Grossman A. B., Nieman L. K. Cushing's syndrome. Lancet 2006; 367: 1605–1617. RV
- Findling J. W., Raff H. Cushing's Syndrome: important issues in diagnosis management. J Clin Endocrinol Metab 2006; 91: 3746–3753. RV
- Strollo P. J., Jr., Rogers R. M. Obstructive sleep apnea. N Engl J Med 1996; 334: 99–104. RV
- Lavie P., Herer P., Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. Br Med J 2000; 320: 479–482. OS
- Grote L., Ploch T., Heitmann J., Knaack L., Penzel T., Peter J. H. Sleep‐related breathing disorder is an independent risk factor for systemic hypertension. Am J Respir Crit Care Med 1999; 160: 1875–1882. OS
- Parati G., Bilo G., Lombardi C., Mancia G. Secondary hypertension: Sleep Apnea. Hypertension: A companion to Braunwald's Heart diseases, H. R Black, W. J Elliott. Saunders‐Elsevier, Amsterdam 2007; 134–143. RV
- Australian National Health Medical Research Council Dietary Salt Study Management Committee. Fall in blood pressure with modest reduction in dietary salt intake in mild hypertension. Lancet 1989; i: 399–402. RT
- Port K., Palm K., Viigimaa M. Daily usage and efficiency of remote home monitoring in hypertensive patients over a one‐year period. J Telemed Telecare 2005; 11((Suppl 1))34–36. OS
- Kearney P. M., Whelton M., Reynolds K., Whelton P. K., He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens 2004; 22: 11–19. RV
- Burt V. L., Cutler J. A., Higgins M., Horan M. J., Labarthe D., Whelton P., Brown C., Roccella E. J. Trends in the prevalence awareness treatment control of hypertension in the adult US population. Data from the Health Examination Surveys 1960 to 1991. Hypertension 1995; 26: 60–69. OS
- Amar J., Chamontin B., Genes N., Cantet C., Salvador M., Cambou J. P. Why is hypertension so frequently uncontrolled in secondary prevention?. J Hypertens 2003; 21: 1199–1205. OS
- Mancia G., Ambrosioni E., Agabiti‐Rosei E., Leonetti G., Trimarco B., Volpe M. Blood pressure control and risk of stroke in untreated and treated hypertensive patients screened from clinical practice: results of the ForLife study. J Hypertens 2005; 23: 1575–1581. OS
APPENDIX:
TASK FORCE MEMBERS
Giuseppe Mancia, Co‐Chairpersona, Guy De Backer Co‐Chairpersonb, Anna Dominiczakc, Renata Cifkovad, Robert Fagarde, Giuseppe Germanof, Guido Grassig, Anthony M. Heagertyh, Sverre E. Kjeldseni, Stephane Laurentj, Krzysztof Narkicwiczk, Luis Ruilopel, Andrzej Rynkiewiczm, Roland E. Schmiedern, Harry A.J. Struijker Boudiero, Alberto Zanchettip
aUniversity of Milano‐Bicocca, Ospedale San Gerardo, Milan, Italy; bDepartment of Public Health, University Hospital, Ghent, Belgium; cUniversity of Glasgow, Glasgow, UK; dInstitute for Clinical Experimental Medicine, Prague, Czech Republic; eCatholic University, Leuven, Belgium; fUniversity La Sapienza, Policlinico Umberto 1, Roma, Italy; gUniversity of Milano‐Bicocca, San Gerardo Hospital, Milan, Italy; hUniversity of Manchester, Manchester, UK; iUllevaal University Hospital, Oslo, Norway; jPharmacology Department, Hopital Europeen Georges Pompidou, Paris, France; kDepartment of Hypertension and Diabetology, Medical University of Gdansk, Gdansk, Poland; lHospital 12 de Octubre, Madrid, Spain; mDepartment of Cardiology, Medical University of Gdansk, Gdansk, Poland; nMedizinische Klinik, University Erlangen Nuernberg, Erlangen, Germany; oDept. of Pharmacology, University of Limburg in Maastricht, Maastricht, The Netherlands; pUniversity of Milan, Istituto Auxologico Italiano, Milan, Italy.