Abstract
Five classes of antihypertensive drugs have proven efficacy in the prevention of cardiovascular morbidity and mortality. Among the remaining antihypertensives, the action of alpha‐1‐blockers is supported by most clinical evidence; however, in combination therapy, the published data concern their use as third‐line drugs at the most. The data from patients with drug‐resistant hypertension remain limited. The aim of our study was to evaluate the efficacy and safety of doxazosin in this clinical setting. Data from 97 patients with resistant hypertension treated by doxazosin were analysed retrospectively. Doxazosin was usually added as the fifth antihypertensive drug in individuals who were either unresponsive to or intolerant of the combination of other antihypertensives. The dose of doxazosin ranged from 2 to 16 mg/day. The mean duration of follow‐up was 21±17 months. Adverse events related to doxazosin treatment were rare and led to discontinuation of the therapy in only five patients (5.2%). Data from 34 patients were subjected to analysis of efficacy. In this subgroup, doxazosin therapy led to the reduction of blood pressure from 159±20/92±14 to 126±16/73±10 mmHg. We found that doxazosin is a well‐tolerated and effective drug for patients with resistant arterial hypertension who require a combination of multiple antihypertensive drugs.
Introduction
In patients with arterial hypertension, adequate control of blood pressure (BP) is essential for improving the long‐term prognosis Citation[1]. Based on large randomized trials, the drugs found to be suitable for antihypertensive therapy consist of diuretics, beta‐blockers, calcium‐channel antagonists, angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) Citation[2]. However, it is not clear which drug should be chosen when the target BP is not reached by the combination therapy using the aforementioned antihypertensives. Drug‐resistant hypertension is a problem not only in clinical practice, but also in clinical studies; the BP of a surprisingly large number of patients remained uncontrolled even in the well‐known studies. Among those remaining antihypertensives, the use of alpha‐1‐adrenergic receptor antagonists is backed by the most clinical data Citation[3],Citation[4]. The available data from the ALLHAT study do not suggest use of these drugs as first‐line antihypertensives Citation[5]. From the ASCOT study, we know that doxazosin is a safe and effective drug as a third add‐on drug in the treatment of hypertension Citation[3]. The effect of doxazosin in the treatment of drug‐resistant hypertension treated unsuccessfully with a combination of several antihypertensives was not described. Therefore, we performed a retrospective study to evaluate the safety and efficacy of doxazosin in patients with drug‐resistant hypertension.
Patients and methods
The data from patients who were referred to our hypertension clinic between 2002 and 2007 were screened for the purpose of this study. The patients were enrolled if they had been treated with a combination of at least four antihypertensive drugs including doxazosin. Records were excluded if doxazosin use was <3 months, with the exception of cases in which use of the drug was discontinued because of adverse events (AEs). The safety of doxazosin therapy was evaluated in all enrolled patients. Because of the retrospective nature of the study, the safety analysis was focused only on the AEs that led to drug withdrawal or a lowering of the dosage. Efficacy analysis was performed only in cases where there was no change in the concomitant medication regimen following the initiation of doxazosin treatment.
Results
Ninety‐seven of 551 screened medical records were selected according to the inclusion criteria. The principal characteristics of the study population are given in . Doxazosin was usually added to the therapeutic regimen when the target BP was not achieved by the combination of a diuretic, a calcium‐channel blocker, a beta‐blocker and an ACEI or ARB. Alternatively, the drug was chosen in the eventuality that some of these antihypertensives were not tolerated. At the time that doxazosin therapy was initiated, an ACEI was used in 75 (77.3%) patients, an ARB in 17 (17.5%), a beta‐blocker in 87 (89.7%), and a calcium‐channel blocker in 95 (97.9%). Diuretics were administered to 92 (94.8%) patients, and combinations of multiple diuretic drugs were given to eight (8.2%).
Table I. Doxazosin in the treatment of resistant arterial hypertension.
At the end of the follow‐up period, doxazosin was typically part of a combination of four to eight antihypertensive drugs, and the dose ranged from 2 to 16 mg daily. The doxazosin dosage was gradually titrated in all patients. The first dose (usually 1 mg) was given in the evening before going to bed in order to prevent symptoms of orthostatic hypotension. Doses up to 4 mg were usually given once daily. Higher daily doses were divided into two doses. The standard formulation of doxazosin was prescribed to all patients; extended release tablets were not used. Details on dosing in individual patients are presented in .
Safety analysis indicated that AEs leading to doxazosin withdrawal or lowering of the dose were noted in six patients (6.2%). The treatment had to be discontinued in five female patients (5.2%). Of these five patients, excessive fatigue was noted for three, and urinary incontinence was reported by two. In one male patient, the dose was lowered from 16 mg to 8 mg because of rhinitis. All of the AEs described above developed shortly after the initiation of doxazosin therapy and resolved when the drug was withdrawn or the dose was decreased.
Heart failure developed in one male patient during the follow‐up period. The symptoms of congestion resolved with an increase in diuretic therapy; doxazosin treatment was not interrupted. A gradual decrease in the left ventricular ejection fraction was observed following the first episode of heart failure. Results of an angiography of his coronary and renal arteries were normal. Before the heart failure was diagnosed, the patient had been using doxazosin for 4 years. In addition to the drug, other risk factors could have contributed to the development of cardiac insufficiency: old age (75 years), severe arterial hypertension that was uncontrolled for decades, a long history of atrial fibrillation, and type 2 diabetes treated with insulin.
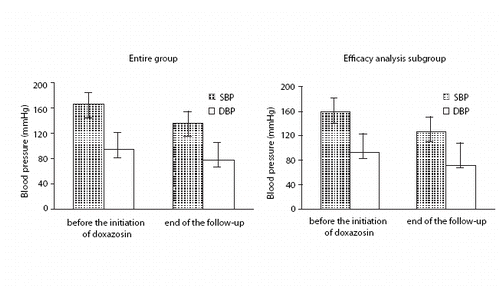
Data from 34 patients were subjected to analysis of efficacy. The doxazosin treatment resulted in a decrease of mean BP from 159±20/92±14 to 126±16/73±10 mmHg (). Doxazosin was given at a mean dose of 6.9±5.3 mg over a mean period of 22±15 months. All but eight patients reached target BP values.
Discussion
Resistant hypertension is defined as BP that remains above the goal BP despite the concurrent use of three antihypertensive drugs of different classes, including a diuretic, prescribed at optimal dosages Citation[6]. The prevalence of resistant hypertension is unknown, but the available data suggest that it is not uncommon in clinical practice. Concerning the treatment recommendations, recent guidelines Citation[6] underscore the importance of effective combination therapy, including the choice and dosing of diuretics. However, data that describe the use of other drug classes in this setting are rather limited. The current study describes our experience with the alpha‐1‐blocker doxazosin in a group of patients with difficult to control arterial hypertension. Although the data were collected retrospectively, the study provides several clinically useful observations.
It is very important to note that doxazosin therapy was well tolerated despite administration of high doses of 12 or 16 mg/day to one third of the patients. The percentage of patients who withdrew from doxazosin because of AEs was low. Fluid retention, a well‐known doxazosin‐related AE, was not noted in our group. This finding may be explained by the diuretic therapy that was administered to almost all patients in our study. Heart failure developed in only one patient during the follow‐up period, and it was unlikely to be related to the doxazosin therapy.
Doxazosin was found to be less effective than chlorthalidone as a first‐line therapy for lowering BP and preventing some cardiovascular events Citation[5]. Data regarding its use in combination therapy of arterial hypertension are limited. Specifically, there is no information concerning the drug's efficacy in the prevention of cardiovascular mortality and morbidity. Several studies have described a decrease in BP when doxazosin was added to other antihypertensive drugs, usually as a second‐ or third‐line agent Citation[3], Citation[4], Citation[7]. Although some of these studies probably also enrolled patients with resistant arterial hypertension Citation[8], Citation[9], to the best of our knowledge, no report has dealt systematically with the use of doxazosin in patients with resistant arterial hypertension.
The efficacy and safety of doxazosin were extensively evaluated in a recently published paper analysing the ASCOT data Citation[3]. In the ASCOT trial, doxazosin was administered at maximal dose 8 mg as a third‐line antihypertensive drug to 11,768 patients. The frequency of adverse reactions to doxazosin reported in the ASCOT study was similar to that in our report (7.5% vs 6.2%). Moreover, in the ASCOT study, no difference in the frequency of heart failure was observed between patients on doxazosin and controls. The mean daily dose of doxazosin used in the ASCOT trial was similar to that given to our patients who were enrolled in the efficacy analysis (7.0 vs 6.9 mg). However, we have observed a more pronounced mean BP decrease (11.7/6.9 vs 33/19 mmHg). This observation might be explained by our different design and study sample. We can also hypothesise that a synergistic effect of doxazosin with the multiple antihypertensives used in our patients contributed to the more marked blood pressure decrease.
The present study is a retrospective analysis of patients who were referred to a tertiary medical centre for uncontrolled or otherwise complicated hypertension. The primary aim of this report is to describe our experience with doxazosin in the context of a real‐life clinical practice, and it is obvious that our results are not free from clinical decision bias. Our data may overestimate the decrease in BP related to doxazosin treatment; the placebo effect can contribute to a BP reduction when a new antihypertensive drug is initiated. The second important point is the selection bias in the analysis of efficacy. Indeed, efficacy analysis did not include patients who switched medications during the follow‐up period. For this reason, we have also reported BP values of the entire group (), even when doxazosin was not the exclusive contributor to the final effect.
We conclude that, despite unquestionable limitations, our analysis suggests that doxazosin is a safe and effective component of combination therapy for patients with resistant arterial hypertension.
Acknowledgement
This work was supported by research projects MZO00179906 and MSM0021620817. No pharma ceutical company was involved.
References
- Turnbull F. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: Results of prospectively‐designed overviews of randomised trials. Lancet 2003; 362: 1527–1535
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42: 1206–1252
- Chapman N, Chang CL, Dahlof B, Sever PS, Wedel H, Poulter NR. Effect of doxazosin gastrointestinal therapeutic system as third‐line antihypertensive therapy on blood pressure and lipids in the Anglo‐Scandinavian Cardiac Outcomes Trial. Circulation 2008; 118: 42–48
- Ohta Y, Tsuchihashi T, Onaka U, Eto K, Ueno M. Usefulness of the alpha1‐blocker doxazosin as a third‐line antihypertensive drug. Hypertens Res 2007; 30: 301–306
- ALLHAT Collaborative Research Group. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). JAMA 2000; 283: 1967–1975
- Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117: 510–526
- Black HR, Sollins JS, Garofalo JL. The addition of doxazosin to the therapeutic regimen of hypertensive patients inadequately controlled with other antihypertensive medications: a randomized, placebo‐controlled study. Am J Hypertens 2000; 13: 468–474
- Pessina AC, Ciccariello L, Perrone F, Stoico V, Gussoni G, Scotti A, et al. Clinical efficacy and tolerability of alpha‐blocker doxazosin as add‐on therapy in patients with hypertension and impaired glucose metabolism. Nutr Metab Cardiovasc Dis 2006; 16: 137–147
- Guzik P, Wykretowicz A, Krauze T, Piskorski J, Adamska K, Milewska A, et al. Add‐on therapy with a nighttime dose of doxazosin in patients with uncontrolled hypertension: effects on autonomic modulation of the cardiovascular system. Hypertens Res 2008; 31: 443–453