Abstract
Aim. Obesity is known to be independently related to left ventricular (LV) hypertrophy (LVH); however, in human hypertension the association of obesity with right ventricular hypertrophy (RVH) is still unsettled. We investigated the relationship of obesity with RVH and biventricular hypertrophy in essential hypertension. Methods. A cohort of untreated and treated uncomplicated essential hypertensives consecutively attending a hospital outpatient hypertension clinic, categorized in three groups according to body mass index (BMI) thresholds (<25, 25–29.9 and ≥30 kg/m2) was considered for the present analysis. RVH was defined by an anterior RV wall thickness equal or higher than 3.1/3.0 mm/m2 in men and women, respectively, and LVH by LV mass index (LVMI) equal or higher than 51 and 47 g/m2.7 in men and women, respectively. Results. A total of 124 patients (37.6%) had normal BMI, 151 patients (45.7%) were overweight and 55 (16.7%) obese. Prevalence rates of biventricular hypertrophy (i.e. LVMI>51 and 47 g/m2.7 and RVWT>3.1 and 3.0 mm) in the three groups were 7.3%, 21.2% and 32.7%, respectively. In a multivariate analysis, BMI (OR=3.58, 95% CI 1.82–7.03, p=0.0002), was the most important correlate of biventricular hypertrophy. Conclusions. Our findings extend previous data on the impact of obesity on cardiac structure by showing that this phenotype is strongly associated with biventricular hypertrophy.
Introduction
A large body of evidence accumulated in the last decades about the prevalence and clinical correlates of left ventricular (LV) hypertrophy (LVH) in human hypertension point out the association of this phenotype with alterations in myocardial texture Citation[1], LV geometry Citation[2] and function Citation[3], as well as with left atrium enlargement Citation[4], impairment of coronary reserve Citation[5] and supraventricular or ventricular arrhythmias Citation[6]. Furthermore, data from prospective observational and intervention trials indicate that LVH is a robust predictor of incident heart failure Citation[7], coronary heart disease Citation[8], stroke Citation[9], cardiovascular (CV) and total mortality Citation[10–12]
High blood pressure (BP) via an increase in peripheral vascular resistances and large arteries stiffness plays a major role in hypertensive myocardial hypertrophy; however, several haemodynamic and non‐haemodynamic factors, such as volume expansion, increased sympathetic nervous system and renin‐angiotensin‐aldosterone activity, excess adiposity, hormones, cytokines and inflammatory factors have been shown to participate to this process Citation[13], Citation[14].
Several myocardial growth factors have been shown to operate in obesity, a condition characterized by a clustering of hypertension, type 2 diabetes mellitus, dyslipidaemia, metabolic syndrome and sleep‐disordered breathing. Whether LVH in obese individuals directly results from the increased adiposity or from these co‐morbidities is hardly determined Citation[15].
Numerous population‐based and hypertensive cohort studies investigating the impact of adiposity on LV structure have shown that LVH is more prevalent in obese individuals than in their lean counterparts, even after adjustment for confounders Citation[16], Citation[17].
The relationship of obesity with the different geometric patterns of LV (i.e. eccentric vs concentric LVH) has been actively investigated, although with conflicting results.
So far, no study has analysed the impact of obesity on the structure of the right ventricle (RV) Citation[18], Citation[19]. Accordingly, we sought to assess the relation of obesity with RV and biventricular hypertrophy in a cohort of essential hypertensives.
Methods
Study population
Three hundred and forty consecutively treated or untreated essential hypertensive patients, most of them referred to our hypertension clinic by general practitioners during a period of 6 months between January and September 2007, were included in the study.
High BP was defined as a systolic BP (SBP) ±140 mmHg and/or diastolic BP (DBP) ±90 mmHg in untreated subjects; treated hypertensives were included regardless of BP values. Main exclusion criteria were history or evidence of congestive heart failure, atrial fibrillation, previous stroke, significant cardiac valve disease (>1+ valvular regurgitation, any degree of valvular stenosis or presence of prosthesis), previous myocardial infarction or coronary bypass, secondary causes of hypertension, neoplastic disease, lung disease and pulmonary arterial hypertension. After an informed consent had been obtained during the initial visit, all patients underwent the following procedures within a week interval: medical history and physical examination, clinic BP measurement, blood and urine sampling, standard 12‐lead electrocardiogram, M‐mode, two‐dimensional and Doppler echocardiographic examination. In all subjects, laboratory tests for secondary hypertension were performed when considered appropriate on clinical grounds. The study protocol was approved by the Ethics Committee of one of the Institutions involved.
Clinic BP measurement
BP was measured by a physician during two different visits at the outpatient clinic using a mercury sphygmomanometer and taking the first and fifth phases of Koroktoff sounds to identify systolic and diastolic values, respectively.
At each visit, three measurements were taken at 1‐min interval after the subjects had rested for 5 min in the sitting position; the average value was used to define clinic SBP and DBP.
Echocardiography
Technical details have been previously reported Citation[20]. Briefly, end‐diastolic and end‐systolic LV internal diameter (LVIDd, LVIDs), interventricular septum thickness (IVSTd, IVSTs) and posterior wall thickness (PWTd, PWTs) were measured on two‐dimensionally guided M‐mode tracings during at least five cycles according to the Penn Convention Citation[21]. LV mass (LVM) was calculated by Devereux's formula and normalized to body height2.7 Citation[22]; LVH was defined by LVM index (LVMI) equal or greater than 51 g/m2.7 in men and 47 g/m2.7 in women Citation[23].
LV myocardial systolic function was assessed as the midwall circumferential shortening and calculated by a two‐shell cylindrical model Citation[24].
LV filling was assessed by recording mitral flow by standard pulsed Doppler technique in apical four‐chamber view; the following parameters were considered: early diastolic peak flow velocity (Em), late diastolic flow velocity (Am), their ratio (E/A)M and Em wave deceleration time (from peak Em‐wave to baseline).
Right ventricular (RV) internal end‐iastolic diameter and RV end‐diastolic thickness were measured in the parasternal long‐axis view (anterior wall) at the outflow tract level as well as in the sub‐costal view at the tips level of the tricuspid valve. RV filling was assessed by recording tricuspid flow by standard pulsed Doppler technique in apical four‐chamber view and the following parameters were considered: early diastolic peak flow velocity (Et), late diastolic flow velocity (At), their ratio (E/A)T and ET wave deceleration time (from peak ET wave to baseline). RV global systolic function was assessed as the tricuspid annular plane systolic excursion (TAPSE).
RV hypertrophy (RVH) was defined by RV anterior thickness ≥3.1 mm/m2 in men and ≥3.0 mm/m2 in women; these cut‐points correspond to the 95th percentile in a group of 90 normotensive, non-obese, healthy adults evaluated in our hospital outpatient clinic for cardiovascular check‐ups in the last 2 years.
Reproducibility of RV wall thickness measurement was evaluated in a subsample of 50 randomly selected patients who underwent a second echocardiographic examination within a 3–5‐day interval; intraobserver coefficient of variation was 7.9% for the parasternal and 11.9% for the subcostal view, respectively.
Both left and right transverse and longitudinal atrial diameters were measured in the apical four‐chamber view at ventricular end-systole.
Statistical analysis
Statistical analysis was performed by the SAS system (version 6.12; SAS Institute Inc., Cary, North Carolina, USA). Patients were categorized in three groups according to body mass index (BMI) thresholds (I: <25 kg/m2, II: 25–29.9 kg/m2 and III: ≥30 kg/m2). Values were expressed as means ±SD or as percentages. Means were compared by the Student's t‐test for independent samples. Analysis of categorical data was carried out with the χ2 test or Fischer's exact test when appropriate. Differences between groups were tested by one‐way analysis of variance (ANOVA). The strength of correlation between variables was tested by linear correlation analysis and multiple regression analysis. The limit of statistical significance was set at p<0.05.
Results
The screening process involved 417 consecutive untreated and treated patients with uncomplicated hypertension. Of these, 11 were excluded because of secondary hypertension, 25 to valvular disease, 16 to chronic pulmonary disease and nine to miscellaneous reasons. Thus, 356 hypertensive subjects met the inclusion criteria, 340 of them completed the study having an echocardiographic examinations of good technical quality. Mean age was 58±13 years, mean SBP and DBP were 139±14 and 88±9 mmHg; gender distribution was 62% and 48%, respectively, for males and females. With regard to other risk factors, 44% of patients fulfilled two or more ATP III Report criteria for metabolic syndrome other than hypertension, 14% were smokers and 10% had type 2 diabetes mellitus.
Untreated patients were 10%, of the treated patients 26% were on monotherapy, 36% on two drugs and 28% on a three or more drugs association. On the whole, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (69%), diuretics (53%) and calcium antagonists (35%) were the most frequently prescribed drugs either on mono‐ or combination therapy.
As for body weight, 37.9% of subjects were lean (group I: BMI <25 kg/m2), 45.3% overweight (group II: BMI 25–29.9 kg/m2) and 16.8% obese (group III: BMI≥30 kg/m2). Clinical and demographic characteristics of the three groups are reported in . Average waist circumference, duration of hypertension, fasting blood glucose, triglycerides, uric acid concentrations, prevalence of men, impaired fasting glucose/type 2 diabetes, metabolic syndrome and multiple antihypertensive therapy (three or more antihypertensive drugs) tended to be higher in obese and overweight than in lean hypertensives; the opposite trend was observed for age and high‐density lipoprotein (HDL)‐cholesterol. The differences were in most instances statistically significant.
Table I. Clinical characteristics of the study population categorized in three groups by body mass index (BMI).
Echocardiographic parameters are shown in . Mean values of LVIDd, LVIDs, IVSTd, PWTd, relative wall thickness, left atrium diameter, absolute LVM and LVMI showed a progressive increase across the groups; both mid-wall shortening fraction and (E/A)M values showed a non-significant opposite trend. As for RV chambers, the following parameters were significantly different among the groups: RV internal diameter and wall thickness in diastole, (E/A)T ratio and deceleration time.
Table II. Echo‐Doppler parameters of the study population categorized in three groups by body mass index (BMI)
Prevalence of LVH, RVH and biventricular hypertrophy
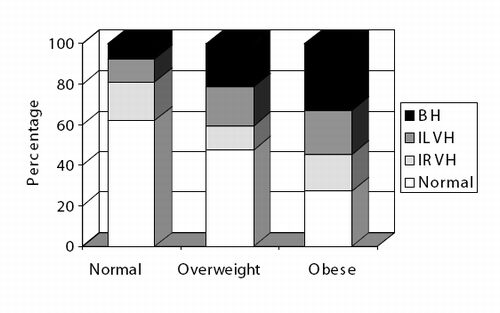
In the population study, 117 (34.4%) and 114 patients (33.5%) fulfilled the gender specific criteria for LVH (i.e. LVMI<51 and 47 g/m2.7) and for RVH (i.e. RVWT<3.1 and 3.0 mm/m2), respectively. According to these thresholds, isolated LVH, isolated RVH and biventricular hypertrophy were found in 16.6%, 15.7% and 17.8% of the sample, respectively.
As shown in , a stepwise increase in prevalence rates of isolated LVH, RVH and biventricular hypertrophy occurred from lean to overwheight and obese hypertensives. Finally, although eccentric LVH was the most common altered LV pattern across the three BMI groups, concentric LVH was approximately four‐fold higher in obese than in lean and overweight patients.
Correlation analyses
In a univariate analysis, BMI was directly related to fasting blood glucose, triglycerides, uric acid, LVM index, LV relative wall thickness, LV midwall shortening and RVWT, and inversely related to HDL‐cholesterol, (E/A)M and (E/A)T ratio. In a multivariate analysis, LVMI, anterior RV wall thickness, (E/A)T ratio and HDL-cholesterol remained independent correlates of BMI ().
Table III. Univariate and multivariate correlations between body mass index and clinical and echocardiographic variables.
The relation of biventricular hypertrophy with clinical variables such as age, BMI, SBP, mid-wall fractional shortening, categorized according to the median value, with duration of hypertension (<10 vs ≥10 years), combination therapy (<3 vs ≥3 drugs), high fasting glucose (no—yes), was analysed by a logistic regression model with stepwise selection. Among these categorical variables, BMI (odds ratio, OR=3.584; 95% confidence interval, 95% CI 1.826–7.036, p=0.0002), high fasting glucose (OR=2.411, 95% CI 1.227–4.378, p=0.0106), mid-wall fractional shortening (OR=2.398, 95% CI 1.270–4.688, p=0.0106) and SBP (OR 2.394, 95% CI 1.187–4.826, p=0.0150), in ranking order, were independent correlates of biventricular hypertrophy.
Discussion
Our study investigated for the first time the relationship between obesity and biventricular hypertrophy in subjects with systemic hypertension; the study, indeed, was performed in a cohort of essential hypertensives attending one hypertension outpatient clinic, most of the patients were on antihypertensive treatment and none manifested overt cardiovascular or pulmonary diseases. Our principal finding is that the prevalence of biventricular hypertrophy (i.e. combined LVH and RVH) in obese individuals was 1.5‐ and 4.5‐fold higher compared with their overweight and lean counterparts, respectively. More importantly, we have been able to show that BMI was the most relevant clinical correlate of biventricular hypertrophy, followed in ranking order by high fasting blood glucose, reduced LV systolic function and high SBP.
A number of points related to our results may deserve some discussion.
Since the pioneer report by Gottdiener et al. published in the mid‐1980s Citation[25], only few studies have examined the impact of systemic hypertension on RV structure. This may be for several reasons, including the fact that the anatomy of RV, a thin‐wall cavity characterized by an irregular geometry, makes an accurate measurement of wall thickness and volume quite difficult. Moreover, no convincing evidence supports the hypothesis that in essential hypertension the pulmonary circulation is exposed to the same dysregulation involving the systemic circulation and leading to a substantial increase in RV after‐load.
Previous studies including a small number of patients (less than 300 hypertensives, overall), have shown that mild or moderate structural RV alterations (i.e. hypertrophy of the anterior free wall) may parallel LV changes (i.e. hypertrophy of interventricular septum/posterior wall and increased LVM) Citation[26–30].
None of these studies, however, provided any information on the clinical correlates of RVH and biventricular hypertrophy and on RV involvement in specific subgroups of hypertensive patients. More recently, by comparing LV and RV echocardiographic characteristics in 286 hypertensives with and without the metabolic syndrome, we have found that alterations in LV geometry and function were more pronounced in patients with the syndrome than in their counterparts; moreover, the greater involvement of LV in these hypertensives was associated with thickening of RV walls and alterations of RV filling Citation[20].
The present study adds a new piece of evidence to previous information by showing that not only LV but also the “non‐stressed ventricle” is affected by obesity. In particular, our results provide the first evidence that prevalence of biventricular hypertrophy in systemic hypertension, as defined by a combined increase in LVMI and RV wall thickness, markedly rises from lean to overweight and obese individuals and this trend is apparently unrelated to differences in well recognized correlates of cardiac hypertrophy such as age, SBP or DBP. It is worth noting that in our obese hypertensives the likelihood of biventricular hypertrophy was 4.5‐fold greater than in their lean counterparts, in spite of a younger age and similar BP values in the former group.
Moreover, our findings contribute to identify clinical, metabolic and echocardiographic variables associated with biventricular hypertrophy in the hypertensive setting. The logistic model, indeed, indicates that BMI is the most important independent variable associated to biventricular involvement, being high fasting glucose, reduced LV myocardial contractility and uncontrolled SBP other clinically relevant contributors to this altered cardiac phenotype. This observation extends to RVH and biventricular hypertrophy previous evidence showing that obesity affects LV morphology by several mechanisms, including not only an increased haemodynamic load (i.e. intravascular volume expansion, elevated stroke volume and blood viscosity) but also a direct effect on cardiac myocytes related to over‐expression of myocardial growth factors.
Despite observations that body size and gender account for up to 50% of adult cardiac dimension variability Citation[31], in previous studies RVH was simply defined according to non‐gender specific, absolute partition values, i.e. RV wall thickness thresholds from 5 to 7 mm in both genders Citation[25], Citation[28], Citation[29]. To overcome this drawback, we adopted, for the first time, sex‐specific criteria normalized for body surface and derived from a non‐obese control group. In particular, RV anterior wall thickness in the parasternal long‐axis view was considered for RVH determination, since in our hands this measurement is more reproducible.
Although a full discussion on the causes of structural and functional RV changes in arterial hypertension is beyond the scope of this paper, some of the mechanisms are (i) mechanical ventricular interaction; (ii) translation of the increased LV filling pressure in the pulmonary circulation; (iii) RV response to tissue growth mediators and hormones operating in systemic hypertension Citation[25]. Furthermore, the effects of obesity on the respiratory system, related to the increased deposits of fat in the chest wall and abdomen, such as decreased lung compliance, hypoventilation and sleep‐apnoea syndrome have been shown to play a central role in RV hypertrophic process Citation[32].
Our work has some limitations that may deserve a comment. First of all, the cross‐sectional design of the study makes any prognostic implication about the association between obesity and biventricular hypertrophy in terms of cardiovascular outcomes unfeasible. It should be pointed out, however, that our primary aim was to investigate the relationship between obesity and structural alterations in both ventricles and to characterize the clinical variables more closely associated with cardiac changes in the hypertensive setting.
This purpose has been clearly accomplished by the finding of the tight link between BMI and biventricular hypertrophy. Secondly, RV systolic pressure could be assessed in less than one third of the study sample (i.e. in 107 patients with minimal or mild tricuspid regurgitation). In these patients, RV systolic BP was still normal, according to inclusion criteria, and no differences were found across the various BMI groups (data not shown). Thirdly, RVH was arbitrarily defined by gender‐specific criteria normalized to BSA and derived from our cohort of healthy normotensive non‐obese individuals; LVH was defined by widely accepted and prognostically validated criteria indexed to height to the allometric 2.7 power. It is quite evident that larger or stricter criteria may have yielded different results. It should be pointed out, however, that not only categorical variables but also continuous variables defining LV and RV structure (i.e. LVMI and RV wall thickness) were independently associated with BMI values. Finally, since our findings refer to a selected group of uncomplicated Caucasian hypertensive patients with low prevalence of obesity attending a single hypertension centre, these results may be invalid in different demographic and ethnic clinical settings.
In conclusion, the present study indicates that obesity is a strong, independent correlate of biventricular hypertrophy and that this cardiac phenotype clusters with major CV risk factors such as reduced LV myocardial performance, impaired fasting glucose and uncontrolled BP. This kind of evidence suggests that strategies aimed at reducing obesity in the population may have a major role in preventing subclinical cardiac damage.
Acknowledgement
The contribution of Arturo Esposito (MD), Cristiana Valerio (MD) and Stefano Meani (MD) in conducting the study is gratefully acknowledged.
Source of funding: this study was supported by a grant from the Italian Society of Hypertension.
Conflict of interest: none.
References
- Rossi MA. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens 1998; 16: 1031–1041
- Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, et al. Patterns of left ventricular geometry and geometric remodelling in essential hypertension. J Am Coll Cardiol 1992; 19: 1550–1558
- Palmiero P, Maiello, Nanda NC. Is echo‐determined left ventricular geometry associated with ventricular filling and midwall shortening in hypertensive ventricular hypertrophy?. Echocardiography 2008; 25: 20–26
- Cuspidi C, Meani S, Fusi V, Valerio C, Catini E, Sala C, et al. Prevalence and correlates of left atrial enlargement in essential hypertension: role of ventricular geometry and the metabolic syndrome. J Hypertens 2005; 23: 875–882
- Kawecka‐Jaszcz K, Czarnecka D, Olszanecka A, Klecha A, Kwiecien‐Sobstel A, Stolarz‐Skrzypek K, et al. Myocardial perfusion in hypertensive patients with normal coronary angiograms. J Hypertens 2008; 26: 1686–1694
- Hennersdorf MG, Strauer BE. Arterial hypertension and cardiac arrhythmias. J Hypertens 2001; 19: 167–177
- de Simone G, Gottdiener JS, Chinali M, Maurer MS. Left ventricular mass predicts heart failure not related to previous myocardial infarction: the Cardiovascular Health Study. Eur Heart J 2008; 29: 741–747
- Bikkina M, Levy D, Evans JC, Larson MG, Benjamin EJ, Wolf PA, et al. Left ventricular mass and the risk of stroke in an elderly cohort: the Framingham Study. JAMA 1994; 272: 33–36
- Ghali JK, Liao Y, Cooper RS. Influence of left ventri‐cular geometric patterns on prognosis in patients with or without coronary artery disease. J Am Coll Cardiol 1998; 31: 1635–1640
- Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Med Intern 1991; 114: 345–352
- Rodriguez CJ, Lin R, Sacco RL, Jin Z, Boden‐Albala B, Homma S, et al. Prognostic implications of left ventricular mass among Hispanics. The Northern Manhattan study. Hypertension 2006; 48: 87–92
- Gerdts E, Cramariuc D, de Simone G, Wacthell, Dalhof B, Devereux RB. Impact of left ventricular geometry on prognosis in hypertensive patients with left ventricular hypertrophy (the LIFE study). Eur J Echocardiogr May 13, 2008, Epub ahead of print.
- Schmieder RE. The role on non‐haemodynamic factors in the genesis of LVH. Nephron Dial Transplant 2005; 20: 2610–2612
- Iwashima Y, Horio T, Kamide K, Rakugi H, Ogikara T, Kawano Y. C‐reactive protein, left ventricular mass index, and risk of cardiovascular disease in essential hypertension. Hypertens Res 2007; 30: 1177–1185
- Owan T, Litwin SE. Is there a cardiomyopathy of obesity. Curr Heart Fail Rep 2007; 4: 221–228
- Lauer MS, Anderson KM, Levy D. Separate and joint influences of obesity and mild hypertension on left ventricular mass and geometry: the Framingham Heart Study. J Am Coll Cardiol 1992; 19: 130–134
- de Simone G, Devereux RB, Roman MJ, Alderman MH, Laragh JH. Relation of obesity and gender to left ventricular hypertrophy in normotensive and hypertensive adults. Hypertension 1994; 23: 600–606
- Fox E, Taylor H, Andrew M, Han H, Mohamed E, Garrison R, et al. Body mass index and blood pressure influences on left ventricular mass and geometry in African Americans. The Atherosclerosis Risk and Communities (ARIC) Study. Hypertension 2004; 44: 55–60
- Toprak A, Wang H, Chen W, Paul T, Srinivasan S, Berenson G. Relation of childhood risk factors to left ventricular hypertrophy (eccentric or concentric) in relatively young adulthood (from the Bogalusa Heart Study). Am J Cardiol 2008; 101: 1621–1625
- Cuspidi C, Valerio C, Sala C, Negri F, Esposito A, Masaidi M, et al. Metabolic syndrome and biventricular hypertrophy in essential hypertension. J Hum Hypertens Sept 18, 2008, Epub ahead of print
- Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977; 55: 613–618
- Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57: 450–458
- de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass; assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol 1995; 25: 1056–1062
- de Simone G, Devereux RB, Roman MJ, Ganau A, Saba PS, Alderman MH, et al. Assessment of left ventricular function by the midwall fractional shortening/end-systolic stress relation in human hypertension. J Am Coll Cardiol 1994; 23: 1444–1451
- Gottdiener JS, Gay JA, Maron BJ, Flectcher RD. Increased right ventricular pressure overload: echocardiographic determination of hypertrophic response of the “nonstressed” ventricle. J Am Coll Cardiol 1985; 6: 550–555
- Nunez BD, Messerli FH, Amodeo C, Garavaglia GE, Schmieder RE, Frohlich ED, et al. Biventricular cardiac hypertrophy in essential hypertension. Am Heart J 1987; 114: 813–818
- Cuspidi C, Sampieri L, Angioni L, Boselli L, Bragato R, Leonetti G, et al. Right ventricular wall thickness and function in hypertensive patients with and without left ventricular hypertrophy: echo-Doppler study. J Hypertens 1989; 7(6)S108–S109
- Myslinski W, Mosiewicz J, Ryczak E, Barud W, Biłan A, Palusinski R, et al. Right ventricular function in systemic hypertension. J Hum Hypertens 1990; 12: 149–155
- Galderisi M, Severino S, Caso P, Cicala S, Petrocelli A, De Simone L, et al. Right ventricular myocardial diastolic dysfunction in different kinds of cardiac hypertrophy: analysis by pulsed Doppler tissue imaging. It Heart J 2001; 2: 912–920
- Cicala S, Galderisi M, Caso P, Petrocelli A, D'Errico A, de Divitiis O, et al. Right ventricular diastolic dysfunction in arterial systemic hypertension: Analysis by pulsed tissue Doppler. Eur J Echocardiography 2001; 3: 135–142
- Dewey FE, Rosenthal D, Murphy DJ, Froelicher VF, Ashley EA. Does size matter? Clinical applications of scaling cardiac size and function for body size. Circulation 2008; 117: 2279–2287
- Kessler R. Pulmonary hypertension in the obstructive sleep apnea syndrome: prevalence, causes and therapeutic consequence. Eur Respir 1996; 9: 787–794