Abstract
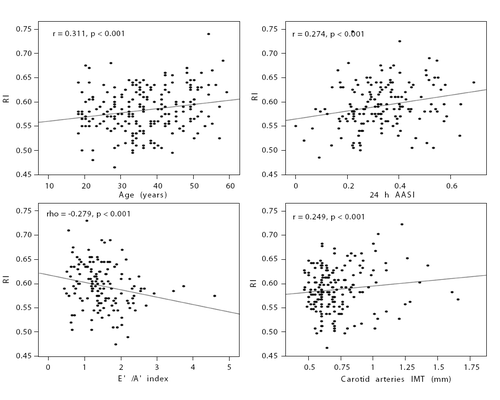
The aim of our study was to evaluate renal resistive index (RI) value in never treated hypertensive patients in relation to ambulatory blood pressure measurement (ABPM) values and early target organ damage. The study included 318 subjects: 223 patients with never treated essential hypertension (mean age 37.1 years) and 95 normotensive healthy subjects (mean age 37.9 years). ABPM, echocardiography and carotid and renal arteries duplex color Doppler examinations were performed. RI values in patients with never treated essential hypertension were no different from the normotensive control group (0.59 ± 0.05 vs 0.59 ± 0.05; NS). In the untreated patients RI correlated significantly with 24‐h pulse pressure (r=0.234; p<0.01) and ambulatory arterial stiffness index (AASI) values (r=0.274; p<0.001), intima‐media thickness (IMT) (r=0.249; p<0.001), E′/A′ (rho= −0.279; p<0.001) and relative wall thickness (RWT; r=0.185; p<0.01). In the multivariate stepwise analysis, RI values correlated independently with carotid IMT (β=0.272; p=0.020) and 24‐h AASI values (β=0.305; p=0.009). In normotensive healthy controls, significant independent correlation between RI and carotid IMT and 24‐h AASI values were also found. Our study may indicate limited value of RI in differentiating patients with uncomplicated hypertension with healthy controls. Renal resistive values were independently correlated with carotid IMT and AASI. These may suggest that renal vascular resistance is related to two markers for cardiovascular events both in the hypertensive and normotensive subjects.
Introduction
The clinical relevance of renal resistive index (RI) has been discussed equivocally. Some data indicate elevated renal resistive indices in patients with diabetic nephropathy, suggesting a prognostic significance of RI for the progression of kidney disease Citation[1–4]
It has also been demonstrated that RI was associated with the severity and duration of essential hypertension. Recently it has been suggested that RI values may be related to increased blood pressure (BP), duration of the disease and early organ damage in patients with essential hypertension with normal renal function or mild renal dysfunction Citation[5–10].
It should be noted that studies focused on the association between RI and target organ damage were mostly performed in patients with previously treated hypertension. The indices of renal perfusion have not been extensively studied in patients with never‐treated essential hypertension (stage 1–2), in particular in comparison with normotensive subjects Citation[5],Citation[6],Citation[8],Citation[9].
Therefore, the aim of our study was to evaluate RI value in patients with untreated essential hypertension in relation to ambulatory BP measurement (ABPM) values, biochemical parameters and early target organ damage. Subjects were required to meet predefined selection criterion‐never treated previously essential hypertension stage 1–2, lack of co‐existing cardiovascular disorders and preserved renal function Citation[11],Citation[12]. We also compared RI values with the age‐matched normotensive healthy subjects.
Material and methods
Patients
The study included 318 subjects: 223 patients with never treated previously essential hypertension (group 1 of mean age: 37.1 years; 162 males, 61 females) and 95 normotensive (age and gender matched for group 1) healthy subjects (group 2 of mean age: 37.9 years; 53 males, 42 females).
In years 2004–2007, consecutive patients with never treated previously essential hypertension attending the outpatient clinic of our Department were asked to enter the study (all subjects were participating in the project 2.16/VII/05 supported by the Institute of Cardiology).
Before entering, the study clinical examination was performed to determine demographics, cardiac history, known duration of hypertension, cardiovascular risk factors and to exclude related co‐morbidities. In untreated patients, hypertension was diagnosed if office systolic and diastolic BP levels were 140 and/or 90 mmHg or more and if the mean daytime ambulatory systolic and diastolic BP levels were higher than 135 and/or 85 mmHg Citation[11]. Subsequently subjects were defined normotensive if office systolic and diastolic BP levels were lower than 140/90 mmHg and if the mean daytime ambulatory systolic and diastolic BP levels were lower than 135/85 mmHg. Thus patients with white‐coat hypertension and masked hypertension were not included into the study.
Subjects were excluded for any of the following reasons: secondary hypertension and/or related comorbidities including positive history or clinical signs of coronary heart disease, cerebrovascular disease, severe obesity, diabetes mellitus, neoplastic, hepatic, renal disease, chronic heart failure, creatinine clearance <60 ml/min, disabling diseases or refusal to provide informed consent.
Age and gender matched healthy subjects (characterized in the ) were asked through a local educational program of the Institute of Cardiology to enter the study at the same time as never‐treated hypertensive patients. Both groups were originating from the same geographical area (Warsaw County). In the control group, hypertension was excluded based on office BP measurements and ambulatory BP measurement as defined above.
Table I. Clinical characteristics and ambulatory blood pressure values of the patients with never treated hypertension (group 1) and healthy controls (group 2).
The study was approved by the Ethics Committee of the National Institute of Cardiology, Warsaw, and informed written consent was obtained from each patient or healthy subject.
Laboratory methods
Blood samples for all biochemical evaluations were taken after overnight fasting and after 60 min rest in the supine position. Creatinine clearance was calculated using the Cockroft‐Gault formula. Concentrations of high‐sensitivity C‐reactive protein (hsCRP), lipids, creatinine, uric acid and glucose were measured using standard laboratory techniques.
Office BP
BP was measured by a trained nurse with a patient in the sitting position after a 5‐min rest, using a mercury sphygmomanometer. Three consecutive readings were performed at two separate visits and the average was recorded.
Ambulatory BP
In all patients, ambulatory BP measurements were recorded using a SpaceLabs 90207 or 90217 (Redmond, Washington, USA). Readings were obtained every 15 min during the day (6:00–22:00 h) and every 30 min during the night (22:00–6:00 h).
Division on sleep and activity periods was made after the recoding based on data from patient diary. Subjects were classified as dippers if the proportional change from awake to asleep BP fell by >10%. From 24‐h BP readings, diastolic BP was plotted against systolic BP and the regression slope was calculated. Ambulatory arterial stiffness index (AASI) was defined as one minus the regression slope as described previously Citation[13].
Echocardiography
Standard transthoracic echocardiographic studies were performed with the GE Vivid 7 using a 2.5–3.5 MHz transducer on the same or the next day when ABPM was recorded. All patients were examined at rest in the left lateral decubitus position. Left ventricle end‐diastolic (LVEDd) and systolic (LVESd) dimensions, septal (IVSd) and posterior wall (PWDd) thickness, and left atrial (LA) size were measured according to the American Society of Echocardiography (ASE) using mostly M‐mode tracings. The left ventricle mass (LVM) was calculated using the modified ASE cube formula proposed by Devereux et al. and was indexed to body surface area (BSA) to obtain the LVM index (LVMI) Citation[14]. Left ventricular hypertrophy was defined according to the ESH/ESC 2003 criteria Citation[11]. The shortening fraction (SF) was computed according to the formula: LVEDd‐LVESd/LVEDd(%). The relative wall thickness (RWT) was calculated as the ratio of the sum of IVSd and LVPWd both measured at end‐diastole divided by the LV end‐diastolic diameter (LVDd). Pulsed Doppler mitral flow inflow at mitral valve leaflet tips for: early (E) and atrial (A) maximal velocities, and deceleration time (DT). Tissue Doppler imaging (TDI) at the lateral and septal mitral annulus for E' and A′ was obtained in the apical four‐chamber view. In the apical five‐chamber view isovolumic relaxation time IVRT was obtained.
Carotid ultrasonography
The carotid ultrasound followed by a duplex color Doppler examination was done in supine position, with a Phillips ATL 5000 and a linear probe at 7.5–12 MHz. Both left and right common carotid arteries were analyzed. Multiple measurements on the distal wall from anterolateral and posterolateral longitudinal views were recorded. Maximal intima‐media thickness (IMT) was measured at three points (3 mm apart) in two segments 1 cm from the flow divider caudally (carotid bulb) and 1 cm caudally from the beginning of the common carotid bulb (common carotid). The IMT value was calculated as an arithmetical mean of 24 measurements from bulb and common carotid segments of both sides.
Renal ultrasound and Doppler studies
For renal ultrasound investigation, a HD11 (Philips, Einthoven, Germany) and ATL 5000 (ATL, USA) both with a multiphase 2–4‐ MHz convex array transducer were used. The intrarenal arteries were visualized in the color duplex mode. Doppler US spectral analysis included mean RI (= peak systolic velocity‐end‐diastolic velocity/peak systolic velocity) and pulsatility index (PI=peak systolic velocity‐end‐diastolic velocity/mean velocity) obtained from three Doppler curves at different sites of the each kidney. For calculation, the software of duplex scanner was used. Measurements were made by two experienced investigators, blinded to the clinical status of patients. Interobserver and intraobserver coefficients of variance of RI were 5.6% and 4.7% respectively (n=12).
Statistical analysis
For comparison of groups’ means and medians, the t‐test for independent samples and Mann‐Whitney test were employed. Comparison of the prevalence rates among groups was performed using the chi‐square test. The results throughout are presented as mean ± standard deviation. The degree of association between variables was assessed using Pearson correlation coefficients (r) or Spearman rank correlation coefficients (rho). Variables associated with RI at <0.1 significance were included into a stepwise multivariate linear regression model to determine the simultaneous effect of several variables on the RI value. P<0.05 was considered statistically significant.
Results
The clinical characteristics of subjects included into group 1 and 2 are shown in the . The group 1 as compared with the group 2 was characterized by higher body mass index (BMI), systolic and diastolic clinic BP levels, higher creatinine clearance, glucose and uric acid concentration and non‐significantly higher incidence of metabolic syndrome.
In our study including 223 patients with never treated essential hypertension, RI and PI values were not different from the age‐matched normotensive control group (0.59 ± 0.05 vs 0.59 ± 0.05; NS and 1.04 ± 0.09 vs 1.05 ± 0.19; NS for RI and PI respectively) (). When subjects were divided according to gender, there were no differences in RI values between hypertensive subjects and normotensive controls (for men 0.58 ± 0.04 vs 0.59 ± 0.05, NS; and for women 0.60 ± 0.06 vs 0.60 ± 0.06, p=NS). Both in groups 1 and 2 there were significant correlations between RI values and age (, ). There were also no differences in PI values between hypertensive subjects and normotensive controls when subjects were divided according to gender.
Figure 1. Resistive index (A) and pulsative index (B) in patients with never treated hypertension and healthy controls. Data are shown as mean ± SD.
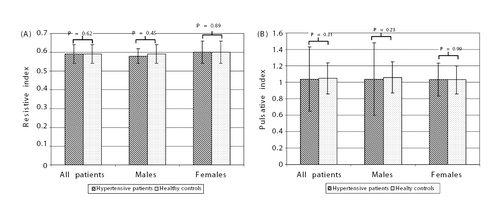
Table II. Pearson’s correlation coefficients for the relationship between resistive index (RI) values and other variables in patients with never treated hypertension and healthy controls.
Figure 2. Correlations between resistive index (RI) and age (A), 24‐h ambulatory arterial stiffness index (AASI) (B), E′/A′ index (C) and carotid arteries intima‐media thickness (IMT) (D) in patients with never treated hypertension.
Patients with untreated essential hypertension were characterized by higher mean 24‐h, daytime and night‐time, systolic and diastolic ambulatory BP values compared with control subjects. No difference was observed for AASI values and systolic and diastolic BP night‐time fall between groups 1 and 2 (). There were no correlations between RI values and systolic and diastolic ambulatory BP values in the group 1. In this group, RI correlated significantly with 24‐h pulse pressure and AASI values and night‐time diastolic BP fall (). In the group 2, there were correlations between diastolic 24‐h BP, 24‐h pulse pressure, AASI values, systolic and diastolic BP night‐time fall and RI values ().
Patients with untreated hypertension compared with healthy controls were characterized by higher IMT (0.72 ± 0.24 vs 0.64 ± 0.13 mm; p<0.001), higher RWT (0.43 ± 0.06 vs 0.40 ± 0.06; p<0.01), lower E′/A′ (1.5 ± 0.7 vs 1.7 ± 0.8; p<0.05) and higher left ventricular mass (155 ± 36 vs 139 ± 29 g; p<0.05) and left ventricular mass index in women (89 ± 18 vs 82 ± 16 m/g2; p=0.06). In the group 1 RI values significantly correlated with IMT, E′/A′, SF and RWT (). In group 2, significant correlations were found between RI values and IMT ().
A multivariate stepwise linear regression model that included all variables correlated with RI values at significance of 0.1 or less was performed. In group 1 (model included age, BMI, 24‐h systolic BP, 24‐h pulse pressure; 24‐h AASI, night‐time systolic and diastolic BP fall, hsCRP concentration, carotid arteries IMT, E′/A′, SF and RWT), RI values correlated independently with carotid arteries IMT (coefficient B=0.051; 95% CI 0.009–0.094; p=0.020; standardized coefficient β=0.272) and 24‐h AASI values (coefficient B=0.120; 95% CI 0.031–0.209; p=0.009; standardized coefficient β=0.305). In group 2 (model included age, BMI, 24‐h diastolic BP, 24‐h AASI, night‐time systolic and diastolic BP fall, carotid arteries IMT), RI values correlated independently with carotid arteries IMT (coefficient B=0.115; 95% CI 0.013–0.217; p=0.028; standardized coefficient β=0.258) and 24‐h AASI values (coefficient B=0.102; 95% CI 0.014–0.191; p=0.025; standardized coefficient β=0.264).
Discussion
The clinical relevance of renal Doppler indices has been discussed extensively Citation[1]. In the present study no difference in RI values were found between patients with never treated essential hypertension and age‐matched normotensive control group.
More recently, it has been suggested that RI values may be related to increased BP, duration of the disease and early organ damage in patients with essential hypertension. It should be noted that our study representing a cohort being evaluated in a single medical institution differs in some ways from the previous reports. We assessed a large group of never treated hypertensive patients, characterized by moderately manifested target organ damage, which was compared with the age‐matched normotensive healthy controls. In other studies, RI was evaluated in patients with essential hypertension who were older, had longer known duration of hypertension and were characterized by higher prevalence of target organ damage.
In a Veglio et al. Citation[10] study including 45 patients with essential hypertension, it has been found that RI was higher in subjects with a long‐standing hypertension compared with normotensive subjects and correlated with the severity and duration of the disease Citation[10]. The authors reported that baseline RI was significantly higher in moderate and severe hypertension when compared with normal subjects and patients with mild hypertension Citation[10].
It should be also noted that some investigators evaluated RI in hypertensive patients but no comparison was performed with normotensive subjects. Also, in the most studied groups never treated hypertension, preserved renal function and lack of cardiovascular disorders as a prespecified criterion were not employed.
Derchi et al. Citation[5] examined renal vascular resistance in 291 untreated patients with essential hypertension. However, the prevalence of mild renal dysfunction was 63% and investigated patients were older, had higher systolic BP and pulse pressure and were characterized by higher renal RI than those with normal renal function.
It is noteworthy that in our group of untreated hypertensive patients no correlation between RI and systolic BP was found. In contrast, Pontremoli et al. Citation[9] and other authors showed a positive correlation with the systolic BP in different groups of hypertensive patients. However, it should be noted that in current study BP measurements were documented by means of ambulatory BP.
In our study in patients with never treated essential hypertension, a positive correlation was found between RI value and 24‐h pulse pressure. Also other investigators reported significant relationship between RI value and pulse pressure, factor known to be associated with vascular stiffness Citation[2],Citation[5],Citation[8],Citation[15]. However, the correlation between RI and pulse pressure could have been expected, given the formula used to calculate RI.
A novel method for evaluating arterial stiffness based on ambulatory BP monitoring was recently proposed. The AASI was found to correlate with other measures of arterial stiffness and to predict cardiovascular mortality in hypertensive patients Citation[16]. However, it is controversial to what extent AASI is a true measure of arterial stiffness. It has been reported that the relation between AASI and pulse wave velocity is weak and affected by other factors Citation[16–18].
Our results showed that both in patients with never treated essential hypertension and in normotensive subjects, a positive correlation was found between RI value and AASI value. Moreover, we have shown in the multivariate analysis that this relations are independent of other variables associated with RI.
Our data are in agreement with the results of Ratto et al. who reported in a large group of untreated patients with essential hypertension that AASI was positively related to the intrarenal RI and urinary albumin excretion Citation[13]. It has been postulated that association between AASI and the renal RI suggests that changes in kidney impedance parallel those observed at the systemic level.
In our study, carotid IMT was significantly greater in patients with never treated hypertension compared with normotensive control group and a positive correlation was found between RI value and IMT. Our data are in agreement with the reports of other authors who described relationship between renal resistive indices and IMT in hypertensives Citation[8],Citation[9]. In the study of Derchi et al. Citation[5] patients with mild renal dysfunction showed increased IMT compared with those with normal renal function and a positive correlation was found between RI value and carotid intima‐media thickness Citation[5]. Also Tedesco et al. Citation[15] showed that hypertensive patients with RI >70 had increased carotid IMT compared with low RI and a positive correlation was found between RI and carotid IMT. It is of relevance that in the current study the independent association between RI and carotid IMT was also found in the healthy controls.
Since the prevalence of microalbuminuria in our group of never treated previously patients with essential hypertension was very low (5.1%), this parameter was not evaluated in relation to RI values.
The present study shows no relationship between RI values and LVH or LVMI. Also Pontremoli et al. Citation[9] and Okura et al. Citation[8] did not find association between renal RI and LVMI and LVH. Okura et al. Citation[8] concluded that this may suggest that the mechanism of hypertension‐mediated progressive damage may differ between vessels and the myocardium. In only one study of Tedesco et al. Citation[15] a positive correlation between RI values and LVMI was found. However, it should be noted that this group differs in some ways from patients included in our study and was distinguishing subjects with RI <70 from those with RI >70. The authors reported that hypertensive patients with RI >70 had increased LVMI with subclinical impairment of left ventricular diastolic function Citation[15].
In our study, RI was positively correlated with RWT in untreated hypertensive patients. This may suggest that concentric remodeling of the ventricle is related to the damage of vessels.
The relationship between RI and echocardiographic diastolic parameters was found for parameters evaluated by TDI. This method reflects the rate of LV myocardial relaxation and is relatively preload and heart rate insensitive Citation[19]. It should be noted that a positive correlation between RI and E′/A′ was found in group 1 but not in the normotensive controls.
In our group of untreated hypertensive patients, no relationship was found between RI values and some cardiovascular risk factors and/or features of the metabolic syndrome including abdominal obesity, dyslipidemia or abnormalities in glucose metabolism.
It should be noted that our results are based on the observational study and longitudinal, prospective studies are needed to evaluate the predictive role of RI in hypertensive patients. It should also be kept in mind that the associations we observed between RI and early organ damage reflect selected hypertensive group characterized by very low prevalence of microalbuminuria and modestly increased IMT compared with healthy controls.
Our study comparing a large cohort of never‐treated patients with essential hypertension and healthy controls may indicate limited value of RI in differentiating patients with uncomplicated hypertension with healthy controls. It may also be suggested that renal vascular resistance is related to two surrogate markers for cardiovascular events‐IMT and AASI‐both in the hypertensive and normotensive subjects.
Acknowledgements
Project 2.15/VII/05 was supported by the Institute of Cardiology, Warsaw, Poland.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hausberg M, Lang D, Barenbrock M, Kosch M. What do Doppler indices of renal perfusion tell us for the evaluation of renal disease. J Hypertens 2005; 23: 1795–1797
- Ohta Y, Fujii K, Arima H, Matsumura K, Tsuchihashi T, Tokumoto M, et al. Increased renal resistive index in atherosclerosis and diabetic nephropathy assessed by Doppler sonography. J Hypertens 2005; 23: 1905–1911
- Petersen LJ, Petersen JR, Ladefoged SD, Mehlsen J, Jensen HA. The pulsatility index and the resistive index in renal arteries in patients with hypertension and chronic renal failure. Nephrol Dial Transplant 1995; 10: 2060–2064
- Petersen LJ, Petersen JR, Talleruphuus U, Ladefoged SD, Mehlsen J, Jensen HA. The pulsatility index and the resistive index in renal arteries. Associations with long-term progression in chronic renal failure. Nephrol Dial Transplant 1997; 12: 1376–1380
- Derchi LE, Leoncini G, Parodi D, Viazzi F, Martinoli C, Ratto E, et al. Mild renal dysfunction and renal vascular resistance in primary hypertension. Am J Hypertens 2005; 18: 966–971
- Galesic K, Brkljacic B, Sabljar‐Matovinovic M, Morovic‐Vergles J, Cvitkovic‐Kuzmic A, Bozikov V. Renal vascular resistance in essential hypertension: Duplex-Doppler ultrasonographic evaluation. Angiology 2000; 51: 667–675
- Jensen GBardelli M, Volkmann R, Caidahl K, Rose G, Aurell M. Renovascular resistance in primary hypertension: experimental variations detected by means of Doppler ultrasound. J Hypertens 1994; 12: 959–964
- Okura T, Watanabe S, Miyoshi K, Fukuoka T, Higaki J. Intrarenal and carotid hemodynamics in patients with essential hypertension. Am J Hypertens 2004; 17: 240–244
- Pontremoli R, Viazzi F, Martinoli C, Ravera M, Nicolella C, Berruti V, et al. Increased renal resistive index in patients with essential hypertension: a marker of target organ damage. Nephrol Dial Transplant 1999; 14: 360–365
- Veglio F, Provera E, Pinna G, Frascisco M, Rabbia F, Melchio R, et al. Renal resistive index after captopril test by echo-Doppler in essential hypertension. Am J Hypertens 1992; 5: 431–436
- 2003 European Society of Hypertension–European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 2003; 21: 1011–1053
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) . J Hypertens 2007; 25: 1105–1187
- Ratto E, Leoncini G, Viazzi F, Vaccaro V, Falqui V, Parodi A, et al. Ambulatory arterial stiffness index and renal abnormalities in primary hypertension. J Hypertens 2006; 24: 2033–2038
- Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57: 450–458
- Tedesco MA, Natale F, Mocerino R, Tassinario G, Calabro R. Renal resistive index and cardiovascular organ damage in a large population of hypertensive patients. J Hum Hypertens 2007; 21: 291–296
- Schillaci G, Parati G. Ambulatory arterial stiffness index: merits and limitations of a simple surrogate measure of arterial compliance. J Hypertens 2008; 26: 182–185
- Gavish B, Ben-Dov IZ, Bursztyn M. Linear relationship between systolic and diastolic blood pressure monitored over 24 h: assessment and correlates. J Hypertens 2008; 26: 199–209
- Schillaci G, Parati G, Pirro M, Pucci G, Mannarino MR, Sperandini L, et al. Ambulatory arterial stiffness index is not a specific marker of reduced arterial compliance. Hypertension 2007; 49: 986–991
- Wang M, Yip GW, Wang AY, Zhang Y, Ho PY, Tse MK, et al. Tissue Doppler imaging provides incremental prognostic value in patients with systemic hypertension and left ventricular hypertrophy. J Hypertens 2005; 23: 183–191
