Abstract
Purpose
Orthostatic hypotension (OH) may predispose older adults to health complications leading to functional impairment. Despite the central role of the kidney in blood pressure control, the contribution of renal function in orthostatic hypotension is poorly investigated. To verify the association between Chronic Kidney Disease (CKD) and OH a population of hospitalised elderly patients with comorbidities was studied.
Materials and methods
174 patients were consecutively admitted to Acute Geriatric Wards. On admission, patients underwent postural systolic (SBP) and diastolic (DBP) blood pressure evaluation by automatic oscillometric device after 10 min rest in lying position, and in standing position at time 0, 1, 3 and 5 min. CKD was assumed for estimated glomerular filtration rate (e-GFR) less than 60 mL/min/1.73 m2.
Results
The mean age of the population enrolled was 74.4 ± 7.0. OH was found in 46.0% and CKD in 56.3% of patients, respectively. A lower e-GFR was observed in patients with (56.1 ± 16.7 mL/min/1.73 m2) than in those without OH (61.1 ± 15.9 mL/min/1.73 m2) (p < 0.05). A greater fall in SBP at 0-min (12.8 ± 6.3 vs. 7.7 ± 3.2 mmHg) and at 1-min (8.4 ± 4.5 vs. 5.7 ± 2.8 mmHg) was found in CKD patients in respect to patients without CKD during active standing test (p < 0.05). Similarly, a DBP reduction at 0-min and at 1-min was observed in CKD patients in respect to patients without CKD (p < 0.05). A multivariate logistic regression analysis showed that CKD was associated to OH (OR 2.426; 95%CI 1.192–4.937; p = 0.014).
Conclusions
CKD is associated to OH in hospitalised older adults.
PLAIN LANGUAGE SUMMARY
What is the context?
Orthostatic hypotension is a frequent disorder in the elderly population. The clinical management of these patients is very difficult due to the multifactorial origin of the disorder. Despite the central role of the kidney in blood pressure control, the contribution of renal function on the development of OH is poorly understood.
What is new?
In this study, we enrolled a population of older hospitalised adults in which we evaluated clinical, biochemical, and pharmacological factors potentially related to OH beyond the known commonest causes such as bed-rest syndrome, advanced heart failure, and autonomic dysfunction. CKD is associated to OH in hospitalised older adults.
What is the impact?
Our study highlights the importance of investigating the relationship between orthostatic hypotension and renal function in older adults. The awareness of this association may facilitate the early recognition of OH among older adults with CKD and improve OH-related healthcare outcomes.
Introduction
Orthostatic hypotension (OH) is a common condition among older adults. The prevalence of OH in hospitalised elderly patients in Internal Medicine wards ranges from 22% to 75% [Citation1]. OH is associated with symptoms such as dizziness, syncope, and falls, and can lead to functional impairment [Citation2–5]. Several studies have reported a correlation between OH and disability, cardiovascular disease, and all-cause mortality [Citation6–9].
It is well-established that certain disorders prevalent in the elderly, such as hypovolemia, anaemia, prolonged bed rest, heart failure, and autonomic dysfunction, may contribute to OH [Citation10]. Additionally, the use of antihypertensive medications, antidepressants, adrenergic blocking agents, and medications for Parkinson’s disease (PD) has been associated with OH, frequently presenting as an iatrogenic condition [Citation11]. Some studies have explored the association of OH with chronic kidney disease (CKD), due to the involvement of the renin-angiotensin-aldosterone system in postural blood pressure regulation [Citation12]. However, the contribution of renal function to OH has been minimally investigated, often remaining a theoretical finding with limited impact on clinical decision-making.
In the elderly population, establishing the contribution of individual factors to the development of OH is challenging, as these factors often coexist, and the aetiology of OH remains elusive, particularly in the context of comorbidities among the elderly population. Therefore, identifying an isolated cause for OH resolution is often ineffective in older individuals.
In this study, we enrolled a population of older adults consecutively admitted to two different geriatric units. We evaluated clinical, biochemical, and pharmacological factors potentially related to OH, beyond the commonly known causes such as bed-rest syndrome, sepsis, advanced heart failure, and autonomic dysfunction secondary to neurological diseases. Our analysis primarily focused on examining the effect of renal function on this disorder.
Materials and methods
Study population
A total of 174 subjects, aged 65 years or older, were consecutively enrolled from February to November 2019 at the Geriatric Units of Federico II – University Hospital (Naples, Italy) and San Giovanni di Dio e Ruggi di Aragona – University Hospital (Salerno, Italy). Traditional risk factors for OH, such as bed-rest syndrome, sepsis with hemodynamic instability, advanced heart failure (NYHA IV), and autonomic dysfunction secondary to neurological diseases, as well as the inability to perform the active standing test, were considered as exclusion criteria. Upon admission, all eligible inpatients underwent a comprehensive evaluation, including medical history collection, physical examination, and electrocardiogram (ECG). Medications were documented and categorised into classes such as calcium antagonists, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), beta-blockers, etc. Blood samples for routine chemistry and haematology studies were collected at admission and every 48 h thereafter. Glomerular filtration rate (eGFR) was estimated from serum creatinine levels using the CKD-EPI equation [Citation13]. Chronic Kidney Disease (CKD) was defined by eGFR values <60 mL/min/1.73 m2 calculated from serum creatinine at admission. The reduction in eGFR values was confirmed by previously recorded values lasting at least three months. Patients showing improved serum creatinine levels during hospitalisation (>1.5 times baseline), indicative of acute kidney injury from intercurrent disease, were excluded [Citation14].
Diagnostic protocol for orthostatic hypotension (OH)
The day following admission, between 8 AM and 10 AM, all eligible inpatients underwent postural blood pressure (BP) evaluation in a designated examination room, maintained at an environmental temperature ranging between 23 °C and 24.5 °C. For this purpose, BP and heart rate (HR) were measured using an automatic oscillometric device (OMRON 6) following a 10-minute period of rest in the supine position, and subsequently in the standing position at time intervals of 0, 1, 3, and 5 min. The difference between the supine BP and standing BP values was defined as delta. A decrease in orthostatic systolic BP (SBP) by 20 mmHg and/or diastolic BP (DBP) by 10 mmHg within 5 min of assuming the upright posture was considered diagnostic for OH, thereby encompassing cases of delayed orthostatic hypotension [Citation15].
Statistical analysis
The baseline characteristics of the sample were presented as mean ± standard deviation for continuous variables, while categorical variables were expressed as percentages. Participants were stratified based on the presence or absence of OH and CKD. Differences between continuous variables, according to the presence or absence of OH and CKD, were analysed using the T-test, while dichotomous data were analysed using the chi-square test. Two-way repeated measures ANOVA was employed to analyse OH values at different time points. Multivariate analysis was conducted using a binary logistic regression with OH as the dependent variable. We incorporated variables that were significant at univariate analysis, as well as those recognised as OH risk factors (age, female sex, diabetes).
A p-value <0.05 was considered statistically significant. Data were collected and analysed using SPSS software (version 23.0, SPSS Inc, Chicago, IL).
Results
Between February and November 2019, a total of 174 inpatients were included in the study, with a mean age of 74.4 ± 7.0 years. OH was identified in 46.0% of patients, while a reduction in renal function was observed in 56.3% of patients. Baseline clinical characteristics stratified by OH and CKD are presented in . Patients with OH (OH+) differed from those without OH (OH−) in terms of lower e-GFR (56.1 ± 16.7 mL/min/1.73 m2 vs. 61.1 ± 15.9 mL/min/1.73 m2; p < 0.05) and higher body mass index. Patients with CKD (CKD) exhibited distinct clinical and biochemical characteristics compared to those without CKD (CKD−). CKD + patients were older, had more comorbidities, and as expected, demonstrated a lower e-GFR (47.6 ± 9.2 mL/min/1.73 m2 vs. 73.2 ± 11.9 mL/min/1.73 m2; p < 0.05). Cardiovascular diseases such as hypertension, heart failure, and consequentially, implantable cardioverter-defibrillator (ICD) and pacemaker, were more prevalent in CKD + patients than in CKD − patients. Urea, uric acid, and potassium levels were higher in CKD + patients compared to CKD − patients, as expected ().
Table 1. Clinical and biochemical characteristics stratified for orthostatic hypotension and chronic kidney disease.
Interestingly, the use of furosemide and spironolactone was more common in OH − patients than in OH + patients (44.6% vs. 29.0% and 19.6% vs. 7.7%; p < 0.05), while the use of tramadol was more frequent in OH + patients than in OH − patients (10.9% vs. 1.1%; p < 0.05). In CKD + patients, the use of furosemide, nitrates, and allopurinol was more prevalent compared to CKD − patients ().
Table 2. Drugs assumption stratified for orthostatic hypotension and chronic kidney disease.
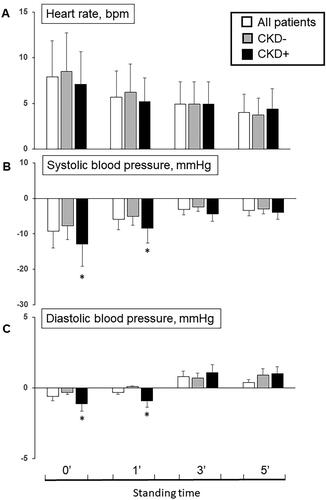
OH + patients exhibited higher supine SBP and DBP values compared to the OH − group (137.4 ± 19.6 mmHg vs 128.3 ± 22.0 mmHg, p < 0.05; 77.2 ± 10.7 mmHg vs 73.6 ± 10.8 mmHg, p < 0.05), along with a lower heart rate (69.2 ± 10.5 bpm vs 72.9 ± 12.4 bpm, p < 0.05). Upon standing (0 min), OH + patients experienced a significant decline in SBP and DBP (115.3 ± 20.5 mmHg vs 128.5 ± 23.4 mmHg, p < 0.05; 71.1 ± 11.7 mmHg vs 77.1 ± 12.7 mmHg, p < 0.05) together with a slight increase in heart rate (76.7 ± 14.3 bpm vs 81.1 ± 13.4 bpm, p < 0.05). After 1 min of standing, OH + patients continued to exhibit a lower SBP and DBP (120.5 ± 20.2 mmHg vs 130.7 ± 24.1 mmHg, p < 0.05; 72.7 ± 10.3 mmHg vs 76.5 ± 12.2 mmHg, p < 0.05) without difference in heart rate between the two groups. At the 3-min mark of standing, a reduction in SBP (124.7 ± 18.6 mmHg vs 132.3 ± 22.5 mmHg, p < 0.05) but not DBP was noted in OH + patients. In both CKD − and CKD + groups, no significant differences in supine SBP, DBP, and heart rate were observed. However, notable reductions in SBP and DBP were observed at 0 min (119.9 ± 21.3 mmHg vs 124.9 ± 24.2 mmHg; 72.8 ± 11.6 mmHg vs 74.8 ± 13.3 mmHg, p < 0.05) and 1 min of standing (121.4 ± 23.5 mmHg vs 127.0 ± 22.0 mmHg; 72.6 ± 11.2 mmHg vs 75.7 ± 12.0 mmHg, p < 0.05) in CKD + patients.
The delta difference in arterial blood pressure and heart rate response from supine to standing positions are illustrated in and . Notably, CKD patients exhibited a greater fall in SBP at 0-min (−12.8 ± 6.3 vs. −7.7 ± 3.2 mmHg) and 1-min (−8.4 ± 4.5 vs. −5.7 ± 2.8 mmHg) compared to patients without CKD during the active standing test (p < 0.05). Similarly, a reduction in DBP at 0-min and 1-min was observed in CKD + patients compared to CKD − patients (p < 0.05).
Figure 1. Difference of heart rate (A), systolic (B) and diastolic (C) blood pressure at 0’, 1’, 3’ and 5’ minutes from lying to standing position among all patients and in patients without (OH−) and with (OH+) orthostatic hypotension (*p < 0.05 OH + vs. OH−).
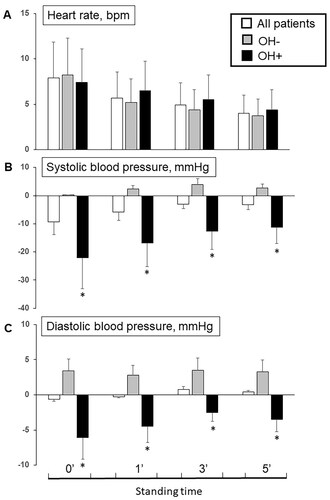
Figure 2. Difference of heart rate (A), systolic (B) and diastolic (C) blood pressure at 0’, 1’, 3’ and 5’ minutes from lying to standing position among all patients and in patients without (CKD−) and with (CKD+) chronic kidney disease (*p < 0.05 CKD + vs. CKD−).
In the multivariate analysis, after incorporating various clinical, pharmacological, and biochemical variables, it was observed that Body mass index (BMI) (OR 1.082; 95% CI 1.004–1.165, p = 0.039) and, notably, CKD (OR 2.426; 95% CI 1.192–4.937; p = 0.014) were clinically associated with OH ()
Table 3. Multivariate binary logistic regression analysis on orthostatic hypotension.
Discussion
Several clinical, biochemical, and pharmacological factors may contribute to the mechanisms leading to OH. The topic is complex and strongly influenced by factors such as the clinical characteristics of the population studied, the diagnostic criteria used, the method of evaluation employed, and the statistical analysis performed. Consequently, despite the abundance of studies in the literature focused on OH risk factors, the results are often inconclusive. In populations with low comorbidity, the causes of OH are more easily identifiable. However, as clinical complexity increases, the presence of multiple factors makes it challenging to determine which of them may be responsible for OH.
In this study, various clinical, biochemical, and pharmacological factors were analysed to assess their contribution to OH. CKD and BMI emerged as independent clinical variables associated with OH in elderly patients with comorbidities.
OH and CKD
The correlation between blood pressure and kidney function is well established in medical literature. Previous studies have emphasised the impact of supine hypertension on the reduction of glomerular filtration rate (GFR). Although the exact mechanism is not fully understood, it involves vascular function and subsequent structural changes in the kidneys [Citation16]. Conversely, the relationship between postural changes in blood pressure and kidney function has received less attention. However, a large sample study found a correlation between OH and renal failure [Citation17]. This relevant study encompassed data from individuals across a wide age range but did not incorporate other biochemical factors or the contribution of medications into the multivariable analysis.
In the SPRINT trial, participants with OH were more likely to have an estimated GFR (eGFR) < 45 mL/min/1.73 m2 (16%) compared to those without OH (9%), and eGFR < 45 mL/min/1.73 m2 was independently associated with a greater decrease in blood pressure upon standing [Citation18]. More recently, another smaller study, while not demonstrating a higher incidence of OH in patients with CKD compared to the control group, found that CKD is a strong predictor for OH when accompanied by advanced comorbidities and polypharmacy [Citation19].
In our study, OH appears to be strongly associated with renal impairment more than with other clinical and pharmacological factors. However, a singular reason for this association is difficult to definitively establish. Renal failure in older adults, more so than in other age groups, may result from a multitude of factors such as dehydration, the effects of medications, and comorbidities. While water and electrolyte balance are typically maintained in the initial stages of CKD and in the ageing kidney, impairment of regulatory and adaptive mechanisms to cope with acute fluid and electrolyte disturbances is observed. Additionally, renal concentrating ability is significantly reduced, leading to a decline in water-conserving capacity by the ageing kidney, as evidenced by reduced maximum urinary osmolality after dehydration [Citation20]. These alterations in volume homeostasis may contribute to the development of OH even in the initial stages of CKD.
Peripheral neuropathy is a common complication of end-stage CKD and is present in half of patients receiving dialysis [Citation21]. The development of uraemic neuropathy has been previously linked to the retention of neurotoxic molecules. However, in patients with CKD, quantifying the contribution of uraemia versus other coexisting causes of neuropathy, such as diabetes, can be challenging. Recent studies have shown that nerves in uraemic patients exist in a chronically depolarised state that correlates with serum potassium concentration [Citation22]. Furthermore, there is ample evidence for enhanced sympathetic activity in CKD patients, partly attributed to the frequent presence of anaemia [Citation23]. The presence of hyperkinetic circulation and alterations in nerve depolarisation can contribute to abnormal cardiovascular responses to postural changes in patients with CKD. However, it should be noted that in our study a preserved chronotropic response during the postural blood pressure evaluation is maintained, indicating a non-neurogenic origin of OH in CKD [Citation24].
In conclusion, although our study does not demonstrate a causal relationship between CKD and OH, several factors may contribute to this association including alterations in volume control, hyperkinetic and hyperadrenergic states associated with secondary anaemia, as well as neuropathy induced both by uraemic state or by associated comorbidities.
OH and BMI
Even within this field of research, results are not entirely consistent. In our study, we observed OH to be associated with higher BMI. However, this finding contrasts with some previous data [Citation25]. The discrepancy could be attributed to differences in the study population. Specifically, our study excluded patients affected by PD and dementia, which were included in the studies. In PD and neurodegenerative diseases, autonomic dysfunction plays a role in BP control, and a lower BMI may result in additional reduction in sympathetic excitability. Conversely, orthostatic tolerance is reduced in young obese individuals compared to age- and sex-matched non-obese subjects and is inversely related to BMI [Citation26]. Valensi et al. found a correlation between obesity and cardiovascular autonomic dysfunction in non-diabetic patients [Citation27]. Accordingly, our results may underline the role of delayed cardiovascular response to initial postural stress in patients with higher BMI.
Obesity is associated with elevated basal sympathetic activity and reduced autonomic function. Studies in obese individuals demonstrate reduced baroreflex sensitivity, which can be reversed with weight loss. Thus, higher resting HR in obese individuals may diminish the reserve to further HR increases to compensate against orthostasis or similar hypotensive challenges, and the findings of our study support these data [Citation28].
OH and drugs
Other studies have suggested an association between OH and specific drugs, such as α-blockers and/or calcium channel blockers, although the results remain inconclusive [Citation11]. In our study population, we observed that the use of furosemide and spironolactone was more common among OH − subjects compared to OH + subjects. Therefore, drugs often considered as potential risk factors for OH did not appear to contribute to the decline in blood pressure in our study. This seemingly paradoxical result, contradicting previous studies, may be attributed either to the exclusion criteria of our study (excluding admissions for severe heart failure) or to potential biases related to the geriatric approach (deprescribing) applied in our sample.
Furthermore, the use of cardioactive drugs does not appear to be correlated with the onset of OH in the elderly population when used correctly [Citation29]. In individuals with heart failure, the pharmacodynamic effects of these drugs may outweigh their adverse effects, such as OH, by restoring hemodynamic stability. Finally, we observe that the use of tramadol is significantly higher in OH + patients, although this result was not confirmed in the multivariate analysis.
Study limitations
Our study provides significant findings regarding the association between OH and CKD in a comorbid elderly population. However, it is essential to consider some study limitations, including the impact of therapy. Firstly, the exclusion criteria, which omitted patients with neurodegenerative diseases like Parkinson’s disease and dementia, as well as conditions known to influence the autonomy of the nervous system and blood pressure regulation, may have limited the representativeness of our study population. Secondly, the exclusion of severe heart failure, often linked with significant disturbances in blood pressure regulation, might have introduced bias into patient selection. Thirdly, medications analysis was conducted independently by dosage, treatment duration, and potential pharmacological interactions. All these findings could influence the OH risk. Furthermore, although we did not find a relationship for some medications known to be OH risk factors, it is important to underline that drugs were examined one by one and not in combinations (e.g. Furosemide + beta-blockers). This approach might have overlooked potential synergistic effects or interactions between medications and OH. Additionally, we were unable to determine the causal direction of the relationship between CKD and OH or to exclude other confounding factors that may influence our results. Furthermore, it is important to highlight that cystatin was not utilised for eGFR assessment, a better method to assess the renal function especially in elderly patients with muscle mass lost. Additionally, albuminuria was not evaluated in our patients as an index of CKD. Lastly, polypharmacy, a potential explanation of the relationship between OH and CKD was not examined.
Conclusions
In our study, we found that OH was associated to CKD. Our findings emphasise the importance of OH diagnosis among patients with CKD. Early identification of OH in CKD patients can timely lead to manage it and improve OH-related clinical outcomes.
Ethical statement
The present study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the University of Naples Federico II “Carlo Romano” (reg. n. 25/12). Each participant signed a written informed consent form.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Tzur I, Izhakian S, Gorelik O. Orthostatic hypotension in internal medicine wards. Curr Med Res Opin. 2019;35(6):947–955. doi:10.1080/03007995.2018.1546679.
- Kario K, Tobin JN, Wolfson LI, et al. Lower standing systolic blood pressure as a predictor of falls in the elderly: a community based prospective study. J Am Coll Cardiol. 2001;38(1):246–252. doi:10.1016/s0735-1097(01)01327-4.
- Galizia G, Convertino G, Testa G, et al. Transient ischemic attack caused by delayed orthostatic hypotension in an elderly hypertensive patient. Geriatr Gerontol Int. 2012;12(3):565–567. doi:10.1111/j.1447-0594.2011.00795.x.
- Mussi C, Galizia G, Abete P, et al. Unexplained falls are frequent in patients with fall-related injury admitted to orthopaedic wards: the UFO study (unexplained falls in older patients). Curr Gerontol Geriatr Res. 2013;2013:928603.
- Ungar A, Mussi C, Ceccofiglio A, et al. Etiology of syncope and unexplained falls in elderly adults with dementia: syncope and dementia (SYD) study. J Am Geriatr Soc. 2016;64(8):1567–1573. doi:10.1111/jgs.14225.
- Masaki KH, Schatz IJ, Burchfiel CM, et al. Orthostatic hypotension predicts mortality in elderly men: the Honolulu Heart Program. Circulation. 1998;98(21):2290–2295. doi:10.1161/01.cir.98.21.2290.
- Ungar A, Galizia G, Morrione A, et al. Two-year morbidity and mortality in elderly patients with syncope. Age Ageing. 2011;40(6):696–702. doi:10.1093/ageing/afr109.
- Luukinen H, Koski K, Laippala P, et al. Prognosis of diastolic and systolic orthostatic hypotension in older persons. Arch Intern Med. 1999;159(3):273–280. doi:10.1001/archinte.159.3.273.
- Luukinen H, Koski K, Laippala P, et al. Orthostatic hypotension and the risk of myocardial infarction in the home-dwelling elderly. J Intern Med. 2004;255(4):486–493. doi:10.1111/j.1365-2796.2004.01313.x.
- Bradley JG, Davis KA. Orthostatic hypotension. Am Fam Physician. 2003;68(12):2393–2398.
- Rivasi G, Rafanelli M, Mossello E, et al. Drug-related orthostatic hypotension: beyond anti-hypertensive medications. Drugs Aging. 2020;37(10):725–738. doi:10.1007/s40266-020-00796-5.
- Jacob G, Robertson D, Mosqueda-Garcia R, et al. Hypovolemia in syncope and orthostatic intolerance role of the renin-angiotensin system. Am J Med. 1997;103(2):128–133. doi:10.1016/s0002-9343(97)00133-2.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006.
- Kidney Disease: improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Inter. 2012;2:1–138.
- Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69–72. doi:10.1007/s10286-011-0119-5.
- Wang F, Zhao H, Yang C, et al. Association of blood pressure in the supine position with target organ damage in subjects over 60 years old. J Int Med Res. 2017;45(1):123–133. doi:10.1177/0300060516677175.
- Canney M, O’Connell MDL, Sexton DJ, et al. Graded association between kidney function and impaired orthostatic blood pressure stabilization in older adults. J Am Heart Assoc. 2017;6(5):e005661.
- Townsend RR, Chang TI, Cohen DL, et al. Orthostatic changes in systolic blood pressure among SPRINT participants at baseline. J Am Soc Hypertens. 2016;10(11):847–856. doi:10.1016/j.jash.2016.08.005.
- Januszko-Giergielewicz B, Gromadziński L, Dudziak M, et al. Orthostatic hypotension in asymptomatic patients with chronic kidney disease. Medicina (Kaunas). 2019;55(4):113. doi:10.3390/medicina55040113.
- Glassock RJ, Rule AD. Aging and the kidneys: anatomy, physiology and consequences for defining chronic kidney disease. Nephron. 2016;134(1):25–29. doi:10.1159/000445450.
- Zifko U, Auinger M, Albrecht G, et al. Phrenic neuropathy in chronic renal failure. Thorax. 1995;50(7):793–794. doi:10.1136/thx.50.7.793.
- Krishnan AV, Phoon RK, Pussell BA, et al. Neuropathy, axonal Na±/K ± pump function and activity-dependent excitability changes in end-stage kidney disease. Clin Neurophysiol. 2006;117(5):992–999. doi:10.1016/j.clinph.2006.02.002.
- Januszko-Giergielewicz B, Dębska-Ślizień A, Górny J, et al. Dobutamine stress echocardiography in the diagnosis of asymptomatic ischemic heart disease in patients with chronic kidney disease–review of literature and single-center experience. Transplant Proc. 2015;47(2):295–303. doi:10.1016/j.transproceed.2014.11.034.
- Norcliffe-Kaufmann L, Kaufmann H, Palma J-A, et al. Orthostatic heart rate changes in patients with autonomic failure caused by neurodegenerative synucleinopathies. Ann Neurol. 2018;83(3):522–531. doi:10.1002/ana.25170.
- Nakamura T, Suzuki M, Ueda M, et al. Lower body mass index is associated with orthostatic hypotension in Parkinson’s disease. J Neurol Sci. 2017;372:14–18. doi:10.1016/j.jns.2016.11.027.
- Grassi G, Seravalle G, Colombo M, et al. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation. 1998;97(20):2037–2042. 97 doi:10.1161/01.cir.97.20.2037.
- Valensi P, Thi BN, Lormeau B, et al. Cardiac autonomic function in obese patients. Int J Obes Relat Metab Disord. 1995;19(2):113–118.
- Lee JF, Harrison ML, Christmas KM, et al. Elevated resting heart rate and reduced orthostatic tolerance in obese humans. Clin Auton Res. 2014;24(1):39–46. doi:10.1007/s10286-013-0222-x.
- Cautela J, Tartiere J-M, Cohen-Solal A, et al. Management of low blood pressure in ambulatory heart failure with reduced ejection fraction patients. Eur J Heart Fail. 2020;22(8):1357–1365. doi:10.1002/ejhf.1835.

