Abstract
Objective
Some brachial cuffs for oscillometric blood pressure (BP) measurement are claimed to cover a wide range of upper-arm circumferences; however, their validation is rarely conducted. Our aim was to compare oscillometric BP measurements obtained with a universal cuff with those obtained with an appropriately sized cuff.
Methods
We utilised the Microlife B6 Connect monitor, conducting oscillometric BP measurements in a random sequence with both a universal cuff (recommended for arm circumferences from 22 to 42 cm) and an appropriately sized cuff (medium for circumference 22–32 cm and large for 32–42 cm). We included 91 individuals with an arm circumference of 22–32 cm and 64 individuals with an arm circumference of 32–42 cm.
Results
For arm circumferences > 32 cm, systolic and diastolic BP measured with the universal cuff was higher than that measured with the large cuff (systolic 6.4 mmHg, 95% confidence interval [CI]). 3.9–8.8, diastolic 2.4 mmHg, 95%CI, 1.2–3.7, p < 0.001 for both). Overestimation of BP with the universal cuff was statistically significant after correcting for the sequence of measurements. No statistical difference was found between the universal cuff and medium cuff for circumferences in the 22–32 cm range. The bladder size in the universal cuff matched the dimensions of the medium-sized cuff; however, the cuff was larger.
Conclusion
Overestimation of BP measured with a universal cuff in persons with large arm circumferences is clinically important. It poses the risk of unnecessary initiation or intensification of antihypertensive medication in persons using the universal cuff.
PLAIN LANGUAGE SUMMARY
What is the context?
Clinical guidelines recommend individualisation of the size of the cuff used for blood pressure measurement according to the circumference of the upper arm.
Many blood pressure monitors are sold with a single “universal” cuff claimed to cover a wide range of upper arm sizes.
We compared blood pressure obtained with the Microlife B6 Connect monitor and a “universal” cuff with the results obtained with individual sized cuffs (medium size for arm circumference between 22 and 32 cm and large size for arm circumference between 32 and 42 cm).
What is new?
In persons with large upper arm circumference is the systolic blood pressure 6.4 mmHg higher and the diastolic blood pressure 2.4 mmHg higher with the universal cuff than with the individual-sized large cuff.
What is the impact?
The universal cuff overestimates blood pressure in persons with large arm circumference.
Introduction
Accurate measurement of blood pressure (BP) is crucial for the diagnosis and management of hypertension. The use of an appropriately sized cuff and bladder is a prerequisite for accurate measurements. O’ Brien extensively reviewed this subject in 1996 [Citation1]. Originally rooted in experiences with auscultatory measurements, extensive documentation supports the notion that a bladder size that is too small in comparison to the upper arm circumference (“undercuffing”) leads to overestimation of BP because the cuff pressure surpasses the pressure at the level of the artery. All guidelines advocate the use of a larger bladder size for arms with larger circumferences, although specific recommendations for dimensions may vary. Apart from specific cases, such as children and pregnancy, oscillometry is the prevailing technique for office BP measurement in the twenty first century. Auscultatory BP measurements require initial total compression of the brachial artery, which creates a no-flow state. In contrast, oscillometry relies on analysing pressure oscillations in the cuff across a pressure interval around the mean BP, corresponding to the peak amplitude of the oscillations [Citation2]. Several attempts have been made to use a conical cuff with standard bladder dimensions for a wide range of upper arm sizes. Manufacturers claim that an internal algorithm can compensate for varying oscillometric signals caused by different arm circumferences [Citation2–4]. The 2023 European Society of Hypertension (ESH) guidelines recommend a bladder length of 75–100% and a bladder width of 37–50% of the middle upper arm circumference for auscultatory measurements [Citation5]. For oscillometric measurements, the guidelines primarily recommend adherence to device instructions. The 2021 ESH guidelines for out-of-office BP monitoring echo this recommendation but add a note that: “some devices have “wide range” cuffs which fit the arms of most adults but require proper validation” [Citation6]. As of 2023, the criteria for the design and function of BP devices include validation of both the devices and the cuffs they utilise [Citation7].
We recently purchased several Microlife B6 Connect oscillometric BP monitors (Widnau, Switzerland). They were all delivered with a so-called universal conical cuff with a semi-rigid shield, and the manufacturer’s instructions stated that these cuffs, designated M-L, could be used for all arm circumferences between 22 and 42 cm. Documentation on the performance of the universal cuff in persons with obese arms, using the Microlife BP A100, was restricted to comparison solely with auscultatory measurements and within a limited number of persons [Citation4,Citation8].
The aim of the present clinical quality control study was to compare oscillometric BP measurements with the universal cuff with those obtained with a medium cuff (for arm circumference 22–32 cm) and a large cuff (32–42 cm), while applying a pragmatic study protocol that could be fitted into a standard clinical routine setting.
Materials and methods
The Microlife B6 Connect is presented by the company as a "a consumer" product, that is, for home BP monitoring. In our clinic, this monitor is used for both office and home BP measurement. Starting in January 2023, we conducted office BP measurements using this monitor in patients scheduled for visits to endocrinological or hypertension clinics at the Silkeborg Regional Hospital, Denmark. Measurements were performed using monitors placed at each of the seven consultation offices used for this purpose. All patients provided informed consent, and their BPs were measured using a universal (M-L) cuff. Patients with an arm circumference between 22 and ≤32 cm were assessed with the medium cuff, while patients with a circumference between >32 cm and ≤42 cm were assessed with a large-sized cuff. M and L cuffs were microlife cuffs purchased from the Danish provider of the Microlife BP monitor. Each patient underwent three measurements conducted in a randomised order using both the universal cuff and the cuff corresponding to their arm circumference. The monitor displays the average of three measurements. In cases of large differences between the individual readings, the monitor presents a weighted average using the so-called “Microlife Average Mode” [Citation9].
Using data from our previous study, we found that the standard deviation (SD) of two single-arm sequential systolic BP examinations (calculated as the average of three measurements) was ± 5.4 mmHg [Citation10]. With a minimal relevant difference of 3 mmHg, a type I error of 5%, and a type II error of 15%, we calculated that 58 persons should have their BP measured in each category of arm circumference.
Statistical analysis
Data are presented as the mean ± SD. BP data were compared using Student’s paired t-test. The distribution of the first measurement with the universal cuff between the two categories of arm circumference and the distribution of sex was assessed using the Chi2 test. Multiple regression was performed with the difference in BP measured with universal cuff-BP measured with the M or L cuff as the dependent variable and the sequence of measurement and category of arm circumference (22 to ≤ 32 or 32 to ≤ 42 cm) as independent variables.
Results
The study was terminated when 155 persons (age 60.6 ± 14.1 years, 112 males) had been included. Of these, 91 had an arm circumference of 22–32 cm (28.8 ± 2.2 cm) and 64 had an arm circumference of 32–42 cm (34.8 ± 2.3 cm). No statistical significant differences was found between the group with arm circumference ≤ 32 cm and the group with circumference > 32 cm with respect to sequence of measurement with universal or appropriate size cuff or clinical data () In persons with an arm circumference > 32 cm, systolic and diastolic BP were significantly higher when measured with the universal cuff than with the appropriately sized L cuff (). No statistical difference was noted between the BP measured with the universal cuff and the M-sized cuff for persons with an arm circumference < 32 cm (). Adjusting for the order of measurement did not change the results (). A Bland-Altman plot of systolic BP (universal cuff) - systolic BP (appropriately sized cuff) vs. the average BP is shown in .
Figure 1. The Bland–Altman plot for persons with a mid-upper arm circumference > 32 cm (n = 64) illustrates the difference between systolic blood pressure measured with the universal cuff (Sy univ) and systolic blood pressure measured with an appropriately sized cuff (Sy appr) plotted against their average. The dashed lines represent mean + 1.96 SD (25.7 mmHg), mean (6.4 mmHg) and mean – 1.96 SD (−13.0 mmHg).
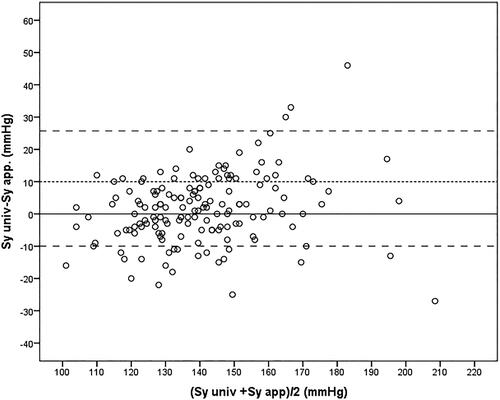
Table 1. Clinical characteristics and sequence of measurements for the persons in the two groups of arm circumference.
Table 2. Comparison of blood pressure measured with the universal cuff and an appropriately sized cuff, irrespective of sequence of measurements.
Table 3. Multiple regression with difference in blood pressure (universal cuff – appropriately sized cuff) as dependent variable and sequence of measurement and category of arm circumference as dependent variable.
Finally, we measured the size of the cuffs and bladders (). In the M and L cuffs, the bladder was easily removed as it was placed in an open pocket in the cuff. The bladder in the universal cuff is sealed within two layers of fabric and can be removed only when the cuff is opened with scissors. In the M and universal cuffs, the bladder size had similar dimensions, while the cuff itself was wider and longer than both the M and L cuffs. The cuff length was measured as the largest circumference, allowing full overlap of the Velcro patches.
Table 4. Bladder size, maximal cuff circumference allowing full overlap of Velcro hooks, cuff width and dimension of flexible plastic shield.
Discussion
We found a significant overestimation of systolic BP in persons with an arm circumference > 32 cm when using the universal cuff compared to the appropriately sized L cuff. This discrepancy has clear clinical implications, posing the risk of inaccurate hypertension diagnosis and unnecessary initiation or intensification of antihypertensive medication.
The clinical characteristics related to arterial stiffness (age, sex, antihypertensive treatment, diabetes status and LDL cholesterol) are evenly distributed between the two groups of arm circumference. Although we have no information about smoking, it seems fair to conclude that the higher BP measured with the universal cuff compared to the appropiately sized cuff in persons with arm circumference >32 cm is related to the arm circumference per se, rather than altered arterial properties. Two previous studies from 2009 and 2010 compared oscillometric measurements using a universal cuff against auscultatory measurements using an appropriately sized cuff. These studies encompassed individuals with a wide range of arm circumferences [Citation4,Citation8]. None of these studies reported any statistically significant differences in BP; however, the studies were underpowered because they included only 16 persons with an arm circumference < 30 cm and 17 persons with an arm circumference > 30 cm. The authors concluded that the universal cuff provides accurate BP readings over a wide range of arm circumferences, and acknowledged that further studies with an adequate number of patients are needed. The universal cuff was validated in a 2021 study involving the Microlife BPA100 Plus device and passed the validation criteria. However, in this study, among the 37 individuals with an arm circumference between 32.5 and 42.0 cm, the specific results for this group were not specified [Citation11].
Given that the bladder dimensions were comparable between the universal cuff and the medium-sized cuff in our study, the observed overestimation of oscillometric BP in arms with a circumference of > 32 cm was not unexpected. A larger dimension of the cuff fabric and a conical semi-rigid shield in the universal cuff apparently cannot compensate for a bladder size matching the arm circumference in the range of 22 to 32 cm. Similarly, it has been shown that oscillometric systolic BP measured with another oscillometric device is overestimated by 4.8 mmHg if a medium-sized cuff is used in persons with an arm circumference demanding a large-size cuff [Citation12]. We advocate that manufacturers of BP monitors consistently provide information on bladder size within their cuffs. Additionally, it is crucial that they thoroughly document the performance of their monitors and recommend cuffs specifically for different categories of arm circumferences [Citation2,Citation3,Citation13].
The strength of our study lies in the much larger number of patients with an arm circumference > 32 cm compared with previous studies, and the fact that we, unlike previous studies, was not funded by the manufacturer. This study has several limitations. It is unknown whether similar results are applicable to many other manufacturers of BP monitors with a universal cuff [Citation14]. The study design was simple and pragmatic, allowing a relatively large number of patients to be examined during routine clinical contact. The recommended study protocol was not applied to validate BP monitoring [Citation15]. This would require a complex protocol with a total of nine single-arm serial measurements with both types of cuffs and auscultatory measurements by two trained investigators using a double stethoscope [Citation16,Citation17]. Therefore, we lack results from auscultatory measurements conducted with an appropriately sized bladder as a reference. Instead, our comparison was based on oscillometric measurements by using an appropriately sized cuff. Although this may be considered a limitation, it prompts consideration of whether auscultatory measurements should remain the only valid reference for assessing the performance of oscillometric devices when considering various cuff size recommendations. Auscultatory measurements are prone to human error and, importantly, most, if not all, large-scale clinical hypertension studies conducted over the past decade have used oscillometric measurements with appropriately sized cuffs.
We measured BP in triplicate using each cuff type, but the final result was a weighted average determined by a proprietary algorithm. While the lack of full insight into this algorithm is unsatisfactory, it is unlikely that it would differentially impact the measurements with different cuffs.
In conclusion, we have reported a clinically significant overestimation of oscillometrically measured BP among persons with large arm circumferences when using a universal cuff compared to oscillometric BP readings obtained with an appropriately sized cuff. Our results underline the importance of using an appropriately sized cuff to obtain accurate BP measurement. This knowledge should also be disseminated to private individuals using a universal cuff for home BP monitoring.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- O’Brien E. Review: a century of confusion; which bladder for accurate blood pressure measurement? J Hum Hypertens. 1996;10(9):1–7.
- Palatini P, Frick GN. Cuff and bladder: overlooked components of BP measurement devices in the modern era? Am J Hypertens. 2012;25(2):136–138. doi: 10.1038/ajh.2011.213.
- Li J, Frick G, Herberigs K, et al. Industry perspectives on the global use of validated blood pressure measuring devices. J Hum Hypertens. 2023;37(2):130–133. doi: 10.1038/s41371-022-00717-6.
- Bonso E, Dorigatti F, Palatini P. Accuracy of the BP A100 blood pressure measuring device coupled with a single cuff with standard-size bladder over a wide range of arm circumferences. Blood Press Monit. 2009;14(5):216–219. doi: 10.1097/MBP.0b013e328330d3f8.
- Mancia G, Kreutz R, Brunström M, et al. 2023 ESH guidelines for the management of arterial hypertension The task force for the management of arterial hypertension of the European society of hypertension: endorsed by the International Society of hypertension (ISH) and the European renal association (ERA). J Hypertens. 2023;41(12):1874–2071. doi: 10.1097/HJH.0000000000003480.
- Stergiou GS, Palatini P, Parati G, et al. 2021 European society of hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens. 2021;39(7):1293–1302. doi: 10.1097/HJH.0000000000002843.
- Stergiou GS, Parati G, Kollias A, et al. Requirements for design and function of blood pressure measuring devices used for the management of hypertension: consensus statement by the european society of hypertension working group on blood pressure monitoring and cardiovascular variability and STRIDE BP. J Hypertens. 2023;41(12):2088–2094. doi: 10.1097/HJH.0000000000003482.
- Bonso E, Saladini F, Zanier A, et al. Accuracy of a single rigid conical cuff with standard-size bladder coupled to an automatic oscillometric device over a wide range of arm circumferences. Hypertens Res. 2010;33(11):1186–1191. doi: 10.1038/hr.2010.146.
- Wilton A, De Greef A, Shennan A. Rapid assessment of blood pressure in the obstetric day unit using microlife MaM technology. Hypertens Pregnancy. 2007;26(1):31–37. doi: 10.1080/10641950601146558.
- Krogager C, Laugesen E, Rossen NB, et al. Evaluation of interarm blood pressure differences using the microlife WatchBP office in a clinical setting. Blood Press Monit. 2017;22(3):161–165. doi: 10.1097/MBP.0000000000000246.
- Beime B, Bramlage C, Krüger R, et al. Validation of the microlife BP B3 AFIB upper arm blood pressure monitor in adults and adolescents according to the ANSI/AAMI/ISO 81060-2:2019 protocol. Blood Press Monit. 2021;26(4):299–304. doi: 10.1097/MBP.0000000000000530.
- Ishigami J, Charleston J, Miller ER, 3rd, et al. Effects of cuff size on the accuracy of blood pressure readings: the cuff(SZ) randomized crossover trial. JAMA Intern Med. 2023;183(10):1061–1068. doi: 10.1001/jamainternmed.2023.3264.
- O’Brien E, Alpert BS, Stergiou GS. Accurate blood pressure measuring devices: influencing users in the 21st century. J Clin Hypertens. 2018;20(7):1138–1141. doi: 10.1111/jch.13278.
- Shahi S, Jackson SL, Streeter TE, et al. Cuff size variation Across manufacturers of home blood pressure devices: a current patient dilemma. Am J Hypertens. 2023;36(10):532–535. doi: 10.1093/ajh/hpad060.
- Stergiou GS, Palatini P, Asmar R, et al. Recommendations and practical guidance for performing and reporting validation studies according to the universal standard for the validation of blood pressure measuring devices by the association for the advancement of medical instrumentation/european society of hypertension/international organization for standardization (AAMI/ESH/ISO). J Hypertens. 2019;37(3):459–466. doi: 10.1097/HJH.0000000000002039.
- Stergiou GS, Alpert B, Mieke S, et al. A universal standard for the validation of blood pressure measuring devices: association for the advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) collaboration statement. J Hypertens. 2018;36(3):472–478. doi: 10.1097/HJH.0000000000001634.
- Non-invasive sphygmomanometers- Part 2: Clinical investigation of intermittent automated measurement type. (ISO 810602:2018). Available from: https://www.iso.org/standard/73339.html.

