Abstract
Purpose
In a prospective open study, with intervention, conducted in Primary Health Care Units by General Practitioners (GPs) in Portugal, the effectiveness of a single pill of candesartan/amlodipine (ARB/amlodipine), as the only anti-hypertension (anti-HTN) medication, in adult patients with uncontrolled HTN (BP > 140/or > 90 mm Hg), either previously being treated with anti-HTN monotherapies (Group I), or combinations with hydrochlorothiazide (HCTZ) (Group II), or not receiving medication at all (Group III), was evaluated across 12-weeks after implementation of the new therapeutic measure.
Materials and methods
A total of 118 GPs recruited patients with uncontrolled HTN who met inclusion/exclusion criteria. Participants were assigned, according to severity, one of 3 (morning) fixed combination candesartan/amlodipine dosage (8/5 or 16/5 or 16/10 mg/day) and longitudinally evaluated in 3 visits (v0, v6 and v12 weeks). Office blood pressure was measured in each visit, and control of HTN was defined per guidelines (BP< 140/90 mmHg).
Results
Of the 1234 patients approached, 752 (age 61 ± 10 years, 52% women) participated in the study and were assigned to groups according to previous treatment conditions. The 3 groups exhibited a statistically significant increased control of blood pressure after receiving the fixed combination candesartan/amlodipine dosage. The overall proportion of controlled HTN participants increased from 0,8% at v0 to 82% at v12. The mean arterial blood pressure values decreased from SBP= 159.0 (± 13.0) and DBP= 91.1 (± 9.6) at baseline to SBP= 132,1 (± 11.3) and DBP= 77,5 (± 8.8) at 12 weeks (p < 0.01). Results remained consistent when controlling for age and sex.
Conclusion
In patients with uncontrolled HTN, therapeutic measures in accordance with guidelines, with a fixed combination candesartan/amlodipine, allowed to overall achieve HTN control at 12 weeks in 82% of previously uncontrolled HTN patients, reinforcing the advantages of these strategies in primary clinical practice.
PLAIN LANGUAGE SUMMARY
What is the context?
Arterial hypertension (HTN) represents the main risk factor for cause of death from cardiovascular disease (CV). Adequate control of hypertension reduces CV risk and significantly prevents CV events and associated morbidity and mortality. This requires patients’ adherence and persistence in implemented treatment and the achievement of tension targets that are related to the reduction of CV risk. The latest international recommendations indicate that hypertension control is insufficient in most countries. In Portugal, hypertension control is <43% and a significant number of patients treated do not comply with the recommendations.
What is new?
In a prospective, interventional, and multicentre study, carried out by General Practitioners (GPs) in Primary Health Care Units across Portugal, the objective was to determine (i) whether the presence of uncontrolled hypertension results from non-compliance with the provisions of the recommendations and the Integrated Care Process (PAI) of the Direção Geral de Saúde (DGS), i.e. inappropriate use of monotherapies or inadequate low doses of combinations of antihypertensives, and (ii) whether the adjustment of hypertension therapies, favouring the schemes provided in the recommendations, allows adequate control of arterial hypertension, in previously uncontrolled patients, when these are closely monitored in a 12-week time period.
What is the impact?
When the guidelines’ therapeutic protocol is followed, as established for each identified group of patients (monotherapy, hydrochlorothiazide, and no medication), results indicate a marked and statistically significant improvements in both SBP and DBP values and hypertension control across time.
Background
Cardiovascular illnesses are widely acknowledged as the leading cause of mortality on a global scale, with arterial hypertension being recognised as a significant risk factor in the development of and progression of cardiovascular disease. The efficacy of hypertension (HTN) medication in mitigating the occurrence of cardiovascular events, such as stroke, coronary disease, cardiac and renal failure, is recognised [Citation1].
National surveys that measure the prevalence, awareness, treatment, and control of HTN are crucial for evaluating its impact on countries, regions, and communities (see [Citation2]), with global HTN disparities large across these indicators [Citation3]. On this, research has shown notable enhancements in the prescription rates of anti-HTN drugs over a span of ten years, resulting in a corresponding reduction in cardiovascular events associated with hypertension (see [Citation4]).
In Portugal, HTN control is insufficient. Specifically, the PHYSA study [Citation4] revealed that the control of HTN in the Portuguese adult population was < 43% and that a significant number of treated patients were non-compliers of the proposed therapeutics. More so, in Portuguese Primary Health Care Centres, 60% of HTN patients, on average, are not controlled [Citation4–7]. On this, the excessive use of monotherapies in Portugal, to the detriment of anti-HTN combinations, may also contribute to lower levels of HTN control in Portugal. In this regard, both several international guidelines [Citation8–11] and the Portuguese ‘Processo Assistencial Integrado’ (Integrated Care Process, PAI) for the ‘Risco cardiovascular (RCV) do Adulto’ (Hypertension Risk in the Adult) of the Direção Geral de Saúde (Portuguese Health Directorate, DGS) [Citation5], propose to initiate the treatment of the majority of hypertensive patients with first-line fixed medication associations, as soon as possible, with first low doses of two components, and increasing the dosage steadily if HTN control is not achieved. On the contrary, due to the heterogeneity of mechanisms underlying HTN, monotherapy is not generally recommended, given that it confers an inadequate control in most patients (and it is only indicated for a small minority of older and/or clinically frail patients). Regarding the first-line anti-hypertensive medications, the hydrochlorothiazide thiazide diuretic, which can be administered either in isolation or in association, is revealed to be inferior in the control of blood pressure, and protection of cardiovascular events, compared to other thiazide-like diuretics (indapamide and chlorthalidone) [Citation12, Citation13], as well as relatively inferior to CCB [Citation14], and was thus withdrawn from the HTN first-line therapeutics and the initial treatment options.
Here, the present study, termed ‘GPHT-PT Study’, sought to strictly comply with HTN medical practice guidelines, particularly at the level of Primary Health Care. The study aimed to verify if in hypertensive patients, either not controlled non-treated, or not controlled treated with therapeutic regimens not aligned with the guidelines, a simple adjustment to the HTN therapeutic strategy allowed to obtain an adequate control of arterial HTN. The research questions were: (i) if the presence of uncontrolled HTN in a large number of patients in Primary Health Care Centres in Portugal results from non-compliance with both multiple international guidelines [Citation8–11] and the Portuguese PAI DGS [Citation5] (i.e. inappropriate use of monotherapies or inadequate low doses of anti-hypertensive associations); (ii) if adjusting HTN therapeutics, by privileging the schemes recommended in the guidelines, here, specifically, fixed combination candesartan/amlodipine dosage 8/5 or 16/5 or 16/10 mg/day, allowed obtaining adequate control of arterial high blood pressure in patients not previously controlled.
Materials and methods
Primary and secondary study goals
The primary goal of the GPHT-PT Study was to evaluate in patients 18-75 years of age, without CV events, and with uncontrolled HTN (BP > 140/or > 90 mm Hg), the effectiveness of a single pill combination of one of 3 doses of candesartan + amlodipine (candesartan/amlodipine, 8/5 or 16/5 or 16/10 mg) as the single anti-HTN medication according to the HTN severity. The secondary goal was to evaluate patient adherence to a systematic hypertension control programme in Primary Care.
Study design
Across Portugal, a total of 118 General Practitioners (GPs) were instructed to recruit the first consecutive 25 to 30 patients without CV events, and with uncontrolled HTN, who met the inclusion and none of the exclusion study criteria. Study enrolment took place across 9 months, starting in November 2021. In the first visit (v0, baseline), study participants were assigned by clinical evaluation to one of 3 study groups according to their current treatment status: Group I, treated with anti-HT monotherapies; Group II, treated with combinations with hydrochlorothiazide (HCTZ); Group III, not taking any anti-HTN medication. Then, patients were clinically assessed by the GP and, according to severity at v0, medicated with one of 3 fixed combination candesartan/amlodipine dosage—8/5 or 16/5 or 16/10 mg/day (morning)—following clinical/HTN guidelines to determine the correct dose for each patient (low, medium, or high, respectively).
Study participants in each of the groups were maintained in their study group throughout the study duration, and were evaluated in 2 more moments [week 6 (v6) and week 12 (v12) after starting treatment]. In each evaluation, Office Blood Pressure (OBP) was measured according to guidelines control of HTN = BP< 140/90 mmHg, mean of 2 of 3 records). The study was based on four international practice guidelines for HTN treatment, namely the: American College of Cardiology/American Heart Association 2017 [Citation8]; European Society of Cardiology (ESC) and European Society of Hypertension (ESH) [Citation9]; 2020 International Society of Hypertension [Citation10]; and World Health Organisation Hypertension 2021 [Citation11]. Summarily, according to these recommendations, HTN treatment should be instituted as soon as possible, with a double combination (specifically, ACEI or ARA II), in association with a thiazide-like diuretic (indapamide or chlorthalidone) or a calcium channel antagonist/blocker (CCB). Of note, although HCT is not exiled from the first line therapy in the 2018 ESH/ESC and 2023 ESH guidelines, there is a controversy in this question between the available hypertension guidelines. Nonetheless, here the decision in this study was to change HCTZ for CCB.
The study was approved by the regional Ethics Committee Comissão de Ética para a Saúde da Unidade Local de Saúde (ULS) Matosinhos and by the Data Protection Officer (reference: 149/CES/JAS, on November 12th 2021). The study participants received information about the study’s procedures and objectives and agreed to participate by signing the voluntary informed consent form. The study was conducted according to the Helsinki Declaration and developed in compliance with the Portuguese General Data Protection Regulation.
Inclusion and exclusion criteria
At recruitment, study participants must had been between 18-75 years of age, without previous CV events, and with uncontrolled (sustained) hypertension (BP > 140/or > 90 mm Hg) as per OBP measurement at v0, being treated with either anti-HT monotherapies, or combinations with hydrochlorothiazide (HCTZ), or receiving no medication for HTN, for at least 2 months. Patients must already had been scheduled for consultation with their GP; that is, consultations were not purposedly scheduled for purposes of study recruitment. In consultation, electronic medical records were used to pre-identify and pre-screen potential study participants regarding inclusion and exclusion criteria, before approaching the patient for participation.
The following inclusion criteria were defined:
Hypertensive patients ≥18 years of age and ≤75 years of age;
Uncontrolled arterial blood pressure (see above) despite previously advised to healthy health styles, and/or medicated with monotherapies or associations of low doses of anti-hypertensives;
With heart rate, heart rhythm and physical conditions that allowed an adequate OBP recording;
Patients could be allocated to one the following categories:
Treated with any monotherapies of anti-hypertensives for at least 2 months and with uncontrolled HTN (BP > 140/or 90 mm Hg, mean of 2nd and 3rd records separated by 1 min);
Treated with a first-line anti-hypertensive association in inadequate regimens for at least 2 months and with uncontrolled HTN (BP > 140/or 90 mm Hg – mean 2nd and 3rd records separated by 1 min);
De novo hypertensive patients without therapeutic treatment and with BP ≥ 160/or ≥100 mm Hg (mean 2nd and 3rd records separated by 1 min).
The following exclusion criteria were defined:
BP ≥ 180/110 mm Hg;
BP <140/90 mm Hg or medicated with ARB/ACEin + Indapamide/Chlorotalidone/Calcium Antag in high dosages (e.g. lisinopril 20-40 mg or equivalent, olmesartan 40 mg or equivalent, amlodipine 10 mg or equivalent, chlorthalidone 25 mg or indapamide 2.5 mg/d);
Diagnosed stroke, congenital heart defects (CHD), dementia or heart failure;
Diagnosed neurological and psychiatric disease;
CKD - GFR <45 ml/min/1.73;
Pregnant, lactating or women of childbearing age without effective contraception;
Intolerance to ARB/ACEin or Calcium Antagonists;
Secondary hypertension (confirmed or suspected);
Arrhythmias that hindered accurate OBP recording;
Refusal to participate in the study;
Inability to sign informed consent;
Prevision to be unable to participate in the 3 evaluation moments.
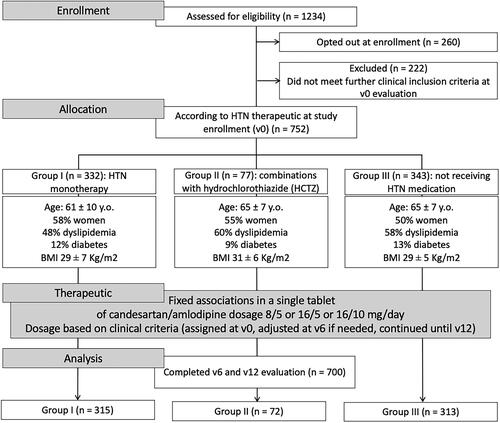
After inclusion and exclusion criteria, of the initial 1234 potential participants approached in consultation for participation in the study, 260 opted to not participate, and 222 who did not meet inclusion criteria upon further clinical evaluation. The remaining participants (752) were assigned to their study groups according to their current treatment: Group I, Monotherapy = 332; Group II, ARBs/ACEin with HCTZ = 77; Group III, Untreated = 343. Of these, a total of 700 completed the study (with assessment at v0, v6 and v12) (), while for 52 information was incomplete for one of more of the assessment points either because the patient missed the appointment, or the GP did not register information according to the study protocol guidelines. None of the patients who did not complete the study cited treatment intolerance as the underlying cause.
Office blood pressure (OBP) measurement
Study investigators (here, GPs) were trained in good practices for assessing blood pressure and in the study implementation protocol. OBP is considered the most common blood pressure measurement method [Citation15]. Since guidelines recommend certain measurement rules to achieve accurate measurements, OBP-procedures and settings were closely followed across health care centres and visits (consultations). In all study participants OBP measurement was carried out in an individual room (GP office), after a rest period, with an automatic device, with 3 repeated measurements.
Treatment protocol
The following exposure conditions were implemented:
Patients on anti-HTN monotherapy for at least 2 months and with BP ≥ 140 or ≥ 90 mm Hg (Group I, anti-HTN monotherapies): in the first assessment (v0) therapy was replaced with fixed associations in a single tablet (1st line anti-hypertensive tablet in the lower dose). In the second assessment (v6), if BP < 140/90 Hg therapy was maintained, and if BP ≥ 140 or ≥ 90 mm Hg therapy was optimised the medium dose;
Patients on anti-HTN low dose combined therapy for at least 2 months and with BP ≥ 140 or ≥ 90 mm Hg (Group II, combinations with HCTZ): in the first assessment (v0) therapy was replaced with fixed associations in a single tablet (1st line anti-hypertensive tablet in the medium dose). In the second assessment (v6), if BP < 140/90 Hg therapy was maintained, and if BP ≥ 140 or ≥ 90 mm Hg therapy was optimised to the higher dose;
Patients not treated with anti-HTN therapy for at least 2 months and with BP ≥ 160/100 mm Hg (Group III, not receiving medication): in the first assessment (v0) therapy was started with fixed associations in a single tablet (1st line anti-hypertensive tablet in the lower dose). In the second assessment (v6), if BP < 140/90 Hg therapy was maintained, and if BP ≥ 140 or ≥ 90 mm Hg therapy was optimised to a medium or higher dose (clinical criteria decision).
Thus, summarily, the following therapeutic adjustment were done in accordance with the best available evidence:
If 6 weeks BP < 140/90 mm Hg, therapy started at visit 0 (v0) was maintained;
If 6 weeks BP ≥ 140/or > = 90 mm Hg, therapy was optimised to the next higher dosage;
Where, low-, medium- and high- fixed combination candesartan/amlodipine dosage are, respectively, 8/5 or 16/5 or 16/10 mg/day (single tablet, morning intake).
Data collection
Each GP collected information, in paper format, in the following way:
The included patients were followed in 3 moments for 12 weeks (visits at 0, 6 and 12 weeks), and in each assessment had 3 in-office HTN measurements;
Arterial Blood Pressure (BP) record:
In the first assessment (v0), the BP was measured once in both arms, with the arm with the highest BP used in all further measurements and assessment moments;
The blood pressure records were carried out by a GP with a validated automatic device with the patient sitting and resting for 5 minutes (per described in [Citation10]);
In each session, 3 BP records were made, with an interval of 1 min in between. The last two records were considered the BP mean;
In each consultation, after the 3 records measures, an additional measurement was carried out after 1 min with the patient standing;
Any BP measures taken, by either a GP or the patient, in the 7 days prior to the consultation were registered.
Other measures:
In moment v0 (baseline) the following data were collected: BP; weight, height and BMI; date of birth, age and gender; habits and allergies; medication in progress; other clinical information and/or measures assessed within the previous 2 months (namely: haematocrit, blood glucose, creatinine, ionogram, uric acid, total Cholesterol, HDH, LDL-C and triglycerides (blood); Urinary sediment, and albumin/creatinine (occasional urine); ECG and Echo TT);
In moment v6 (week 6 after implementing protocol/starting treatment) the following data were collected: BP; weight, height and BMI; medication in progress; any adverse reactions to the implemented protocol; other clinical information and/or measures assessed within the previous 6 weeks;
In moment v12 (week 12 after implementing protocol/starting treatment) the following data were collected: BP; weight, height and BMI; medication in progress; any adverse reactions to the implemented protocol; other clinical information and/or measures assessed within the previous 6 weeks.
Data management and statistical analyses
Data for the GPHT-PT Study were collected through clinical evaluations, blood pressure measurements, patient characteristics, medication records, and other clinical measurements at three time points: baseline (v0), week 6 (v6), and week 12 (v12). The data underwent thorough cleaning procedures to rectify inconsistencies and missing values. Categorical variables were coded as necessary, and data were securely stored while maintaining patient confidentiality and adhering to data protection regulations.
Statistical analysis was performed using IBM SPSS Statistics (version 26). Descriptive statistics were computed to summarise the study population characteristics. The variation in control hypertension was analysed with Cochran’s Q test, which is used to determine if there are differences in a dichotomous dependent variable between three or more related groups.
Changes in blood pressure over time within each treatment group (v0, v6, v12) were analysed by a General Linear Model (GLM) with a Repeated Measures Multivariate Analysis of Covariance (MANCOVA) to assess the impact of the treatment across time while controlling for age and sex.
Results
Overall, across the three groups (GI, GII and GIII), there was an average decrease in blood pressure scores over time ().
Table 1. Systolic and diastolic blood pressure at baseline at baseline and at weeks 6 and 12 after study enrolment and implementation of the new therapeutic measures with one of 3 fixed combination ARB/amlodipine dosage (8/5 or 16/5 or 16/10 mg/day).a
The number of participants with controlled HTN increased from 0.8% to 81.6%. The non-parametric Cochran’s Q test suggests this is a statistically significant difference X2(2, N = 752) = 945.6, p < .001 ().
Table 2. Number (N) and percentage (%) of study participants with uncontrolled and controlled hypertension (HTN) at baseline and at weeks 6 and 12 after study enrolment and implementation of the new therapeutic measures with one of 3 fixed combination candesartan/amlodipine dosage (8/5 or 16/5 or 16/10 mg/day).
A Repeated Measures Multivariate Analysis of Covariance (MANCOVA) was performed to investigate the effectiveness of treatment in differences in arterial blood pressure across time while controlling for age and sex. Two dependent variables were used: Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP). The independent variable was group treatment (Group I, Group II, Group III) across time. Preliminary assumption testing was conducted to check for normality, linearity, univariate and multivariate outliers, homogeneity of variance-covariance matrices, and multicollinearity, with no serious violations noted. There was a statistically significant difference across time, F (4, 719) = 25.6, p < .001, Wilks’ Lambda = .88, indicating an overall decrease in blood pressure across groups with a large effect size (partial eta squared = .125). When the results for the two dependent variables were considered separately, both reached statistical significance for a decreasing linear trend: i) Systolic Blood Pressure, F (4, 719) = 27.2, p < .001, partial eta squared = .036; and ii) Diastolic Blood Pressure F (4, 719) = 78.4, p < .001, partial eta squared = .098.
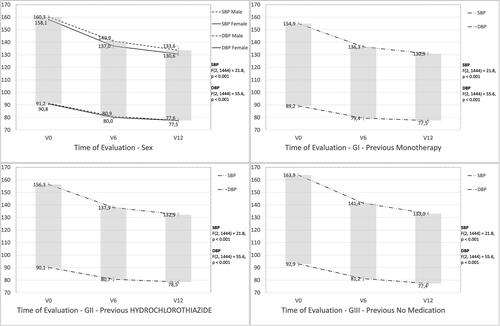
These results suggest the effectiveness of treatment across time, regardless of previous treatment group, age and sex (). An inspection of the estimated mean scores of the multivariate model indicated that Systolic Blood Pressure (SBP) dropped from M = 159.1 (± 12.9) to M = 131.9 (±11.1) and Diastolic Blood Pressure (DBP) dropped from M = 90.9 (± 9.7) to M = 77.6 (±8.8). A difference that is statistically and clinically significant.
Figure 2. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) across time by sex and study group.
Side effects and adverse reactions
No side effects or serious adverse reactions were reported during the study. None of the patients who did not complete the study cited treatment intolerance as the underlying cause.
Discussion
In the present study, in patients with uncontrolled HTN (under monotherapy, combinations with HCTZ or non-medicated), followed by GPs across primary health care units in Portugal, therapeutic measures in accordance with guidelines with a fixed combination candesartan/amlodipine allowed to achieve HTN control at 12 weeks in 82% of previously uncontrolled patients, reinforcing the advantages of implementing these strategies in primary clinical practice. Results also indicate that the presence of uncontrolled HTN may stem from non-compliance with the provisions of the recommendations and the Integrated Care Process (PAI) of the Direção Geral de Saúde (DGS), i.e. inappropriate use of monotherapies or inadequate low doses of combinations of antihypertensives. More so, under the present therapeutic scheme, which included close monitoring at fixed intervals, participant retention was 93% also pointing towards how relevant it is for the adjustment of HTN therapies, favouring the schemes provided in the recommendations, to be accompanied by close monitoring so that the patient remains engaged.
The study follows other findings that stress the need of effective, practical, and low-cost interventions, including in primary care settings, to promote better HTN control [Citation1] as are, for example, the larger reaching ‘Measure accurately, Act rapidly, and Partner with patients (MAP)’ study [Citation16] and the ‘Ayushman Bharat’ initiative [Citation17]. While it is widely recognised that treatment of HTN is highly effective [Citation18], numerous barriers may account for the low rates of blood pressure control in Portugal.
Regardless of the underlying cause for uncontrolled HTN in Portugal, a nation-wide survey of hypertension conducted in 2003 showed an age-adjusted prevalence of hypertension of 42.1%, and a level of hypertension control of 11.2% [Citation19]. A decade later, a second country-wide study (the ‘Portuguese HYpertension and SAlt (PHYSA) Study’ [Citation4]) showed that the overall prevalence of hypertension remained similar at 42.2%; where, among the hypertensive patients, 76.6% were aware of the hypertension condition, 74.9% were treated and 42.5% were controlled, that is, respectively, 1.7, 1.9 and 3.8 times higher than the previous data in 2003. More so, the percentage of hypertensive patients under treatment who were controlled almost doubled from 2003 to 2012, and patients with adequate control of hypertension were more frequently treated with combination of antihypertensive drugs (65% fixed combinations), indicating that progress can be attained with an efficient and methodical treatment protocols. Such measures should include, but not be limited to, an increase in use of antihypertensive medications, or introduction of new therapeutic approaches, as our present study indicates, not dismissing the importance of health literacy and access to primary healthcare.
Of relevance, the American as well as the ESH guidelines still mention as initial therapies to include at least one of four major classes: angiotensin-converting enzyme inhibitors (ACEIs), angiotensin-receptor blockers (ARBs), thiazide or thiazide-like diuretics, and CCBs. Some other guidelines indeed stress really stress the preference of first CCB and then thiazide-like. More so, in people with tendency to edoema it is clinical practice to choose HCTZ/HCTZ-like diuretic rather than for CCB, with many combination pills still containing hydrochloorthiazide. Nonetheless, here we have opted for CCB. Furthermore, several study limitations are of care. Albeit longitudinal, prospective, and experimental, the GPHT-PT Study had an open design and, due to ethical considerations, control groups were not established. That is, groups of participants similar in age, sex, and clinical status, to groups GI, GII or GIII, followed throughout the 12-week study period, but not started on the fixed combination candesartan/amlodipine therapeutic protocol, particularly relevant when knowing that team-based care is among the most effective interventions for controlling blood pressure [Citation20]. More so, the study sample may not be representative of the total adult Portuguese population for duration of HTN, including controlled and uncontrolled periods, and/or present HTN age-prevalence (in 2012 it was 6.8, 46.9 and 74.9% in people below 35 years, 35–64 years and above 64 years [Citation4]), as present country data does not allow to completely analyse for this, and it did not include certain age-groups (<18 and >75 years of age) or clinical conditions. It is also of consideration that adhesion to the medication scheme could only be ascertained from pharmacy record (medication dispensed) and what was voiced by the participant as his/her daily adherence. Finally, the ‘white coat’ effect could be a confounder. Finally, the authors recognise that close monitoring and patient engagement are crucial, but that while here the results are of relevance for the study time frame (3 months), a longer study would be pertinent and necessary, as many therapies fail in the first year. Nonetheless, study strengths are of note. The GPHT-PT Study involved a total of 118 GPs, across local health care units in Portugal, without any significant difference between GPs, centres, sex and age. Results were consistent throughout observations (time and biological gradient, dose-response), with an increasing, and significant, achievement of HTN control as per guidelines, upon the start of the candesartan/amlodipine therapeutic protocol. Further studies should aim to scale in time, further enhancing hypertension beyond 12 weeks, maintaining the strategy to measure accurately, comply with the recommendations and the Integrated Care Process (PAI) of the Portuguese DGS, and partnering with hypertensive patients in their care through close monitoring.
Take-home message
In patients with uncontrolled HTN (under monotherapies, or combinations with HCTZ, or non-medicated) followed by GPs, therapeutic measures implemented in accordance with guidelines with a fixed combination candesartan/amlodipine allowed to achieve HTN control at 12 weeks in 82% of previously uncontrolled patients;
Results reinforce the advantages of implementing guideline-based strategies in primary clinical practice;
Easy to follow therapeutic strategies, by both doctors and patients, along with more frequent than usual medical visits, may have contributed to the observed success.
List of investigators on behalf of the GPHT-PT study
Alexandra Soares; Ana Catarina Silva; Ana Elisa Serodio; Ana Ernesto; Ana Gonçalves; Ana Inês Letra; Ana Laura Serranito; Ana Macedo; Ana Rita Faustino; Ana Sofia Carvalho; Anabela Pena; André Coelho Santos; Andreína Fernandes; Ângelo Afonso; António Rodrigues; Arturas Slidziauskas; Bruno Cerca; Casimiro Correlo; Catarina Avillez; Catarina Duarte Silva; Catarina Oliveira; Catarina Silva; Cátia Matos; Celine Mendes; Célia Barros; Cláudia Bessa; Cláudia Silva; David Fontinha; Diana Rato; Diogo Amaral; Diogo C. Tavares; Ecaterina Mereacre; Eduarda Martins; Ermelinda Alves; Elisa Serôdio; Fábio Leite Costa; Fernanda Luís; Fernando Ribeiro; Filipa Faria; Filipe Melo; Francisca Mateus; Francisco Costa; Halane Tiny; Helena Ribeiro; Idalina Lima; Inês Calvinho; Isabel Faria; Ivo Carneiro; Joana Azeredo; Joana Cirne; Joana F. L. Ribeiro; Joana Gonçalves; Joana Paço; Joana Freitas Sanches; João Dias; João Girão; João Lima; João Palas; João Vitor Dinis; Jorge da Cunha; Jorge Isaías; José Augusto Santos; José Bastos Silva; José Mário Costa; José Tiago Teixeira; Juan Hernandez; Júlio Becker; Liliana Moita; Madalena Rodrigues; Mafalda Macedo; Marcelo Alfar; Maria Luís; Maria João Augusto; Maria João Loureiro; Maria João Martins; Maria José Correia; Mariana Amaral; Mariana Pinho Pereira; Mariana Rocha Silva; Marisa Gonçalves; Marta Ribeiro; Mercedes Bravo; Micaela Oliveira; Miguel Cancela; Miguel Pereira; Nelylena Costa; Nuno Amaral; Nuno Namora; Nuno Pinto; Oleh Yaremiy; Olena Lourenço; Patricia Peixoto Oliveira; Paulo Colunas; Pedro Azevedo; Pedro Barreira; Pedro Capelo; Pedro Oliveira; Pedro Seabra; Pedro Silva; Punit Naguindás; Raquel Pinto; Rayda Hernandez; Ricardo Silva; Rita João Caiado; Rita Xavier; Roberto Rodrigues; Rui Cabral Monteiro; Rui Guedes; Rui Santos; Rute Marques; Sara Correia; Sara Loureiro Brandão; Sara Robalo Santos; Sebastian Pena; Silvia Silva; Sónia Moreira; Susana Corte Real; Susana Fernandes; Tania Mendes Serrão; Teresa Cruz; Tiago Fernandes; Vadym Sosnovskyi; Valter Alves dos Santos; Valter Moreira.
Acknowledgments
We express our gratitude to the Association P5 Digital Medical Center (ACMP5) for their valuable assistance in the writing and data analysis of the manuscript. Additionally, we extend our thanks to all the General Practitioners (GPs) who actively participated in patient recruitment and data collection, as well to all study participants.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Blood Pressure Lowering Treatment Trialists’ Collaboration. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021;397(10285):1625–1636. doi:10.1016/S0140-6736(21)00590-0. Erratum in: lancet 2021; 397(10288):1884.
- Joffres M, Falaschetti E, Gillespie C, et al. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: a cross-sectional study. BMJ Open. 2013;3(8):e003423. doi:10.1136/bmjopen-2013-003423.
- Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies From 90 countries. Circulation. 2016;134(6):441–450. doi:10.1161/CIRCULATIONAHA.115.018912.
- Polonia J, Martins L, Pinto F, et al. Prevalence awareness, treatment and control of hypertension and salt intake in Portugal: changes over a decade. The PHYSA study. J Hypertens. 2014;32(6):1211–1221. doi:10.1097/HJH.0000000000000162.
- Processo Assistencial Integrado do Risco Vascular no Adulto. Direção-Geral da Saúde (DGS), Departamento da Qualidade na Saúde 2014. ISBN 978-972-675-212-7. Available at: https://www.aenfermagemeasleis.pt/2015/01/05/processo-assistencial-integrado-do-risco-cardiovascular-no-adulto/amp/.
- Guideline for the pharmacological treatment of hypertension in adults. Geneva: World Health Organization; 2021. ISBN-13: 978-92-4-003398-6ISBN-13: 978-92-4-003397-9
- Lopes E, Alarcão V, Simões R, et al. Hypertension control at the primary health care: a comparison among Portuguese natives and Portuguese speaking African coutries immigrants. Acta Med Port. 2016;29(3):193–204. doi:10.20344/amp.6714.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71(6):e13–e115. doi:10.1161/HYP.0000000000000065.
- Williams B, Mancia G, Spiering W, Authors/Task Force Members., et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). J Hypertens. 2018;36(10):1953–2041. doi:10.1097/HJH.0000000000001940.
- Unger T, Borghi C, Charchar F, et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi:10.1161/HYPERTENSIONAHA.120.15026.
- Campbell NRC, Paccot Burnens M, Whelton PK, et al. 2021 World health organization guideline on pharmacological treatment of hypertension: policy implications for the region of the americas. Lancet Reg Health Am. 2022;9. doi:10.1016/j.lana.2022.100219.
- Roush GC, Buddharaju V, Ernst ME. Is chlorthalidone better than hydrochlorothiazide in reducing cardiovascular events in hypertensives? Curr Opin Cardiol. 2013;28(4):426–432. doi:10.1097/HCO.0b013e3283622075.
- DiNicolantonio JJ, Bhutani J, Lavie CJ, et al. Evidence-based diuretics: focus on chlorthalidone and indapamide. Future Cardiol. 2015;11(2):203–217. doi:10.2217/fca.14.83.
- Jamerson K, Weber MA, Bakris GL; ACCOMPLISH Trial Investigators., et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417–2428. doi:10.1056/NEJMoa0806182.
- Stergiou GS, Palatini P, Parati G; European Society of Hypertension Council and the European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability., et al. 2021 European society of hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens. 2021;39(7):1293–1302. doi:10.1097/HJH.0000000000002843.
- Egan BM, Sutherland SE, Rakotz M, et al. Improving hypertension control in primary care with the measure accurately, act rapidly, and partner with patients protocol. Hypertension. 2018;72(6):1320–1327. doi:10.1161/HYPERTENSIONAHA.118.11558.
- Satish P, Khetan A, Raithatha S, et al. Standardizing hypertension management in a primary care setting in India through a protocol based model. Indian Heart J. 2019;71(5):375–380. doi:10.1016/j.ihj.2019.11.257.
- Pfeffer MA, McMurray JJ. Lessons in uncertainty and humility—clinical trials involving hypertension. N Engl J Med. 2016;375(18):1756–1766. doi:10.1056/NEJMra1510067.
- Macedo ME, Lima MJ, Silva AO, et al. Prevalence, awareness, treatment and control of hypertension in Portugal: the PAP study. J Hypertens. 2005;23(9):1661–1666. doi:10.1097/01.hjh.0000179908.51187.de.
- Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care. 2006;44(7):646–657. doi:10.1097/01.mlr.0000220260.30768.32.